Abstract
Mast cells (MCs) occupy a central role in immunological as well as non-immunological processes as reflected in the variety of the mediators by which MCs influence other cells. Published lists of MC mediators have all shown only subsets—usually quite small—of the full repertoire. The full repertoire of MC mediators released by exocytosis is comprehensively compiled here for the first time. The compilation of the data is essentially based on the largely cytokine-focused database COPE®, supplemented with data on the expression of substances in human MCs published in several articles, plus extensive research in the PubMed database. Three hundred and ninety substances could be identified as mediators of human MCs which can be secreted into the extracellular space by activation of the MC. This number might still be an underestimate of the actual number of MC mediators since, in principle, all substances produced by MCs can become mediators because of the possibility of their release by diffusion into the extracellular space, mast cell extracellular traps, and intercellular exchange via nanotubules. When human MCs release mediators in inappropriate manners, this may lead to symptoms in any or all organs/tissues. Thus, such MC activation disorders may clinically present with a myriad of potential combinations of symptoms ranging from trivial to disabling or even life-threatening. The present compilation can be consulted by physicians when trying to gain clarity about MC mediators which may be involved in patients with MC disease symptoms refractory to most therapies.
Supplementary information
The online version contains supplementary material available at 10.1007/s00210-023-02545-y.
Keywords: Mast cell, Mast cell mediators, Systemic mast cell activation disease, Mastocytosis
Introduction
Mast cells (MCs) are round, about 20 μm diameter cells of the immune system containing cytoplasmic granules variably filled with many messenger substances (mediators). They originate in hematopoietic tissue; white adipose tissue has been identified as a reservoir of MC precursors, too (Poglio et al. 2010). They are resident in all vascularized organs and tissues; the majority are located at the interfaces to the outside world, such as mucous membranes and skin. At these sites, MCs are best positioned to sense when tissues are under attack by potentially harmful pathogens (parasites, bacteria, viruses, venoms) and can act accordingly. In addition, MCs likely have many more underappreciated roles in the human homeostasis of organs that undergo continuous growth and remodeling such as hair follicles and bones, wound healing, disease response, tissue repair, and angiogenesis. They are sensors of hypoxemia, air pressure, vibratory stimuli, and light. In addition, MCs are an integral component of the stress response system (Afrin et al. 2016).
MCs developed more than 500 million years ago (Crivellato et al. 2015), i.e., before the development of adaptive immunity, suggesting that MCs act as effector immune cells and as regulatory immune cells and play central roles in both innate and adaptive immunity (Gri et al. 2012). Through evolution, MCs have become optimized for their already discovered functions such as regulatory control of homeostasis of the organism, potent effector cells of the immune system, and regulation of the functional interaction of the innate and adaptive immune system (Norrby 2022). It seems likely that many other MC mediators with their associated functions remain to be discovered.
The aim of the present survey is to provide all those working in the field of MCs, scientifically and clinically, with a comprehensive compilation of human MC mediators released by exocytosis that can be used as a reference work.
Methods
The compilation of the data is essentially based on the COPE® database (Ibelgaufts 2023), which also contains references that go beyond the references given in the tables herein. This database was last accessed in March 2023. These data were supplemented with data on the expression of proteins in human MCs which had been investigated by, and were published in, Liang et al. (2018), Motakis et al. (2014), Haenisch et al. (2013), Okayama (2005), Halloran et al. (2019), and Babina et al. (2004). In addition, the PubMed database was searched with the phrase human “mast cell*” mediator*. The selective information on the potential effects of the compiled mediators were taken from the GeneCards® database (https://www.genecards.org/).
Results
On the basis of the analyzed databases, 390 substances could be identified (Online Resource 1 and 2) which are formed intracellularly by human MCs and can be secreted by exocytosis into the extracellular space by activation of the MC and can induce effects in effector cells. In studies on murine MCs, another 55 substances have been identified (data not shown) as potential mediators. However, since these substances have not yet been detected in human MCs, they are not further considered as human MC mediators in the following. Each of the 390 potential mediators is able to induce several effects on effector cells (GeneCards®). Selected manifestations of MC activation have been linked to specific mediators (Table 1) as an example of using the data from Online Resource 1.
Table 1.
Selected manifestations of mast cell activation (MCA)
| Symptoms | Potential mediators |
|---|---|
| Dermatologic manifestations | |
| Urticaria | Histamine |
| Flush, erythema | Histamine |
| Angioedema | Histamine, Bradykinin |
| Hemangiomas, telangiectasias, cherry angiomata, arteriovenous malformations, hemorrhoids, aneurysms, etc. | Probably multiple angiogenic mediators |
| Wound healing process and keloid formation | Angiopoietin Like 6; Epiregulin |
| Desquamation in the epidermis | Kallikrein Related Peptidase 5 |
| Respiratory manifestations | |
| Cough, wheezing | Histamine |
| Airway inflammation and obstructive dyspnea due to potent smooth muscle contracting activity and proinflammatory activity | Leukotriene C4, D4, E4 |
| Induction of sneezing following exposure to chemical irritants or allergens | Neuromedin B |
| Anticholinergic symptoms | Acetylcholinesterase |
| Cardiovascular manifestations | |
| Hypotension | Adrenomedullin |
| Hypotension - vasodilator and anti-proliferation agent, counterbalancing the actions of the vasoconstrictor angiotensin II | Angiotensin Converting Enzyme 2 |
| Hypotension - vasodilation and hypotension via bradykinin | Kallikrein 1, Kallikrein Related Peptidase 2, 8, 9, Kininogen 1 |
| Hypotension - plays a key role in mediating cardio-renal homeostasis and vasodilation | Natriuretic Peptide A |
| Hypotension - vasodilation | Nitric oxide |
| Hypotension - vasodilation | Platelet activating factor |
| Hypotension/hypertension - vasodilation at low doses and vasoconstriction at high doses | Prostaglandin D2 |
| Hypertension - a potent vasoconstrictor, affects cardiac contractility and heart rate through its action on the sympathetic nervous system | Angiotensin II, Angiotensinogen |
| Hypertension - responsible for converting angiotensin I to the vasoactive peptide angiotensin II | chymase 1 |
| Hypertension - potent vasoconstriction | Endothelin 1, 3 |
| Hypertension - vasoconstrictive action | Peptide YY |
| Hypertension - generation of angiotensin I from angiotensinogen in the plasma, initiating a cascade of reactions which produce hypertension and increased sodium retention by the kidney | Renin |
| Regulation of heart function | Triiodothyronine, 3-Iodothyroacetic acid; 3-Iodothyroanamine |
| Atherosclerosis and aortic valve stenosis | Biglycan |
| Atherosclerosis - disturbed plasma and tissues lipid homeostasis | Apolipoprotein E |
| Increased erythropoiesis | Erythropoietin, Inhibin subunit α (=activin A) |
| Gastrointestinal manifestations | |
| Gastritis – increased gastric acid secretion | Histamine |
| Anticholinergic symptoms | Acetylcholinesterase |
| Protective effect - stabilization of the protective mucous gel overlying the gastrointestinal mucosa | Trefoil Factor 1 |
| Enteritis/colitis - important role in the maintenance of intestinal epithelial homeostasis and the promotion of mucosal healing | Milk Fat Globule EGF And Factor V/VIII Domain Containing |
| Diarrhea – stimulation of colonic smooth muscle contraction | Neuromedin B |
| Obstipation/dyspepsia - inhibits exocrine pancreatic secretion and inhibitis jejunal and colonic mobility | Peptide YY |
| Obstipation/dyspepsia/gastroparesis– inhibition of gastrointestinal motility and gastric acid secretion | Trefoil Factor 2 |
| Weight gain or loss - regulator of most hormones of the gastrointestinal tract | Somatostatin |
| Weight gain or loss - key regulator of energy balance and body weight control | Leptin |
| Weight gain or loss - disturbed plasma and tissues lipid homeostasis | Apolipoprotein E |
| Neurologic manifestations | |
| Increased amyloid precursor protein | A Disintegrin And a Metalloprotease (=ADAM) Domain 9 |
| Increased neuroendocrine stress responses | Adenylate Cyclase Activating Polypeptide 1 |
| Influences on cortical excitability, stress response, food intake, circadian rhythms, and cardiovascular function | Neuropeptide Y |
| Neurotransmitter and neuromodulator | Neurotensin |
| Influence on neurogenesis and neuroplasticity associated with learning, memory, depression and chronic pain | VGF Nerve Growth Factor Inducible |
| Coagulopathic manifestations | |
| Increased bleeding | |
| Cleavage of the von Willebrand Factor | ADAM Metallopeptidase With Thrombospondin Type 1 Motif 13 |
| Inhibiting prothrombin activation | Alpha-1-Microglobulin/Bikunin Precursor |
| May prevent activation of the intrinsic blood coagulation cascade by binding to phospholipids on the surface of damaged cells | Apolipoprotein H |
| Anticoagulant | Heparin |
| Inhibition of collagen-induced platelet aggregation | Leukocyte Associated Immunoglobulin Like Receptor 2 |
| Conversion of plasminogen to the fibrinolytic enzyme plasmin | Plasminogen Activator, Tissue Type; Plasminogen Activator, Urokinase |
| Decreased bleeding/thrombophilia | |
| Activation of factor XIII | Cathepsin C |
| Polymerization to form an insoluble fibrin matrix as one of the primary components of blood clots | Fibrinogen α, β, γ-chains |
| High-molecular-weight kininogenis essential for blood coagulation and assembly of the kallikrein-kinin system | Kininogen 1 |
| Platelet action | Platelet activating factor |
| Neutralization of heparin on the endothelial surface of blood vessels, thereby inhibiting local antithrombin activity and promoting coagulation | Platelet Factor 4 |
| As well as | |
| Bind coagulation factor XII leading to its autoactivation | Complement C1q Binding Protein |
| Inhibition of thrombin, trypsin, plasminogen activator and urokinase | Serpin family A, B, E members |
| Skeletal manifestations | |
| Osteolysis | |
| Increased osteoclast formation | ADAM Metallopeptidase Domain 12 |
| Stimulation of osteoclasts and inhibition of osteoblasts | Activin-A |
| Autocrine factor which heightens osteoclast formation and bone resorption | Annexin A2 |
| Thiol protease involved in osteoclastic bone resorption | Cathepsin K |
| Antagonistic effect on osteogenesis due to its direct binding to BMP proteins | DAN (=differential screening-selected gene in neuroblastoma) Domain BMP (=bone morphogenic protein) Antagonist Family Member 5 |
| Negative regulator of bone mineralization | Extracellular Matrix Protein 1 |
| Osteogenesis | |
| Increased bone growth | Biglycan |
| Increased osteogenesis | Bone Morphogenetic Protein 2 |
| Ectopic bone formation and promotion of fracture healing | Bone Morphogenetic Protein 7 |
| Regulation of calcium and bone homeostasis | Bone Morphogenetic Protein 8b |
| Promotes osteogenesis by stimulating the differentiation of mesenchymal progenitors into mature osteoblasts | C-Type Lectin Domain Containing 11A |
| Stimulates the growth of chondrocytes and osteoblasts | Leukocyte Cell Derived Chemotaxin 2 |
| Pain manifestation | |
| Inducing pain | |
| Nociception | Galanin And GMAP Prepropeptide |
| Headache | Histamine |
| Direct activation of pain nerve fibers; in the posterior horn of the spinal cord amplification or weakening of pain impulses | Serotonin (5-hydroxytryptamine) |
| Preprotein of the pain-inducing tachykinin peptide hormone family: substance P, neurokinin A, neuropeptide K, neuropeptide gamma | Tachykinin Precursor 1 |
| Chronic pain | VGF Nerve Growth Factor Inducible |
| Induction of acute itch | Neuromedin B |
| Neuromodulation | Nitric oxide |
| Inhibition of pain | |
| Preproprotein for the formation of the secreted endogenous opioid peptides beta-neoendorphin, dynorphin, leu-enkephalin, rimorphin, and leumorphin | Prodynorphin |
| Precursor of β-Endorphin | Proopiomelanocortin |
| Neurologic manifestations | |
| Myasthenia | Acetylcholinesterase |
| Neuroendocrine modulator of pituitary corticotroph function | Cardiotrophin Like Cytokine Factor 1 |
| Mediating the autonomic, behavioral and neuroendocrine responses to stress | Corticotropin Releasing Hormone |
| Depression of neuronal activity | Cortistatin |
| Elevated expression of alpha-B crystallin occurs in many neurological diseases | Crystallin Alpha B |
| Acts as neurotransmitter | Histamine |
| Modulatory effects on the immune system | |
| Reduced T-cell activation and proliferation; numbers of hematopoietic stem cells in bone marrow | Activated Leukocyte Cell Adhesion Molecule |
| Control of the immune response | ADAM Like Decysin 1; Macrophage Migration Inhibitory Factor |
| Upregulated in multiple inflammatory diseases | Angiopoietin-2 |
| B-cell stimulatory agent | Cardiotrophin Like Cytokine Factor 1 |
| Important role in innate immunity defense against bacteria and viruses | Cathelicidin Antimicrobial Peptide |
| Activates serine proteases such as elastase, cathepsin G and granzymes A and B | Cathepsin C |
| Probably involved in the processing of antigenic peptides during MHC class II-mediated antigen presentation; may play a role in activation-induced lymphocyte depletion in the thymus | Cathepsin D |
| Participates in the killing and digestion of engulfed pathogens; it has bacteriocidal activity | Cathepsin G |
| Chemotaxis | C-C Motif Chemokine Ligand 1, 2, 3, 4, 4L1, 5,7, 8, 11, 13, 15, 17, 18, 19, 20, 22-25, 28; C17orf99; Ninjurin 1; X-C Motif Chemokine Ligand 1and 2 |
| Controlling the production, differentiation, and function of white cell populations of the blood, the granulocytes and mononuclear phagocytes; promotes the release of pro-inflammatory chemokines | Colony Stimulating Factor 1, 2, 3 |
| Triggering of the complement cascade |
Complement C1q A Chain, Complement C1q Binding Protein, Complement C3, C5 Complement Factor D, Complement Factor Properdin |
| Chemoattractants for various immune cells |
C-X3-C Motif Chemokine Ligand 1; C-X-C Motif Chemokine Ligand 1, 2, 3, 5, 8, 9, 10, 11, 12, 14, 16, 17, ISG15 Ubiquitin Like Modifier, Leukocyte Cell Derived Chemotaxin 2, Leukotrien B4 |
| Antibacterial, fungicide and antiviral activities | Defensin Alpha 1, 4, 5 ,6 Beta 1, 4A, 108B, 119; Granulysin; Lysozyme |
| Inducing cytokine production | High Mobility Group Box 1 |
| Enhances all basic T-cell responses to a foreign antigen | Inducible T Cell Costimulator |
| Key part of the innate immune response with potent antiviral, antiproliferative and immunomodulatory properties | Interferon Alpha 1, Beta 1, Gamma, Lambda 1-3 |
| Immunoregulation | Interleukin (IL)-1 Alpha, 1 Beta, Interleukin-1 Receptor Antagonist, IL 2-7, 9-11, 12B, 13, 15, 16, 17A, 17C, 17D, 17F, 18, 22, 23 Subunit Alpha, 24, 25, 27, 31, 32, 37 |
| An important component of the non-specific immune system with an antimicrobial activity | Lactotransferrin |
| Tumor progression/regression by MCA | |
| Progression | |
| Important role in tumor progression due to its effect on mRNA production and angiogenesis | Angiogenin |
| Has been implicated in tumor invasion and metastasis | Cathepsin B, F |
| Expressed in a significant fraction of human breast cancers, where it could contribute to tumor invasiveness | Cathepsin K |
| Stimulates the motility of tumor cells and has angiogenic properties, and its expression is upregulated in several kinds of carcinomas | Ectonucleotide Pyrophosphatase/Phosphodiesterase 2 |
| Involved in the growth and proliferation of tumor cells by inducing vasculogenesis | Epidermal Growth Factor-Like Domain Multiple 7 |
| Promotes cancer invasion and metastasis | Kallikrein Related Peptidase 7 |
| Elevated expression of this protein may be associated with cancer cachexia | Inhibin Subunit Beta A |
| Regression | |
| Can prevent metastasis by inhibiting vascular growth and tumor cell invasion due to its role as an apoptosis survival factor for vascular endothelial cells | Angiopoietin Like 4 |
| Tumor suppression by stimulation of autophagy and inflammation and an inhibition of angiogenesis and tumorigenesis | Decorin |
| Inhibits the proliferation of tumor cell | Oncostatin M |
Understanding the autocrine/paracrine activation of MCs (Fig. 1) is essential for understanding the development of an acute MC mediator release episode (He et al. 2012). Therefore, Table 2 lists all mediators which are likely to induce, via 30 distinct receptor classes, autocrine activation of the releasing MC, and paracrine activation of other MCs in the proximity of the releasing MC. This finding agrees well with the clinical observation of acute to subacute activation phases of MCs beyond anaphylactic reactions. These 30 activating mechanisms are opposed only by seven autocrine/paracrine receptors that can inhibit MC activation (Table 2).
Fig. 1.
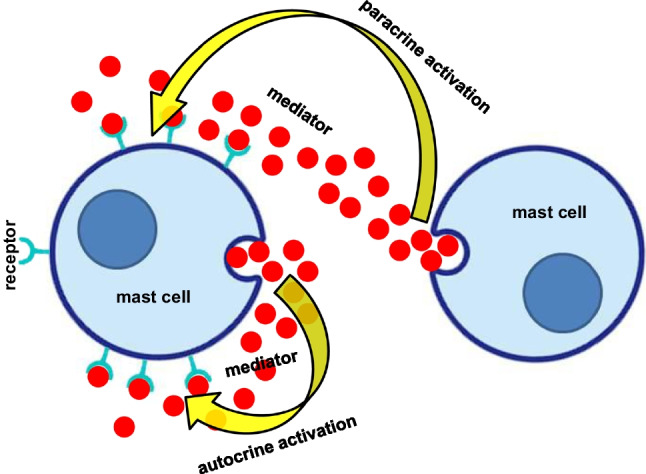
Mast cell activation after mediator (red circles) exocytosis by autocrine and paracrine stimulation of mast cell receptors for this specific released mediator
Table 2.
Facilitatory and inhibitory autocrine regulation of mast cells
| Stimulatory receptor | Autocrine ligand |
|---|---|
| Muscarinic acetylcholine receptor (Haenisch et al. 2013) | Acetylcholine (choline acetyltransferase protein has been found in human skin mast cells; Reinheimer et al. 1998) |
| Adenosine A2A, A2B, A3 receptors (Haenisch et al. 2013) | Adenosine (Marquardt et al. 1984) |
| Adrenocorticotropic hormone receptor | Adrenocorticotrophin |
| Angiotensin-converting enzyme 2 | Angiotensin II |
| C3a, C5a receptors | Complement C3, C5 |
| CXCR1-4 receptors | Chemokines |
| CD47 | Amyloid ß peptides (Niederhoffer et al. 2009) |
| KIT | Stem cell factor (KIT-ligand) |
| CD226 | Nectin-2 (Bachelet et al. 2006) |
| CD300 | Eosinophilic cationic protein (encoded bei RNASE3) |
| CRHR-1, 2 receptors | Corticotropin-releasing hormone, urocortin |
| Cysteinyl receptor 1 and 2 (Jiang et al. 2006) | Leukotrienes |
| Endothelin receptors types A, B | Endothelin 1, 3 |
| Histamine H1- H2- H4 receptors | Histamine |
| Interleukin 1 Receptor Type 1 (Jayapal et al. 2006) | Interleukin 1 beta |
| Interleukin 4 receptor (Haenisch et al. 2013) | Interleukin 4 |
| Interleukin 6 receptor (McHale et al. 2018) | Interleukin 6 |
| Interleukin 10 receptor (Liang et al. 2018) | Interleukin 10 |
| Interleukin 17 receptor (Liang et al. 2018) | Interleukin 17 |
| Interleukin 18 receptor (Haenisch et al. 2013) | Interleukin 18 |
| Low-density lipoprotein-, Very low-density lipoprotein-receptors | Apolipoprotein E |
| Neurotrophic Receptor Tyrosine Kinase 1 (Peng et al. 2013) | Nerve growth factor |
| Tachykininreceptor 1, 2 (Le et al. 2016) | Substance P |
| Neurotensin receptor 1,2 (Alysandratos et al. 2012) | Neurotensin |
| MRGPRX2 receptor | Opioid peptides |
| F2R Like Trypsin Receptor 1 (Haenisch et al. 2013) | Proteases |
| P2Y-, P2X-purinoceptors (Schulman et al. 1999) | ATP |
| S1P1- S1P2-receptors (Oskeritzian et al. 2008) | S1P |
| Somatostatin receptor 2 (Haenisch et al. 2013) | Somatostatin |
| Transforming growth factor beta receptor 1-3 (Haenisch et al. 2013) | Transforming growth factor beta1, beta2 |
| Inhibitory receptors | |
| Cannabinoid CB2 (CB1) receptor | Anandamide (Bisogno et al. 1997; Braile et al. 2021; ) |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Haenisch et al. 2013)) | Lactoferrin |
| Interleukin10 receptor (Haenisch et al. 2013) | Il-10 |
| Interleukin 1 Receptor Accessory Protein Like 1 | Il-37 |
| Nucleotide converting ectoenzyme E-NPP3 (Tsai and Takeda 2016) | ATP |
| Peroxisome proliferator-activated receptor gamma (PPAR-γ; Paruchuri et al. 2008) | 15-Deoxy-Δ12,14-Prostaglandin J2 (a metabolite of PGD2) |
| Sialic Acid Binding Ig Like Lectin 9 (Miralda et al. 2023) | Sialic Acid Binding Ig Like Lectin 9 ligand |
Two further phenomena could be important for MC activation: first, the possibility of reuptake of mediators released by the MCs for later re-exocytosis. Such reuptake of released mediators may not be accompanied by stimulation of the corresponding receptor because (1) the receptor may still be inactivated due to previous autocrine activation, and (2) reuptake may take place via receptor-independent specific reuptake mechanisms (e.g., transporters). Second, substances originally formed and released by other cells which were taken up and stored by the MCs can potentially act as MC mediators when subsequently released from the MCs (Table 3). The possibility of reuptake or uptake of substances or groups of substances into the MCs which then can act as mediators could be identified for 15 compounds (Table 3).
Table 3.
Uptake of substances as potential mediators into human mast cells
| Lactoferrin (He et al. 2003) | |
| IL-17A (Noordenbos et al. 2016) | |
| Extracellular vesicles (Shefler et al. 2021) | |
| Exosomes (Ekström et al. 2012) | |
| Fullerene (Dellinger et al. 2010) | |
| Histamine (Huszti 2003) | |
| Heparanase (Higashi et al. 2019) | |
| Thyroid hormones* (via Solute Carrier Family 3 Member 2; Solute Carrier Organic Anion Transporter Family Member 4A1; Solute Carrier Family 16 Member 10; Haenisch et al. 2013) | |
| Choline, guanidine, histamine, epinephrine, norepinephrine, dopamine* (via Solute Carrier Family 22 Member 1; Haenisch et al. 2013) | |
| Adenosine* (via Solute Carrier Family 28 Member 3 and Solute Carrier Family 29 Member 1; Haenisch et al. 2013) | |
| Biogenic amines including serotonin, dopamine, norepinephrine and epinephrine* (via Solute Carrier Family 29 Member 4; Haenisch et al. 2013) | |
| Gamma-aminobutyric acid* (via Solute Carrier Family 6 Member 13; Solute Carrier Family 36 Member 1; Haenisch et al. 2013) | |
| Choline* (via Solute Carrier Family 44 Member 1 and 4; Haenisch et al. 2013) | |
| Prostaglandins D2, E1 and E2, leukotriene C4, thromboxane B2* (via Solute Carrier Organic Anion Transporter Family Member 2B1; Haenisch et al. 2013) | |
| Prostaglandins E1 and E2, thyroxine and vasopressin* (via Solute Carrier Organic Anion Transporter Family Member 3A1; Haenisch et al. 2013) |
*Deduced from the expression of the respective carrier in HMC1 cells
Discussion
The central role of MCs in immunological as well as non-immunological processes is reflected by the large number of mediators by which MCs may influence other cells (Lundequist and Pejler 2011). The profile of mediators and cytokines stored or produced de novo in MCs can markedly differ between and even within organs/tissues depending upon a wide array of macro- and micro-environmental factors including antigenic and physical stimuli. Although the number of MC mediators has been assumed to be large, there has not yet been any comprehensive compilation of human MC mediators. In this article, the known human MC mediators are comprehensively compiled for the first time. And indeed, the number of mediators, at least 390, turns out to be extraordinarily high compared to the number of messenger substances known to be formed and released by other cells. However, this number still might substantially underestimate the actual number of MC mediators, once one takes into consideration broader definitions of “mediatorˮ and broader definitions of effector mechanisms than we consider for our present purposes.
MC actions can be targeted very precisely. Occasionally, MCs release pre-stored mediators via classic non-selective whole-MC degranulation (as in anaphylaxis), but this is the exception, not the rule, in MC activation (Theoharides et al. 2007, 2023). Otherwise, anaphylactic reaction would occur consistently in every episode of MC activation, but this is obviously not the case. Rather than wholly degranulate, MCs much more commonly selectively release specific mediators, referred to as differential release (Table 4), i.e., release of the content of individual secretory granules or individual mediators without whole-MC degranulation (Theoharides et al. 1982). This process is distinct from “piecemeal degranulation” that has additionally been reported (Dvorak 2005). MCs can also form synapses for targeted secretion (Table 4).With regard to the possibility that, under certain circumstances, almost all molecules that can be produced by a MC might be able to act as mediators, four release options are of particular interest: (1) diffusion of substances into the extracellular space; (2) release of mRNA, microRNA, and proteins expressed in the MC by secretion of exosomes and vesicles (Savage et al. 2023), some of them containing KIT (Pfeiffer et al. 2022); (3) formation of nanotubules with exchange of intracellular material which seems to be involved in inducing apoptosis in cancer cells (Ahani et al. 2022); and (4) formation of MC extracellular traps (Möllerherm et al. 2016; Table 4). These four mechanisms, by which MCs can use almost any molecule as a mediator, underline the extraordinary role of these cells in our immune system. At the same time, this creates an almost insurmountable hurdle for precisely attributing specific clinical symptoms to specific messenger substances. This problem of assigning (a) certain MC mediator(s) to symptoms is further complicated by the fact that released MC mediators can maintain and enhance MC activation in autocrine and paracrine manners (Fig. 1), and additionally by the possibility of MCs taking up substances from their immediate environment and then re-releasing them. In this context, it has to be noted that MCs are able to survive even complete degranulation followed by regranulation (Iskarpatyoti et al. 2022). Interestingly, MCs have altered granule contents and structure after regranulation, likely depending on the trigger that had induced the degranulation (Friend et al. 1996; Iskarpatyoti et al. 2022, further references therein).
Table 4.
Forms of communication between mast cells and effector cells
| • Mediator release by degranulation | |
| • Selective exocytotic mediator release | |
| Untargeted piecemeal degranulation (Theoharides et al. 1982) | |
| Untargeted by differential release (Theoharides and Douglas 1978; Theoharides et al. 1982; Moon et al. 2014) | |
| Targeted by synaptic contact with with the target cell (Carroll-Portillo et al. 2012) | |
| Targeted by mast cell extracellular traps of DNA (Möllerherm et al. 2016; Garcia-Rodriguez et al. 2020) | |
| • Release of exosomes containing mRNA, microRNA,and proteins (D’Incà and Pucillo 2015; Liang et al. 2018; Kim et al. 2018; Klein and Sagi-Eisenberg 2019; Shefler et al. 2021) | |
| • Diffusion of mediators into the extracellular space (Kritikou et al. 2016; Chen and Popel 2007) | |
| • Formation of nanotubules with exchange of intracellular material (Elishmereni et al. 2011; Ahani et al. 2022) |
Clinical impact
It does not require a great imagination to envision that the very same mechanisms which enable MCs to protect the organism can wreak focused or multisystem havoc when uncontrolled, potentially causing a vast array of diseases, some of which might be quite severe. In this context, primary systemic MC disease (dominantly MC activation syndrome (MCAS)) is of particular interest for at least two reasons: (1) its prevalence of about 20% (Molderings et al. 2013; Maitland et al. 2020) represents a significant socio-economic problem; and (2) due to its epigenetic causation with transgenerational transmission (Molderings 2022), it tends to manifest in successive generations more severely and at steadily earlier ages, creating increasing treatment challenges. Systemic mast cell disease (also presently termed mast cell activation disease (MCAD)), in its assorted variants (including systemic mastocytosis and MCAS), is usually driven, at the level of the individual, by multiple stem cell germline and somatic mutations (emerging out of complex interactions between stressor-induced cytokine storms and a genome rendered insufficiently robust, by the aforementioned epigenetic variants, at repairing or eradicating induced mutations) leading directly or indirectly to inappropriate chronic constitutive and reactive activation of the affected MCs (Weinstock et al. 2021). Due to both their widespread distribution and the great heterogeneity of aberrant mediator expression patterns, symptoms may occur in all organs and tissues. Hence, the clinical presentation of MCAD disease is very diverse, with a myriad of combinations of symptoms, ranging in the severity of illness from trivial to disabling and even life-threatening (Afrin et al. 2016).
Perspective
The present survey of the potential MC mediators in the narrower sense (Online Resource 1 and 2) and broader sense (Table 4), together with the findings of autocrine and paracrine stimulation and the ability of the MC to (re)use substances it takes up as mediators, are not of interest merely to researchers. These tables can be consulted by attending physicians, too, when trying to gain clarity about MC mediators which may be involved in patients with MC disease symptoms which are often resistant to therapy, such as hyper-/hypotension, transient tachyarrhthmias, or migrating pain. Such a procedure might be extraordinarily effective if, based on the available tables and with the help of special computer programs to be developed, all the information contained in relevant databases such as GeneCards®, PubMed, EMBL’s European Bioinformatics Institute, Embase, Cochrane Library, and others could help link the symptoms in a patient to given mediator expression profiles, thereby hopefully providing personalized therapeutic insights. This might enable the selection of treatments (Molderings et al. 2016) more likely to help patients exhibiting specific MC-mediator-induced symptoms. Ultimately, though, routine performance in the clinical laboratory of MC-specific genome sequencing (using pipelines already in place in many laboratories for sequencing the tumor cells in biopsies, but re-tuned, likely based on strong CD117 expression, to select the MCs in the sample) will be needed to discover not only which mutational profiles reliably correlate with which symptom profiles but also which treatments will best address the phenotypes driven by particular mutational profiles.
Supplementary information
(XLSX 51 kb)
(DOCX 35 kb)
Author contributions
G.J.M. and L.B.A. conceived and designed the research project and contributed to the analysis of the data. Both G.J.M. and L.B.A. wrote the manuscript. Both authors read and approved the manuscript. The authors confirm that no paper mill and artificial intelligence was used.
Funding
Open Access funding enabled and organized by Projekt DEAL. The investigations were supported by a grant of the Förderclub Mastzellforschunge.V.
Data availability
Not applicable
Declarations
Ethical approval
Not applicable
Competing interests
Dr. Molderings is chief medical officer of the start-up company MC Sciences, Ltd. Dr. Afrin is an uncompensated volunteer medical advisor to the start-up company MC Sciences Ltd.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afrin LB, Butterfield JH, Raithel M, Molderings GJ. Often seen, rarely recognized: mast cell activation disease--a guide to diagnosis and therapeutic options. Ann Med. 2016;48:190–201. doi: 10.3109/07853890.2016.1161231. [DOI] [PubMed] [Google Scholar]
- Ahani E, Fereydouni M, Motaghed M, Kepley CL. Identification and characterization of tunneling nanotubes involved in human mast cell FcεRI-mediated apoptosis of cancer cells. Cancers (Basel) 2022;14:2944. doi: 10.3390/cancers14122944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alysandratos KD, Asadi S, Angelidou A, Zhang B, Sismanopoulos N, Yang H, Critchfield A, Theoharides TC. Neurotensin and CRH interactions augment human mast cell activation. Plos One. 2012;7:e48934. doi: 10.1371/journal.pone.0048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babina M, Guhl S, Stärke A, Kirchhof L, Zuberbier T, Henz BM. Comparative cytokine profile of human skin mast cells from two compartments--strong resemblance with monocytes at baseline but induction of IL-5 by IL-4 priming. J Leukoc Biol. 2004;75:244–252. doi: 10.1189/jlb.0403157. [DOI] [PubMed] [Google Scholar]
- Bachelet I, Munitz A, Mankutad D, Levi-Schaffer F. Mast cell costimulation by CD226/CD112 (DNAM-1/Nectin-2): a novel interface in the allergic process. J Biol Chem. 2006;281:27190–27196. doi: 10.1074/jbc.M602359200. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Maurelli S, Melck D, De Petrocellis L, Di Marzo V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J Biol Chem. 1997;272:3315–3323. doi: 10.1074/jbc.272.6.3315. [DOI] [PubMed] [Google Scholar]
- Braile M, Marcella S, Marone G, Galdiero MR, Varricchi G, Loffredo S. The interplay between the immune and the endocannabinoid systems in cancer. Cells. 2021;10:1282. doi: 10.3390/cells10061282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll-Portillo A, Surviladze Z, Cambi A, Lidke DS, Wilson BS. Mast cell synapses and exosomes: membrane contacts for information exchange. Front Immunol. 2012;3:46. doi: 10.3389/fimmu.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Popel AS. Vascular and perivascular nitric oxide release and transport: biochemical pathways of neuronal nitric oxide synthase (NOS1) and endothelial nitric oxide synthase (NOS3) Free Radic Biol Med. 2007;42:811–822. doi: 10.1016/j.freeradbiomed.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivellato E, Travan L, Ribatti D. The phylogenetic profile of mast cells. Methods Mol Biol. 2015;1220:11–27. doi: 10.1007/978-1-4939-1568-2_2. [DOI] [PubMed] [Google Scholar]
- D’Incà F, Pucillo CE. Exosomes: tiny clues for mast cell communication. Front Immunol. 2015;6:73. doi: 10.3389/fimmu.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger A, Zhou Z, Norton SK, Lenk R, Conrad D, Kepley CL. Uptake and distribution of fullerenes in human mast cells. Nanomed. 2010;6:575–582. doi: 10.1016/j.nano.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak AM. Piecemeal degranulation of basophils and mast cells is effected by vesicular transport of stored secretory granule contents. Chem Immunol Allergy. 2005;85:135–184. doi: 10.1159/000086516. [DOI] [PubMed] [Google Scholar]
- Ekström K, Valadi H, Sjöstrand M, Malmhäll C, Bossios A, Eldh M, Lötvall J (2012) Characterization of mRNA and microRNA in human mast cell-derived exosomes and their transfer to other mast cells and blood CD34 progenitor cells. J Extracell Vesicles 1:18389. 10.3402/jev.v1i0.18389 [DOI] [PMC free article] [PubMed]
- Elishmereni M, Alenius HT, Bradding P, Mizrahi S, Shikotra A, Minai-Fleminger Y, Mankuta D, Eliashar R, Zabucchi G, Levi-Schaffer F. Physical interactions between mast cells and eosinophils: a novel mechanism enhancing eosinophil survival in vitro. Allergy. 2011;66:376–385. doi: 10.1111/j.1398-9995.2010.02494.x. [DOI] [PubMed] [Google Scholar]
- Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol. 1996;135:279–290. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rodriguez KM, Bahri R, Sattentau C, Roberts IS, Goenka A, Bulfone-Paus S. Human mast cells exhibit an individualized pattern of antimicrobial responses. Immun Inflamm Dis. 2020;8:198–210. doi: 10.1002/iid3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gri G, Frossi B, D'Inca F, Danelli L, Betto E, Mion F, Sibilano R, Pucillo C. Mast cell: an emerging partner in immune interaction. Front Immunol. 2012;3:120. doi: 10.3389/fimmu.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch B, Herms S, Molderings GJ. The transcriptome of the human mast cell leukemia cells HMC-1.2: an approach to identify specific changes in the gene expression profile in KitD816V systemic mastocytosis. Immunol Res. 2013;56:155–162. doi: 10.1007/s12026-013-8391-1. [DOI] [PubMed] [Google Scholar]
- Halloran KM, Parkes MD, Chang J, Timofte IL, Snell GI, Westall GP, Hachem R, Kreisel D, Trulock E, Roux A, Juvet S, Keshavjee S, Jaksch P, Klepetko W, Halloran PF. Molecular assessment of rejection and injury in lung transplant biopsies. J Heart Lung Transplant. 2019;38:504–513. doi: 10.1016/j.healun.2019.01.1317. [DOI] [PubMed] [Google Scholar]
- He S, McEuen AR, Blewett SA, Li P, Buckley MG, Leufkens P, Walls AF. The inhibition of mast cell activation by neutrophil lactoferrin: uptake by mast cells and interaction with tryptase, chymase and cathepsin G. Biochem Pharmacol. 2003;65:1007–1015. doi: 10.1016/s0006-2952(02)01651-9. [DOI] [PubMed] [Google Scholar]
- He S, Zhang H, Zeng X, Yang P. Self-amplification mechanisms of mast cell activation: a new look in allergy. Curr Mol Med. 2012;12:1329–1339. doi: 10.2174/156652412803833544. [DOI] [PubMed] [Google Scholar]
- Higashi N, Maeda R, Sesoko N, Isono M, Ishikawa S, Tani Y, Takahashi K, Oku T, Higashi K, Onishi S, Nakajima M, Irimura T. Chondroitin sulfate E blocks enzymatic action of heparanase and heparanase-induced cellular responses. Biochem Biophys Res Commun. 2019;520:152–158. doi: 10.1016/j.bbrc.2019.09.126. [DOI] [PubMed] [Google Scholar]
- Huszti Z. Histamine uptake into non-neuronal brain cells. Inflamm Res. 2003;52(Suppl 1):S03–S06. doi: 10.1007/s000110300028. [DOI] [PubMed] [Google Scholar]
- Ibelgaufts H (2023) COPE -Cytokines & Cells Online Pathfinder Encyclopaedia. http://www.cells-talk.com/index.php/page/about. Last accessed March 2023
- Iskarpatyoti JA, Shi J, Abraham MA, Rathore APS, Miao Y, Abraham SN. Mast cell regranulation requires a metabolic switch involving mTORC1 and a glucose-6-phosphate transporter. Cell Rep. 2022;40:111346. doi: 10.1016/j.celrep.2022.111346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayapal M, Tay HK, Reghunathan R, Zhi L, Chow KK, Rauff M, Melendez AJ. Genome-wide gene expression profiling of human mast cells stimulated by IgE or FcepsilonRI-aggregation reveals a complex network of genes involved in inflammatory responses. BMC Genomics. 2006;7:210. doi: 10.1186/1471-2164-7-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Kanaoka Y, Feng C, Nocka K, Rao S, Boyce JA. Cutting edge: Interleukin 4-dependent mast cell proliferation requires autocrine/intracrine cysteinyl leukotriene-induced signaling. J Immunol. 2006;177:2755–2759. doi: 10.4049/jimmunol.177.5.2755. [DOI] [PubMed] [Google Scholar]
- Kim DK, Cho YE, Komarow HD, Bandara G, Song BJ, Olivera A, Metcalfe DD. Mastocytosis-derived extracellular vesicles exhibit a mast cell signature, transfer KIT to stellate cells, and promote their activation. Proc Natl Acad Sci U S A. 2018;115:E10692–E10701. doi: 10.1073/pnas.1809938115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein O, Sagi-Eisenberg R. Anaphylactic degranulation of mast cells: focus on compound exocytosis. J Immunol Res. 2019;2019:9542656. doi: 10.1155/2019/9542656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritikou E, Kuiper J, Kovanen PT, Bot I. The impact of mast cells on cardiovascular diseases. Eur J Pharmacol. 2016;778:103–115. doi: 10.1016/j.ejphar.2015.04.050. [DOI] [PubMed] [Google Scholar]
- Le DD, Schmit D, Heck S, Omlor AJ, Sester M, Herr C, Schick B, Daubeuf F, Fähndrich S, Bals R, Frossard N, Al Kadah B, Dinh QT. Increase of mast cell-nerve association and neuropeptide receptor expression on mast cells in perennial allergic rhinitis. Neuroimmunomodulation. 2016;023:261–270. doi: 10.1159/000453068. [DOI] [PubMed] [Google Scholar]
- Liang Y, Qiao L, Peng X, Cui Z, Yin Y, Liao H, Jiang M, Li L. The chemokine receptor CCR1 is identified in mast cell-derived exosomes. Am J Transl Res. 2018;10:352–367. [PMC free article] [PubMed] [Google Scholar]
- Lundequist A, Pejler G. Biological implications of preformed mast cell mediators. Cell Mol Life Sci. 2011;68:965–975. doi: 10.1007/s00018-010-0587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland A, Brock I, Reed W (2020) Immune dysfunction, both mast cell activation disorders and primary immune deficiency, is common among patients with hypermobile spectrum disorder (HSD) or hypermobile type Ehlers Danlos Syndrome (hEDS). In Proceedings of the EDS ECHO Summit, October 2, 2020; session 12, poster number 001, available online: https://www.ehlers-danlos.com/wp-content/uploads/2020/09/Poster-001-FINAL-A-Maitland-et-al-EDS-ECHO-SUMMIT-Oct-2020.pdf
- Marquardt DL, Gruber HE, Wasserman SI. Adenosine release from stimulated mast cells. Proc Natl Acad Sci U S A. 1984;81:6192–6196. doi: 10.1073/pnas.81.19.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale C, Mohammed Z, Deppen J, Gomez G. Interleukin-6 potentiates FcεRI-induced PGD2 biosynthesis and induces VEGF from human in situ-matured skin mast cells. Biochim Biophys Acta Gen Subj. 2018;1862:1069–1078. doi: 10.1016/j.bbagen.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralda I, Samanas NB, Seo AJ, Foronda JS, Sachen J, Hui Y, Morrison SD, Oskeritzian CA, Piliponsky AM. Siglec-9 is an inhibitory receptor on human mast cells in vitro. J Allergy Clin Immunol. 2023;S0091-6749(23):00511-0. doi: 10.1016/j.jaci.2023.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molderings GJ. Systemic mast cell activation disease variants and certain genetically determined comorbidities may be consequences of a common underlying epigenetic disease. Med Hypotheses. 2022;163:110862. doi: 10.1016/j.mehy.2022.110862. [DOI] [Google Scholar]
- Molderings GJ, Haenisch B, Bogdanow M, Fimmers R, Nöthen MM. Familial occurrence of systemic mast cell activation disease. Plos One. 2013;8:e76241. doi: 10.1371/journal.pone.0076241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molderings GJ, Haenisch B, Brettner S, Homann J, Menzen M, Dumoulin FL, Panse J, Butterfield J, Afrin LB. Pharmacological treatment options for mast cell activation disease. Naunyn Schmiedeberg's Arch Pharmacol. 2016;389:671–694. doi: 10.1007/s00210-016-1247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möllerherm H, von Köckritz-Blickwede M, Branitzki-Heinemann K. Antimicrobial activity of mast cells: role and relevance of extracellular DNA traps. Front Immunol. 2016;7:265. doi: 10.3389/fimmu.2016.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon TC, Befus AD, Kulka M. Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol. 2014;5:569. doi: 10.3389/fimmu.2014.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motakis E, Guhl S, Ishizu Y, Itoh M, Kawaji H, de Hoon M, Lassmann T, Carninci P, Hayashizaki Y, Zuberbier T, Forrest AR, Babina M, FANTOM consortium Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood. 2014;123:e58–e67. doi: 10.1182/blood-2013-02-483792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhoffer N, Levy R, Sick E, Andre P, Coupin G, Lombard Y, Gies JP. Amyloid beta peptides trigger CD47-dependent mast cell secretory and phagocytic responses. Int J Immunopathol Pharmacol. 2009;22:473–483. doi: 10.1177/039463200902200224. [DOI] [PubMed] [Google Scholar]
- Noordenbos T, Blijdorp I, Chen S, Stap J, Mul E, Cañete JD, Lubberts E, Yeremenko N, Baeten D. Human mast cells capture, store, and release bioactive, exogenous IL-17A. J Leukoc Biol. 2016;100:453–462. doi: 10.1189/jlb.3HI1215-542R. [DOI] [PubMed] [Google Scholar]
- Norrby K. Do mast cells contribute to the continued survival of vertebrates? APMIS. 2022;130:618–624. doi: 10.1111/apm.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama Y. Mast cell-derived cytokine expression induced via Fc receptors and Toll-like receptors. Chem Immunol Allergy. 2005;87:101–110. doi: 10.1159/000087574. [DOI] [PubMed] [Google Scholar]
- Oskeritzian CA, Alvarez SE, Hait NC, Price MM, Milstien S, Spiegel S. Distinct roles of sphingosine kinases 1 and 2 in human mast-cell functions. Blood. 2008;111:4193–4200. doi: 10.1182/blood-2007-09-115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruchuri S, Jiang Y, Feng C, Francis SA, Plutzky J, Boyce JA. Leukotriene E4 activates peroxisome proliferator-activated receptor gamma and induces prostaglandin D2 generation by human mast cells. J Biol Chem. 2008;283:16477–16487. doi: 10.1074/jbc.M705822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng WM, Maintz L, Allam JP, Raap U, Gütgemann I, Kirfel J, Wardelmann E, Perner S, Zhao W, Fimmers R, Walgenbach K, Oldenburg J, Schwartz LB, Novak N. Increased circulating levels of neurotrophins and elevated expression of their high-affinity receptors on skin and gut mast cells in mastocytosis. Blood. 2013;122:1779–1788. doi: 10.1182/blood-2012-12-469882. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Petersen JD, Falduto GH, Anderson DE, Zimmerberg J, Metcalfe DD, Olivera A. Selective immunocapture reveals neoplastic human mast cells secrete distinct microvesicle- and exosome-like populations of KIT-containing extracellular vesicles. J Extracell Vesicles. 2022;11:e12272. doi: 10.1002/jev2.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poglio S, De Toni-Costes F, Arnaud E, Laharrague P, Espinosa E, Casteilla L, Cousin B. Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells. 2010;28:2065–2072. doi: 10.1002/stem.523. [DOI] [PubMed] [Google Scholar]
- Reinheimer T, Vogel P, Racké K, Bittinger F, Kirkpatrick CJ, Saloga J, Knop J, Wessler I. Non-neuronal acetylcholine is increased in chronic inflammation like atopic dermatitis. Naunyn-Schmiedeberg’s Arch Pharmacol (Suppl) 1998;358:R87. [Google Scholar]
- Savage A, Risquez C, Gomi K, Schreiner R, Borczuk AC, Worgall S, Silver RB. The mast cell exosome-fibroblast connection: a novel pro-fibrotic pathway. Front Med (Lausanne) 2023;10:1139397. doi: 10.3389/fmed.2023.1139397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman ES, Glaum MC, Post T, Wang Y, Raible DG, Mohanty J, Butterfield JH, Pelleg A. ATP modulates anti-IgE-induced release of histamine from human lung mast cells. Am J Respir Cell Mol Biol. 1999;20:530–537. doi: 10.1165/ajrcmb.20.3.3387. [DOI] [PubMed] [Google Scholar]
- Shefler I, Salamon P, Mekori YA. Extracellular vesicles as emerging players in intercellular communication: relevance in mast cell-mediated pathophysiology. Int J Mol Sci. 2021;22:9176. doi: 10.3390/ijms22179176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Bondy PK, Tsakalos ND, Askenase PW. Differential release of serotonin and histamine from mast cells. Nature. 1982;297:229–231. doi: 10.1038/297229a0. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Douglas WW. Secretion in mast cells induced by calcium entrapped within phospholipid vesicles. Science. 1978;201:1143–1145. doi: 10.1126/science.684435. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Perlman AI, Twahir A, Kempuraj D (2023) Mast cell activation: beyond histamine and tryptase. Expert Rev Clin Immunol 1-16. 10.1080/1744666X.2023.2200936. Epub ahead of print [DOI] [PubMed]
- Tsai SH, Takeda K. Regulation of allergic inflammation by the ectoenzyme E-NPP3 (CD203c) on basophils and mast cells. Semin Immunopathol. 2016;38:571–579. doi: 10.1007/s00281-016-0564-2. [DOI] [PubMed] [Google Scholar]
- Weinstock LB, Pace LA, Rezaie A, Afrin LB, Molderings GJ. Mast cell activation syndrome: a primer for the gastroenterologist. Dig Dis Sci. 2021;66:965–982. doi: 10.1007/s10620-020-06264-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX 51 kb)
(DOCX 35 kb)
Data Availability Statement
Not applicable


