Abstract
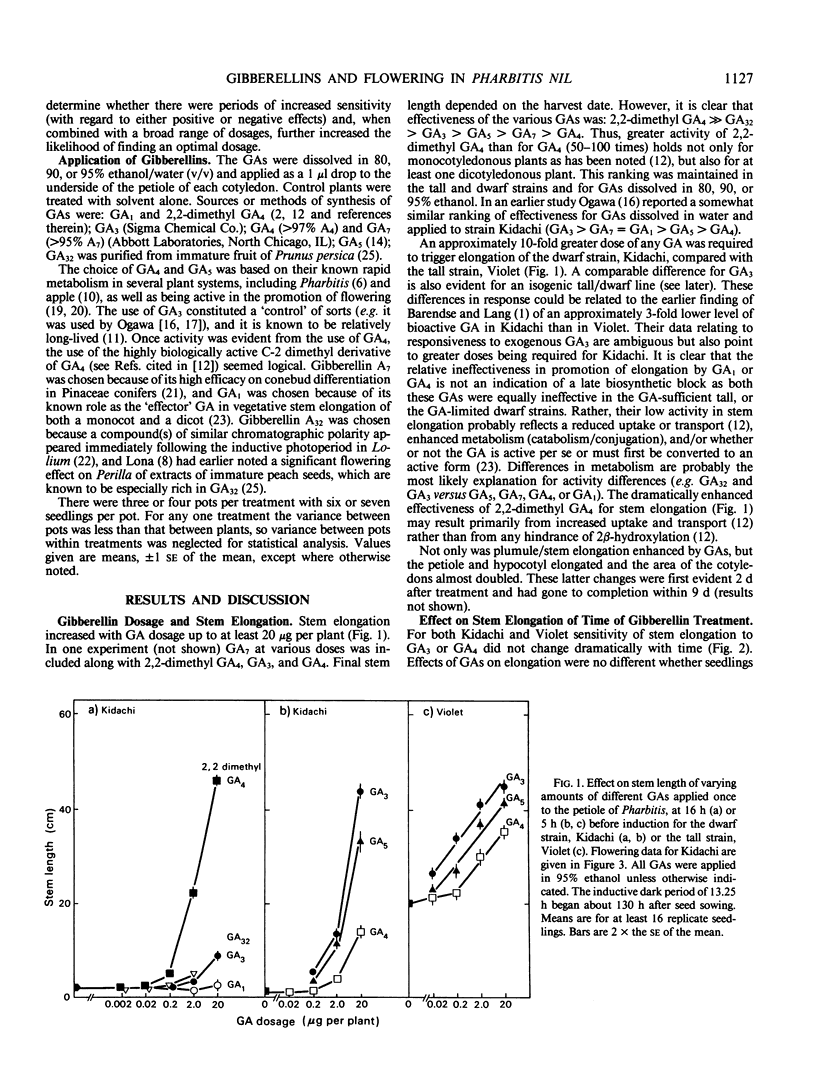
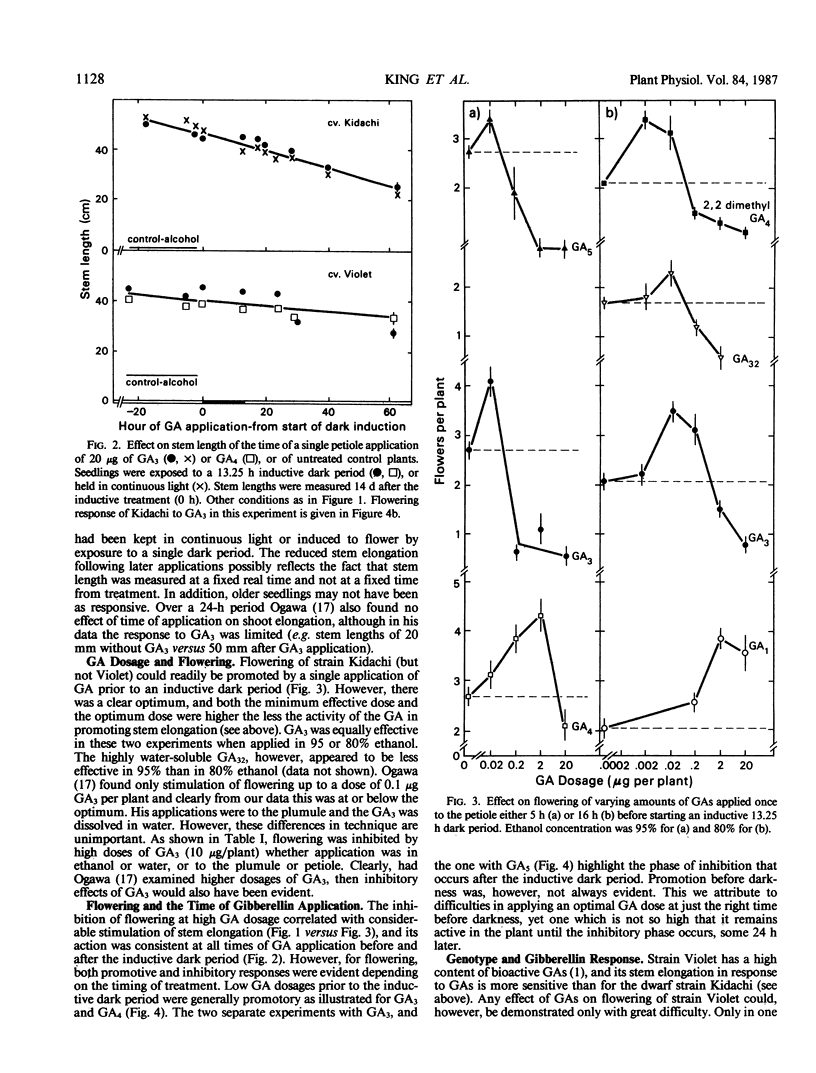
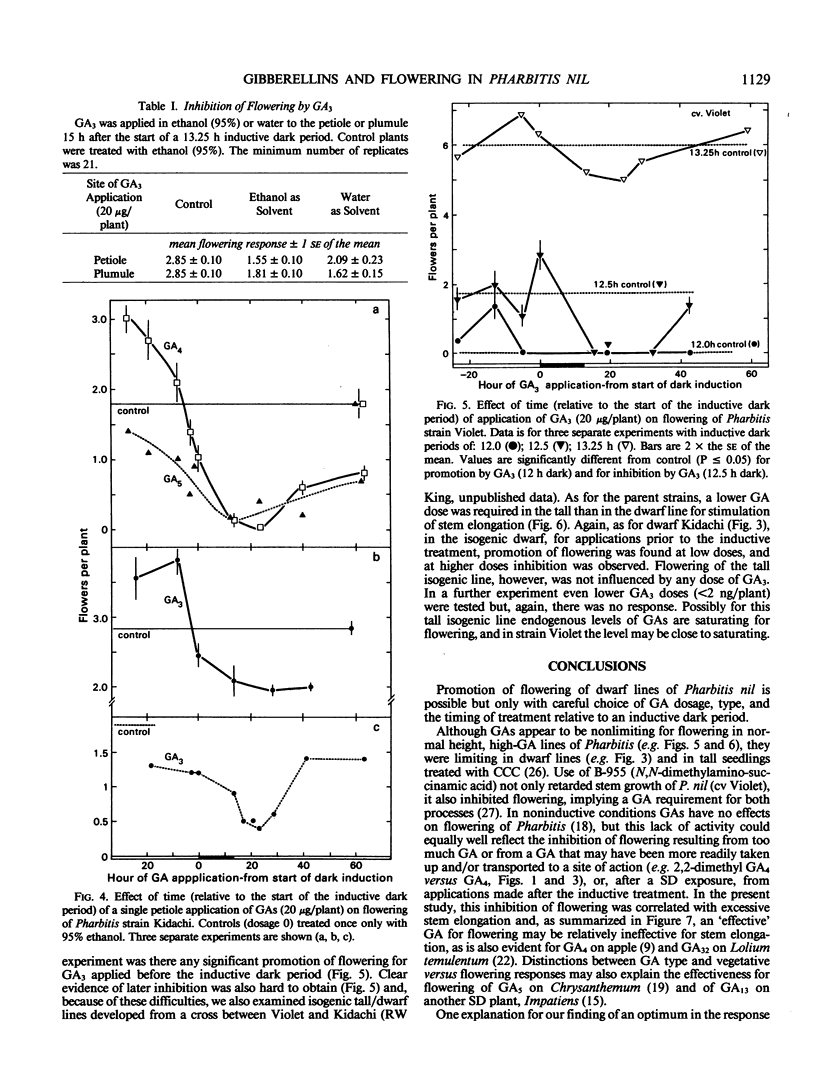
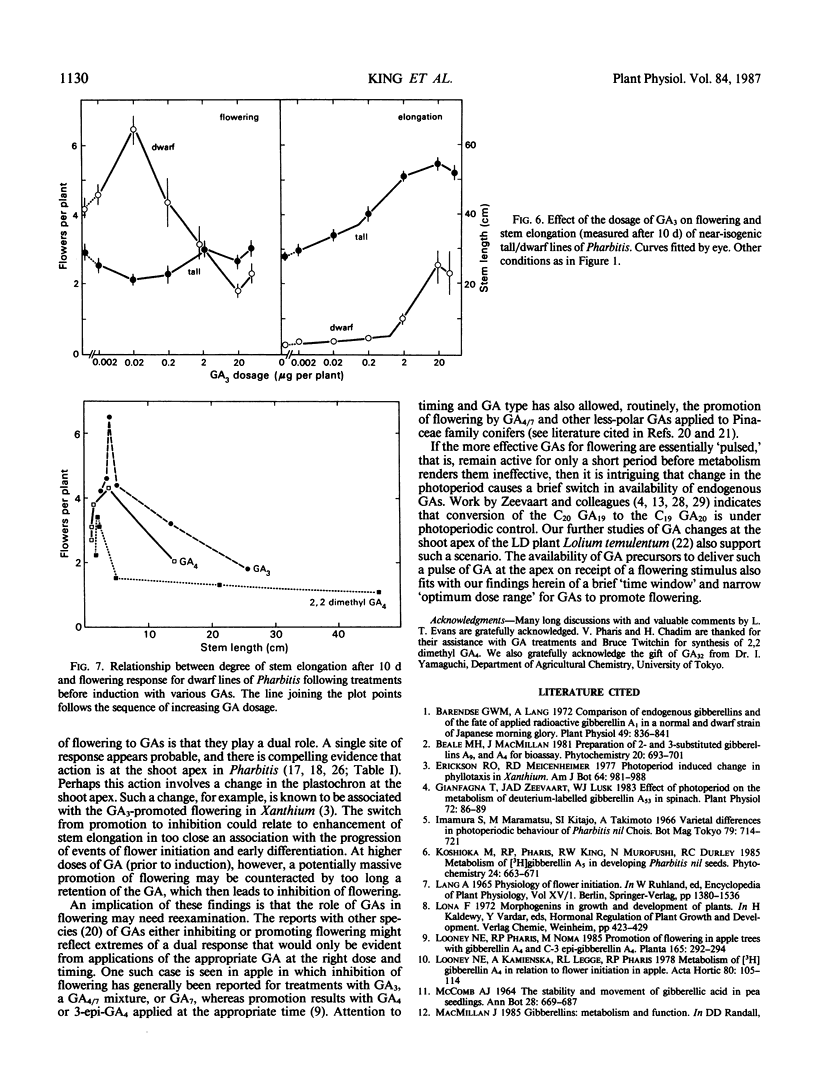
Flowering can be modified by gibberellins (GAs) in Pharbitis nil Chois. in a complex fashion depending on GA type, dosage, and the timing of treatment relative to a single inductive dark period. Promotion of flowering occurs when GAs are applied 11 to 17 hours before a single inductive dark period. When applied 24 hours later the same GA dosage is inhibitory. Thus, depending on their activity and the timing of application there is an optimum dose for promotion of flowering by any GA, with an excessive dose resulting in inhibition. Those GAs highly promotory for flowering at low doses are also most effective for stem elongation (2,2-dimethyl GA4 ≫ GA32 > GA3 > GA5 > GA7 > GA4). However, the effect of GAs on stem elongation contrasts markedly with that on flowering. A 10- to 50-fold greater dose is required for maximum promotion of stem elongation, and the response is not influenced by time of application relative to the inductive dark period. These differing responses of flowering and stem elongation raise questions about the use of relatively stable, highly bioactive GAs such as GA3 to probe the flowering response. It is proposed that the `ideal' GAs for promoting flowering may be highly bioactive but with only a short lifetime in the plant and, hence, will have little or no effect on stem elongation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barendse G. W., Lang A. Comparison of endogenous gibberellins and of the fate of applied radioactive gibberellin a(1) in a normal and a dwarf strain of Japanese morning glory. Plant Physiol. 1972 May;49(5):836–841. doi: 10.1104/pp.49.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfagna T., Zeevaart J. A., Lusk W. J. Effect of photoperiod on the metabolism of deuterium-labeled gibberellin a(53) in spinach. Plant Physiol. 1983 May;72(1):86–89. doi: 10.1104/pp.72.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. D., Zeevaart J. A. Effect of Photoperiod on the Levels of Endogenous Gibberellins in Spinach as Measured by Combined Gas Chromatography-selected Ion Current Monitoring. Plant Physiol. 1980 Nov;66(5):844–846. doi: 10.1104/pp.66.5.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharis R. P., Evans L. T., King R. W., Mander L. N. Gibberellins, Endogenous and Applied, in Relation to Flower Induction in the Long-Day Plant Lolium temulentum. Plant Physiol. 1987 Aug;84(4):1132–1138. doi: 10.1104/pp.84.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Effects of photoperiod on growth rate and endogenous gibberellins in the long-day rosette plant spinach. Plant Physiol. 1971 Jun;47(6):821–827. doi: 10.1104/pp.47.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Effects of the Growth Retardant CCC on Floral Initiation and Growth in Pharbitis nil. Plant Physiol. 1964 May;39(3):402–408. doi: 10.1104/pp.39.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]


