Abstract
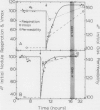
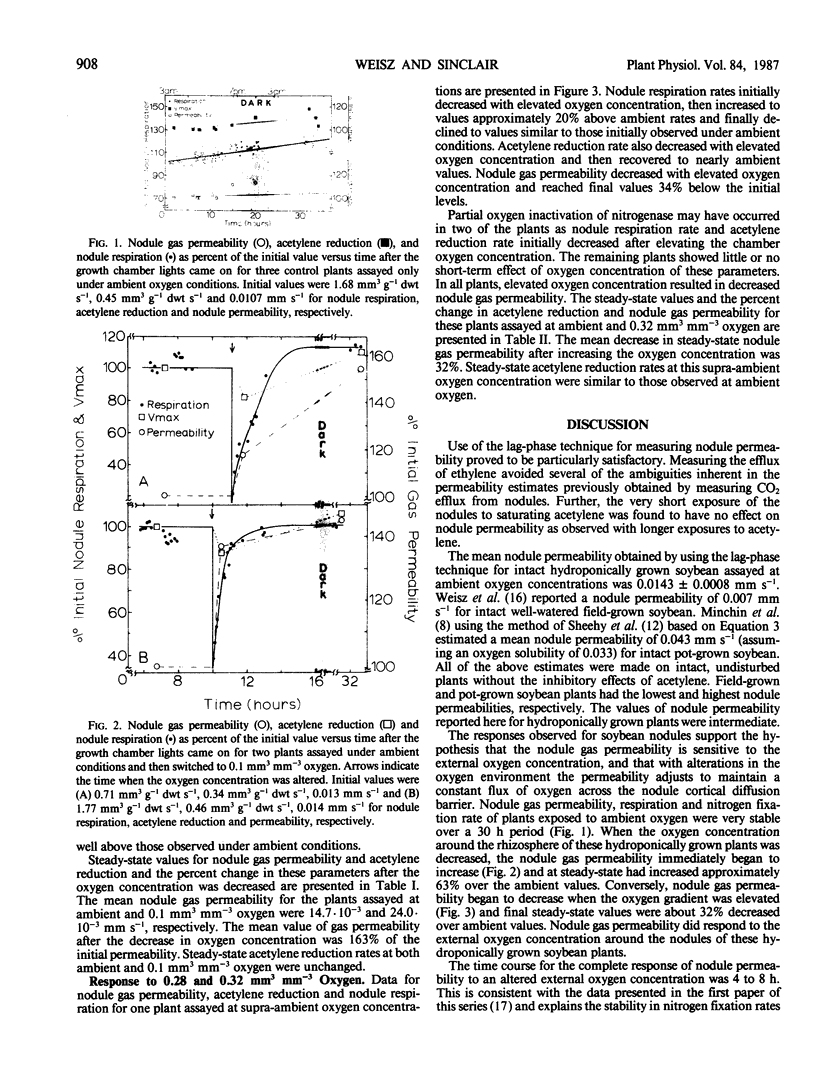
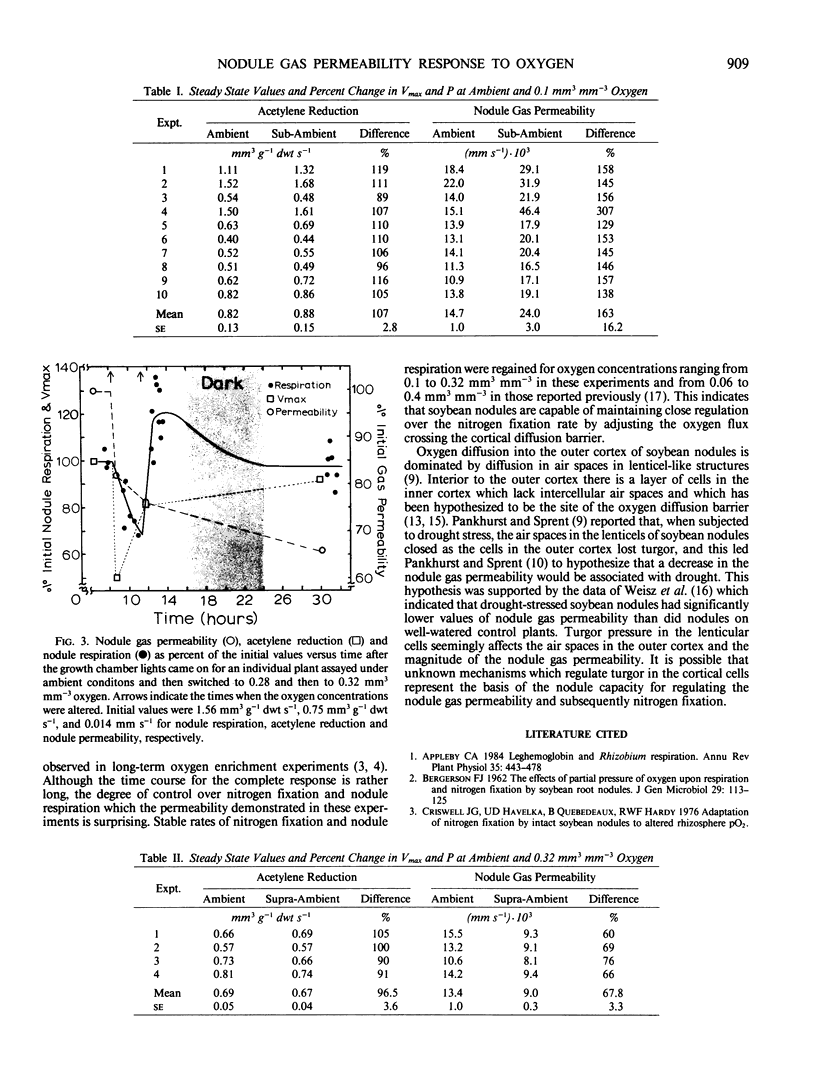
Nodule nitrogen fixation rates are regulated by a mechanism which is responsive to the rhizosphere oxygen concentration. In some legumes, this oxygen-sensitive mechanism appears to involve changes in the gas permeability of a diffusion barrier in the nodule cortex. In soybean evidence for such a mechanism has not been found. The purpose of this research was to make quantitative measurements of soybean nodule gas permeability to test the hypothesis that soybean nodule gas permeability is under physiological control and responsive to the rhizosphere oxygen concentration. Intact hydroponically grown soybean plants were exposed to altered rhizosphere oxygen concentrations, and the nodule gas permeability, acetylene reduction and nodule respiration rates were repeatedly assayed. After a change in the external oxygen concentration, nitrogenase activity and nodule respiration rates displayed a short-term transient response after which the values returned to rates similar to those observed under ambient oxygen conditions. In contrast to steady-state nitrogenase activity and nodule respiration, nodule gas permeability was dramatically affected by the change in oxygen concentration. Decreasing the external oxygen concentration to 0.1 cubic millimeter per cubic millimeter resulted in a mean increase in nodule gas permeability of 63%. Increasing the rhizosphere oxygen concentration resulted in decreased nodule gas permeability. These data are consistent with the hypothesis that soybean nodules are capable of regulating nitrogen fixation and nodule respiration rates in response to changes in the rhizosphere oxygen concentration and indicate that the regulatory mechanism involves physiological control of the nodule gas permeability.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Imsande J., Ralston E. J. Hydroponic growth and the nondestructive assay for dinitrogen fixation. Plant Physiol. 1981 Dec;68(6):1380–1384. doi: 10.1104/pp.68.6.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Sinclair T. R., Goudriaan J. Physical and morphological constraints on transport in nodules. Plant Physiol. 1981 Jan;67(1):143–145. doi: 10.1104/pp.67.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz P. R., Denison R. F., Sinclair T. R. Response to drought stress of nitrogen fixation (acetylene reduction) rates by field-grown soybeans. Plant Physiol. 1985 Jul;78(3):525–530. doi: 10.1104/pp.78.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz P. R., Sinclair T. R. Regulation of soybean nitrogen fixation in response to rhizosphere oxygen: I. Role of nodule respiration. Plant Physiol. 1987 Jul;84(3):900–905. doi: 10.1104/pp.84.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]