Abstract
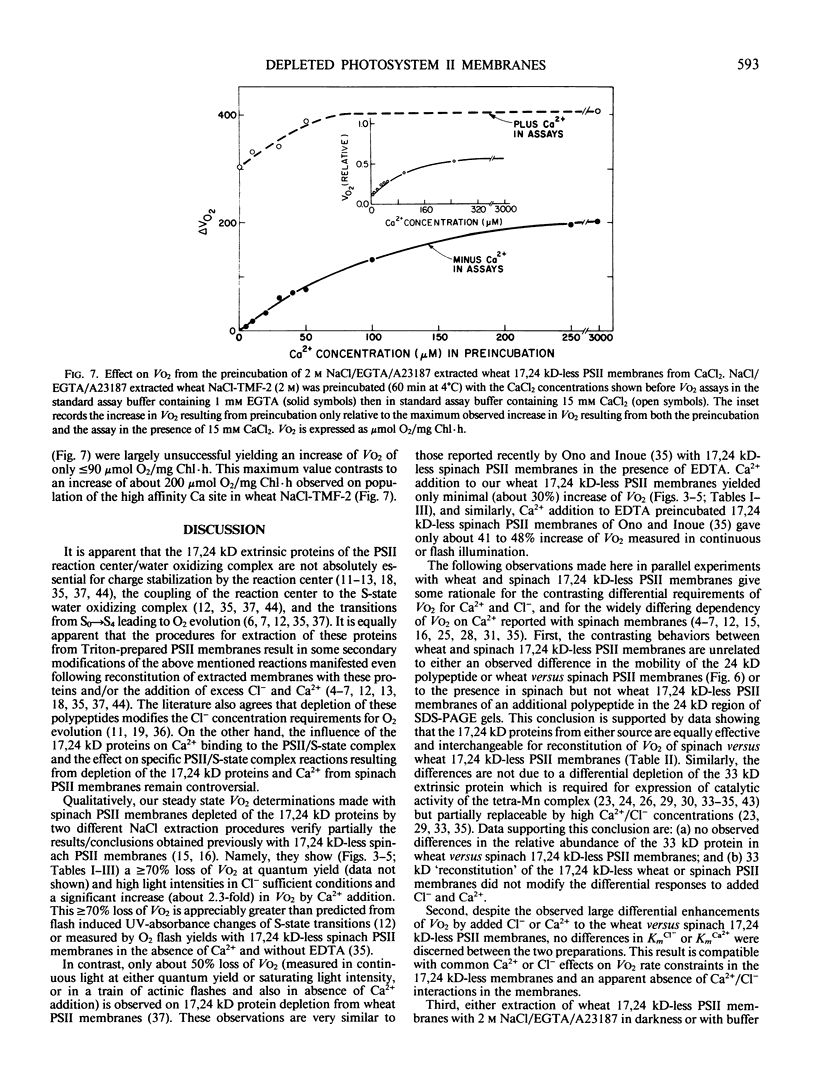
Analyses were made of the effects of extraction of the 17,24 kilodalton extrinsic proteins from spinach versus wheat photosystem II (PSII) membranes on Ca abundance and O2 evolution capacity determined in the absence and presence of either Cl− or Ca2+. Extraction of these proteins from spinach PSII routinely diminished steady state O2 evolution by about 70% when assayed in the presence of sufficient Cl−. Additionally, O2 evolution of 17,24 kilodalton-less spinach PSII membranes showed about 2-fold more enhancement by Ca2+ than by Cl− during assay. When the same extraction and assay procedures were applied to wheat PSII membranes, we observed, in contrast to 17,24 kilodalton-less spinach PSII, only about 50% inhibition of O2 evolution and about 2-fold greater enhancement by Cl− than by Ca2+. Irrespective of differences in the magnitude of enhancement of O2 evolution by Ca2+versus Cl− in spinach versus wheat, the Km values for Cl− (about 1.7 millimolar) and Ca2+ (about 1.5 millimolar) were similar for both type preparations. The abundance of Ca specifically associated with fully functional PSII (about 2 and about 3 Ca/200 chlorophyll for spinach and wheat, respectively) was diminished to about 1 per 200 chlorophyll upon 17.24 kilodalton protein depletion. Further treatment of wheat 17,24 kilodalton-less PSII in darkness with 2 molar NaCl/1 millimolar ethyleneglycol-bis(β-aminoethyl ether)-N,N′-tetraacetic acid/20 micromolar A231872 made O2 evolution highly dependent on Ca2+ addition, much like the 17,24 kilodalton-less spinach PSII. Analyses of this Ca2+ effect on O2 evolution revealed both high (Km about 65 micromolar) and low (Km about 1.5 millimolar) affinity Ca2+ sites in wheat 17,24 kilodalton-less PSII. The results suggest that during 17,24 kilodalton extraction by NaCl, spinach PSII is more susceptible than wheat PSII to loss of high affinity Ca and irreversible inhibition of O2 evolution.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blough N. V., Sauer K. The effects of mono- and divalent salts on the O2-evolution activity and low temperature multiline EPR spectrum of Photosystem II preparations from spinach. Biochim Biophys Acta. 1984 Nov 26;767(2):377–381. doi: 10.1016/0005-2728(84)90208-1. [DOI] [PubMed] [Google Scholar]
- Boska M., Blough N. V., Sauer K. The effect of mono- and divalent salts on the rise and decay kinetics of EPR signal II in Photosystem II preparations from spinach. Biochim Biophys Acta. 1985 Jun 26;808(1):132–139. doi: 10.1016/0005-2728(85)90035-0. [DOI] [PubMed] [Google Scholar]
- Govindjee, Kambara T., Coleman W. The electron donor side of photosystem II: the oxygen evolving complex. Photochem Photobiol. 1985 Aug;42(2):187–210. doi: 10.1111/j.1751-1097.1985.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Jansson C., Hansson O., Akerlund H. E., Andreasson L. E. EPR studies on the photosystem II donor side in salt-washed and reconstituted inside-out thylakoids. Biochem Biophys Res Commun. 1984 Oct 15;124(1):269–276. doi: 10.1016/0006-291x(84)90947-1. [DOI] [PubMed] [Google Scholar]
- Kok B., Forbush B., McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem Photobiol. 1970 Jun;11(6):457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Nakatani H. Y. Photosynthetic oxygen evolution does not require the participation of polypeptides of 16 and 24 kilodaltons. Biochem Biophys Res Commun. 1984 Apr 16;120(1):299–304. doi: 10.1016/0006-291x(84)91448-7. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]