Abstract
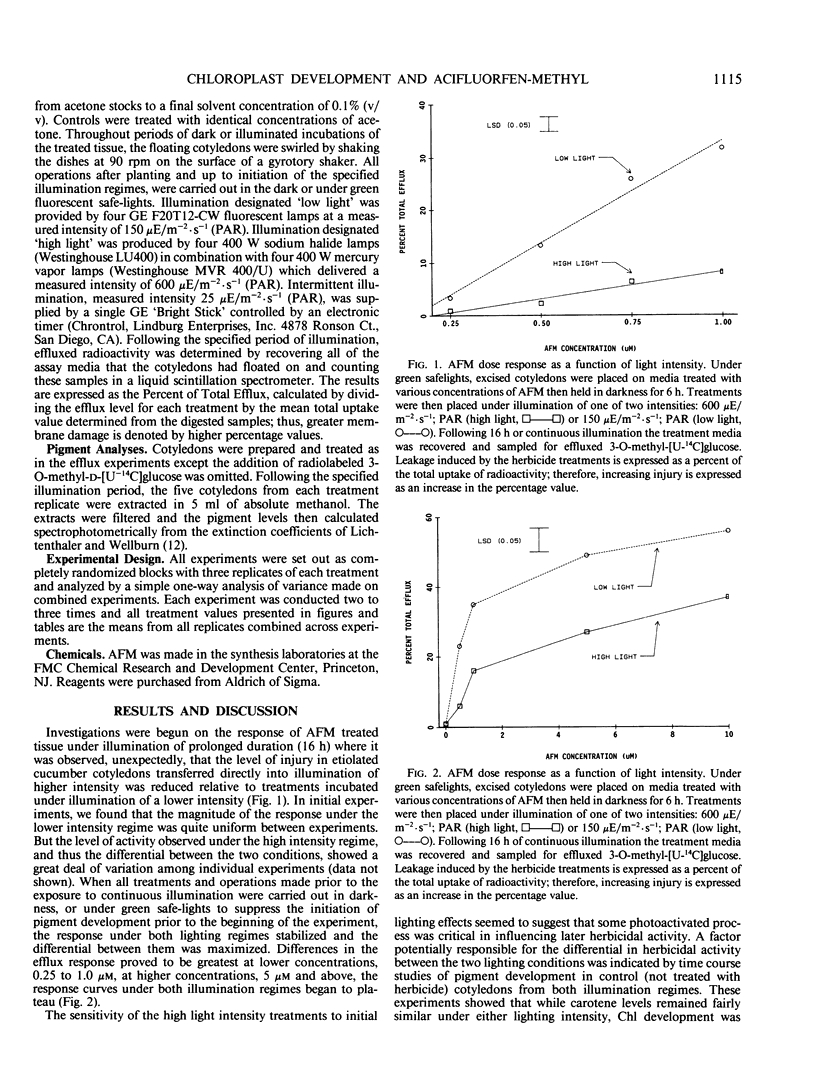
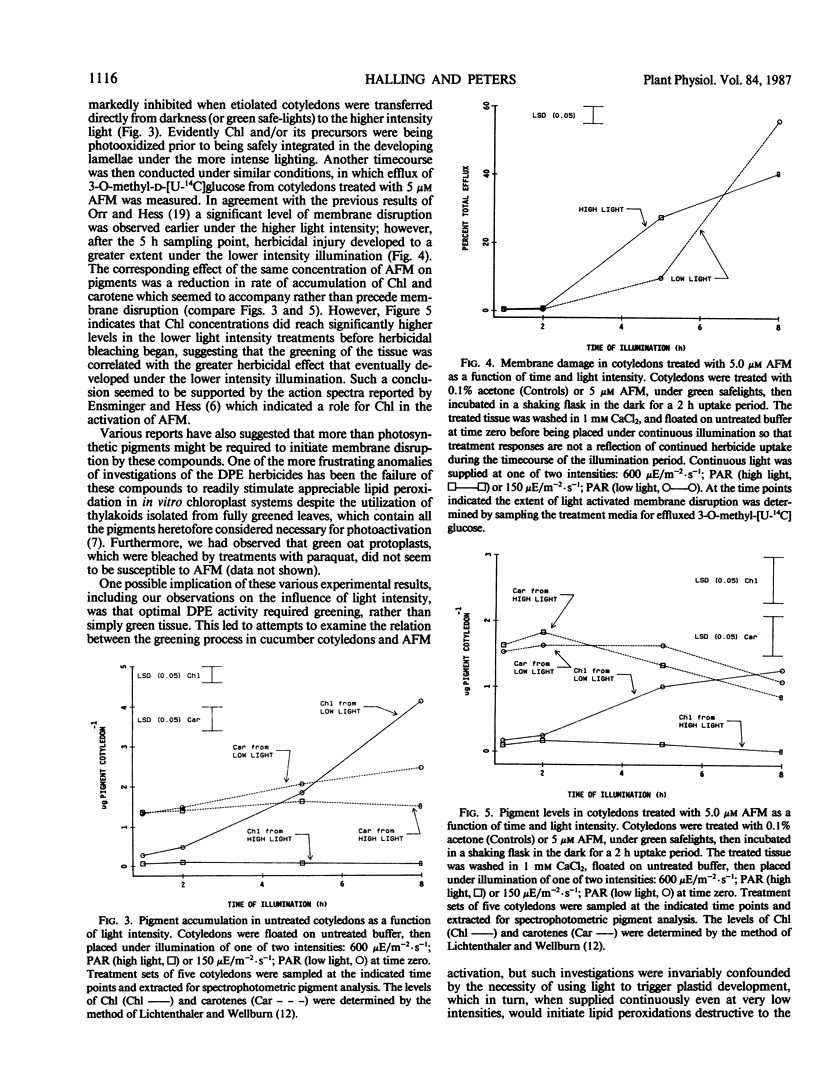
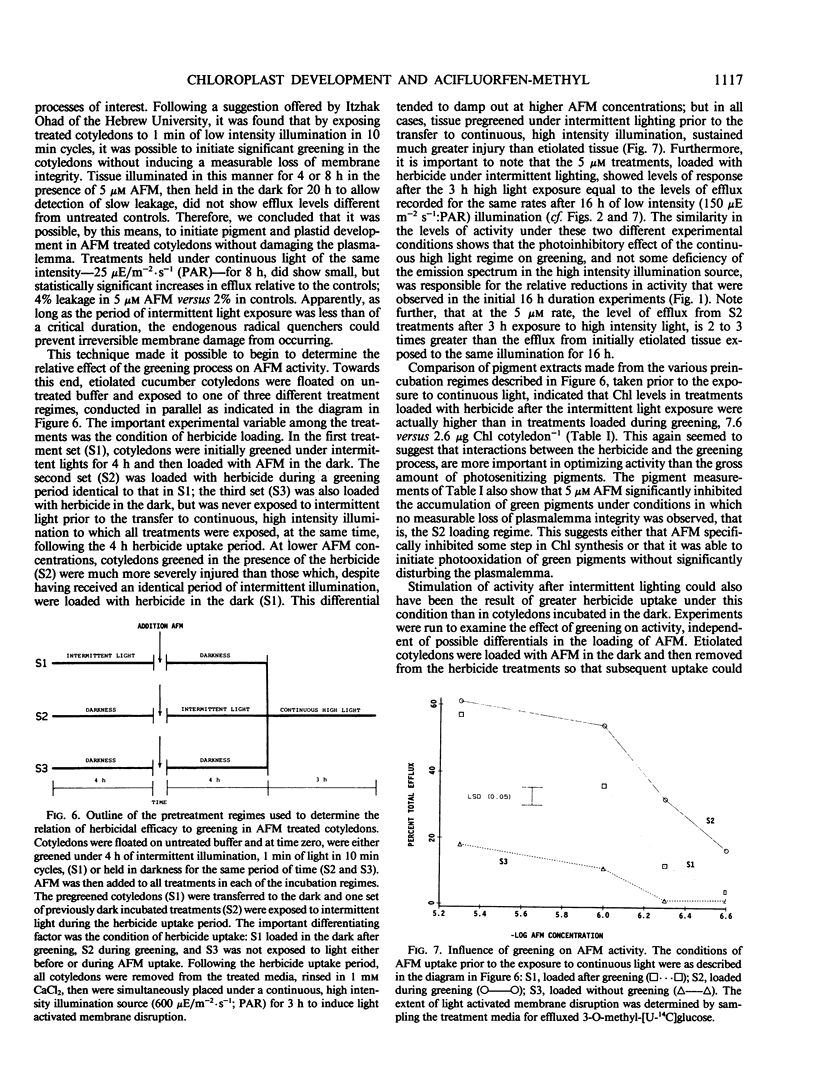
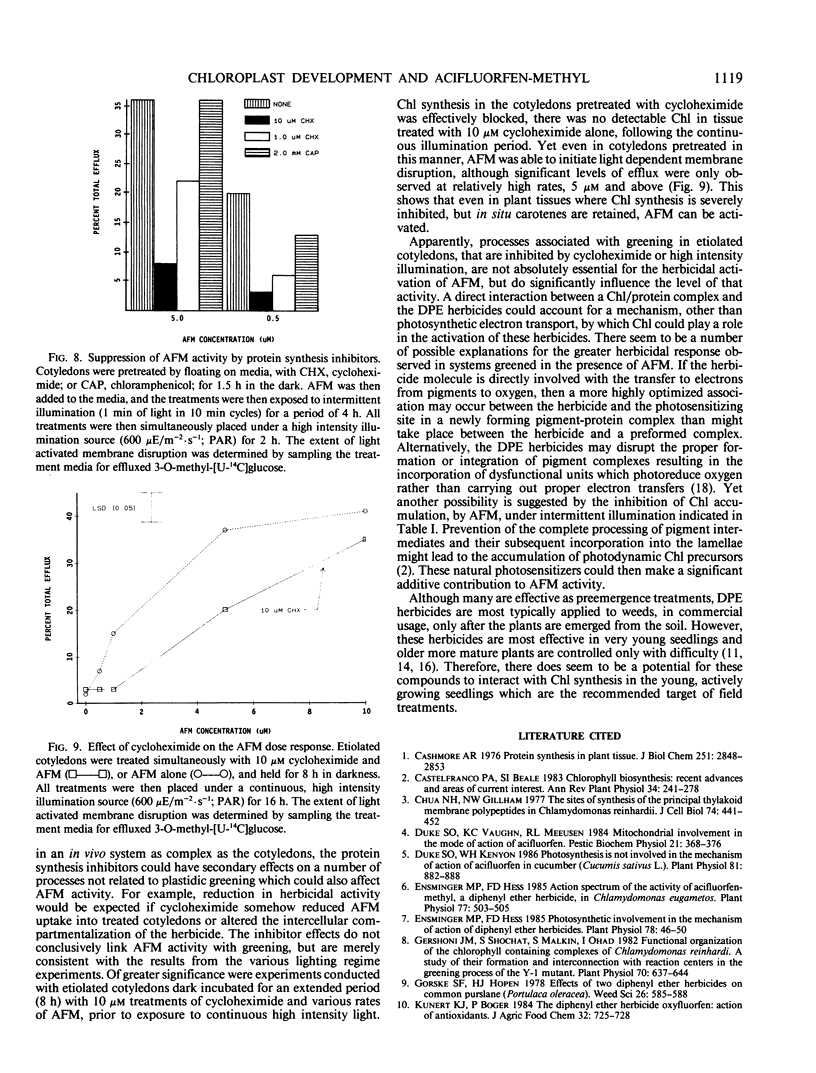
The activity of acifluorfen-methyl (AFM); methyl 5-(2-chloro-4-[trifluoromethyl] phenoxy)-2-nitrobenzoate in excised cucumber cotyledons (Cucumis sativus L.) was examined. AFM induced membrane disruption, was significantly greater when etiolated cotyledons were illuminated 16 hours at 150 microeinsteins per square meter per second photosynthetically active radiation versus incubation under illumination of 4-fold greater intensity. These results were unexpected since the loss of membrane integrity is initiated by photodynamic reactions. Untreated, etiolated cotyledons were not able to accumulate chlorophyll under the higher light intensity while control and herbicide treated cotyledons greened significantly under the lower intensity illumination suggesting that some process associated with greening stimulated AFM activity. Inhibition of greening by cycloheximide also reduced AFM activity. Intermittent lighting induced greening in AFM treated cotyledons without causing any detectable loss of plasmalemma integrity. Utilization of this system for pretreatment of cotyledons prior to continuous illumination revealed that activity was greater when tissue was greened in the presence of AFM than when herbicide treatments were made after a greening period of the same duration. The results indicate that the pigments in situ in etiolated tissue are sufficient, without greening, to initiate membrane disruption by AFM. However, greening increases the herbicidal efficacy greatly. Furthermore, the stimulation appears to be due to specific interactions between AFM and the developing plastid and is not attributable solely to an increase in endogenous photosensitizers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashmore A. R. Protein synthesis in plant leaf tissue. The sites of synthesis of the major proteins. J Biol Chem. 1976 May 10;251(9):2848–2853. [PubMed] [Google Scholar]
- Chua N. H., Gillham N. W. The sites of synthesis of the principal thylakoid membrane polypeptides in Chlamydomonas reinhardtii. J Cell Biol. 1977 Aug;74(2):441–452. doi: 10.1083/jcb.74.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. O., Kenyon W. H. Photosynthesis Is Not Involved in the Mechanism of Action of Acifluorfen in Cucumber (Cucumis sativus L.). Plant Physiol. 1986 Jul;81(3):882–888. doi: 10.1104/pp.81.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger M. P., Hess F. D. Action Spectrum of the Activity of Acifluorfen-methyl, a Diphenyl Ether Herbicide, in Chlamydomonas eugametos. Plant Physiol. 1985 Feb;77(2):503–505. doi: 10.1104/pp.77.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger M. P., Hess F. D. Photosynthesis involvement in the mechanism of action of diphenyl ether herbicides. Plant Physiol. 1985 May;78(1):46–50. doi: 10.1104/pp.78.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Shochat S., Malkin S., Ohad I. Functional Organization of the Chlorophyll-Containing Complexes of Chlamydomonas reinhardi: A Study of Their Formation and Interconnection with Reaction Centers in the Greening Process of the y-1 Mutant. Plant Physiol. 1982 Sep;70(3):637–644. doi: 10.1104/pp.70.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold O., Aurich O. Sites of synthesis of chloroplast lamellar proteins in Vicia faba. Biochim Biophys Acta. 1972 Sep 29;281(1):103–112. doi: 10.1016/0005-2787(72)90192-x. [DOI] [PubMed] [Google Scholar]
- Nadler K., Granick S. Controls on chlorophyll synthesis in barley. Plant Physiol. 1970 Aug;46(2):240–246. doi: 10.1104/pp.46.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N. C., Smillie R. M., Henningsen K. W., Von Wettstein D. Composition and Function of Thylakoid Membranes from Grana-rich and Grana-deficient Chloroplast Mutants of Barley. Plant Physiol. 1979 Jan;63(1):174–182. doi: 10.1104/pp.63.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr G. L., Elliott C. M., Hogan M. E. Activity in vivo and redox States in vitro of nitro- and chlorodiphenyl ether herbicide analogs. Plant Physiol. 1983 Dec;73(4):939–944. doi: 10.1104/pp.73.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr G. L., Hess F. D. Mechanism of Action of the Diphenyl Ether Herbicide Acifluorfen-Methyl in Excised Cucumber (Cucumis sativus L.) Cotyledons : LIGHT ACTIVATION AND THE SUBSEQUENT FORMATION OF LIPOPHILIC FREE RADICALS. Plant Physiol. 1982 Feb;69(2):502–507. doi: 10.1104/pp.69.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo A. D., Chereskin B. M., Castelfranco P. A., Franceschi V. R., Wezelman B. E. ATP requirement for mg chelatase in developing chloroplasts. Plant Physiol. 1980 May;65(5):956–960. doi: 10.1104/pp.65.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley S. M. Interaction of chloroplasts with inhibitors: effects of two diphenylether herbicides, fomesafen and nitrofluorfen, on electron transport, and some comparisons with dibromothymoquinone, diuron, and paraquat. Plant Physiol. 1983 Jun;72(2):461–468. doi: 10.1104/pp.72.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Castelfranco P. A. Mg-protoporphyrin-IX and delta-aminolevulinic acid synthesis from glutamate in isolated greening chloroplasts. delta-Aminolevulinic acid sysnthesis. Arch Biochem Biophys. 1978 Mar;186(2):376–382. doi: 10.1016/0003-9861(78)90448-4. [DOI] [PubMed] [Google Scholar]


