Abstract
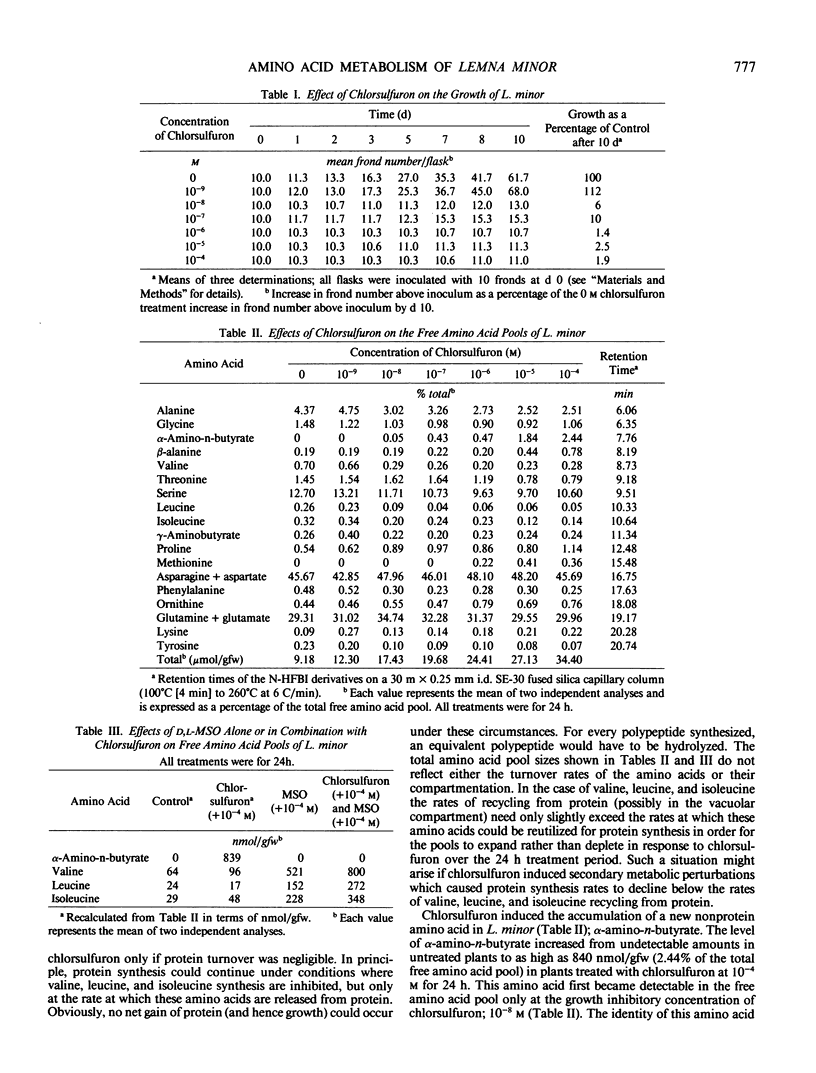
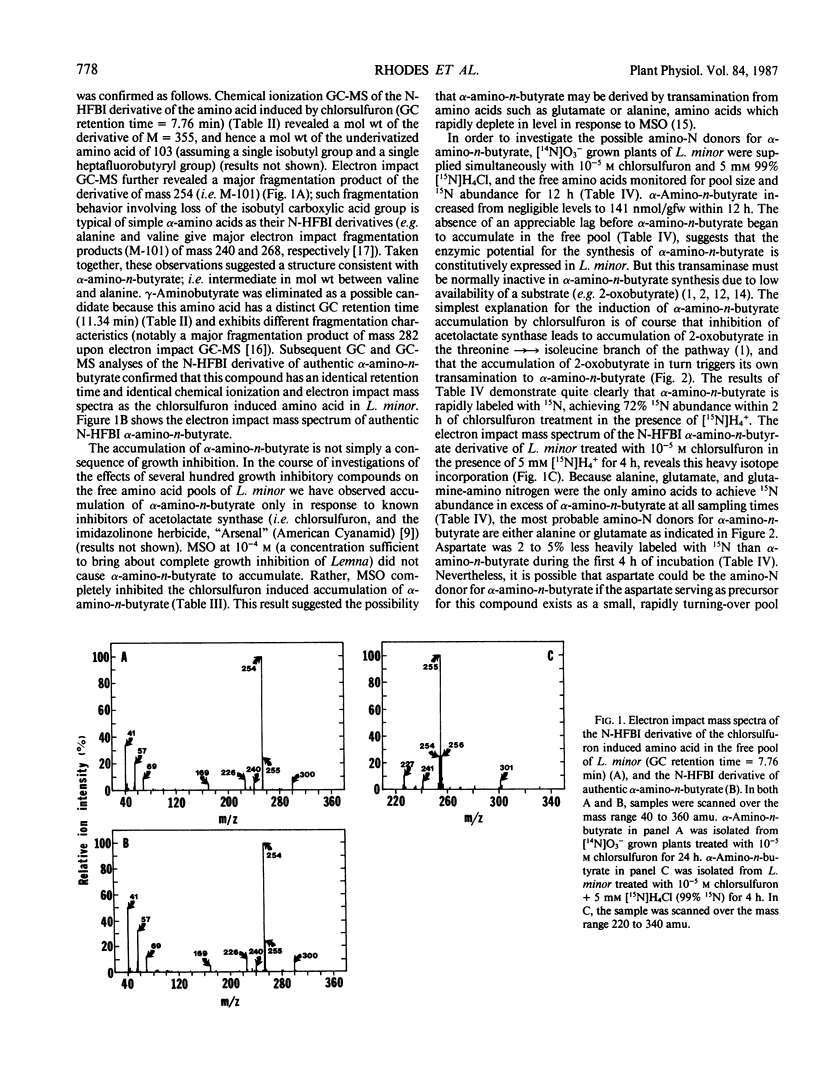
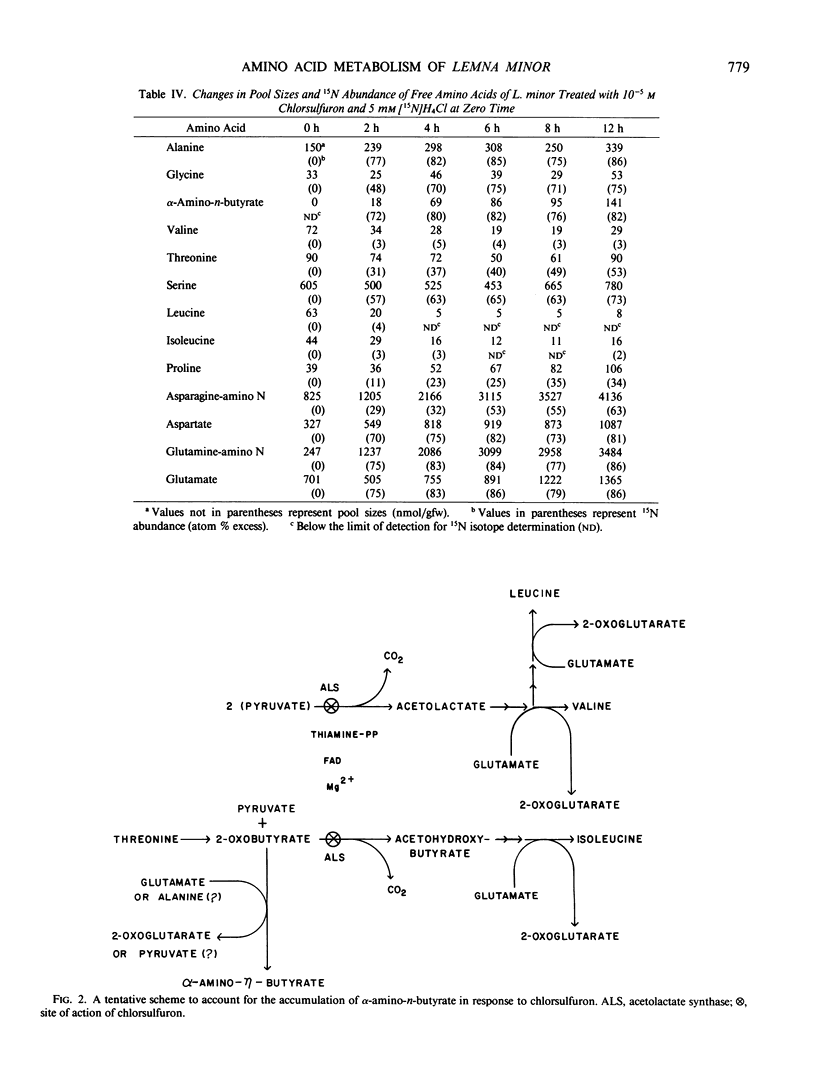
Chlorsulfuron, an inhibitor of acetolactate synthase (EC 4.1.3.18) (TB Ray 1984 Plant Physiol 75: 827-831), markedly inhibited the growth of Lemna minor at concentrations of 10−8 molar and above, but had no inhibitory effects on growth at 10−9 molar. At growth inhibitory concentrations, chlorsulfuron caused a pronounced increase in total free amino acid levels within 24 hours. Valine, leucine, and isoleucine, however, became smaller percentages of the total free amino acid pool as the concentration of chlorsulfuron was increased. At concentrations of chlorsulfuron of 10−8 molar and above, a new amino acid was accumulated in the free pool. This amino acid was identified as α-amino-n-butyrate by chemical ionization and electron impact gas chromatography-mass spectrometry. The amount of α-amino-n-butyrate increased from undetectable levels in untreated plants, to as high as 840 nanomoles per gram fresh weight (2.44% of the total free pool) in plants treated with 10−4 molar chlorsulfuron for 24 hours. The accumulation of this amino acid was completely inhibited by methionine sulfoximine. Chlorsulfuron did not inhibit the methionine sulfoximine induced accumulations of valine, leucine, and isoleucine, supporting the idea that the accumulation of the branched-chain amino acids in methionine sulfoximine treated plants is the result of protein turnover rather than enhanced synthesis. Protein turnover may be primarily responsible for the failure to achieve complete depletion of valine, leucine, and isoleucine even at concentrations of chlorsulfuron some 104 times greater than that required to inhibit growth. Tracer studies with 15N demonstrate that chlorsulfuron inhibits the incorporation of 15N into valine, leucine, and isoleucine. The α-amino-n-butyrate accumulated in the presence of chlorsulfuron and [15N]H4+ was heavily labeled with 15N at early time points and appeared to be derived by transamination from a rapidly labeled amino acid such as glutamate or alanine. We propose that chlorsulfuron inhibition of acetolactate synthase may lead to accumulation of 2-oxobutyrate in the isoleucine branch of the pathway, and transamination of 2-oxobutyrate to α-amino-n-butyrate by a constitutive transaminase utilizing either glutamate or alanine as α-amino-N donors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaleff R. S., Mauvais C. J. Acetolactate synthase is the site of action of two sulfonylurea herbicides in higher plants. Science. 1984 Jun 29;224(4656):1443–1445. doi: 10.1126/science.224.4656.1443. [DOI] [PubMed] [Google Scholar]
- Chaleff R. S., Ray T. B. Herbicide-resistant mutants from tobacco cell cultures. Science. 1984 Mar 16;223(4641):1148–1151. doi: 10.1126/science.223.4641.1148. [DOI] [PubMed] [Google Scholar]
- Davies D. D., Humphrey T. J. Amino Acid recycling in relation to protein turnover. Plant Physiol. 1978 Jan;61(1):54–58. doi: 10.1104/pp.61.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco S. C., Dumas K. S. Genetic analysis of mutants of Saccharomyces cerevisiae resistant to the herbicide sulfometuron methyl. Genetics. 1985 Jan;109(1):21–35. doi: 10.1093/genetics/109.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn G. A., Brown J. W. Properties of a methionyl-tRNA synthetase from Sarcina lutea. Biochim Biophys Acta. 1967 Sep 12;146(1):264–271. doi: 10.1016/0005-2744(67)90093-9. [DOI] [PubMed] [Google Scholar]
- Kacser H. The control of enzyme systems in vivo: elasticity analysis of the steady state. Biochem Soc Trans. 1983 Jan;11(1):35–40. doi: 10.1042/bst0110035. [DOI] [PubMed] [Google Scholar]
- LaRossa R. A., Schloss J. V. The sulfonylurea herbicide sulfometuron methyl is an extremely potent and selective inhibitor of acetolactate synthase in Salmonella typhimurium. J Biol Chem. 1984 Jul 25;259(14):8753–8757. [PubMed] [Google Scholar]
- Miflin B. J. Cooperative feedback control of barley acetohydroxyacid synthetase by leucine, isoleucine, and valine. Arch Biochem Biophys. 1971 Oct;146(2):542–550. doi: 10.1016/0003-9861(71)90159-7. [DOI] [PubMed] [Google Scholar]
- Ray T. B. Site of action of chlorsulfuron: inhibition of valine and isoleucine biosynthesis in plants. Plant Physiol. 1984 Jul;75(3):827–831. doi: 10.1104/pp.75.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Deal L., Haworth P., Jamieson G. C., Reuter C. C., Ericson M. C. Amino Acid Metabolism of Lemna minor L. : I. Responses to Methionine Sulfoximine. Plant Physiol. 1986 Dec;82(4):1057–1062. doi: 10.1104/pp.82.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Handa S., Bressan R. A. Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol. 1986 Dec;82(4):890–903. doi: 10.1104/pp.82.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Myers A. C., Jamieson G. Gas Chromatography-Mass Spectrometry of N- Heptafluorobutyryl Isobutyl Esters of Amino Acids in the Analysis of the Kinetics of [N]H(4) Assimilation in Lemna minor L. Plant Physiol. 1981 Nov;68(5):1197–1205. doi: 10.1104/pp.68.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekura R., Meister A. gamma-Glutamylcysteine synthetase. Further purification, "half of the sites" reactivity, subunits, and specificity. J Biol Chem. 1977 Apr 25;252(8):2599–2605. [PubMed] [Google Scholar]
- Yadav N., McDevitt R. E., Benard S., Falco S. C. Single amino acid substitutions in the enzyme acetolactate synthase confer resistance to the herbicide sulfometuron methyl. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4418–4422. doi: 10.1073/pnas.83.12.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]


