Abstract
Antibiotic chemotherapy is widely regarded as one of the most significant medical advancements in history. However, the continued misuse of antibiotics has contributed to the rapid rise of antimicrobial resistance (AMR) globally. Staphylococcus aureus , a major human pathogen, has become synonymous with multidrug resistance and is a leading antimicrobial-resistant pathogen causing significant morbidity and mortality worldwide. This review focuses on (1) the targets of current anti-staphylococcal antibiotics and the specific mechanisms that confirm resistance; (2) an in-depth analysis of recently licensed antibiotics approved for the treatment of S. aureus infections; and (3) an examination of the pre-clinical pipeline of anti-staphylococcal compounds. In addition, we examine the molecular mechanism of action of novel antimicrobials and derivatives of existing classes of antibiotics, collate data on the emergence of resistance to new compounds and provide an overview of key data from clinical trials evaluating anti-staphylococcal compounds. We present several successful cases in the development of alternative forms of existing antibiotics that have activity against multidrug-resistant S. aureus . Pre-clinical antimicrobials show promise, but more focus and funding are required to develop novel classes of compounds that can curtail the spread of and sustainably control antimicrobial-resistant S. aureus infections.
Keywords: antimicrobial resistance, antibiotic therapy, Staphylococcus aureus
Introduction
The World Health Organization (WHO) has identified antimicrobial resistance (AMR) as one of the gravest threats to global public health due to the significant morbidity, mortality and economic costs associated with multidrug-resistant (MDR) infections [1]. For example, in the USA, over 2.8 million people suffer from antibiotic-resistant infections annually, resulting in more than 35 000 deaths [2]. Furthermore, disease caused by antibiotic-resistant pathogens results in extra healthcare costs of approximately US $20 billion to US hospitals. Future predictions estimate that if left unchecked, AMR could lead to 10 million deaths per year and negatively impact global GDP to the tune of US $100 trillion [3].
Staphylococcus aureus is a major human pathogen and leading cause of bacteraemia and infective endocarditis, as well as bone, skin and soft tissue, pleuropulmonary and implant-related infections [4]. The range and severity of diseases caused by S. aureus are in part determined by the myriad of virulence factors, including toxins, adhesins, immune evasins, proteases and other tissue-degrading enzymes. Combined with the impressive ability to gain resistance to multiple classes of antibiotics, these attributes culminate in a pathogen that can be incredibly difficult to treat. At present, there is no available vaccine to prevent S. aureus infection. All previous vaccination attempts have failed at clinical trials, particularly those designed to generate high titres of opsonic antibodies directed against staphylococcal surface antigens [5, 6]. The complex host–pathogen interaction has led to several hypotheses for the disappointing results observed in human trials, which have been extensively reviewed [5–7]. Importantly, lack of effective vaccines significantly enhances the clinical use of antibiotics for treatment of S. aureus infections, increasing the selective pressure for resistance. Worryingly, recent reports have estimated that S. aureus ranks second in global AMR burden, with 100 000 deaths attributed to, and 1 million deaths associated with, resistant infections caused by this bacterium [8].
Current anti-staphylococcal treatment options rely on antibiotics with well-defined bacterial targets that are described in detail below along with the mechanisms that S. aureus uses to resist their activity. The treatment regimen to combat S. aureus depends on several factors, including disease severity and site of infection, antibiogram of the causative isolate and patient antibiotic allergies, and has been recently reviewed [4, 9].
S. aureus AMR mechanisms fall into four main categories: (1) limiting drug uptake, (2) modifications of the target site, (3) drug inactivation and (4) active drug efflux. These resistance mechanisms can either be intrinsic, the resistance genes are chromosomally located and expressed or induced in all species, or they can be acquired. The most common examples of intrinsic resistance in S. aureus include limiting drug uptake, typically through cell wall modifications, or drug efflux. Acquired resistance arises either from the gain of genetic material through horizontal gene transfer or chromosomal mutations. Genotypic evidence indicates that clinically derived S. aureus isolates utilize combinations of these resistance mechanisms that have been employed to withstand the activity of all known classes of antibiotics [10, 11]. S. aureus can be considered MDR by virtue of harbouring the mecA gene or when resistant to ≥1 antibiotic agent in ≥3 antibiotic categories. The increase in the antibiotic resistance coverage of S. aureus has resulted in a new antimicrobial category called extensively drug-resistant (XDR), which can be classified as an isolate that is resistant to ≥1 antibiotic agent in all but ≤2 antibiotic categories [12]. While still relatively rare, XDR S. aureus isolates are increasingly being reported and represent a significant health concern [13, 14]. Pan-drug resistance (PDR) has currently not been reported in S. aureus, potentially explained by the fitness cost associated with such resistance. This is in stark contrast to the Gram-negative organisms Klebsiella pneumoniae , Acinetobacter baumanii and Pseudomonas aeruginosa, whereby PDR is increasingly being documented worldwide and is associated with high mortality [15, 16].
Compounding the problem surrounding S. aureus antibiotic resistance, is the lack of innovation in antibiotic development, where few novel antibiotic classes have been introduced successfully into the clinic since the turn of the century. Indeed, the last truly novel Gram-positive antibiotics introduced were daptomycin (DAP) and linezolid (LZD) in the early 2000s [17]. Accordingly, this review aims to provide a background to recently licensed anti-staphylococcal antibiotics, while providing an assessment of the current pipeline and promising prospects. We aim to provide a critical analysis of the antibiotics that are currently in the mid to late stage of the clinical development process, indicating their mechanism of action, where applicable how they differ from previous structures, whether resistance has been observed, and an overview of the current clinical trial data. Importantly, while numerous antibiotics in development have anti-staphylococcal activity, we are focused on those that are specifically being developed to combat S. aureus .
Current anti-staphylococcal antibiotics: targets and resistance mechanisms
The cell wall
β-lactams, such as penicillin (PEN), bear a structural resemblance to the d-Ala-d-Ala-terminating pentapeptide of lipid II, which is the natural substrate for transpeptidase penicillin-binding proteins (PBPs). Thus β-lactams interrupt cell wall formation by covalently binding to PBPs and preventing the transpeptidase reaction from occurring, resulting in cell death [18]. The resistance of S. aureus to this class of antibiotic has garnered this bacterium much notoriety and it has become a global health burden. Resistance first emerged against PEN in the 1940s, a few years after it was introduced into the clinic [19]. Resistant strains acquired a plasmid encoding a penicillinase called blaZ, which was able to hydrolyse the β-lactam ring, rendering it unable to bind to PBPs [20]. PEN resistance spread rapidly; by the 1960s more than 90 % of strains produced this enzyme regardless of clinical isolation source [21, 22]. As a response, a semi-synthetic PEN, containing a dimethoxyphenyl group called methicillin (MET), insensitive to penicillinase activity, was introduced. S. aureus resistance to this modified β-lactam emerged rapidly and strains were termed methicillin-resistant S. aureus (MRSA) [23]. A low-affinity PBP called PBP2A/PBP2′ encoded by the mecA gene harboured on a mobile genetic element, the staphylococcal chromosomal cassette mec (SCCmec), was discovered to confer resistance to MET and certain cephalosporins [24–26].
Glycopeptides similarly inhibit cell wall synthesis; however, these antibiotics bind to the d-Ala-d-Ala residue of lipid II, which results in a cessation of the transpeptidase reaction through substrate sequestration but also a reduction in transglycosylase activity through additional steric hindrance [27, 28]. Glycopeptides can also be called lipoglycopeptides when a lipophilic side chain is present in the structure, as is the case with teicoplanin and telavancin [29]. The unprecedented spread of MRSA globally led clinics to rely on glycopeptides such as vancomycin (VAN), which were previously considered ‘last resort’ antimicrobials. This increased selective pressure eventually led to the emergence of hetero-vancomycin intermediate S. aureus (hVISA), which served as a precursor to vancomycin intermediate S. aureus (VISA) with minimal inhibitory concentrations (MICs) of ≥4 µg ml−1 [30, 31]. The emergence of VISA is attributed to chromosomal mutations in cell wall stress stimulon genes such as walRK, yycH, vraRS, graRS, rpoB and tcaA [32]. These mutations serve to decrease the level of crosslinking present within the cell wall, resulting in an increase in the number of free d-Ala-d-Ala residues. Thus, VAN is sequestered within the cell wall instead of reaching its lipid II target at the cell membrane. This mechanism of resistance has been referred to as ‘drug capture ‘or ‘clogging’ [32]. It was feared that VISA could in turn lead to the emergence of vancomycin-resistant S. aureus (VRSA). Unfortunately, this has since come to fruition due to the acquisition of the vanA gene, mobilized on the Tn1536 transposable element, from Enterococcus faecium [33]. The expression of this gene alters the early stages of peptidoglycan synthesis, replacing the terminal d-Ala on lipid II with d-lactate. This change causes a >1000-fold decrease in VAN binding affinity [33, 34]. So far, VRSA has not spread in the same way as penicillin-resistant S. aureus or MRSA. Instead, only isolated reports have been described and these strains do not seem to have established themselves in healthcare facilities [35]. The reasoning for this is threefold. Firstly, PBP2A struggles to utilize d-Ala-d-Lac as a substrate for peptidoglycan transpeptidation. Secondly, VRSA strains exhibit an extremely long lag phase before growth in inhibitory concentrations of VAN is initiated. Finally, the plasmids harbouring vanA were found to be unstable in S. aureus [11].
The folate pathway
TMP–SMX is a synergistic combination of two antibiotics that work by inhibiting the formation of folic acid, an essential nutrient for nucleic acid synthesis [36]. Sulfamethoxazole (SMX) is an antibiotic that is structurally analogous to para-aminobenzoic acid (PABA). This enables it to bind to the dihydropteroate synthase enzyme (DHPS) and prevent the conversion of PABA to dihydropteroate (DHP) [37]. SMX resistance is achieved through chromosomal overproduction of PABA [38] or through mutational changes in the folP gene, which encodes DHPS, which presumably reduces the binding affinity of SMX [39, 40]. Trimethoprim (TMP) acts later in the folic acid biosynthesis pathway, instead inhibiting the dihydrofolate reductase (DHFR) enzyme, thereby preventing the formation of tetrahydrofolate (THF) from dihydrofolate (DHF) [41]. TMP resistance is achieved through a single point mutation F98Y in the chromosomal dfrB gene, which codes for the DHFR enzyme. This confers intermediate resistance, with MICs ≤256 µg ml−1 [42]. For high-level resistance, MICs of ≥512 µg ml−1, S. aureus must acquire alternative naturally occurring resistant dfr genes such as dfrA, dfrG and dfrK from Bacteroides , Clostridium and Neisseria species, which can be housed on plasmids or transposable elements [43–45]. A further novel diaminopyrimidine resistance determinant, designated dfrL, was found recently in a livestock-associated MRSA strain of clonal complex 398. This variant conferred a TMP MIC of 1024 µg ml−1. The ancestry of the dfrL is unclear, but the authors suggested that it is of recent staphylococcal origin [46].
The cell membrane
The mechanism of action of the lipopeptide DAP is not fully understood. DAP requires Ca2+ ions for antimicrobial activity facilitating the formation of an amphiphilic complex, similar to cationic antimicrobial peptides (cAMPs). As cAMPs target the negatively charged phospholipids of the Gram-positive bacterial membrane, researchers initially postulated that DAP would have a similar mechanism of action [47]. Indeed, numerous membrane perturbation effects have been described for DAP, including altered membrane curvature, membrane depolarization and leakage [48]. However, kill kinetic analysis has suggested this to be the result of rather than the cause of death [49]. More recent reports describe a role for DAP in cell wall inhibition. It was shown that DAP interacts with undecaprenyl-coupled cell wall precursors and phosphatidylglycerol to form a tripartite complex resulting in delocalization of peptidoglycan machinery and significant membrane rearrangement [50]. Thus, DAP could equally be categorized as an antibiotic that targets the cell wall. Resistance to DAP is typically achieved through point mutations in MprF, which catalyses the synthesis and translocation of the positively charged phospholipid lysyl-phosphatidylglycerol into the outer leaflet of the membrane [51]. It is unclear how these mutations impact on DAP resistance. Initial observations suggested that single-nucleotide polymorphisms (SNPs) were gain of function, causing an increase in the membrane levels of lysyl-phosphatidylglycerol that would cause electrostatic repulsion of DAP [52]. In agreement with this, a change in surface charge can often be detected for strains harbouring mprF mutations. However, this is not always the case and some mprF mutations, although causing an increase in DAP MIC, are not associated with an increase in lysyl-phosphatidylglycerol levels [53, 54]. More recently, crosstalk between MprF and cell wall biosynthesis machinery was observed where mutations in mprF stimulated the expression of the vraRS two-component system, resulting in a thicker cell wall [55, 56]. Accordingly, MprF-mediated DAP resistance appears to be dual functional, altering the architecture of both the cell wall and the cell membrane. Resistance to DAP can also occur through an increase in d-alanylation of teichoic acids, a process that decorates the teichoic acid polymers with positive charges, which serves to repel DAP [57, 58]. Other genes involved in resistance to DAP include the cardiolipin synthase cls, the phospholipid synthase pgsA and rpoB which codes for the β-subunit of the DNA-dependant RNA polymerase [59].
Nucleic acid biosynthesis
Fluoroquinolones, such as levofloxacin (LVX), comprise the first entirely synthetic antibiotic class that work by targeting two enzymes involved in DNA replication. The first enzyme, DNA gyrase, is formed of a heterotetramer of GyrA and GyrB, and catalyses the ATP-dependent negative supercoiling of DNA [60]. The second enzyme, DNA topoisomerase IV, consists of a heterotetramer of GrlA and GrlB (also referred to as ParC and ParE), which promotes chromosome decatenation following replication [60]. Together these enzymes work to relieve the enormous torsional stress and topological constraints present during DNA replication. Inhibition of these enzymes results in DNA shearing preventing replication [60, 61]. Resistance to fluoroquinolones in S. aureus occurs through two main mechanisms. The first is alteration of the target enzymes caused by single or multiple point mutations of either gyrase or topoisomerase, which reduce the binding affinity of the fluoroquinolone [62–64]. The second is increased efflux; overexpression of the NorA [65], NorB [66] and NorC [67] efflux pumps has been shown to cause a four- to eightfold increase in MIC against various fluoroquinolones. These efflux pumps belong to the major facilitator superfamily (MFS) of transporters that utilize the proton gradient across the bacterial membrane to expel substrates. NorA confers resistance against hydrophilic fluroquinolones such as ciprofloxacin (CIP). The NorB and NorC efflux are less stringent and can expel both hydrophilic and hydrophobic fluoroquinolones [65–68]. A number of other S. aureus MFS transporters have also been shown to contribute to fluoroquinolone resistance but are less well studied than the Nor efflux pumps. Hyper-production of MdeA and QacB reduces the susceptibility towards CIP and norfloxacin (NOR) [69, 70]. SdrM and LmrS also reduce susceptibility towards NOR and gatifloxacin, respectively [71, 72]. MepA, a member of the multiple antibiotic and toxin extrusion (MATE) family of secondary transporters, also confers elevated MICs against CIP, NOR, moxifloxacin (MOX) and sparfloxacin [73]. Highly resistant strains will likely employ both of these mechanisms, often possessing multiple gyrase and topoisomerase IV mutations as well as being hyper-producers of one or more efflux pumps.
Ansamycins such as rifampicin (RIF) inhibit RNA synthesis by targeting the DNA-dependent RNA polymerase, binding within the DNA/RNA channel of the RNA polymerase β-subunit in close vicinity to the enzyme active site [74]. Rather than preventing RNA polymerase from binding to the promoter and initiating transcription, RIF inhibits the formation of the first ribonucleotide phosphodiester bond, halting mRNA production [75]. RIF resistance occurs through target site modification; mutations of the rpoB gene occur in a hotspot region termed the rifampicin resistance-determining region. By far the most encountered rpoB resistant mutations are H481Y and L466S [76, 77]. Highly resistant strains may harbour both these mutations along with others such as A477D and S486L [76–78]. Some rpoB mutations are associated with increased cell wall thickness, can promote the conversion of strains from hVISA to VISA and small colony variant (SCV) formation, significantly influencing the chronicity of S. aureus infections [79, 80].
Protein synthesis
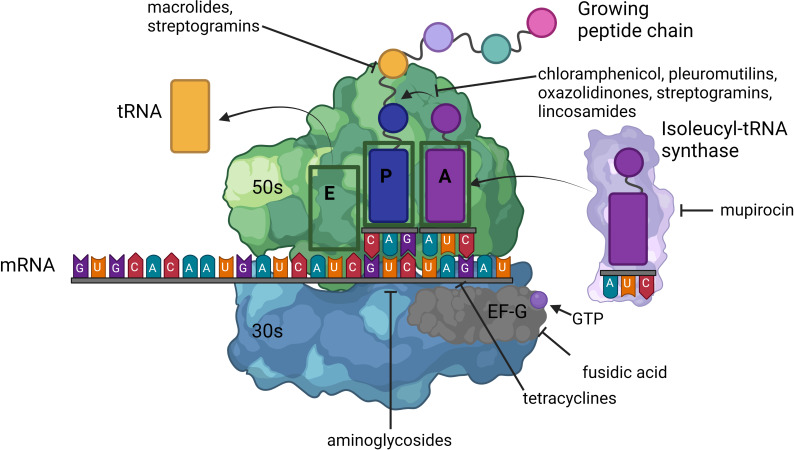
Multiple antibiotic classes inhibit protein synthesis. Antibiotics can be categorized into those that inhibit the 30S subunit of the ribosome such as the aminoglycosides and tetracyclines, compounds that inhibit the 50S subunit of the ribosome such as oxazolidinones, macrolides, streptogramins, lincosamides and pleuromutilins, and drugs with distinct mechanisms of action independent of these subunits, such as mupirocin and fusidic acid (Fig. 1). Several resistance mechanisms that prevent protein synthesis inhibition, particularly those directed against the 50S subunit, have substantial overlap (Table 1).
Fig. 1.
Ribosomal structure and antibiotics that interfere with protein synthesis. Ribosomes are central to the process of protein synthesis. In S. aureus , the ribosome is a 70S ribonucleoprotein complex consisting of a large 50S and small 30S subunit. The 50S subunit is formed from 23S and 5S ribosomal RNA (rRNA) and is responsible for initial amino acyl-tRNA binding, peptidyl transfer and regulation of the elongation factor. Three amino acyl-tRNA-binding sites are present within the 50S subunit. The 30S subunit is made from 16s rRNA and through binding to mRNA, initiates the translation process. Protein synthesis can be divided into four steps: initiation, elongation, termination and recycling. Binding of the 30S subunit to mRNA triggers the formation of the 70S complex and recruitment of the initiator tRNA (typically fMet-tRNA). Elongation follows and involves the addition of amino acids to a growing chain in a stepwise manner through the acceptor (A), peptidyl (P) and exit (E) amino acyl-tRNA-binding sites. Antibiotics that target the 50S subunit either block the polypeptide exit channel (macrolides and streptogramins) or disrupt amino acyl-tRNA binding at the peptidyl transferase centre (PTC) (lincosamides, streptogramins, phenicols, pleuromutilins and oxazolidinones). Tetracyclines bind to the 30S subunit, close to the decoding centre, and cause amino acyl-tRNA to dissociate from the A site. Aminoglycosides cause misreading of mRNA. Mupirocin inhibits the isoleucyl-tRNA synthase. Fusidic acid inhibits elongation factor G (EF-G), preventing the ribosome from traversing along the mRNA [11, 83, 288].
Table 1.
Summary of resistance mechanisms used to evade protein synthesis inhibitors
|
Mechanism |
Determinant |
Spectrum |
|---|---|---|
|
30S ribosomal inhibitors | ||
|
Drug inactivation |
Ant, Aph, Aac |
A |
|
Efflux |
TetK, TetL |
T |
|
Ribosomal protection |
TetO, TetM |
T |
|
50S ribosomal inhibitors | ||
|
rRNA methyltransferase |
Erm |
MLSB |
|
Cfr |
PhLOPSA |
|
|
Vga |
LSA |
|
|
Lsa |
LSAP |
|
|
ABC-F ribosomal protection |
Sal |
LSAP |
|
Msr |
MSB |
|
|
OptrA |
OPh |
|
|
Efflux |
Fex |
Ph |
|
Drug modification |
VgB |
SB |
|
Vat |
SA |
|
|
Cat |
Ph |
|
|
Target site mutation:23srRNA, L3 and L4 |
O |
|
|
Target site modification |
RluC |
O |
|
RlmN |
O |
|
|
Ribosome-independent protein synthesis inhibitors | ||
|
Target site mutation |
FusA, FusE |
FA |
|
Target site modification |
FusB, FusC |
FA |
|
Target bypass |
MupA |
Mu |
A, aminoglycosides; FA, fusidic acid; L, lincosamide; M, macrolide; Mu, mupirocin; O, oxazolidinones; P, pleuromutilins; Ph, chloramphenicol; SA, streptogramin A; SB, streptogramin B; T, tetracycline.
Aminoglycosides are the only ribosome-targeting antibiotics that are bactericidal. This is due to a distinctive mechanism of action that causes the misreading of codons. Consequently, the translational error rate is inflated from <1 in 1000 to around 1 in 100, so that almost every protein has multiple incorrect amino acids [81]. The bactericidal effect associated with aminoglycosides is thought to stem from the result of faulty membrane proteins that cause membrane channels to form and the leakage of intracellular contents [82]. The binding to the negatively charged phosphate groups of the 16S rRNA A site within the 30S subunit is facilitated by the presence of several amino and guanido groups within the aminoglycoside structure, which confers polycationic properties at physiological pH [83]. S. aureus resistance to aminoglycosides relies on the acquisition of aminoglycoside-modifying enzymes (AMEs), aac (6′)-Ie+aph (2″), ant (4′)-Ia, aph (3′)-IIIa and ant (6)-Ia genes, which serve to inactivate the antibiotic [84–86].
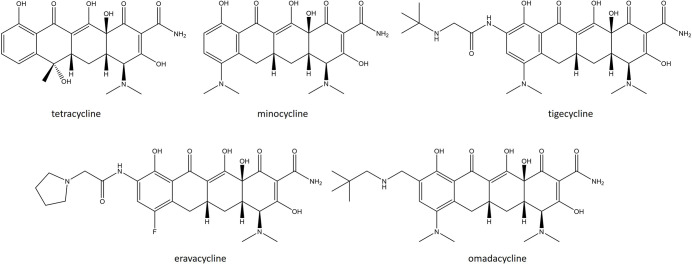
Tetracyclines prevent the binding of amino acyl tRNA through interactions with the 16S rRNA A site. Two main mechanisms are used by S. aureus to evade their killing. The first is active expulsion of the antibiotic through plasmid-mediated TetK and TetL MFS efflux pumps. TetK is typically housed on the small multicopy pT181 plasmid within the SCCmecIII of MRSA strains [87, 88]. A novel MFS efflux pump, SA09310, has also recently been shown to mediate tetracycline (TET) resistance; a Δsa09310 mutant displays a 64-fold and 8-fold reduction in MIC against TET and doxycycline (DOX) [89]. The second is through the expression of ribosomal protection proteins (RPPs), TetO and TetM, which bind to the ribosome and prevent the access of TET to their target. These proteins possess GTPase activity like elongation factor G (EF-G) but cannot function as an elongation factor and can either be chromosomally encoded or present on transposable elements such as Tn916 and Tn1545 [88, 90]. A third-generation semisynthetic TET called tigecycline (TGC) was introduced into the clinic in 2005. This newer derivative can be categorized separately to archetypal tetracyclines and referred to as a glycylcycline. It was designed by altering minocycline (MIN), specifically via the addition of a 9-tert-butyl-glycylamido side chain at the ninth position of the d-ring (Fig. 2) [91]. This side chain helps to overcome these two existing TET resistance mechanisms. The resulting molecule is substantially more potent than TET and MIN and active against strains harbouring tetracycline efflux pumps and RPPs [11]. However, TGC-resistant MRSA strains have since been reported and are caused by mutations in mepR, causing overexpression of MepA, and rpsJ [92].
Fig. 2.
Structures of recently approved tetracyclines.
Chloramphenicol (CHL) functions by binding reversibly to the L16 protein of the 50S ribosomal subunit. L16 interacts directly with the 23S rRNA in the 50S peptidyltransferase centre and thus CHL specifically disrupts peptide bond formation [11, 93]. In S. aureus, resistance occurs through several mechanisms. Acquisition of the chloramphenicol florfenicol resistance cfr gene encoding a ribosomal RNA methyltransferase that methylates an adenine residue at position 2503 in domain V of the 23S rRNA prevents CHL activity. Importantly, this gene confers an extended spectrum of resistance that includes oxazolidinones, phenicols, lincosamides, pleuromutilins and streptogramin A, commonly referred to as the PhLOPSA group of antibiotics [94]. In addition, acquisition of the cat gene, encoding a CHL acetyltransferase that transfers an acetyl group from acetyl coenzyme A to CHL, results in drug inactivation. S. aureus may acquire the oxazolidinone phenicol resistance determinant optrA, conferring resistance to CHL and oxazolidinones. OptrA shares phylogenetic similarities with two ATP-binding site ABC-F proteins and is thought to displace the antibiotic from the target site. Lastly, resistance to CHL may occur through acquisition of fexA, which functions as an MFS transporter to expel CHL [95, 96].
Macrolides, lincosamides and streptogramins are often grouped together and referred to as the MLS group of antibiotics due to having a similar mechanism of action that involves targeting the 23S rRNA of the 50S ribosomal subunit and having overlapping resistance mechanisms [11]. Antibiotic binding at this site prevents the exit path of the growing peptide chain, which leads to a dissociation of the peptidyl-tRNA during the translocation reaction [97]. Resistance to MLS antibiotics is determined through the expression of either erm or msr genes. The erm genes (ermA, ermB and ermC) each encode adenyl-N-methyltransferase enzymes, which serve to alter the target site by specifically methylating a highly conserved adenine 2058 residue in domain V of the 23S rRNA. This triggers a conformational change in the ribosome, facilitating resistance to MLS antibiotics [98, 99]. The mechanism by which the msr genes msrA and msrB confer resistance is a contentious topic. Msr genes encode ATP-binding cassette (ABC) transporters, but it is unclear whether they function as dedicated efflux pumps or RPPs [100–102]. An additional mechanism of resistance against macrolides includes esterases encoded by empC, ereA and ereB, which hydrolyse the macrocylic lactone ring, promoting high-level erythromycin (ERY) resistance [100, 103]. Additional streptogramin/lincosamide resistance mechanisms include an alternative ABC transporter ribosomal protection protein called Sal [104]. Finally, virginiamycin B lyases (Vgb) can linearize streptogramin B molecules and virginiamycin acetyltransferases (Vat) O-acetylate the hydroxyl group at position O8 of streptogramin A molecules, leading to drug inactivation [105].
Oxazolidinones such as LZD bind to the 23S rRNA of the 50S ribosomal subunit close to the macrolide- and CHL-binding site but with a separate mechanism of action. These antibiotics interfere with the formation of the ribosomal–fMet–tRNA initiation complex. LZD is the first fully synthetic ribosomal-targeting antibiotic and, along with DAP, the only new antibiotic class to be introduced since 2000. Since its inception in the clinic, it has become invaluable in the treatment of complicated MRSA infections [106]. Along with resistance mediated by Cfr and OptrA, resistance to oxazolidinones can also be achieved through target site modification of the ribosome. In S. aureus, the most clinically relevant ribosomal mutation is G2576U, but U2500A and G2447U may also be encountered, although all three seem to have a direct impact on drug binding [107, 108]. Other oxazolidinone resistance mechanisms include upregulation of the RluC enzyme responsible for pseudouridylation of U2504, loss of RlmN methyltransferase activity and mutations in the ribosomal proteins L3 and L4 [109]. It is unclear whether L3/L4 mutations alone can cause LZD mutations, as they are typically present in strains already harbouring ribosomal mutations [108].
Pleuromutilins inhibit protein synthesis by binding close to the peptidyl transferase centre of the 50S ribosomal subunit. This interferes with the transfer of amino acyl-tRNAs from the A to the P site during peptidyl transfer, inhibiting peptide bond formation [110]. Along with Cfr, resistance to pleuromutilins is achieved through mutations in the rplC and rplD genes that encode the L3 and L4 ribosomal proteins. These proteins do not interact directly with pleuromutilins but can cause conformational changes in the peptidyl transferase centre, preventing correct positioning of the drug. A third cause of pleuromutilin resistance in S. aureus is through the vga family of genes that encode ABC-F transporters that lack transmembrane domains. Rather than contributing to efflux, it is thought that these proteins instead act as ribosomal protectors. These RPPs also confer resistance to streptogramin A and lincosamides [110, 111].
Fusidic acid (FA) is unusual in that it inhibits protein synthesis independent of ribosome binding, peptide bond or translocation inhibition, instead interfering with EF-G function [112]. EF-G functions as a GTPase that promotes the translocation of amino acyl-tRNA through the three ribosomal tRNA-binding sites, allowing deacylated tRNA to be expelled through the E site [112]. FA does not bind to free EF-G, instead it binds to an intermediary ribosome–EF-G complex, specifically at an inter domain pocket close to the GTPase active site. Thus, FA prevents EF-G dissociation from the ribosome and consequently the turnover of EF-G [113, 114]. There are two mechanisms by which S. aureus gains resistance to FA. The first is mutations of the gene encoding EF-G (fusA) or the gene encoding the ribosome protein L6 (fusE), which interacts with EF-G upon ribosome binding. Single mutations of the former are largely responsible for fusidic acid resistance in S. aureus [115, 116]. The second is horizontal gene acquisition of fusB and fusC, typically housed on SCCmec elements. These two genes encode proteins that bind to EF-G–fusidic acid complex that stimulates a conformational change within EF-G, allowing it to be released and for translation to continue [117, 118].
Mupirocin (MUP) also has a unique mechanism of action, inhibiting protein synthesis by targeting isoleucyl-tRNA synthase activity. This enzyme is responsible for the formation of a covalent linkage between the isoleucine amino acid and the respective tRNA for isoleucine tRNAile [119]. In the presence of mupirocin there is no tRNA carrying isoleucine available, thus protein synthesis halts at a codon for isoleucine. Low-level resistance to mupirocin is achieved through mutations in the isoleucyl-tRNA synthase that reduce the binding affinity of the drug [120]. Higher level resistance can be achieved through the gain of the plasmid housed determinant MupA. MupA encodes an insensitive isoleucyl-tRNA synthase, reminiscent of PBP2A with MET, which mupirocin is unable to bind [121, 122].
Approved anti-staphylococcal antibiotics since 2010
Since 2010 there have been 15 antibiotics approved for clinical use that show excellent anti-staphylococcal activity (Table 2). Importantly, none were first-in-class antibiotics. Instead, these compounds were modifications of existing, well-established pharmacophores such as the cephalosporin, quinolone, oxazolidinone, tetracycline, pleuromutilin and lipoglycopeptide classes of antibiotic. Despite this seemingly impressive number of new antibiotics hitting the market, there is a marked disparity in their availability, with most only approved for use by US, EU, or UK medicinal governing bodies.
Table 2.
Antibiotics launched since 2010. Antibiotics are ordered from oldest to newest and include trade name, company, approval year, drug class, route of administration, indications and countries (or regions) with approval
|
Antibiotic |
Trade name |
Company |
Approval year |
Drug class |
Route of administration |
Indications |
Approval |
|---|---|---|---|---|---|---|---|
|
Ceftaroline |
Teflaro/Zinfloro |
Allergen |
2010 |
Cephalosporin |
IV |
ABSSSIs, CABP |
FDA, EMA, MHRA |
|
Dalbavancin |
Dalvance |
Allergen |
2014 |
Lipoglycopeptide |
IV |
ABSSSIs |
FDA, EMA, MHRA |
|
Oritavancin |
Orbactiv/Kirmyrsa |
Melinta |
2014 |
Lipoglycopeptide |
IV |
ABSSSIs |
FDA. EMA, MHRA |
|
Tedizolid |
Sivextro |
Merck |
2014 |
Oxazolidinone |
IV, po |
ABSSSIs |
FDA, EMA, MHRA |
|
Finafloxacin |
Xtoro |
MerLion Pharmaceuticals |
2014 |
Quinolone |
Drop suspension |
Titis externa |
FDA, Health Canada |
|
Nemonoxacin |
Taigexyn |
TaiGen Biotechnology |
2016 |
Quinolone |
IV, po |
ABSSSIs, CABP |
Taiwan ROC, Russian Federation, CFDA |
|
Zabofloxacin |
Zabolante |
Dong Wha Pharmaceutical |
2016 |
Quinolone |
po |
COPD |
Republic of Korea, CFDA, Middle East, Africa |
|
Delafloxacin |
Baxdela/Quofenix |
Melinta |
2017 |
Quinolone |
IV, po |
ABSSSIs, CABP |
FDA, EMA, MHRA |
|
Ozenoxacin |
Ozanex /Ozewid/Xepi |
Medimetriks Pharmaceuticals |
2017 |
Quinolone |
Topical |
Impetigo |
EMA, PMDA, FDA, MHRA |
|
Eravacycline |
Xereva |
Tetraphase |
2018 |
Tetracycline |
IV |
cIAIs |
FDA, EMA, MHRA |
|
Omadacycline |
Nurzyra |
Paratek |
2018 |
Tetracycline |
IV, po |
ABSSSIs, CABP |
FDA |
|
Lefamulin |
Xenleta |
Nabriva |
2019 |
Pleuromutilin |
IV, po |
CABP |
FDA, EMA |
|
Lascufloxacin |
Lasvic |
Kyorin Pharmaceuticals |
2019 |
Quinolone |
IV, po |
CABP |
PMDA |
|
Levonadifloxacin |
Emrok |
Wockhardt |
2020 |
Quinolone |
IV, po |
ABSSSIs |
DCGI |
|
Contezolid |
Youxitai |
MicuRx |
2021 |
Oxazolidinone |
IV, po |
cSSSIs |
CFDA |
ABSSSI, acute bacterial skin and skin structure infections; CABP, community acquired bacterial pneumonia; CFDA, China Food and Drug Administration ; cIAI, complicated intra-abdominal infection; COPD, chronic obstructive pulmonary disease; cSSSI, complicated bacterial skin and skin structure infections; cUTI, complicated urinary tract infections; DCGI, Drugs Controller General of India; EMA, European medicines agency EU ; FDA, Food and Drug Administration US; HABP, hospital acquired bacterial pneumonia; IV, intra-venous; MHRA, Medicines and Health Products Regulatory Agency UK; PMDA, Pharmaceuticals and Medical Devices Agency Japan; po, per os (oral administration); VABP, ventilator associated bacterial pneumonia.
Cephalosporins
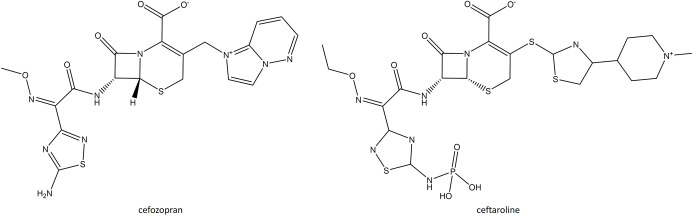
One novel fifth-generation oxyimino-cephalosporin, ceftaroline fosamil (CFT), has been approved in the last decade and retains activity against MRSA and VRSA by binding covalently to PBP2A [123, 124]. It was developed by modifying the structure of the fourth-generation cephalosporin, cefozopran (Fig. 3). This includes a 1,3-thiazole ring at the 3-position of the cephalosporin nucleus as well as an oxime group in the C-7 acyl moiety and phosphono group, which together are responsible for the improved anti-MRSA activity and solubility of CFT [123]. CFT is marketed as a IV drug for use against S. aureus and other Gram-positive organisms and includes Gram-negative coverage. In phase III clinical trials, CANVAS 1 (NCT00424190) and CANVAS 2 (NCT00423657), CFT was compared with VAN/aztreonam (ATM) to treat complicated bacterial skin and skin structure infections (cSSSIs) caused by MRSA and other common cSSSI pathogens. In CANVAS 1, 703 patients suffering from cSSSIs were treated with either CFT (353) or VAN/ATM (349). The microbial cure rate for MRSA cSSSIs was 95.1 % for CFT and 95.2 % for VAN/ATM [125]. In CANVAS 2, clinical cure rates were similar between CFT and the comparator agents. MRSA cSSSIs were cured in 91.4 % of patients for CFT, and 93.3 % of patients treated with VAN/ATM. In both trials, similar rates of adverse events (AEs) were observed, with diarrhoea, nausea, headaches and pruritis the most frequently encountered. Overall, CFT demonstrated high clinical cure rates for both MRSA cSSSIs and cSSSIs caused by alternative pathogens whilst being well tolerated [126]. CFT was also evaluated in two FOCUS phase III studies (NCT00621504 and NCT00509106), assessing its potential in the treatment of community-acquired bacterial pneumonia (CABP). The clinical cure rates for S. aureus CABP was 72 % for CFT and 60 % for ceftriaxone and again it was well tolerated with minimal AEs [127]. Following the success of these trials, CFT gained US Food and Drug Administration (FDA) clinical approval in 2010 to treat CABP and acute bacterial skin and skin structure infections (ABSSSIs) and achieved European Medicines Agency (EMA) approval in 2012 for CABP and cSSSIs. Initial in vitro studies indicated that staphylococcal spp. acquired resistance at very low rates [128]. Despite this initial optimism, CFT-resistant strains of S. aureus have subsequently emerged and can be attributed to novel mutations in the CFT-binding pocket of PBP2A, including Y446N, E447K and E239K [129, 130].
Fig. 3.
Structure of ceftaroline and its progenitor cefozopran.
Quinolones
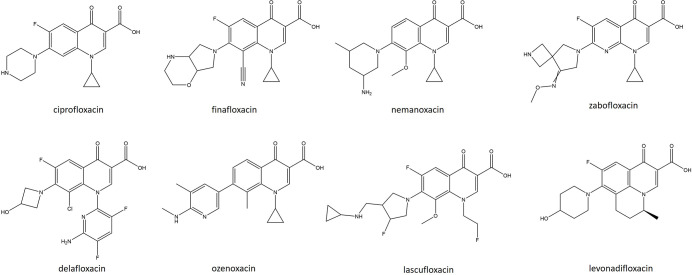
Seven new fluoroquinolone derivatives have been approved for clinical use since 2010. Despite initially being developed as anti-Gram-negative therapeutics, next-generation fluoroquinolones have been shown to have excellent anti-staphylococcal activity. The first of these, finafloxacin (FIN), is a novel fourth-generation fluoroquinolone harbouring an 8-cyano and 7-pyrrolo-oxazinyl substituent (Fig. 4) [131]. It has been shown to be effective in the clearance of infections caused by S. aureus, Acinetobacter baumanii and a range of other Gram-positive and -negative organisms [132]. Notably, this derivative was shown to have superior activity, when compared with CIP, in low-pH environments and infection-relevant conditions, likely due to its propensity to accumulate rapidly within bacterial cells combined with low rates of efflux [133–135]. In normal microbroth dilution assays FIN had a twofold lower MIC than CIP against community acquired MRSA [134]. When tested in synthetic urine, FIN demonstrated a 4–32-fold lower MIC than CIP against MRSA and methicillin-susceptible S. aureus (MSSA) [132]. FIN, as a topical formulation, has been evaluated in two randomized multicentre phase III trials for the treatment of otitis externa (NCT01535560, NCT01535599). Across both trials, pathogen eradication was achieved in 67 % of patients receiving FIN versus 13 % of patients receiving a vehicle control. The eradication rate for S. aureus was 89 % compared with 33 % for the placebo. Time to cure was also significantly shortened for patients receiving FIN, 3.5 days versus 6.8 days. The most frequently encountered AEs for topical FIN were nausea and pruritis of the ear canal, although this only occurred in 1 % of patients [131]. As a result, this topical suspension, under the trade name Xtoro, gained FDA and Health Canada approval as a drop suspension for the treatment of otitis externa in 2014. FIN has also completed a phase II trial (NCT01928433), administered by IV and orally for the treatment of complicated urinary tract infections (cUTIs). In this multicentre, randomized, double-blind, double-dummy, active-control trial FIN was administered for either 5 (FIN5) or 10 (FIN10) days compared with CIP for 10 days (CIP10). Clinical cure responses were observed for 70 % of FIN5 patients, 68 % of FIN10 patients and 57 % of CIP10 patients. FIN was generally well tolerated. Overall, 43.4, 42.7 and 54.2 % of patients in the FIN5, FIN10 and CIP10 groups experienced mild AEs. Together these results indicated that a 5 day course of FIN resulted in improved eradication and clinical outcomes compared with CIP [136]. Resistance studies involving this antibiotic against S. aureus are sparse, as the resistance breakpoints for this antibiotic have not yet been defined. However, in vitro studies involving finafloxacin noted that elevated MICs because of spontaneous mutation were rare. It has been noted that MIC measurements improve drastically when tested in acidic environments [131, 135].
Fig. 4.
. Structures of recently approved quinolones (finafloxacin, nemanoxacin, zabofloxacin, delafloxacin, ozenoxacin, lascufloxacin, levonadifloxacin) and ciprofloxacin.
Nemonoxacin (NEM) is a non-fluorinated C-8 methoxy quinolone targeting both DNA gyrase and topoisomerase IV (Fig. 4) [137]. C-8 methoxy substituents have previously been associated with an improved spectrum of activity and reduced mutant selection rates [74]. Indeed, NEM has been demonstrated to have broad-spectrum activity in both in vitro and in vivo studies against both Gram-positive and -negative pathogens but with a particular proclivity against MRSA and penicillin-resistant S. pneumoniae (PRSP). Removal of the fluorine side chain is believed to reduce toxicity [137, 138]. In a phase III, multicentre, randomized, double-blind, double-dummy, active-controlled, non-inferiority trial (NCT01529476), 500 mg of oral NEM was compared with 500 mg of LVX for the treatment of CABP [139]. Of a total of 527 patients, 356 received NEM and 171 received LVX, resulting in clinical cure rates of 94.3 and 93.5%, respectively. The incidence of AEs was also comparable between NEM (33.1 %) and LVX (33.3 %). Combined with the results of earlier phase II trials (NCT00434291 and NCT01537250), NEM reached non-inferiority versus LVX whilst maintaining a satisfactory safety profile [138, 139]. Following these trials, NEM gained Chinese approval in 2016 for the treatment of CABP and ABSSSIs and has since gone on to receive a qualified infectious disease product and It-track designation for CABP and ABSSSIs by the FDA. Due to its limited use, resistance has not currently been reported in the clinic, but in vitro studies have demonstrated resistance in S. pneumoniae strains harbouring three mutations within the quinolone resistance-determining regions (QRDRs): S82Y in gyrA, S494T in gyrB and P454S in parC . Thus, resistance to nemonoxacin is less likely to develop as resistance to other fluoroquinolones can occur through only two mutations in the QRDRs [140]. In another study involving clinical isolates of S. pneumoniae, S. aureus, E. faecium and Enterococcus faecalis, serial passage with nemonoxacin resulted in a fourfold increase in MIC against the S. pneumoniae isolate. However, this increase in MIC was found to be independent of mutations in the QRDRs. Contrastingly, the MICs against S. aureus , E. faecium and E. faecalis did not increase, demonstrating that nemonoxacin has a low potential for inducing resistance [141].
Zabofloxacin (ZBO) is a novel fluoronaphthyridone quinolone with a 7-pyrrolidone substituent (Fig. 4) that exhibits excellent in vitro broad-spectrum activity targeting both DNA gyrase and topoisomerase IV. It demonstrates impressive activity against a range of major respiratory pathogens, including fluoroquinolone-resistant S. aureus , S. pneumoniae , Haemophilus influenzae and Moraxhella catarrhalis [142]. In a phase III multicentre, double-blinded, randomized non-inferiority clinical trial (NCT01658020), oral administration of 367 mg (once daily for 5 days) of ZBO was compared with 400 mg of MOX (once daily for 7 days). The clinical cure rates were 88.2 % for ZBO and 89.1 % for MOX. No significant difference was observed, indicating that ZBO exhibited an equivalent clinical outcome for the treatment of chronic obstructive pulmonary disease (COPD). A sub-study in this trial also examined ZBO for the treatment of lower respiratory tract infection (LRTI) without the manifestation of chronic bronchitis. In this patient group the cure rate was 85.9 % for ZBO and 84.2 % for MOX, similarly without a significant difference being reached [143]. ZBO was approved for clinical use in the Republic of Korea in 2016 as an oral formulation for the treatment of acute bacterial exacerbation of COPD and later by countries in the Middle East and North Africa. Epidemiological studies in Egypt have shown that of 116 MRSA strains, 61.2 % were susceptible and 37.9 % were resistant, performing better than MOX, CIP and LVX. The resistant strains were found to have mutations in the QRDRs, namely S84L in gyrA, S80F in parC and P451S in parE [144].
Delafloxacin (DLX) possesses a unique, highly anionic scaffold that targets both DNA gyrase and topoisomerase IV of Gram-positive and -negative organisms with equal affinity (Fig. 4). DLX is the only non-zwitterionic fluoroquinolone currently available in the clinic [145, 146]. This molecule has an intriguing chemical structure, making it a weak acid, which stays uncharged in acidic environments, promoting transfer across the bacterial membrane and rapid accumulation within the cell [147]. Accordingly, DLX has increased activity in acidic environments such as the phagolysosome, within biofilms, abscesses and in the skin [147, 148]. It has broad-spectrum, bactericidal activity against multiple staphylococci (including MRSA), streptococci and enterococci, as well as many Gram-negative organisms [145, 146, 149]. A phase III multicentre, randomized, double-blind trial (NCT01984684) with VAN/ATM as a comparator was performed to determine the efficacy of DLX in the treatment of ABSSSIs. Eight hundred and fifty adults received either DLX (300 mg IV every 12 h for 3 days followed by a switch to 400 mg oral) or VAN/ATM (15 mg kg−1 for 5–14 days). Clinical objective responses were 83.7 % in the DLX treatment group and 80.6 % for the VAN/ATM combination. Treatment AE rates were similar between DLX and the comparator, but VAN/ATM was associated with a higher rate of drug-related discontinuation (1.2 vs 2.4 %). Thus, DLX reached non-inferiority for the treatment of ABSSSIs, which promoted its approval by the FDA in 2017 [150]. In contrast to other quinolones and due to the dual targeting ability of gyrase and topoisomerase, delafloxacin has increased stability and efficacy against gyrase and topoisomerase mutations [151]. Like NEM, double or triple mutations of the QRDR are required for the development of DLX resistance. However, DLX-resistant MRSA strains have been isolated from healthcare-associated infections. Genome sequence analysis of six DLX resistant isolates identified double mutations in gyrA (either S84L, Q88K or S85P) and a parC mutation (either S80Y/K, Q84G, or D36G) [152].
Ozenoxacin (OZE) is a novel non-fluorinated quinolone (Fig. 4). Like NEM, its non-fluorination grants a better safety profile than classical fluorinated quinolones, precluding the development of quinolone-induced chondrotoxicity and transdermal absorption [153]. Like DLX, OZE inhibits both gyrase and topoisomerase IV with equal affinity, conferring activity against CIP- and LVX-resistant S. aureus, S. pyogenes, Staphylococcus epidermidis and Streptococcus agalactiae [154, 155]. OZE's anti-staphylococcal activity has been further examined; in a study of 1031 S . aureus skin and soft tissue infection isolates, OZE exhibited an MIC90 of ≤0.05 µg ml−1, performing better than LVX [156]. Two multicentre, randomized, double-blind, vehicle-controlled phase III clinical trials (NCT01397461 and NCT02090764) have been performed to evaluate a 1 % topical OZE formulation for the treatment of impetigo. In the first trial, patients received either OZE, placebo or retapamulin (RET). Microbial clearance was 70.8 % for OZE versus 38.2 % for the placebo after 3–4 days and 79.2 versus 56.6 % after 6–7 days. OZE also contributed to more rapid microbial clearance than RET and was well tolerated [157]. In the second, 411 patients received either OZE or a placebo. OZE demonstrated superior cure rates after 5 days (54.9 versus 37.9 %). A notable superior microbial eradication was also noted after only 2 days (87.2 versus 63.9 %). It was similarly well tolerated, with only eight patients experiencing AEs, and only one potentially as a result of OZE administration [158]. Subsequently, this topical quinolone was approved in 12 European countries, the USA and Canada for the treatment of non-bullous impetigo in children. Like other dual targeting quinolones, OZE is less likely to result in the selection of resistant mutants. In vitro studies have shown that OZE maintains activity against isolates harbouring as many as four mutations in gyrA/grlA and is not affected by the efflux pump inhibitor reserpine [154].
Lascufloxacin (LSFX) is a novel 8-methoxy fluoroquinolone displaying a unique pharmacophore at the first and seventh positions of the quinoline nucleus (Fig. 4) [159]. Although it harbours some Gram-negative activity, its most potent activity was observed when tested against Gram-positive pathogens, including MRSA and S. pneumoniae, with an MIC90 of 0.06 µg ml−1 [159]. LSFX is well tolerated and achieves rapid distribution to the epithelial lining fluid and alveolar macrophages and achieves drug concentrations exceeding the MIC90 values for most MSSA and MRSA isolates [160]. In a double-blind, randomized comparative phase III study, LSFX (75 mg) was compared with LVX (500 mg) in patients suffering from CABP. The clinical efficacy rate following cessation of treatment was 96.0 % for LSFX and 95.8 % for LVX. The incidence of AEs was similar in both patient populations, 17.9 % for LSFX and 19.0 % for LVX with no serious AEs noted for either drug [161]. This drug was first approved for clinical use in Japan in 2019 where it has been used to treat CABP and other Gram-positive respiratory tract infections. Elevated MICs of 2 µg ml−1 (approximately a 16-fold increase) against LSFX have been reported and are due to double mutations in both gyrA and parC, namely S84L E88V or S84L E88K for gyrA, and S80F E84K for parC. These same mutations result in MICs of 32–128 µg ml−1 (a 64–256-fold increase) against CIP and LVX, therefore lascufloxacin shows increased activity against wild-type and mutated topoisomerase IV and gyrase enzymes and has a lower tendency to select for resistant mutants [159, 160, 162, 163].
The most recent quinolone to gain market approval is levonadifloxacin (LEV). This belongs to a new benzoquinolizine subclass of fluoroquinolones that is an arginine salt of the active S(−) isomer of the commonly prescribed topical fluoroquinolone nadifloxacin (Fig. 4). Unlike most archetypal fluoroquinolones, which preferentially target topoisomerase IV over DNA gyrase, LEV preferentially targets DNA gyrase [164, 165]. Due to the presence of a non-basic side chain, it remains unionized at an acidic pH, which allows for superior activity in acidic environments [166, 167]. It has broad-spectrum activity but shows particular potency against quinolone-resistant MRSA strains, hVISA, VRSA and DAP-resistant S. aureus [168]. It was evaluated in a phase III multicentre, randomized, open-label, active-comparator study with 500 subjects suffering from ABSSSIs (NCT03405064). Patients received either 1000 mg of oral LEV with 600 mg of oral LZD as a comparator, or 800 mg of IV LEV with 600 mg of IV LZD as a comparator. The clinical cure rate for LEV was higher for both the oral (91.0 vs 87.8 %) and IV (95.2 vs 93.6 %) formulations. At the lower bound of the 95 % confidence interval the treatment difference was greater than 15 %, meaning that both oral and IV LEV reached non-inferiority against oral and IV LZD. Approximately 30 % of patients tested positive for an MRSA infection, and LEV exhibited higher clinical cure rates in these patients (95.0 vs 89.3 %). AE rates were similar for IV treatment groups (20.8 vs 22.4 %, for LEV and LZD) and oral treatment groups (16.0 vs 13.5 %, respectively) and were all mild in nature [169]. This antibiotic recently gained approval and is available as an IV and oral tablet formation for use against ABSSSIs and diabetic foot ulcers in the USA. LEV is associated with resistance suppression properties. For example, while overexpression of the NorA efflux pump can lead to fluoroquinolone resistance, this has no effect on LEV activity, since it is not a substrate for NorA. Additionally, its higher affinity for DNA gyrase seems to come with a lower frequency of mutations [170]. This allows LEV to maintain activity against strains with multiple mutations in the QRDR with strains harbouring gyrA S84P and S85L, and grlA S80F mutations presenting with a LEV MIC of 2 µg ml−1 [171].
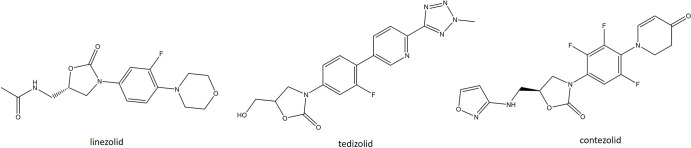
Oxazolidinones
Two second-generation oxazolidinones have been approved clinically. Although structurally similar to LZD, tedizolid (TDZ) harbours two modifications that improve potency, increase binding affinity, and reduce its susceptibility to horizontally acquired resistance (Fig. 5). The first is the presence of a C-5 hydroxymethyl group replacing the acetamide group in LZD. The second is the addition of a C- and d-ring system, the incorporation of pyridine and tetrazole rings, respectively, enabling TDZ to form additional binding interactions within the upper region of the ribosomal peptidyl transferase centre [172, 173]. Together these modifications result in TDZ being four–eightfold more potent than LZD against MRSA and hVISA strains of S. aureus , vancomycin-resistant E. faecium (VRE) and streptococci [174]. Additionally, it has been shown to have improved oral bioavailability and a lower risk of drug interactions, culminating in an antibiotic with better safety and pharmacodynamics [175]. Two phase III clinical trials, ESTABLISH-1 and ESTABLISH-2, evaluated TDZ compared with LZD for the treatment of ABSSSIs. ESTABLISH-1 (NCT01170221) was a randomized, double-blind, noninferiority trial where either 200 mg of oral TDZ or 600 mg of oral LZD was administered every 12 h for 10 days [176]. ESTABLISH-2 (NCT01421511) was also a randomized, double-blind, noninferiority trial that compared patients receiving IV TDZ (200 mg for 6 days) with IV LZD (600 mg twice daily for 10 days) [177]. The result of both studies found TDZ reaching the non-inferiority threshold compared to LZD. Gastrointestinal (GI)-related AEs and myelotoxicity were also encountered less frequently in patients receiving TDZ [176, 177]. TDZ gained FDA approval in 2014 for the treatment of Gram-positive ABSSSIs. It is also being investigated in two phase IV trials for the treatment of S. aureus infections in cystic fibrosis (NCT02444234) and for the treatment of prosthetic joint infections (NCT03746327) and two phase II trials for the treatment of Gram-positive bone and joint infections (NCT03009045) and for the treatment of MDR Mycobacterium tuberculosis (NCT05534750). As with LZD, the spontaneous frequency of mutation against TDZ is low and the modified side chain at the C-5 position helps maintain activity, with MICs of 0.5–1 µg ml−1 against strains harbouring the horizontally transmissible cfr gene [178]. A recent study that sought to investigate the capacity of TDZ to select for cross-resistance to other antimicrobials found a A1345G single nucleotide variant in the rpoB gene after 10 days of serial passage that gave a TDZ MIC of 4 µg ml−1. This mutation also conferred elevated MICs against LZD, CHL, RET and quinupristin/dalfopristin [179]. Subsequent serial passaging experiments with TDZ and LZD showed an unchanged TDZ MIC of 0.5 µg ml−1 for MSSA ATCC 29213 but an elevated TDZ MIC from 0.25 µg ml−1 to 2 µg ml−1 for MRSA ATCC 33591. For LZD the MIC increased 32- and 64-fold for the MSSA and MRSA strains, respectively. The stepwise increase in TDZ MIC against MRSA ATCC 33591 was found to require multiple mutations in the 23S rRNA, but only single mutations were required to confer LZD resistance [180].
Fig. 5.
Structures of recently approved oxazolidinones (tedizolid and contezolid) compared with linezolid.
Contezolid (CZD) is a novel ortho-fluoro dihydropyridone second-generation oxazolidinone (Fig. 5). It differs from LZD by replacing the morpholine group with a piperidinone group [181]. This modification helps address the myelosuppression and monoamine oxidase (MAO) inhibition counterindications, which are known to be associated with LZD. LZD has been shown to bind and inhibit both A and B isoforms of the MAO enzyme and can therefore cause serotonin syndrome, especially when co-administered with serotonin re-uptake inhibitors, various inhibitors, or other MAO inhibitors [182]. CZD on the other hand has exhibited between 2- and 148-fold reduction in MAO-A and MAO-B inhibition in vitro. This was deemed to be of potential clinical significance, warranting the investigation of CZD in clinical trials [183]. The spectrum of CZD covers most Gram-positive pathogens, including MRSA, PRSP and VRE, although the potency of this drug is considered to have a similar efficacy to LZD. Furthermore, strains that are resistant to LZD are generally considered to be cross-resistant to CZD [181, 184, 185]. In a double-blind, multicentre, phase III study conducted in PR China, CZD was compared to LZD for the treatment of complicated skin and soft tissue infections (cSSTIs). In total, 596 patients received either oral CZD (800 mg every 12 h for 7–14 days) or oral LZD (600 mg every 12 h for 7–14 days). The clinical cure rate was 92.8 % for CZD and 93.4 % for LZD. The clinical AEs for CZD were comparable to those for LZD; nausea, vomiting, abdominal discomfort and increases in alanine aminotransferase, aspartate aminotransferase, blood uric acid and blood bilirubin levels were most the most frequently encountered AEs and were considered mild to moderate in their severity [184]. CZD was approved by the National Medical Products Administration of China in 2021 for the treatment of cSSSIs caused by a range of Gram-positive organisms, including MRSA, VRE, S. pyogenes and S. agalactiae . CZD is also under investigation in the USA in an effort to accelerate global approval; NCT03747497, a phase II trial for treatment of ABSSSIs, and NCT05369052, a phase III trial for the treatment of diabetic foot ulcers.
Tetracyclines
Two tetracycline antibiotics entered the clinic in 2018. Eravacycline (ERV) is a broad-spectrum, fully synthetic fluorocycline that has been developed to treat various MDR organisms, such as MRSA, VRE and carbapenem-resistant Enterobacteriaceae (CRE) (Fig. 2). It was designed to overcome two mechanisms of TET resistance, namely drug efflux and ribosomal protection, by modifications to the C7 and C9 positions of the TET core [186]. Prior structural modifications of the C7 and C9 positions has led to the discovery and development of clinically important TET derivatives such as MIN and TGC [91, 187]. These antibiotics showed improved activity against tetracycline-specific efflux but remained susceptible to RPPs. The substitutions present in ERV are a pyrrolidine group on the C9 side chain and a fluoro group at C7. Together these improve the antibacterial potency against strains harbouring both TET efflux and RPP resistance determinants. ERV is largely unaffected by the presence of tetM, tetK, or tetA with a 4–16-fold increase in potency (MIC90 0.016–0.05 µg ml−1) against S. aureus , E. faecalis, Escherichia coli and Acinetobacter baumannii compared to TGC. This in vitro activity has carried over to impressive in vivo efficacy in murine models, including a septicaemia model challenged with SHV-producing E. coli , and a neutropenic thigh model challenged with a tetM-producing S. aureus strain [186, 188]. Encouraging in vitro and in vivo data directed an examination of the clinical efficacy of ERV. The clinical trials IGNITE1 (NCT01844856) and IGNITE4 (NCT01844856) focused on the treatment of complicated intrabdominal infections (cIAI). IGNITE1 was a randomized, double-blind, double-dummy, comparative, noninferiority, multicentre, multinational trial consisting of 541 patients. Subjects received either 1 mg kg−1 IV ERV every 12 h or 1 g of IV ertapenem every 24 h, both for a median of 7 days. Isolated organisms were a mix of Gram-negative aerobes and Gram-positive anaerobes/aerobes. Clinical cure rates were 87.0 % for ERV and 88.8 % for ertapenem and reached non-inferiority criteria. There were three deaths in the ERV group and six in the ertapenem group but these were not believed to be a result of either drug treatment. More AEs occurred in the ERV group, specifically the incidence of nausea and phlebitis [189, 190]. IGNITE4 was a second randomized, double-blind, double-dummy, comparative, noninferiority, multicentre, multinational trial investigating cIAIs in a further 500 patients. Participants received either 1 mg kg−1 IV ERV every 12 h or 1 g of IV meropenem every 8 h, for 4–14 days. ERV similarly reached non-inferiority, with clinical cure rates of 91.2 % (ERV) and 90.8 % (meropenem). AEs were mostly gastrointestinal in nature and occurred in 37.2 % of the ERV group and 30.9 % of the meropenem group [191]. This was followed by the approval of this antibiotic by the EMA and FDA for the IV administered treatment of cIAIs. S. aureus resistance to eravacycline has not been reported, but MIC creeping verging on hetero resistance has been observed. Elevated MICs of 1–4 µg ml−1 were reported more frequently for MSSA strains. These were reversed when combined with efflux pump inhibitors, suggesting the involvement of efflux in this phenotype [192, 193].
Omadacycline (OMA) is a semisynthetic 9-aminomethylcycline derivative of MIN. Like the prototypical semisynthetic tetracyclines MIN and TGC, it harbours a C9 substitution to the tetracycline core (Fig. 2). Specifically, OMA has a an aminoethyl group present at the C9 position that overcomes TET resistance mechanisms commonly employed against MIN and DOX, including efflux and ribosomal protection [194]. These features allow OMA to maintain activity in the presence of S. aureus strains with tetO and tetK [195]. In addition, this structural modification increases the antimicrobial potency of this drug whilst reducing the unwanted side effects frequently encountered with TGC administration, such as nausea and emesis [195, 196]. OMA is an impressively broad-spectrum drug with coverage against aerobic and anaerobic Gram-positives, Gram-negatives and other atypical pathogens. For S. aureus it exhibits excellent in vitro activity against MRSA isolates with an MIC90 of between 0.25–0.5 µg ml−1 [193, 194, 197]. OMA has completed three phase III trials: OPTIC (NCT02531438) for the treatment of CABP and OASIS (NCT02378480) and OASIS-2 (NCT02877927) for the treatment of ABSSSIs. In OPTIC, a randomized, double-blind, multicentre study, 744 patients received either 100 mg IV OMA (initially twice daily but reduced to once daily) or 100 mg of IV MOX for 3 days. Clinical success at 5–10 days post-administration was 92.9 % for OMA and 90.4 % for MOX in the clinically evaluable population, reaching the non-inferiority threshold [198]. OASIS and OASIS-2 were randomized, double-blind, multicentre studies comparing the safety and efficacy of OMA to LZD. In OASIS, patients received IV OMA (100 mg twice a day for the first 2 days followed by 100 mg/day) or IV LZD (600 mg twice daily). Post treatment clinical success for the clinically evaluable (CE) population was comparable between OMA (96.3 %) and LZD (93.5 %). Success rates for S. aureus were also similar (OMA, 83.3 %; LZD, 83.4 %) [199]. In OASIS-2, patients received oral OMA (450 mg/day for days 1–2, then 300 mg/day thereafter) or oral LZD (600 mg twice daily). OMA again performed similarly to LZD, with success rates of 97.9 and 95.5%, respectively. In this study OMA was superior to LZD for the treatment of MSSA (82.7 vs 79.8 %) and MRSA (85.6 vs 79.4 %) [200]. In all phase III trials OMA was deemed safe and to have high tolerability, with only minor GI issues noted [198–200]. It also reached non-inferiority vs its comparator in all cases, leading the FDA to approve it for the treatment of CABP and ABSSSIs. The EMA approved OMA for use against ABSSSIs, requiring a further phase III CABP study (NCT04779242). OMA heteroresistance has been reported for clinical isolates in vitro. Isolates with elevated MICs of ≥1 µg ml−1 were reduced to as low as ≤0.03 when performed in the presence of PAβN or CCCP. The authors suggested that, as is the case with TGC, tetK overexpression was the culprit for the elevated MICs. However, further analysis indicated that the overexpression of two putative genes, RS01625 and RS00550, contributes to omadacycline heteroresistance. Next-generation sequencing also found that heteroresistant strains harboured mutations in fibronectin-binding protein (FnBP), which the authors concluded may impede the penetration of OMA [201].
Pleuromutilins
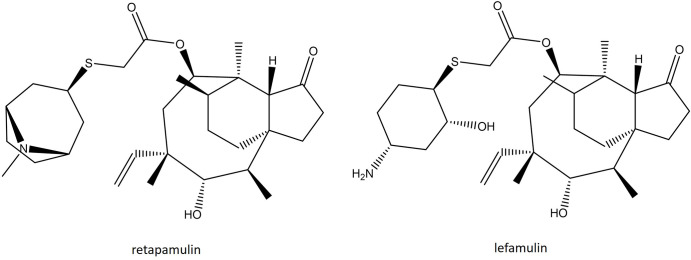
Lefamulin (LEF) is a novel semisynthetic pleuromutilin consisting of a tricyclic mutilin core that helps facilitate an interaction with domain V of the 23S rRNA subunit through additional hydrophobic bonds and van der Waal forces (Fig. 6). In addition, this molecule possesses a large C14 extensional group that is the main driver of the pharmacodynamic and antimicrobial properties. This C14 side chain harbours a thioether bond that significantly improves solubility and metabolic stability, both of which enable LEF to be administered orally and intravenously [202, 203] and increases the number of hydrogen bonds with the target site, which lowers the impact of ribosomal mutations [202, 204]. LEF has a distinct mechanism of protein synthesis inhibition that involves binding at the A- and P-site, preventing peptidyl transferase reaction. It shares some similarities with the mechanism of action of oxazolidinones, but LEF employs an ‘induced-fit’ strategy to close the binding pocket of the ribosome. This mechanism is incredibly effective at preventing the formation of the first peptide bond, but once elongation has started, LEF is ineffective [202–205]. Thus, the activity of LEF is believed to be bacteriostatic against most bacterial species, with the exception of Mycoplasma pneumoniae [206]. Its spectrum of activity includes all Gram-positive aerobes except E. faecalis, as well as certain fastidious Gram-negative aerobes, such as H. influenzae and M. catarrhalis. Importantly, it retains activity against MRSA, VISA, hVISA and VRSA strains of S. aureus with an MIC90 of 0.12 µg ml−1, as well as MDR S. pneumoniae and VRE [207–209]. LEF has completed two phase III trials, LEAP 1 and LEAP 2. LEAP 1 was a multicentre, randomized, double-blind, double-dummy, active-controlled, parallel-group study (NCT02559310) that evaluated the efficacy of IV-to-oral LEF with MOX with or without LZD as a comparator in adults with CABP. A total of 551 patients received either 150 mg IV LEF (n=276) every 12 h, or 400 mg IV MOX (n=275) every 24 h. After six doses, this could be switched to 600 mg oral LEF every 12 h or 400 mg oral MOX every 24 h. Under this treatment regimen LEF reached non-inferiority to MOX, with an early clinical response of 87.3 vs 90.2 %, respectively. The rates of discontinuation due to AEs were 2.9 % for LEF and 4.4 % for MOX, with the most frequently reported AEs being hypokalaemia, insomnia, or pain during administration [210]. LEAP 2 (NCT02813694) was a further double-blind, double-dummy, parallel-group randomized clinical trial comparing oral LEF with oral MOX for the treatment of CABP. Patients received 600 mg oral LEF every 12 h for 5 days or 400 mg oral MOX every 24 h for 7 days. The clinical success response was 89.7 % (LEF) and 93.6 % (MOX) in the clinically evaluable population, reaching the non-inferiority threshold. AE rates were encountered in 12.1 % of the LEF group and 1.1 % of the MOX group, but were mild in nature [211]. Following these studies, LEF was deemed safe and effective for the treatment of CABP and gained approval by the FDA and EMA, making it the first pleuromutilin approved for systemic use. There is evidence of low frequency of spontaneous mutation (<10−9), making resistance developing during administration unlikely [202, 203]. Nevertheless, in vitro resistance has been described and is a result of 23S, rplC and rplD mutations, or acquisition of the Tn5406-encoded Vga(Av) or plasmid-borne Vga(A) [212].
Fig. 6.
Structure of the recently approved pleuromutilin lefamulin compared with the first in class retapamulin.
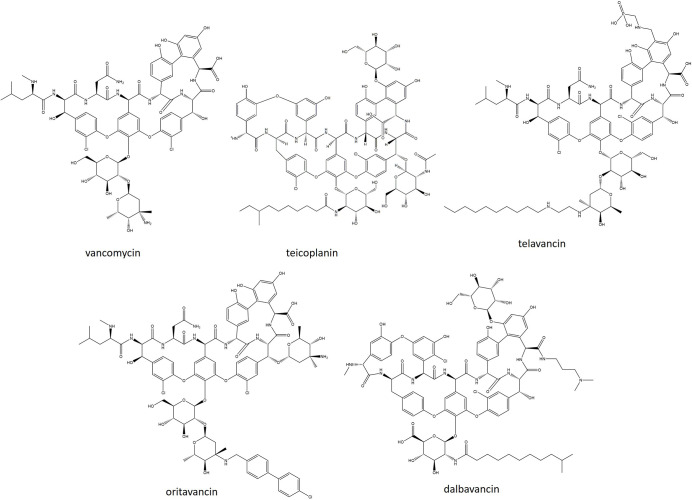
Lipoglycopeptides
In the last 9 years two synthetic lipoglycopeptides have been clinically used. Conventional glycopeptides inhibit late-stage peptidoglycan synthesis via their aglycone group, which forms five hydrogen bonds with the terminal d-Ala-d-Ala residue of lipid II [213]. Early work focussing on enhancing the activity of VAN suggested that lipid II binding could be enhanced through two mechanisms: (1) the formation of glycopeptide dimers that lock the binding pocket in the correct configuration and promote cooperative binding and (2) the anchoring of the glycopeptide to the membrane, which may help to maintain the drug in close proximity to its target and cause membrane disruption [214, 215]. Lipoglycopeptides retain the aglycone group and therefore conserve this primary mode of action. However, the addition of a lipophilic side chain linked by a disaccharide moiety anchors the molecule to the membrane and causes membrane-induced bacteriolysis [214].
Oritavancin (ORI) is a chemically synthesized derivative of the naturally occurring lipoglycopeptide chloroeremomycin. It differs from chloroeremomycin through the addition of a chlorobiphenylmethyl side chain to the disaccharide moiety, which increases the amphipathicity of the molecule (Fig. 7) [216]. The presence of a 4-epi-vancosamine sugar in both ORI and chloroeremomycin contributes to dimer formation by putting the molecules in a back-to-back orientation [216, 217]. ORI has been demonstrated to disrupt membrane integrity and reverse membrane polarity [218, 219]. The lipophilic side chain also possesses an additional lipid II binding site that interacts with the pentaglycine bridge. This feature permits inhibition of both transglycosylase and transpeptidase reactions, explaining its improved activity against VAN-resistant strains [220]. Like all glycopeptides, ORI is only active against Gram-positive species. However, against VAN-susceptible staphylococci, streptococci and enterococci, it is more potent than VAN with an MIC90 of 0.06 µg ml−1 against S. aureus . ORI is the only lipoglycopeptide to be active against VRE harbouring vanA [221]. The suitability of oritavancin as a treatment option for ABSSSIs has been investigated in two phase III trials, SOLO 1 and SOLO 2, with VAN as a comparator. SOLO 1 was an international, randomized, double-blind study (NCT01252719) where participants were given a single IV dose of 1200 mg ORI followed by either IV placebo (n=475) or an IV dose of VAN (1 g or 15 mg kg−1) every 12 h for 7–10 days (n=479). All three efficacy end points (primary end point, assessed clinical cure and proportion of patients with a reduction in lesion size of ≥20 %) met the specified non-inferiority margin of 10 %. Overall, the frequency of AEs was similar between patient groups, but nausea was more common with ORI [222]. SOLO 2 was a global, multicentre, randomized, double-blind, comparative efficacy and safety study using the same dosing regimen as SOLO 1 (NCT01252732). Similarly, in this study all three efficacy endpoints met the 10 % non-inferiority margin. ORI was well tolerated with only mild AEs reported (nausea, vomiting, headache, cellulitis and increased alanine aminotransferase levels) [223]. Following the success of both trials, the FDA and EMA approved it for use against patients suffering from ABSSSIs. Resistance following clinical use has not been described for ORI, but elevated MICs of ≤16 µg ml−1 have been observed in a laboratory setting. This was achieved through mutations of the vanS(B) sensor gene, as well as hyperproduction of VanH, VanA and VanX. The presence of the vanZ gene also confers an MIC of 8 µg ml−1 through an unknown mechanism [224].
Fig. 7.
. Structures of recently approved lipoglycopeptides compared with vancomycin, teicoplanin and telavancin.
Dalbavancin (DAL) is a semi-synthetic derivative of the naturally occurring lipoglycopeptide A40926 [225]. The peptide carbonyl group present in the structure of A40926 is replaced by a 3,3-dimethylaminopropylamide group in DAL (Fig. 7) [226]. DAL has been shown to dimerize in solution but without the need for a ligand, due to the presence of an extended lipophilic side chain. However, unlike ORI, this type of dimerization is not believed to be co-operative [227]. Membrane anchoring has been demonstrated for DAL, which is hypothesized to increase the lipid II binding affinity, leading to increased antimicrobial potency [228]. Its spectrum activity is similar to that of other glycopeptides, displaying bactericidal activity against a variety of Gram-positive pathogens. It has 8–16-fold greater activity than VAN against staphylococci and streptococci. A recent examination of over 1100 MRSA isolates has shown the MIC50 of DAL to be 0.06 µg ml−1, compared with 1 µg ml−1 and 0.5 µg ml−1 for VAN and teicoplanin, respectively. Only three S. aureus isolates possessed MICs above the currently proposed FDA breakpoint [229]. In addition to its improved antimicrobial potency, DAL has an extended half-life, allowing it to be dosed once weekly [230]. Two phase III randomized, double-blind, multicentre, noninferiority studies clinical trials, DISCOVER 1 and DISCOVER 2 (NCT01339091 and NCT01431339), investigated the efficacy of DAL for the treatment of ABSSSIs. In both trials patients received either 1000 mg IV DAL on day 1 followed by 500 mg IV on day 8 or 1000 mg IV (or 15 mg kg−1) VAN every 12 h for 3 days. The VAN-treated group had the option to the switch, on day 3, to 600 mg orally administered LZD every 12 h for 12–14 days. Across DISCOVER 1 and 2, the primary endpoint indicated non-inferiority of DAL vs its comparator. Early clinical response rates were 79.7 % for DAL and 79.8 % for VAN–LZD. For patients infected with S. aureus, clinical success was seen in 90.6 % of the DAL treatment group and 93.8 % of the VAN–LZD group. The most commonly encountered AEs were nausea, diarrhoea and pruritis, but these were noted to be lower in the DAL treatment group [231]. DAL is also being investigated in several other trials, including a phase IV trial investigating osteomyelitis (NCT03426761), a phase IV trial investigating DAL over prolonged IV therapy (NCT03982030), a phase III trial investigating the suitability of DAL in the treatment of ABSSSIs in children (NCT02814916) and a phase II trial investigating S. aureus bacteraemia (NCT04775953). Resistance to DAL remains rare, occurring in only 1 % of isolates tested [232]. Similar to VAN, reduced DAL susceptibility is seen in hVISA and VISA strains. DAL does not retain activity against strains harbouring VanA or VanB, thus VRSA strains are similarly described as DAL-resistant [233, 234]. A recent case study of a patient with end-stage renal disease that developed S. aureus infective endocarditis found the causative MRSA isolate to be resistant to VAN, DAP and DAL due to walk and scrA mutations [235].
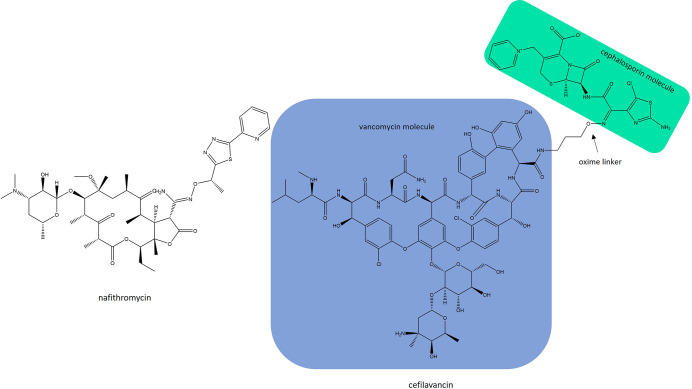
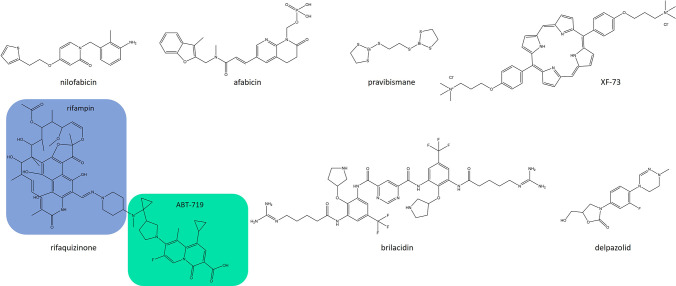
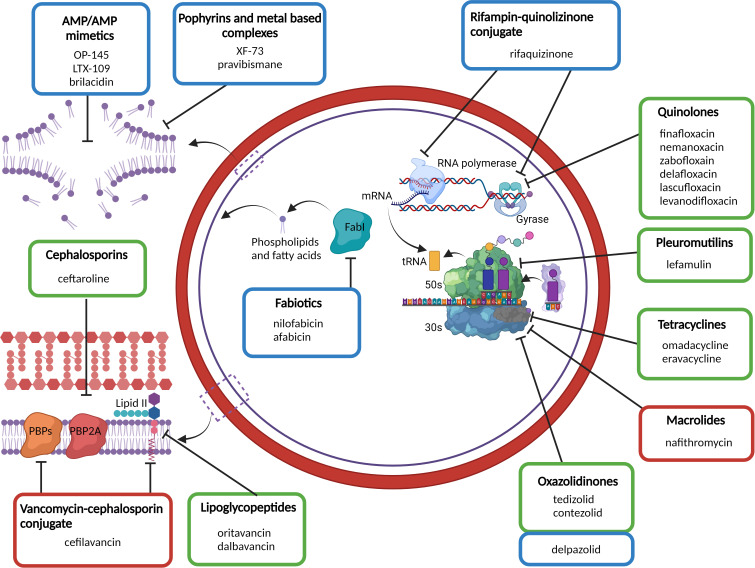
Anti-staphylococcal compounds in clinical development
Here we review anti-staphylococcal compounds currently engaged in phase III and phase II clinical trials, representing novel, first-in-class antibiotics, heterodimer antibiotic conjugates, peptidomimetic AMPs and improved derivatives of existing antibiotics. Antibiotics that have since been dropped by pharmaceutical companies, or that have not had an update for the last 5 years, are not included for comment. Also not included are alternatives to antibiotics, such as vaccines, neutralizing antibodies, bacteriophage and anti-virulence strategies. Recent reviews on alternatives to antibiotics currently in the clinical pipeline are provided by Ghosh et al., Douglas et al. and Czaplewski et al. [236–238]
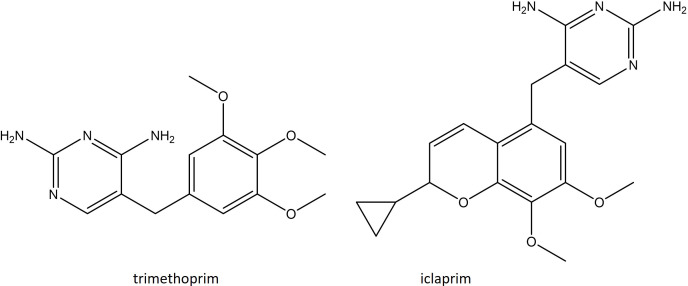
Iclaprim: a cautionary tale
Iclaprim is only the second DHFR inhibitor that has reached the new drug application (NDA) stage of clinical development (Fig. 8). Iclaprim was developed following an in-depth enzymatic, direct binding and x-ray crystallographic analysis of compound interaction with S. aureus TMP-resistant DHFR (F98Y variant), as well as the S. pneumoniae TMP-resistant DHFR (I100L variant) isolates [239]. Enhanced hydrophobic interactions of iclaprim within the substrate-binding pocket of DHFR are essential for increased affinity and antibacterial activity, which correspond to a 20-fold increase in potency compared with TMP [239], allowing for the administration of iclaprim without the need for a sulphonamide. Therefore, sulphonamide-associated safety issues such as rashes, hypersensitivity, blood dyscrasias and life-threatening hyperkalaemia, can be avoided [240]. This drug has had a long and arduous development process spanning more than 20 years. Initially developed by Roche, Arpida subsequently obtained exclusive ownership and submitted an NDA for iclaprim in 2009 that was ultimately rejected, with the FDA citing that the most recent phase III trial did not demonstrate the efficacy of iclaprim in the treatment of ABSSSIs within an acceptable non-inferiority margin. Iclaprim changed hands several times before Motif Bio became owners and completed two phase II trials, REVIVE 1 (NCT02600611) and REVIVE 2 (NCT02607618), comparing the efficacy of iclaprim vs VAN for the treatment of ABSSSIs [241, 242]. A second NDA was submitted in 2018 but this was again rejected, citing additional toxicity testing requirements. Unfortunately, not gaining NDA approval resulted in the collapse of the company and forced this promising antibiotic to be shelved for the foreseeable future.
Fig. 8.
Structure of iclaprim compared with the first-in-class trimethoprim.
Phase III clinical trials
There are two antibiotics in phase III clinical development for the treatment of S. aureus infections (Table 3). Nafithromycin is a novel lactone ketolide derived from ERY that lacks the l-cladinose moiety present in earlier macrolides, instead having a ketone in its place (Fig. 9). This increases the number of binding positions with the ribosome, which allows nafithromycin to maintain activity against certain macrolide-resistant organisms [243]. Nafithromycin differs from other members of the ketolide class, namely solithromycin and telithromycin (TEL), by having a lactone group at positions C11 and C12 instead of a carbamate. Furthermore, this lactone group is attached to a 2-pyridine-1,3,4-thiadiazole side chain, which enables nafithromycin to demonstrate activity against TEL-resistant S. pneumoniae, as well as promote oral availability [244]. A global surveillance study has shown that nafithromycin has improved activity against S. aureus compared with ERY, clindamycin (CLI) and TEL. Nafithromycin exhibited MIC50/90 of 0.06/>2 µg ml−1 against 716 MRSA and MSSA isolates. The MIC50/90 against ERY-resistant strains with an inducible phenotype was 0.06/0.06 µg ml−1. However, nafithromycin exhibited limited activity against TEL-resistant and CLI-resistant strains where the resistance phenotype was constitutively expressed. Nevertheless, the study highlighted the improved activity of nafithromycin where only 11.4 % of strains were resistant compared with 37.2 % for ERY[244]. Having shown favourable clinical outcomes in a phase II, randomized, double-blind, comparative study evaluating oral nafithromycin vs oral MOX for the treatment of CABP in adults (NCT02903836), a phase III trial has now been initiated. This will be a randomized, multicentre, double‐blind, comparative study (CTRI/2019/11/021964) investigating the oral administration of nafithromycin vs MOX in CABP patients in India.
Table 3.
Antibiotics in phase III clinical trials. Antibiotics are described by company, drug class, route of administration and indications
|
Antibiotic |
Company |
Drug class |
Route of administration |
Indication |
Trial no. |
|---|---|---|---|---|---|
|
Nafithromycin |
Wockhardt |
Macrolide |
po |
CABP |
CTRI/2019/11/021964 |
|
Cefilavancin |
Theravance/R-pharm |
Glycopeptide-cephalosporin heterodimer |
IV |
cSSSIs |
CABP, community acquired bacterial pneumonia ; cSSSI, complicated skin and skin structure infections; IV, intra-venous; po, per os (oral administration).
Fig. 9.
Structures of phase III anti-staphylococcal compounds.
Cefilavancin (TD-1792) is a VAN–third-generation cephalosporin conjugate. The VAN molecule is covalently bonded from its terminal carboxyl group to the cephalosporin lactam amine group via a stable oxime linker (Fig. 9) [245]. The two in one nature of cefilavancin enables it to target both d-Ala-d-Ala-containing peptidoglycan precursors, as well as the active sites of PBPs, which culminates in a dual-pronged mode of action. Promisingly, the activity of cefilavancin is largely unaffected by pre-existing resistance mechanisms [246]. Moreover, the antibacterial effect could not be recreated by using individual components, consistent with a multivalent mechanism [247]. This molecule exhibits potent in vitro activity against S. aureus , with MSSA strains exhibiting an MIC90 of 0.015 µg ml−1 and MRSA/hVISA strains an MIC90 of 0.03 µg ml−1. Time–kill studies also showed bactericidal activity of cefilavancin at concentrations of ≤0.12 µg ml−1 and a post-antibiotic effect of >2 h [246]. In phase I studies, plasma concentrations following IV injection of cefilavancin at 2 mg kg−1 were consistently maintained for 24 h above the MIC at which 100 % of MRSA strains were killed [246]. A randomized, double-blind, active-controlled, phase II trial (NCT00442832) exploring the safety and efficacy of cefilavancin in patients with cSSSIs caused by suspected or confirmed Gram-positive organisms was conducted [247]. The clinical cure rates were 91.7 % for cefilavancin and 90.7 % for VAN. AEs occurred at similar frequencies, except for itching, which was more common in the VAN treatment group. Currently, the manufacturer is in the process of launching a phase III trial for the treatment of cSSSIs throughout Russia and Georgia.
Phase II clinical trials
A total of nine anti-staphylococcal compounds are currently in phase II clinical trials (Table 4). OP-145 (previously known as P60.4Ac) is a synthetically modified LL-37 antimicrobial peptide (AMP) that was constructed to maintain its Gram-positive/-negative antimicrobial activity whilst minimizing the secondary pro-inflammatory response of LL-37 [248]. It contains several structural modifications, including a more favourable amphipathic helix formation as well as N- and C-terminal alterations to protect against proteolytic degradation, which improves its in vivo stability. OP-145 has been shown to be effective at killing MRSA in both planktonic and biofilm in vitro assays, as well as an MRSA-infected thermally wounded human skin equivalent model. OP-145 also shows no toxicity and displays higher or equal activity compared to LL-37 [249]. Preliminary investigation of OP-145 as a local topical therapeutic has shown that it retains activity within formulations suitable for skin and nasal mucosa administration [250]. This helped to spur the clinical development pathway with a randomized, double-blinded, placebo-controlled multicentre phase II study that investigated the suitability of OP-145 as an ototopical solution for the treatment of chronic suppurative otitis media (CSOM). In this study, the application of OP-145 to the ear canal of patients was safe and well tolerated with only mild to moderate AEs reported that were most likely unrelated to the study treatment. OP-145 treatment also demonstrated a higher treatment success compared to the placebo group (47 vs 6 %) [251]. OP-145 has also undergone follow-up modifications, yielding a new peptide SAAP-148 that demonstrated a superior activity profile. This peptide is currently under pre-clinical investigation [252, 253]. The discovery and improved potency of SAAP-148 may cause the developer to call a halt to the development of OP-145 and instead focus on SAAP-148.
Table 4.
Antibiotics in phase II clinical trials. Antibiotics are described by company, drug class, route of administration and indications. Previous developmental names are indicated in parentheses
|
Antibiotic |
Company |
Drug class |
Route of administration |
Indication |
Trial no. |
|---|---|---|---|---|---|
|
OP-145 (P60.4Ac) |
OctoPlus |
Antimicrobial peptide |
Ototopical solution |
Chronic otitis media |
|
|
LTX-109 (Lytixar) |
Lytix Biopharma |
Antimicrobial peptide |
Topical |
Impetigo |
|
|
Nilofabicin (CG400549) |
Crystal Genomics |
Benzyl-piridinone† |
po |
ABSSSIs |
|
|
Pravibismane (MBN 101) |
Microbion corporation |
Bismuth-ethanedithiol* |
Topical |
Diabetic foot infections |
|
|
XF-73 (exeporfinium chloride) |
DestinyPharma |
Di-cationic porphyrin* |
Nasal gel/topical |
Nasal decolonization |
|
|
Afabicin (Debio-1450) |
Debiopharm |
Pyrido-enamide† |
IV, po |
ABSSSIs, BJIs |
|
|
Rifaquizinone (TNP-2092) |
TenNor Therapeutics |
Rifamycin-quino lizinone hybrid |
IV,po |
PJIs, ABSSSIs |
|
|
Brilacidin |
Innovation Pharmaceuticals |
Defensin mimetic* |
IV |
ABSSSIs |
|
|
Delpazolid |
LegoChem Biosciences |
Oxazolidinone |
po |
Bacteraemia |
*denotes a first in class antibiotic
†denotes a first in class antibiotic with a novel target
ABSSSI, acute bacterial skin and skin structure infections; BJI, bone and joint infections ; BSI, bloodstream infections; IV, intra-venous; PJI, prosthetic joint infections; po, per os (oral administration).
LTX-109 is another naturally inspired peptidomimetic therapeutic. It was designed through initial identification of essential residues within lactoferricin B, through a full alanine scan, and subsequent substitution of individual residues. The resulting structure of LTX-109 contains cationic and lipophilic groups as well as nongenetically encoded amino acids, a modified tryptophan residue and an ethyl phenyl group at the C-terminus [254]. It is broad spectrum and displays a rapid bactericidal effect against both MRSA and VRSA strains, with an MIC range of 2–4 µg ml−1. Like OP-145, it also maintains its antimicrobial activity in the presence of host endoproteases [254, 255]. Thus far, LTX-109 has not been associated with cross-resistance to other natural and synthetic AMPs and has been shown to have a low propensity for the development of resistance, likely related to its rapid accumulation within bacterial membranes [256, 257]. An initial phase I/IIa clinical trial assessing nasal decolonization in MRSA and MSSA carriers has demonstrated the clinical efficacy of LTX-109 (NCT01158235). In this study, patients were treated for 3 days with either placebo, 1%, 2 % or 5 % LTX-109. In the 2 and 5% treatment groups, a statistically significant decrease in S. aureus burden was observed, measured by colony-forming units (c.f.u.) recovered from nasal swabs. Systemic levels of LTX-109 was very low and no safety concerns were encountered following a 9 week period [258]. A further phase II, double-blind, placebo-controlled, randomized study (NCT04767321) designed to evaluate the safety, tolerability and exploratory efficacy of 3 % LTX-109 gel in persistent S. aureus carriers was completed in 2021, but the results have not been published.
Nilofabicin (CG400549) is a novel benzyl-pyridone FabI inhibitor (Fig. 10). The bacterial fatty acid biosynthesis (FAS) pathway provides essential precursors for the assembly of numerous important cellular components, including phospholipids, lipoproteins and teichoic acids. The final step of this pathway is catalysed by the enoyl-ACP reductase (FabI), which is responsible for the reduction of enoyl-ACP derivative. This is an important reaction, as the reduction of enoyl-ACP derivatives dictates the ratio of saturated : unsaturated fatty acids, coordinates fatty acid and phospholipid synthesis and play a central role in the final stages of fatty acid elongation [259]. In S. aureus, FabI is the only enoyl-ACP reductase and is therefore essential [260]. Nilofabicin has an impressive spectrum of activity against MDR S. aureus , which includes MRSA and MSSA, as well as quinolone-resistant isolates. Against a panel of clinical isolates, it demonstrated an MIC90 of 0.25 µg ml−1. It has proven to be effective in eradicating systemic infections in mice, and through time–kill analysis has been shown to be a bacteriostatic agent. A FabI-overexpressing S. aureus strain resulted in higher MICs against nilofabicin. Higher MICs were also seen during in vitro selection of nilofabicin-resistant mutants, revealing a unique FabI (F204L) mutation, which confirmed FabI as the primary antibacterial target [261]. The pharmacokinetics, tolerability and efficacy of nilofabicin were demonstrated in a recent phase IIa open label exploratory study in test subjects with complications (NCT01593761).
Fig. 10.
. Structures of phase II anti-staphylococcal compounds.
Afabicin desphosphono (Debio 1452; AFN-1252) and its prodrug afabicin (Debio 1450; AFN-1720) is a second FabI inhibitor currently in phase II clinical development (Fig. 10). It is structurally different from nilofabicin, composed of a 3-methylbenzofuran and an oxotetrahydronaphthyridine linked through a N-methylpropenamide group. Afabicin is a narrow-spectrum drug, with potent anti-staphylococcal activity – an MIC90 of 0.015 µg ml−1 against clinically relevant MSSA and MRSA isolates [262] – and has proven to be more effective than LZD in mouse models of septicaemia [263]. These authors also noted a low dose-dependant propensity for resistance development, with an average frequency of 4.8×10−10. The FabI mutation M99T corresponded to a twofold increase in afabicin MIC but had no effect on the activity of triclosan. A second Y147H mutation that mapped to the FabI active site corresponded to an 8- to 16-fold increase in afabicin MIC, and a 64- to 128-fold increase in triclosan MIC [263]. An open label phase II proof-of-concept study (NCT01519492) to evaluate the efficacy of a daily dose of 400 mg afabicin in the treatment of ABSSSIs caused by staphylococci was completed in 2012. In this study the early response rate at day 3 was 97.3 %. More specifically, 82.9 % of patients had a ≥20 % decrease in erythema area, and 77.9 % of patients had a ≥20 % decrease in the area of induration. Afabicin was generally well tolerated, with drug-related AEs mostly mild or moderate in nature [264]. A second phase II multicentre, randomized, parallel-group, double-blind, double-dummy study has also recently been completed that investigated the efficacy, safety and tolerability of IV afabicin vs IV VAN with oral LZD in the treatment of ABSSSIs (NCT02426918). Both low- and high-dose afabicin reached non-inferiority and were well tolerated. The most common AEs reported were headache and nausea [265]. Debiopharm has recently started recruiting for another phase II randomized, active-controlled, open-label study (NCT03723551) to assess the safety and tolerability of afabicin in the treatment of subjects with bone and joint infection due to S. aureus.
Many metal-based co-ordinated complexes have played key roles in human medicine; salvarsan, an organoarsenic compound used for the treatment of syphilis and gold-complexed auranofin for rheumatoid arthritis therapy are some notable examples. More recently, bismuth-containing complexes have emerged as attractive antimicrobial candidates. Pravisbismane is a bismuth–thiol compound that is currently under development for the topical treatment of diabetic foot infections (Fig. 10). Although its exact mechanism of action and structure are undisclosed, the developer Microbion corporation states that pravibismane “is the first in a new class of bioenergetic inhibitors”https://microbioncorp.com/_news/microbion-corporation-announces-generic-name-for-lead-antimicrobial-compound-pravibismane. It serves to inhibit membrane-dependent ATP biosynthesis, resulting in a cessation of downstream processes. A study investigating the antibacterial effects against E. coli found that pravibismane acts rapidly, within 30 min, to completely shut down cell growth, rather than cause cell lysis, in a way that is reminiscent of non-specific cell-damaging agents such as detergents [266]. This study also revealed the effect of pravibismane on the bioenergetics of the cell membrane. Through the use of the membrane potential-sensitive dye DiBAC4(5), pravibismane was shown to depolarize the bacterial cells in a dose-dependent manner [266]. Furthermore, a luciferase-based assay determined that incubation with pravibismane caused a decrease in luminescence, showing that intracellular ATP levels were depleted [266]. This was unlike other test antibiotics, which had limited to no effect on intracellular ATP. Although performed on E. coli , it is likely that similar effects are observed with S. aureus [266]. A phase II randomized, open-label, controlled, multicentre study to assess the safety, tolerability and efficacy of topically applied pravibismane in patients with diabetic foot infections is currently in the recruitment process (NCT05174806).
Naturally occurring porphyrins such as haeme have long been noted for their antibacterial activity. Much of this antibacterial activity is based on their ability to catalyse peroxidase and oxidase reactions, resulting in the formation of reactive oxygen species (ROS) [267]. XF-73 is a leading drug candidate being developed by Destiny Pharma and is based on the porphyrin moiety (Fig. 10). First synthesized in 2009, XF-73 is a di-cationic porphyrin that has been shown in vitro to kill MSSA, MRSA, DAP-resistant, LZD-resistant and VISA strains. XF-73 exhibited an MIC of 1 µg ml−1 against S. aureus strain SH1000, portraying a more rapid killing kinetics than all other comparator agents. Investigation into the mechanism of action of XF-73 found that it interacted with the bacterial membrane, but rather than causing lysis, it instead causes membrane perturbation, resulting in the intercellular leakage of ATP and K+. It was also noted that a 10 min exposure of S. aureus to XF-73 caused a complete inhibition of DNA, RNA and protein synthesis [268]. The clinical investigation of XF-73 began with a phase I (NCT01592214) randomized, open-label study that tested the suitability and tolerability of two nasal formations: a 0.5 mg g−1/2 % gel and a 2 mg g−1/2 % gel. No systemic absorption of XF-73 was detected, and treatment was associated with a rapid reduction in S. aureus. Similarly, XF-73 was well tolerated, with only rhinorrhoea and nasal dryness noted as AEs [269]. A further multicentre, randomized, placebo-controlled, phase 2 study (NCT03915470) assessed the effect of nasal XF-73 on the nasal burden of S. aureus in patients undergoing cardiac surgery. After three applications of XF-73 over a period of 24 h, a greater reduction in nasal S. aureus c.f.u. was noted vs the placebo. No application site reactions or other treatment-emergent AEs were noted [270]. XF-73 is also in pre-clinical development for the treatment of superficial skin infections in PR China, the USA and the EU.
Rifaquizinone (TNP-2092) is heterodimer antibiotic that links together rifampin and the quinolizinone ABT-719 (Fig. 10), with the aim of maintaining the potent activity of rifampin while limiting the generation of rifampin resistance [271]. Rifampin is currently the antibiotic of choice for staphylococcal prosthetic joint infections due to its potent disruption of bacterial biofilms [272]. However, rifampin is recommended to be administered alongside oral antibiotics such as LVX and CIP to minimize the occurrence of point mutations in RNA polymerase, which can cause high level resistance [273]. As such, TNP-2092 was designed to improve the therapeutic options for these persistent infections and negate the need for the administration of multiple antibiotics. TNP-2092 has been shown to retain its rifampin-mediated potency for RNA polymerase whilst simultaneously exhibiting equipotent activity against DNA gyrase and topoisomerase IV. It has a much lower propensity for resistance than its constituent molecules, as it is not a substrate for quinolone efflux. Furthermore, it is active against strains harbouring parC and gyrA mutations (responsible for quinolone resistance) and rpoB mutations (responsible for rifampin resistance). A TNP-2092-resistant mutant has been selected for following 26 days of serial passage. The resulting strains with MICs of >16 µg ml−1 and 4 µg ml−1 harboured five mutations: rpoB R484H, gyrA ΔL520, S84L, parC<R236> and H103Y [271]. TNP-2092 shows in vitro activity against MSSA and MRSA isolates (MIC90 of 0.015 µg ml−1) [274] and has completed a phase II double-blind, randomized, multicentre, parallel, controlled study demonstrating the safety, tolerability, appropriate pharmacokinetics and efficacy of TNP-2092 vs VAN in adults with ABSSSIs (NCT03964493). More, recently a phase I open-label study to evaluate the tissue distribution, plasma pharmacokinetics, safety and tolerability of a single 300 mg IV dose of TNP-2092 in patients undergoing total hip or knee arthroplasty was completed; however, the results have not been posted (NCT04294862).
Brilacidin (PMX30063) is a small arylamide foldamer that mimics naturally occurring AMPs (Fig. 10). It was optimized for its activity against S. aureus and is composed of a planar scaffold decorated with two trifluoromethane hydrophobic substitutions and four positive guanadinyl and pyridinyl substitutions [275]. It has been shown that brilacidin exposure causes a rapid depolarization of the membrane at a comparable rate to that of the clinically used lipopeptide DAP. Further transcriptomic analysis showed that, like DAP, brilacidin causes induction of the cell-wall-stress two-component systems VraRS and WalRK, a phenomenon strongly associated with lipid II-targeting antibiotics [275–277]. A serial passage resistance study showed that no significant increase in MIC occurred over a period of 16 days, demonstrating that S. aureus struggles to evolve resistance to brilacidin. Two phase II clinical trials have evaluated the efficacy and safety of brilacidin compared with DAP in subjects with ABSSSIs (NCT02052388 and NCT01211470). The primary endpoint of early clinical response, defined as a >20 % reduction in lesion size, was high (>90 %) in patients treated with IV brilacidin and comparable to that of the DAP-treated group. No serious AEs were documented, and all treatment-emergent AEs were mild or transient in nature, indicating an appropriate safety profile [278]. In 2015, the developer, Innovation Pharmaceuticals, announced a phase III trial further investigating brilacidin for treatment of ABSSSIs, but this has been delayed. Therapeutic use of brilacidin is being investigated in phase II trials for oral mucositis (NCT02324335), coronavirus disease 2019 (COVID-19) (NCT04784897), phase I investigation for colonic delivery (NCT04240223) and pre-clinical development for fungal infections.
Delpazolid (previously LCB01-0371) is a second-generation oxazolidinone containing an amidrazone moiety. This novel substituent to the oxazolidinone scaffold was incorporated to circumvent mitochondrial toxicity associated with prolonged LZD administration. Delpazolid subsequently proved in vitro to be less toxic, a feat that was mirrored in early clinical trials [279, 280]. The developer, LegoChem Biosciences, initially examined the utility of delpazolid as an anti-tuberculosis agent. An early bactericidal activity study in patients with uncomplicated tuberculosis (TB) showed promise, where delpazolid exhibited 25 % of the reduction in the log c.f.u. of the study control drug (NCT02836483) [281]. Multiple studies have shown the impressive in vitro and in vivo potency of delpazolid against S. aureus and enterococci [282]. The activity of delpazolid as an effective anti-MRSA agent was assessed; 100 bloodstream MRSA isolates were challenged with delpazolid and 3 comparators (VAN, DAP and LZD). The MIC50 and MIC90 for delpazolid and LZD were identical, 1 µg ml−1 and 2 µg ml−1, respectively. However, delpazolid showed a greater cumulative percentage of MICs up to 1 µg ml−1 than LZD (74 vs 52 %) [283]. This work directed the creation of a phase IIa study (NCT05225558) comparing the efficacy of oral delpazolid vs IV VAN in patients suffering from MRSA bacteraemia, which is currently at the recruitment stage. There has been limited investigation of the resistance potential of delpazolid in S. aureus . However, no significant differences in resistance rates were observed between LZD and delpazolid against XDR TB [284].
Concluding remarks
The burden of S. aureus antimicrobial resistance is a severe global problem. MRSA remains on the high-priority pathogen list due to the remarkable ability of this pathogen to evolve resistance to antimicrobials, disseminate widely and cause life-threatening infections. In this review, we examine the current and future pipeline of anti-staphylococcal antimicrobials (Fig. 11). Overall, there are promising signs; we discuss several novel versions of existing antibiotic classes that are active against MDR S. aureus . For example, significant advancements in quinolone antibiotic research have resulted in the development of drugs with enhanced stability and improved toxicity profiles, and activity against S. aureus strains harbouring mutations within the QRDRs and expressing efflux pumps. Second-generation oxazolidinones have shown improve potency, safety and pharmacodynamics, and a decreased propensity for resistance to emerge following prolonged in vitro exposure. Although successful, both examples are based on improved versions of existing classes of antibiotics and thus eventual resistance to these derivatives is more likely to emerge.
Fig. 11.
Summary of current and future anti-staphylococcal compounds. Colour groupings indicate recently approved antibiotics (green), antibiotics undergoing phase III trials (red) and antimicrobials undergoing phase II trials (blue).
A number of the antimicrobials in phases II–III offer first-in-class compounds, but only the fabiotics afabicin and nilofabicin have a truly novel, previously unexploited target. The
diaryldiamine CRS3123 (methionyl-tRNA synthase inhibitor) and the distamycin MGB-BP-3 (DNA minor grove binder) also have novel targets but are currently under clinical investigation for Clostridium difficile infections (Table S1, available in the online version of this article). However, the landscape and direction of investigatory antibiotics is ever changing. Delpazolid, initially developed as an anti-TB drug, has since started phase II trials for MRSA bacteraemia. Therefore, we have also included phase II and III antibiotics that have been shown to have impressive in vitro activity against S. aureus (Table S1), and could, like delpazolid, become anti-staphylococcal antibiotics in the future. Another compound that warrants close monitoring is the FtsZ-targeting benzamide TXA709, which is currently at the beginning of its clinical journey, having recently completed phase I trials (Table S2). This is the only other anti-staphylococcal antibiotic in clinical development with a unique target.
Improvements in conjugate chemistry have ushered in a novel therapeutic approach with the development of heterodimer antibiotics with dual active targets. Cefilavancin is a covalently linked glycopeptide–cephalosporin conjugate with excellent potency against MRSA/hVISA strains. Similarly, rifaquizinone is a rifampin–quinolizinone heterodimer that simultaneously targets DNA gyrase and RNA polymerase. Along with enhanced activity, dual-acting heterodimer antibiotics may result in a lower propensity for resistance emergence during therapy. This is proving to be a popular area of antibiotic development as a third heterodimer antibiotic, DNV3837, an oxazolidinone–quinolone conjugate, is currently in phase II trials to treat C. difficile infections (Table S1). Future work will determine the optimal antibiotic classes to partner; current heterodimers include quinolone–macrolide, quinolone–tetracycline, quinolone–benzylpyramidine (trimethoprim) and quinolone–cephalosporin [285]. The latter combination has been used successfully to preferentially eradicate pathogenic β-lactamase-producing bacteria, over beneficial members of the microbiome [286].
Antibiotic development is an incredibly costly venture. Current estimates put the cost of developing a new antimicrobial at US $1.5 billion [287]. If we are to overcome the antibiotic resistance pandemic, small–medium-sized pharmaceutical companies that are currently the drivers of antibiotic development cannot be expected to shoulder this burden alone. This strategy will undoubtedly result in more casualties of the antibiotic development process like the hugely promising iclaprim. Fortunately, governmental healthcare systems and charitable organizations are establishing economic strategies and incentives to drive the development of novel antibiotics. These include, Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X), the Global Antibiotic Research and Development Partnership (GARDP) and the Innovative Medicines Initiative (IMI), as well as a $1 billion AMR action fund. Going forward, we need to preserve the integrity of these antibiotics. Proper antibiotic stewardship, appropriate prescribing and consumption, surveillance and increased stringency in antibiotic use in agriculture will aid in the fight to combat antibiotic resistance.
Supplementary Data
Funding information
This work was supported by the Academy of Medical Sciences Springboard Award (SBF006\1023: M. L.).
Acknowledgement
Figures 1 and 11 were made with Biorender.com
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: ABC, ATP binding cassette; ABSSSIs, acute bacterial skin and skin structure infections; AEs, adverse events; AFN, afabicin; AMEs, aminoglycoside modifying enzymes; AMP, antimicrobial peptide; AMR, antimicrobial resistance; ATM, aztreonam; CABP, community acquired bacterial pneumonia; cAMP, cationic antimicrobial peptide; CARB-X, combating antibiotic-resistant bacteria biopharmaceutical accelerator; CE, clinically evaluable; CFT, ceftaroline fosamil; CHL, chloramphenicol; cIAIs, complicated intrabdominal infections; CIP, ciprofloxacin; CLI, clindamycin; COPD, chronic obstructive pulmonary disease; CRE, carbapenem resistant Enterobacteriaceae; CSOM, chronic suppurative otitis media; cSSSIs, complicated bacterial skin and skin structure infections; cSSTIs, complicated skin and soft tissue infections; cUTIs, complicated urinary tract infections; DAL, dalbavancin; DAP, daptomycin; DHF, dihydrofolate; DHFR, dihydrofolate reductase; DHP, dihydropteroate; DHPS, dihydropteroate synthase enzyme; DLX, delafloxacin; DOX, doxycyline; EF-G, elongation factor G; EMA, European Medicines Agency; ERV, eravacycline; ERY, erythromycin; FA, fusidic acid; FabI, enoyl-ACP reductase; FAS, fatty acid biosynthesis; FDA, Food and Drug Administration; FIN, finafloxacin; FnBP, fibronectin-binding protein; GARDP, Global Antibiotic Research and Development Partnership; GI, gastrointestinal; hVISA, hetero-vancomcin intermediate S. aureus; IMI, innovative medicines initiative; IV, intra-venous; LEF, lefamulin; LEV, levonadifloxacin; LRTI, lower respiratory tract infection; LSFX, lascufloxacin; LVX, levofloxacin; LZD, linezolid; MAO, monoamine oxidase; MATE, multiple antibiotic and toxin extrusion; MDR, multidrug-resistant; MET, methicillin; MFS, major facilitator superfamily; MIC, minimal inhibitory concentration; MIN, minocycline; MLS, macrolide lincosamide streptogramin; MOX, moxifloxacin; MRSA, methicillin resistant S. aureus; MSSA, methicillin susceptible S. aureus; MUP, mupirocin; NDA, new drug application; NEM, nemonoxacin; NOR, norfloxacin; OMA, omadacycline; ORI, oritavancin; OZE, ozenoxacin; PABA, para-aminobenzoic acid; PBP, penicillin binding protein; PDR, pan drug-resistant; PEN, penicillin; PRSP, penicillin resistant S. pneumoniae; PTC, peptidyl transferase centre; QRDR, quinolone resistance determining regions; RET, retapamulin; RIF, rifampicin; ROS, reactive oxygen species; RPPs, ribosomal protection proteins; rRNA, ribosomal RNA; SCCmec, staphylococcal chromosomal cassette mec; SCV, small colony variant; SMX, sulfamethoxazole; SNPs, single nucleotide polymorphisms; TB, tuberculosis; TDZ, tedizolid; TEL, telithromycin; TET, tetracycline; TGC, tigecycline; THF, tetrahydrofolate; TMP, trimethoprim; TMP-SMX, trimethoprim / sulfamethoxazole; VAN, vancomycin; VISA, vancomycin intermediate S. aureus; VRE, vancomycin-resistant E. faecium; VRSA, vancomycin resistant S. aureus; WHO, World Health Organisation; XDR, extensively drug-resistant; ZBO, zabofloxacin.
Two supplementary tables are available with the online version of this article.
References
- 1.Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109:309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC Antibiotic resistance threats in the United States. Atlanta, GA: U.S. Department of Health and Human Services: CDC; 2019. [Google Scholar]
- 3.Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. 2019;12:3903–3910. doi: 10.2147/IDR.S234610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology. Clin Microbiol Rev. 2015;3 doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clegg J, Soldaini E, McLoughlin RM, Rittenhouse S, Bagnoli F, et al. Staphylococcus aureus vaccine research and development: the past, present and future, including novel therapeutic strategies. Front Immunol. 2021;12:705360. doi: 10.3389/fimmu.2021.705360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller LS, Fowler VG, Shukla SK, Rose WE, Proctor RA. Development of a vaccine against Staphylococcus aureus invasive infections: evidence based on human immunity, genetics and bacterial evasion mechanisms. FEMS Microbiol Rev. 2020;44:123–153. doi: 10.1093/femsre/fuz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proctor RA. Development of a Staphylococcus aureus vaccine: an ongoing journey. Clin Infect Dis. 2023;77:321–322. doi: 10.1093/cid/ciad217. [DOI] [PubMed] [Google Scholar]
- 8.Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter CF and GN Johns Hopkins ABX Guide. Baltimore, MD; 2019. [Google Scholar]
- 10.Pantosti A, Sanchini A, Monaco M. Mechanisms of antibiotic resistance in Staphylococcus aureus . Future Microbiol. 2007;2:323–334. doi: 10.2217/17460913.2.3.323. [DOI] [PubMed] [Google Scholar]
- 11.Foster TJ. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol Rev. 2017;41:430–449. doi: 10.1093/femsre/fux007. [DOI] [PubMed] [Google Scholar]
- 12.Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 13.Guo D, Liu Y, Han C, Chen Z, Ye X. Staphylococcus aureus isolated from pigs: implication for livestock-association markers and vaccine strategies. Infect Drug Resist. 2018;Volume 11:1299–1307. doi: 10.2147/IDR.S173624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moosavian M, Baratian Dehkordi P, Hashemzadeh M. Characterization of Sccmec, SpA types and multidrug resistant of methicillin-resistant Staphylococcus aureus isolates in Ahvaz, Iran. Infect Drug Resist. 2020;13:1033–1044. doi: 10.2147/IDR.S244896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abat C, Fournier PE, Jimeno MT, Rolain JM, Raoult D. Extremely and pandrug-resistant bacteria extra-deaths: myth or reality? Eur J Clin Microbiol Infect Dis. 2018;37:1687–1697. doi: 10.1007/s10096-018-3300-0. [DOI] [PubMed] [Google Scholar]
- 16.Karakonstantis S, Kritsotakis EI, Gikas A. Pandrug-resistant Gram-negative bacteria: a systematic review of current epidemiology, prognosis and treatment options. J Antimicrob Chemother. 2020;75:271–282. doi: 10.1093/jac/dkz401. [DOI] [PubMed] [Google Scholar]
- 17.Brown ED, Wright GD. Antibacterial drug discovery in the resistance era. Nature. 2016;529:336–343. doi: 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- 18.Tipper DJ, Strominger JL. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci. 1965;54:1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barber M, Rozwadowska-Dowzenko M. Infection by penicillin-resistant staphylococci. The Lancet. 1948;252:641–644. doi: 10.1016/S0140-6736(48)92166-7. [DOI] [PubMed] [Google Scholar]
- 20.Kirby WM. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944;99:452–453. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- 21.Rounteee PM, Freeman BM. Infections caused by a particular phage type of Staphylococcus aureus . Med J Aust. 1955;2:157–161. doi: 10.5694/j.1326-5377.1955.tb35001.x. [DOI] [PubMed] [Google Scholar]
- 22.Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus . J Clin Invest. 2003;111:1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barber M. Methicillin-resistant staphylococci. J Clin Pathol. 1961;14:385–393. doi: 10.1136/jcp.14.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus . Antimicrob Agents Chemother. 2000;44:1549–1555. doi: 10.1128/AAC.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartman BJ, Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus . J Bacteriol. 1984;158:513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Utsui Y, Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 1985;28:397–403. doi: 10.1128/AAC.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammes WP, Neuhaus FC. On the mechanism of action of vancomycin: inhibition of peptidoglycan synthesis in Gaffkya homari . Antimicrob Agents Chemother. 1974;6:722–728. doi: 10.1128/AAC.6.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieto M, Perkins HR. Physicochemical properties of vancomycin and iodovancomycin and their complexes with diacetyl-L-lysyl-D-alanyl-D-alanine. Biochem J. 1971;123:773–787. doi: 10.1042/bj1230773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng D, Debabov D, Hartsell TL, Cano RJ, Adams S, et al. Approved glycopeptide antibacterial drugs: mechanism of action and resistance. Cold Spring Harb Perspect Med. 2016;6:a026989. doi: 10.1101/cshperspect.a026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. The Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 31.Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus . Science. 2003;302:1569–1571. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 32.Hiramatsu K, Kayayama Y, Matsuo M, Aiba Y, Saito M, et al. Vancomycin-intermediate resistance in Staphylococcus aureus . J Glob Antimicrob Resist. 2014;2:213–224. doi: 10.1016/j.jgar.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Périchon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2009;53:4580–4587. doi: 10.1128/AAC.00346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arthur M, Reynolds P, Courvalin P. Glycopeptide resistance in enterococci. Trends Microbiol. 1996;4:401–407. doi: 10.1016/0966-842X(96)10063-9. [DOI] [PubMed] [Google Scholar]
- 35.Cong Y, Yang S, Rao X. Vancomycin resistant Staphylococcus aureus infections: a review of case updating and clinical features. J Adv Res. 2020;21:169–176. doi: 10.1016/j.jare.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wormser GP, Keusch GT, Heel RC. Co-trimoxazole (Trimethoprim-sulfamethoxazole) an updated review of its antibacterial activity and clinical efficacy. Drugs. 1982;24:459–518. doi: 10.2165/00003495-198224060-00002. [DOI] [PubMed] [Google Scholar]
- 37.Kalkut G. Sulfonamides and trimethoprim. Cancer Invest. 1998;16:612–615. doi: 10.3109/07357909809032892. [DOI] [PubMed] [Google Scholar]
- 38.Grim SA, Rapp RP, Martin CA, Evans ME. Trimethoprim-sulfamethoxazole as a viable treatment option for infections caused by methicillin-resistant Staphylococcus aureus . Pharmacotherapy. 2005;25:253–264. doi: 10.1592/phco.25.2.253.56956. [DOI] [PubMed] [Google Scholar]
- 39.Hampele IC, D’Arcy A, Dale GE, Kostrewa D, Nielsen J, et al. Structure and function of the dihydropteroate synthase from Staphylococcus aureus . J Mol Biol. 1997;268:21–30. doi: 10.1006/jmbi.1997.0944. [DOI] [PubMed] [Google Scholar]
- 40.Griffith EC, Wallace MJ, Wu Y, Kumar G, Gajewski S, et al. The structural and functional basis for recurring sulfa drug resistance mutations in Staphylococcus aureus dihydropteroate synthase. Front Microbiol. 2018;9:1369. doi: 10.3389/fmicb.2018.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hitchings GH, Burchall JJ. Inhibition of folate biosynthesis and function as a basis for chemotherapy. Adv Enzymol Relat Areas Mol Biol. 2006:417–468. doi: 10.1002/9780470122723.ch9. [DOI] [PubMed] [Google Scholar]
- 42.Dale GE, Broger C, D’Arcy A, Hartman PG, DeHoogt R, et al. A single amino acid substitution in Staphylococcus aureus dihydrofolate reductase determines trimethoprim resistance. J Mol Biol. 1997;266:23–30. doi: 10.1006/jmbi.1996.0770. [DOI] [PubMed] [Google Scholar]
- 43.Sekiguchi J, Tharavichitkul P, Miyoshi-Akiyama T, Chupia V, Fujino T, et al. Cloning and characterization of a novel trimethoprim-resistant dihydrofolate reductase from a nosocomial isolate of Staphylococcus aureus CM.S2 (IMCJ1454) Antimicrob Agents Chemother. 2005;49:3948–3951. doi: 10.1128/AAC.49.9.3948-3951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadlec K, Schwarz S. Identification of a novel trimethoprim resistance gene, dfrK, in a methicillin-resistant Staphylococcus aureus ST398 strain and its physical linkage to the tetracycline resistance gene tet(L) Antimicrob Agents Chemother. 2009;53:776–778. doi: 10.1128/AAC.01128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rouch DA, Messerotti LJ, Loo LSL, Jackson CA, Skurray RA. Trimethoprim resistance transposon Tn4003 from Staphylococcus aureus encodes genes for a dihydrofolate reductase and thymidylate synthetase flanked by three copies of IS257. Mol Microbiol. 1989;3:161–175. doi: 10.1111/j.1365-2958.1989.tb01805.x. [DOI] [PubMed] [Google Scholar]
- 46.Kime L, Waring T, Mohamad M, Mann BF, O’Neill AJ. Resistance to antibacterial antifolates in multidrug-resistant Staphylococcus aureus: prevalence estimates and genetic basis. J Antimicrob Chemother. 2023;78:1201–1210. doi: 10.1093/jac/dkad063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Straus SK, Hancock REW. Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: comparison with cationic antimicrobial peptides and lipopeptides. Biochim Biophys Acta. 2006;1758:1215–1223. doi: 10.1016/j.bbamem.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Silverman JA, Perlmutter NG, Shapiro HM. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus . Antimicrob Agents Chemother. 2003;47:2538–2544. doi: 10.1128/AAC.47.8.2538-2544.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung D, Rozek A, Okon M, Hancock REW. Structural transitions as determinants of the action of the calcium-dependent antibiotic daptomycin. Chem Biol. 2004;11:949–957. doi: 10.1016/j.chembiol.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 50.Grein F, Müller A, Scherer KM, Liu X, Ludwig KC, et al. Ca2+-Daptomycin targets cell wall biosynthesis by forming a tripartite complex with undecaprenyl-coupled intermediates and membrane lipids. Nat Commun. 2020;11:1455. doi: 10.1038/s41467-020-15257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ernst CM, Staubitz P, Mishra NN, Yang S-J, Hornig G, et al. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog. 2009;5:e1000660. doi: 10.1371/journal.ppat.1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ernst CM, Slavetinsky CJ, Kuhn S, Hauser JN, Nega M, et al. Gain-of-function mutations in the phospholipid flippase MprF confer specific daptomycin resistance. mBio. 2018;9:e01659-18. doi: 10.1128/mBio.01659-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thitiananpakorn K, Aiba Y, Tan X-E, Watanabe S, Kiga K, et al. Association of mprF mutations with cross-resistance to daptomycin and vancomycin in methicillin-resistant Staphylococcus aureus (MRSA) Sci Rep. 2020;10:16107. doi: 10.1038/s41598-020-73108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayer AS, Mishra NN, Chen L, Kreiswirth BN, Rubio A, et al. Frequency and distribution of single-nucleotide polymorphisms within mprF in methicillin-resistant Staphylococcus aureus clinical isolates and their role in cross-resistance to daptomycin and host defense antimicrobial peptides. Antimicrob Agents Chemother. 2015;59:4930–4937. doi: 10.1128/AAC.00970-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehta S, Cuirolo AX, Plata KB, Riosa S, Silverman JA, et al. VraSR two-component regulatory system contributes to mprF-mediated decreased susceptibility to daptomycin in in vivo-selected clinical strains of methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2012;56:92–102. doi: 10.1128/AAC.00432-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Douglas EJA, Alkhzem AH, Wonfor T, Li S, Woodman TJ, et al. Antibacterial activity of novel linear polyamines against Staphylococcus aureus . Front Microbiol. 2022;13:948343. doi: 10.3389/fmicb.2022.948343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bertsche U, Yang S-J, Kuehner D, Wanner S, Mishra NN, et al. Increased cell wall teichoic acid production and D-alanylation are common phenotypes among daptomycin-resistant methicillin-resistant Staphylococcus aureus (MRSA) clinical isolates. PLoS One. 2013;8:e67398. doi: 10.1371/journal.pone.0067398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cafiso V, Bertuccio T, Purrello S, Campanile F, Mammina C, et al. dltA overexpression: a strain-independent keystone of daptomycin resistance in methicillin-resistant Staphylococcus aureus . Int J Antimicrob Agents. 2014;43:26–31. doi: 10.1016/j.ijantimicag.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Tran TT, Munita JM, Arias CA. Mechanisms of drug resistance: daptomycin resistance. Ann N Y Acad Sci. 2015;1354:32–53. doi: 10.1111/nyas.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fournier B, Zhao X, Lu T, Drlica K, Hooper DC. Selective targeting of topoisomerase IV and DNA gyrase in Staphylococcus aureus: different patterns of quinolone-induced inhibition of DNA synthesis. Antimicrob Agents Chemother. 2000;44:2160–2165. doi: 10.1128/AAC.44.8.2160-2165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Helgesen E, Sætre F, Skarstad K. Topoisomerase IV tracks behind the replication fork and the SeqA complex during DNA replication in Escherichia coli . Sci Rep. 2021;11:474. doi: 10.1038/s41598-020-80043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmitz FJ, Jones ME, Hofmann B, Hansen B, Scheuring S, et al. Characterization of grlA, grlB, gyrA, and gyrB mutations in 116 unrelated isolates of Staphylococcus aureus and effects of mutations on ciprofloxacin MIC. Antimicrob Agents Chemother. 1998;42:1249–1252. doi: 10.1128/AAC.42.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ito H, Yoshida H, Bogaki-Shonai M, Niga T, Hattori H, et al. Quinolone resistance mutations in the DNA gyrase gyrA and gyrB genes of Staphylococcus aureus . Antimicrob Agents Chemother. 1994;38:2014–2023. doi: 10.1128/AAC.38.9.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang T, Tanaka M, Sato K. Detection of grlA and gyrA mutations in 344 Staphylococcus aureus strains. Antimicrob Agents Chemother. 1998;42:236–240. doi: 10.1128/AAC.42.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ubukata K, Itoh-Yamashita N, Konno M. Cloning and expression of the norA gene for fluoroquinolone resistance in Staphylococcus aureus . Antimicrob Agents Chemother. 1989;33:1535–1539. doi: 10.1128/AAC.33.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Truong-Bolduc QC, Dunman PM, Strahilevitz J, Projan SJ, Hooper DC. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus . J Bacteriol. 2005;187:2395–2405. doi: 10.1128/JB.187.7.2395-2405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Truong-Bolduc QC, Strahilevitz J, Hooper DC. NorC, a new efflux pump regulated by MgrA of Staphylococcus aureus . Antimicrob Agents Chemother. 2006;50:1104–1107. doi: 10.1128/AAC.50.3.1104-1107.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu JL, Grinius L, Hooper DC. NorA functions as a multidrug efflux protein in both cytoplasmic membrane vesicles and reconstituted proteoliposomes. J Bacteriol. 2002;184:1370–1377. doi: 10.1128/JB.184.5.1370-1377.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang J, O’Toole PW, Shen W, Amrine-Madsen H, Jiang X, et al. Novel chromosomally encoded multidrug efflux transporter MdeA in Staphylococcus aureus . Antimicrob Agents Chemother. 2004;48:909–917. doi: 10.1128/AAC.48.3.909-917.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakaminami H, Noguchi N, Sasatsu M. Fluoroquinolone efflux by the plasmid-mediated multidrug efflux pump QacB variant QacBIII in Staphylococcus aureus . Antimicrob Agents Chemother. 2010;54:4107–4111. doi: 10.1128/AAC.01065-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamada Y, Hideka K, Shiota S, Kuroda T, Tsuchiya T. Gene cloning and characterization of SdrM, a chromosomally-encoded multidrug efflux pump, from Staphylococcus aureus . Biol Pharm Bull. 2006;29:554–556. doi: 10.1248/bpb.29.554. [DOI] [PubMed] [Google Scholar]
- 72.Floyd JL, Smith KP, Kumar SH, Floyd JT, Varela MF. LmrS is a multidrug efflux pump of the major facilitator superfamily from Staphylococcus aureus . Antimicrob Agents Chemother. 2010;54:5406–5412. doi: 10.1128/AAC.00580-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaatz GW, DeMarco CE, Seo SM. MepR, a repressor of the Staphylococcus aureus MATE family multidrug efflux pump MepA, is a substrate-responsive regulatory protein. Antimicrob Agents Chemother. 2006;50:1276–1281. doi: 10.1128/AAC.50.4.1276-1281.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andersson MI, MacGowan AP. Development of the quinolones. J Antimicrob Chemother. 2003;51 Suppl 1:1–11. doi: 10.1093/jac/dkg212. [DOI] [PubMed] [Google Scholar]
- 75.Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, et al. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell. 2001;104:901–912. doi: 10.1016/s0092-8674(01)00286-0. [DOI] [PubMed] [Google Scholar]
- 76.O’Neill AJ, Huovinen T, Fishwick CWG, Chopra I. Molecular genetic and structural modeling studies of Staphylococcus aureus RNA polymerase and the fitness of rifampin resistance genotypes in relation to clinical prevalence. Antimicrob Agents Chemother. 2006;50:298–309. doi: 10.1128/AAC.50.1.298-309.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sekiguchi J-I, Fujino T, Araake M, Toyota E, Kudo K, et al. Emergence of rifampicin resistance in methicillin-resistant Staphylococcus aureus in tuberculosis wards. J Infect Chemother. 2006;12:47–50. doi: 10.1007/s10156-005-0417-8. [DOI] [PubMed] [Google Scholar]
- 78.Huang Y-T, Liao C-H, Chen S-Y, Yang C-J, Hsu H-S, et al. Characterization of rifampin-resistant Staphylococcus aureus nasal carriage in patients receiving rifampin-containing regimens for tuberculosis. Infect Drug Resist. 2018;11:1175–1182. doi: 10.2147/IDR.S163634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loss G, Simões PM, Valour F, Cortês MF, Gonzaga L, et al. Staphylococcus aureus Small Colony Variants (SCVs): news from a chronic prosthetic joint infection. Front Cell Infect Microbiol. 2019;9:363. doi: 10.3389/fcimb.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsuo M, Hishinuma T, Katayama Y, Cui L, Kapi M, et al. Mutation of RNA polymerase beta subunit (rpoB) promotes hVISA-to-VISA phenotypic conversion of strain Mu3. Antimicrob Agents Chemother. 2011;55:4188–4195. doi: 10.1128/AAC.00398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCarter YS. Antibiotics: challenges, mechanisms, opportunities written by Christopher Walsh, PhD and Timothy Wencewicz, PhD. Lab Med. 2017;48:e42. doi: 10.1093/labmed/lmw070. [DOI] [Google Scholar]
- 82.Davis BD, Chen LL, Tai PC. Misread protein creates membrane channels: an essential step in the bactericidal action of aminoglycosides. Proc Natl Acad Sci. 1986;83:6164–6168. doi: 10.1073/pnas.83.16.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Champney WS. Antibiotics targeting bacterial ribosomal subunit biogenesis. J Antimicrob Chemother. 2020;75:787–806. doi: 10.1093/jac/dkz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rahimi F. Characterization of resistance to aminoglycosides in methicillin-resistant Staphylococcus aureus strains isolated from a tertiary care hospital in Tehran, Iran. Jundishapur J Microbiol. 2016;9:e29237. doi: 10.5812/jjm.29237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hauschild T, Sacha P, Wieczorek P, Zalewska M, Kaczyńska K, et al. Aminoglycosides resistance in clinical isolates of Staphylococcus aureus from a University Hospital in Bialystok, Poland. Folia Histochem Cytobiol. 2008;46:225–228. doi: 10.2478/v10042-008-0034-3. [DOI] [PubMed] [Google Scholar]
- 86.Schmitz FJ, Fluit AC, Gondolf M, Beyrau R, Lindenlauf E, et al. The prevalence of aminoglycoside resistance and corresponding resistance genes in clinical isolates of staphylococci from 19 European hospitals. J Antimicrob Chemother. 1999;43:253–259. doi: 10.1093/jac/43.2.253. [DOI] [PubMed] [Google Scholar]
- 87.Jensen SO, Lyon BR. Genetics of antimicrobial resistance in Staphylococcus aureus . Future Microbiol. 2009;4:565–582. doi: 10.2217/fmb.09.30. [DOI] [PubMed] [Google Scholar]
- 88.Schmitz FJ, Krey A, Sadurski R, Verhoef J, Milatovic D, et al. Resistance to tetracycline and distribution of tetracycline resistance genes in European Staphylococcus aureus isolates. J Antimicrob Chemother. 2001;47:239–240. doi: 10.1093/jac/47.2.239. [DOI] [PubMed] [Google Scholar]
- 89.Li D, Ge Y, Wang N, Shi Y, Guo G, et al. Identification and characterization of a novel major facilitator superfamily efflux pump, SA09310, mediating tetracycline resistance in Staphylococcus aureus . Antimicrob Agents Chemother. 2023;67:e0169622. doi: 10.1128/aac.01696-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dönhöfer A, Franckenberg S, Wickles S, Berninghausen O, Beckmann R, et al. Structural basis for TetM-mediated tetracycline resistance. Proc Natl Acad Sci. 2012;109:16900–16905. doi: 10.1073/pnas.1208037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peterson LR. A review of tigecycline--the first glycylcycline. Int J Antimicrob Agents. 2008;32 Suppl 4:S215–22. doi: 10.1016/S0924-8579(09)70005-6. [DOI] [PubMed] [Google Scholar]
- 92.Dabul ANG, Avaca-Crusca JS, Van Tyne D, Gilmore MS, Camargo ILBC. Resistance in in vitro selected tigecycline-resistant methicillin-resistant Staphylococcus aureus sequence type 5 is driven by mutations in mepR and mepA genes. Microb Drug Resist. 2018;24:519–526. doi: 10.1089/mdr.2017.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lade H, Kim JS. Bacterial targets of antibiotics in methicillin-resistant Staphylococcus aureus . Antibiotics. 2021;10:398. doi: 10.3390/antibiotics10040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, Vester B. The Cfr rRNA methyltransferase confers resistance to Phenicols, Lincosamides, Oxazolidinones, Pleuromutilins, and Streptogramin A antibiotics. Antimicrob Agents Chemother. 2006;50:2500–2505. doi: 10.1128/AAC.00131-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y, Lv Y, Cai J, Schwarz S, Cui L, et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother. 2015;70:2182–2190. doi: 10.1093/jac/dkv116. [DOI] [PubMed] [Google Scholar]
- 96.Wu C, Zhang X, Liang J, Li Q, Lin H, et al. Characterization of florfenicol resistance genes in the coagulase-negative Staphylococcus (CoNS) isolates and genomic features of a multidrug-resistant Staphylococcus lentus strain H29. Antimicrob Resist Infect Control. 2021;10 doi: 10.1186/s13756-020-00869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tenson T, Lovmar M, Ehrenberg M. The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J Mol Biol. 2003;330:1005–1014. doi: 10.1016/s0022-2836(03)00662-4. [DOI] [PubMed] [Google Scholar]
- 98.Westh H, Hougaard DM, Vuust J, Rosdahl VT. erm genes in erythromycin-resistant Staphylococcus aureus and coagulase-negative staphylococci. APMIS. 1995;103:225–232. [PubMed] [Google Scholar]
- 99.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miklasińska-Majdanik M. Mechanisms of resistance to macrolide antibiotics among Staphylococcus aureus . Antibiotics. 2021;10:1406. doi: 10.3390/antibiotics10111406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharkey LKR, Edwards TA, O’Neill AJ. ABC-F proteins mediate antibiotic resistance through ribosomal protection. mBio. 2016;7:e01975. doi: 10.1128/mBio.01975-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fyfe C, Grossman TH, Kerstein K, Sutcliffe J. Resistance to macrolide antibiotics in public health pathogens. Cold Spring Harb Perspect Med. 2016;6:a025395. doi: 10.1101/cshperspect.a025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leclercq R. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin Infect Dis. 2002;34:482–492. doi: 10.1086/324626. [DOI] [PubMed] [Google Scholar]
- 104.Hot C, Berthet N, Chesneau O. Characterization of sal(A), A novel gene responsible for lincosamide and streptogramin A resistance in Staphylococcus sciuri . Antimicrob Agents Chemother. 2014;58:3335–3341. doi: 10.1128/AAC.02797-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lipka M, Filipek R, Bochtler M. Crystal structure and mechanism of the Staphylococcus cohnii virginiamycin B lyase (Vgb) Biochemistry. 2008;47:4257–4265. doi: 10.1021/bi7015266. [DOI] [PubMed] [Google Scholar]
- 106.Brickner SJ, Barbachyn MR, Hutchinson DK, Manninen PR. Linezolid (ZYVOX), the first member of a completely new class of antibacterial agents for treatment of serious gram-positive infections. J Med Chem. 2008;51:1981–1990. doi: 10.1021/jm800038g. [DOI] [PubMed] [Google Scholar]
- 107.Locke JB, Zurenko GE, Shaw KJ, Bartizal K. Tedizolid for the management of human infections: in vitro characteristics. Clin Infect Dis. 2014;58 Suppl 1:S35–42. doi: 10.1093/cid/cit616. [DOI] [PubMed] [Google Scholar]
- 108.Long KS, Vester B. Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob Agents Chemother. 2012;56:603–612. doi: 10.1128/AAC.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stefani S, Bongiorno D, Mongelli G, Campanile F. Linezolid resistance in staphylococci. Pharmaceuticals. 2010;3:1988–2006. doi: 10.3390/ph3071988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paukner S, Riedl R. Pleuromutilins: potent drugs for resistant bugs-mode of action and resistance. Cold Spring Harb Perspect Med. 2017;7:a027110. doi: 10.1101/cshperspect.a027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Watkins RR, Holubar M, David MZ. Antimicrobial resistance in methicillin-resistant Staphylococcus aureus to newer antimicrobial agents. Antimicrob Agents Chemother. 2019;63:e01216-19. doi: 10.1128/AAC.01216-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bodley JW, Zieve FJ, Lin L, Zieve ST. Formation of the ribosome-G factor-GDP complex in the presence of fusidic acid. Biochem Biophys Res Commun. 1969;37:437–443. doi: 10.1016/0006-291x(69)90934-6. [DOI] [PubMed] [Google Scholar]
- 113.Moazed D, Noller HF. Intermediate states in the movement of transfer RNA in the ribosome. Nature. 1989;342:142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- 114.Ramrath DJF, Lancaster L, Sprink T, Mielke T, Loerke J, et al. Visualization of two transfer RNAs trapped in transit during elongation factor G-mediated translocation. Proc Natl Acad Sci. 2013;110:20964–20969. doi: 10.1073/pnas.1320387110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fernandes P. Fusidic acid: a bacterial elongation factor inhibitor for the oral treatment of acute and chronic staphylococcal infections. Cold Spring Harb Perspect Med. 2016;6:a025437. doi: 10.1101/cshperspect.a025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Howden BP, Grayson ML. Dumb and dumber--the potential waste of a useful antistaphylococcal agent: emerging fusidic acid resistance in Staphylococcus aureus . Clin Infect Dis. 2006;42:394–400. doi: 10.1086/499365. [DOI] [PubMed] [Google Scholar]
- 117.Tomlinson JH, Thompson GS, Kalverda AP, Zhuravleva A, O’Neill AJ. A target-protection mechanism of antibiotic resistance at atomic resolution: insights into FusB-type fusidic acid resistance. Sci Rep. 2016;6:19524. doi: 10.1038/srep19524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cox G, Thompson GS, Jenkins HT, Peske F, Savelsbergh A, et al. Ribosome clearance by FusB-type proteins mediates resistance to the antibiotic fusidic acid. Proc Natl Acad Sci. 2012;109:2102–2107. doi: 10.1073/pnas.1117275109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thomas CM, Hothersall J, Willis CL, Simpson TJ. Resistance to and synthesis of the antibiotic mupirocin. Nat Rev Microbiol. 2010;8:281–289. doi: 10.1038/nrmicro2278. [DOI] [PubMed] [Google Scholar]
- 120.Antonio M, McFerran N, Pallen MJ. Mutations affecting the Rossman fold of isoleucyl-tRNA synthetase are correlated with low-level mupirocin resistance in Staphylococcus aureus . Antimicrob Agents Chemother. 2002;46:438–442. doi: 10.1128/AAC.46.2.438-442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hodgson JE, Curnock SP, Dyke KG, Morris R, Sylvester DR, et al. Molecular characterization of the gene encoding high-level mupirocin resistance in Staphylococcus aureus J2870. Antimicrob Agents Chemother. 1994;38:1205–1208. doi: 10.1128/AAC.38.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Udo EE, Jacob LE, Mathew B. Genetic analysis of methicillin-resistant Staphylococcus aureus expressing high- and low-level mupirocin resistance. J Med Microbiol. 2001;50:909–915. doi: 10.1099/0022-1317-50-10-909. [DOI] [PubMed] [Google Scholar]
- 123.Ishikawa T, Matsunaga N, Tawada H, Kuroda N, Nakayama Y, et al. TAK-599, a novel N-phosphono type prodrug of anti-MRSA cephalosporin T-91825: synthesis, physicochemical and pharmacological properties. Bioorg Med Chem. 2003;11:2427–2437. doi: 10.1016/s0968-0896(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 124.Jones RN, Deshpande LM, Mutnick AH, Biedenbach DJ. In vitro evaluation of BAL9141, a novel parenteral cephalosporin active against oxacillin-resistant staphylococci. J Antimicrob Chemother. 2002;50:915–932. doi: 10.1093/jac/dkf249. [DOI] [PubMed] [Google Scholar]
- 125.Wilcox MH, Corey GR, Talbot GH, Thye D, Friedland D, et al. CANVAS 2: the second Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother. 2010;65 Suppl 4:iv53–iv65. doi: 10.1093/jac/dkq255. [DOI] [PubMed] [Google Scholar]
- 126.Wilcox MH, Corey GR, Talbot GH, Thye D, Friedland D, et al. CANVAS 2: the second Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother. 2010;65 Suppl 4:iv53–iv65. doi: 10.1093/jac/dkq255. [DOI] [PubMed] [Google Scholar]
- 127.File TM, Wilcox MH, Stein GE. Summary of ceftaroline fosamil clinical trial studies and clinical safety. Clin Infect Dis. 2012;55 Suppl 3:S173–80. doi: 10.1093/cid/cis559. [DOI] [PubMed] [Google Scholar]
- 128.Duplessis C, Crum-Cianflone NF. Ceftaroline: a new cephalosporin with activity against methicillin-resistant Staphylococcus aureus (MRSA) Clin Med Rev Ther. 2011;3:1–17. doi: 10.4137/CMRT.S1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Long SW, Olsen RJ, Mehta SC, Palzkill T, Cernoch PL, et al. PBP2a mutations causing high-level Ceftaroline resistance in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2014;58:6668–6674. doi: 10.1128/AAC.03622-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nigo M, Diaz L, Carvajal LP, Tran TT, Rios R, et al. Ceftaroline-resistant, daptomycin-tolerant, and heterogeneous vancomycin-intermediate methicillin-resistant Staphylococcus aureus causing infective endocarditis. Antimicrob Agents Chemother. 2017;61:e01235-16. doi: 10.1128/AAC.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McKeage K. Finafloxacin: first global approval. Drugs. 2015;75:687–693. doi: 10.1007/s40265-015-0384-z. [DOI] [PubMed] [Google Scholar]
- 132.Dalhoff A, Stubbings W, Schubert S. Comparative in vitro activities of the novel antibacterial finafloxacin against selected Gram-positive and Gram-negative bacteria tested in mueller-hinton broth and synthetic urine. Antimicrob Agents Chemother. 2011;55:1814–1818. doi: 10.1128/AAC.00886-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barnes KB, Richards MI, Laws TR, Núñez A, Thwaite JE, et al. Finafloxacin is an effective treatment for inhalational tularemia and plague in mouse models of infection. Antimicrob Agents Chemother. 2021;65 doi: 10.1128/AAC.02294-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lemaire S, Van Bambeke F, Tulkens PM. Activity of finafloxacin, a novel fluoroquinolone with increased activity at acid pH, towards extracellular and intracellular Staphylococcus aureus, Listeria monocytogenes and Legionella pneumophila . Int J Antimicrob Agents. 2011;38:52–59. doi: 10.1016/j.ijantimicag.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 135.Emrich NC, Heisig A, Stubbings W, Labischinski H, Heisig P. Antibacterial activity of finafloxacin under different pH conditions against isogenic strains of Escherichia coli expressing combinations of defined mechanisms of fluoroquinolone resistance. J Antimicrob Chemother. 2010;65:2530–2533. doi: 10.1093/jac/dkq375. [DOI] [PubMed] [Google Scholar]
- 136.Wagenlehner F, Nowicki M, Bentley C, Lückermann M, Wohlert S, et al. Explorative randomized Phase II clinical study of the efficacy and safety of finafloxacin versus ciprofloxacin for treatment of complicated urinary tract infections. Antimicrob Agents Chemother. 2018;62:e02317-17. doi: 10.1128/AAC.02317-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Adam HJ, Laing NM, King CR, Lulashnyk B, Hoban DJ, et al. In vitro activity of nemonoxacin, a novel nonfluorinated quinolone, against 2,440 clinical isolates. Antimicrob Agents Chemother. 2009;53:4915–4920. doi: 10.1128/AAC.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kırmusaoğlu S. Staphylococcus and Streptococcus. IntechOpen; 2020. Nemonoxacin (Taigexyn ®): a new non-fluorinated quinolone. [DOI] [Google Scholar]
- 139.Yuan J, Mo B, Ma Z, Lv Y, Cheng S-L, et al. Safety and efficacy of oral nemonoxacin versus levofloxacin in treatment of community-acquired pneumonia: a phase 3, multicenter, randomized, double-blind, double-dummy, active-controlled, non-inferiority trial. J Microbiol Immunol Infect. 2019;52:35–44. doi: 10.1016/j.jmii.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 140.Roychoudhury S, Makin K, Twinem T, Leunk R, Hsu MC. In vitro resistance development to nemonoxacin in Streptococcus pneumoniae: a unique profile for a novel nonfluorinated quinolone. Microb Drug Resist. 2016;22:578–584. doi: 10.1089/mdr.2016.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang JJ, Cheng A, Tai HM, Chang LW, Hsu MC, et al. Selected mutations by nemonoxacin and fluoroquinolone exposure among relevant Gram-positive bacterial strains in Taiwan. Microb Drug Resist. 2020;26:110–117. doi: 10.1089/mdr.2019.0048. [DOI] [PubMed] [Google Scholar]
- 142.Kocsis B, Gulyás D, Szabó D. Delafloxacin, finafloxacin, and zabofloxacin: novel fluoroquinolones in the antibiotic pipeline. Antibiotics. 2021;10:1506. doi: 10.3390/antibiotics10121506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rhee CK, Chang JH, Choi EG, Kim HK, Kwon Y-S, et al. Zabofloxacin versus moxifloxacin in patients with COPD exacerbation: a multicenter, double-blind, double-dummy, randomized, controlled, Phase III, non-inferiority trial. Int J Chron Obstruct Pulmon Dis. 2015;10:2265–2275. doi: 10.2147/COPD.S90948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mohamed NM, Zakaria AS, Edward EA, Abdel-Bary A. In Vitro and In Vivo activity of zabofloxacin and other fluoroquinolones against MRSA isolates from a university hospital in Egypt. Pol J Microbiol. 2019;68:59–69. doi: 10.21307/pjm-2019-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nilius AM, Shen LL, Hensey-Rudloff D, Almer LS, Beyer JM, et al. In vitro antibacterial potency and spectrum of ABT-492, a new fluoroquinolone. Antimicrob Agents Chemother. 2003;47:3260–3269. doi: 10.1128/AAC.47.10.3260-3269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Harnett SJ, Fraise AP, Andrews JM, Jevons G, Brenwald NP, et al. Comparative study of the in vitro activity of a new fluoroquinolone, ABT-492. J Antimicrob Chemother. 2004;53:783–792. doi: 10.1093/jac/dkh180. [DOI] [PubMed] [Google Scholar]
- 147.Saravolatz LD, Stein GE. Delafloxacin: a new anti-methicillin-resistant Staphylococcus aureus fluoroquinolone. Clin Infect Dis. 2019;68:1058–1062. doi: 10.1093/cid/ciy600. [DOI] [PubMed] [Google Scholar]
- 148.Tulkens PM, Van Bambeke F, Zinner SH. Profile of a novel anionic fluoroquinolone-delafloxacin. Clin Infect Dis. 2019;68:S213–S222. doi: 10.1093/cid/ciy1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pfaller MA, Sader HS, Rhomberg PR, Flamm RK. In Vitro activity of delafloxacin against contemporary bacterial pathogens from the United States and Europe, 2014. Antimicrob Agents Chemother. 2017;61:e02609-16. doi: 10.1128/AAC.02609-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.O’Riordan W, McManus A, Teras J, Poromanski I, Cruz-Saldariagga M, et al. A comparison of the efficacy and safety of intravenous followed by oral delafloxacin with vancomycin plus aztreonam for the treatment of acute bacterial skin and skin structure infections: a Phase 3, multinational, double-blind, randomized study. Clin Infect Dis. 2018;67:657–666. doi: 10.1093/cid/ciy165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mogle BT, Steele JM, Thomas SJ, Bohan KH, Kufel WD. Clinical review of delafloxacin: a novel anionic fluoroquinolone. J Antimicrob Chemother. 2018;73:1439–1451. doi: 10.1093/jac/dkx543. [DOI] [PubMed] [Google Scholar]
- 152.Iregui A, Khan Z, Malik S, Landman D, Quale J. Emergence of delafloxacin-resistant Staphylococcus aureus in Brooklyn, New York. Clin Infect Dis. 2020;70:1758–1760. doi: 10.1093/cid/ciz787. [DOI] [PubMed] [Google Scholar]
- 153.González Borroto JI, Awori MS, Chouinard L, Smith SY, Tarragó C, et al. Studies on articular and general toxicity of orally administered ozenoxacin in juvenile rats and dogs. Future Microbiol. 2018;13:31–40. doi: 10.2217/fmb-2017-0291. [DOI] [PubMed] [Google Scholar]
- 154.Vila J, Hebert AA, Torrelo A, López Y, Tato M, et al. Ozenoxacin: a review of preclinical and clinical efficacy. Expert Rev Anti Infect Ther. 2019;17:159–168. doi: 10.1080/14787210.2019.1573671. [DOI] [PubMed] [Google Scholar]
- 155.López Y, Tato M, Espinal P, Garcia-Alonso F, Gargallo-Viola D, et al. In vitro activity of Ozenoxacin against quinolone-susceptible and quinolone-resistant gram-positive bacteria. Antimicrob Agents Chemother. 2013;57:6389–6392. doi: 10.1128/AAC.01509-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Canton R, Morrissey I, Vila J, Tato M, García-Castillo M, et al. Comparative in vitro antibacterial activity of ozenoxacin against Gram-positive clinical isolates. Future Microbiol. 2018;13:3–19. doi: 10.2217/fmb-2017-0289. [DOI] [PubMed] [Google Scholar]
- 157.Gropper S, Albareda N, Chelius K, Kruger D, Mitha I, et al. Ozenoxacin 1% cream in the treatment of impetigo: a multicenter, randomized, placebo- and retapamulin-controlled clinical trial. Future Microbiol. 2014;9:1013–1023. doi: 10.2217/fmb.14.78. [DOI] [PubMed] [Google Scholar]
- 158.Rosen T, Albareda N, Rosenberg N, Alonso FG, Roth S, et al. Efficacy and safety of ozenoxacin cream for treatment of adult and pediatric patients with impetigo: a randomized clinical trial. JAMA Dermatol. 2018;154:806–813. doi: 10.1001/jamadermatol.2018.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kishii R, Yamaguchi Y, Takei M. In Vitro activities and spectrum of the novel fluoroquinolone lascufloxacin (KRP-AM1977) Antimicrob Agents Chemother. 2017;61:e00120-17. doi: 10.1128/AAC.00120-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Furuie H, Tanioka S, Shimizu K, Manita S, Nishimura M, et al. Intrapulmonary pharmacokinetics of lascufloxacin in healthy adult volunteers. Antimicrob Agents Chemother. 2018;62:e02169-17. doi: 10.1128/AAC.02169-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Miki M, Umemoto Y. Phase III double-blind comparative study of Lascufloxacin versus levofloxacin in patients with community-acquired pneumonia. Japanese J Chemother. 2020 [Google Scholar]
- 162.Kishii R, Yamaguchi Y, Takei M. In Vitro activities and spectrum of the novel fluoroquinolone lascufloxacin (KRP-AM1977) Antimicrob Agents Chemother. 2017;61:e00120-17. doi: 10.1128/AAC.00120-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thakare R, Singh S, Dasgupta A, Chopra S. Lascufloxacin hydrochloride to treat bacterial infection. Drugs Today. 2020;56:365–376. doi: 10.1358/dot.2020.56.6.3137167. [DOI] [PubMed] [Google Scholar]
- 164.Appalaraju B, Baveja S, Baliga S, Shenoy S, Bhardwaj R, et al. In vitro activity of a novel antibacterial agent, levonadifloxacin, against clinical isolates collected in a prospective, multicentre surveillance study in India during 2016-18. J Antimicrob Chemother. 2020;75:600–608. doi: 10.1093/jac/dkz493. [DOI] [PubMed] [Google Scholar]
- 165.Bakthavatchalam YD, Shankar A, Muniyasamy R, Peter JV, Marcus Z, et al. Levonadifloxacin, a recently approved benzoquinolizine fluoroquinolone, exhibits potent in vitro activity against contemporary Staphylococcus aureus isolates and Bengal Bay clone isolates collected from a large Indian tertiary care hospital. J Antimicrob Chemother. 2020;75:2156–2159. doi: 10.1093/jac/dkaa142. [DOI] [PubMed] [Google Scholar]
- 166.Bhagwat SS, McGhee P, Kosowska-Shick K, Patel MV, Appelbaum PC. In vitro activity of the quinolone WCK 771 against recent U.S. hospital and community-acquired Staphylococcus aureus pathogens with various resistance types. Antimicrob Agents Chemother. 2009;53:811–813. doi: 10.1128/AAC.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.de Souza NJ, Gupte SV, Deshpande PK, Desai VN, Bhawsar SB, et al. A chiral benzoquinolizine-2-carboxylic acid arginine salt active against vancomycin-resistant Staphylococcus aureus . J Med Chem. 2005;48:5232–5242. doi: 10.1021/jm050035f. [DOI] [PubMed] [Google Scholar]
- 168.Jacobs MR, Bajaksouzian S, Windau A, Appelbaum PC, Patel MV, et al. In vitro activity of the new quinolone WCK 771 against staphylococci. Antimicrob Agents Chemother. 2004;48:3338–3342. doi: 10.1128/AAC.48.9.3338-3342.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bhatia A, Mastim M, Shah M, Gutte R, Joshi P, et al. Efficacy and safety of a novel broad-spectrum anti-MRSA agent levonadifloxacin compared with linezolid for acute bacterial skin and skin structure infections: a Phase 3, openlabel, randomized study. J Assoc Physicians India. 2020;68:30–36. [PubMed] [Google Scholar]
- 170.Bhagwat SS, Nandanwar M, Kansagara A, Patel A, Takalkar S, et al. Levonadifloxacin, a novel broad-spectrum anti-MRSA benzoquinolizine quinolone agent: review of current evidence. Drug Des Devel Ther. 2019;13:4351–4365. doi: 10.2147/DDDT.S229882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bhagwat SS, Mundkur LA, Gupte SV, Patel MV, Khorakiwala HF. The anti-methicillin-resistant Staphylococcus aureus quinolone WCK 771 has potent activity against sequentially selected mutants, has a narrow mutant selection window against quinolone-resistant Staphylococcus aureus, and preferentially targets DNA gyrase. Antimicrob Agents Chemother. 2006;50:3568–3579. doi: 10.1128/AAC.00641-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhanel GG, Love R, Adam H, Golden A, Zelenitsky S, et al. Tedizolid: a novel oxazolidinone with potent activity against multidrug-resistant gram-positive pathogens. Drugs. 2015;75:253–270. doi: 10.1007/s40265-015-0352-7. [DOI] [PubMed] [Google Scholar]
- 173.Michalska K, Karpiuk I, Król M, Tyski S. Recent development of potent analogues of oxazolidinone antibacterial agents. Bioorg Med Chem. 2013;21:577–591. doi: 10.1016/j.bmc.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 174.Barber KE, Smith JR, Raut A, Rybak MJ. Evaluation of tedizolid against Staphylococcus aureus and enterococci with reduced susceptibility to vancomycin, daptomycin or linezolid. J Antimicrob Chemother. 2016;71:152–155. doi: 10.1093/jac/dkv302. [DOI] [PubMed] [Google Scholar]
- 175.Salavert Lletí M, García-Bustos V, Morata Ruiz L, Cabañero-Navalon MD. Tedizolid: new data and experiences for clinical practice. Rev Esp Quimioter. 2021;34 Suppl 1:22–25. doi: 10.37201/req/s01.06.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Prokocimer P, De Anda C, Fang E, Mehra P, Das A. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA. 2013;309:559–569. doi: 10.1001/jama.2013.241. [DOI] [PubMed] [Google Scholar]
- 177.Moran GJ, Fang E, Corey GR, Das AF, De Anda C, et al. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2014;14:696–705. doi: 10.1016/S1473-3099(14)70737-6. [DOI] [PubMed] [Google Scholar]
- 178.Locke JB, Finn J, Hilgers M, Morales G, Rahawi S, et al. Structure-activity relationships of diverse oxazolidinones for linezolid-resistant Staphylococcus aureus strains possessing the cfr methyltransferase gene or ribosomal mutations. Antimicrob Agents Chemother. 2010;54:5337–5343. doi: 10.1128/AAC.00663-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shen T, Penewit K, Waalkes A, Xu L, Salipante SJ, et al. Identification of a novel tedizolid resistance mutation in rpoB of MRSA after in vitro serial passage. J Antimicrob Chemother. 2021;76:292–296. doi: 10.1093/jac/dkaa422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Locke JB, Hilgers M, Shaw KJ. Novel ribosomal mutations in Staphylococcus aureus strains identified through selection with the oxazolidinones linezolid and torezolid (TR-700) Antimicrob Agents Chemother. 2009;53:5265–5274. doi: 10.1128/AAC.00871-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Meng J, Zhong D, Li L, Yuan Z, Yuan H, et al. Metabolism of MRX-I, a novel antibacterial oxazolidinone, in humans: the oxidative ring opening of 2,3-Dihydropyridin-4-one catalyzed by non-P450 enzymes. Drug Metab Dispos. 2015;43:646–659. doi: 10.1124/dmd.114.061747. [DOI] [PubMed] [Google Scholar]
- 182.Shouan A, Kumar R, Lal V, Grover S. Linezolid-induced serotonin syndrome. Ind Psychiatry J. 2020;29:345–348. doi: 10.4103/ipj.ipj_37_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang W, Voss KM, Liu J, Gordeev MF. Nonclinical evaluation of antibacterial oxazolidinones contezolid and contezolid acefosamil with low serotonergic neurotoxicity. Chem Res Toxicol. 2021;34:1348–1354. doi: 10.1021/acs.chemrestox.0c00524. [DOI] [PubMed] [Google Scholar]
- 184.Hoy SM. Contezolid: first approval. Drugs. 2021;81:1587–1591. doi: 10.1007/s40265-021-01576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang S, Cai C, Shen Y, Sun C, Shi Q, et al. In vitro activity of contezolid against methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococcus, and strains with linezolid resistance genes from China. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.729900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xiao X-Y, Hunt DK, Zhou J, Clark RB, Dunwoody N, et al. Fluorocyclines. 1. 7-fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline: a potent, broad spectrum antibacterial agent. J Med Chem. 2012;55:597–605. doi: 10.1021/jm201465w. [DOI] [PubMed] [Google Scholar]
- 187.Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337–352. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee YR, Burton CE. Eravacycline, a newly approved fluorocycline. Eur J Clin Microbiol Infect Dis. 2019;38:1787–1794. doi: 10.1007/s10096-019-03590-3. [DOI] [PubMed] [Google Scholar]
- 189.Alosaimy S, Abdul‐Mutakabbir JC, Kebriaei R, Jorgensen SCJ, Rybak MJ. Evaluation of eravacycline: a novel fluorocycline. Pharmacotherapy. 2020;40:221–238. doi: 10.1002/phar.2366. [DOI] [PubMed] [Google Scholar]
- 190.Solomkin J, Evans D, Slepavicius A, Lee P, Marsh A, et al. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the investigating Gram-negative infections treated with eravacycline (IGNITE 1) trial. JAMA Surg. 2017;152:224. doi: 10.1001/jamasurg.2016.4237. [DOI] [PubMed] [Google Scholar]
- 191.Solomkin JS, Gardovskis J, Lawrence K, Montravers P, Sway A, et al. IGNITE4: results of a Phase 3, randomized, multicenter, prospective trial of eravacycline vs meropenem in the treatment of complicated intraabdominal infections. Clin Infect Dis. 2019;69:921–929. doi: 10.1093/cid/ciy1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang F, Bai B, Xu G, Lin Z, Li G, et al. Eravacycline activity against clinical S. aureus isolates from China: in vitro activity, MLST profiles and heteroresistance. BMC Microbiol. 2018;18 doi: 10.1186/s12866-018-1349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zeng W, Zhang X, Liu Y, Zhang Y, Xu M, et al. In vitro antimicrobial activity and resistance mechanisms of the new generation tetracycline agents, eravacycline, omadacycline, and tigecycline against clinical Staphylococcus aureus isolates. Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.1043736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Macone AB, Caruso BK, Leahy RG, Donatelli J, Weir S, et al. In Vitro and In vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob Agents Chemother. 2014;58:1127–1135. doi: 10.1128/AAC.01242-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tanaka SK, Steenbergen J, Villano S. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg Med Chem. 2016;24:6409–6419. doi: 10.1016/j.bmc.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 196.Honeyman L, Ismail M, Nelson ML, Bhatia B, Bowser TE, et al. Structure-activity relationship of the aminomethylcyclines and the discovery of omadacycline. Antimicrob Agents Chemother. 2015;59:7044–7053. doi: 10.1128/AAC.01536-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pfaller MA, Huband MD, Shortridge D, Flamm RK. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe: report from the SENTRY antimicrobial surveillance program, 2016 to 2018. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.02488-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Stets R, Popescu M, Gonong J, Mitha I, Nseir W, et al. A Phase 3 randomized, double-blind, multi-center study to compare the safety and efficacy of IV to oral omadacycline to moxifloxacin for the treatment of adult subjects with CABP (The OPTIC Study) Open Forum Infect Dis. 2017;4:S543–S544. doi: 10.1093/ofid/ofx163.1413. [DOI] [Google Scholar]
- 199.Markham A, Keam SJ. Omadacycline: first global approval. Drugs. 2018;78:1931–1937. doi: 10.1007/s40265-018-1015-2. [DOI] [PubMed] [Google Scholar]
- 200.O’Riordan W, Cardenas C, Shin E, Sirbu A, Garrity-Ryan L, et al. Once-daily oral omadacycline versus twice-daily oral linezolid for acute bacterial skin and skin structure infections (OASIS-2): a phase 3, double-blind, multicentre, randomised, controlled, non-inferiority trial. Lancet Infect Dis. 2019;19:1080–1090. doi: 10.1016/S1473-3099(19)30275-0. [DOI] [PubMed] [Google Scholar]
- 201.Bai B, Lin Z, Pu Z, Xu G, Zhang F, et al. /Pi cotransporter family protein, and fibronectin-binding protein. Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Novak R. Are pleuromutilin antibiotics finally fit for human use? Ann N Y Acad Sci. 2011;1241:71–81. doi: 10.1111/j.1749-6632.2011.06219.x. [DOI] [PubMed] [Google Scholar]
- 203.Paukner S, Riedl R. Pleuromutilins: potent drugs for resistant bugs-mode of action and resistance. Cold Spring Harb Perspect Med. 2017;7:a027110. doi: 10.1101/cshperspect.a027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Novak R, Shlaes DM. The pleuromutilin antibiotics: a new class for human use. Curr Opin Investig Drugs. 2010;11:182–191. [PubMed] [Google Scholar]
- 205.Eyal Z, Matzov D, Krupkin M, Wekselman I, Paukner S, et al. Structural insights into species-specific features of the ribosome from the pathogen Staphylococcus aureus . Proc Natl Acad Sci. 2015;112:E5805–14. doi: 10.1073/pnas.1517952112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Waites KB, Crabb DM, Duffy LB, Jensen JS, Liu Y, et al. In vitro activities of lefamulin and other antimicrobial agents against macrolide-susceptible and macrolide-resistant Mycoplasma pneumoniae from the United States, Europe, and China. Antimicrob Agents Chemother. 2017;61:e02008-16. doi: 10.1128/AAC.02008-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mendes RE, Farrell DJ, Flamm RK, Talbot GH, Ivezic-Schoenfeld Z, et al. In vitro activity of lefamulin tested against Streptococcus pneumoniae with defined serotypes, including multidrug-resistant isolates causing lower respiratory tract infections in the United States. Antimicrob Agents Chemother. 2016;60:4407–4411. doi: 10.1128/AAC.00627-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sader HS, Paukner S, Ivezic-Schoenfeld Z, Biedenbach DJ, Schmitz FJ, et al. Antimicrobial activity of the novel pleuromutilin antibiotic BC-3781 against organisms responsible for community-acquired respiratory tract infections (CARTIs) J Antimicrob Chemother. 2012;67:1170–1175. doi: 10.1093/jac/dks001. [DOI] [PubMed] [Google Scholar]
- 209.Sader HS, Biedenbach DJ, Paukner S, Ivezic-Schoenfeld Z, Jones RN. Antimicrobial activity of the investigational pleuromutilin compound BC-3781 tested against Gram-positive organisms commonly associated with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother. 2012;56:1619–1623. doi: 10.1128/AAC.05789-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.File TM, Jr, Goldberg L, Das A, Sweeney C, Saviski J, et al. Efficacy and safety of intravenous-to-oral lefamulin, a pleuromutilin antibiotic, for the treatment of community-acquired bacterial pneumonia: the Phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) trial. Clin Infect Dis. 2019;69:1856–1867. doi: 10.1093/cid/ciz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Alexander E, Goldberg L, Das AF, Moran GJ, Sandrock C, et al. Oral lefamulin vs moxifloxacin for early clinical response among adults with community-acquired bacterial pneumonia. JAMA. 2019;322:1661. doi: 10.1001/jama.2019.15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Paukner S, Sader HS, Ivezic-Schoenfeld Z, Jones RN. Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY antimicrobial surveillance program in 2010. Antimicrob Agents Chemother. 2013;57:4489–4495. doi: 10.1128/AAC.00358-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang F, Zhou H, Olademehin OP, Kim SJ, Tao P. Insights into key interactions between vancomycin and bacterial cell wall structures. ACS Omega. 2018;3:37–45. doi: 10.1021/acsomega.7b01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Van Bambeke F, Van Laethem Y, Courvalin P, Tulkens PM. Glycopeptide antibiotics. Drugs. 2004;64:913–936. doi: 10.2165/00003495-200464090-00001. [DOI] [PubMed] [Google Scholar]
- 215.Beauregard DA, Williams DH, Gwynn MN, Knowles DJ. Dimerization and membrane anchors in extracellular targeting of vancomycin group antibiotics. Antimicrob Agents Chemother. 1995;39:781–785. doi: 10.1128/AAC.39.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cooper RDG, Snyder NJ, Zweifel MJ, Staszak MA, Wilkie SC, et al. Reductive alkylation of glycopeptide antibiotics: synthesis and antibacterial activity. J Antibiot. 1996;49:575–581. doi: 10.7164/antibiotics.49.575. [DOI] [PubMed] [Google Scholar]
- 217.Allen NE, Nicas TI. Mechanism of action of oritavancin and related glycopeptide antibiotics. FEMS Microbiol Rev. 2003;26:511–532. doi: 10.1111/j.1574-6976.2003.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 218.Domenech O, Dufrêne YF, Van Bambeke F, Tukens PM, Mingeot-Leclercq M-P. Interactions of oritavancin, a new semi-synthetic lipoglycopeptide, with lipids extracted from Staphylococcus aureus . Biochim Biophys Acta. 2010;1798:1876–1885. doi: 10.1016/j.bbamem.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 219.Belley A, McKay GA, Arhin FF, Sarmiento I, Beaulieu S, et al. Oritavancin disrupts membrane integrity of Staphylococcus aureus and vancomycin-resistant enterococci to effect rapid bacterial killing. Antimicrob Agents Chemother. 2010;54:5369–5371. doi: 10.1128/AAC.00760-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kim SJ, Cegelski L, Stueber D, Singh M, Dietrich E, et al. Oritavancin exhibits dual mode of action to inhibit cell-wall biosynthesis in Staphylococcus aureus . J Mol Biol. 2008;377:281–293. doi: 10.1016/j.jmb.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Saravolatz LD, Pawlak J, Johnson LB. In vitro activity of oritavancin against community-associated meticillin-resistant Staphylococcus aureus (CA-MRSA) Int J Antimicrob Agents. 2010;36 doi: 10.1016/j.ijantimicag.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 222.Corey GR, Kabler H, Mehra P, Gupta S, Overcash JS, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370:2180–2190. doi: 10.1056/NEJMoa1310422. [DOI] [PubMed] [Google Scholar]
- 223.Corey GR, Good S, Jiang H, Moeck G, Wikler M, et al. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60:254–262. doi: 10.1093/cid/ciu778. [DOI] [PubMed] [Google Scholar]
- 224.Arthur M, Depardieu F, Reynolds P, Courvalin P. Moderate-level resistance to glycopeptide LY333328 mediated by genes of the vanA and vanB clusters in enterococci. Antimicrob Agents Chemother. 1999;43:1875–1880. doi: 10.1128/AAC.43.8.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yushchuk O, Andreo-Vidal A, Marcone GL, Bibb M, Marinelli F, et al. New molecular tools for regulation and improvement of A40926 glycopeptide antibiotic production in Nonomuraea gerenzanensis ATCC 39727. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Malabarba A, Ciabatti R, Scotti R, Goldstein BP, Ferrari P, et al. New semisynthetic glycopeptides MDL 63,246 and MDL 63,042, and other amide derivatives of antibiotic A-40,926 active against highly glycopeptide-resistant VanA enterococci. J Antibiot. 1995;48:869–883. doi: 10.7164/antibiotics.48.869. [DOI] [PubMed] [Google Scholar]
- 227.Cheng M, Ziora ZM, Hansford KA, Blaskovich MA, Butler MS, et al. Anti-cooperative ligand binding and dimerisation in the glycopeptide antibiotic dalbavancin. Org Biomol Chem. 2014;12:2568–2575. doi: 10.1039/C3OB42428F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Streit JM, Fritsche TR, Sader HS, Jones RN. Worldwide assessment of dalbavancin activity and spectrum against over 6,000 clinical isolates. Diagn Microbiol Infect Dis. 2004;48:137–143. doi: 10.1016/j.diagmicrobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 229.Candiani G, Abbondi M, Borgonovi M, Romanò G, Parenti F. In-vitro and in-vivo antibacterial activity of BI 397, a new semi-synthetic glycopeptide antibiotic. J Antimicrob Chemother. 1999;44:179–192. doi: 10.1093/jac/44.2.179. [DOI] [PubMed] [Google Scholar]
- 230.Leighton A, Gottlieb AB, Dorr MB, Jabes D, Mosconi G, et al. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob Agents Chemother. 2004;48:940–945. doi: 10.1128/AAC.48.3.940-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, et al. Once-weekly Dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370:2169–2179. doi: 10.1056/NEJMoa1310480. [DOI] [PubMed] [Google Scholar]
- 232.Jones RN, Sader HS, Flamm RK. Update of dalbavancin spectrum and potency in the USA: report from the SENTRY Antimicrobial Surveillance Program (2011) Diagn Microbiol Infect Dis. 2013;75:304–307. doi: 10.1016/j.diagmicrobio.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 233.Ortwine JK, Werth BJ, Sakoulas G, Rybak MJ. Reduced glycopeptide and lipopeptide susceptibility in Staphylococcus aureus and the “seesaw effect”: taking advantage of the back door left open? Drug Resist Updat. 2013;16:73–79. doi: 10.1016/j.drup.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 234.Smith JR, Roberts KD, Rybak MJ. Dalbavancin: a novel lipoglycopeptide antibiotic with extended activity against Gram-positive infections. Infect Dis Ther. 2015;4:245–258. doi: 10.1007/s40121-015-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhang R, Polenakovik H, Barreras Beltran IA, Waalkes A, Salipante SJ, et al. Emergence of dalbavancin, vancomycin, and daptomycin nonsusceptible Staphylococcus aureus in a patient treated with dalbavancin: case report and isolate characterization. Clin Infect Dis. 2022;75:1641–1644. doi: 10.1093/cid/ciac341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Czaplewski L, Bax R, Clokie M, Dawson M, Fairhead H, et al. Alternatives to antibiotics-a pipeline portfolio review. Lancet Infect Dis. 2016;16:239–251. doi: 10.1016/S1473-3099(15)00466-1. [DOI] [PubMed] [Google Scholar]
- 237.Ghosh C, Sarkar P, Issa R, Haldar J. Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol. 2019;27:323–338. doi: 10.1016/j.tim.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 238.Douglas EJA, Wulandari SW, Lovell SD, Laabei M. Novel antimicrobial strategies to treat multi-drug resistant Staphylococcus aureus infections. Microb Biotechnol. 2023;16:1456–1474. doi: 10.1111/1751-7915.14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Oefner C, Bandera M, Haldimann A, Laue H, Schulz H, et al. Increased hydrophobic interactions of iclaprim with Staphylococcus aureus dihydrofolate reductase are responsible for the increase in affinity and antibacterial activity. J Antimicrob Chemother. 2009;63:687–698. doi: 10.1093/jac/dkp024. [DOI] [PubMed] [Google Scholar]
- 240.Ho JMW, Juurlink DN. Considerations when prescribing trimethoprim-sulfamethoxazole. Can Med Assoc J. 2011;183:1851–1858. doi: 10.1503/cmaj.111152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Holland TL, O’Riordan W, McManus A, Shin E, Borghei A, et al. A Phase 3, randomized, double-blind, multicenter study to evaluate the safety and efficacy of intravenous iclaprim versus vancomycin for treatment of acute bacterial skin and skin structure infections suspected or confirmed to be due to Gram-positive pathogens (REVIVE-2 Study) Antimicrob Agents Chemother. 2018;62:e02580-17. doi: 10.1128/AAC.02580-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Huang DB, O’Riordan W, Overcash JS, Heller B, Amin F, et al. A Phase 3, randomized, double-blind, multicenter study to evaluate the safety and efficacy of intravenous iclaprim vs vancomycin for the treatment of acute bacterial skin and skin structure infections suspected or confirmed to be due to gram-positive pathogens: REVIVE-1. Clin Infect Dis. 2018;66:1222–1229. doi: 10.1093/cid/cix987. [DOI] [PubMed] [Google Scholar]
- 243.Zhanel GG, Walters M, Noreddin A, Vercaigne LM, Wierzbowski A, et al. The ketolides. Drugs. 2002;62:1771–1804. doi: 10.2165/00003495-200262120-00006. [DOI] [PubMed] [Google Scholar]
- 244.Flamm RK, Rhomberg PR, Sader HS. In Vitro activity of the novel lactone ketolide nafithromycin (WCK 4873) against contemporary clinical bacteria from a global surveillance program. Antimicrob Agents Chemother. 2017;61:e01230-17. doi: 10.1128/AAC.01230-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Long DD, Aggen JB, Chinn J, Choi S-K, Christensen BG, et al. Exploring the positional attachment of glycopeptide/β-lactam heterodimers. J Antibiot. 2008;61:603–614. doi: 10.1038/ja.2008.80. [DOI] [PubMed] [Google Scholar]
- 246.Blais J, Lewis SR, Krause KM, Benton BM. Antistaphylococcal activity of TD-1792, a multivalent glycopeptide-cephalosporin antibiotic. Antimicrob Agents Chemother. 2012;56:1584–1587. doi: 10.1128/AAC.05532-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Stryjewski ME, Potgieter PD, Li Y-P, Barriere SL, Churukian A, et al. TD-1792 versus vancomycin for treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother. 2012;56:5476–5483. doi: 10.1128/AAC.00712-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Piller P, Wolinski H, Cordfunke RA, Drijfhout JW, Keller S, et al. Membrane activity of LL-37 derived antimicrobial peptides against Enterococcus hirae: superiority of SAAP-148 over OP-145. Biomolecules. 2022;12:523. doi: 10.3390/biom12040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nell MJ, Tjabringa GS, Wafelman AR, Verrijk R, Hiemstra PS, et al. Development of novel LL-37 derived antimicrobial peptides with LPS and LTA neutralizing and antimicrobial activities for therapeutic application. Peptides. 2006;27:649–660. doi: 10.1016/j.peptides.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 250.Haisma EM, Göblyös A, Ravensbergen B, Adriaans AE, Cordfunke RA, et al. Antimicrobial peptide P60.4Ac-containing creams and gel for eradication of methicillin-resistant Staphylococcus aureus from cultured skin and airway epithelial surfaces. Antimicrob Agents Chemother. 2016;60:4063–4072. doi: 10.1128/AAC.03001-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Peek NFAW, Nell MJ, Brand R, Jansen-Werkhoven T, van Hoogdalem EJ, et al. Ototopical drops containing a novel antibacterial synthetic peptide: safety and efficacy in adults with chronic suppurative otitis media. PLoS One. 2020;15:e0231573. doi: 10.1371/journal.pone.0231573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Scheper H, Wubbolts JM, Verhagen JAM, de Visser AW, van der Wal RJP, et al. SAAP-148 eradicates MRSA persisters within mature biofilm models simulating prosthetic joint infection. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.625952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.de Breij A, Riool M, Cordfunke RA, Malanovic N, de Boer L, et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci Transl Med. 2018;10:eaan4044. doi: 10.1126/scitranslmed.aan4044. [DOI] [PubMed] [Google Scholar]
- 254.Isaksson J, Brandsdal BO, Engqvist M, Flaten GE, Svendsen JSM, et al. A synthetic antimicrobial peptidomimetic (LTX 109): stereochemical impact on membrane disruption. J Med Chem. 2011;54:5786–5795. doi: 10.1021/jm200450h. [DOI] [PubMed] [Google Scholar]
- 255.Saravolatz LD, Pawlak J, Johnson L, Bonilla H, Saravolatz LD, 2nd, et al. In vitro activities of LTX-109, a synthetic antimicrobial peptide, against methicillin-resistant, vancomycin-intermediate, vancomycin-resistant, daptomycin-nonsusceptible, and linezolid-nonsusceptible Staphylococcus aureus . Antimicrob Agents Chemother. 2012;56:4478–4482. doi: 10.1128/AAC.00194-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Isaksson J, Brandsdal BO, Engqvist M, Flaten GE, Svendsen JSM, et al. A synthetic antimicrobial peptidomimetic (LTX 109): stereochemical impact on membrane disruption. J Med Chem. 2011;54:5786–5795. doi: 10.1021/jm200450h. [DOI] [PubMed] [Google Scholar]
- 257.Haug BE, Stensen W, Kalaaji M, Rekdal Ø, Svendsen JS. Synthetic antimicrobial peptidomimetics with therapeutic potential. J Med Chem. 2008;51:4306–4314. doi: 10.1021/jm701600a. [DOI] [PubMed] [Google Scholar]
- 258.Nilsson AC, Janson H, Wold H, Fugelli A, Andersson K, et al. LTX-109 is a novel agent for nasal decolonization of methicillin-resistant and -sensitive Staphylococcus aureus . Antimicrob Agents Chemother. 2015;59:145–151. doi: 10.1128/AAC.03513-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Heath RJ, Rock CO. Regulation of fatty acid elongation and initiation by acyl-acyl carrier protein in Escherichia coli . J Biol Chem. 1996;271:1833–1836. doi: 10.1074/jbc.271.4.1833. [DOI] [PubMed] [Google Scholar]
- 260.Yin D, Fox B, Lonetto ML, Etherton MR, Payne DJ, et al. Identification of antimicrobial targets using a comprehensive genomic approach. Pharmacogenomics. 2004;5:101–113. doi: 10.1517/phgs.5.1.101.25679. [DOI] [PubMed] [Google Scholar]
- 261.Park HS, Yoon YM, Jung SJ, Kim CM, Kim JM, et al. Antistaphylococcal activities of CG400549, a new bacterial enoyl-acyl carrier protein reductase (FabI) inhibitor. J Antimicrob Chemother. 2007;60:568–574. doi: 10.1093/jac/dkm236. [DOI] [PubMed] [Google Scholar]
- 262.Karlowsky JA, Kaplan N, Hafkin B, Hoban DJ, Zhanel GG. AFN-1252, a FabI inhibitor, demonstrates a Staphylococcus-specific spectrum of activity. Antimicrob Agents Chemother. 2009;53:3544–3548. doi: 10.1128/AAC.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kaplan N, Albert M, Awrey D, Bardouniotis E, Berman J, et al. Mode of action, in vitro activity, and in vivo efficacy of AFN-1252, a selective antistaphylococcal FabI inhibitor. Antimicrob Agents Chemother. 2012;56:5865–5874. doi: 10.1128/AAC.01411-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hafkin B, Kaplan N, Murphy B. Efficacy and safety of AFN-1252, the first Staphylococcus-specific antibacterial agent, in the treatment of acute bacterial skin and skin structure infections, including those in patients with significant comorbidities. Antimicrob Agents Chemother. 2015;60:1695–1701. doi: 10.1128/AAC.01741-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wittke F, Vincent C, Chen J, Heller B, Kabler H, et al. Afabicin, a first-in-class antistaphylococcal antibiotic, in the treatment of acute bacterial skin and skin structure infections: clinical noninferiority to vancomycin/Linezolid. Antimicrob Agents Chemother. 2020;64:e00250-20. doi: 10.1128/AAC.00250-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Baker B. Pravibismane is a potent, broad spectrum anti-infective small molecule that rapidly disrupts bacterial bioenergetics and HALTS bacterial growth. Open Forum Infect Dis. 2020;7:S659–60. [Google Scholar]
- 267.Stojiljkovic I, Evavold BD, Kumar V. Antimicrobial properties of porphyrins. Expert Opin Investig Drugs. 2001;10:309–320. doi: 10.1517/13543784.10.2.309. [DOI] [PubMed] [Google Scholar]
- 268.Ooi N, Miller K, Hobbs J, Rhys-Williams W, Love W, et al. XF-73, a novel antistaphylococcal membrane-active agent with rapid bactericidal activity. J Antimicrob Chemother. 2009;64:735–740. doi: 10.1093/jac/dkp299. [DOI] [PubMed] [Google Scholar]
- 269.Yendewa GA, Griffiss JM, Jacobs MR, Fulton SA, O’Riordan MA, et al. A two-part phase 1 study to establish and compare the safety and local tolerability of two nasal formulations of XF-73 for decolonisation of Staphylococcus aureus: a previously investigated 0.5mg/g viscosified gel formulation versus a modified formulation. J Glob Antimicrob Resist. 2020;21:171–180. doi: 10.1016/j.jgar.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mangino JE, Firstenberg MS, Milewski RKC, Rhys-Williams W, Lees JP, et al. Exeporfinium chloride (XF-73) nasal gel dosed over 24 hours prior to surgery significantly reduced Staphylococcus aureus nasal carriage in cardiac surgery patients: safety and efficacy results from a randomized placebo-controlled phase 2 study. Infect Control Hosp Epidemiol. 2023;44:1171–1173. doi: 10.1017/ice.2023.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ma Z, Lynch AS. Development of a dual-acting antibacterial agent (TNP-2092) for the treatment of persistent bacterial infections. J Med Chem. 2016;59:6645–6657. doi: 10.1021/acs.jmedchem.6b00485. [DOI] [PubMed] [Google Scholar]
- 272.Ma Z, Morris TW, Combrink KD. Therapeutic Opportunities for the Treatment of Biofilm-Associated Infections. 2004. pp. 197–210. [Google Scholar]
- 273.Zimmerli W. Role of rifampin for treatment of orthopedic implant–related Staphylococcal infections. JAMA. 1998;279 doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]
- 274.Fisher CR, Schmidt-Malan SM, Ma Z, Yuan Y, He S, et al. In vitro activity of TNP-2092 against periprosthetic joint infection-associated staphylococci. Diagn Microbiol Infect Dis. 2020;97:115040. doi: 10.1016/j.diagmicrobio.2020.115040. [DOI] [PubMed] [Google Scholar]
- 275.Choi S, Isaacs A, Clements D, Liu D, Kim H, et al. De novo design and in vivo activity of conformationally restrained antimicrobial arylamide foldamers. Proc Natl Acad Sci. 2009;106:6968–6973. doi: 10.1073/pnas.0811818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mascher T, Zimmer SL, Smith TA, Helmann JD. Antibiotic-inducible promoter regulated by the cell envelope stress-sensing two-component system LiaRS of Bacillus subtilis . Antimicrob Agents Chemother. 2004;48:2888–2896. doi: 10.1128/AAC.48.8.2888-2896.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Mensa B, Howell GL, Scott R, DeGrado WF. Comparative mechanistic studies of brilacidin, daptomycin, and the antimicrobial peptide LL16. Antimicrob Agents Chemother. 2014;58:5136–5145. doi: 10.1128/AAC.02955-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Jorgensen DM SROWTK Presented at: 25th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) Copenhagen: 2015. A randomized, double-blind study comparing single-dose and short-course brilacidin to daptomycin in the treatment of acute bacterial skin & skin structure infections (ABSSSI) [Google Scholar]
- 279.Cho YL, Jang J. Development of delpazolid for the treatment of tuberculosis. Appl Sci. 2020;10:2211. doi: 10.3390/app10072211. [DOI] [Google Scholar]
- 280.Douros A, Grabowski K, Stahlmann R. Drug-drug interactions and safety of linezolid, tedizolid, and other oxazolidinones. Expert Opin Drug Metab Toxicol. 2015;11:1849–1859. doi: 10.1517/17425255.2015.1098617. [DOI] [PubMed] [Google Scholar]
- 281.Kim JS, Kim Y-H, Lee SH, Kim YH, Kim J-W, et al. Early bactericidal activity of delpazolid (LCB01-0371) in patients with pulmonary tuberculosis. Antimicrob Agents Chemother. 2022;66:e0168421. doi: 10.1128/AAC.01684-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Jeong J-W, Jung S-J, Lee H-H, Kim Y-Z, Park T-K, et al. In vitro and in vivo activities of LCB01-0371, a new oxazolidinone. Antimicrob Agents Chemother. 2010;54:5359–5362. doi: 10.1128/AAC.00723-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Park JS, Choi YJ, Kwon K, Choi SJ, Moon SM, et al. In-vitro activity of delpazolid and comparator agents against methicillin-resistant Staphylococcus aureus involved in bloodstream infection. Ann Lab Med. 2023;43:389–391. doi: 10.3343/alm.2023.43.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zong Z, Jing W, Shi J, Wen S, Zhang T, et al. Comparison of In Vitro activity and MIC distributions between the novel oxazolidinone delpazolid and linezolid against multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis in China. Antimicrob Agents Chemother. 2018;62:e00165-18. doi: 10.1128/AAC.00165-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Tevyashova AN, Olsufyeva EN, Preobrazhenskaya MN. Design of dual action antibiotics as an approach to search for new promising drugs. Russ Chem Rev. 2015;84:61–97. doi: 10.1070/RCR4448. [DOI] [Google Scholar]
- 286.Evans LE, Krishna A, Ma Y, Webb TE, Marshall DC, et al. Exploitation of antibiotic resistance as a novel drug target: development of a β-lactamase-activated antibacterial prodrug. J Med Chem. 2019;62:4411–4425. doi: 10.1021/acs.jmedchem.8b01923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Towse A, Hoyle CK, Goodall J, Hirsch M, Mestre-Ferrandiz J, et al. Time for a change in how new antibiotics are reimbursed: development of an insurance framework for funding new antibiotics based on a policy of risk mitigation. Health Policy. 2017;121:1025–1030. doi: 10.1016/j.healthpol.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 288.Chellat MF, Raguž L, Riedl R. Targeting antibiotic resistance. Angew Chem Int Ed Engl. 2016;55:6600–6626. doi: 10.1002/anie.201506818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.