Abstract
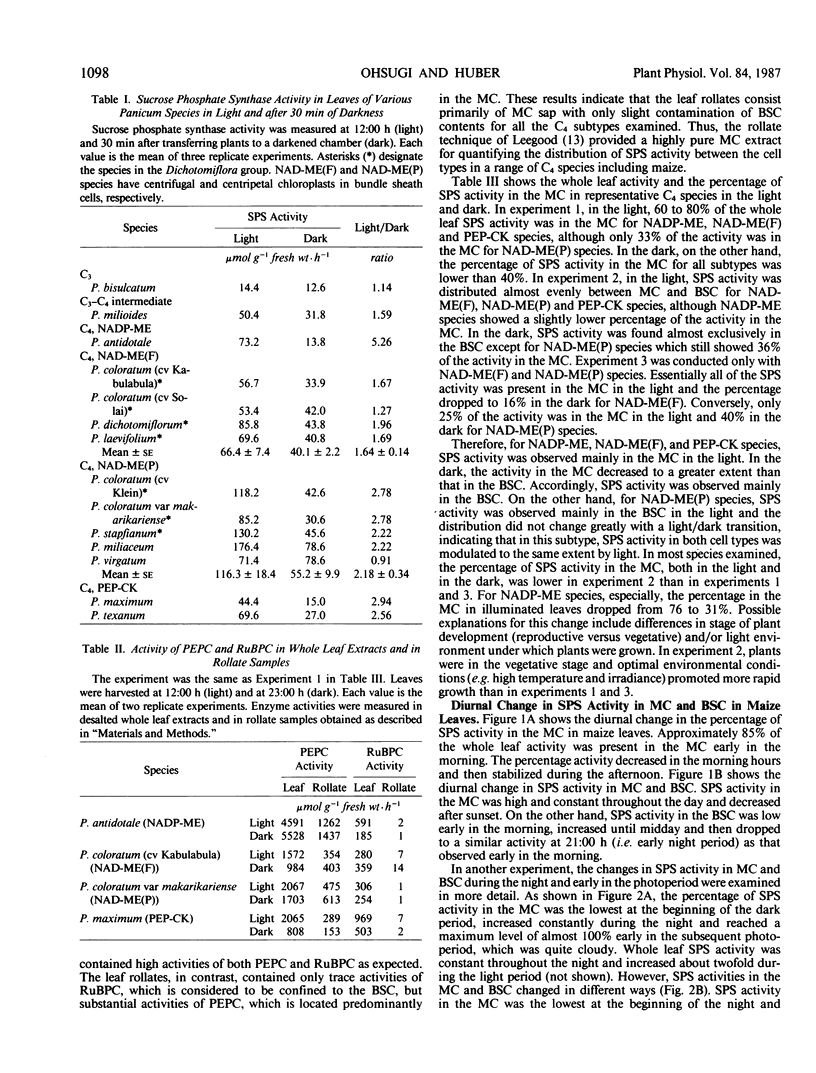
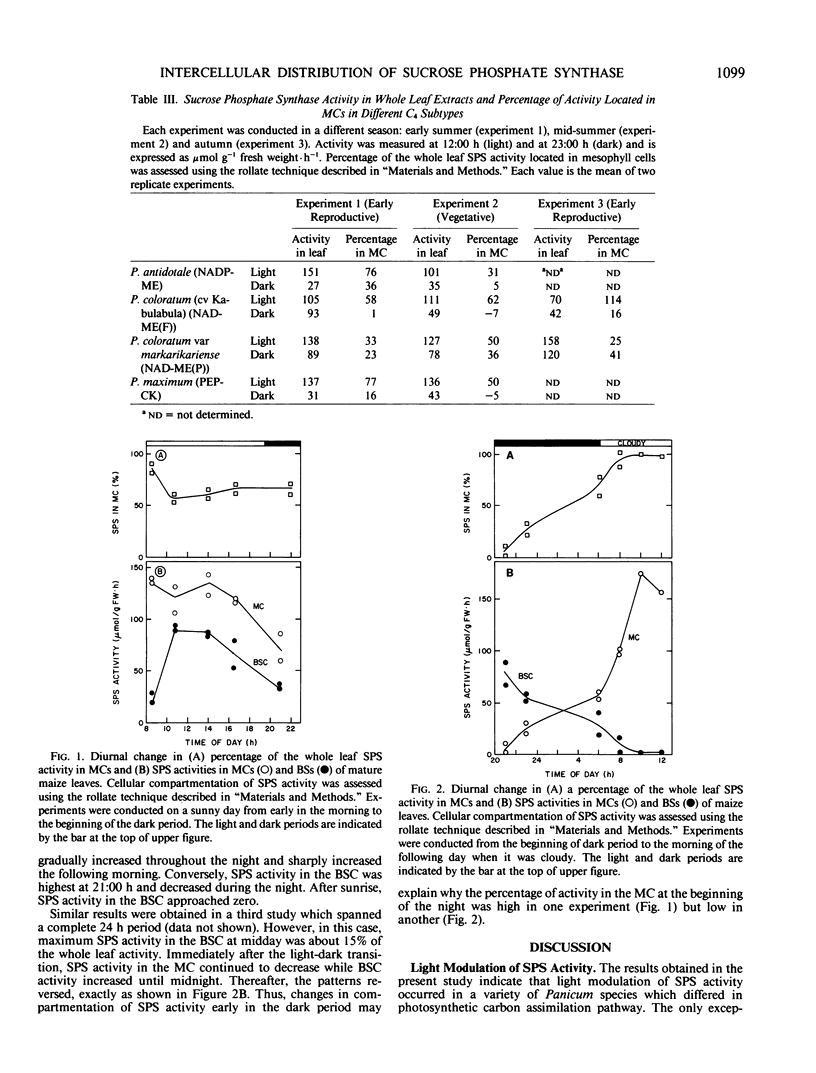
Experiments were conducted with several Panicum species (representing the different C4 subtypes) to examine the light modulation of sucrose phosphate synthase (SPS) activity and the effect of illumination on the distribution of SPS activity between mesophyll cells (MC) and bundle sheath cells (BSC). Activity of SPS in the light decreased in the order: C4 > C3-C4 intermediate > C3. In illuminated leaves, SPS activities were similar among the three C4 subtypes, but SPS activity was higher for NAD-malic enzyme (NAD-ME) species with centripetal chloroplasts in BSC (NAD-ME(P) species) than for NAD-ME species with centrifugal chloroplasts in BSC (NAD-ME(F) species). Transfer of plants into darkness for 30 minutes resulted in decreased SPS activity for all species tested except Panicum bisulcatum (C3 species) and Panicum virgatum (NAD-ME(P) species) which showed little or no change. All C4 subtypes had some SPS activity both in MC and BSC. In the light, SPS activity was mainly in the MC for NADP-ME, NAD-ME(F) and phosphoenolpyruvate carboxykinase species, while it was mainly in the BSC for NAD-ME(P) species. In the dark, for all C4 subtypes, SPS activity in the MC was decreased to a greater extent than that in the BSC. It is intriguing that NAD-ME(F) and NAD-ME(P) species differed in the activity and distribution of SPS activity between MC and BSC, although they are otherwise identical in the photosynthetic carbon assimilation pathway. Diurnal changes in SPS activity in the MC and BSC were also examined in maize leaves. SPS activity in the MC in maize leaves was high and relatively constant throughout the middle of the light period, dropped rapidly after sunset and increased again prior to the light period. On the other hand, SPS activity in the BSC was lower and changed more coincidently with light intensity than that in the MC. The results suggested that light activation of SPS activity located in the BSC may require higher irradiance for saturation than the SPS in the MC. We conclude that SPS may function in both MC and BSC for sucrose synthesis in the light, particularly at high light intensity, while in the dark, the major function may be in the BSC during starch degradation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. M., Dittrich P., Campbell W. H., Black C. C. Metabolism of epidermal tissues, mesophyll cells, and bundle sheath strands resolved from mature nutsedge leaves. Arch Biochem Biophys. 1974 Jul;163(1):246–262. doi: 10.1016/0003-9861(74)90475-5. [DOI] [PubMed] [Google Scholar]
- Helmerhorst E., Stokes G. B. Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Anal Biochem. 1980 May 1;104(1):130–135. doi: 10.1016/0003-2697(80)90287-0. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Sugiyama T., Akazawa T. Light modulation of maize leaf phosphoenolpyruvate carboxylase. Plant Physiol. 1986 Oct;82(2):550–554. doi: 10.1104/pp.82.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalt-Torres W., Kerr P. S., Usuda H., Huber S. C. Diurnal changes in maize leaf photosynthesis : I. Carbon exchange rate, assimilate export rate, and enzyme activities. Plant Physiol. 1987 Feb;83(2):283–288. doi: 10.1104/pp.83.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. An improved spectrophotometric assay for ribulosebisphosphate carboxylase. Biochim Biophys Acta. 1974 Jul 17;358(1):226–229. doi: 10.1016/0005-2744(74)90274-5. [DOI] [PubMed] [Google Scholar]
- Mbaku S. B., Fritz G. J., Bowes G. Photosynthetic and Carbohydrate Metabolism in Isolated Leaf Cells of Digitaria pentzii. Plant Physiol. 1978 Oct;62(4):510–515. doi: 10.1104/pp.62.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Kerr P. S., Huber S. C. Characterization of diurnal changes in activities of enzymes involved in sucrose biosynthesis. Plant Physiol. 1983 Oct;73(2):428–433. doi: 10.1104/pp.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher R. C., Kremer D. F. Changes of Sucrose-Phosphate Synthase Activity in Barley Primary Leaves during Light/Dark Transitions. Plant Physiol. 1984 Dec;76(4):910–912. doi: 10.1104/pp.76.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher R. C., Kremer D. F. Possible control of maize leaf sucrose-phosphate synthase activity by light modulation. Plant Physiol. 1985 Nov;79(3):695–698. doi: 10.1104/pp.79.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J., Wötzel C., Buchanan B. B. Enzyme regulation in c(4) photosynthesis : identification and localization of activities catalyzing the synthesis and hydrolysis of fructose-2,6-bisphosphate in corn leaves. Plant Physiol. 1985 Apr;77(4):999–1003. doi: 10.1104/pp.77.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H., Edwards G. E. Localization of glycerate kinase and some enzymes for sucrose synthesis in c(3) and c(4) plants. Plant Physiol. 1980 May;65(5):1017–1022. doi: 10.1104/pp.65.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]


