Abstract
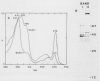
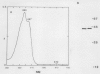
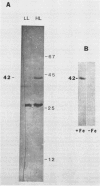
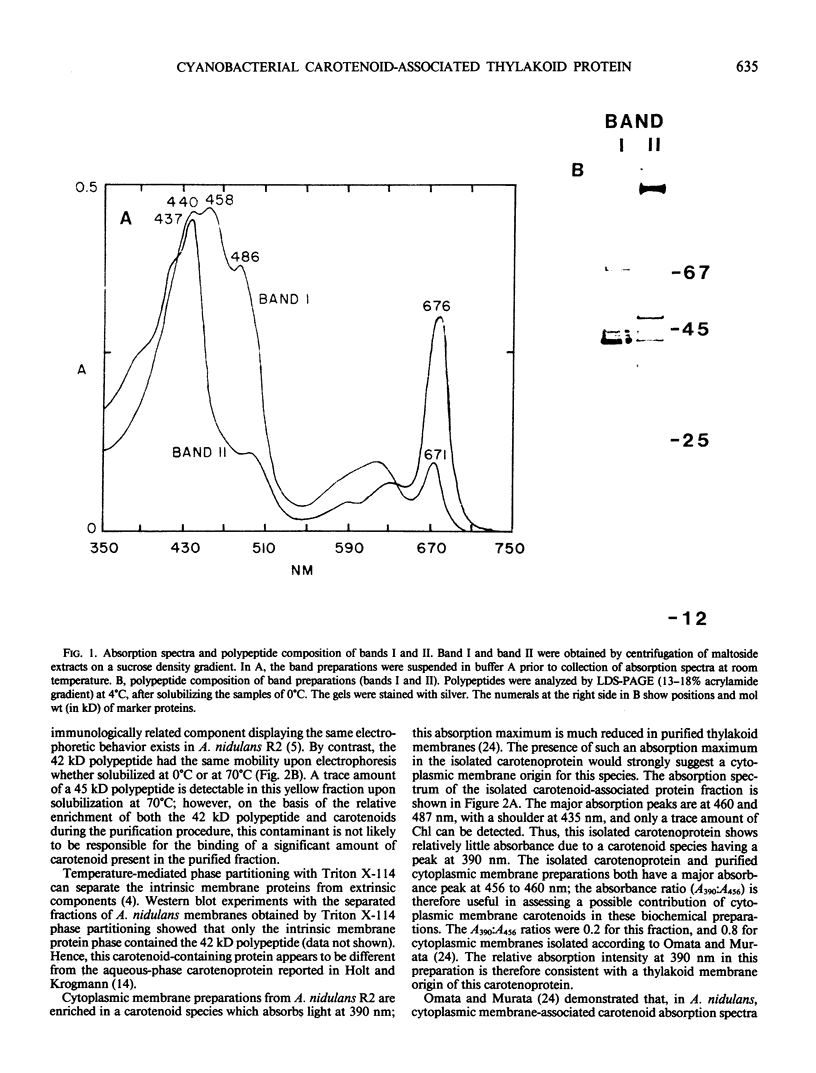
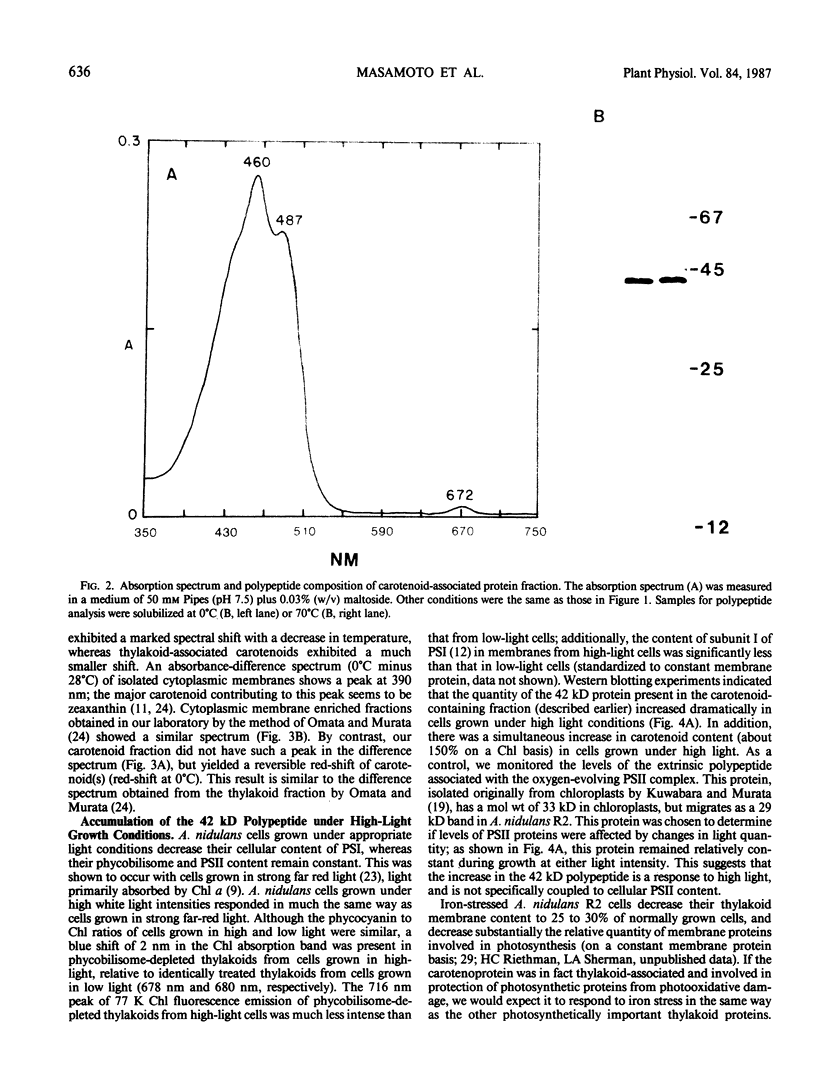
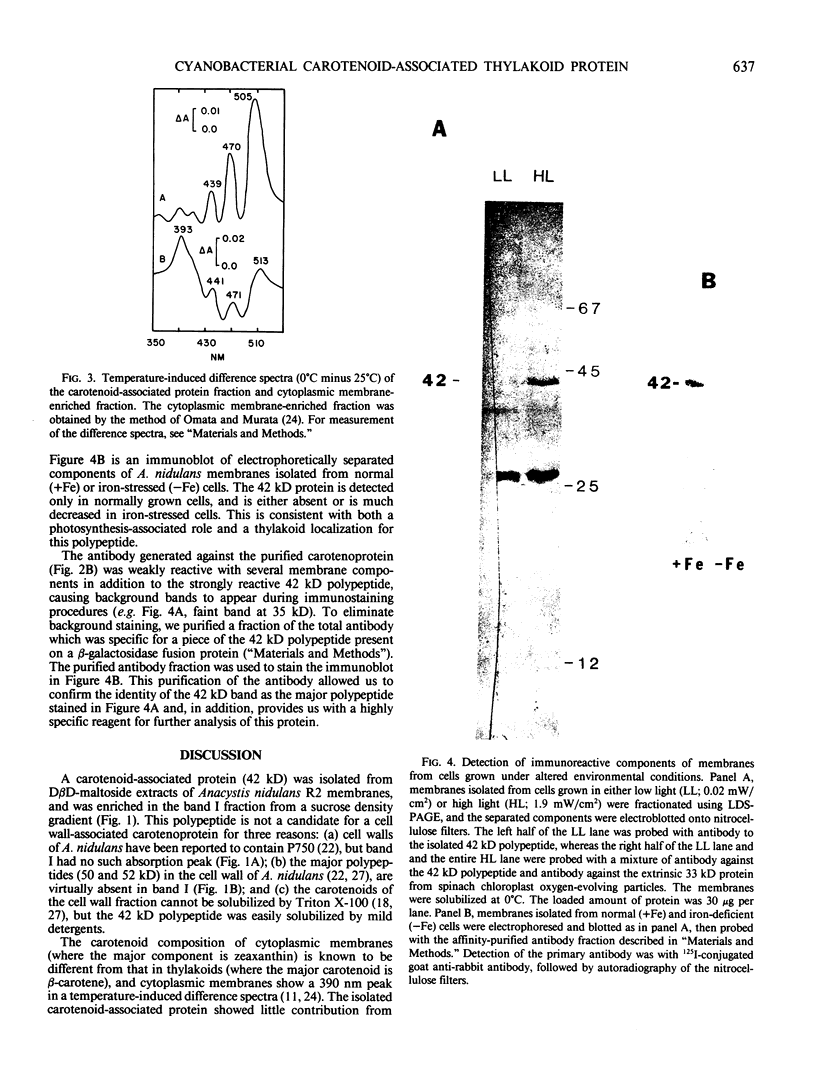
A carotenoid-associated membrane protein was isolated from Anacystis nidulans R2 thylakoids. Sodium pyrophosphate and sodium bromide washed thylakoids were solubilized with the nonionic detergents dodecyl-β-D-maltoside and octyl-β-D-glucopyranoside, and these detergent extracts were fractionated on a sucrose density gradient. A yellow fraction from the sucrose gradient was further purified by anion-exchange and organomercuric-affinity column chromatography to yield a fraction virtually free of chlorophyll and highly enriched in both carotenoids and a 42 kilodalton polypeptide. Evidence presented in this paper suggests that the carotenoid-containing 42 kilodalton protein is thylakoid associated rather than cytoplasmic membrane associated. The purified 42 kilodalton polypeptide was used to raise polyclonal antibodies in rabbits. Immuno-chemical detection of the 42 kilodalton polypeptide on Western blots demonstrated an increased accumulation of this polypeptide in cells grown under high-light conditions relative to cells grown under low light.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., McSwain B. D., Tsujimoto H. Y., Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974 Aug 23;357(2):231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Bricker T. M., Sherman L. A. Triton X-114 phase fractionation of membrane proteins of the cyanobacterium Anacystis nidulans R2. Arch Biochem Biophys. 1984 Nov 15;235(1):204–211. doi: 10.1016/0003-9861(84)90269-8. [DOI] [PubMed] [Google Scholar]
- Bullerjahn G. S., Sherman L. A. Identification of a carotenoid-binding protein in the cytoplasmic membrane from the heterotrophic cyanobacterium Synechocystis sp. strain PCC6714. J Bacteriol. 1986 Jul;167(1):396–399. doi: 10.1128/jb.167.1.396-399.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Blomberg F. Immunochemical studies of thylakoid membrane polypeptides from spinach and Chlamydomonas reinhardtii. A modified procedure for crossed immunoelectrophoresis of dodecyl sulfate.protein complexes. J Biol Chem. 1979 Jan 10;254(1):215–223. [PubMed] [Google Scholar]
- Gombos Z., Vigh L. Primary Role of the Cytoplasmic Membrane in Thermal Acclimation Evidenced in Nitrate-Starved Cells of the Blue-Green Alga, Anacystis nidulans. Plant Physiol. 1986 Feb;80(2):415–419. doi: 10.1104/pp.80.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Influence of Iron Deprivation on the Membrane Composition of Anacystis nidulans. Plant Physiol. 1984 Jan;74(1):90–95. doi: 10.1104/pp.74.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens U. J., Weckesser J. Carotenoid-containing outer membrane of Synechocystis sp. strain PCC6714. J Bacteriol. 1985 Oct;164(1):384–389. doi: 10.1128/jb.164.1.384-389.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T., Murata N. Purification and characterization of 33 kilodalton protein of spinach chloroplasts. Biochim Biophys Acta. 1979 Dec 14;581(2):228–236. doi: 10.1016/0005-2795(79)90242-3. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Myers J., Graham J. R., Wang R. T. Light Harvesting in Anacystis nidulans Studied in Pigment Mutants. Plant Physiol. 1980 Dec;66(6):1144–1149. doi: 10.1104/pp.66.6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi H. B., Riethman H. C., Sherman L. A. Organization of pigment proteins in the photosystem II complex of the cyanobacterium Anacystis nidulans R2. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6903–6907. doi: 10.1073/pnas.82.20.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch C. M., Gibson J. Isolation of the carotenoid-containing cell wall of three unicellular cyanobacteria. J Bacteriol. 1983 Jul;155(1):345–350. doi: 10.1128/jb.155.1.345-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Filamentous phage pre-coat is an integral membrane protein: analysis by a new method of membrane preparation. Cell. 1982 Jan;28(1):177–184. doi: 10.1016/0092-8674(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Sherman D. M., Sherman L. A. Effect of iron deficiency and iron restoration on ultrastructure of Anacystis nidulans. J Bacteriol. 1983 Oct;156(1):393–401. doi: 10.1128/jb.156.1.393-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons M., Swysen C., Sybesma C. The light-induced carotenoid absorbance changes in Rhodopseudomonas sphaeroides: an analysis and interpretation of the band shifts. Biochim Biophys Acta. 1977 Dec 23;462(3):706–717. doi: 10.1016/0005-2728(77)90112-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]