Abstract
Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) is a serious clinical syndrome with a high morbidity and mortality. Presently, therapeutic approaches for ALI/ARDS primarily revolve around symptomatic supportive care encompassing mechanical ventilation and fluid management. Regrettably, the prognosis for most ALI/ARDS patients remains bleak due to the absence of effective treatment strategies. Even survivors of ALI/ARDS may have long-term pulmonary dysfunction and cognitive impairment. The quality of life has been seriously compromised. The emergence of mesenchymal stem cells (MSCs) and their exosomes has opened up an expansive realm of potential and optimism for addressing the plight of ALI/ARDS patients, as MSCs and their derived exosomes exhibit multifaceted capabilities, including anti-inflammatory properties, facilitation of tissue repair and regeneration, and apoptosis inhibition. Therefore, future research should focus on the possible mechanisms of MSCs and their derived exosomes for the treatment of ALI/ARDS and open up new avenues for their clinical applications.
Keywords: MSCs, Exosomes, Acute lung injury, ARDS (acute respiratory disease syndrome)
1. Acute lung injury
Acute lung injury (ALI) or its severe form acute respiratory distress syndrome (ARDS) stands as a prevailing and intricate clinical syndrome observed in critically ill patients. It is an acute respiratory failure caused by non-cardiogenic intrapulmonary and extrapulmonary factors, and its hallmark clinical presentation involves the onset of acute or progressive hypoxic respiratory failure (Fig. 1) [1]. Currently, there is no specific treatment for ALI/ARDS, the main therapeutic strategies approaches encompass supportive measures such as mechanical ventilation, administration of glucocorticoids, and controlled fluid intake [2]. The mortality rate among patients remains notably high, particularly in severe cases, with reported mortality reaching 46.1% [3]. The quest for an efficacious intervention strategy for ALI/ARDS remains a pressing concern.
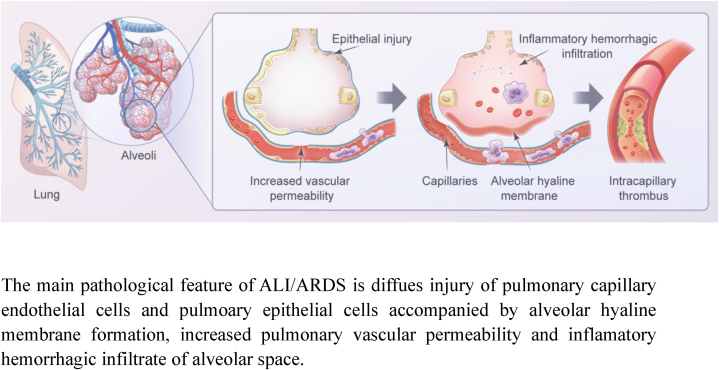
Fig. 1.
The pathological features of ALI/ARDS.
The main pathological feature of ALI/ARDS is diffues injury of pulmonary capillary endothelial cells and pulmoary epithelial cells accompanied by alveolar hyaline membrane formation, increased pulmonary vascular permeability and inflamatory hemorrhagic infiltrate of alveolar space.
2. MSCs
Mesenchymal stem cells (MSCs) represent a class of pluripotent stem cells derived from a variety of organs and tissues, such as bone marrow, adipose tissue, muscle, umbilical cord, and placenta [4]. According to the minimal defining criteria of human MSCs established by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) [5]: (1). MSCs demonstrate plastic adherence, appearing as fibroblasts, and possess the capacity for osteogenic, chondrogenic, and adipogenic differentiation. (2). In addition, MSCs exhibit the presence of cell surface markers such as CD73, CD90, and CD105, while lacking the expression of hematopoietic and endothelial antigens (CD14 or CD11b, CD34, CD45, HLA-DR) and MSCs must be plastically adherent under standard culture conditions. The field of regenerative medicine has increasingly recognized MSCs due to their convenient procurement, in vitro self-renewal, multidirectional differentiation, and migration potential [6,7]. Notably, MSCs exhibit attributes characterized by anti-inflammatory and immunomodulatory properties, fostering tissue and organ regeneration, which play an indispensable role in various inflammatory and autoimmune diseases [[8], [9], [10]].
3. MSCs and ALI/ARDS
3.1. MSCs can reduce inflammation response in ALI/ARDS
MSCs emerge as a promising avenue for ALI/ARDS intervention (Fig. 2). MSCs exhibit the capacity to mitigate alveolar epithelium permeability, dampen the inflammatory cytokine response to injury, heighten the expression of anti-inflammatory cytokines, notably IL-10, and curtail the infiltration of inflammatory cells [11]. These advantageous outcomes primarily stem from the paracrine effects of MSCs. Through paracrine secretion of keratinocyte growth factor (KGF), MSCs facilitate the restoration of impaired alveolar fluid clearance by type II alveolar epithelial cells [12]. Remarkably, KGF also exerts a protective role in ALI induced by E. coli endotoxin, manifesting in the reduction of pulmonary edema and lung inflammation [13].
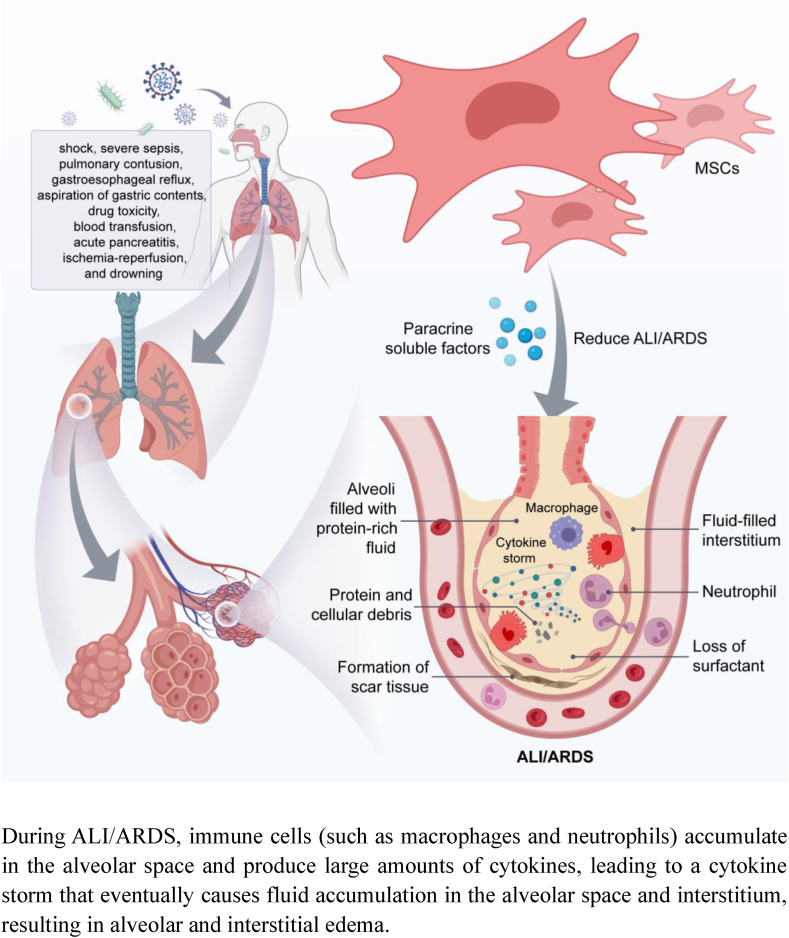
Fig. 2.
MSCs attenuate ALI/ARDS through paracrine soluble factors
During ALI/ARDS, immune cells (such as macrophages and neutrophils) accumulate in the alveolar space and produce large amounts of cytokines, leading to a cytokine storm that eventually causes fluid accumulation in the alveolar space and interstitium, resulting in alveolar and interstitial edema.
3.2. MSCs can protect endothelial barrier and epithelial injury in ALI/ARDS
Within the context of ALI/ARDS, an investigation illuminated MSCs' potential to safeguard both the endothelial barrier and epithelial well-being [14]. Notably, the reparative influence of hepatocyte growth factor (HGF), secreted by MSCs, was identified in the context of LPS-triggered pulmonary microvascular endothelial barrier dysfunction.The paracrine dynamics of MSCs further emerge as crucial in immune modulation. Evidently, MSCs, through paracrine HGF, orchestrate the conversion of mature dendritic cells (DCs) into tolerogenic DCs, thereby mitigating pathological lung injury [15].
3.3. Clinical application of MSCs in ALI/ARDS
Furthermore, beyond their efficacy in preclinical investigations concerning ALI, the therapeutic potential of MSCs has been substantiated in patients afflicted with ALI/ARDS (Table 1). In a multicenter clinical trial of MSCs for the treatment of ALI caused by novel coronavirus, the administration of MSCs yielded notable benefits. Specifically, patients subjected to MSC treatment exhibited significant advancements in lung lesion absorption when juxtaposed against the placebo-receiving cohort [8]. Similarly, an exploration of MSC therapy in severe COVID-19 cases unveiled a 10.8% enhancement in overall lung lesion volume within the MSC-treated group, coupled with an augmentation in the 6-min walk distance, a significant indicator of functional improvement. This contrasted with outcomes within the placebo group [16]. Lanzoni et al. performed another double-blind, randomized, controlled trial (Twenty-four subjects were randomized 1:1 to either MSCs treatment or the control group) and found that MSCs infusions significantly decreased cytokine levels at day 6 and improved survival in COVID-19 patients with ARDS [17]. Monsel A et al. found no significant difference in the ratio change of the partial pressure of oxygen to fractional inspired oxygen (PaO2/FiO2) at baseline and day 7 in ARDS patients with intravenous Umbilical cord-derived MSCs compared with the placebo group [18]. The research undertaken by Kaffash Farkhad N et al. echoed favorable outcomes.Intravenous MSC infusion significantly ameliorated the SPO2/FiO2 ratio and lowered levels of serum CRP, IL-6, IFN-g, TNF-α, and IL-17A. Regrettably, discernible alterations in patients' chest CT scan images eluded detection [19]. Notwithstanding MSCs' pivotal and advantageous contributions to ALI mitigation, the safety profile of their clinical deployment necessitates further evaluation. This arises from their tumorigenic predisposition and the potential to incite vascular embolism [20,21].
Table 1.
Selected studies on the therapeutic effect of MSCs in the clinical application of ALI/ARDS.
| Author | Publication time | MSCs source | Results | Reference |
|---|---|---|---|---|
| Shi et al. | 2021 | Human umbilical cord (UC)-derived MSCs | Improvement in whole lung lesion and improved restoration of the integrated reserve capability of patients. No significant improvement in lymphocyte count subpopulations, oxygen therapy status, or mMRC dyspnea score in patients | [8] |
| Shi et al. | 2022 | Human UC-MSCs | UC-MSCs improved total lung lesions in severe COVID-19 patients, restored pulmonary reserve capacity, and did not affect the production and maintenance of neutralizing antibodies in patients. | [16] |
| Lanzoni G et al. | 2021 | Human UC-MSCs | UC-MSCs reduced the levels of inflammatory factors such as GM-CSF, IFNg, IL-5, IL-6, IL-7, TNFa, TNFb, PDGF-BB and improved the survival of ARDS patients | [17] |
| Monsel A et al. | 2022 | Human UC-MSCs | No significant between-group secondary-endpoint differences were observed for SOFA scores, PaO2/FiO2 ratios, compliance, organ-failure–free days, ventilation-free days, duration of ventilation. Later decreases of inflammatory markers in the UC-MSCs-treated group were the only significant difference found | [18] |
| Kaffash Farkhad N et al. | 2022 | Human UC-MSCs | Intravenous UC-MSCs significantly improved SPO2/FiO2 and the levels of serum CRP, IL-6, IFN-g, TNF-α, and IL-17A. No significant changes were observed in CT scan images of patients during the study period. | [19] |
4. The characteristics of MSCs-derived extracellular vesicles and exosome biogenesis
4.1. Classification of MSCs-EV
MSCs-derived extracellular vesicles (EVs) have garnered significant attention as a pivotal research focal point within the realm of ALI repair. The International Society of Extracellular Vesicles (ISEV) offers a contemporary classification for EVs based on several criteria: (1). Physical characteristics encompassing density density (low, middle, high, with each range defined), or size [“small EVs” (<200 nm) and “medium/large EVs” (>200 nm)]; (2). Biochemical composition: CD63+/CD81+-EVs, Annexin A5-stained EVs, etc. (3). Cell of origin: podocyte EVs, hypoxic EVs, large oncosomes, apoptotic bodies [22]. In light of their diameter size and biogenesis pathway, EVs are conventionally grouped into three subtypes, apoptotic bodies (50–5000 nm), microvesicles (100–1000 nm), and exosomes (30–150 nm) (Fig. 3) [23]. The prevalent method for EV isolation and concentration is ultracentrifugation [24]. However, ultracentrifugation does not yield highly pure EVs. Although serial washing can increase purity, they also diminish yield [25]. Size-based fractionation methods are faster and easier to implement than conventional methods, while producing EVs with comparable or superior purity and functional activity [26]. Certain markers can be used to identify exosomes, microvesicles (MVs), and apoptotic bodies. For example, CD9, CD63, CD81, TSG101, and Alix are specific markers of exosomes; CD63, CD9, and CD81 are members of the tetraspanins family and they are directly involved in the sorting of extracellular vesicle contents. TSG101 is an endosomal sorting complex required for transport (ESCRT) complex-associated protein. Alix is directly involved in the process of membrane vesicle separation from the plasma membrane to form independent membrane structures. ARF6, VCAMP3, P-selectin, and Integrins are MVs markers. Apoptotic bodies, in turn, exhibit markers such as TSP and C3b [[27], [28], [29], [30]]. Most MSCs-EVs preparations are characterized according to the Minimal Information for Studies of EVs (MISEV2014), published by the International Society for Extracellular Vesicles (ISEV) in 2014 [31]. There's a need for MSCs-EVs to be defined by quantifiable metrics. These metrics should encompass the identification of EV cellular origin, the presence of lipid-membrane vesicles, and the extent of physical and biochemical vesicle integrity. Various potential metrics have been proposed for MSCs-EVs, including the ratio of MSCs to non-MSCs surface antigens, the ratio of membrane lipids to proteins, the ratio of specific lipids, the concentration of membrane lipid vesicles, vesicle integrity, and biological activity [32]. Given the unlikelihood of achieving global uniform standardization for MSCs-EVs production, the challenges lie in quantifying and validating each metric and developing analytical methods to predict the therapeutic efficiency of MSCs-EVs. This necessitates the establishment of quantifiable, robust, and reproducible parameters, addressing the critical need for standardization in order to ensure the reliability and reproducibility of EVs research.
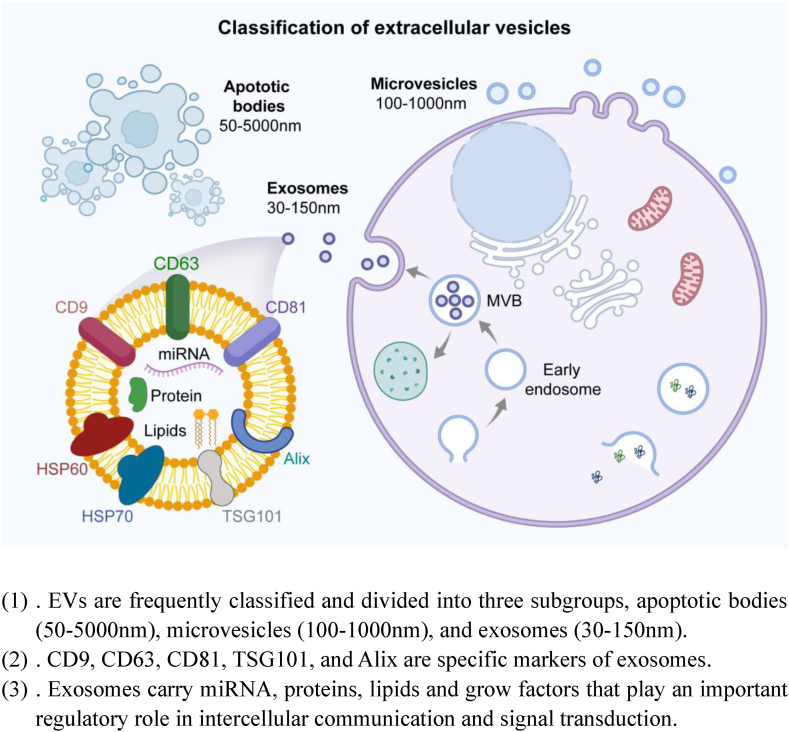
Fig. 3.
Classification of MSCs-EV
(1). EVs are frequently classified and divided into three subgroups, apoptotic bodies (50–5000 nm), microvesicles (100–1000 nm), and exosomes (30–150 nm).
(2). CD9, CD63, CD81, TSG101, and Alix are specific markers of exosomes.
(3). Exosomes carry miRNA, proteins, lipids and grow factors that play an important regulatory role in intercellular communication and signal transduction.
4.2. Biogenesis of MSCs-EV
Exosomes, the most diminutive subtype of extracellular vesicles (EVs), exhibit a diameter ranging from 30 to 150 nm. They originate from the endosomal pathway, where the membranes of multivesicular bodies (MVBs) invaginate inwards to generate intraluminal vesicles (ILVs) within these MVBs. Upon the fusion of MVBs with the plasma membrane, the enclosed ILVs are extruded into the extracellular milieu as exosomes [33,34]. Microvesicles (MVs) are shedding vesicles that budded directly from the plasma membrane [35]. Apoptotic bodies, the largest EVs, materialize as a consequence of blebbing from apoptotic cells [36]. Various molecules and mechanisms contribute to exosome biogenesis, loading, and intracellular transport. Molecules alone or in the form of different complexes called endosomal sorting complexes required for transport (ESCRT) machinery mediate exosome biogenesis [37].
4.3. Exosomes biogenesis and application
Exosome biogenesis from virus-infected cells may contribute to promoting virus infection and suppression of immune cell response, as the receptors (CD9 and ACE2) located on exosomes accelerate viral entry and escape from immune cell recognition [38]. Exosome biogenesis has also been shown to reduce the inflammatory response during aging [39]. Exosomes, furthermore, serve as vital tools for inducing autophagy flow within target cells through the transfer of autophagy activators and autophagy-related molecules. The interplay between exosome biogenesis and autophagy plays a central role in maintaining cellular homeostasis [40]. The investigators found that in hydrogen peroxide-treated human umbilical vein cells, expression levels of factors linked to exosome biogenesis and secretion were markedly heightened, while the autophagic pathway faced substantial inhibition [41]. The inhibition of autophagy also promoted the secretion and biogenesis of exosomes from CD146+ cells [42]. The investigators also identified increased exosome biogenesis in polyhedral oligomeric silsesquioxane nanoparticles-stimulated human endothelial cells (HUVECs) consistent with upregulation of angiogenic factors such as VEGRR-2, Ang-1, and Ang-2, and proposed the hypothesis that exosomes may enhance angiogenesis [43]. Exosomes derived from hypoxic tumor cells have the potential to promote tumorigenesis by facilitating metastasis, angiogenesis, and the modulation of immune responses. Inhibition of the biogenesis of these exosomes could offer a therapeutic avenue to mitigate tumorigenesis [44]. Moreover, a study showed that metformin could promote exosome biogenesis and secretion in human multiform glioblastoma cells. The enhanced exosome biogenesis and secretion may be a protective method against drug stress conditions or a strategy allowing cells for cells to modulate the cytotoxic effects of metformin, thereby enhancing the therapeutic resistance of neighboring cells [45]. In rat models of asthma, a pro-inflammatory response evoked by the upregulation of IL-4 and TNF-a expression could promote exosome biogenesis, which may alter the paracrine activity within lung tissue during asthmatic states [46].
5. MSCs-derived EVs and ALI/ARDS
5.1. Biological function of exosomes
Exosomes serve as transporters of miRNA, proteins, lipids, and growth factors, thereby playing a pivotal role in governing intercellular communication and signal transduction., and exosomes be used as an alternative to stem cells for the treatment of various diseases [[47], [48], [49]]. An intriguing discovery emerged from a study showcasing shifts in specific exosomal cargo within senescent cells—namely, augmented miRNA-21 and diminished miRNA-126 levels. These alterations hold potential as aging biomarkers for age-related conditions [50].
Presenting a cell-free therapeutic approach, exosomes adeptly circumvent immune responses and the propensity for tumor formation. Their non-immunogenic nature and robust biostability enable them to penetrate deeply into tissues while evading the risk of organ embolization [[51], [52], [53]]. Due to these advantages, the prospect of MSCs-derived exosomes in ALI has been underestimated. Indeed, MSCs-derived exosomes exhibit the capability to elevate oxidative phosphorylation levels within alveolar macrophages, thereby reinstating macrophage metabolism, nurturing immune homeostasis, and mitigating lung inflammation. This is achieved through the transfer of mitochondrial components [54]. Additionally, MSCs-derived exosomes participate in the transmission of cellular contents, including miRNA, to recipient cells. This transfer leads to a reduction in inflammatory responses and oxidative stress, thereby diminishing lung injury (Table 1) [55].
5.2. Mechanism of ALI/ARDS mitigation by MSCs-MVs
Due to partial overlap in the size of exosomes and MVs, MVs may also play a reparative role in lung injury (Table 2). Particularly, MSCs-derived MVs showcase the potential to bolster alveolar fluid clearance, diminish lung protein permeability, and curtail bacterial presence in compromised alveoli. This feat is achieved through the transfer of keratinocyte growth factor (KGF) mRNA from MVs to recipient cells, thereby instigating a restorative process [56]. Additionally, in murine models of acute lung injury (ALI), MVs derived from MSCs exert a beneficial influence by tempering lung inflammation provoked by lipopolysaccharide (LPS), and reinstating the integrity of alveolar capillaries. Notably, this effect is partly orchestrated by angiopoietin-1 (Ang1) mRNA [57].
Table 2.
The role of MSCs-EV in acute lung injury (ALI).
| Author | Publication time | Animal type | Lung injury model | MSCs source | The subtype of MSCs-EVs | Result | Reference |
|---|---|---|---|---|---|---|---|
| Tang et al. | 2017 | C57BL/6 male mice | LPS-induced ALI | Bone marrow | MSCs-MVs | MSCs-MVs ameliorated the lung inflammation induced by LPS in mice, including the influx of WBCs and neutrophils, and MIP-2 secretion, Meanwhile, MSC-MVs restored the pulmonary capillary permeability. Ang-1 mRNA partly mediated the therapeutic effects of MSC MVs | [56] |
| Monsel A et al. | 2015 | C57BL/6 mice | E. coli Pneumonia–induced ALI | Bone marrow | MSCs-MVs | Administration of MVs derived from human MSCs improved survival in part through keratinocyte growth factor secretion and decreased the influx of inflammatory cells, cytokines, protein, and bacteria in mice injured with bacterial pneumonia. | [57] |
| Islam MN et al. | 2012 | C57BL/6J mice | LPS-Induced ALI | Bone marrow | MSCs-MVs | MSCs can release mitochondria-containing MVs that are phagocytosed by damaged alveolar epithelium, thereby improving bioenergetics in the alveolar epithelium. | [58] |
| Morrison TJ et al. | 2017 | C57BL/6 mice | LPS-induced ALI | Bone marrow | MSCs-EVs | The transfer of functional mitochondria in EVs is responsible for anti-inflammatory and phagocytosis-enhancing effects on macrophages in the inflammatory environment through the promotion of oxidative phosphorylation, thus ameliorating lung injury. | [59] |
| Li et al. | 2019 | C57/BL6 mice | Lung ischemia/reperfusion (I/R) model | Bone marrow | MSCs-Exos | MSC-Exo could reduce oxidative stress-induced apoptosis while partially reducing the pro-inflammatory polarization of alveolar macrophage induced by hypoxia/reoxygenation in an exosomal miR-21-5p-dependent manner, thus alleviating IRI in lung. | [60] |
| Liu et al. | 2021 | Sprague-Dawley rats | LPS-induced ALI | Bone marrow | MSCs-Exos | Exosomal miR-384–5p resulted in relieving LPS-injured autophagy disorder in alveolar macrophages by targeting Beclin-1. | [61] |
| Tian et al. | 2021 | C57BL/6 mice | CLP-induced ALI | Adipose | MSCs-Exos | Exosomal miR-16–5p from ADSCs promotes macrophage polarization and attenuates septic lung injury in mice via suppressing TLR4. | [62] |
| Xu et al. | 2022 | C57Bl/6J mice | LPS-induced ALI | Bone marrow | MSCs-Exos | Exosomes and miR-150 partially attenuated lung inflammation, including total cells, neutrophils, macrophages, TNF-α, IL-6, and IL-1β and microvascular endothelial cell injury through the MAPK pathways. | [63] |
| Wu et al. | 2021 | SD rats | Hyperoxia-induced ALI (HILI) | Bone marrow | MSCs-Exos | MSCs-Exos derived-miR-425 targeted PTEN mRNA and upregulated PI3K/AKT axis, and promoted lung epithelial cell survival to inhibit HILI. | [64] |
| Xiao et al. | 2020 | C57BL/6 mice | LPS-induced ALI | Bone marrow | MSCs-Exos | MSC-exosome transmitted miR-23a-3p and miR-182–5p reversed the progression of LPS-induced lung injury and fibrosis through inhibiting NF-κB and hedgehog pathways via silencing Ikbkb and destabilizing IKKβ. | [65] |
Not to be overlooked, MSCs-derived MVs demonstrate their reparative efficacy in lung injury induced by severe bacterial pneumonia. This is primarily achieved through the attenuation of inflammatory cell infiltration and cytokine activity. The therapeutic significance of this process is underscored by KGF's pivotal role in orchestrating the outcome [58]. In addition, MVs could also increase intracellular ATP levels in damaged type 2 alveolar epithelial cells [58].
Revealing a fascinating facet, a study showcased that MSCs derived from bone marrow released MVs carrying mitochondria. These MVs were phagocytosed by compromised alveolar epithelial cells, thereby ameliorating bioenergetics in these cells [59]. Phagocytosed mitochondria-containing MVs increased the survival of LPS-induced ALI mice, which was decreased if MSCs contained dysfunctional mitochondria or lacked Connexin 43 [59]. Another study demonstrated that MSCs could promote an anti-inflammatory and highly phagocytic macrophage phenotype through EV-mediated mitochondrial transfer [60].
5.3. MSCs-exosomes can attenuate ALI/ARDS by transferring miRNA
Li et al. conducted a study that unveiled the crucial involvement of exosomal miRNA-21–5p in ALI repair. This miRNA fosters M2 macrophage polarization while simultaneously mitigating the production of pro-inflammatory cytokines, thereby contributing to the repair process [61]. The miRNA-384–5p capsuled in bone marrow MSCs-derived exosomes could attenuate ALI-induced impairment of alveolar macrophage autophagy by targeting Beclin-1, thus reducing the inflammatory responses [62]. Furthermore, exosomes derived from adipose MSCs exhibit the ability to curb Toll-like receptor 4 (TLR4) activity by transporting miRNA-16–5p. This action promotes M2 macrophage polarization, ultimately ameliorating sepsis-induced ALI in mice [63].
Exosomal miRNA-150 could attenuate pulmonary microvascular endothelial cell injury via the MAPK signaling pathway [64]. In a model of hyperoxia-induced acute lung injury, miRNA-425 in MSCs-derived exosomes could reduce apoptosis of alveolar epithelial cells by targeting PTEN and upregulating the PI3K/AKT axis [65]. A previous research effort conducted by our team substantiated that miRNA enclosed in MSCs-derived exosomes effectively counteracts epithelial-mesenchymal transition in alveolar epithelial cells. Moreover, it mitigates LPS-induced lung injury and lung fibrosis by suppressing NF-kB and Hedgehog signaling pathways [69].
It's paramount to recognize that a growing body of research suggests that miRNAs are often not present in sufficient functional concentrations and may not be efficiently delivered to recipient cells. Some researchers have engineered extracellular vesicles (EVs) to be fusogenic, aiming to enhance their ability to carry functional messenger RNAs. Nevertheless, such engineering efforts haven't conclusively demonstrated the alteration of cellular functionality when exposed to miRNA-containing EVs [70].
Crucially, establishing the role of exosomal miRNA in mediating the therapeutic effects of MSCs-derived exosomes necessitates the prerequisite of biologically relevant miRNA concentrations [71]. It was reported that only a fraction of the miRNA identified in MSCs were secreted in MSCs-derived exosomes [72]. Similarly, research has indicated that a majority of individual exosomes within standard preparations do not carry biologically significant quantities of miRNA. Consequently, the functionality of individual exosomes in miRNA communication remains uncertain [73]. Thus, further examination is warranted to determine whether MSCs-derived EVs indeed exert their biological effects through miRNA transfer.
5.4. MSCs-exosomes may play a role in ALI/ARDS through the proteome
MSCs-derived exosomes exhibit a multifaceted influence on various cellular processes, encompassing migration, proliferation, matrix synthesis, and macrophage infiltration, all culminating in the facilitation of tissue repair. Researchers have attributed this positive effect to the CD73 protein contained in exosomes [74]. Remarkably, MSCs-derived exosomes have demonstrated efficacy in diminishing the extent of myocardial infarction within a mouse model of myocardial ischemia-reperfusion injury, an outcome attributed to a protein-mediated mechanism [75]. Milk fat globule-epidermal growth factor-factor 8 (MFG-E8) contained in exosomes promoted M2 polarization of macrophages via the SOCS3/STAT3 signaling pathway, consequently, this action improves the inflammatory microenvironment and curtails neuronal cell apoptosis [76]. The ubiquitin carboxyl-terminal hydrolase isozyme L3 (UCHL3) in cell-derived exosomes induced by mechanical stimulation promoted osteogenesis in MSCs, thereby facilitating alveolar bone remodeling during orthodontic tooth movement [77]. Furthermore, heightened expression of galactoglucosan-1 in exosomes derived from TNF-α pre-treated MSCs augments the anti-inflammatory phenotypic transformation of macrophages via the JAK-STAT signaling pathway. This sequence of events leads to reduced intrauterine adhesions and endometrial fibrosis [78]. Zhu et al. found that MSCs-derived exosomes could alleviate Escherichia coli endotoxin-induced ALI, wherein KGF carried by exosomes was deemed essential [13]. The therapeutic effect of MSCs-exosomes on neonatal rat models of hyperoxia lung injury (HLI) showed that the mechanism of MSCs-exosomes in reducing HLI in neonatal rats was mainly mediated by VEGF encapsulated within the exosome [79]. In addition, MSCs-derived exosomes could profoundly ameliorate pulmonary inflammation and alveolar-capillary leakage via exosomal factor TSG-6 (a secreted anti-inflammatory protein) [80]. Interestingly, MSCs have been revealed to modulate intracellular oxidative stress by employing microvesicles mediated by arrestin domain-containing protein 1, enabling the targeting of depolarized mitochondria to the plasma membrane. This orchestration ultimately mitigates silica-induced lung injury [81]. Collectively, the cited studies illuminate the pivotal role of MSCs-derived exosomal proteome in modulating inflammatory responses and promoting tissue damage repair. However, there remains a need for future research to delve into the precise impact of MSCs-derived exosomal proteome within the context of ALI/ARDS. Furthermore, exosomal proteome contents hold promise as diagnostic biomarkers and therapeutic targets for various diseases. For example, Yao et al. found that retinol-binding protein 4 (RBP4), which is enriched in serum exosomes. RBP4's activation of the NF-kB pathway and promotion of reactive oxygen species (ROS) accumulation were associated with M1 polarization of Kupffer cells and hepatic lipid accumulation [82].
5.5. Clinical application of MSCs and MSCs-exosomes in ALI/ARDS
MSCs-derived exosomes hold the capacity to effectively curtail cytokine storms and mitigate tissue damage by virtue of their anti-inflammatory and immunomodulatory properties [83].The promise they exhibit for clinical application in the context of ALI/ARDS can be attributed to several factors, including their reduced tumorigenicity, straightforward storage requirements, ready availability, safety profile, and the ability to downregulate cytokine storms [[66], [84], [85], [86]]. Sengupta et al. found that bone marrow-derived exosomes were used to treat severe COVID-19 patients. The treatment's effectiveness was evaluated over a 14-day period, during which the survival rate of patients reached 83%. Concurrently, laboratory results indicated a reduction in C-reactive protein and ferritin levels, alongside substantial improvements in the counts of neutrophils and CD4+ and CD8+ positive lymphocytes [66] (see Table 3). Results of a trial of nebulized treatment with MSCs-exosomes given to seven patients with COVID-19 pneumonia showed that exosomes promoted the absorption of lung lesions, shorten the duration of hospitalization, and did not cause secondary allergic reactions [67]. In addition, Zarrabi M et al. found that intravenous administration of MSCs-derived exosomes effectively lowered serum inflammatory markers such as IL-6, TNF-α and IFN-γ in ARDS patients without serious adverse effects [68]. Despite the beneficial therapeutic results achieved with MSCs-EVs in patients with COVID-19, their approval for use remains pending. This is owing to the need for appropriate manufacturing and quality control regulations, as well as the requisite reliance on preclinical data derived from animal models and clinical data extracted from MSCs-related clinical trials. A comprehensive evaluation of the potential benefits and risks of MSCs-derived EVs for COVID-19 is imperative. Achieving this balance necessitates a rigorous assessment of EVs through well-designed clinical trials guided by rational protocols and meticulous oversight [87].
Table 3.
Selected studies on the therapeutic effect of MSCs-exosomes in the clinical application of ALI/ARDS.
| Author | Publication time | MSCs-exosomes source | Results | Reference |
|---|---|---|---|---|
| Sengupta V et al. | 2020 | Bone marrow-derived MSCs-exosomes | The laboratory results suggested a decrease in C-reactive protein and ferritin levels and a significant improvement in the number of neutrophils and CD4+ and CD8+ positive lymphocytes | [66] |
| Chu M et al. | 2022 | UC-MSCs-exosomes | MSCs-exosomes could promote the absorption of lung lesions, shorten the duration of hospitalization, and did not cause secondary allergic reactions. | [67] |
| Zarrabi M et al. | 2023 | Perinatal tissue-derived MSCs-exosomes | MSCs-derived exosomes reduced serum inflammatory markers such as IL-6, TNF-α and IFN-γ in ARDS patients. | [68] |
6. Conclusion and perspectives
The promising outcomes observed in the realm of MSCs-derived exosomes herald a bright future for ALI treatment. Their mechanism of action predominantly hinges on the transfer of bioactive components like miRNA to target cells, thereby instigating immunomodulation and damage repair. Nonetheless, the potential multifaceted mechanisms of exosomes require further exploration through extensive studies.
The domain of EV application, however, is riddled with challenges. The contribution of EVs in the communication between tumor cells and the secondary microenvironment supports the formation of the pre-metastatic niche and intensification of tumor development [88]. Meanwhile, tumor-derived exosomes are involved in tumorigenesis through the induction of niche formation, metastasis, angiogenesis, and immune regulation [88,89]. In addition, tumor cell-derived exosomes and their exosomal CircRNAs hold promise as diagnostic and prognostic markers for cancer, reshaping the landscape of targeted cancer therapy [90,91]. Tumor cell and immune cell-derived exosomes also assume pivotal roles in host defense against pathogen invasion by modulating innate immune signaling and reprogramming immune responses [92]. Plant-derived exosomes also have promising clinical applications as cell-free therapies due to their heightened biocompatibility and biodegradability [93]. As the field advances, it becomes crucial to delve into not only EV biogenesis, characterization, and normal condition definitions but also to scrutinize inhibitory effects of specific EV types under pathological conditions. Although a large number of experiments have been performed, the variety of methods used to isolate and characterize EVs makes it challenging to analyze and draw conclusions. Therefore, more advanced and efficient techniques are urgently needed. Moreover, the challenge of large-scale exosome production remains, as achieving global standardization for MSCs and MSCs-derived EV production is unfeasible. Few protocols comply with Good Manufacturing Practice (GMP) standards, and only a few have been issued [94]. Establishing GMP-compliant exosome production protocols, characterized by precise characterization and identification methods, is pivotal for their use in clinical trials [95]. Furthermore, the differing characteristics of MSCs-derived exosomes from various tissue sources or culture conditions need to be addressed to determine optimal treatment conditions for ALI through preclinical studies. The development of accurate exosome loading methods for detection is also essential. Defining stringent “identity” and “efficacy” criteria and addressing potential side effects are prerequisites for the therapeutic use of MSCs-derived EVs [32]. As research advances, MSCs-derived exosomes, as an innovative cell-free treatment approach, hold potential to emerge as a powerful therapeutic alternative to stem cells, contributing to swift and substantial developments in the field.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors approved the publication of the manuscript.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Chang Liu, Email: 1471780945@qq.com.
Mei Yang, Email: 3148882@qq.com.
References
- 1.Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N. Engl. J. Med. 2017;377(6):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 2.Saguil A., Fargo M.V. Acute respiratory distress syndrome: diagnosis and management. Am. Fam. Physician. 2020;101(12):730–738. [PubMed] [Google Scholar]
- 3.Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 4.Brown C., McKee C., Bakshi S., Walker K., Hakman E., Halassy S., et al. Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13(9):1738–1755. doi: 10.1002/term.2914. [DOI] [PubMed] [Google Scholar]
- 5.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Fuentes D.E., Fernández-Garza L.E., Samia-Meza J.A., Barrera-Barrera S.A., Caplan A.I., Barrera-Saldaña H.A. Mesenchymal stem cells current clinical applications: a systematic review. Arch. Med. Res. 2021;52(1):93–101. doi: 10.1016/j.arcmed.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Han Y., Li X., Zhang Y., Han Y., Chang F., Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8) doi: 10.3390/cells8080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi L., Huang H., Lu X., Yan X., Jiang X., Xu R., et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Targeted Ther. 2021;6(1):58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan X., Yan F., Mohammed H.A.G., Liu O. Maxillofacial-derived mesenchymal stem cells: characteristics and progress in tissue regeneration. Stem Cell. Int. 2021;2021 doi: 10.1155/2021/5516521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbaspanah B., Reyhani S., Mousavi S.H. Applications of umbilical cord derived mesenchymal stem cells in autoimmune and immunological disorders: from literature to clinical Practice. Curr. Stem Cell Res. Ther. 2021;16(4):454–464. doi: 10.2174/1574888X16999201124153000. [DOI] [PubMed] [Google Scholar]
- 11.Laffey J.G., Matthay M.A. Fifty years of research in ARDS. Cell-Based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am. J. Respir. Crit. Care Med. 2017;196(3):266–273. doi: 10.1164/rccm.201701-0107CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J.W., Wu X. Mesenchymal stem cells ameliorate LPS-induced acute lung injury through KGF promoting alveolar fluid clearance of alveolar type II cells. Eur. Rev. Med. Pharmacol. Sci. 2015;19(13):2368–2378. [PubMed] [Google Scholar]
- 13.Zhu Y.G., Feng X.M., Abbott J., Fang X.H., Hao Q., Monsel A., et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cell. 2014;32(1):116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng S.S., Guo F.M., Huang L.L., Huang Y.Z., Xie J.F., Yang C.S., et al. mTORC2 activation mediated by mesenchymal stem cell-secreted hepatocyte growth factors for the recovery of lipopolysaccharide-induced vascular endothelial barrier. Stem Cell. Int. 2021;2021 doi: 10.1155/2021/9981589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Z., Chang W., Meng S., Xu X., Xie J., Guo F., et al. Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res. Ther. 2019;10(1):372. doi: 10.1186/s13287-019-1488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi L., Yuan X., Yao W., Wang S., Zhang C., Zhang B., et al. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanzoni G., Linetsky E., Correa D., Messinger Cayetano S., Alvarez R.A., Kouroupis D., et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10(5):660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monsel A., Hauw-Berlemont C., Mebarki M., Heming N., Mayaux J., Nguekap Tchoumba O., et al. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit. Care. 2022;26(1):48. doi: 10.1186/s13054-022-03930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaffash Farkhad N., Sedaghat A., Reihani H., Adhami Moghadam A., Bagheri Moghadam A., Khadem Ghaebi N., et al. Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: a successful phase 1, control-placebo group, clinical trial. Stem Cell Res. Ther. 2022;13(1):283. doi: 10.1186/s13287-022-02920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu W., Huang L., Li Y., Qian H., Shan X., Yan Y., et al. Mesenchymal stem cell-secreted soluble signaling molecules potentiate tumor growth. Cell Cycle. 2011;10(18):3198–3207. doi: 10.4161/cc.10.18.17638. [DOI] [PubMed] [Google Scholar]
- 21.Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7(1) doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abreu S.C., Lopes-Pacheco M., Weiss D.J., Rocco P.R.M. Mesenchymal stromal cell-derived extracellular vesicles in lung diseases: current status and perspectives. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.600711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardiner C., Di Vizio D., Sahoo S., Théry C., Witwer K.W., Wauben M., et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J. Extracell. Vesicles. 2016;5 doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webber J., Clayton A. How pure are your vesicles? J. Extracell. Vesicles. 2013;2 doi: 10.3402/jev.v2i0.19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busatto S., Vilanilam G., Ticer T., Lin W.L., Dickson D.W., Shapiro S., et al. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells. 2018;7(12) doi: 10.3390/cells7120273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akers J.C., Gonda D., Kim R., Carter B.S., Chen C.C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro Oncol. 2013;113(1):1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaiswal R., Sedger L.M. Intercellular vesicular transfer by exosomes, microparticles and oncosomes - implications for cancer biology and treatments. Front. Oncol. 2019;9:125. doi: 10.3389/fonc.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandham S., Su X., Wood J., Nocera A.L., Alli S.C., Milane L., et al. Technologies and standardization in research on extracellular vesicles. Trends Biotechnol. 2020;38(10):1066–1098. doi: 10.1016/j.tibtech.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kugeratski F.G., Hodge K., Lilla S., McAndrews K.M., Zhou X., Hwang R.F., et al. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat. Cell Biol. 2021;23(6):631–641. doi: 10.1038/s41556-021-00693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lötvall J., Hill A.F., Hochberg F., Buzás E.I., Di Vizio D., Gardiner C., et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 2014;3 doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witwer K.W., Van Balkom B.W.M., Bruno S., Choo A., Dominici M., Gimona M., et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles. 2019;8(1) doi: 10.1080/20013078.2019.1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kowal J., Tkach M., Théry C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Gurunathan S., Kang M.H., Jeyaraj M., Qasim M., Kim J.H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4) doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camussi G., Deregibus M.C., Bruno S., Cantaluppi V., Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78(9):838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 36.Record M., Silvente-Poirot S., Poirot M., Wakelam M.J.O. Extracellular vesicles: lipids as key components of their biogenesis and functions. J. Lipid Res. 2018;59(8):1316–1324. doi: 10.1194/jlr.E086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juan T., Fürthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin. Cell Dev. Biol. 2018;74:66–77. doi: 10.1016/j.semcdb.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Hassanpour M., Rezaie J., Nouri M., Panahi Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmadi M., Rezaie J. Ageing and mesenchymal stem cells derived exosomes: molecular insight and challenges. Cell Biochem. Funct. 2021;39(1):60–66. doi: 10.1002/cbf.3602. [DOI] [PubMed] [Google Scholar]
- 40.Hassanpour M., Rezabakhsh A., Rezaie J., Nouri M., Rahbarghazi R. Exosomal cargos modulate autophagy in recipient cells via different signaling pathways. Cell Biosci. 2020;10:92. doi: 10.1186/s13578-020-00455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahbubfam S., Rezaie J., Nejati V. Crosstalk between exosomes signaling pathway and autophagy flux in senescent human endothelial cells. Tissue Cell. 2022;76 doi: 10.1016/j.tice.2022.101803. [DOI] [PubMed] [Google Scholar]
- 42.Hassanpour M., Rezaie J., Darabi M., Hiradfar A., Rahbarghazi R., Nouri M. Autophagy modulation altered differentiation capacity of CD146(+) cells toward endothelial cells, pericytes, and cardiomyocytes. Stem Cell Res. Ther. 2020;11(1):139. doi: 10.1186/s13287-020-01656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feghhi M., Rezaie J., Akbari A., Jabbari N., Jafari H., Seidi F., et al. Effect of multi-functional polyhydroxylated polyhedral oligomeric silsesquioxane (POSS) nanoparticles on the angiogenesis and exosome biogenesis in human umbilical vein endothelial cells (HUVECs) Mater. Des. 2021;197 [Google Scholar]
- 44.Jafari R., Rahbarghazi R., Ahmadi M., Hassanpour M., Rezaie J. Hypoxic exosomes orchestrate tumorigenesis: molecular mechanisms and therapeutic implications. J. Transl. Med. 2020;18(1):474. doi: 10.1186/s12967-020-02662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soraya H., Sani N.A., Jabbari N., Rezaie J. Metformin increases exosome biogenesis and secretion in U87 MG human glioblastoma cells: a possible mechanism of therapeutic resistance. Arch. Med. Res. 2021;52(2):151–162. doi: 10.1016/j.arcmed.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Almohammai A., Rahbarghazi R., Keyhanmanesh R., Rezaie J., Ahmadi M. Asthmatic condition induced the activity of exosome secretory pathway in rat pulmonary tissues. J. Inflamm. 2021;18(1):14. doi: 10.1186/s12950-021-00275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu. Rev. Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 48.Liu C., Xiao K., Xie L. Advances in the use of exosomes for the treatment of ALI/ARDS. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.971189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joo H.S., Suh J.H., Lee H.J., Bang E.S., Lee J.M. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int. J. Mol. Sci. 2020;21(3) doi: 10.3390/ijms21030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaban S.A., Rezaie J., Nejati V. Exosomes derived from senescent endothelial cells contain distinct pro-angiogenic miRNAs and proteins. Cardiovasc. Toxicol. 2022;22(6):592–601. doi: 10.1007/s12012-022-09740-y. [DOI] [PubMed] [Google Scholar]
- 51.Riazifar M., Pone E.J., Lötvall J., Zhao W. Stem cell extracellular vesicles: extended messages of regeneration. Annu. Rev. Pharmacol. Toxicol. 2017;57:125–154. doi: 10.1146/annurev-pharmtox-061616-030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., Bi J., Huang J., Tang Y., Du S., Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020;15:6917–6934. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikfarjam S., Rezaie J., Zolbanin N.M., Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J. Transl. Med. 2020;18(1):449. doi: 10.1186/s12967-020-02622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia L., Zhang C., Lv N., Liang Z., Ma T., Cheng H., et al. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics. 2022;12(6):2928–2947. doi: 10.7150/thno.69533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng Y., Liu J., Chen P., Lin L., Luo Y., Ma X., et al. Exosomal miR-22-3p from human umbilical cord blood-derived mesenchymal stem cells protects against lipopolysaccharid-induced acute lung injury. Life Sci. 2021;269 doi: 10.1016/j.lfs.2020.119004. [DOI] [PubMed] [Google Scholar]
- 56.Park J., Kim S., Lim H., Liu A., Hu S., Lee J., et al. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax. 2019;74(1):43–50. doi: 10.1136/thoraxjnl-2018-211576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang X.D., Shi L., Monsel A., Li X.Y., Zhu H.L., Zhu Y.G., et al. Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by ang-1 mRNA. Stem Cell. 2017;35(7):1849–1859. doi: 10.1002/stem.2619. [DOI] [PubMed] [Google Scholar]
- 58.Monsel A., Zhu Y.G., Gennai S., Hao Q., Hu S., Rouby J.J., et al. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am. J. Respir. Crit. Care Med. 2015;192(3):324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Islam M.N., Das S.R., Emin M.T., Wei M., Sun L., Westphalen K., et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morrison T.J., Jackson M.V., Cunningham E.K., Kissenpfennig A., McAuley D.F., O'Kane C.M., et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 2017;196(10):1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J.W., Wei L., Han Z., Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur. J. Pharmacol. 2019;852:68–76. doi: 10.1016/j.ejphar.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 62.Liu X., Gao C., Wang Y., Niu L., Jiang S., Pan S. BMSC-derived exosomes ameliorate LPS-induced acute lung injury by miR-384-5p-controlled alveolar macrophage autophagy. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/9973457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tian J., Cui X., Sun J., Zhang J. Exosomal microRNA-16-5p from adipose mesenchymal stem cells promotes TLR4-mediated M2 macrophage polarization in septic lung injury. Int. Immunopharm. 2021;98 doi: 10.1016/j.intimp.2021.107835. [DOI] [PubMed] [Google Scholar]
- 64.Xu J., Xu D., Yu Z., Fu Z., Lv Z., Meng L., et al. Exosomal miR-150 partially attenuated acute lung injury by mediating microvascular endothelial cells and MAPK pathway. Biosci. Rep. 2022;42(1) doi: 10.1042/BSR20203363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y., Li J., Yuan R., Deng Z., Wu X. Bone marrow mesenchymal stem cell-derived exosomes alleviate hyperoxia-induced lung injury via the manipulation of microRNA-425. Arch. Biochem. Biophys. 2021;697 doi: 10.1016/j.abb.2020.108712. [DOI] [PubMed] [Google Scholar]
- 66.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cell. Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chu M., Wang H., Bian L., Huang J., Wu D., Zhang R., et al. Nebulization therapy with umbilical cord mesenchymal stem cell-derived exosomes for COVID-19 pneumonia. Stem Cell Rev Rep. 2022;18(6):2152–2163. doi: 10.1007/s12015-022-10398-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zarrabi M., Shahrbaf M.A., Nouri M., Shekari F., Hosseini S.E., Hashemian S.R., et al. Allogenic mesenchymal stromal cells and their extracellular vesicles in COVID-19 induced ARDS: a randomized controlled trial. Stem Cell Res. Ther. 2023;14(1):169. doi: 10.1186/s13287-023-03402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiao K., He W., Guan W., Hou F., Yan P., Xu J., et al. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020;11(10):863. doi: 10.1038/s41419-020-03034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Albanese M., Chen Y.A., Hüls C., Gärtner K., Tagawa T., Mejias-Perez E., et al. MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. PLoS Genet. 2021;17(12) doi: 10.1371/journal.pgen.1009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toh W.S., Lai R.C., Zhang B., Lim S.K. MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 2018;46(4):843–853. doi: 10.1042/BST20180079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen T.S., Lai R.C., Lee M.M., Choo A.B., Lee C.N., Lim S.K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38(1):215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chevillet J.R., Kang Q., Ruf I.K., Briggs H.A., Vojtech L.N., Hughes S.M., et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. U.S.A. 2014;111(41):14888–14893. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang S., Chuah S.J., Lai R.C., Hui J.H.P., Lim S.K., Toh W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 75.Lai R.C., Yeo R.W., Tan K.H., Lim S.K. Mesenchymal stem cell exosome ameliorates reperfusion injury through proteomic complementation. Regen. Med. 2013;8(2):197–209. doi: 10.2217/rme.13.4. [DOI] [PubMed] [Google Scholar]
- 76.Ren J., Zhu B., Gu G., Zhang W., Li J., Wang H., et al. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. 2023;14(1):70. doi: 10.1038/s41419-023-05607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pu P., Wu S., Zhang K., Xu H., Guan J., Jin Z., et al. Mechanical force induces macrophage-derived exosomal UCHL3 promoting bone marrow mesenchymal stem cell osteogenesis by targeting SMAD1. J. Nanobiotechnol. 2023;21(1):88. doi: 10.1186/s12951-023-01836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J., Pan Y., Yang J., Wang J., Jiang Q., Dou H., et al. Tumor necrosis factor-α-primed mesenchymal stem cell-derived exosomes promote M2 macrophage polarization via Galectin-1 and modify intrauterine adhesion on a novel murine model. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.945234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Braun R.K., Chetty C., Balasubramaniam V., Centanni R., Haraldsdottir K., Hematti P., et al. Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia. Biochem. Biophys. Res. Commun. 2018;503(4):2653–2658. doi: 10.1016/j.bbrc.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chaubey S., Thueson S., Ponnalagu D., Alam M.A., Gheorghe C.P., Aghai Z., et al. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res. Ther. 2018;9(1):173. doi: 10.1186/s13287-018-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phinney D.G., Di Giuseppe M., Njah J., Sala E., Shiva S., St Croix C.M., et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yao J.M., Ying H.Z., Zhang H.H., Qiu F.S., Wu J.Q., Yu C.H. Exosomal RBP4 potentiated hepatic lipid accumulation and inflammation in high-fat-diet-fed mice by promoting M1 polarization of Kupffer cells. Free Radic. Biol. Med. 2023;195:58–73. doi: 10.1016/j.freeradbiomed.2022.12.085. [DOI] [PubMed] [Google Scholar]
- 83.Xu R., Feng Z., Wang F.S. Mesenchymal stem cell treatment for COVID-19. EBioMedicine. 2022;77 doi: 10.1016/j.ebiom.2022.103920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mitrani M.I., Bellio M.A., Sagel A., Saylor M., Kapp W., VanOsdol K., et al. Case report: administration of amniotic fluid-derived nanoparticles in three severely ill COVID-19 patients. Front. Med. 2021;8 doi: 10.3389/fmed.2021.583842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yousefi Dehbidi M., Goodarzi N., Azhdari M.H., Doroudian M. Mesenchymal stem cells and their derived exosomes to combat Covid-19. Rev. Med. Virol. 2022;32(2) doi: 10.1002/rmv.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khanh V.C., Fukushige M., Chang Y.H., Hoang N.N., Yamashita T., Obata-Yasuoka M., et al. Wharton's jelly mesenchymal stem cell-derived extracellular vesicles reduce SARS-CoV2-induced inflammatory cytokines under high glucose and uremic toxin conditions. Stem Cell. Dev. 2021;30(15):758–772. doi: 10.1089/scd.2021.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Börger V., Weiss D.J., Anderson J.D., Borràs F.E., Bussolati B., Carter D.R.F., et al. International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: considerations for potential therapeutic agents to suppress coronavirus disease-19. Cytotherapy. 2020;22(9):482–485. doi: 10.1016/j.jcyt.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rezaie J., Ahmadi M., Ravanbakhsh R., Mojarad B., Mahbubfam S., Shaban S.A., et al. Tumor-derived extracellular vesicles: the metastatic organotropism drivers. Life Sci. 2022;289 doi: 10.1016/j.lfs.2021.120216. [DOI] [PubMed] [Google Scholar]
- 89.Rezaie J., Akbari A., Rahbarghazi R. Inhibition of extracellular vesicle biogenesis in tumor cells: a possible way to reduce tumorigenesis. Cell Biochem. Funct. 2022;40(3):248–262. doi: 10.1002/cbf.3695. [DOI] [PubMed] [Google Scholar]
- 90.Vahabi A., Rezaie J., Hassanpour M., Panahi Y., Nemati M., Rasmi Y., et al. Tumor Cells-derived exosomal CircRNAs: novel cancer drivers, molecular mechanisms, and clinical opportunities. Biochem. Pharmacol. 2022;200 doi: 10.1016/j.bcp.2022.115038. [DOI] [PubMed] [Google Scholar]
- 91.Rahbarghazi R., Jabbari N., Sani N.A., Asghari R., Salimi L., Kalashani S.A., et al. Tumor-derived extracellular vesicles: reliable tools for Cancer diagnosis and clinical applications. Cell Commun. Signal. 2019;17(1):73. doi: 10.1186/s12964-019-0390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rezaie J., Etemadi T., Feghhi M. The distinct roles of exosomes in innate immune responses and therapeutic applications in cancer. Eur. J. Pharmacol. 2022;933 doi: 10.1016/j.ejphar.2022.175292. [DOI] [PubMed] [Google Scholar]
- 93.Nemati M., Singh B., Mir R.A., Nemati M., Babaei A., Ahmadi M., et al. Plant-derived extracellular vesicles: a novel nanomedicine approach with advantages and challenges. Cell Commun. Signal. 2022;20(1):69. doi: 10.1186/s12964-022-00889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lener T., Gimona M., Aigner L., Börger V., Buzas E., Camussi G., et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J. Extracell. Vesicles. 2015;4 doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rezaie J., Feghhi M., Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun. Signal. 2022;20(1):145. doi: 10.1186/s12964-022-00959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.