Abstract
Antibody (Ab) validation is the procedure in which an Ab is thoroughly assayed for sensitivity and specificity in a given application. Validation of Abs against post-translationally modified (PTM) targets is particularly challenging because it requires specifically prepared antigen. Here we describe a novel validation method using surrogate proteins in a Western blot. The surrogate protein, which we termed ‘MILKSHAKE,’ is a modified maltose binding protein enzymatically conjugated to a peptide from the chosen target that is either modified or nonmodified at the residue of interest. The certainty of the residue’s modification status can be used to confirm Ab specificity. This method also allows for Ab validation even in the absence or limited availability of treated cell lysates.
Keywords: antibody, post-translational modification, sortase, validation, Western blot
METHOD SUMMARY
We have developed a novel method for validating Abs in a Western blot using modified maltose-binding protein enzymatically conjugated to a target peptide mixed with mammalian cell lysate as the antigen. This method can be used to confirm the primary antibody’s specificity for the target sequence and as a Western blot process control specific to the primary antibody used. We will demonstrate the utility of MILKSHAKE for post-translationally modified (PTM) targets and for determining specificity at an amino acid polymorphic site, but it should be apparent that it can also be used for non-PTMs.
The sometimes poor reproducibility of published research has been a major focus of the scientific community in recent years [1]. The sources of such problematic research can include insufficient description of the methods used in a study and the absence of internal controls that can be used to compare results across different experiments [2,3]. A major cause for concern is proper reagent validation and interexperimental consistency, including that of the antibodies (Abs) used in experiments. Many of these Ab-specific concerns have been incorporated into detailed recommendations for Ab validation before beginning experimental work [4,8]. Ab binding can vary between vendors and lots, and their performance can also depend on the conditions used in assays that measure their binding to their target. Even small changes in assay conditions such as a different blocking buffer or incubation time can change results [5].
This reproducibility issue increases in complexity when validating Abs against post-translationally modified (PTM) targets. Ab-based methods of study for these targets rely on the availability of Abs that can specifically recognize a modified amino acid residue within a protein or peptide. Antigens for PTM studies involve special preparation aimed at induction, reduction or retention of modification at a desired residue [9]. The methods that produce a site-specific modification are not always known, nor are these methods reliably reproducible themselves. Even when a modification treatment is discovered for a particular PTM, other variables such as batch-to-batch variation, cell health, kinase activity, storage conditions and researcher technique persist.
The most reliable method for validation for PTM-specific Abs is one in which each antigen sample contains the target sequence with either 100% modification or 100% nonmodification at the residue of interest. The method described in this study provides that certainty in a Western blot assay.
Transpeptidases like the sortase A enzyme have become important tools in protein modification. Researchers can achieve site-specific protein modification with desired substituents that cannot be installed by standard genetic approaches [6]. In this study, we took advantage of previous work showing that sortase A can accept substrates of varying structure [7]. We have developed a system in which a modified maltose binding protein (modMBP) is fused to a peptide containing our moiety of interest using sortase A conjugation. The resulting protein, which we call MILKSHAKE, can be used in Western blot to validate the specificity of binders to the modified or nonmodified epitope of interest. We believe this method will work with many types of PTMs, including, for example, acetylation, methylation, glycosylation and phosphorylation. The work in the research presented in this article will be focused on phosphoproteins because they are the most common PTM analyzed in Western blot. Positive binding to MILKSHAKE in Western blot correlates with binding to full length target proteins found in treated cell lysates. In addition, comparing the binding of an Ab to MILKSHAKE and to a treated cell lysate in Western blot can verify the quality of the lysate treatment itself.
Materials & methods
Source of enzymes
Enzymes (sortase, Nde I, Bam HI, ligase) were purchased from the manufacturer as indicated and used under the conditions specified.
Plasmid DNA construction
A plasmid expressing modified maltose binding protein (modMBP) was constructed by inserting C-terminal transglutaminase and sortase conjugation sites into the maltose binding protein expression vector pMAL-c5x (New England Biolabs [NEB], MA, USA; inserted sequence {PKPQQFMGGGGSLPETG}, using the Nde I and Bam HI restriction sites, also from NEB).
Protein purification
modMBP was purified from Escherichia coli cultures (NEBExpress, NEB). Cultures were grown at 37°C until an OD600 = 0.5 was reached. Cultures were then induced with 0.3 mM IPTG (AmericanBio, Inc., MA, USA) for an additional 1 h. After centrifugation, freeze–thaw and sonication, protein was purified from pellets via gravity flow using amylose resin (NEB) for immobilization and 10 mM maltose (MilliporeSigma, MA, USA) for elution. Protein was stored at −80°C in 15% glycerol.
Peptide design
Peptides (Biopeptek Pharmaceuticals LLC, PA, USA) conjugated to a modMBP contain an N-terminal sortase conjugation site {GGGSGSS}, a target-specific sequence (either phosphorylated or nonphosphorylated at a single amino acid) and a C-terminal hemagglutinin (HA) tag {YPYDVPDYA}.
Sortase conjugation reaction
The following components (per reaction) were mixed in a 96-well deep-well plate: 0.145 mg purified modMBP, 10x Sortase buffer [200 mM Tris-HCl, 1.5 M NaCl, 50 mM CaCl2, 2 mM betamercaptoethanol], 0.04-mg target peptide (design described above), 3-μg sortase enzyme [Moradec, CA, USA] and 1x phosphate-buffered saline (PBS) to final volume of 113 μl. One well without target peptide was used as a negative ‘unconjugated’ control. The plate was incubated with shaking at 250 rpm at 37°C for 2 h. Reactions were then mixed with equal volume of 2x Laemmli Sample Buffer (Bio-Rad Laboratories, CA, USA) containing 200 mM dithiothreitol (AmericanBio) and denatured at 95°C for 10 min.
Preparation of cell lysates
Thirty milliliters of HEK293-6E cells (National Research Council of Canada, Ottawa, Canada) at 1 × 106 cells/ml were pelleted by centrifugation and washed with 5 ml chilled 1x PBS. Each pellet was resuspended in 300 μl lysis buffer (RIPA buffer [MilliporeSigma] containing 3.2 mM sodium orthovanadate [NEB] and 10 μg each leupeptin, pepstatin, aprotinin and chymostatin [MilliporeSigma]), and incubated on ice for 15 min. Pellets were then combined and sonicated three times for 2 s each time and kept on ice. Resulting lysates were centrifuged for 10 min at 8000×g and quantified at A280 using a microplate reader. Lysates were then mixed with equal volume of 2x Laemmli Sample Buffer (Bio-Rad Laboratories) containing 200 mM dithiothreitol (AmericanBio) and denatured at 95°C for 10 min.
Polyacrylamide gel electrophoresis
Each lane of a 4–20% polyacrylamide gel (Bio-Rad Laboratories) was loaded with MILKSHAKE (modMBP conjugated to a target peptide) and prepared HEK lysate (as described earlier). Gels were run at 180V for 40 min in SDS buffer.
Western blot
After electrophoresis, proteins were transferred to nitrocellulose membranes using the Trans-Blot Turbo system (Bio-Rad Laboratories). Membranes were washed with 1X Tris buffered saline (TBS) for 5 min at room temperature. Membranes were incubated in blocking buffer (3% milk-TBST) for 1 h at room temperature followed by washing three times for 5 min each with 1X TBS with 0.1% Tween 20 (TBST). Primary Abs were added at a concentration of 1 μg/ml (unless otherwise specified) in 5% BSA-TBST and incubated shaking overnight at 4°C. The following day, membranes were washed with 1x TBST three times for 5 min each. Secondary HRP conjugated Abs were added using 3% milk-TBST at vendor recommended concentrations and incubated at room temperature for 1 h. Membranes were again washed with 1x TBST three times for 5 min each. Blots were visualized using chemiluminescence with exposure times ranging from 0.5 to 15 s (Bio-Rad Laboratories).
Rabbit immunization & Ab isolation
All rabbits were housed, maintained and inoculated by Covance Inc. (PA, USA). Covance’s Institutional Animal Care and Use Committee approved this study and is Association for Accreditation of Laboratory Animal Care accredited. Rabbits were immunized (subcutaneous route) with 0.5-mg target peptide conjugated to KLH carrier protein (Day 0) and boosted with 0.25 mg target peptide conjugated to Keyhole limpet hemocyanin carrier protein on days 14 and 28. Abs were generated from cultured B cells isolated from whole blood collection.
Results
Target peptide design
Peptides were designed for use in this method with three elements (Figure 1A). The first is an N-terminal sequence (GGGSGSS) that is essential for recognition of the peptide by the sortase enzyme. The second is the target sequence from a protein of interest. This sequence contains the modified residue in the center, flanked by 8 to 9 residues from the target protein. The third element is the C-terminal hemagglutinin (HA) tag that allows for downstream detection of the target peptide in a successfully conjugated MILKSHAKE protein.
Figure 1. Sortase-mediated conjugation of modified maltose binding protein with target peptides.
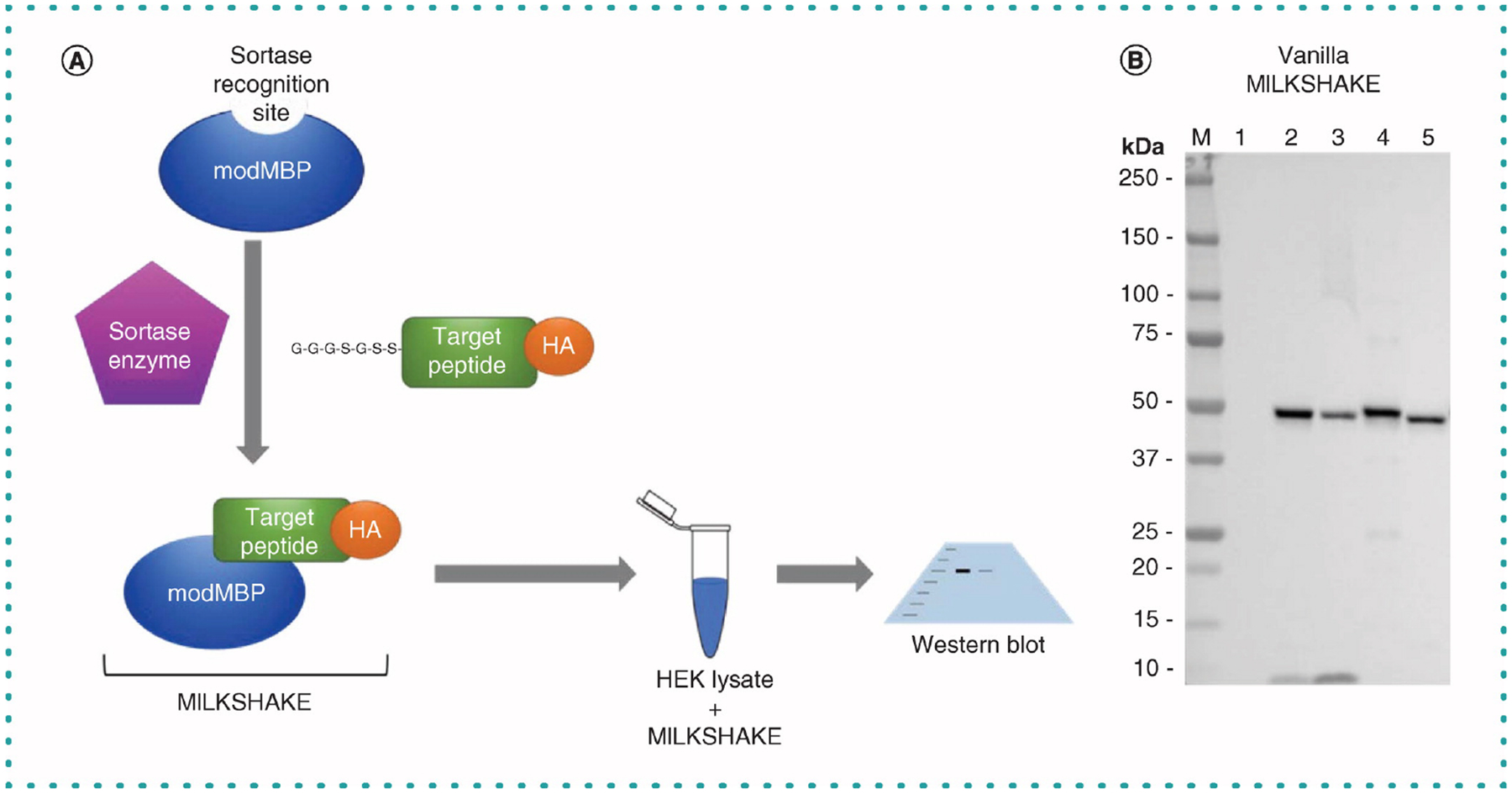
(A) Schematic of sortase A mediating the conjugation of modMBP to target peptides and subsequent use in western blot. (B) HA tag present only in the target peptide is detected in lanes loaded with MILKSHAKE but not in lane loaded with modMBP ‘unconjugate’ negative control. The expected size of MILKSHAKE is 42 kDa. Blot was incubated with anti-HA tag HRP Ab (Abcam ab128131; Cambridge, UK) at 1:5000 dilution. Exposure 15 s. Each lane contains 500 ng MILKSHAKE and 15 μg HEK lysate. Lanes: M, Precision Plus Protein Standard (BioRad); 1, unconjugated modMBP negative control; 2, phospho-conjugated MILKSHAKE Target A; 3, non-phospho conjugated MILKSHAKE Target A; 4, phospho-conjugated MILKSHAKE Target B, 5, nonphospho-conjugated MILKSHAKE Target B.
MILKSHAKE surrogate protein is produced by sortase conjugation
The sortase A enzyme recognizes a specific amino acid sequence near the C-terminus of our purified modified maltose binding protein (modMBP). The enzyme can then be used to conjugate modMBP to the N-terminus of the target peptide present in the reaction (Figure 1A). Maltose binding protein was chosen for this study because it was predicted to have high protein yield during purification, is easily purified on a column and has a midsize molecular, mass, making it easy visualize in Western blot. We found that both the purification and conjugation steps are high yield and robust. We are routinely able to purify 60 mg of modMBP per liter of culture. To confirm the conjugation of our target peptide to modMBP, we used an Ab against the C-terminal HA-tag present in the peptide. Completed sortase reactions of modMBP with peptides were tested in Western blot with an HRP-conjugated anti-HA Ab. In Figure 1B, the tag is shown to be present in four target peptide-conjugated MILKSHAKE proteins but not in the unconjugated modMBP control. MILKSHAKE proteins are typically used by us in two experiment types: Western blots in which lanes are loaded only with the MILKSHAKE protein as a test of the Ab’s specificity (which we have termed ‘vanilla’) and Western blots in which lanes are loaded with a mixture of MILKSHAKE protein and HEK cell lysate as a more stringent test of the Ab’s sensitivity (which we have termed ‘strawberry’).
Many commercially available phospho-specific antibodies bind MILKSHAKE as expected
Using purified modMBP and specifically designed peptides as described earlier, we were able to generate MILKSHAKE protein containing the phosphorylated or nonphosphorylated epitopes from desired proteins. With catalog published Abs from three vendors, we are able to detect binding to our phospho-peptide MILKSHAKE and not to nonphospho-peptide MILKSHAKE nor to an irrelevant phospho-peptide MILKSHAKE (Figure 2A). These data also match the phospho-specificity as measured in endogenous treated cell lysates in the vendors’ product datasheets (data not shown). To validate the MILKSHAKE method, we have tested 25 commercially available phospho-specific Abs from seven vendors and have observed the majority of them (22 of 25 Abs tested) to bind as expected to the phospho-peptide MILKSHAKE and not to the nonphospho MILKSHAKE (some data not shown). In Figure 2B, we demonstrate with a phospho-specific Ab for one target, GSK3B_Serine9, the correlation between MILKSHAKE binding specificity and binding specificities found in endogenous treated lysates. One endogenous lysate utilizes treatment with Calyculin A, a potent phosphatase inhibitor, to induce the phosphorylation of Serine 9 in GSK3B. The complementary lysate treatment utilizes LY294002, a selective inhibitor of phosphatidylinositol 3-kinase, to produce a nonphosphorylated Serine 9 in GSK3B. Taken together these data support that MILKSHAKE acts as a representative surrogate for full-length endogenous PTM antigens in Western blot. Going a step further, researchers can use MILKSHAKE binding specificity of a particular Ab in Western blot as a control to verify the treatment conditions for induction or reduction of a PTM are appropriate for that particular PTM.
Figure 2. Published phospho-specific antibodies recognize phospho-peptide MILKSHAKE.
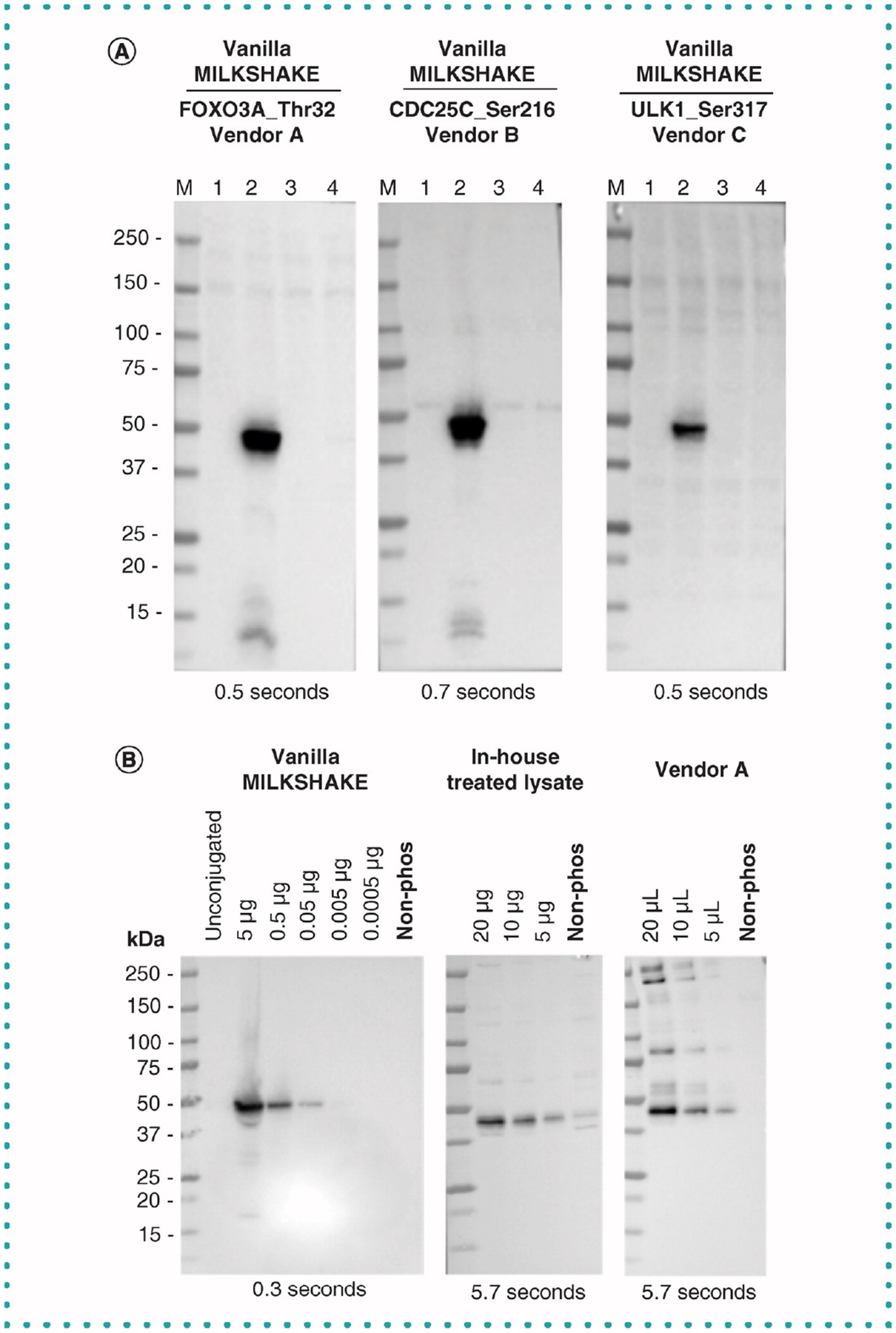
(A) Blots were incubated with primary Abs diluted 1:1000 from three vendors. Each IgG Ab binds the phosphorylated residues listed for each target present in the conjugated modMBP in Lane 2. Each lane contains 500 ng MILKSHAKE and 15 μg HEK lysate. Exposure times are listed on each blot. Lane M: Precision Plus Protein Standard (BioRad) Lane 1: unconjugated modMBP Lane 2: modMBP conjugated to phosphorylated target peptide specific to the primary Ab tested Lane 3: modMBP conjugated to nonphosphorylated target peptide specific to the primary Ab tested Lane 4: modMBP conjugated to an irrelevant phosphorylated peptide, not related to the primary Ab tested. Note: the Abs from Vendors A and B and C are polyclonal rabbit IgGs. (B) Binding specificity for the target GSK-3β_Ser9 in MILKSHAKE compared with in-house treated lysates (Non-phosphorylated treatment: PC-3 cells serum starved overnight and treated with 10 μM LY294002 for 30 min (Cell Signaling Technology 9901); Phosphorylated treatment: PC-3 cells serum starved overnight) and treated lysates purchased from Vendor A (Phosphorylated condition: HeLa whole cell extracts treated with Calyculin A (50 nM) for 3 h, serum-free media; nonphosphorylated condition: vehicle treated HeLa cells, serum free media). Note: Serum starvation is used to synchronize all cells to the same phase of the cell cycle before inhibitor treatments. Lysates were serially diluted and blots were incubated with a phospho specific Ab from Vendor B which recognizes GSK-3β phosphorylated at Serine 9 at 1:1000 dilution. Lysate from Vendor A with an unknown concentration was added using volumes comparable to in-house treated lysates. Ab binds to the phosphorylated residue or condition in MILKSHAKE, in-house treated lysates and purchased lysates. No binding was detected to the nonphosphorylated residue or condition in MILKSHAKE or treated lysates. Exposure times are listed on each blot.
Using MILKSHAKE to validate Ab PTM specificity & sensitivity
As previously discussed, validating Abs before experimental use is crucial for obtaining meaningful data. It is important to validate these Abs on appropriately modified/nonmodified antigen material. Researchers have used various approaches to develop this material. One method is to genetically introduce a phospho-like modification by replacing the residue of interest with aspartic acid or glutamic acid, mimicking the phospho group’s negative charge; however, this method falls short of a truly functional phosphorylated residue [10]. Other methods include full or partial chemical synthesis of the protein to incorporate the modified residue; however, these methods have their own challenges, including cost [10].
For validation of Western blot Abs, the most commonly used antigen materials are mammalian cell lysates that have undergone treatment (e.g., chemical exposure, UV radiation) to induce (or reduce) modification of residues on a protein of interest. Several stages during the generation of this material can pose challenges. First, it can be difficult to identify the appropriate treatment and cell line for the residue of interest. Orphan PTM sites also may have unknown phospho-induction or reduction conditions. Second, treated mammalian cell lysates can be technically challenging to generate reproducibly. Even with careful adherence to a protocol, these lysates may have varying degrees of modification present in ‘treated’ and ‘untreated’ samples. Many proteins also contain more than one modifiable residue leading to uncertainty as to which site the Ab is binding. Any difficulty in production of the treated antigen material can lead to potentially incorrect specificity determination for the Ab under testing.
Using target peptides conjugated to modMBP as the surrogate antigen allows a researcher to test the desired quantity of correctly modified or unmodified target in each experiment. It also enables researchers to verify the specificity and sensitivity of the Ab they are testing. To demonstrate the variation in quality of commercially available PTM Abs, we tested Abs from a variety of vendors described as binding specifically to a phosphorylated target. We tested Abs available for binding AKT phosphorylated at Serine473 (Figure 3). Antibodies from Vendors B and F bind strongly to the phospho MILKSHAKE protein as expected. The Abs for Vendors B and F do also show some faint binding to the nonphospho MILKSHAKE protein at the concentration loaded (0.5 μg), which is more pronounced in the vanilla version where the only protein present in each lane is MILKSHAKE. However, the phospho MILKSHAKE binding is more intense for the same concentration loaded (0.5 μg) pointing to stronger Ab binding in the presence of the phosphorylated residue. It is notable as well that Abs from Vendors B and F bind with marked difference in sensitivity to the same amount of target protein loaded per lane. In contrast, Vendor E’s Ab for the same target is not specific to the phospho MILKSHAKE protein but also binds the nonphospho MILKSHAKE with similar intensity (indicated by arrow).
Figure 3. Published phospho-specific antibodies show varying degrees of specificity to phospho-AKT1_Ser473 MILKSHAKE.
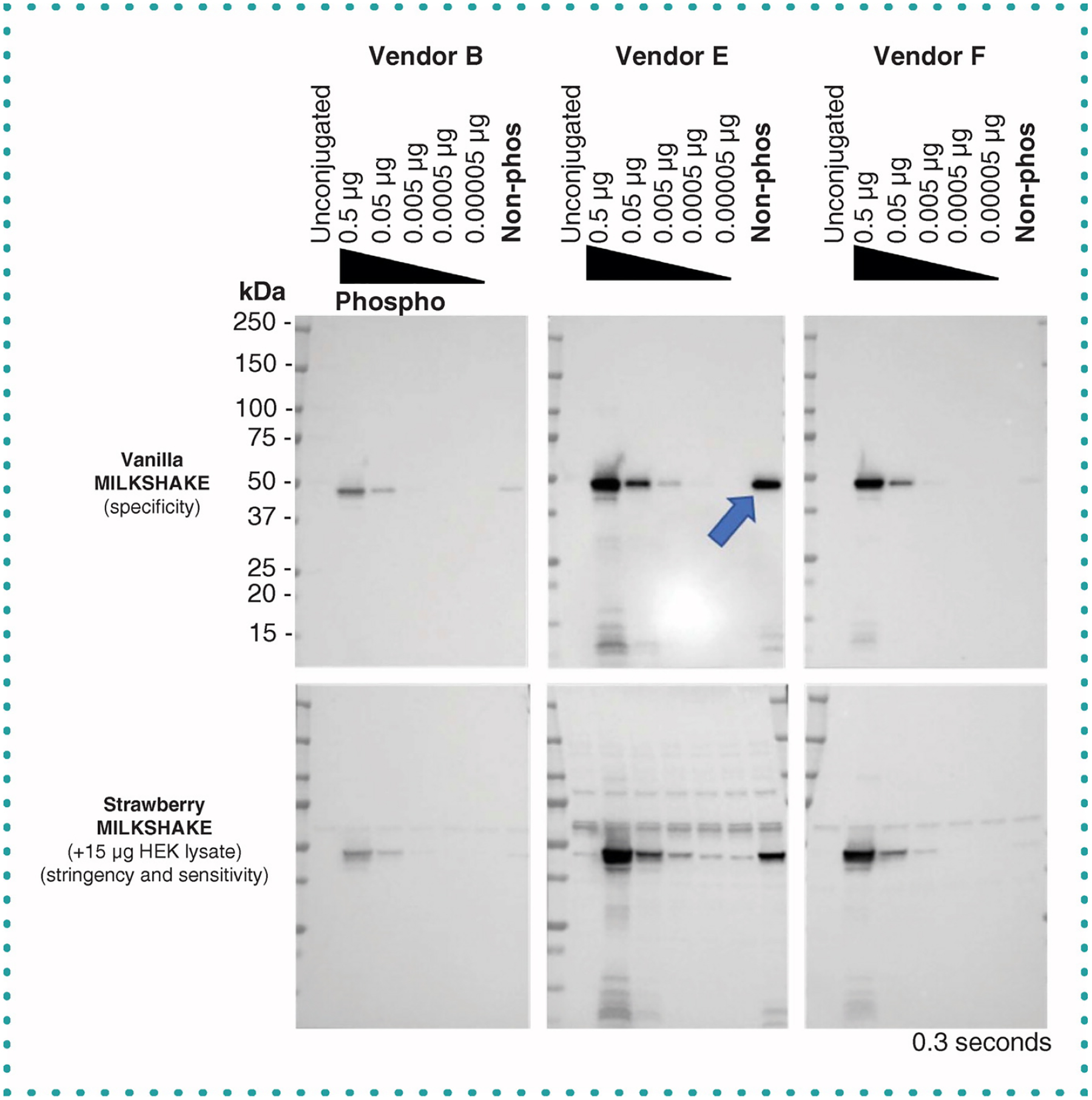
(Upper panels) Blots containing MILKSHAKE proteins were incubated with primary Abs diluted 1:1000 from three vendors. IgGs from Vendors B and F show binding specific to phosphorylated AKT1_Ser473. IgG Ab from Vendor E binds to the phosphorylated as well as the non-phosphorylated residue (blue arrow). (Lower panels) Vendors B and F maintain phospho-specificity when MILKSHAKE is mixed with HEK-lysate (strawberry condition). MILKSHAKE concentrations and exposure time are displayed on each blot. Phospho-MILKSHAKE conjugations were serially diluted. Unconjugated modMBP and non-phosphorylated MILKSHAKE were loaded at 0.5 μg per lane.
In Figure 4, we demonstrate the range of sensitivity of PTM Abs for their MILKSHAKE protein targets. As the amount of MILKSHAKE loaded per lane decreases, more sensitive Abs are still able to produce stronger bands. For example, Vendor A’s Ab for EIF2S1_Serine51 is more sensitive at lower dilutions than Vendor D’s Ab (Figure 4B). This type of data could be critical for a researcher choosing an Ab for a low abundance target. Another insight gained via this method is the ability to visualize nonspecific binding to other cellular proteins in the HEK lysate (present in ‘strawberry’ version in the lower panels). In Figure 4A (lower panels), we show that Vendor E’s Ab targeting GSK3B_Serine9 suffers from this nonspecific binding whereas Abs from competitors are highly specific. Access to this type of data would enable researchers to choose the best Abs available for their own experiments.
Figure 4. Published phospho-specific Abs show varying degrees of sensitivity to phospho-GSK3β_Ser9 and EIF2S1_Ser51 MILKSHAKE.
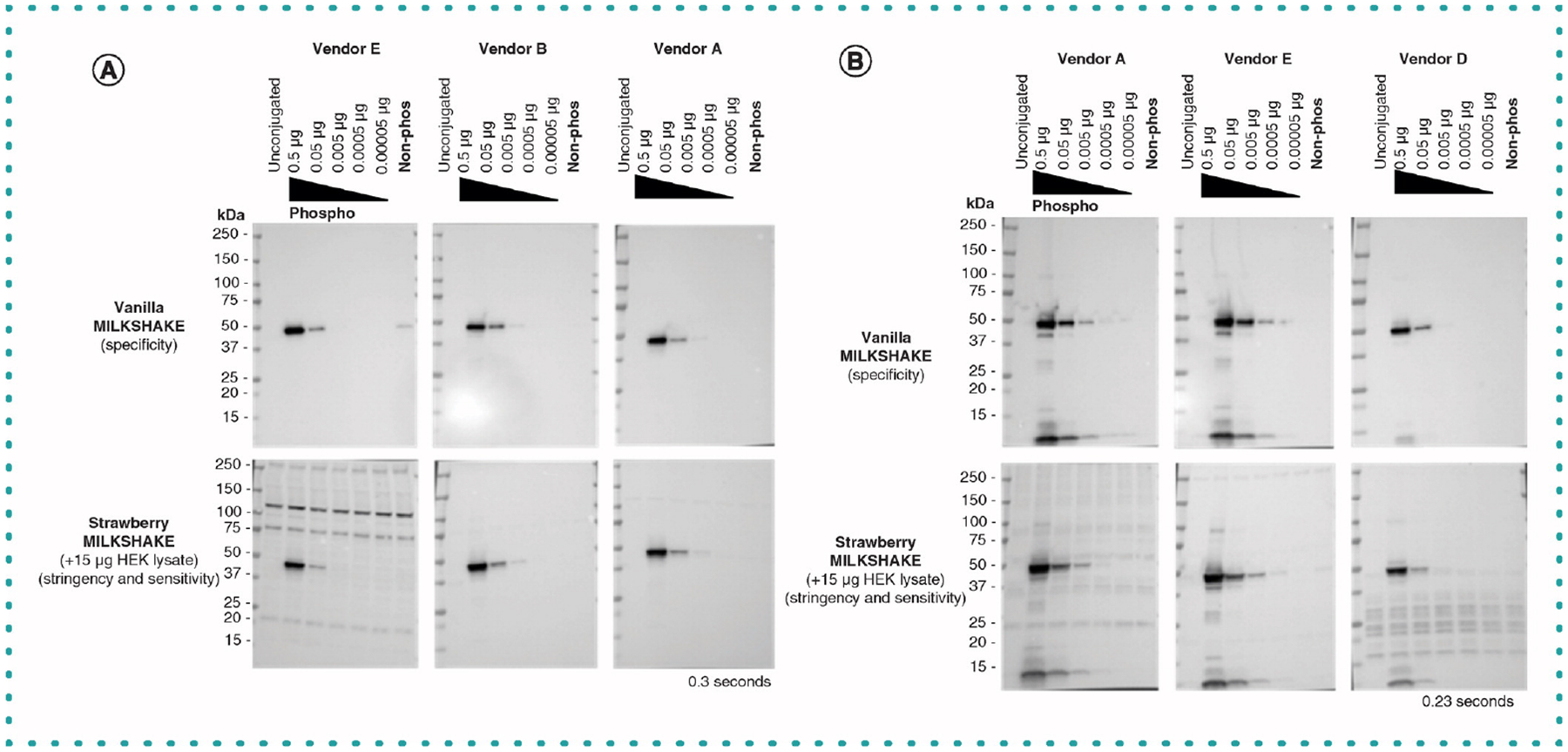
(A) Blots were incubated with primary Abs specific to GSK3β_Ser9 from three vendors and tested using MILKSHAKE alone and mixed with 15-μg HEK lysate. Abs were diluted based on vendor specifications: Vendor E, 1:500; Vendor B, 1:1000 and Vendor A, 1:500. IgG Abs from all three vendors were specific to the phospho-GSK3β_Ser9 MILKSHAKE peptide. Vendor E exhibited some binding to the nonphosphorylated MILKSHAKE and displayed nonspecific bands when tested in Strawberry MILKSHAKE. (B) Blots incubated with primary Abs specific to EIF2S1_Ser51 from three vendors and tested in MILKSHAKE and Strawberry MILKSHAKE. Abs were diluted based on vendor specifications: Vendor A, 1:500; Vendor E, 1:500 and Vendor D, 1:200. Abs from all three vendors showed specific binding to the phospho-MILKSHAKE only. Vendors A and E detected phosphorylated residues in MILKSHAKE loading concentrations as low as 0.005 μg. In contrast, Vendor D detected phospho-MILKSHAKE with a loading concentration as low as 0.05 μg. MILKSHAKE concentrations and exposure times are displayed on each blot. Phospho-MILKSHAKE was serially diluted. Unconjugated modMBP and nonphosphorylated MILKSHAKE were loaded at 0.5 μg per lane.
MILKSHAKE validation clarifies antigen sequence discrepancies
Commercially available PTM Abs are most often named for the target protein and residue number to which they bind. The residue position numbers used in these names are not always straightforward. Some numbering schemes may be derived from full-length sequences, while others may be based on processed, spliced or cleaved versions of the same protein. In Figure 5, we test MILKSHAKE proteins containing phospho and nonphospho versions of residues surrounding Tyrosine 1068 from the EGF receptor protein (target sequence: FLQRYSSDPTGAL). This sequence location was determined using full length EGFR sequences provided by the Universal Protein Resource and the UniProt ID P00533 [11]. However, when these MILKSHAKE proteins were tested with an Ab labeled by Vendor B as a specific binder to phospho-EGFR_Tyr1068 we observed no binding (Figure 5). When these same MILKSHAKE proteins were tested again with an Ab labeled as a binder to phospho-EGFR_Tyr1045 we observed phospho-specific binding. The EGFR protein undergoes a processing event that cleaves an N-terminal signal peptide resulting in two residue numbering schemes. The tyrosine found in the MILKSHAKE protein sequence is at position 1068 in the full-length sequence and at position 1045 in the cleaved sequence. Without specific antigen sequences provided by the Ab manufacturer, researchers may choose to purchase the wrong Ab for their study. MILKSHAKE proteins contain known sequences and known modifications providing researchers confidence the Ab they select is binding to their site of interest.
Figure 5. Western blot analysis of two phospho-EGFR Abs from the same vendor using MILKSHAKE.
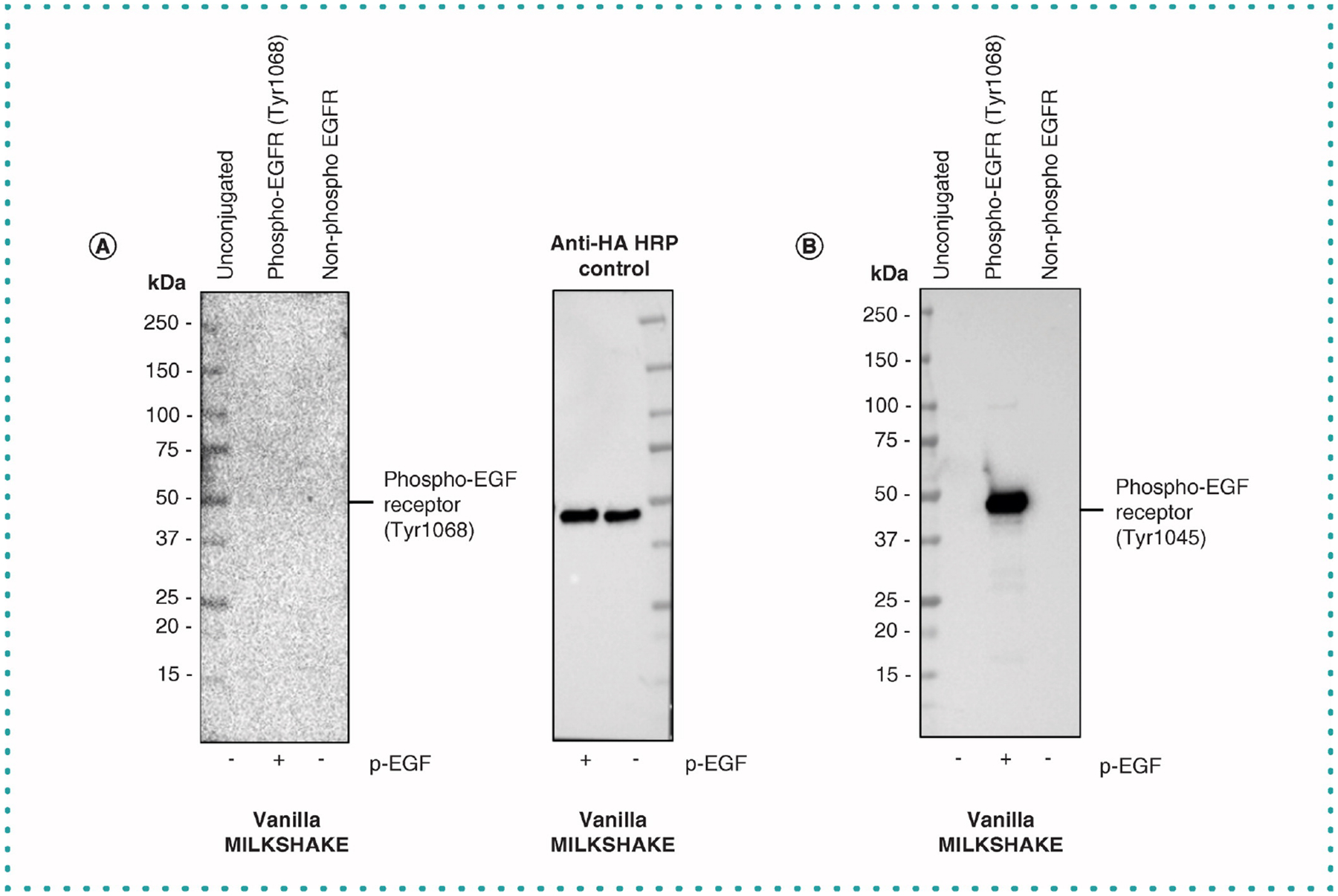
(A) Blot with lanes containing modMBP (unconjugated), phospho-EGFR_Tyr1068 MILKSHAKE and non-phospho-EGFR MILKSHAKE were incubated with Phospho-EGF Receptor (Tyr1068) (D7A5) XP Rabbit mAb (Cell Signaling Technology #3777) at 1:1000 dilution. Phospho and non-phospho MILKSHAKE proteins were also incubated with anti-HA tag HRP Ab (Abcam ab128131) as a loading control. (B) Blot with lanes containing modMBP (unconjugated), phospho-EGFR_Tyr1068 and non-phospho-EGFR_Tyr1068 MILKSHAKE proteins incubated with Phospho-EGF Receptor_Tyr1045 Ab (Cell Signaling Technology #2237). This Ab recognized the phospho-EGFR residue present in MILKSHAKE. A protein sequence numbering discrepancy results in two different position numbers (1068 and 1045) being used for the same tyrosine at this location (FLQRYSSDPTGAL). Each lane contains 0.5 μg of MILKSHAKE. Abs used are polyclonal. Blots were exposed for 10, 6.2 and 0.4 s respectively.
Validation of phospho-specific Abs during discovery & development using MILKSHAKE
Another important use for the MILKSHAKE method is as a surrogate Western blot assay during the screening phases of Ab discovery and development. MILKSHAKE allows researchers to identify candidate Abs with the desired PTM binding specificity and eliminate those Abs with nonspecific background binding to other cellular proteins (which are present in the ‘strawberry’ version). Validating with MILKSHAKE can save the expense and effort of generating large batches of treated cell lysates for intermediate validation steps. Figure 6A shows purified scFv intermediates generated from a phage display pipeline binding to MILKSHAKE with varying sensitivity and specificity; the assay identifies a top performer (scFv 102) with regard to phospho-specificity and reduced nonspecific binding to move forward for further development. Figure 6B shows MILKSHAKE binding specificity for polyclonal intermediates produced following a rabbit immunization with a target peptide. Here in addition to phospho and non-phospho MILKSHAKE types, a third variation containing a single residue change (not a PTM) is added to the assay. The results show it is possible to use this method to differentiate Ab binding specificities to antigens with only a single amino acid difference from the target sequence. We have also observed that it is possible to validate Abs in Western blot against other target types whose sequences are difficult to produce such as those with splice junctions using a MILKSHAKE-like protein generated by genetically encoding the desired splice junction to the C-terminal end of modMBP (data not shown).
Figure 6. Intermediate validation using MILKSHAKE to select pipeline candidate Abs for further development.
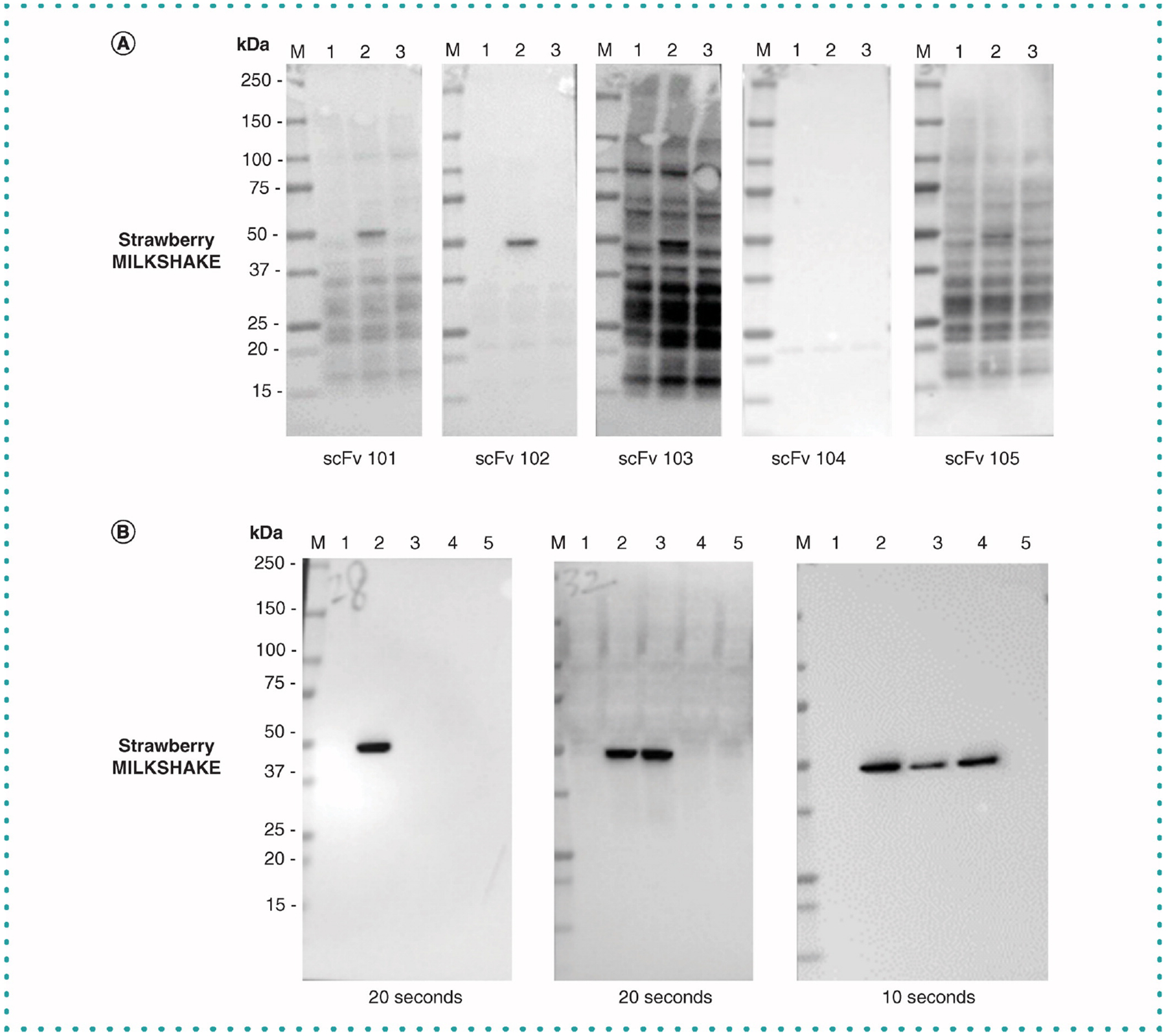
(A) Strawberry MILKSHAKE blots were incubated with phage display pipeline intermediate scFvs as primary Abs at 1 μg/ml. Each Ab binds the phosphorylated MILKSHAKE in lane 2 with varying affinity and specificity. ScFvs 103 and 105 demonstrate strong nonspecific binding to off target cellular proteins. ScFv 102 demonstrates the strongest phospho-specific binding with the least non-specific binding. Each lane contains 0.5-μg MILKSHAKE and 15-μg HEK lysate. Blots were exposed for 0.8 s. Lane M: Precision Plus Protein Standard (BioRad) Lane 1: unconjugated modMBP Lane 2: modMBP conjugated to phosphorylated Target Peptide specific to the primary Ab tested Lane 3: modMBP conjugated to nonphosphorylated Target Peptide specific to the primary Ab tested. (B) Strawberry MILKSHAKE Blots were incubated with polyclonal IgG supernatant (0.2 μg/ml) derived from isolated B cells following rabbit immunization with a target peptide. Each polyclonal IgG binds either the phosphorylated MILKSHAKE protein in lane 2 alone or in addition binding the nonphosphorylated MILKSHAKE in lane 3. The polyclonal IgG in the third panel also binds a nonphosphorylated MILKSHAKE containing a single polymorphism in Lane 4. Each lane contains 0.5-μg MILKSHAKE and 15-μg HEK lysate. Blots were exposed for 20, 20 and 10 s, respectively. Lane M: Precision Plus Protein Standard (BioRad). Lane 1: unconjugated modMBP. Lane 2: modMBP conjugated to phosphorylated target peptide specific to the primary Ab tested. Lane 3: modMBP conjugated to nonphosphorylated target peptide specific to the primary Ab tested. Lane 4: modMBP conjugated to nonphosphorylated target peptide specific to the primary Ab tested but with a single amino acid change compared with Lane 3. Lane 5: modMBP conjugated to an irrelevant phosphorylated peptide, not related to the primary Ab tested.
Discussion
It is crucial to validate the specificity and sensitivity of Abs before using them on experimental samples. Abs with inappropriate or inadequate validation contribute to overall poor reproducibility in research. The method described here can improve reproducibility by allowing researchers to choose the best Ab for their experiment. It should be noted that even in a small sample of target sites that we examined for this article (25) that three of the Abs tested deviated from the binding profiles indicated by their vendors (Figures 3–5) due to misidentified residue numbers, PTM-independent binding or nonspecific binding to other cellular proteins.
The MILKSHAKE method provides a level playing field to compare Abs from a variety of sources. Instead of comparing different product datasheets, each with different validation methods (i.e., different cell lines, treatments, concentration of material used) researchers can choose the concentration of MILKSHAKE that best fits their needs and directly compare Ab performance. In addition, the assay could be further customized by swapping the accompanying HEK lysate used in this manuscript to one which closely matches their experimental sample types (i.e., a glioblastoma lysate for neuronal targets). This change could reveal the most accurate binding profile to nonspecific cellular proteins or lack thereof for the Ab under testing.
A further expansion of this method can be aimed at other PTM types such as methylation or glycosylation. Researchers’ efforts to develop affinity reagents binding these diverse modifications must be matched with assays like MILKSHAKE that provide certainty of residues present in the antigen sample and the modification status of each residue. Future MILKSHAKE-like methods can also be adapted for other Ab applications. The MILKSHAKE method described here is appropriate for Western blot, an assay that interrogates denatured epitopes. When developing new methods where conformational epitopes are present, the MILKSHAKE-like proteins used will need to be designed with epitope conformation in mind. Both these developments will further strengthen MILKSHAKE’s role as a validation tool.
Future perspective
The surrogate Western blot method described here will increase the value of published PTM Western blot results. By ensuring the Ab used in an experimental study is indeed modification specific, residue-position specific and free of excessive nonspecific cellular protein binding, researchers can reach conclusions with confidence.
The challenges in validating PTM-specific Abs also persist in assays outside of Western blot such as flow cytometry, immunocytochemistry and immunohistochemistry. We foresee an expansion of the use of MILKSHAKE-like proteins in these assays as well, both for validating the specificity of PTM and non-PTM Abs and as a process control for antigen preparation for these technically challenging assays.
Summary points.
The authors have developed a surrogate protein (‘MILKSHAKE’) using modified maltose binding protein (modMBP) and a target protein peptide containing a modified or nonmodified residue of interest for use in Western blot.
MILKSHAKE proteins are used as surrogates for target proteins that undergo post translational modification in Western blot either alone (‘vanilla’ MILKSHAKE) or mixed with mammalian cell lysate for increased sensitivity testing (‘strawberry’ MILKSHAKE).
Many commercially available phospho-specific Abs bind as expected in MILKSHAKE Western blots.
MILKSHAKE Western blot data correlate closely with data obtained using modification-specific treated mammalian cell lysates for commercially available Abs.
MILKSHAKE Western blot data can help researchers identify Abs with poor specificity or sensitivity and be used as a Western blot process control.
MILKSHAKE Western blot data for commercially available Abs are modification and sequence specific unlike current validation methods or product labeling.
MILKSHAKE Western blots can identify candidate Abs during development that perform best in Western blot and can specifically bind sequence polymorphisms which may be present in different target types.
Acknowledgments
The authors thank Tyler Schlagetter for cell maintenance and Malik Blackwell and Soweto Thomas for reagent preparation.
Footnotes
Financial & comspeting interests disclosure
Names of antibody manufacturers have been omitted to remove any potential competing interests with Abcam or Abbratech Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Baker M 1,500 scientists lift the lid on reproducibility. Nature. 533, 452–454 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Lithgow GJ, Driscoll M, Phillips P. A long journey to reproducible results. Nature. 548, 387–388 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Biological Standards Institute. The case for standards in life science research: seizing opportunities at a time of critical need. (2013).
- 4.Uhlen M, Bandrowski A, Carr S et al. A proposal for validation of antibodies. Nat. Methods 13, 823–827 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordeaux J, Welsh AW, Agarwal S et al. Antibody Validation. BioTechniques. 48(3), 197–209 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article outlines Ab validation standards.
- 6.Mao H, Hart SA, Schink A, Pollok BA. Sortase-mediated protein ligation: a new method for protein engineering. J. Am. Chem. Soc 126(9), 2670–2671 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Guimaraes C, Witte MD, Theile CS et al. Site-specific C-terminal internal loop labeling of proteins using sortase-mediated reactions. Nat. Protoc 8(9), 1787–1799 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillai-Kastoori L, Heaton S, Shiflett SD et al. Antibody validation for Western blot: by the user, for the user. J. Biol. Chem 295(4), 926–939 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article outlines Ab validation standards for western blot.
- 9.Mandell JW. Phosphorylation state-specific antibodies. Am. J. Pathol 163(5), 1687–1698 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Cole PA. Synthetic approaches to protein phosphorylation. Curr. Opin. Chem. Biol 28, 115–122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UniProtKB - P00533 (EGFR_HUMAN). www.uniprot.org/uniprot/P00533#sequences


