Key Points
Question
What were transfusion practices worldwide among patients admitted to the intensive care unit (ICU)?
Findings
In this international, prospective, cohort study of 3643 patients from 233 ICUs in 30 countries, 25% of patients received 1 or more red blood cell (RBC) transfusion during their ICU stay, with a median total of 2 units per patient. The proportion of patients transfused ranged from 0% to 100% across centers, from 0% to 80% across countries, and from 19% to 45% across continents.
Meaning
RBC transfusion was common in patients admitted to ICUs worldwide, with high variability across centers in transfusion practices.
Abstract
Importance
Red blood cell (RBC) transfusion is common among patients admitted to the intensive care unit (ICU). Despite multiple randomized clinical trials of hemoglobin (Hb) thresholds for transfusion, little is known about how these thresholds are incorporated into current practice.
Objective
To evaluate and describe ICU RBC transfusion practices worldwide.
Design, Setting, and Participants
International, prospective, cohort study that involved 3643 adult patients from 233 ICUs in 30 countries on 6 continents from March 2019 to October 2022 with data collection in prespecified weeks.
Exposure
ICU stay.
Main Outcomes and Measures
The primary outcome was the occurrence of RBC transfusion during ICU stay. Additional outcomes included the indication(s) for RBC transfusion (consisting of clinical reasons and physiological triggers), the stated Hb threshold and actual measured Hb values before and after an RBC transfusion, and the number of units transfused.
Results
Among 3908 potentially eligible patients, 3643 were included across 233 ICUs (median of 11 patients per ICU [IQR, 5-20]) in 30 countries on 6 continents. Among the participants, the mean (SD) age was 61 (16) years, 62% were male (2267/3643), and the median Sequential Organ Failure Assessment score was 3.2 (IQR, 1.5-6.0). A total of 894 patients (25%) received 1 or more RBC transfusions during their ICU stay, with a median total of 2 units per patient (IQR, 1-4). The proportion of patients who received a transfusion ranged from 0% to 100% across centers, from 0% to 80% across countries, and from 19% to 45% across continents. Among the patients who received a transfusion, a total of 1727 RBC transfusions were administered, wherein the most common clinical indications were low Hb value (n = 1412 [81.8%]; mean [SD] lowest Hb before transfusion, 7.4 [1.2] g/dL), active bleeding (n = 479; 27.7%), and hemodynamic instability (n = 406 [23.5%]). Among the events with a stated physiological trigger, the most frequently stated triggers were hypotension (n = 728 [42.2%]), tachycardia (n = 474 [27.4%]), and increased lactate levels (n = 308 [17.8%]). The median lowest Hb level on days with an RBC transfusion ranged from 5.2 g/dL to 13.1 g/dL across centers, from 5.3 g/dL to 9.1 g/dL across countries, and from 7.2 g/dL to 8.7 g/dL across continents. Approximately 84% of ICUs administered transfusions to patients at a median Hb level greater than 7 g/dL.
Conclusions and Relevance
RBC transfusion was common in patients admitted to ICUs worldwide between 2019 and 2022, with high variability across centers in transfusion practices.
This cohort study evaluates and describes red blood cell transfusion practices in intensive care units worldwide.
Introduction
Red blood cell (RBC) transfusion is common in patients admitted to the intensive care unit (ICU). During 2021, more than 1.7 million RBC units were transfused in a critical care setting in the United States alone.1 RBC transfusion is a potentially lifesaving treatment, but may be limited by the increasing scarcity of blood products and poses risks to patients including transfusion-associated circulatory overload and transfusion-related acute lung injury.2
Multiple randomized clinical trials (RCTs) in various critically ill populations have suggested that a more restrictive transfusion regimen (ie, lower hemoglobin [Hb] level of 7 to 8 g/dL as threshold for RBC transfusion) is safe,3,4,5,6 and the latest guidelines reflect a restraint in RBC transfusion.7,8,9 However, there is considerable uncertainty regarding the best strategies for transfusion management among different patient populations. Further uncertainty exists about current transfusion practice, and the clinical reasons and physiologic triggers that inform the decision to transfuse.8,9
To address this knowledge gap, a worldwide, prospective, observational study was conducted to describe the occurrence rate, reasons, triggers, and between-center heterogeneity of RBC transfusion across 6 continents and 30 countries over 3 years.
Methods
Study Design and Oversight
The International Point Prevalence Study of Intensive Care Unit Transfusion Practices (InPUT) was an international, multicenter, prospective, observational cohort study of transfusion practice. Recruitment of participating centers was directed by national coordinators, intensive care societies, and through direct contact by the steering committee members (eTables 1, 2, and 3 in Supplement 1). The study was approved by the institutional review board of the Amsterdam University Medical Centers and, thereafter, by national and local ethical committees. Study procedures aligned with the Declaration of Helsinki. Informed consent procedures were guided by national regulations and consisted of either written or oral informed consent by the patient and/or legally authorized representative, and in some countries, informed consent was waived due to the observational and noninvasive nature of the study. Income status of each participating country was extracted from the 2023 World Bank Classification System.10 The protocol and standard operating procedure can be found in Supplement 2.
Informed by a pilot feasibility study,11 participating centers aimed to recruit all adult patients newly admitted to their ICU during a predefined week. For every included patient during the study week, physiological data were collected daily during the ICU stay up to day 28. For each unit of transfused blood, the indications for transfusion were collected, as were outcome data through day 28.
Data collection was scheduled between March 2020 and February 2021. Due to the COVID-19 pandemic, 2 significant changes were implemented. First, the prescheduled 8 weeks of inclusion were extended to 16 weeks, finalizing data collection in January 2022 (eTable 4 in Supplement 1). Second, due to the delayed COVID-19 wave in Australia and New Zealand, data collection was rescheduled from January 2022 to October 2022.
All patients admitted to the ICU during study weeks were included if they were 18 years or older (Table 1). Patients were excluded from further data analyses if the available data did not fulfill the minimum quality standards (further described in the Statistical Analyses section) or if informed consent was not provided by the patient or legally authorized representative in case this was required by local or national legislation.
Table 1. Demographics, Stratified by RBC Transfusion Status.
| Variable | Transfusion (n = 894)a | No transfusion (n = 2749)a |
|---|---|---|
| Age, y | 61 (17) | 61 (16) |
| Sex | ||
| Female | 351 (39.3) | 1025 (37.3) |
| Male | 543 (61.7) | 1724 (62.7) |
| Medical history (multiple options possible) | ||
| Solid tumor | 128 (14.3) | 357 (13.0) |
| Chronic kidney failure | 107 (12.0) | 222 (8.1) |
| Acute coronary syndrome | 106 (11.9) | 275 (10.0) |
| Heart failure | 104 (11.6) | 323 (11.7) |
| Chronic obstructive pulmonary disease | 73 (8) | 339 (12.3) |
| Liver failure | 49 (6) | 61 (2) |
| Hematologic malignancy | 32 (4) | 63 (2) |
| Organ transplant | 21 (2) | 33 (1) |
| Benign hematologic disease | 10 (1) | 25 (1) |
| Bone marrow transplant | 3 (<1) | 6 (<1) |
| Otherb | 231 (25.8) | 728 (26.5) |
| Code status | ||
| Do not resuscitate | 40 (5) | 135 (4.9) |
| Do not intubate | 28 (3) | 108 (3.9) |
| Do not transfuse | 7 (<1.0) | 6 (<1.0) |
| APACHE IV scorec | 57 (33-80) | 44 (27-66) |
| EuroSCORE IId | 2.58 (1.26-5.14) | 1.62 (0.88-3.56) |
| Elective admission | 316 (35.3) | 955 (34.7) |
| Referred from | ||
| Operation theater/OR | 441 (49.4) | 1072 (39.0) |
| General ward | 179 (20.0) | 444 (16.2) |
| Emergency department | 195 (21.8) | 950 (34.6) |
| Other hospital | 73 (8) | 240 (8.7) |
| Other (ie, home, other ICU) | 5 (<1) | 42 (2) |
| Referring specialty | ||
| Cardiothoracic surgery | 208 (23.3) | 330 (12.0) |
| Internal medicine | 158 (17.7) | 534 (19.4) |
| Surgery | 114 (12.8) | 326 (11.9) |
| Gastrointestinal surgery | 92 (10) | 223 (8.1) |
| Trauma surgery | 57 (6) | 94 (3) |
| Pulmonology | 48 (5) | 353 (12.8) |
| Orthopedic surgery | 47 (5) | 61 (2) |
| Neurosurgery | 43 (5) | 248 (9.0) |
| Cardiology | 37 (4) | 258 (9.4) |
| Gynecology | 30 (3) | 50 (2) |
| Urology | 24 (3) | 70 (3) |
| Neurology | 21 (2) | 128 (4.7) |
| Other (ie, emergency, ENT) | 15 (2) | 74 (3) |
| Surgery <24 h before ICU admission | 491 (54.9) | 1111 (40.4) |
| Reason for ICU admission | ||
| Postoperative monitoring | 345 (38.7) | 960 (35.0) |
| Shock | 170 (19.1) | 262 (9.6) |
| Respiratory failure | 134 (15.0) | 657 (24.0) |
| Other | 70 (8) | 238 (8.7) |
| Trauma | 63 (7) | 106 (3.9) |
| Metabolic disturbances | 49 (6) | 219 (8.0) |
| Acute brain injury | 32 (4) | 197 (7.2) |
| In- or out-of-hospital cardiac arrest | 29 (3) | 104 (3.8) |
| Shock present | 325 (36.4) | 527 (19.2) |
| Supportive therapies at admission (multiple options possible) | ||
| Invasive mechanical ventilation | 500 (55.9) | 1119 (40.7) |
| Kidney replacement therapy | 54 (6) | 77 (3) |
| Other supporte | 49 (6) | 92 (3) |
| Other mechanical cardiac support | 18 (2) | 18 (<1) |
| ECMO | 11 (1) | 4 (<1) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ECMO, extracorporeal membrane oxygenation; ENT, ear, nose, and throat; EuroSCORE, European System for Cardiac Operative Risk Evaluation; ICU, intensive care unit; OR, operating room; RBC, red blood cell.
Presented as No. (%) for frequency data, median (IQR, shown first-third quartile borders) for nonparametric, and mean (SD) for parametric numerical variables.
Other included epilepsy, dementia, cerebrovascular ischemia or hemorrhage, peripheral vascular disease, diabetes, and obstructive sleep apnea.
The APACHE IV score is used to reflect the patient’s severity of illness, expressed by a 0-286 range, in which higher severity of illness is reflected by a higher score.
The EURO II score is used to predict mortality in cardiac surgery patients, expressed by a 0-100 percentage of in-hospital mortality.
Other supportive therapies included noninvasive mechanical ventilation methods, kidney replacement therapy, and temporary cardiac pacing.
Data Collection
Data were collected at ICU admission, followed by daily collection during ICU stay up to 28 days or until death or discharge, whichever came first. Daily data included laboratory values (nadir Hb, nadir platelet count, coagulation parameters) and organ support treatment (such as respiratory and kidney support). In addition, newly occurring complications during ICU stay were reported by the clinical team daily including bleeding, cardiac, pulmonary, and kidney complications. The patient outcomes were evaluated up to day 28 after ICU admission. Transfusion events were defined as the administration of RBC, platelet, plasma, whole blood, vitamin K, prothrombin complex concentrate, fibrinogen, cryoprecipitate, and tranexamic acid, as well as the option for massive transfusion protocol. When multiple transfusions were administered during the day, a separate transfusion event was created for each transfusion. For each transfusion event, the factors contributing to the decision to transfuse were collected and categorized as clinical reasons (eg, Hb level or age) and physiological triggers (eg, hypotension or high lactate levels).
Outcomes
The primary outcome was the occurrence rate of RBC transfusion during ICU stay, defined as receiving 1 or more RBC units. This included massive transfusion protocol or administration of whole blood in countries where RBC transfusions were not available as a separate product. Secondary outcomes included (1) the clinical reasons for RBC transfusion, (2) physiological triggers for RBC transfusion, (3) the Hb values around an RBC transfusion, and (4) the number of units transfused in total and per ICU day. Hb values around an RBC transfusion included the last value measured before the transfusion was administered (before transfusion), the first value after the transfusion was administered (post transfusion), and the Hb threshold that was stated in the center’s protocol, or, in the absence of a protocol, the expert’s opinion for that patient (threshold).
Statistical Analyses
Descriptive statistics were reported as mean (SD) or median (IQR), when appropriate. Missing data were evaluated for count and correlation, and patients were excluded if their data did not fulfill the quality standards. Minimum quality standards included the requirement that (1) the number of questionnaires had to match the expected or stated number of questionnaires (ie, when for a patient 6 transfusion events were stated, 6 separate transfusion questionnaires had to be created), and (2) all variables assessed as mandatory had to be available. The details of these quality standards are described in eMethods 1 in Supplement 1, next to an overview of missing data in the cleaned data set (eTable 5 in Supplement 1).
Statistical analyses included 4 comparisons: (1) characteristics of patients who did or did not receive transfusion; (2) the primary and secondary outcomes across centers, countries, and continents; (3) the primary and secondary outcomes of postsurgical vs non-postsurgical patients; and (4) the secondary outcomes among the different stated thresholds in the transfusion events.
First, the Mann-Whitney U or χ2 test was used to compare patients who did vs did not receive transfusion, with a Bonferroni correction applied. Primary and secondary outcomes across centers, countries, and continents, as well as postsurgical indication, were reported using descriptive statistics. Second, a subgroup analysis was performed comparing secondary outcomes among transfusion thresholds as stated in the transfusion questionnaire, divided into restrictive (Hb level <7 g/dL), intermediate (Hb level, 7-9 g/dL), and liberal (Hb level >9 g/dL). No multiplicity correction was performed in this subgroup analysis and, as such, should be interpreted as hypothesis-generating. Third, the median Hb level at the time of transfusion per center was plotted in relation to the absolute number and proportion of ICUs.
All analyses were performed in R (version 4.2.1) using the R studio interface.12 A Bonferroni-adjusted 2-sided P value of less than .05 was considered statistically significant.
Results
A total of 3908 patients were eligible from 233 centers in 30 countries and 6 continents. After excluding the patients for whom no informed consent was obtained or waived and patients who did not fulfill the data quality standards, 3643 patients (93% of eligible patients) remained for further analyses (eFigure 1 in Supplement 1). Per ICU, a median of 11 patients were included for analyses (IQR, 5-20). The 6 continents included Africa (n = 150), Asia (n = 182), Europe (n = 2167), South America (n = 50), North America (n = 167), and Oceania (n = 927). Of the 30 participating countries, 19 were classified as high income, 5 as upper middle income, and 6 as lower middle income (eTable 6 in Supplement 1).
The baseline characteristics of the study population are shown in Table 1. The mean (SD) age was 61 (16) years, and 62% were male (n = 2267). Thirteen patients had a do-not-transfuse order. Thirty-five percent of patients were admitted electively (n = 1271), with the main reasons for admission consisting of postoperative monitoring (n = 1042 [82%]) and respiratory failure (n = 90 [7%]). The median length of stay was 3 days (IQR, 2-6), during which the median Sequential Organ Failure Assessment score was 3.2 (IQR, 1.5-6.0). Twenty-eight–day mortality was 17%, of which the majority died during ICU stay (443/618 [72%]). Approximately 81% of all patients who survived the ICU stay at 28-day follow-up were discharged from the hospital (eTable 7 in Supplement 1).
RBC Transfusion
The proportion of patients who received a transfusion ranged from 0% to 100% across centers: no transfusion was administered in 22 centers from 11 countries, whereas all patients received a transfusion in 14 centers from 8 countries. A total of 894 patients (25%) received 1 or more RBC transfusions during their ICU stay (Table 2). A total of 1727 transfusion events were recorded, with a median of 2 events (IQR, 1-4) per patient and a median of 1 RBC unit (IQR, 1-2) per event. In addition, a patient who underwent transfusion received a median of 0.5 RBC units (IQR, 0.25-1) per day spent in the ICU, adding up to a total of 2 units (IQR, 1-4) during their ICU stay.
Table 2. Characteristics During Intensive Care Unit Stay.
| Variable | Transfusion (n = 894)a | No transfusion (n = 2749)a |
|---|---|---|
| Daily forms | 4 (3-9) | 3 (2-5) |
| Mean daily blood loss, mL | 82 (0-275) | 0 (0-46) |
| Median SOFA score during ICU stayb | 5 (2.5-7.85) | 3 (1.40-5.33) |
| Invasive mechanical ventilation during ICU stay | 537 (60.1) | 980 (35.6) |
| Kidney replacement therapy during ICU stay | 142 (15.9) | 149 (5.4) |
| Newly developed complications during ICU stay | ||
| Acute kidney injury | 260 (29.1) | 351 (12.8) |
| Sepsis | 257 (28.7) | 495 (18.0) |
| ARDS | 141 (15.8) | 370 (13.5) |
| Gastrointestinal bleed | 114 (12.8) | 45 (2) |
| Failure to wean | 104 (11.6) | 117 (4.3) |
| Acute coronary syndrome | 95 (10.6) | 223 (8.1) |
| Liver failure | 86 (10) | 80 (2.9) |
| Cerebrovascular event | 62 (7) | 222 (8.1) |
| Bone marrow failure | 27 (3) | 13 (<1) |
| Retinal hemorrhage | 6 (<1) | 4 (<1) |
| Admission laboratory values | ||
| Hb level, g/dL | 10.0 (2.7) | 12.5 (2.3) |
| Anemiac | ||
| Male | 385/543 (70.9) | 708/1724 (41.1) |
| Female | 238/351 (67.8) | 415/1025 (40.5) |
| Platelet count, ×109/L | 205 (133-283) | 227 (170-291) |
| Laboratory values during ICU stay | ||
| Mean Hb level during ICU stay | 8.7 (1.4) | 11.4 (2.0) |
| Nadir Hb | 7.6 (1.6) | 10.8 (2.1) |
| Anemia during ICU stayc | 882/891 (99.0) | 2162/2697 (80.2) |
| Received RBC | 894 (100) | 0 |
| No. of RBC units transfused, total during ICU stay | 2 (1-4) | |
| No. of RBC units transfused per transfused dayd | 1 (1-1.5) | |
| No. of RBC units per ICU daye | 0.5 (0.25-1) | |
| No. of RBC transfusion events | 2 (1-4) | |
| Received platelet transfusion | 163 (18.2) | 45 (2) |
| Received plasma transfusion | 254 (28.4) | 83 (3) |
| Received massive transfusion protocol | 34 (4) | 0 (0) |
| Received coagulation product | 147 (16.4) | 61 (2) |
| Use of VHA, No. of patients | 91 (10) | 53 (2) |
| EPO administered | 35 (4) | 43 (2) |
Abbreviations: ARDS, acute respiratory distress syndrome; EPO, erythropoietin; Hb, hemoglobin; ICU, intensive care unit; RBC, red blood cell; SOFA, Sequential Organ Failure Assessment; VHA, viscoelastic hemostatic assay.
Presented as No. (%) for frequency data, median (shown first-third quartile borders) for nonparametric, and mean (SD) for parametric numerical variables.
The SOFA score is a measure that reflects the course of in-ICU organ dysfunction, expressed by a 0-24 range, in which severe organ dysfunction is reflected by a higher score.
Anemia defined as <12 g/dL for females and <13 g/dL for males, according to the definition by the World Health Organization.
Calculated as the sum of RBC transfused divided by the days a transfusion was administered per patient.
Calculated as the sum of RBC transfused divided by the ICU length of stay.
Of the postsurgical patients, 31% received an RBC transfusion with a median total of 2 units (IQR, 1-4), whereas 20% of the non-postsurgical patients underwent transfusion, receiving a median total of 2 units (IQR, 1-3) (eTable 8 in Supplement 1). The daily proportion of patients who received transfusion ranged from 11% (n = 416/3643) on admission to 5% on day 5 (n = 46/973) (eFigure 2 in Supplement 1). Most transfusions were administered during daytime hours (n = 1063 [63%]).
The proportion of patients transfused ranged from 0% to 80% across countries and from 19% to 45% across continents. The highest transfusion incidence was in Africa (45% of patients), whereas the highest numbers of events and units per patient were in South America (1 event [IQR, 1-6], 4 units [IQR, 3-6] per patient; eTable 9 in Supplement 1).
An overview of clinical reasons and physiological triggers is presented in Table 3. Across all patients, the main stated clinical reasons for transfusion were low Hb level in 81.8%, active bleeding in 27.7%, and hemodynamic instability in 23.5%. The main stated physiological triggers were hypotension in 42.2%, tachycardia in 27.4%, and increased lactate level in 17.8%. In 39.5% of transfusion events, no physiological trigger was cited to support the decision to transfuse.
Table 3. Characteristics of RBC Transfusion Events.
| Variable | Total RBC events (n = 1727)a |
|---|---|
| Time of administration during daytime hours (07:30 am-6:00 pm) | 1063 (62.9) |
| Certification level of transfusion requestor | |
| Intensivist | 1055 (61.1) |
| Resident, specialist in training | 326 (18.8) |
| Specialist nonintensivist practicing ICU | 252 (14.6) |
| Specialist nonintensivist outside ICU | 74 (4) |
| Nurse | 12 (<1) |
| Other | 8 (<1) |
| Student | 0 |
| Primary medical specialty of transfusion requestor | |
| Anesthesiology | 818 (47.7) |
| Intensivistb | 390 (22.7) |
| Internal medicine | 236 (13.8) |
| Surgery | 180 (10.5) |
| Pulmonology | 48 (3) |
| Cardiology | 27 (2) |
| Other (ie, emergency medicine, neurology) | 17 (1) |
| No. of RBC units per event, median (IQR) | 1 (1-2) |
| Hemoglobin levels | |
| Threshold predefined, median (IQR), g/dLc | 8.0 (7.0-9.0) |
| Restrictive (<7 g/dL) | 299 (17.3) |
| Intermediate (7-9 g/dL) | 430 (24.9) |
| Liberal (>9 g/dL) | 459 (26.6) |
| No threshold stated | 539 (31.2) |
| Before transfusion, g/dLd | 7.7 (1.6) |
| Post transfusion, g/dLe | 9.1 (1.5) |
| Hb level increase after transfusion, median (IQR), g/dLf | 1.2 (0.7-2.0) |
| Change in Hb level (between Hb prior and stated Hb threshold), g/dLg | −0.7 (1.5) |
| As part of massive transfusion protocol | 43 (3) |
| Whole blood (no isolated RBC available) | 54 (3) |
| Reason for RBC transfusion (multiple options possible)h | |
| Low Hb value | 1412 (81.8) |
| Active bleeding | 479 (27.7) |
| Hemodynamic instability | 406 (23.5) |
| Improving general state | 142 (8.2) |
| Improving peripheral oxygen | 115 (6.7) |
| (New) coronary ischemia | 69 (4) |
| Presurgery | 67 (4) |
| Age of patient | 60 (4) |
| Other | 47 (3) |
| Improving weaning | 43 (3) |
| Part of clinical trial | 6 (<1) |
| Exchange transfusion | 2 (<1) |
| No. of reasons | 1 (1-2) |
| Trigger for RBC transfusion (multiple options possible)h | |
| Hypotension | 728 (42.2) |
| No physiological trigger affected the decision to transfuse | 682 (39.5) |
| Tachycardia | 474 (27.4) |
| Increased lactate levels (>2 mmol/L) | 308 (17.8) |
| Acidosis | 204 (11.8) |
| Arrhythmia | 70 (4) |
| Other | 66 (4) |
| Venous oxygen saturation <65% | 36 (2) |
| Central venous oxygen saturation <65% | 30 (2) |
| ECG changes | 23 (1) |
| No. of triggers | 1 (1-2) |
Abbreviations: ECG, electrocardiogram; ED, emergency department; Hb, hemoglobin; ICU, intensive care unit; RBC, red blood cell.
Presented as No. (%) for frequency data, median (IQR shown 1st-3rd quartile borders) for non-parametric and mean (SD) for parametric numerical variables.
Intensivist training differs worldwide; whereas in some countries, training starts with another medical specialty (ie, surgery, anesthesiology, internal medicine), some countries offer a special intensivist training program. The latter is hereby noted as intensivist.
The Hb value at which an RBC transfusion is advised as stated in the ICU’s protocol or, in the absence of a protocol, the expert’s opinion for that patient.
The most recent Hb value before the RBC transfusion was administered, within 4 hours prior to the decision to transfuse.
The first Hb level measured after RBC transfusion within 24 hours.
Calculated as the difference between the measured Hb level before and after RBC transfusion.
Calculated as the difference between the measured Hb level before and the Hb threshold. A negative change in Hb level may insinuate protocol adherence (ie, the measured Hb level was below the stated threshold), whereas in case of a positive value, the threshold had not yet been reached.
Factors that contributed to the decision to transfuse, consisting of either clinical reasoning (reasons) or physiological parameters (triggers).
By continent, the main stated clinical reasons for transfusion were low Hb value, active bleeding, and hemodynamic instability in all regions except Africa, where 33% of RBC transfusions were listed to improve the patient’s general state. The main stated physiological triggers were hypotension and tachycardia in all continents but South America, where increased lactate level (>2 mmol/L) was one of the main physiological triggers (39%) listed for RBC transfusion. The highest number of reasons per transfusion event were listed in Africa and North America (2 [IQR, 1-3]), whereas the highest number of triggers per event were listed in South America (2 [IQR, 0-2]) (eTable 10 and eFigure 3 in Supplement 1).
Course and Thresholds of Hemoglobin
At ICU admission, 60% of the patients had anemia (<12 g/dL for female [n = 653 {60%}] and <13 g/dL for male [N = 1093 {60%}] patients; n = 1746/2917). During their ICU stay, this increased to 85% (n = 3044/3588). Almost all patients receiving an RBC transfusion had anemia during their ICU stay (n = 882/891 [99%]). Patients who received a transfusion, compared with those who did not, had a lower Hb level on admission and lower mean and lower nadir Hb during their ICU stay (all P < .001).
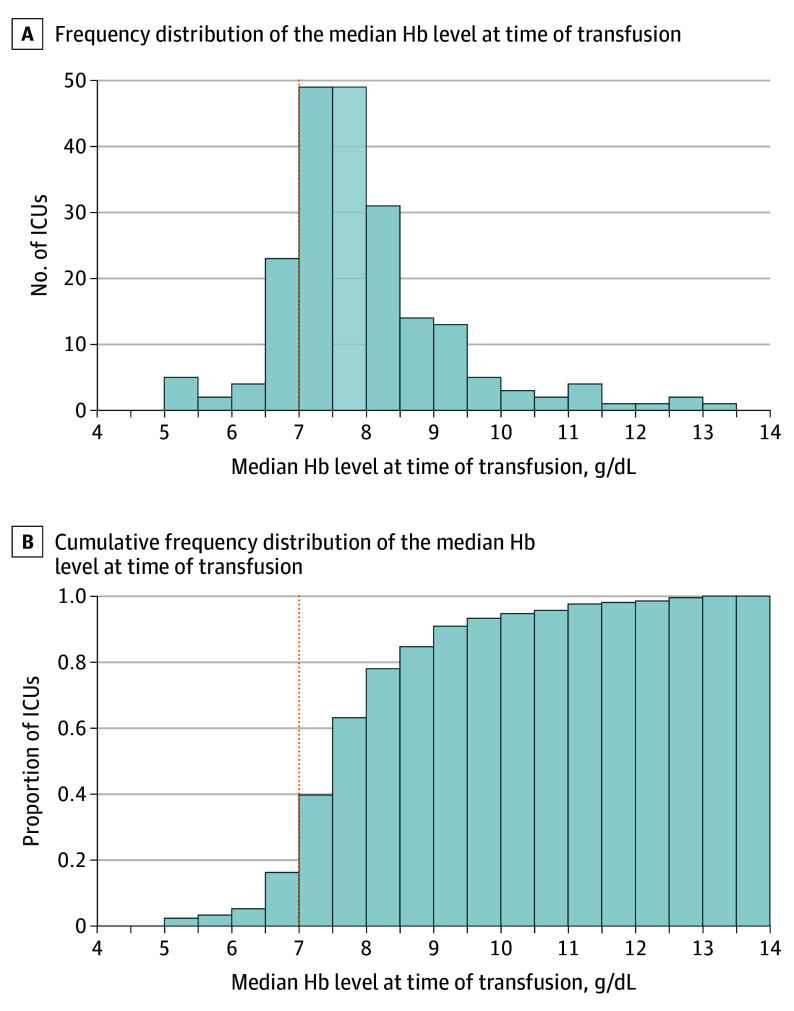
The Figure presents the median Hb level at the time of transfusion across centers, which ranged from 5.2 g/dL to 13.1 g/dL. As visualized in panel B, around 16% of ICUs recorded a median Hb level below 7 g/dL at the time of transfusion. Median pretransfusion Hb level ranged from 5.3 g/dL to 9.1 g/dL across countries and from 7.2 g/dL to 8.7 g/dL across continents (eFigure 4 in Supplement 1). Across all RBC transfusion events, the median Hb level increase after transfusion was 1.2 g/dL (IQR, 0.7-2.0). For the events in which low Hb level was a reason to transfuse, the mean (SD) lowest measured Hb level before transfusion was 7.4 (1.2) g/dL.
Figure. Median Hemoglobin (Hb) Level Before Red Blood Cell (RBC) Transfusion in Patients Undergoing Transfusion During Intensive Care Unit (ICU) Stay.
For patients with multiple RBC transfusions, the mean of the pretransfusion Hb level across different transfusions was calculated. In panels A and B, the dotted vertical line at 7 g/dL represents the current guideline for RBC transfusion. Approximately 84% of ICUs transfused their patients at a median Hb level above 7 g/dL.
In 81% of the transfusion events, independent of the indication to transfuse, the Hb level before transfusion was below or equal to the stated threshold (n = 963/1188). Among the events with a stated transfusion threshold (n = 1188), the mean (SD) Hb level before transfusion was 8.5 (1.5) g/dL within a liberal threshold (n = 459) and 6.95 (1.4) g/dL within a restrictive threshold (n = 299). The proportion of events with a restrictive threshold among the regions was highest in North America (89%) and Europe (24%). Further, among those threshold categories, the same top 3 clinical reasons and physiological triggers for transfusion were reported (eTable 11 and eFigure 5 in Supplement 1).
Discussion
In this prospective, international study conducted in 233 ICUs across 30 countries on 6 continents, 25% of patients received 1 or more RBC transfusions during their ICU stay. A wide range in transfusion occurrence rates was found across centers, countries, and continents. Although many different clinical reasons and triggers were stated for RBC transfusion, the 3 most common reasons (low Hb level, active bleeding, hemodynamic instability) and triggers (hypotension, tachycardia, no physiological trigger affected the decision to transfuse) were largely overlapping in all regions. There was also considerable variation between centers in the lowest Hb level before RBC transfusion.
The incidence of RBC transfusion reported in this study is in line with the results of the ICON study, which was performed over a decade ago and demonstrated an in-ICU transfusion rate of 26%.13 Although a decreasing trend in transfusion rates had been described earlier, the similarity between the ICON study and the current results could imply that this trend has reached a plateau.14 An explanation for the initial decrease could be increased awareness of possible adverse effects or the uptake of evidence generated in several large RCTs. The extensive number of reasons and triggers stated for transfusion, and the high variance in Hb levels at the time of transfusion, confirms the existing heterogeneity in transfusion behavior worldwide. It may be the case that harmonizing guidelines and education could further decrease RBC transfusion. Of note, in the course of this study, 2 guidelines were published by the European Society of Intensive Medicine on transfusion management in bleeding and nonbleeding patients, of which the degree of implementation cannot be assessed in the current results.8,9
A low Hb level was the most commonly stated reason for RBC transfusion. In recent decades, multiple RCTs have concluded that using a restrictive Hb threshold to trigger an RBC transfusion is safe in different patient populations.3,5,6,15 Compared with the ICON study, the Hb value before RBC transfusion in the current study was lower and had a smaller standard deviation (mean [SD], ICON: 8.3 [2.2] g/dL vs InPUT: 7.7 [1.6] g/dL).13 Previous surveys showed a wide variance in the Hb threshold stated by physicians working in the ICU.16,17 In the present study, substantial between-center variation in the mean lowest Hb level was observed on the day of a transfusion and in the proportion of patients that received a transfusion.
There is ongoing discussion about the appropriateness and feasibility of individualized RBC transfusion thresholds and triggers.18,19,20 Although a wide range of markers and methods have been studied, inconclusive results for their use limit the development of an optimal personalized approach. The current study observed a wide range of stated clinical reasons and physiological triggers for transfusion. The European Society of Intensive Medicine transfusion guidelines advise to use Hb or hematocrit over alternative triggers, such as venous oxygen saturation, in nonbleeding patients, whereas in bleeding patients, alternative triggers and reasons are not mentioned at all. The absence of alternative triggers and reasons is consistent with other international guidelines.7,21 In this context, it is noteworthy that most transfusion events were at least partly motivated by 1 or more physiological (ie, non-Hb) triggers.
RBC transfusion has been linked to mortality, which has been used as an end point in most RCTs.5,6,15,22,23 In addition, observational studies have reported an increased risk of mortality in patients with severe anemia without transfusing RBCs.24 In the current study cohort, the mortality rate aligned with other large cohorts of ICU patients.25 Although it was found that a larger proportion of the patients who received a transfusion had died by day 28, this should not be interpreted to mean that receiving a transfusion led to a higher probability of death, as potential confounders were not corrected for. Previous studies reported conflicting results for the association between transfusion and mortality, which may also differ between subgroups.13,26,27 This research question is beyond the scope of this article, but these and other subquestions will be studied in the future (eMethods 2 in Supplement 1).
To our knowledge, this study is one of the largest prospective cohorts designed to study this topic to date and the first study to provide an extensive overview of clinical reasons and physiological triggers that were used in the decision to transfuse. It was performed in an international setting with a wide range of centers and countries. Due to the inclusion of all patients admitted to the ICU and a very high eligibility-to-inclusion ratio, selection bias was low.
Limitations
This study had several limitations. First, although international recruitment took place, the majority of centers that participated reflect middle- to high-income countries, which potentially limits the generalizability to lower-income countries. Second, transfusion variables were only collected for patients who received 1 or more transfusions. Therefore, exact transfusion thresholds as stated in the center’s protocol for patients who did not receive a transfusion were unknown. Third, no data were collected regarding racial and ethnic composition of the population. Fourth, during data collection, the COVID-19 pandemic started. Although no center reported a resulting scarcity of blood products nor COVID-19 as a reason (not) to transfuse, additional post hoc analyses on the influence of the pandemic on transfusion behavior was not able to be performed. Fifth, no hospital characteristics, including hospital type (academic vs peripheral) and number of beds, were collected.
Conclusions
Among an international sample of patients admitted to ICUs from 2019 to 2022, RBC transfusion was common, with variability across centers in transfusion occurrences and indications.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
eTable 1. Steering Committee
eTable 2. National Coordinators
eTable 3. Participating Centers
eTable 4. Inclusion Weeks
eMethods 1. Data Cleaning, Missing Data Patterns and Prerequisites
eTable 5. Missing Data Frequencies in Cleaned Dataset, Stratified per Continent
eFigure 1. Flowchart
eTable 6. Details Participating Countries
eTable 7. Patient Outcomes Stratified by Transfusion Status
eTable 8. Post-Surgical vs. Non-Post-Surgical Patients
eFigure 2. Absolute Count and Proportion Receiving an RBC Transfusion per Admission Day
eTable 9. Daily + Transfusion Details per Continent
eTable 10. Transfusion Events per Continent
eFigure 3. Reasons and Triggers per Continent
eFigure 4. Hemoglobin Before Transfusion in Transfused Patients, by Continent
eTable 11. Transfusion Reasons + Triggers as Stratified per Threshold
eFigure 5. Reasons and Triggers per Threshold
eMethods 2. Predefined Sub-questions and Secondary Analyses
Trial Protocol
Nonauthor Collaborators. InPUT Study Group
Data Sharing Statement
References
- 1.Free RJ, Sapiano MRP, Chavez Ortiz JL, Stewart P, Berger J, Basavaraju SV. Continued stabilization of blood collections and transfusions in the United States: findings from the 2021 National Blood Collection and Utilization Survey. Transfusion. 2023;(January):1-11. doi: 10.1111/trf.17360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosboom JJ, Klanderman RB, Zijp M, et al. Incidence, risk factors, and outcome of transfusion-associated circulatory overload in a mixed intensive care unit population: a nested case-control study. Transfusion. 2018;58(2):498-506. doi: 10.1111/trf.14432 [DOI] [PubMed] [Google Scholar]
- 3.Mazer CD, Whitlock RP, Fergusson DA, et al. ; TRICS Investigators and Perioperative Anesthesia Clinical Trials Group . Restrictive or liberal red-cell transfusion for cardiac surgery. N Engl J Med. 2017;377(22):2133-2144. doi: 10.1056/NEJMoa1711818 [DOI] [PubMed] [Google Scholar]
- 4.Murphy GJ, Pike K, Rogers CA, et al. ; TITRe2 Investigators . Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372(11):997-1008. doi: 10.1056/NEJMoa1403612 [DOI] [PubMed] [Google Scholar]
- 5.Ducrocq G, Gonzalez-Juanatey JR, Puymirat E, et al. ; REALITY Investigators . Effect of a restrictive vs liberal blood transfusion strategy on major cardiovascular events among patients with acute myocardial infarction and anemia: the REALITY randomized clinical trial. JAMA. 2021;325(6):552-560. doi: 10.1001/jama.2021.0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care: Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417. doi: 10.1056/NEJM199902113400601 [DOI] [PubMed] [Google Scholar]
- 7.Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316(19):2025-2035. doi: 10.1001/jama.2016.9185 [DOI] [PubMed] [Google Scholar]
- 8.Vlaar AP, Oczkowski S, de Bruin S, et al. Transfusion strategies in non-bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. 2020;46(4):673-696. doi: 10.1007/s00134-019-05884-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlaar APJ, Dionne JC, de Bruin S, et al. Transfusion strategies in bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. 2021;47(12):1368-1392. doi: 10.1007/s00134-021-06531-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Bank Country and Lending Groups . The World Bank. Accessed August 28, 2023. https://data.worldbank.org/
- 11.de Bruin S, Alders MY, van Bruggen R, et al. ; Cardiovascular Dynamics Section and Transfusion Task Force of the ESICM; Collaborators . International point prevalence study of Intensive care unit transfusion practices: pilot study in the Netherlands. Transfus Clin Biol. 2019;26(4):202-208. doi: 10.1016/j.tracli.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 12.The R Project for Statistical Computing . The R Project for Statistical Computing. Accessed October 3, 2023. http://www.r-project.org/
- 13.Vincent JL, Jaschinski U, Wittebole X, et al. ; ICON Investigators . Worldwide audit of blood transfusion practice in critically ill patients. Crit Care. 2018;22(1):102. doi: 10.1186/s13054-018-2018-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavalcante Dos Santos E, Bakos P, Vincent JL. How have red blood transfusion practices changed in critically ill patients? a comparison of the ICON and ABC studies conducted 13 years apart. Transfusion. 2020;60(12):2801-2806. doi: 10.1111/trf.16048 [DOI] [PubMed] [Google Scholar]
- 15.Holst LB, Haase N, Wetterslev J, et al. ; TRISS Trial Group; Scandinavian Critical Care Trials Group . Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371(15):1381-1391. doi: 10.1056/NEJMoa1406617 [DOI] [PubMed] [Google Scholar]
- 16.de Bruin S, Scheeren TWL, Bakker J, van Bruggen R, Vlaar APJ; Cardiovascular Dynamics Section and Transfusion Guideline Task Force of the ESICM . Transfusion practice in the non-bleeding critically ill: an international online survey-the TRACE survey. Crit Care. 2019;23(1):309. doi: 10.1186/s13054-019-2591-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Bruin S, Eggermont D, van Bruggen R, et al. ; Cardiovascular Dynamics Section and Transfusion Task Force of the ESICM . Transfusion practice in the bleeding critically ill: an international online survey: the TRACE-2 survey. Transfusion. 2022;62(2):324-335. doi: 10.1111/trf.16789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holst LB, Carson JL, Perner A. Should red blood cell transfusion be individualized? no. Intensive Care Med. 2015;41(11):1977-1979. doi: 10.1007/s00134-015-3948-1 [DOI] [PubMed] [Google Scholar]
- 19.Docherty A, Walsh TS. Should blood transfusion be individualised? we are not sure. Intensive Care Med. 2015;41(11):1980-1982. doi: 10.1007/s00134-015-4034-4 [DOI] [PubMed] [Google Scholar]
- 20.Sakr Y, Vincent JL. Should red cell transfusion be individualized? yes. Intensive Care Med. 2015;41(11):1973-1976. doi: 10.1007/s00134-015-3950-7 [DOI] [PubMed] [Google Scholar]
- 21.Tibi P, McClure RS, Huang J, et al. STS/SCA/AmSECT/SABM update to the clinical practice guidelines on patient blood management. Ann Thorac Surg. 2021;112(3):981-1004. doi: 10.1016/j.athoracsur.2021.03.033 [DOI] [PubMed] [Google Scholar]
- 22.Carson JL, Terrin ML, Noveck H, et al. ; FOCUS Investigators . Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462. doi: 10.1056/NEJMoa1012452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergamin FS, Almeida JP, Landoni G, et al. Liberal versus restrictive transfusion strategy in critically III oncologic patients: the transfusion requirements in critically III oncologic patients randomized controlled trial. Crit Care Med. 2017;45(5):766-773. doi: 10.1097/CCM.0000000000002283 [DOI] [PubMed] [Google Scholar]
- 24.Shander A, Javidroozi M, Naqvi S, et al. An update on mortality and morbidity in patients with very low postoperative hemoglobin levels who decline blood transfusion (CME). Transfusion. 2014;54(10, pt 2):2688-2695. doi: 10.1111/trf.12565 [DOI] [PubMed] [Google Scholar]
- 25.Vincent JL, Sakr Y, Singer M, et al. ; EPIC III Investigators . Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478-1487. doi: 10.1001/jama.2020.2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincent JL, Baron J-F, Reinhart K, et al. ; ABC (Anemia and Blood Transfusion in Critical Care) Investigators . Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499-1507. doi: 10.1001/jama.288.12.1499 [DOI] [PubMed] [Google Scholar]
- 27.Corwin HL, Gettinger A, Pearl RG, et al. The CRIT Study: anemia and blood transfusion in the critically ill—current clinical practice in the United States. Crit Care Med. 2004;32(1):39-52. doi: 10.1097/01.CCM.0000104112.34142.79 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Steering Committee
eTable 2. National Coordinators
eTable 3. Participating Centers
eTable 4. Inclusion Weeks
eMethods 1. Data Cleaning, Missing Data Patterns and Prerequisites
eTable 5. Missing Data Frequencies in Cleaned Dataset, Stratified per Continent
eFigure 1. Flowchart
eTable 6. Details Participating Countries
eTable 7. Patient Outcomes Stratified by Transfusion Status
eTable 8. Post-Surgical vs. Non-Post-Surgical Patients
eFigure 2. Absolute Count and Proportion Receiving an RBC Transfusion per Admission Day
eTable 9. Daily + Transfusion Details per Continent
eTable 10. Transfusion Events per Continent
eFigure 3. Reasons and Triggers per Continent
eFigure 4. Hemoglobin Before Transfusion in Transfused Patients, by Continent
eTable 11. Transfusion Reasons + Triggers as Stratified per Threshold
eFigure 5. Reasons and Triggers per Threshold
eMethods 2. Predefined Sub-questions and Secondary Analyses
Trial Protocol
Nonauthor Collaborators. InPUT Study Group
Data Sharing Statement