Abstract
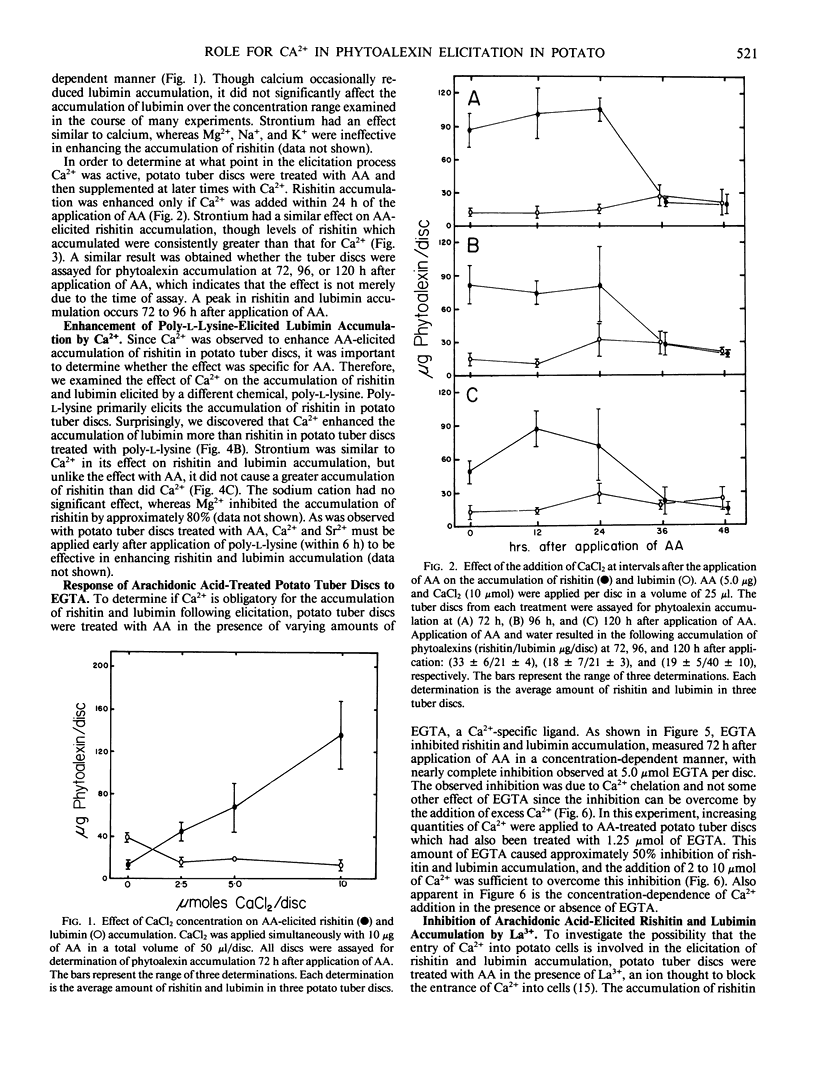
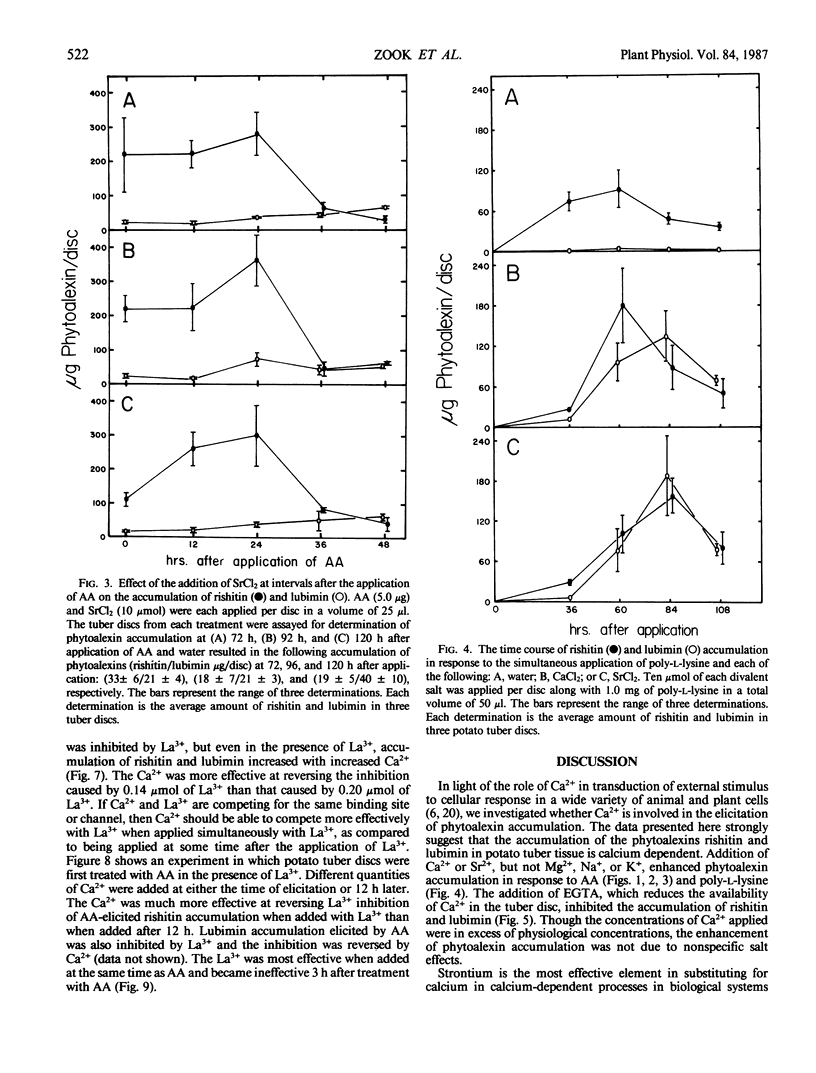
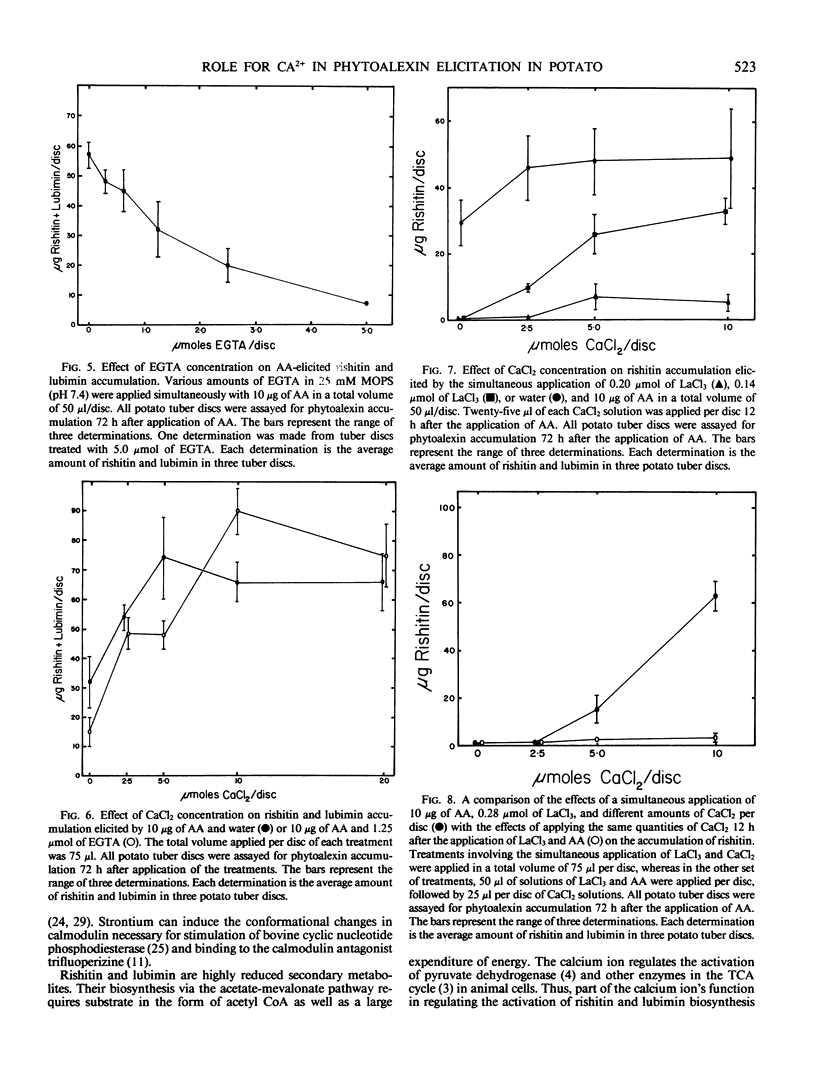
Calcium and strontium ions enhanced rishitin but not lubimin accumulation in tuber tissue of potato (Solanum tuberosum cv Kennebec) treated with arachidonic acid (AA). The same cations in the presence of poly-l-lysine (PL) enhanced the accumulation of lubimin more than rishitin. In contrast, Mg2+ did not affect AA-elicited rishitin and lubimin accumulation and inhibited the accumulation of these compounds following application of PL. AA-elicited potato tuber tissue remained sensitive to the stimulatory effects of Ca2+ and Sr2+ up to 24 h after application of AA, but PL-elicited tuber tissue was sensitive to Ca2+ and Sr2+ for only 6 hours after PL application. Ethyleneglycol-bis (β-aminoethyl ether)-N,N′-tetraacetic acid and La3+ both inhibited rishitin and lubimin accumulation elicited by AA. The inhibition by either agent was overcome by the addition of Ca2+. Calcium was more effective in overcoming lanthanum inhibition when applied simultaneously than when applied 12 hours later. Lanthanum was only effective in inhibiting rishitin and lubimin accumulation when applied within 3 hours of the application of AA. Inhibition of phytoalexin accumulation was greater when La3+ was applied simultaneously with AA compared to La3+ application after AA application to discs. These observations suggest that the mobilization of calcium may play a central regulatory role in the expression of phytoalexin accumulation following elicitation in potato tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Charbonneau H., Jones H. P., McCann R. O., Cormier M. J. Characterization of the plant nicotinamide adenine dinucleotide kinase activator protein and its identification as calmodulin. Biochemistry. 1980 Jun 24;19(13):3113–3120. doi: 10.1021/bi00554a043. [DOI] [PubMed] [Google Scholar]
- Bostock R. M., Kuc J. A., Laine R. A. Eicosapentaenoic and Arachidonic Acids from Phytophthora infestans Elicit Fungitoxic Sesquiterpenes in the Potato. Science. 1981 Apr 3;212(4490):67–69. doi: 10.1126/science.212.4490.67. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Edgell N. J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980 Jul 15;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman F. R., Weiss G. B. Contractile responses and 45Ca movements in monkey ileal smooth muscle. Arch Int Pharmacodyn Ther. 1974 May;209(1):14–25. [PubMed] [Google Scholar]
- Hansford R. G., Castro F. Role of Ca2+ in pyruvate dehydrogenase interconversion in brain mitochondria and synaptosomes. Biochem J. 1985 Apr 1;227(1):129–136. doi: 10.1042/bj2270129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakschik B. A., Sun F. F., Lee L., Steinhoff M. M. Calcium stimulation of a novel lipoxygenase. Biochem Biophys Res Commun. 1980 Jul 16;95(1):103–110. doi: 10.1016/0006-291x(80)90710-x. [DOI] [PubMed] [Google Scholar]
- Kauss H. Volume regulation in poterioochromonas: involvement of calmodulin in the ca-stimulated activation of isofloridoside-phosphate synthase. Plant Physiol. 1983 Jan;71(1):169–172. doi: 10.1104/pp.71.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Köhle H., Jeblick W., Poten F., Blaschek W., Kauss H. Chitosan-elicited callose synthesis in soybean cells as a ca-dependent process. Plant Physiol. 1985 Mar;77(3):544–551. doi: 10.1104/pp.77.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol Pharmacol. 1977 Jul;13(4):690–697. [PubMed] [Google Scholar]
- Ranjeva R., Refeno G., Boudet A. M., Marmé D. Activation of plant quinate:NAD 3-oxidoreductase by Ca and calmodulin. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5222–5224. doi: 10.1073/pnas.80.17.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Saunders M. J., Hepler P. K. Calcium antagonists and calmodulin inhibitors block cytokinin-induced bud formation in Funaria. Dev Biol. 1983 Sep;99(1):41–49. doi: 10.1016/0012-1606(83)90252-x. [DOI] [PubMed] [Google Scholar]
- Saunders M. J., Hepler P. K. Calcium ionophore a23187 stimulates cytokinin-like mitosis in funaria. Science. 1982 Sep 3;217(4563):943–945. doi: 10.1126/science.217.4563.943. [DOI] [PubMed] [Google Scholar]
- Serlin B. S., Roux S. J. Modulation of chloroplast movement in the green alga Mougeotia by the Ca2+ ionophore A23187 and by calmodulin antagonists. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6368–6372. doi: 10.1073/pnas.81.20.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo T. S., Wang J. H. Mechanism of activation of a cyclic adenosine 3':5'-monophosphate phosphodiesterase from bovine heart by calcium ions. Identification of the protein activator as a Ca2+ binding protein. J Biol Chem. 1973 Sep 10;248(17):5950–5955. [PubMed] [Google Scholar]


