Abstract
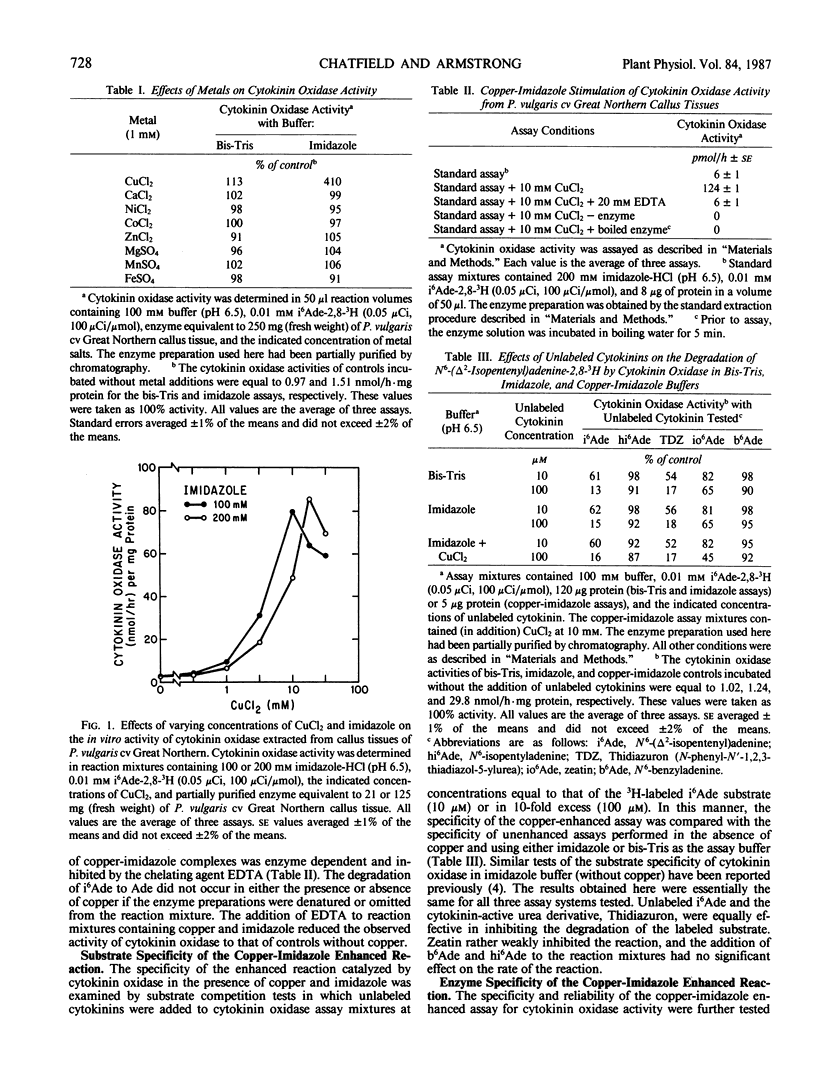
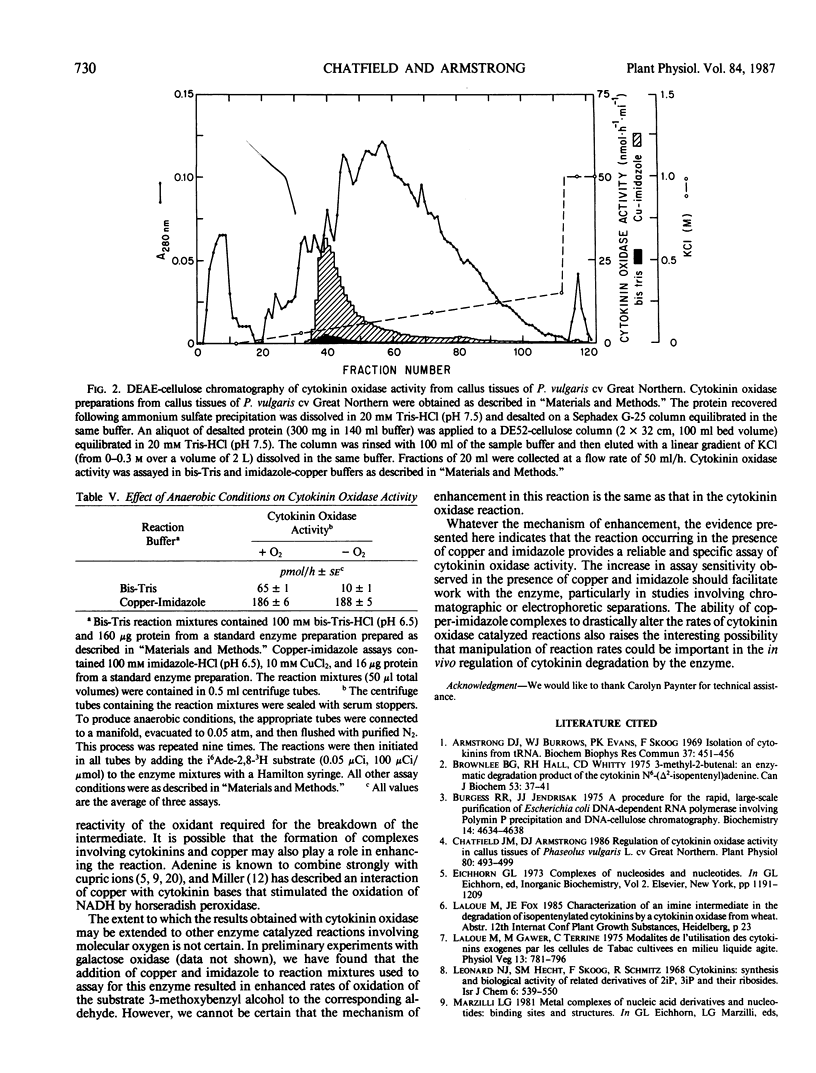
The effects of metal ions on cytokinin oxidase activity extracted from callus tissues of Phaseolus vulgaris L. cv Great Northern have been examined using an assay based on the oxidation of N6-(Δ2-isopentenyl)-adenine-2,8-3H (i6 Ade) to adenine (Ade). The addition of cupric ions to reaction mixtures containing imidazole buffer markedly enhanced cytokinin oxidase activity. In the presence of optimal concentrations of copper and imidazole, cytokinin oxidase activity was stimulated more than 20-fold. The effect was enzyme dependent, specific for copper, and observed only in the presence of imidazole. The substrate specificity of the copper-imidazole enhanced reaction, as judged by substrate competition tests, was the same as that observed in the absence of copper and imidazole. Similarly, in tests involving DEAE-cellulose chromatography, elution profiles of cytokinin oxidase activity determined using a copper-imidazole enhanced assay were identical to those obtained using an assay without copper and imidazole. On the basis of these results, the addition of copper and imidazole to reaction mixtures used to assay for cytokinin oxidase activity is judged to provide a reliable and specific assay of greatly enhanced sensitivity for the enzyme. The mechanism by which copper and imidazole enhance cytokinin oxidase activity is not certain, but the reaction catalyzed by the enzyme was not inhibited by anaerobic conditions when these reagents were present. This observation suggests that copper-imidazole complexes are substituting for oxygen in the reaction mechanism by which cytokinin oxidase effects cleavage of the N6-side chain of i6Ade.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. J., Burrows W. J., Evans P. K., Skoog F. Isolation of cytokinins from tRNA. Biochem Biophys Res Commun. 1969 Oct 22;37(3):451–456. doi: 10.1016/0006-291x(69)90936-x. [DOI] [PubMed] [Google Scholar]
- Brownlee B. G., Hall R. H., Whitty C. D. 3-Methyl-2-butenal: an enzymatic degradation product of the cytokinin, N-6-(delta-2 isopentenyl)adenine. Can J Biochem. 1975 Jan;53(1):37–41. doi: 10.1139/o75-006. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Chatfield J. M., Armstrong D. J. Regulation of Cytokinin Oxidase Activity in Callus Tissues of Phaseolus vulgaris L. cv Great Northern. Plant Physiol. 1986 Feb;80(2):493–499. doi: 10.1104/pp.80.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. O. Possible Regulatory Roles of Cytokinins : NADH Oxidation by Peroxidase and a Copper Interaction. Plant Physiol. 1985 Nov;79(3):908–910. doi: 10.1104/pp.79.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok M. C., Mok D. W., Dixon S. C., Armstrong D. J., Shaw G. Cytokinin structure-activity relationships and the metabolism of N-(delta-isopentenyl)adenosine-8-C in phaseolus callus tissues. Plant Physiol. 1982 Jul;70(1):173–178. doi: 10.1104/pp.70.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paces V., Werstiuk E., Hall R. H. Conversion of N-(Delta-Isopentenyl)adenosine to Adenosine by Enzyme Activity in Tobacco Tissue. Plant Physiol. 1971 Dec;48(6):775–778. doi: 10.1104/pp.48.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitty C. D., Hall R. H. A cytokinin oxidase in Zea mays. Can J Biochem. 1974 Sep;52(9):789–799. doi: 10.1139/o74-112. [DOI] [PubMed] [Google Scholar]


