Abstract
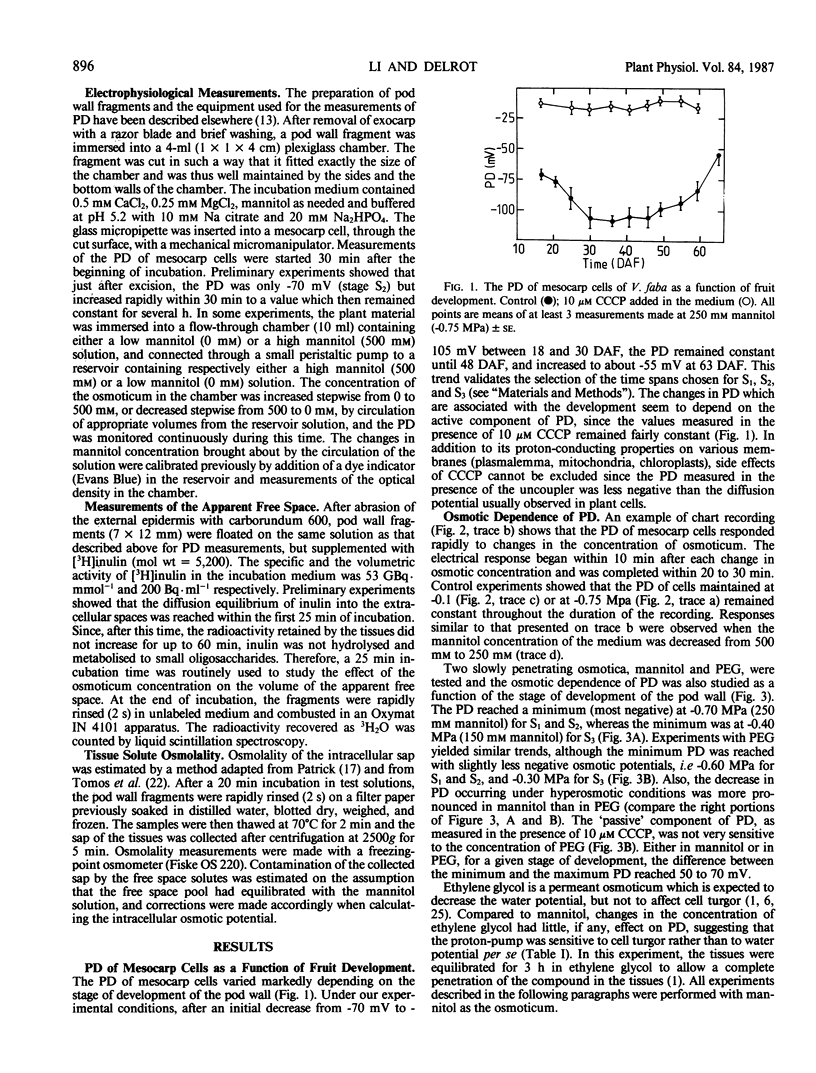
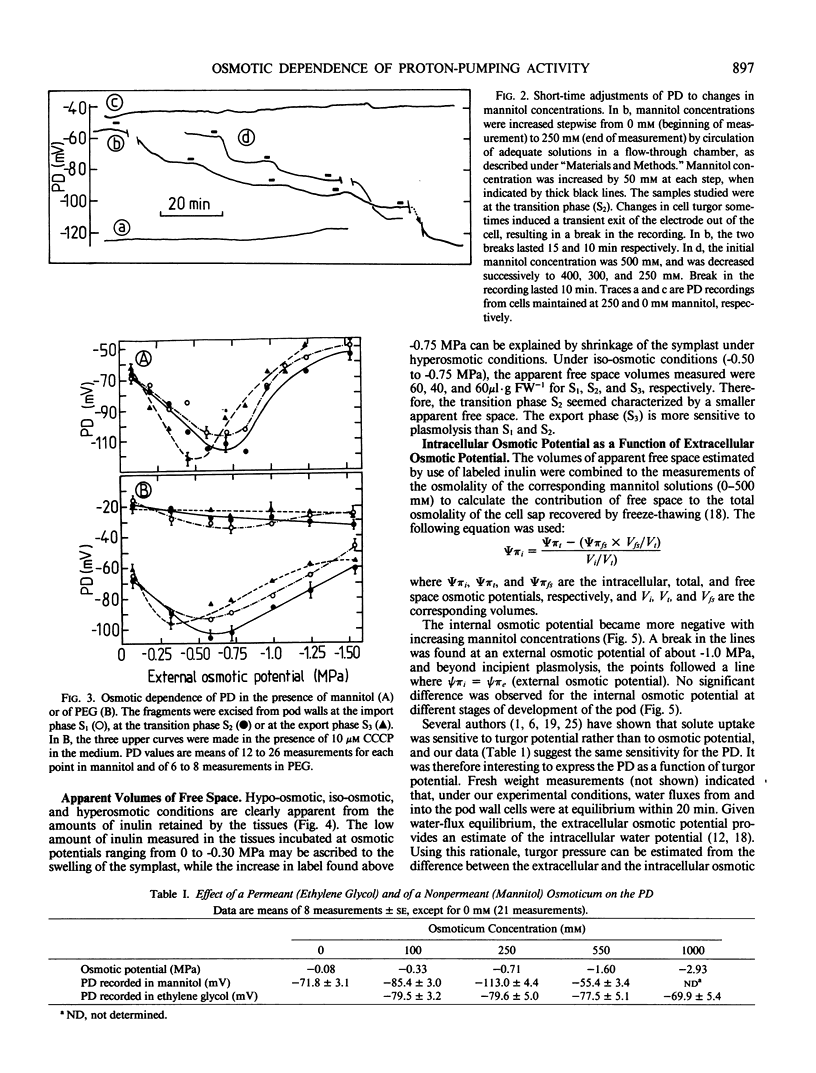
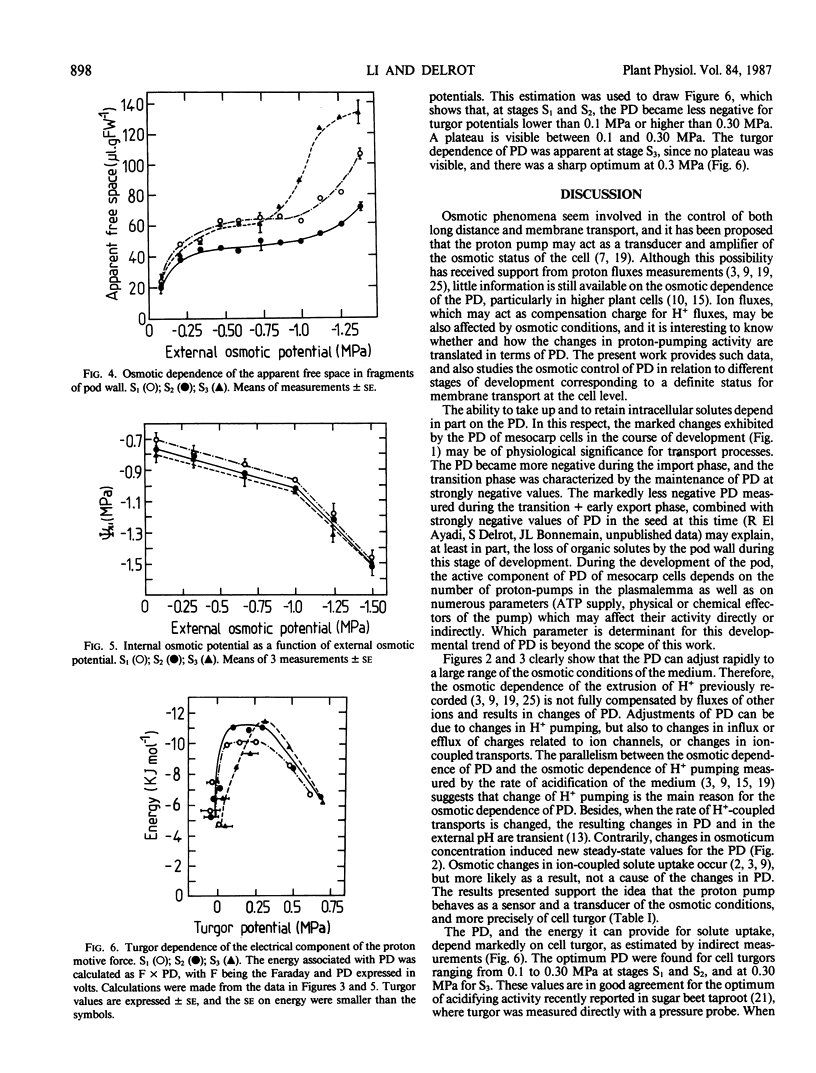
Pod walls of broadbean (Vicia faba L. cv Aguadulce) were harvested at the import (S1), at the transition (S2) or at the export (S3) phase for assimilate transport. Measurements of the transmembrane potential difference (PD) of mesocarp cells were made under various osmotic conditions. Internal osmotic potentials and cell turgor were calculated from osmolality measurements of cell saps recovered by freeze-thawing, after correction for the contribution of the free-space solution. Changes in the mannitol concentration of the medium altered the PD within a few minutes, and new stable values of PD were reached within 20 minutes after the osmotic change. With mannitol as the osmoticum, the most negative PD was measured at an external osmotic potential of -0.70 megapascals (MPa) for S1 and S2, while the most negative was at -0.40 MPa for S3. Ethylene glycol, a permeant osmoticum, had little effect on PD, showing that the PD was sensitive to turgor, not to solute potential per se. For S1 and S2, the PD was less negative for turgor potentials lower than 0.1 MPa or greater than 0.3 MPa. S3 samples exhibited a different turgor dependence, with a sharp optimum of the negativity of the PD at 0.3 MPa. The data are consistent with the proposal that the proton pump acts as a transducer of the osmotic conditions. They show that the osmotic sensitivity of the PD of mesocarp cells of broadbean changes with the stage of development of the pod.
Full text
PDF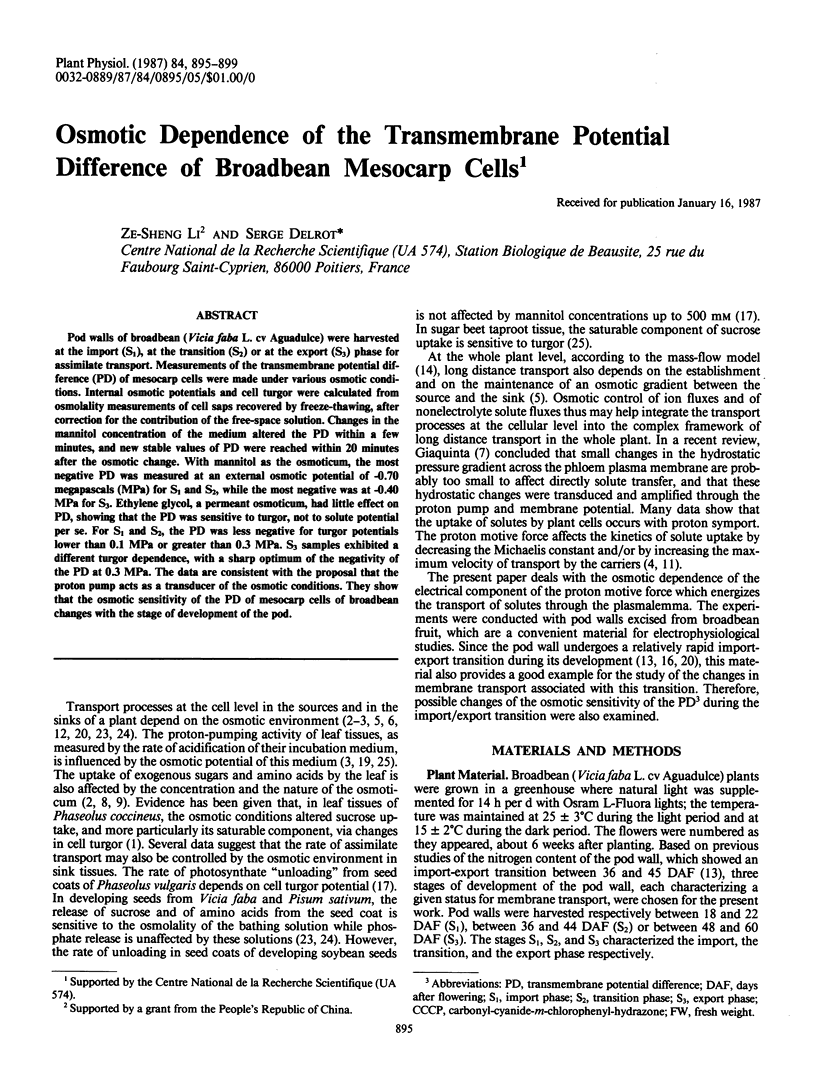

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Delrot S., Bonnemain J. L. Involvement of Protons as a Substrate for the Sucrose Carrier during Phloem Loading in Vicia faba Leaves. Plant Physiol. 1981 Mar;67(3):560–564. doi: 10.1104/pp.67.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford R. M., Thorne J. H. Sucrose Concentration at the Apoplastic Interface between Seed Coat and Cotyledons of Developing Soybean Seeds. Plant Physiol. 1985 Apr;77(4):863–868. doi: 10.1104/pp.77.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Wyse R. E. Electrical evidence for turgor inhibition of proton extrusion in sugar beet taproot. Plant Physiol. 1986 Dec;82(4):1148–1150. doi: 10.1104/pp.82.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Tanner W. The hexose-proton cotransport system of chlorella. pH-dependent change in Km values and translocation constants of the uptake system. J Gen Physiol. 1974 Nov;64(5):568–581. doi: 10.1085/jgp.64.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold L., Seiden A., Volokita M. Is modulation of the rate of proton pumping a key event in osmoregulation? Plant Physiol. 1984 Jul;75(3):846–849. doi: 10.1104/pp.75.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H., Rainbird R. M. An in vivo technique for the study of Phloem unloading in seed coats of developing soybean seeds. Plant Physiol. 1983 May;72(1):268–271. doi: 10.1104/pp.72.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse R. E., Zamski E., Tomos A. D. Turgor regulation of sucrose transport in sugar beet taproot tissue. Plant Physiol. 1986 Jun;81(2):478–481. doi: 10.1104/pp.81.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]


