Abstract
Heat stress can cause systemic immune dysregulation and threaten the health of broilers. Guanidinoacetic acid (GAA) has been shown to be effective against heat stress, but whether it is beneficial for immunity is unclear.
Therefore, the effects of dietary GAA supplementation on the immunity of chronic heat-stressed broilers were evaluated. A total of 192 Arbor Acres male broilers (28-day old) were randomly allocated to 4 treatments: the normal control group (NC, 22°C, ad libitum feeding), the heat stress group (HS, 32°C, ad libitum feeding), the pair-fed group (PF, kept at 22°C and received food equivalent to that consumed by the HS group on the previous day), and the GAA group (HG, 32°C, ad libitum feeding; basal diet supplemented with 0.6 g/kg GAA). Samples were collected on d 7 and 14 after treatment. Results showed that broilers exposed to heat stress exhibited a decrease (P < 0.05) in ADG, ADFI, thymus and bursa of Fabricius indexes, and an increase (P < 0.05) in feed conversion ratio and panting frequency, compared to the NC group. Levels of corticotropin-releasing factor, corticosterone (CORT), heat shock protein 70 (HSP70), IL-6, and TNF-α were elevated (P < 0.05) while lysozyme and IgG concentration was decreased (P < 0.05) in the HS group compared with the NC group after 7 d of heat exposure. The concentrations of IgG and IL-2 were decreased (P < 0.05) and CORT was increased (P < 0.05) in the HS group compared with the NC group after 14 d of heat exposure. Noticeably, GAA supplementation decreased the levels of CORT (P < 0.05) and increased the IL-2, IgG, and IgM concentrations (P < 0.05) compared with the HS group. In conclusion, chronic heat stress increased CORT release, damaged immune organs, and impaired the immunity of broilers. Dietary supplementation of 0.6 g/kg GAA can reduce the CORT level and improve the immune function of broilers under heat stress conditions.
Key words: chronic heat stress, guanidinoacetic acid, corticosterone, immunity, broiler
INTRODUCTION
Broilers are highly susceptible to heat stress induced by high ambient temperatures as they cannot dissipate heat efficiently due to their surface covering of feathers and lacking skin sweat glands. In light of global warming, this problem has grown to be a significant barrier to broiler production (Chauhan et al., 2021; Liu et al., 2021). Studies have shown that physiological disorders, such as systemic immune dysregulation, occur in broilers exposed to high temperatures, and consequently lead to poor growth and increased mortality of broilers (Quinteiro-Filho et al., 2012; Abdelqader and Al-Fataftah, 2014). Heat stress-induced intestinal barrier injury promotes the release of endotoxin from commensal bacteria into the blood (Alhenaky et al., 2017), which elevates the levels of proinflammatory cytokines (De Boever et al., 2008; Liu et al., 2021), challenging the weakened immune system of broilers. Therefore, finding a solution for chronic heat stress is crucial.
The hypothalamus-pituitary-adrenal (HPA) axis plays a vital role in stimulating and integrating various physiological and neural responses to adverse stimuli (Wang et al., 2022). When heat serves as a stressor, it initiates the synthesis of corticotropin-releasing factor (CRF) or hormone from the paraventricular nucleus of the hypothalamus, which acts on the anterior pituitary to promote the release of adrenocorticotropic hormone (ACTH). Subsequently, ACTH activates adrenocortical cells, leading to the synthesis and release of glucocorticoids (GCs). In chickens, the primary glucocorticoid is corticosterone (CORT) (Beckford et al., 2020). Chronic stimulation of the HPA axis has been shown to slow animal growth and suppress immune responses, thus making them more susceptible to infection (Bagath et al., 2019; Nelson et al., 2020). In addition, during chronic heat stress, ATP generation is reduced (Azad et al., 2010). High circulating CORT shifts metabolic processes toward catabolism (Nelson et al., 2020), promoting the utilization of muscle energy reserves in the form of glycogen (Gonzalez-Esquerra and Leeson, 2006). It also suppresses muscle growth and protein synthesis while simultaneously elevating liver gluconeogenesis to meet energy demands (Ma et al., 2021).
Nutritional intervention is considered an effective strategy for alleviating organ damage caused by heat stress in broilers (Chauhan et al., 2021). Guanidinoacetic acid (GAA) has been authorized for use as a nutritional additive in commercial diets in several regions around the world (Portocarero and Braun, 2021). GAA has been shown to improve feed conversion (Majdeddin et al., 2020), intestinal morphology, and mucosal barrier function (Peng et al., 2023) in broilers subjected to heat stress. Amiri et al. (2019) reported that GAA supplementation at a rate of 0.06% could be an effective feed additive for preventing the negative effects of heat stress on growth performance, mortality rate, antioxidant status, and gut morphology in broilers. The beneficial effects of GAA on heat stress are primarily due to improved cellular energy status and arginine-sparing functions (Majdeddin et al., 2020, 2023). GAA is the precursor of creatine (Cr), a high-energy molecule that generates ATP through the creatine/phosphocreatine system (Dayan et al., 2023). Dietary GAA supplementation enhances muscle accumulation of Cr and PCr, allowing creatine-loaded muscles to increase growth or work capacity (Michiels et al., 2012; Zhang et al., 2019). GAA effectively reduces the metabolic drain on arginine (Arg) and glycine (Gly) for Cr synthesis, leaving more Arg available for muscle growth and other important physiological processes (DeGroot et al., 2019; Portocarero and Braun, 2021). Arginine supplementation is widely reported to modulate or enhance humoral and cellular immune responses (Jahanian, 2009; Munir et al., 2009; Burin Junior et al., 2019) and attenuate inflammation (Tan et al., 2014). Importantly, Arg plays a key role as a nitrogenous precursor for the endogenous synthesis of nitric oxide (NO). In this regard, NO has been shown to act via the central nervous system to regulate body temperature, stimulate peripheral blood flow, and promote autonomic heat dissipation in broilers (Wu et al., 2010; Uyanga et al., 2020).
Altogether, given the beneficial effects of GAA described above, this study aimed to examine the effectiveness of GAA supplementation in mediating chronic heat stress through its regulatory functions on the HPA axis hormones and immunity.
MATERIALS AND METHODS
Experimental Design
All experimental procedures and bird management were approved by Nanjing Agricultural University Institutional Animal Care and Use Committee under protocol number SYXK 2021-0014.
A total of 192 twenty-eight-day-old Arbor Acres male broilers (body weight, 1,442.3 ± 2.8 g) were used in this study and were randomly allocated to 4 treatments: the normal control group (NC, kept at 22°C), the heat stress group (HS, kept at 32°C), the pair-fed group (PF, kept at 22°C and received food equivalent to that consumed by the HS group on the previous day), and the GAA group (HG, kept at 32°C). GAA group given a basal diet supplemented with 0.6 g/kg GAA (Michiels et al., 2012; Amiri et al., 2019; Peng et al., 2023). Each group included 6 replicates with 8 birds per cage (1.2 m × 0.8 m × 0.45 m). The chickens were reared in the environment control rooms for 7 or 14 d at 22°C or 32°C, 24 h/d. The lighting was programmed to produce 18 h of light and 6 h of darkness throughout the experimental period. The GAA (Purity ≥ 99%) additive was acquired from Tianjin Tiancheng Pharmaceutical Co., Ltd. (Tianjin, China). The basal diet composition and nutrient levels are shown in Table 1. In addition to the restricted feed intake of chickens in the PF group, the rest of the birds had ad libitum access to feed and water throughout the study period.
Table 1.
Ingredients and nutrient composition of experimental diets.
| Ingredients (%) | 28-42 d |
|---|---|
| Corn | 59.37 |
| Soybean meal1 | 31.90 |
| Soybean oil | 5.00 |
| Limestone | 1.23 |
| Dicalcium phosphate | 1.50 |
| L-lysine·HCl | 0.11 |
| DL-methionine | 0.27 |
| Salt | 0.30 |
| Vitamin premix2 | 0.03 |
| Mineral premix3 | 0.20 |
| 70% Choline chloride | 0.09 |
| Calculated nutrients | |
| Metabolizable energy (MJ/kg) | 12.98 |
| Crude protein | 19.00 |
| Calcium | 0.90 |
| Total phosphorus | 0.56 |
| Nonphytate phosphorus | 0.35 |
| Lysine | 1.00 |
| Methionine | 0.46 |
| Methionine + cysteine | 0.80 |
| Threonine | 0.60 |
| Tryptophan | 0.20 |
| Arginine | 1.32 |
Crude protein level of soybean meal: 44.2%.
Vitamin premix provided per kilogram of diet: Vitamin A, 12,000 IU; vitamin D3, 2,500 IU; vitamin E, 20 IU; menadione, 1.3 mg; thiamin, 2.21 mg; riboflavin, 7.8 mg; nicotinamide, 40 mg; calcium pantothenate, 16.5 mg; pyridoxine HCl, 4 mg; biotin, 0.04 mg; folic acid, 1.2 mg; vitamin B12, 0.015 mg.
Mineral premix provided per kilogram of diet: iron, 80 mg; copper, 8.0 mg; manganese, 110 mg, zinc 65 mg; iodine, 1.1 mg; selenium, 0.3 mg.
Growth Performance and Sample Collection
Body weight and feed consumption were recorded at d 28, 35, and 42. The average daily gain (ADG), average daily feed intake (ADFI), and the feed-to-gain ratio (F/G) were calculated. On d 35 and 42, video of each cage was recorded for the measurement of panting frequency, and then 2 birds per replicate with weight close to average weight of the cage was selected and euthanized by electrical stunning and exsanguinations (Peng et al., 2023). Blood samples were immediately collected from the jugular vein of each bird, loaded into heparinized tubes, and centrifuged for 15 min at 1,500 × g. The plasma samples were collected and stored at −20°C for subsequent analysis of CRF, ACTH, CORT, heat shock protein 70 (HSP70), immunoglobulin A (IgA), IgG, IgM, interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), IL-2, and lysozyme. The spleen, thymus, and bursa of Fabricius were weighted to calculate the organ index.
Panting Frequency
Panting frequency defined as the number of breaths per minute. Panting frequency was assessed for 2 birds randomly selected by each cage during 1 min interval, based on video recordings.
Detection of Plasma HPA Axis Hormone and HSP70
The plasma levels of CRF (H288-1), ACTH (H097-1), CORT (H205-1), and HSP70 (H264-2) were measured using commercial ELISA kits (Nanjing Jiancheng Bioengineering Institute Co., Ltd., Nanjing, China) following the individual manufacturer's protocols. In brief, the procedure can be summarized as follows: Preparing of reagents, sample and standards; add prepared plasma samples and standards 50 μL, Bio Ab reacting 30 min at 37°C; plate washed 5 times, adding HRP Conjugate Reagent 50 μL, reacting 30 min at 37°C; washed 5 times, adding Chromogen solution A, B 50 μL, reacting 10 min at 37°C; add stop solution 50 μL; measure the optical density values of each well under 450 nm wavelength within 10 min; according to the concentration and OD values, calculate the standard curve linear regression equation with apply ELISAcalc and apply logistic curve for fitting model (4 parameters). The main apparatus was an incubator of 37°C, and a standard enzyme reader.
Detection of Immune-Related Indicators and Lysozyme
IL-6 (H007-1), TNF-α (H052-1), IL-2 (H003-1), IgA (H108-1), IgG (H106-1), IgM (H109-1) were determined by ELISA (Nanjing Jiancheng Bioengineering Institute Co., Ltd., Nanjing, China). Lysozyme (A050-1) concentration was detected using a lysozyme assay kit (Nanjing Jiancheng Bioengineering Institute Co., Ltd., Nanjing, China). Lysozyme was detected as follows: The prepared application bacterial solution, the standard application solution, and the required samples were prewarmed to 37°C; add 0.2 mL sample to the tube, take 2 mL of application bacteria solution quickly into the tube, immediately mix and time; measure the transmittance value at 530 nm in the visible spectrophotometer, 15 s to read the transmittance value T0, at 2 min 15 s to read the transmittance value T2; calculate the differences in transmittance, △T(measured value) = T2 − T0. The main apparatus was a spectrophotometer.
C(standard) is the standard concentration and N is the sample dilution multiple.
Statistical Analysis
Statistical Analysis System with the SPSS (version 25.0, SPSS Inc., Chicago, IL, USA) was used to analyze the data for the NC, HS, and PF groups using 1-way analysis of variance and a Tukey multiple range test. T tests for independent samples were performed between the HS and HG groups, exploring the functions of GAA under condition of heat stress. Results are presented as mean ± standard error (SE) and the statistical significance was considered at P < 0.05. The data of growth performance were analyzed with the cage as the experimental replicate and other indices were analyzed by the mean of 2 sampled chickens per cage as a replicate (n = 6).
RESULTS
Growth Performance
The changes in growth performance of broilers caused by heat stress and the GAA intervention effects are presented in Table 2. After 7 d and 14 d of heat exposure, the ADFI of the chickens in the HS group significantly decreased (P < 0.05) compared to the NC group. The HS group had a lower (P < 0.05) ADG than the NC and PF groups after 7 d and 14 d of heat exposure. Additionally, heat exposure increased (P < 0.05) the F/G compared to the NC and PF groups after 7 and 14 d. The ADFI, ADG and F/G in the HG group did not differ (P > 0.05) from the HS group after 7 d and 14 d treatments.
Table 2.
Effects of heat stress on the growth performance and the guanidinoacetic acid (GAA) intervention effects in broilers.
| Treatment1 |
P value3 |
|||||
|---|---|---|---|---|---|---|
| Items2 | NC | HS | PF | HG | ANOVA | T test |
| Heat exposure for 7 d | ||||||
| ADFI (g/bird/d) | 148.38 ± 5.89a | 125.70 ± 5.40b | 129.22 ± 5.12b | 132.89 ± 2.59 | 0.027 | 0.277 |
| ADG (g/bird/d) | 86.56 ± 2.04a | 57.60 ± 4.84b | 76.57 ± 3.52a | 67.00 ± 3.22 | <0.001 | 0.365 |
| F/G (g/g) | 1.67 ± 0.03b | 2.22 ± 0.12a | 1.70 ± 0.02b | 1.90 ± 0.08 | <0.001 | 0.261 |
| Heat exposure for 14 d | ||||||
| ADFI (g/bird/d) | 168.06 ± 4.62a | 125.62 ± 4.87b | 128.66 ± 8.47b | 143.16 ± 4.90 | 0.001 | 0.727 |
| ADG (g/bird/d) | 87.68 ± 3.51a | 53.65 ± 3.08c | 70.37 ± 6.43b | 66.12 ± 3.67 | 0.001 | 0.640 |
| F/G (g/g) | 1.87 ± 0.04b | 2.43 ± 0.12a | 1.95 ± 0.06b | 2.17 ± 0.04 | 0.046 | 0.307 |
NC, normal control group; HS, heat stress group; PF, pair-fed group; HG, GAA group. Values are mean ± SE of 6 replicates.
F/G, feed to gain ratio.
One-way ANOVA and Tukey's test were conducted for the NC, HS, and HG groups; T test was conducted for the HS and HG groups.
Means with different superscripts within each row are significantly different among the NC, HS, and PF groups (P < 0.05).
Panting Frequency
Figure 1 illustrates the effects of heat stress on panting frequency and the GAA intervention effects in broilers. When compared to the NC and PF groups, the chickens in the HS group showed a significant increase in panting (P < 0.05) after 7 d and 14 d of treatment. In addition, the chickens in the HG group also experienced heavy panting, with no significant difference (P > 0.05) observed between the HG and HS groups.
Figure 1.
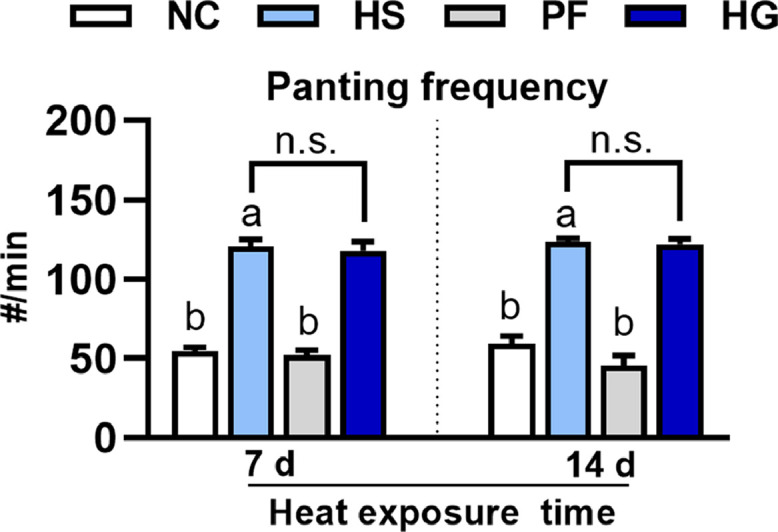
Effects of heat stress on the panting frequency and the GAA intervention effects in broilers. Data are presented as mean ± SE (n = 6). One-way ANOVA and Tukey's test were conducted for the NC, HS, and HG groups; T test was conducted for the HS and HG groups. a–bDifferent letters indicate significant difference among the NC, HS and PF groups (P < 0.05). n.s.means no significant.
Plasma HPA Axis Hormone Concentration
Table 3 presents the effects of heat stress on HPA axis hormone levels and the GAA intervention effects in broilers. In comparison to the NC group, the CRF level in the HS and PF groups was significantly increased (P < 0.05) after 7 d of heat exposure. The CORT levels were significantly elevated (P < 0.05) after 7 d and 14 d of heat exposure in the HS group, when compared to the NC and PF groups. However, after 7 and 14 d of treatment, the GAA treatment significantly decreased (P < 0.05) the CORT level in the HG group compared to the HS group.
Table 3.
Effects of heat stress on the level of hypothalamus-pituitary-adrenal (HPA) axis and the guanidinoacetic acid (GAA) intervention effects in broilers.
| Treatment1 |
P value3 |
|||||
|---|---|---|---|---|---|---|
| Items2 | NC | HS | PF | HG | ANOVA | T test |
| Heat exposure for 7 d | ||||||
| CRF (ng/mL) | 63.30 ± 6.42b | 101.77 ± 8.26a | 117.27 ± 7.38a | 114.82 ± 8.28 | <0.001 | 0.661 |
| ACTH (ng/L) | 63.60 ± 10.67 | 43.21 ± 5.05 | 49.00 ± 7.91 | 44.21 ± 10.82 | 0.103 | 0.336 |
| CORT (ng/mL) | 8.19 ± 0.14b | 9.35 ± 0.31a | 8.58 ± 0.24b | 7.83 ± 0.31⁎⁎ | 0.015 | 0.009 |
| Heat exposure for 14 d | ||||||
| CRF (ng/mL) | 107.16 ± 10.83 | 132.32 ± 9.71 | 127.75 ± 11.96 | 167.33 ± 17.30 | 0.139 | 0.456 |
| ACTH (ng/L) | 42.68 ± 1.25 | 37.60 ± 4.91 | 36.87 ± 4.25 | 49.05 ± 4.70 | 0.322 | 0.381 |
| CORT (ng/mL) | 8.52 ± 0.21b | 9.92 ± 0.21a | 8.96 ± 0.32b | 8.01 ± 0.32⁎⁎ | 0.047 | 0.007 |
NC, normal control group; HS, heat stress group; PF, pair-fed group; HG, GAA group. Values are mean ± SE of 6 replicates.
CRF, corticotropin-releasing factor; ACTH, adrenocorticotropic hormone; CORT, corticosterone.
One-way ANOVA and Tukey's test were conducted for the NC, HS, and HG groups; T test was conducted for the HS and HG groups.
Means with different superscripts within each row are significantly different among the NC, HS, and PF groups (P < 0.05).
Means with asterisks indicate a significant difference between HS and HG group (P < 0.01).
Plasma HSP70 Concentration
Figure 2 illustrates the effects of heat stress on plasma HSP70 levels and the GAA intervention effects in broilers. Heat exposure notably elevated (P < 0.05) the levels of HSP70 in the HS group after 7 and 14 d of treatment, as compared to the NC and PF groups. No substantial difference (P > 0.05) in HSP70 levels was observed between the HG and HS groups.
Figure 2.
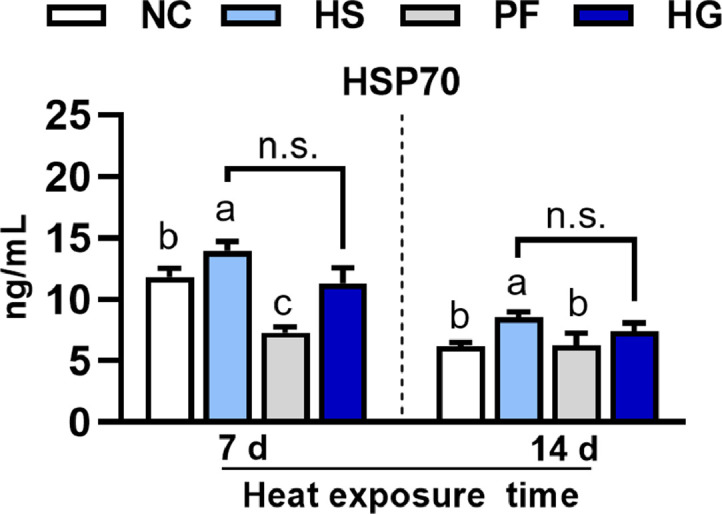
Effects of heat stress on the plasma HSP70 level and the GAA intervention effects in broilers. Data are presented as mean ± SE (n = 6). One-way ANOVA and Tukey's test were conducted for the NC, HS, and HG groups; T test was conducted for the HS and HG groups. a–cDifferent letters indicate significant difference among the NC, HS, and PF groups (P < 0.05). n.s.means no significant.
Immune Organ Indexes
Table 4 shows the effects of heat stress on the immune organ indexes and the GAA intervention effects in broilers. After 14 d of heat exposure, the HS group exhibited lower (P < 0.05) organ indexes for the thymus and bursa of Fabricius in comparison to both the NC and PF groups. After 7 d of heat exposure, the HS group and PF group presented a decreased (P < 0.05) thymus index when compared to the NC group. No significant difference (P > 0.05) in immune organ indexes was observed between the HG and HS groups.
Table 4.
Effects of heat stress on the immune organ indexes and the guanidinoacetic acid (GAA) intervention effects in broilers.
| Treatment1 |
P value2 |
|||||
|---|---|---|---|---|---|---|
| Items | NC | HS | PF | HG | ANOVA | T test |
| Heat exposure for 7 d | ||||||
| Spleen (g/100 g) | 0.13 ± 0.01 | 0.10 ± 0.01 | 0.14 ± 0.03 | 0.10 ± 0.01 | 0.292 | 0.195 |
| Thymus (g/100 g) | 0.34 ± 0.04a | 0.14 ± 0.01b | 0.16 ± 0.02b | 0.12 ± 0.01 | <0.001 | 0.951 |
| Bursa of Fabricius (g/100 g) | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.114 | 0.306 |
| Heat exposure for 14 d | ||||||
| Spleen (g/100 g) | 0.12 ± 0.02 | 0.10 ± 0.02 | 0.13 ± 0.01 | 0.09 ± 0.01 | 0.579 | 0.636 |
| Thymus (g/100 g) | 0.30 ± 0.04a | 0.14 ± 0.02b | 0.28 ± 0.03a | 0.11 ± 0.03 | 0.001 | 0.841 |
| Bursa of Fabricius (g/100 g) | 0.06 ± 0.01a | 0.04 ± 0.01b | 0.06 ± 0.01a | 0.06 ± 0.01 | 0.027 | 0.084 |
NC, normal control group; HS, heat stress group; PF, pair-fed group; HG, GAA group. Values are mean ± SE of 6 replicates.
One-way ANOVA and Tukey's test were conducted for the NC, HS, and HG groups; T test was conducted for the HS and HG groups.
Means with different superscripts within each row are significantly different among the NC, HS and PF groups (P < 0.05).
Plasma Proinflammatory Cytokines Levels
Table 5 displays the impacts of heat stress on plasma proinflammatory cytokine levels and the GAA intervention effects in broilers. After 14 d of heat exposure, the HS group exhibited a significant decrease (P < 0.05) in IL-2 levels compared to the NC and PF groups. After 7 d of heat exposure, chickens in the HS group exhibited higher (P < 0.05) levels of IL-6 and TNF-α compared to the NC and PF groups. TNF-α levels were also increased in the PF group compared to the NC group after 7 d of treatment. In contrast to the HS group, the HG group showed a considerable increase (P < 0.05) in IL-2 levels after 14 d of heat exposure.
Table 5.
Effects of heat stress on the plasma proinflammatory cytokines and the guanidinoacetic acid (GAA) intervention effects in broilers.
| Treatment1 |
P value3 |
|||||
|---|---|---|---|---|---|---|
| Items2 | NC | HS | PF | HG | ANOVA | T test |
| Heat exposure for 7 d | ||||||
| IL-2 (ng/L) | 39.83 ± 5.46 | 48.54 ± 7.83 | 37.78 ± 4.64 | 71.07 ± 6.90 | 0.242 | 0.870 |
| IL-6 (ng/L) | 95.46 ± 11.61b | 177.16 ± 16.14a | 118.23 ± 7.31b | 156.00 ± 6.12 | 0.001 | 0.120 |
| TNF-α (ng/L) | 45.94 ± 1.37b | 62.40 ± 3.61a | 59.44 ± 5.40a | 62.75 ± 6.47 | 0.027 | 0.539 |
| Heat exposure for 14 d | ||||||
| IL-2 (ng/L) | 63.08 ± 3.85a | 49.26 ± 2.78b | 62.78 ± 5.39a | 74.24 ± 3.39* | 0.048 | 0.011 |
| IL-6 (ng/L) | 141.86 ± 1.25 | 174.41 ± 14.58 | 157.82 ± 13.81 | 171.04 ± 12.71 | 0.095 | 0.767 |
| TNF-α (ng/L) | 77.61 ± 7.40 | 98.79 ± 6.96 | 94.59 ± 11.61 | 99.87 ± 8.07 | 0.114 | 0.744 |
NC, normal control group; HS, heat stress group; PF, pair-fed group; HG, GAA group. Values are mean ± SE of 6 replicates.
IL-2, interleukin 2; IL-6, interleukin 6; TNF-α, tumor necrosis factor α.
One-way ANOVA and Tukey's test were conducted for the NC, HS, and HG groups; T test was conducted for the HS and HG groups.
Means with different superscripts within each row are significantly different among the NC, HS, and PF groups (P < 0.05).
Means with asterisk indicate a significant difference between HS and HG group (P < 0.05).
Plasma Immunoglobulin and Lysozyme Levels
Table 6 shows the effects of heat stress on plasma immunoglobulin and lysozyme levels and the GAA intervention effects in broilers. After 7 and 14 d of heat exposure, the IgG level was significantly lower (P < 0.05) in the HS group compared to the NC group. Additionally, the IgG level decreased in the PF group after 7 d of treatment when compared to the NC group. In addition, the lysozyme concentration significantly decreased (P < 0.05) in the HS group compared to the NC and PF groups after 7 d of heat exposure. Interestingly, after 14 d of treatment, the IgG and IgM levels in the HG group showed significant increases (P < 0.05) compared to those in the HS group. Moreover, the HG group exhibited a significant increase (P < 0.05) in the lysozyme level compared to the HS group after 7 d of heat exposure.
Table 6.
Effects of heat stress on the plasma immunoglobulin and lysozyme and the guanidinoacetic acid (GAA) intervention effects in broilers.
| Treatment1 |
P value3 |
|||||
|---|---|---|---|---|---|---|
| Items2 | NC | HS | PF | HG | ANOVA | T test |
| Heat exposure for 7 d | ||||||
| IgA (mg/mL) | 8.36 ± 0.40 | 7.51 ± 0.96 | 6.59 ± 0.59 | 8.03 ± 0.54 | 0.087 | 0.287 |
| IgG (mg/mL) | 11.77 ± 0.78a | 8.26 ± 1.22b | 10.45 ± 1.09ab | 12.14 ± 0.84 | 0.016 | 0.529 |
| IgM (mg/mL) | 8.05 ± 0.54 | 7.15 ± 0.45 | 6.75 ± 0.37 | 6.59 ± 0.65 | 0.082 | 0.197 |
| Lysozyme (μg/mL) | 0.52 ± 0.06a | 0.14 ± 0.05b | 0.52 ± 0.03a | 0.21 ± 0.02* | <0.001 | 0.001 |
| Heat exposure for 14 d | ||||||
| IgA (mg/mL) | 8.99 ± 0.25 | 8.07 ± 0.25 | 8.50 ± 0.86 | 9.54 ± 0.26 | 0.165 | 0.687 |
| IgG (mg/mL) | 16.11 ± 2.91a | 9.80 ± 1.09b | 8.59 ± 1.02b | 15.42 ± 2.72* | 0.029 | 0.001 |
| IgM (mg/mL) | 8.53 ± 0.40 | 7.73 ± 0.23 | 7.83 ± 0.69 | 9.33 ± 0.23* | 0.299 | 0.012 |
| Lysozyme (μg/mL) | 0.34 ± 0.04 | 0.39 ± 0.05 | 0.45 ± 0.04 | 0.40 ± 0.27 | 0.190 | 0.098 |
NC, normal control group; HS, heat stress group; PF, pair-fed group; HG, GAA group. Values are mean ± SE of 6 replicates.
IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M.
One-way ANOVA and Tukey's test were conducted for the NC, HS, and HG groups; T test was conducted for the HS and HG groups.
Means with different superscripts within each row are significantly different among the NC, HS, and PF groups (P < 0.05).
Means with asterisk indicate a significant difference between HS and HG group (P < 0.05).
DISCUSSION
The hypothalamus-pituitary-adrenal axis is one of the most important systems that integrate the body during stress and/or homeostatic disturbances, and the long-term release of the key hormone corticosterone can compromise the immune system and threaten the health of the organism (Honda et al., 2015). This study highlights the harm caused by heat stress to the immune system of broilers and evaluates the effectiveness of dietary GAA supplementation in mediating the HPA axis and its immune damage in alleviating chronic heat stress.
The detrimental effects of heat stress on broiler growth performance have been extensively documented (Hu et al., 2022; Sun et al., 2023). In our study, broilers exposed to heat stress exhibited a decrease in ADG and ADFI, and an increase in F/G, compared to broilers of the NC group. The results that chicken experienced heavy pant in the HS and HG groups are similar to those reported by Majdeddin et al. (2020), who reported broilers panted heavily during episodes of heat stress. Here, the high panting frequency of broilers at high temperatures confirmed heat stress. Alhenaky et al. (2017) reported that decreased performance in heat-stressed broilers may be related to increased CORT concentrations because activation of the HPA axis redistributes energy to maintain and adapt to stressful conditions, and less energy is available for growth, resulting in decreased growth performance. Heat stress activates the neuroendocrine system, leading to the activation of the HPA axis and subsequent release of glucocorticoids, and CORT is the major glucocorticoid in broilers (Ghulam Mohyuddin et al., 2022; Li et al., 2022). In agreement with previous studies, our findings showed that CORT levels in blood were significantly increased after chronic heat stress despite no consistent changes in CRF and ACTH (Quinteiro-Filho et al., 2017; Nawaz et al., 2021). Notably, GAA treatment was found to effectively reduce CORT content in our results. Similarly, Amiri et al. (2019) demonstrated a decrease in blood cortisol which birds grown in heat stress fed with GAA supplemented diet. In fact, dietary GAA can be recognized as an efficacious replacement for arginine (Baker, 2009). Though the specific mechanism by which GAA reduces CORT has not been reported, one possible reason is that GAA supplementation may reduce metabolic stress (i.e., protein degradation) benefiting by from its arginine-sparing functions (Amiri et al., 2019). Also, GAA enables more NO synthesis and enhanced autonomic heat dissipation of broilers reduces the stimulation of the body by heat, and decreases the stimulation of the HPA axis (Uyanga et al., 2020). During chronic stress, CORT secretion has been associated with immune suppression, which makes animals more vulnerable to disease challenges due to the broken immune system (Bagath et al., 2019). Therefore, the reduction of CORT under GAA addition conditions may contribute to the reduction of the immunosuppressive effect of CORT.
Heat shock protein belongs to the family of HSP, and heat is the foremost inducer of HSP-related genes. The expression of HSP70 has been widely reported as a marker of heat stress in chickens. As a chaperone protein, rapidly synthesized circulating HSP70 has been reported in broilers in the condition of heat stress (Hu et al., 2022). Consistent with the elevated levels of CORT, increased plasma HSP70 was also detected in broilers after exposed to heat stress for 7 and 14 d, suggesting that chickens were negatively affected by heat stress.
Previous studies have demonstrated that heat stress impairs the development of immune organs in broilers (Quinteiro-Filho et al., 2010; Sun et al., 2023). However, the underlying mechanisms responsible for such impairments are multifaceted and not yet fully understood. Chronically stressful conditions lead to excessive production of corticosterone, which inhibits the activity of immune cells (Coutinho and Chapman, 2011) and induces degeneration of immune organs (Aschbacher et al., 2013). Inconsistent, the immune organ indexes of the thymus and bursa of Fabricius were found to be decreased in the HS group in our study. Moreover, insufficient nutrient intake due to reduced feed intake under heat stress is an important cause of the bursa of Fabricius, spleen, and thymus organs impairment (Sun et al., 2023). In the present study, the decreased thymus index in PF treatment directly confirms this point. Unfortunately, coping with heat stress through the GAA did not protect the immune organs from heat stress damage, suggesting that the GAA has limitations in alleviating heat stress. However, whether the alleviating effect of GAA on heat stress in broilers can be enhanced by appropriate addition dose warrants further investigation.
Cytokines as the markers of immunity that can enhance host defenses for controlling infection (Akinyemi and Adewole, 2022). Heat stress leads to integrated intestinal barrier injury and induces the release of endotoxin from commensal bacteria into blood (Alhenaky et al., 2017), increasing proinflammatory cytokines (De Boever et al., 2008; Liu et al., 2021), which presumably activates immune functions through the enhanced proliferation of lymphocytes and macrophages. Previous studies have shown increased levels of proinflammatory cytokines, such as IL-6, TNF-α, IL-2, IL-1b, and IL-8, in broilers subjected to heat stress (Hu et al., 2022). Our findings align with the above results, showing a significant increase in IL-6 and TNF-α in plasma after 7 d of heat stress. The cytokines are related to T-helper cells, and the Th1 cells mainly secrete IL-2 (Romagnani, 2000). IL-2 is an important cytokine for reducing viral or bacterial infection, and the production of IL-2 is increased by endotoxins (Costalonga and Zell, 2007). With the prolongation of heat stress for 14 d, chickens presented a lower level of IL-2 in the HS group, which is consistent with the results of immune organ damage. Differently, we showed that GAA supplement increased IL-2 concentration in the HG group, a fact that might be attributable to the improved intestinal mucosal barrier function by GAA, and the enhanced vulnerable intestinal barrier ability to resist the invasion of endotoxin (Amiri et al., 2019; Peng et al., 2023). Besides that, the arginine-sparing effect of GAA might increase IL-2 production, as Li et al. (2007) reported dietary supplementation with 1 or 2% arginine to tumor-bearing or septic rats increased T-lymphocyte proliferation, IL-2 production, IL-2 receptor expression on T lymphocytes, etc.
Honda et al. (2015) found the vaccinated chicken presented a lower level of IgG in serum after 19 d in the condition of heat stress. Similar results were found in our study, HS significantly reduced IgG levels after 7 and 14 d of heat stress. Moreover, IgG level also decreased in the PF group, suggesting feed intake was involved in the secretion of IgG. Lysozyme is a protein that functions as a bacteriostatic and anti-inflammatory agent. It is essential for maintaining homeostasis in normal defense mechanisms and nonspecific immunity (Abu Hafsa et al., 2022). In our study, chickens in the HS group severely decreased lysozyme concentration in plasma after 7 d of heat stress. The results confirm the severe blow of heat stress on broiler immunity. Differently, dietary supplements with GAA appear to contribute to alleviating the deleterious effect of heat stress on humoral immune response and lysozyme level in chickens, and GAA addition increased lysozyme, IgM, and IgG concentration in the HG group. Serum immunoglobulins secreted by B cells are reflective of the actual humoral immunity of the bird. It is reported Arg regulates the production of antibodies by B-cells and B-cell development (De Jonge et al., 2002), and is an essential substrate in the immune system (Uyanga et al., 2020). These findings indicate that GAA may be involved in the regulation of plasma immunoglobulin concentration.
In conclusion, chronic heat stress increases CORT release, which leads to damage to immune organs and impairs immunity in broilers. Supplementation of 0.6 g/kg GAA displays a beneficial function on immunity in conditions of heat stress, which improves immunity by suppressing the synthesis of CORT and increasing plasma levels of IL-2, IgG, and IgM in broilers.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (32072780), the National Key Research and Development Program of China (2016YFD0500501), and the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS [2023]).
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the present study.
REFERENCES
- Abdelqader A., Al-Fataftah A.R. Thermal acclimation of broiler birds by intermittent heat exposure. J. Therm. Biol. 2014;39:1–5. [Google Scholar]
- Abu Hafsa S.H., Mahmoud A.E.M., Fayed A.M.A., Abdel-Azeem A.S. The effect of exogenous lysozyme supplementation on growth performance, caecal fermentation and microbiota, and blood constituents in growing rabbits. Animals (Basel) 2022;12:899. doi: 10.3390/ani12070899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemi F., Adewole D. Effects of brown seaweed products on growth performance, plasma biochemistry, immune response, and antioxidant capacity of broiler chickens challenged with heat stress. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhenaky A., Abdelqader A., Abuajamieh M., Al-Fataftah A.R. The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. J. Therm. Biol. 2017;70:09–14. doi: 10.1016/j.jtherbio.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Amiri M., Ghasemi H.A., Hajikhodadadi I., Farahani A.H.K. Efficacy of guanidinoacetic acid at different dietary crude protein levels on growth performance, stress indicators, antioxidant status, and intestinal morphology in broiler chickens subjected to cyclic heat stress. Anim. Feed Sci. Technol. 2019;254 [Google Scholar]
- Aschbacher K., O'Donovan A., Wolkowitz O.M., Dhabhar F.S., Su Y., Epel E. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology. 2013;38:1698–1708. doi: 10.1016/j.psyneuen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad M.A.K., Kikusato M., Maekawa T., Shirakawa H., Toyomizu M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010;155:401–406. doi: 10.1016/j.cbpa.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Bagath M., Krishnan G., Devaraj C., Rashamol V.P., Pragna P., Lees A.M., Sejian V. The impact of heat stress on the immune system in dairy cattle: A review. Res. Vet. Sci. 2019;126:94–102. doi: 10.1016/j.rvsc.2019.08.011. [DOI] [PubMed] [Google Scholar]
- Baker D.H. Advances in protein-amino acid nutrition of poultry. Amino Acids. 2009;37:29–41. doi: 10.1007/s00726-008-0198-3. [DOI] [PubMed] [Google Scholar]
- Beckford R.C., Ellestad L.E., Proszkowiec-Weglarz M., Farley L., Brady K., Angel R., Liu H.C., Porter T.E. Effects of heat stress on performance, blood chemistry, and hypothalamic and pituitary mRNA expression in broiler chickens. Poult. Sci. 2020;99:6317–6325. doi: 10.1016/j.psj.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burin Junior A.M., Fernandes N.L.M., Snak A., Fireman A., Horn D., Fernandes J.I.M. Arginine and manganese supplementation on the immune competence of broilers immune stimulated with vaccine against Salmonella Enteritidis. Poult. Sci. 2019;98:2160–2168. doi: 10.3382/ps/pey570. [DOI] [PubMed] [Google Scholar]
- Chauhan S.S., Rashamol V., Bagath M., Sejian V., Dunshea F.R. Impacts of heat stress on immune responses and oxidative stress in farm animals and nutritional strategies for amelioration. Int. J. Biometeorol. 2021;65:1231–1244. doi: 10.1007/s00484-021-02083-3. [DOI] [PubMed] [Google Scholar]
- Costalonga M., Zell T. Lipopolysaccharide enhances in vivo interleukin-2 production and proliferation by naive antigen-specific CD4 T cells via a Toll-like receptor 4-dependent mechanism. Immunology. 2007;122:124–130. doi: 10.1111/j.1365-2567.2007.02620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A.E., Chapman K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan J., Melkman-Zehavi T., Reicher N., Braun U., Inhuber V., Mabjeesh S.J., Halevy O., Uni Z. Supply and demand of creatine and glycogen in broiler chicken embryos. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1079638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boever S., Beyaert R., Vandemaele F., Baert K., Duchateau L., Goddeeris B., De Backer P., Croubels S. The influence of age and repeated LPS administration on body temperature and the relation with interleukin-6 and IgM antibodies in broiler chickens. Avian Pathol. 2008;37:39–44. doi: 10.1080/03079450701784875. [DOI] [PubMed] [Google Scholar]
- DeGroot A.A., Braun U., Dilger R.N. Guanidinoacetic acid is efficacious in improving growth performance and muscle energy homeostasis in broiler chicks fed arginine-deficient or arginine-adequate diets. Poult. Sci. 2019;98:2896–2905. doi: 10.3382/ps/pez036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonge W.J., Kwikkers K.L., te Velde A.A., van Deventer S.J., Nolte M.A., Mebius R.E., Ruijter J.M., Lamers M.C., Lamers W.H. Arginine deficiency affects early B cell maturation and lymphoid organ development in transgenic mice. J. Clin. Invest. 2002;110:1539–1548. doi: 10.1172/JCI16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghulam Mohyuddin S., Khan I., Zada A., Qamar A., Arbab A.A.I., Ma X.B., Yu Z.C., Liu X.X., Yong Y.H., Ju X.H., Zhang-Ping Y., Yong-Jiang M. Influence of heat stress on intestinal epithelial barrier function, tight junction protein, and immune and reproductive physiology. Biomed. Res. Int. 2022;2022 doi: 10.1155/2022/8547379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Esquerra R., Leeson S. Concentrations of putrescine, spermidine, and spermine in duodenum and pancreas as affected by the ratio of arginine to lysine and source of methionine in broilers under heat stress. Poult. Sci. 2006;85:1398–1408. doi: 10.1093/ps/85.8.1398. [DOI] [PubMed] [Google Scholar]
- Honda B.T., Calefi A.S., Costola-de-Souza C., Quinteiro-Filho W.M., da-Silva-Fonseca J.G., de-Paula V.F., Palermo-Neto J. Effects of heat stress on peripheral T and B lymphocyte profiles and IgG and IgM serum levels in broiler chickens vaccinated for Newcastle disease virus. Poult. Sci. 2015;94:2375–2381. doi: 10.3382/ps/pev192. [DOI] [PubMed] [Google Scholar]
- Hu J.Y., Mohammed A.A., Murugesan G.R., Cheng H.W. Effect of a synbiotic supplement as an antibiotic alternative on broiler skeletal, physiological, and oxidative parameters under heat stress. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanian R. Immunological responses as affected by dietary protein and arginine concentrations in starting broiler chicks. Poult. Sci. 2009;88:1818–1824. doi: 10.3382/ps.2008-00386. [DOI] [PubMed] [Google Scholar]
- Li P., Yin Y.L., Li D., Kim S.W., Wu G. Amino acids and immune function. Br. J. Nutr. 2007;98:237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- Li Q., Zhou H., Ouyang J., Guo S., Zheng J., Li G. Effects of dietary tryptophan supplementation on body temperature, hormone, and cytokine levels in broilers exposed to acute heat stress. Trop. Anim. Health Prod. 2022;54:164. doi: 10.1007/s11250-022-03161-3. [DOI] [PubMed] [Google Scholar]
- Liu W.C., Ou B.H., Liang Z.L., Zhang R., Zhao Z.H. Algae-derived polysaccharides supplementation ameliorates heat stress-induced impairment of bursa of Fabricius via modulating NF-κB signaling pathway in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Zhang L., Li J., Xing T., Jiang Y., Gao F. Heat stress alters muscle protein and amino acid metabolism and accelerates liver gluconeogenesis for energy supply in broilers. Poult. Sci. 2021;100:215–223. doi: 10.1016/j.psj.2020.09.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdeddin M., Braun U., Lemme A., Golian A., Kermanshahi H., De Smet S., Michiels J. Guanidinoacetic acid supplementation improves feed conversion in broilers subjected to heat stress associated with muscle creatine loading and arginine sparing. Poult. Sci. 2020;99:4442–4453. doi: 10.1016/j.psj.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdeddin M., Braun U., Lemme A., Golian A., Kermanshahi H., De Smet S., Michiels J. Effects of feeding guanidinoacetic acid on oxidative status and creatine metabolism in broilers subjected to chronic cyclic heat stress in the finisher phase. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels J., Maertens L., Buyse J., Lemme A., Rademacher M., Dierick N.A., Smet S.D. Supplementation of guanidinoacetic acid to broiler diets: effects on performance, carcass characteristics, meat quality, and energy metabolism. Poult. Sci. 2012;91:402–412. doi: 10.3382/ps.2011-01585. [DOI] [PubMed] [Google Scholar]
- Munir K., Muneer M.A., Masaoud E., Tiwari A., Mahmud A., Chaudhry R.M., Rashid A. Dietary arginine stimulates humoral and cell-mediated immunity in chickens vaccinated and challenged against hydropericardium syndrome virus. Poult. Sci. 2009;88:1629–1638. doi: 10.3382/ps.2009-00152. [DOI] [PubMed] [Google Scholar]
- Nawaz A.H., Amoah K., Leng Q.Y., Zheng J.H., Zhang W.L., Zhang L. Poultry response to heat stress: its physiological, metabolic, and genetic implications on meat production and quality including strategies to improve broiler production in a warming world. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.699081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J.R., Sobotik E.B., Athrey G., Archer G.S. Effects of supplementing yeast fermentate in the feed or drinking water on stress susceptibility, plasma chemistry, cytokine levels, antioxidant status, and stress- and immune-related gene expression of broiler chickens. Poult. Sci. 2020;99:3312–3318. doi: 10.1016/j.psj.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X.Y., Xing T., Li J.L., Zhang L., Jiang Y., Gao F. Guanidinoacetic acid supplementation improves intestinal morphology, mucosal barrier function of broilers subjected to chronic heat stress. J. Anim. Sci. 2023;101:skac355. doi: 10.1093/jas/skac355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portocarero N., Braun U. The physiological role of guanidinoacetic acid and its relationship with arginine in broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Calefi A.S., Cruz D.S.G., Aloia T.P.A., Zager A., Astolfi-Ferreira C.S., Piantino Ferreira J.A., Sharif S., Palermo-Neto J. Heat stress decreases expression of the cytokines, avian β-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella Enteritidis. Vet. Immunol. Immunopathol. 2017;186:19–28. doi: 10.1016/j.vetimm.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Gomes A.V., Pinheiro M.L., Ribeiro A., Ferraz-de-Paula V., Astolfi-Ferreira C.S., Ferreira A.J., Palermo-Neto J. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella Enteritidis. Avian Pathol. 2012;41:421–427. doi: 10.1080/03079457.2012.709315. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sá L.R., Ferreira A.J., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Romagnani S. T-cell subsets (Th1 versus Th2) Ann. Allergy Asthma Im. 2000;85:9–21. doi: 10.1016/S1081-1206(10)62426-X. [DOI] [PubMed] [Google Scholar]
- Sun S., Li B., Wu M., Deng Y., Li J., Xiong Y., He S. Effect of dietary supplemental vitamin C and betaine on the growth performance, humoral immunity, immune organ index, and antioxidant status of broilers under heat stress. Trop. Anim. Health Prod. 2023;55:96. doi: 10.1007/s11250-023-03500-y. [DOI] [PubMed] [Google Scholar]
- Tan J., Liu S., Guo Y., Applegate T.J., Eicher S.D. Dietary L-arginine supplementation attenuates lipopolysaccharide-induced inflammatory response in broiler chickens. Br. J. Nutr. 2014;111:1394–1404. doi: 10.1017/S0007114513003863. [DOI] [PubMed] [Google Scholar]
- Uyanga V.A., Jiao H., Zhao J., Wang X., Lin H. Dietary L-citrulline supplementation modulates nitric oxide synthesis and anti-oxidant status of laying hens during summer season. J. Anim. Sci. Biotechnol. 2020;11:103. doi: 10.1186/s40104-020-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Sun W., Wu E., Wang K., Chen X., Cui Y., Zhang G., Lv F., Wang Y., Peng X., Si H. Polysaccharides from Abruscantoniensis hance modulate intestinal microflora and improve intestinal mucosal barrier and liver oxidative damage induced by heat stress. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.868433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Burghardt R.C., Johnson G.A., Kim S.W., Li X.L., Satterfield M.C., Spencer T.E. Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J. Anim. Sci. 2010;88:E195–E204. doi: 10.2527/jas.2009-2446. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li J.L., Wang X.F., Zhu X.D., Gao F., Zhou G.H. Attenuating effects of guanidinoacetic acid on preslaughter transport-induced muscle energy expenditure and rapid glycolysis of broilers. Poult. Sci. 2019;98:3223–3232. doi: 10.3382/ps/pez052. [DOI] [PubMed] [Google Scholar]


