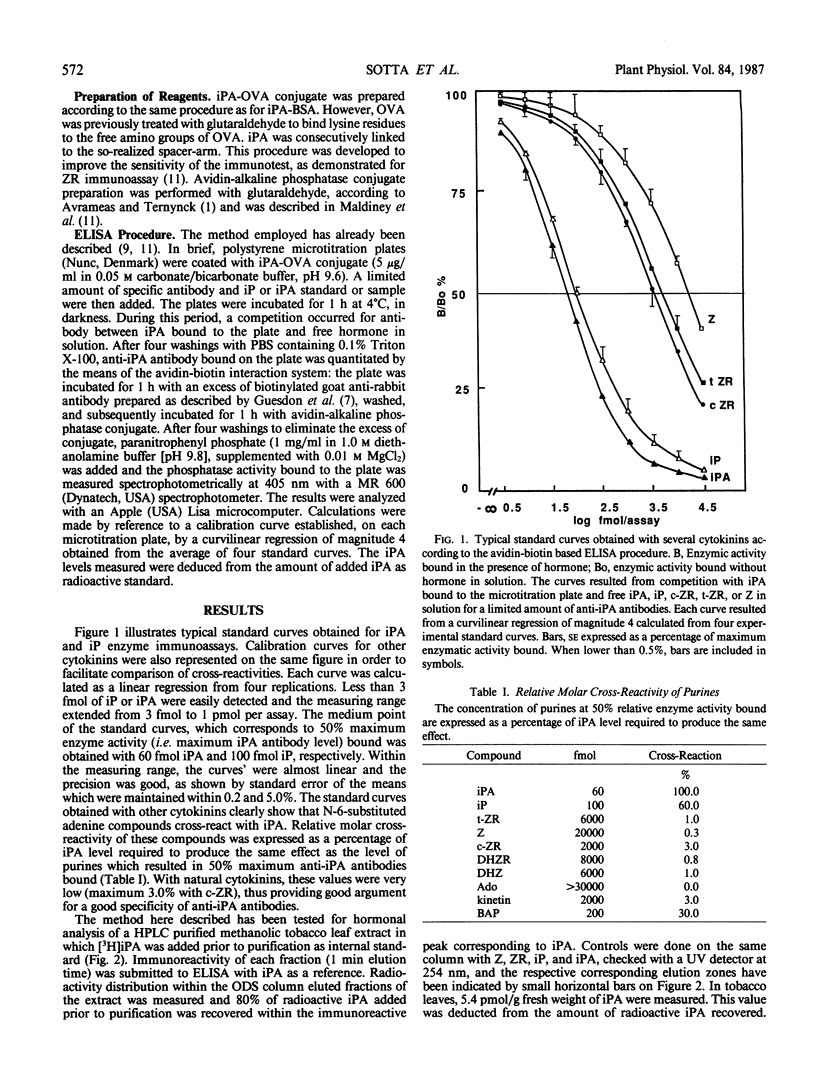
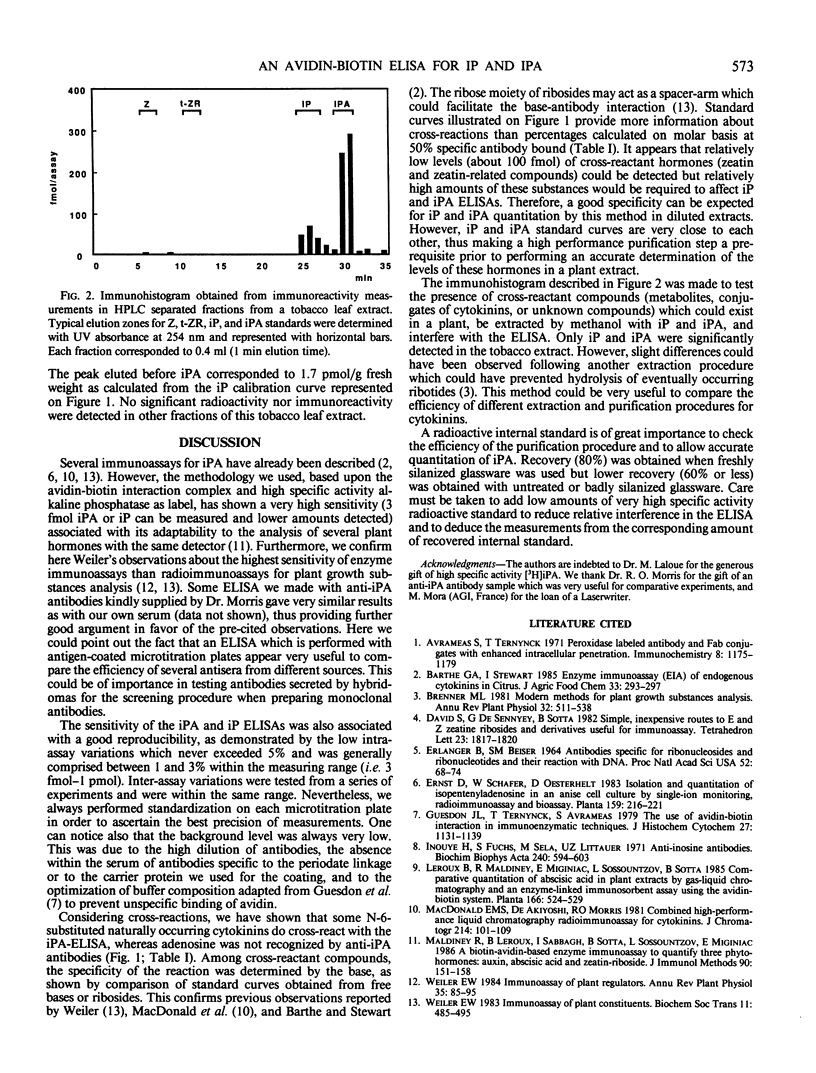
Abstract
A solid phase enzyme immunoassay was developed for isopentenyladenine (iP) and isopentenyladenosine (iPA) quantitation in HPLC purified plant extracts. It was performed on antigen-coated microtitration plates on which bound antibodies were indirectly labeled by the means of a biotinylated goat anti-rabbit antibody and an avidin-alkaline phosphatase conjugate. Less than 3 femtomoles of iP or iPA were easily detected and the measuring range extended from 3 femtomole to 1 picomole. The reproducibility has been tested and intra- and interassay variations did not exceed 5.0%. The specificity of iPA antibodies was good, as determined by cross-reactivity measurements with other adenylic compounds. The specificity of the measurements for iP and iPA was demonstrated by analysis of the immunoreactivity of fractions obtained by HPLC separation of a methanolic tobacco leaf extract.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Bartolf M., Brennan E., Price C. A. Partial Characterization of a Cadmium-binding Protein from the Roots of Cadmium-treated Tomato. Plant Physiol. 1980 Sep;66(3):438–441. doi: 10.1104/pp.66.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANGER B. F., BEISER S. M. ANTIBODIES SPECIFIC FOR RIBONUCLEOSIDES AND RIBONUCLEOTIDES AND THEIR REACTION WITH DNA. Proc Natl Acad Sci U S A. 1964 Jul;52:68–74. doi: 10.1073/pnas.52.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw B. A., Johnson M. A. The effect of glutathione on development in wild carrot suspension cultures. Biochem Biophys Res Commun. 1985 Dec 31;133(3):988–993. doi: 10.1016/0006-291x(85)91233-1. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Grill E., Winnacker E. L., Zenk M. H. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci U S A. 1987 Jan;84(2):439–443. doi: 10.1073/pnas.84.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E., Winnacker E. L., Zenk M. H. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science. 1985 Nov 8;230(4726):674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Hallenbeck W. H. Human health effects of exposure to cadmium. Experientia. 1984 Feb 15;40(2):136–142. doi: 10.1007/BF01963576. [DOI] [PubMed] [Google Scholar]
- Inouye H., Fuchs S., Sela M., Littauer U. Z. Anti-inosine antibodies. Biochim Biophys Acta. 1971 Jul 29;240(4):594–603. doi: 10.1016/0005-2787(71)90717-9. [DOI] [PubMed] [Google Scholar]
- Jackson P. J., Roth E. J., McClure P. R., Naranjo C. M. Selection, Isolation, and Characterization of Cadmium-Resistant Datura innoxia Suspension Cultures. Plant Physiol. 1984 Aug;75(4):914–918. doi: 10.1104/pp.75.4.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murasugi A., Wada C., Hayashi Y. Cadmium-binding peptide induced in fission yeast, Schizosaccharomyces pombe. J Biochem. 1981 Nov;90(5):1561–1564. doi: 10.1093/oxfordjournals.jbchem.a133627. [DOI] [PubMed] [Google Scholar]
- Rauser W. E. Isolation and Partial Purification of Cadmium-Binding Protein from Roots of the Grass Agrostis gigantea. Plant Physiol. 1984 Apr;74(4):1025–1029. doi: 10.1104/pp.74.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R. N., Wagner G. J. Properties of tobacco (Nicotiana tabacum) cadmium-binding peptide(s). Unique non-metallothionein cadmium ligands. Biochem J. 1987 Feb 1;241(3):641–647. doi: 10.1042/bj2410641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock J. C. Cadmium in foods and the diet. Experientia. 1984 Feb 15;40(2):152–156. doi: 10.1007/BF01963578. [DOI] [PubMed] [Google Scholar]
- Steffens J. C., Hunt D. F., Williams B. G. Accumulation of non-protein metal-binding polypeptides (gamma-glutamyl-cysteinyl)n-glycine in selected cadmium-resistant tomato cells. J Biol Chem. 1986 Oct 25;261(30):13879–13882. [PubMed] [Google Scholar]
- Wagner G. J. Characterization of a cadmium-binding complex of cabbage leaves. Plant Physiol. 1984 Nov;76(3):797–805. doi: 10.1104/pp.76.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. J., Trotter M. M. Inducible cadmium binding complexes of cabbage and tobacco. Plant Physiol. 1982 Apr;69(4):804–809. doi: 10.1104/pp.69.4.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel H. J., Jäger H. J. Subcellular distribution and chemical form of cadmium in bean plants. Plant Physiol. 1980 Mar;65(3):480–482. doi: 10.1104/pp.65.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler E. W. Immunoassay of plant constituents. Phytochemical society Tate and Lyle lecture. Biochem Soc Trans. 1983 Oct;11(5):485–495. doi: 10.1042/bst0110485. [DOI] [PubMed] [Google Scholar]


