Abstract
Environmental triggers often work via signal transduction cascades that modulate the epigenome and transcriptome of cell types involved in the disease process. Multiple sclerosis (MS) is an autoimmune disease affecting the central nervous system being characterized by a combination of recurring inflammation, demyelination and progressive loss of axons. The mechanisms of MS onset are not fully understood and genetic variants may explain only some 20% of the disease susceptibility. From the environmental factors being involved in disease development low vitamin D levels have been shown to significantly contribute to MS susceptibility. The pro-hormone vitamin D3 acts via its metabolite 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) as a high affinity ligand to the transcription factor VDR (vitamin D receptor) and is a potent modulator of the epigenome at thousands of genomic regions and the transcriptome of hundreds of genes. A major target tissue of the effects of 1,25(OH)2D3 and VDR are cells of innate and adaptive immunity, such as monocytes, dendritic cells as well as B and T cells. Vitamin D induces immunological tolerance in T cells and reduces inflammatory reactions of various types of immune cells, all of which are implicated in MS pathogenesis. The immunomodulatory effects of 1,25(OH)2D3 contribute to the prevention of MS. However, the strength of the responses to vitamin D3 supplementation is highly variegated between individuals. This review will relate mechanisms of individual’s vitamin D responsiveness to MS susceptibility and discuss the prospect of vitamin D3 supplementation as a way to extinguish the autoimmunity in MS.
Keywords: multiple sclerosis, vitamin D, vitamin D response index, immune system, genetics, epigenetics
1. Introduction
The central nervous system (CNS) autoimmune disease MS is the leading cause of disability in younger and middle-aged adults that is not caused by a trauma [1]. The clinically most common form of the disease is RRMS (relapsing remitting MS), i.e., an alternation between symptomatic attacks (relapses) and periods of recovery (remissions), which is found in up to 90% of patients in the age range of 20–40 years [2]. When RRMS progresses, the patient accumulates irreversible neurological disabilities. The main pathological feature of MS are focal lesions in the CNS, which are created by B and T cells from the periphery that pass a disrupted blood-brain barrier and act against an antigen in the CNS [3]. The best candidate for the latter is myelin, which is a protein that insulates and protects axons, so that they can efficiently conduct nerve impulses. The loss of myelin as well as the prevention of remyelination due to increasingly disabled myelin-producing oligodendrocytes leads to axon destruction and neurodegeneration. This process is associated with waves of inflammation, i.e., the massive production of pro-inflammatory cytokines. The latter is triggered by CD4+ cells that are polarized as T helper (TH) cells of types 1 and 17 [4]. Thus, MS is an inflammatory autoimmune disease that is based on the interaction of (i) T and B cells of adaptive immunity, (ii) dendritic cells, monocytes, natural killer cells and microglia of innate immunity and (iii) oligodendrocytes, astrocytes and neurons of the CNS. Although several successful clinical trials have been completed in MS demonstrating that immunosuppressing and immunomodulatory therapies can lead to slowing down this disease, a fully effective treatment is still lacking. Accordingly, MS is currently still an incurable disease and even newest therapies may fail in stopping disease progression or lead to the severe complications [5]. Therefore, maximal attention should be taken on preventing disease onset.
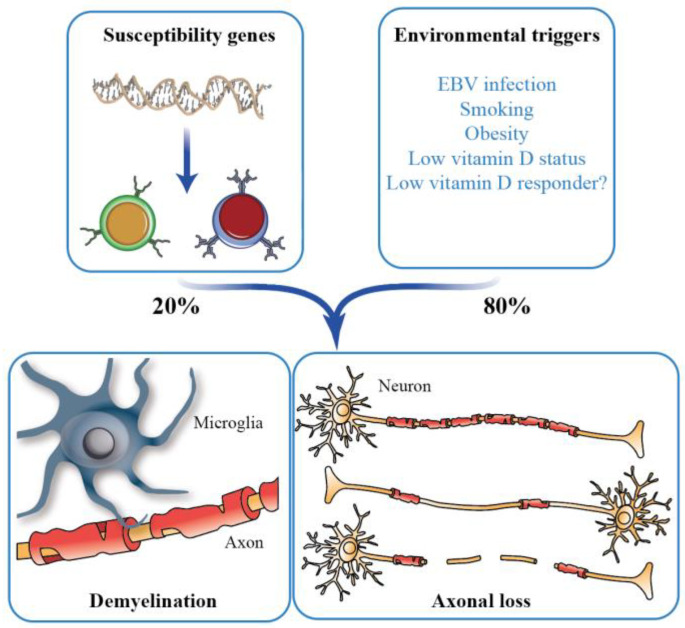
In general, the susceptibility for most common, non-communicable diseases like MS is based on a complex interplay of genetics and environment [6]. This means that both inherited or acquired genetic variants as well as environmental signals that via signal transduction pathways affect the function of transcription factors and chromatin modifiers [7] contribute to the disease risk. SNVs (single nucleotide variants) associated with MS susceptibility highlight genes in their vicinity that have key roles in immunity [8]. The cluster of HLA (human leukocyte antigen) genes, which encode for type I and type II MHC (major histocompatibility complex) proteins, are a “hotspot” for MS-related genes. The most prominent genetic risk factor for MS is the HLA-DRB*15:01 gene variant [9]. The gene encodes for a MHC type II protein, which is expressed on antigen-presenting cells and binds endogenous myelin autoantigens and probably also MS-associated foreign antigens [10]. Although currently more than 200 identified MS-related SNVs have been described, together they explain only some 20% of the disease risk [8]. Therefore, environmental factors seem to play a major role in MS susceptibility, such as EBV (Epstein Barr virus) infection. In addition, the lifestyle of the individual, such as smoking (promoting via lung inflammation systemic activation of proinflammatory pathways), adolescent obesity (causing systemic low grade chronic inflammation) and the frequency of outdoor activities (increasing endogenous vitamin D3 production via sun exposure), increases or decreases the risk of MS onset [11,12,13] (Figure 1).
Figure 1.
MS and vitamin D. MS is a complex autoimmune disorder, the onset of which is triggered by a combination of genetic (with estimated contribution 20%) and environmental factors (with estimated contribution 80%). MS is characterized by inflammatory, demyelinating lesions in the CNS leading to heterogeneous axonal loss.
A low vitamin D status, which often relates to low sun exposure, is another important environmental risk for MS [2,9,14]. In contrast, sufficient vitamin D levels, in particular within the first decades of life, are protective against disease onset [15]. Vitamin D3 is a pro-hormone being produced endogenously in UV-B exposed human skin [16,17] (Figure 2). However, the seasonal variation of sun exposure affects in particular to those individuals living above a latitude of 38° N (15% of world population) [18,19]. In addition, in modern lifestyle most individuals spend long times indoors, while for climatic and cultural reasons they are covering most of our skin outdoors. Therefore, in particular in Europe, vitamin D3 needs to be supplemented during winter times, in order not to develop vitamin D deficiency.
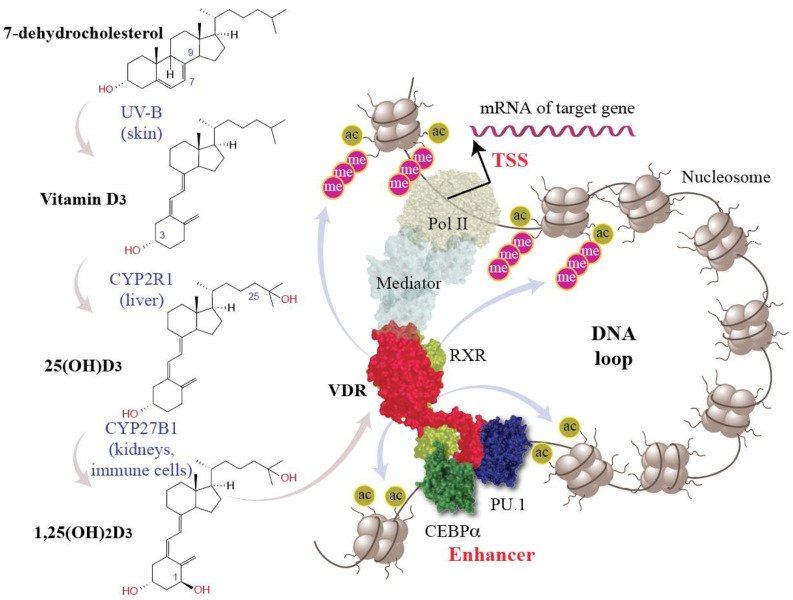
Figure 2.
Principles of vitamin D signaling. 7-dehydrolesterol is a direct precursor of cholesterol but under exposure with UV-B can also convert into vitamin D3 (left). CYP2R1 (cytochrome P450 family 2 subfamily R member 1) hydroxylates vitamin D3 into 25(OH)D3 (25-hydroxyvitamin D3) and CYP27B1 adds a further hydroxy group to C1. 1,25(OH)2D3 is a nuclear hormone that activates VDR (red). The pioneer transcription factors PU.1 (purine-rich box 1, dark blue) and CEBPα (CCAAT enhancer binding protein α, green) support the DNA binding of VDR-RXR (retinoid X receptor, light green) heterodimers. VDR-bound enhancers activate via DNA looping and the Mediator complex Pol II (RNA polymerase II) waiting on the TSS (transcription start site) region of a vitamin D target gene. This finally changes the expression of hundreds of vitamin D target genes (top right), a number of which are involved in the regulation of the immune system and have an impact on MS (Section 4).
Vitamin D is best known for its regulation of calcium homeostasis, which is important for proper bone mineralization [20,21], but in addition 1,25(OH)2D3 and VDR have a modulatory role on the immune system. Ligand-activated VDR stimulates innate immune cells like monocytes and macrophages, which fight against pathogenic microbes [22]. In parallel this inhibits overboarding responses of cells of the adaptive immune system [23,24], such as B and T cells, i.e., and prevents in this way the onset of autoimmune diseases like MS [25], rheumatoid arthritis [26], inflammatory bowel disease [27] and type I diabetes [28]. Importantly, the VITAL RCT (randomized controlled trial) with more than 25,000 participants indicated that vitamin D3 supplementation (2000 IU/day) over 5 years reduced the risk of autoimmune diseases by 22% [29]. This is an important finding, since for the primary target of the VITAL trial, the prevention of cancer and cardiovascular disease, there was a null result [30]. Furthermore, Mendelian randomization studies reported a significantly increased risk of a low vitamin D status for MS onset, while comparable studies focusing on other outcomes had null effects [31]. Thus, vitamin D seems to have more prominent effects on autoimmunity and in particular on MS than on many other diseases.
The ability of vitamin D to modulate the activity of immune system seems to be the key in understanding how its lack may cause a malfunction of immune cells in the periphery. Some of these incorrectly programmed immune cells then migrate to the CNS, where they target myelin. This short review describes current views on the role of vitamin D in MS, with a focus on its impact on the immune function and epigenetic landscape of the cells as a mechanism contributing to autoimmune demyelination.
2. Vitamin D Status versus Responsiveness
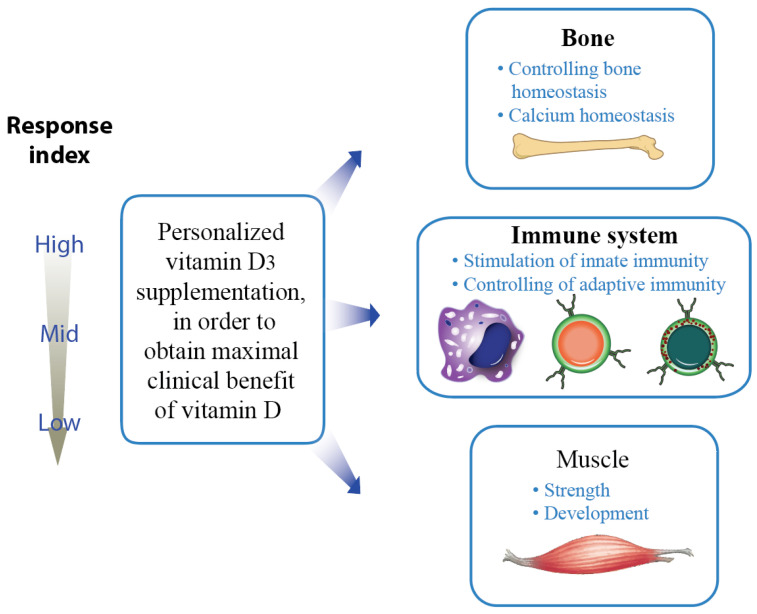
The serum levels of the abundant vitamin D3 metabolite 25(OH)D3, which has a half-life of more than 14 days, are a biomarker for the vitamin D status [32]. Below a 25(OH)D3 level of 50 nM (20 ng/mL) an individual is considered as vitamin D deficient [33], because his/her risk for musculoskeletal disorders like rickets in children as well as osteomalacia and fractures in adults like is significantly increased [34]. Therefore, on the population level a vitamin D status in the range of 75–100 nM (30–40 ng/mL) 25(OH)D3 is recommended [35], in order to get musculoskeletal as well as non-skeletal benefits of vitamin D. For the average person, deficiency can be prevented by a supplementation of 25 µg (1000 IU) vitamin D3 per day [36], but a dose of 1 µg (40 IU)/kg body weight may be most appropriate, in order to fulfill also the needs of low vitamin D responders (Figure 3). In cases of severe deficiency, a bolus of 1000 IU vitamin D3/kg body weight can speed up reaching appropriate 25(OH)D3 levels. However, long-term overdosing of vitamin D3 or its metabolites should be avoided, in order not to cause tissue calcification through hypercalcemia.
Figure 3.
Knowing an individual’s vitamin D response index allows personalized supplementation with vitamin D3. In this way, clinical benefits of vitamin D are optimized.
Since there is significant genetic and epigenetic variation between human individuals [37], recommendations for vitamin D3 supplementation for the whole population may not be suited best for everyone. The concept of the vitamin D response index was developed on the basis of the vitamin D intervention studies VitDmet (NCT01479933, ClinicalTrials.gov (accessed on 14 June 2023)) [38,39,40,41] and VitDbol (NCT02063334) [42,43], which were designed more as a medical experiment than an RCT. In the VitDmet study, 71 elderly pre-diabetic individuals were supplemented daily with either 0, 40, or 80 µg vitamin D3 over 5 months during winter in Finland. Blood samples were collected at the beginning and end of the intervention. In contrast, the VitDbol study used 35 young healthy individuals, who obtained a single bolus of 80,000 IU vitamin D3. Samples were gathered at days 0, 1, 2, and 30. PBMCs (peripheral blood mononuclear cells) were isolated, and RNA was prepared without any further in vitro culture. The supplemented vitamin D3 is endogenously converted to 25(OH)D3 and 1,25(OH)2D3, which results in changes of gene expression being measured by qPCR (quantitative polymerase chain reaction) or RNA-seq (RNA sequencing). Thus, the interventions represent safe human in vivo experiments.
Based on these studies, individuals can be distinguished via vitamin D-triggered parameters into high, mid and low responders to vitamin D (Figure 4). Importantly, 25% of the study participants showed a low vitamin D response index [44,45]. These individuals should take higher doses of vitamin D3 than suggested by guidelines for the general population (maximal 1000 IU, but in many countries far less). In contrast, persons with a high vitamin D response index are better prepared to cope with European winters characterized by low or no endogenous vitamin D3 production. This suggests that low vitamin D responders may suffer more frequently from autoimmune diseases [46], infections [47] and/or cancer [48], while high vitamin D responders have lower susceptibilities for these diseases. So far, there is no standardized procedure to determine the vitamin D response index. However, this may not be necessary, if all individuals are treated as potential low responders, i.e., supplemented with 4000 IU vitamin D3/day.
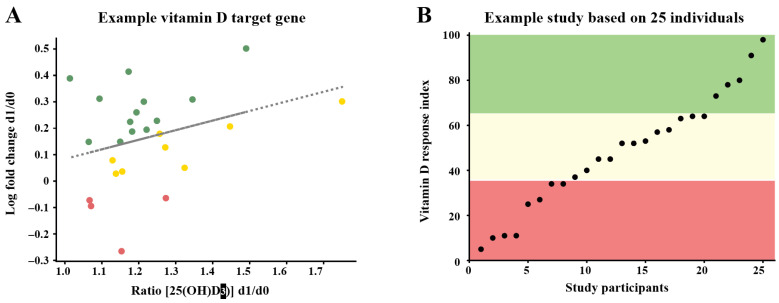
Figure 4.
Principles of determining the vitamin D response index. Data are analyzed by relating the changes in the expression of vitamin D target genes at day 1 (d1) and day 0 (d0) to the ratio of the 25(OH)D3 serum levels at d1 and d0 (A). In this way, the response of the target genes is calculated comparably to in vitro studies [23]. The responsiveness of each the study participants is scored for every selected vitamin D target gene as no (red), weak (yellow) or strong responders (green) (A). The sums of the scores of all tested genes is ranked and the individuals are segregated by the method k-means into low, mid and high responders [44] (B).
Like most other traits, the vitamin D response index is based on genetics, epigenetics and environment. Variants in the genes controlling vitamin D transport and metabolism, such as DHCR7 (7-dehydrocholesterol reductase), CYP2R1, CYP24A1 and GC (GC vitamin D binding protein), contribute to differences in the vitamin D status of individuals [49]. Interestingly, SNVs in the loci of the genes CYP24A1, CYP2R1 and DHCR7 contribute to MS susceptibility [50,51,52]. However, a person’s vitamin D response index is independent from his/her vitamin D status. Accordingly, high responders can have a low 25(OH)D3 serum levels and handle it well, while low responders should have a high vitamin D status, since at low 25(OH)D3 levels they cannot benefit from the physiological actions of vitamin D (Figure 3). In fact, high responders can handle a low vitamin D status, while low responders need to have a high vitamin D status, in order to benefit from vitamin D. Thus, genetic variants contributing to the vitamin D status and to the vitamin D response index are expected to be largely different.
On the molecular level the vitamin D response index is not well understood. In the case of the anti-coagulant drug warfarin interindividual differences in the response to its administration are found to be based on SNVs in the genes CYP2C9 and VKORC1 (vitamin K epoxide reductase complex subunit 1) [53]. In contrast, so far no SNVs related to the vitamin D response index have been described. This makes it likely that epigenetics and environment rather than genetics can explain this trait. It had been observed that persons differ in their sets of vitamin D target genes, which may be part of the explanation of differences in the vitamin D response index [54]. Moreover, vitamin D target genes have different EC50-value for their activation by vitamin D [55]. While some genes respond already at 0.1 nM 1,25(OH)2D3, others require levels of 1 nM and higher. The difference in the response of vitamin D target genes relates to their epigenetic status suggesting that also interindividual differences in the vitamin D response index are based on epigenetics.
3. Vitamin D Regulates the Epigenome and Transcriptome of Immune Cells
Chromatin is a complex of genomic DNA with nuclear proteins, such as histones, and serves as the physical expression of the epigenome. The epigenetic status of a gene, i.e., primarily the accessibility of its TSS and enhancer regions, determines, whether it can be transcribed or not. Thus, the epigenome regulates the transcriptome [56,57]. Interestingly, a number of attributes of the epigenome, such as (i) the accessibility of chromatin, (ii) the binding of transcription factors like VDR and the pioneer factors PU.1 and CEBPα, (iii) the binding of chromatin organizing factors, such as CTCF (CCCTC-binding factor), as well as changes in histone modifications are vitamin D sensitive [58,59] (Figure 2). There are some 10,000 VDR binding loci per VDR gene expressing cell type [60], i.e., there are thousands of sites within the epigenome where vitamin D may have an effect. However, only a minority of VDR-bound genomic regions contact via DNA looping TSS regions, i.e., they have an effect on the transcriptome [61]. Thus, the vast majority of genomic VDR binding sites seem to contribute to epigenetic memory of a cell but not directly to gene transcription.
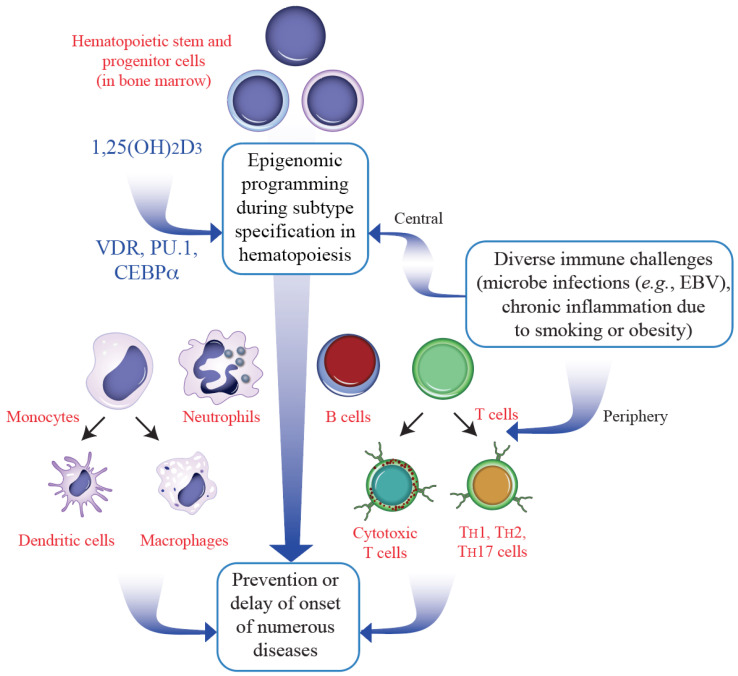
Hematopoiesis generates out of stem cell in the bone marrow more than 100 different cell types of the immune system and the blood. This involves epigenetic programming of terminally differentiated cells and creates a dominant form of epigenetic memory. Together with PU.1 and CEBPα VDR acts as a key regulator of myeloid line of hematopoiesis generating innate cells like granulocytes and monocytes [62] (Figure 5). Since most immune cell types have a rapid turnover, they can respond more flexibly to environmental changes than other tissues. Perturbations like antigen encounter and DAMPs (danger associated molecular patterns) exposure induce signal transduction cascades, the end of which are chromatin modifiers and transcription factors. This leads to changes in the epigenome and transcriptome of the respective immune cells representing another form of epigenetic memory known as trained immunity [63,64]. Thus, immune challenges are linked to epigenetic memory preparing the cells to react more effectively to a repeated encounter [65]. This may explain epidemiological observations that supplementation with vitamin D3 [66] or cod liver oil [67] at young age significantly reduces MS susceptibility later in life. Since the immune system takes about the first 10 years of life to mature, vitamin D sufficiency ensuring proper immune cell programming reduces the risk of MS onset (Figure 5).
Figure 5.
Hematopoietic differentiation triggered by vitamin D. The epigenome modulating effect of vitamin D (via the VDR with the help of PU.1 and CEBPα) modulates the differentiation of immune cells during hematopoiesis (top). In parallel, many immune challenges influence this differentiation process in the bone marrow as well as in the periphery. The stabilization of the epigenomes of the subtypes of monocytes and T cells (bottom) contributes to the prevention or delaying the onset of diseases including MS.
4. Vitamin D Target Genes with Impact for MS
The cells of the immune system that are most responsive to vitamin D are monocytes [44], which via their differentiated forms, dendritic cells and macrophages, do not only modulate inflammation, but also metabolic pathways. The evolutionary oldest function of VDR is the regulation of genes involved of energy metabolism [68,69], such as FBP1 (fructose-bisphosphatase 1) [70] and PFKFB4 (6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4) [71]. The innate as well as the adaptive immunity both use a lot of energy for growth and differentiation, VDR became also an important regulator of immunity [72]. Thus, vitamin D connects (energy) metabolism with immunity [73,74].
Ligand-activated VDR inhibits the maturation, differentiation and stimulatory capacity of dendritic cells [75]. This involves reprogramming of glucose metabolism via upregulating the PFKFB4 gene and changing the functional profile of dendritic cells. These epigenetic changes result in the reduction of TH1 cell counts and the increase of Treg (T regulatory) and TH2 cells [25,76], which are critical for immunological tolerance. Moreover, during the development of autoimmune diseases like MS, the TH17/Treg ratio gets into misbalance [77]. Vitamin D can (i) inhibit the polarization of T cells into the subtype TH17 by downregulating the genes IL17 (interleukin 17), IL22, RORC (RAR related orphan receptor C) and IL23R (interleukin 23 receptor) [78] and (ii) support the production of Treg cells by upregulating the genes FOXP3 (forkhead box P3), IL10 and CTLA4 (cytotoxic T-lymphocyte associated protein 4) [79]. In early stages of MS, TH1 cell numbers are high and mediate chronic inflammation causing demyelination [80,81]. In contrast, ligand-activated VDR antagonizes the pro-inflammatory transcription factors NFκB (nuclear receptor κB) and NFAT (nuclear receptor activated T cells) so that the IL2 gene, which is the major growth factor adaptive immunity, is produced in lower amounts [82].
Short-term vitamin D3 bolus supplementations can be used as safe human in vivo experiments. In this context of such experiments, at some 800 genomic regions significant changes in chromatin accessibility were observed [83]. Interestingly, the HLA gene cluster, shows a high density of vitamin D-triggered chromatin accessibility changes.
Furthermore, 10 of the 12 genes within the HLA class II subcluster were classified as vitamin D targets in PBMCs [23]. In addition, in monocytes also the class I genes HLA-A and HLA-C respond to vitamin D [84]. Thus, not only SNVs like HLA-DRB*15:01 [9] highlight the link between the HLA gene cluster and MS susceptibility, but vitamin D-triggered changes in the epigenome and transcriptome are also important.
Since MS is a complex multigenic disease, there are many other vitamin D target genes related to the disease. For example, the effects of vitamin D on the growth of hematopoietic stem cells is mediated by the family of CXCL (C-X-C motif chemokine ligand) genes [85], of which CXCL1, CXCL5, CXCL7, CXCL8, CXCL9, CXCL10, PARM1 (prostate androgen-regulated mucin-like protein 1) and EREG (epiregulin) are vitamin D targets [23]. Other vitamin D target genes related to autoimmunity are ACVRL1 (activin A receptor like type 1), NINJ1 (ninjurin 1), SRGN (serglycin), CD93 (CD93 molecule) and CEBPB [86]. Interestingly, the latter genes are highly expressed in immune cells but show only low inducibility. The long-term response of these genes corresponds with the low vitamin D responsiveness of their enhancers [86]. These observations suggest that vitamin D target genes involved in autoimmunity have a different set of vitamin D-triggered enhancers than those that contributing to rapid responses to microbe infections [87]. All these data strongly implicate a causal link between vitamin D induced transcriptional changes and the development of MS.
5. Relating MS Pathogenesis to Vitamin D
The pathology of MS can be subdivided into the processes (i) activation of self-reactive B and T cells, (ii) invasion of the CNS by these immune cells through the disruption of the blood-brain barrier, (iii) effector function of the invaded cells and (iv) progressive neurodegeneration [2]. The main role of vitamin D is to prevent the onset of MS through a lifelong support of proper epigenetic programing of immune cells both during hematopoiesis in the bone marrow as well as during microbe and DAMP exposure in the periphery. Thus, appropriate levels of circulating vitamin D should prevent the activation of self-reactive lymphocytes (step 1).
Improving the vitamin D status via vitamin D3 supplementation after MS diagnosis seems to be beneficial, since it results on the cellular level in the reduction of TH1 and TH17 blood counts [88] as well as in higher levels of Treg cells [89]. This effect is also seen by the reduction in IL17 levels and an increase in the IL10 concentrations, which are the major cytokines produced by both cell types. Thus, the clinical result of improved 25(OH)D3 serum levels are reduced effector function of TH1 and TH17 cells, such as less destruction of the blood-brain barrier (step 2), less chronic inflammation and increased immunological tolerance, i.e., controlled effector functions of immune cell inactivating the CNS (step 3) and reduced hindering of oligodendrocyte precursor cell survival and differentiation for axon repair (step 4). Intervention studies performed in different countries with in total more than 1000 MS patients indicated a 50–70% decreased number of relapses, when the vitamin D status was above 50 nM [15]. A very recent systematic review of 15 RCTs summarized that although supplementation with vitamin D3 has a beneficial effect against new lesions, there is no significant evidence that it is effective for preventing relapses or the progression of MS [90]. However, all of these studies were shorter than the 5 years of the VITAL trial [29]. Moreover, there was no personalized daily vitamin D3 supplementation dose based on a vitamin D response index segregation. Therefore, there is a need for new vitamin D3 supplementation trials in MS where dosing is individualized and based on the responsiveness to the vitamin.
6. Conclusions and Perspectives
Vitamin D3 controls via its biologically active metabolite 1,25(OH)2D3 the VDR cistrome. The action of VDR results in epigenetic memory in respective cell types, such as T cells and monocytes. A smaller subset of these VDR binding sites represent enhancer regions that regulate the expression of target genes with key impact on immune functions like those of the HLA or CXCL gene cluster. In persons with a sufficient vitamin D status in relation to their vitamin D response index, VDR will be optimally activated. These individuals will benefit from a well-functioning immune system and therefore have a low risk in developing MS (Table 1). In contrast, in insufficiently supplemented low vitamin D responders immune cells function suboptimal and/or differentiate into MS promoting subtypes, such as TH1 and TH17 instead of TH2 and Treg cells.
Table 1.
Previous clinical trials on vitamin D in MS (summarized in [90]) have not taken into the account the need for a dose adjustment according to the individual response index. Therefore, results were of mixed clinical efficacy. New vitamin D3 supplementation trials in MS should consider different dosing based on the patients response index to this allowing thus allowing a proper assessment of the biological and clinical effects.
| Vitamin D Response Index | Daily Vitamin D3 Supplementation | Immune System Balance Restored | Clinical Effect |
|---|---|---|---|
| High | Standard (1000 IU) | + | Present |
| Mid | Standard (1000 IU) | +/− | Partial |
| Mid | Mid (2000 IU) | + | Present |
| Low | Standard (1000 IU) | − | Absent |
| Low | High (4000 IU) | + | Present |
Some 25% of the general population are low vitamin D responders [45] (Section 2) and have an increased risk of developing MS. Therefore, future RCTs should consider the segregation into high, mid and low vitamin D responders. In addition, the guidelines for vitamin D supplementation should take into account that an individual may be a low vitamin D responder, i.e., higher daily doses should be recommended.
It is likely that low responsiveness to vitamin D is one of many approaches to characterize a subgroup of people that shows a susceptibility to multiple types of diseases including MS that is significantly higher than that of other subgroups. Thus, a low vitamin D index is a warning sign for the respective individual to better adapt his/her lifestyle to the given environmental conditions, such as (i) exposure to sunlight, (ii) sufficient physical activity outdoors and (iii) vitamin D3 supplementation in optimal amounts.
Author Contributions
C.C. and M.P.M. wrote and edited the manuscript. C.C. created the figures. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This publication is part of a project that has received funding from the European Union’s Horizon2020 research and innovation program under grant agreement no. 952601 and from the David and Amy Fulton Foundation, Seattle, US (both to C.C.). M.P.M. was supported by the National Science Centre Poland grant OPUS 2016/23/B/NZ6/02541 and a grant by the University of Warmia and Mazury in Olsztyn.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Walton C., King R., Rechtman L., Kaye W., Leray E., Marrie R.A., Robertson N., La Rocca N., Uitdehaag B., van der Mei I., et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020;26:1816–1821. doi: 10.1177/1352458520970841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charabati M., Wheeler M.A., Weiner H.L., Quintana F.J. Multiple sclerosis: Neuroimmune crosstalk and therapeutic targeting. Cell. 2023;186:1309–1327. doi: 10.1016/j.cell.2023.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reich D.S., Lucchinetti C.F., Calabresi P.A. Multiple Sclerosis. N. Engl. J. Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Quinn D.B., Palmer M.T., Lee Y.K., Weaver C.T. Emergence of the Th17 pathway and its role in host defense. Adv. Immunol. 2008;99:115–163. doi: 10.1016/S0065-2776(08)00605-6. [DOI] [PubMed] [Google Scholar]
- 5.Bierhansl L., Hartung H.P., Aktas O., Ruck T., Roden M., Meuth S.G. Thinking outside the box: Non-canonical targets in multiple sclerosis. Nat. Rev. Drug Discov. 2022;21:578–600. doi: 10.1038/s41573-022-00477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlberg C., Velleuer E., Molnar F. Molecular Medicine: How Science Works. Springer; Berlin/Heidelberg, Germany: 2023. [Google Scholar]
- 7.Ordovas J.M., Ferguson L.R., Tai E.S., Mathers J.C. Personalised nutrition and health. BMJ. 2018;361:bmj.k2173. doi: 10.1136/bmj.k2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Multiple Sclerosis Genetics C. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365:eaav7188. doi: 10.1126/science.aav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramagopalan S.V., Maugeri N.J., Handunnetthi L., Lincoln M.R., Orton S.M., Dyment D.A., Deluca G.C., Herrera B.M., Chao M.J., Sadovnick A.D., et al. Expression of the multiple sclerosis-associated MHC class II allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet. 2009;5:e1000369. doi: 10.1371/journal.pgen.1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., Jelcic I., Muhlenbruch L., Haunerdinger V., Toussaint N.C., Zhao Y., Cruciani C., Faigle W., Naghavian R., Foege M., et al. HLA-DR15 molecules jointly shape an autoreactive T cell repertoire in Multiple Sclerosis. Cell. 2020;183:1264–1281.e20. doi: 10.1016/j.cell.2020.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsson T., Barcellos L.F., Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017;13:25–36. doi: 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- 12.Hedstrom A.K., Olsson T., Kockum I., Hillert J., Alfredsson L. Low sun exposure increases multiple sclerosis risk both directly and indirectly. J. Neurol. 2020;267:1045–1052. doi: 10.1007/s00415-019-09677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostkamp P., Salmen A., Pignolet B., Gorlich D., Andlauer T.F.M., Schulte-Mecklenbeck A., Gonzalez-Escamilla G., Bucciarelli F., Gennero I., Breuer J., et al. Sunlight exposure exerts immunomodulatory effects to reduce multiple sclerosis severity. Proc. Natl. Acad. Sci. USA. 2021;118:e2018457118. doi: 10.1073/pnas.2018457118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sintzel M.B., Rametta M., Reder A.T. Vitamin D and multiple sclerosis: A comprehensive review. Neurol. Ther. 2018;7:59–85. doi: 10.1007/s40120-017-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierrot-Deseilligny C., Souberbielle J.C. Vitamin D and multiple sclerosis: An update. Mult. Scler. Relat. Disord. 2017;14:35–45. doi: 10.1016/j.msard.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Bendik I., Friedel A., Roos F.F., Weber P., Eggersdorfer M. Vitamin D: A critical and essential micronutrient for human health. Front. Physiol. 2014;5:248. doi: 10.3389/fphys.2014.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holick M.F. Vitamin D. 3rd ed. Academic Press; Cambridge, MA, USA: 2011. Photobiology of vitamin D; pp. 13–22. [DOI] [Google Scholar]
- 18.Pierrot-Deseilligny C., Souberbielle J.C. Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis? Brain A J. Neurol. 2010;133:1869–1888. doi: 10.1093/brain/awq147. [DOI] [PubMed] [Google Scholar]
- 19.Simpson S., Jr., Blizzard L., Otahal P., Van der Mei I., Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: A meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2011;82:1132–1141. doi: 10.1136/jnnp.2011.240432. [DOI] [PubMed] [Google Scholar]
- 20.van de Peppel J., van Leeuwen J.P. Vitamin D and gene networks in human osteoblasts. Front. Physiol. 2014;5:137. doi: 10.3389/fphys.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmeliet G., Dermauw V., Bouillon R. Vitamin D signaling in calcium and bone homeostasis: A delicate balance. Best Pract. Research. Clin. Endocrinol. Metab. 2015;29:621–631. doi: 10.1016/j.beem.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Chun R.F., Liu P.T., Modlin R.L., Adams J.S., Hewison M. Impact of vitamin D on immune function: Lessons learned from genome-wide analysis. Front. Physiol. 2014;5:151. doi: 10.3389/fphys.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanel A., Carlberg C. Time-resolved gene expression analysis monitors the regulation of inflammatory mediators and attenuation of adaptive immune response by vitamin D. Int. J. Mol. Sci. 2022;23:911. doi: 10.3390/ijms23020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malmberg H.R., Hanel A., Taipale M., Heikkinen S., Carlberg C. Vitamin D treatment sequence is critical for transcriptome modulation of immune challenged primary human cells. Front. Immunol. 2021;12:754056. doi: 10.3389/fimmu.2021.754056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu M., McComish B.J., Burdon K.P., Taylor B.V., Körner H. The association between vitamin D and multiple sclerosis risk: 1,25(OH)2D3 induces super-enhancers bound by VDR. Front. Immunol. 2019;10:488. doi: 10.3389/fimmu.2019.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeffery L.E., Raza K., Hewison M. Vitamin D in rheumatoid arthritis-towards clinical application. Nat. Rev. Rheumatol. 2016;12:201–210. doi: 10.1038/nrrheum.2015.140. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher J., Cooper S.C., Ghosh S., Hewison M. The role of vitamin D in Inflammatory bowel disease: Mechanism to management. Nutrients. 2019;11:1019. doi: 10.3390/nu11051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Infante M., Ricordi C., Sanchez J., Clare-Salzler M.J., Padilla N., Fuenmayor V., Chavez C., Alvarez A., Baidal D., Alejandro R., et al. Influence of vitamin D on islet autoimmunity and beta-cell function in type 1 diabetes. Nutrients. 2019;11:2185. doi: 10.3390/nu11092185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn J., Cook N.R., Alexander E.K., Friedman S., Walter J., Bubes V., Kotler G., Lee I.M., Manson J.E., Costenbader K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ. 2022;376:e066452. doi: 10.1136/bmj-2021-066452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manson J.E., Cook N.R., Lee I.M., Christen W., Bassuk S.S., Mora S., Gibson H., Gordon D., Copeland T., D’Agostino D., et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouillon R., Manousaki D., Rosen C., Trajanoska K., Rivadeneira F., Richards J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022;18:96–110. doi: 10.1038/s41574-021-00593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollis B.W. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 33.Del Valle H.B., Yaktine A.L., Taylor C.L., Ross A.C., editors. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; Washington, DC, USA: 2011. [PubMed] [Google Scholar]
- 34.Bouillon R., Carmeliet G., Verlinden L., van Etten E., Verstuyf A., Luderer H.F., Lieben L., Mathieu C., Demay M. Vitamin D and human health: Lessons from Vitamin D receptor null mice. Endocr. Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pludowski P., Holick M.F., Pilz S., Wagner C.L., Hollis B.W., Grant W.B., Shoenfeld Y., Lerchbaum E., Llewellyn D.J., Kienreich K., et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013;12:976–989. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M., Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 37.Genomes Project C., Abecasis G.R., Auton A., Brooks L.D., DePristo M.A., Durbin R.M., Handsaker R.E., Kang H.M., Marth G.T., McVean G.A. An integrated map of genetic variation from 1092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlberg C., Seuter S., de Mello V.D., Schwab U., Voutilainen S., Pulkki K., Nurmi T., Virtanen J., Tuomainen T.P., Uusitupa M. Primary Vitamin D target genes allow a categorization of possible benefits of vitamin D3 supplementation. PLoS ONE. 2013;8:e71042. doi: 10.1371/journal.pone.0071042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilfinger J., Seuter S., Tuomainen T.-P., Virtanen J.K., Voutilainen S., Nurmi T., de Mello V.D.F., Uusitupa M., Carlberg C. Primary vitamin D receptor target genes as biomarkers for the vitamin D3 status in the hematopoietic system. J. Nutr. Biochem. 2014;25:875–884. doi: 10.1016/j.jnutbio.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Ryynänen J., Neme A., Tuomainen T.P., Virtanen J.K., Voutilainen S., Nurmi T., de Mello V.D., Uusitupa M., Carlberg C. Changes in vitamin D target gene expression in adipose tissue monitor the vitamin D response of human individuals. Mol. Nutr. Food Res. 2014;58:2036–2045. doi: 10.1002/mnfr.201400291. [DOI] [PubMed] [Google Scholar]
- 41.Saksa N., Neme A., Ryynänen J., Uusitupa M., de Mello V.D., Voutilainen S., Nurmi T., Virtanen J.K., Tuomainen T.P., Carlberg C. Dissecting high from low responders in a vitamin D3 intervention study. J. Steroid Biochem. Mol. Biol. 2015;148:275–282. doi: 10.1016/j.jsbmb.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 42.Vukic M., Neme A., Seuter S., Saksa N., de Mello V.D., Nurmi T., Uusitupa M., Tuomainen T.P., Virtanen J.K., Carlberg C. Relevance of vitamin D receptor target genes for monitoring the Vitamin D responsiveness of primary human cells. PLoS ONE. 2015;10:e0124339. doi: 10.1371/journal.pone.0124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seuter S., Virtanen J.K., Nurmi T., Pihlajamäki J., Mursu J., Voutilainen S., Tuomainen T.P., Neme A., Carlberg C. Molecular evaluation of vitamin D responsiveness of healthy young adults. J. Steroid Biochem. Mol. Biol. 2017;174:314–321. doi: 10.1016/j.jsbmb.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Carlberg C. Molecular approaches for optimizing Vitamin D supplementation. Vitam. Horm. 2016;100:255–271. doi: 10.1016/bs.vh.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Carlberg C., Haq A. The concept of the personal vitamin D response index. J. Steroid Biochem. Mol. Biol. 2018;175:12–17. doi: 10.1016/j.jsbmb.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Salzer J., Hallmans G., Nystrom M., Stenlund H., Wadell G., Sundstrom P. Vitamin D as a protective factor in multiple sclerosis. Neurology. 2012;79:2140–2145. doi: 10.1212/WNL.0b013e3182752ea8. [DOI] [PubMed] [Google Scholar]
- 47.Mangin M., Sinha R., Fincher K. Inflammation and vitamin D: The infection connection. Inflamm. Res. 2014;63:803–819. doi: 10.1007/s00011-014-0755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleet J.C., DeSmet M., Johnson R., Li Y. Vitamin D and cancer: A review of molecular mechanisms. Biochem. J. 2012;441:61–76. doi: 10.1042/BJ20110744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang X., O’Reilly P.F., Aschard H., Hsu Y.H., Richards J.B., Dupuis J., Ingelsson E., Karasik D., Pilz S., Berry D., et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 2018;9:260. doi: 10.1038/s41467-017-02662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramasamy A., Trabzuni D., Forabosco P., Smith C., Walker R., Dillman A., Sveinbjornsdottir S., North American Brain Expression Consortium. UK Brain Expression Consortium. Hardy J., et al. Genetic evidence for a pathogenic role for the vitamin D3 metabolizing enzyme CYP24A1 in multiple sclerosis. Mult. Scler. Relat. Disord. 2014;3:211–219. doi: 10.1016/j.msard.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manousaki D., Dudding T., Haworth S., Hsu Y.H., Liu C.T., Medina-Gomez C., Voortman T., van der Velde N., Melhus H., Robinson-Cohen C., et al. Low-frequency synonymous coding variation in CYP2R1 has large efects on vitamin D levels and risk of multiple sclerosis. Am. J. Hum. Genet. 2017;101:227–238. doi: 10.1016/j.ajhg.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alloza I., Otaegui D., de Lapuente A.L., Antiguedad A., Varade J., Nunez C., Arroyo R., Urcelay E., Fernandez O., Leyva L., et al. ANKRD55 and DHCR7 are novel multiple sclerosis risk loci. Genes Immun. 2012;13:253–257. doi: 10.1038/gene.2011.81. [DOI] [PubMed] [Google Scholar]
- 53.Rieder M.J., Reiner A.P., Gage B.F., Nickerson D.A., Eby C.S., McLeod H.L., Blough D.K., Thummel K.E., Veenstra D.L., Rettie A.E. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N. Engl. J. Med. 2005;352:2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 54.Hanel A., Neme A., Malinen M., Hamalainen E., Malmberg H.R., Etheve S., Tuomainen T.P., Virtanen J.K., Bendik I., Carlberg C. Common and personal target genes of the micronutrient vitamin D in primary immune cells from human peripheral blood. Sci. Rep. 2020;10:21051. doi: 10.1038/s41598-020-78288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanel A., Veldhuizen C., Carlberg C. Gene-regulatory potential of 25-hydroxyvitamin D3 and D2. Front. Nutr. 2022;9:910601. doi: 10.3389/fnut.2022.910601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haberle V., Stark A. Eukaryotic core promoters and the functional basis of transcription initiation. Nat. Rev. Mol. Cell Biol. 2018;19:621–637. doi: 10.1038/s41580-018-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rando O.J., Chang H.Y. Genome-wide views of chromatin structure. Annu. Rev. Biochem. 2009;78:245–271. doi: 10.1146/annurev.biochem.78.071107.134639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carlberg C. Molecular endocrinology of Vitamin D on the epigenome level. Mol. Cell. Endocrinol. 2017;453:14–21. doi: 10.1016/j.mce.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 59.Carlberg C. Genome-wide (over)view on the actions of vitamin D. Front. Physiol. 2014;5:167. doi: 10.3389/fphys.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tuoresmäki P., Väisänen S., Neme A., Heikkinen S., Carlberg C. Patterns of genome-wide VDR locations. PLoS ONE. 2014;9:e96105. doi: 10.1371/journal.pone.0096105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neme A., Seuter S., Carlberg C. Selective regulation of biological processes by vitamin D based on the spatio-temporal cistrome of its receptor. Biochim. Et Biophys. Acta (BBA)-Gene Regul. Mech. 2017;1860:952–961. doi: 10.1016/j.bbagrm.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Novershtern N., Subramanian A., Lawton L.N., Mak R.H., Haining W.N., McConkey M.E., Habib N., Yosef N., Chang C.Y., Shay T., et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell. 2011;144:296–309. doi: 10.1016/j.cell.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Netea M.G., Joosten L.A., Latz E., Mills K.H., Natoli G., Stunnenberg H.G., O’Neill L.A., Xavier R.J. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saeed S., Quintin J., Kerstens H.H., Rao N.A., Aghajanirefah A., Matarese F., Cheng S.C., Ratter J., Berentsen K., van der Ent M.A., et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Novakovic B., Habibi E., Wang S.-Y., Arts R.J., Davar R., Megchelenbrink W., Kim B., Kuznetsova T., Kox M., Zwaag J., et al. β-Glucan reverses the epigenetic state of LPS-Induced immunological tolerance. Cell. 2016;167:1354–1368.e14. doi: 10.1016/j.cell.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munger K.L., Zhang S.M., O’Reilly E., Hernan M.A., Olek M.J., Willett W.C., Ascherio A. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.WNL.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 67.Cortese M., Riise T., Bjornevik K., Holmoy T., Kampman M.T., Magalhaes S., Pugliatti M., Wolfson C., Myhr K.M. Timing of use of cod liver oil, a vitamin D source, and multiple sclerosis risk: The EnvIMS study. Mult. Scler. 2015;21:1856–1864. doi: 10.1177/1352458515578770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Escriva H., Bertrand S., Laudet V. The evolution of the nuclear receptor superfamily. Essays Biochem. 2004;40:11–26. doi: 10.1042/bse0400011. [DOI] [PubMed] [Google Scholar]
- 69.Krasowski M.D., Ni A., Hagey L.R., Ekins S. Evolution of promiscuous nuclear hormone receptors: LXR, FXR, VDR, PXR, and CAR. Mol. Cell. Endocrinol. 2011;334:39–48. doi: 10.1016/j.mce.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heikkinen S., Väisänen S., Pehkonen P., Seuter S., Benes V., Carlberg C. Nuclear hormone 1α,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res. 2011;39:9181–9193. doi: 10.1093/nar/gkr654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vanherwegen A.S., Eelen G., Ferreira G.B., Ghesquiere B., Cook D.P., Nikolic T., Roep B., Carmeliet P., Telang S., Mathieu C., et al. Vitamin D controls the capacity of human dendritic cells to induce functional regulatory T cells by regulation of glucose metabolism. J. Steroid Biochem. Mol. Biol. 2019;187:134–145. doi: 10.1016/j.jsbmb.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 72.Vanherwegen A.S., Gysemans C., Mathieu C. Vitamin D endocrinology on the cross-road between immunity and metabolism. Mol. Cell. Endocrinol. 2017;453:52–67. doi: 10.1016/j.mce.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 73.Verstuyf A., Carmeliet G., Bouillon R., Mathieu C. Vitamin D: A pleiotropic hormone. Kidney Int. 2010;78:140–145. doi: 10.1038/ki.2010.17. [DOI] [PubMed] [Google Scholar]
- 74.Carlberg C. The physiology of vitamin D-far more than calcium and bone. Front. Physiol. 2014;5:335. doi: 10.3389/fphys.2014.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barragan M., Good M., Kolls J.K. Regulation of dendritic cell function by vitamin D. Nutrients. 2015;7:8127–8151. doi: 10.3390/nu7095383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dankers W., Colin E.M., van Hamburg J.P., Lubberts E. Vitamin D in autoimmunity: Molecular mechanisms and therapeutic potential. Front. Immunol. 2016;7:697. doi: 10.3389/fimmu.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kleinewietfeld M., Hafler D.A. Seminars in immunology. Volume 25. Academic Press; Cambridge, MA, USA: 2013. The plasticity of human Treg and Th17 cells and its role in autoimmunity; pp. 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palmer M.T., Lee Y.K., Maynard C.L., Oliver J.R., Bikle D.D., Jetten A.M., Weaver C.T. Lineage-specific effects of 1,25-dihydroxyvitamin D3 on the development of effector CD4 T cells. J. Biol. Chem. 2011;286:997–1004. doi: 10.1074/jbc.M110.163790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeffery L.E., Burke F., Mura M., Zheng Y., Qureshi O.S., Hewison M., Walker L.S., Lammas D.A., Raza K., Sansom D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Munger K.L., Levin L.I., Hollis B.W., Howard N.S., Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 81.Limketkai B.N., Mullin G.E., Limsui D., Parian A.M. Role of vitamin D in inflammatory bowel disease. Nutr. Clin. Pract. 2017;32:337–345. doi: 10.1177/0884533616674492. [DOI] [PubMed] [Google Scholar]
- 82.Zeitelhofer M., Adzemovic M.Z., Gomez-Cabrero D., Bergman P., Hochmeister S., N’Diaye M., Paulson A., Ruhrmann S., Almgren M., Tegner J.N., et al. Functional genomics analysis of vitamin D effects on CD4+ T cells in vivo in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 2017;114:E1678–E1687. doi: 10.1073/pnas.1615783114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carlberg C., Seuter S., Nurmi T., Tuomainen T.P., Virtanen J.K., Neme A. In vivo response of the human epigenome to Vitamin D: A proof-of-principle study. J. Steroid Biochem. Mol. Biol. 2018;180:142–148. doi: 10.1016/j.jsbmb.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 84.Neme A., Seuter S., Malinen M., Nurmi T., Tuomainen T.P., Virtanen J.K., Carlberg C. In vivo transcriptome changes of human white blood cells in response to vitamin D. J. Steroid Biochem. Mol. Biol. 2019;188:71–76. doi: 10.1016/j.jsbmb.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 85.Cortes M., Chen M.J., Stachura D.L., Liu S.Y., Kwan W., Wright F., Vo L.T., Theodore L.N., Esain V., Frost I.M., et al. Developmental vitamin D availability impacts hematopoietic stem cell production. Cell Rep. 2016;17:458–468. doi: 10.1016/j.celrep.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koivisto O., Hanel A., Carlberg C. Key Vitamin D target genes with functions in the immune system. Nutrients. 2020;12:1140. doi: 10.3390/nu12041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hanel A., Malmberg H.R., Carlberg C. Genome-wide effects of chromatin on Vitamin D signaling. J. Mol. Endocrinol. 2020;64:R45–R56. doi: 10.1530/JME-19-0246. [DOI] [PubMed] [Google Scholar]
- 88.da Costa D.S., Hygino J., Ferreira T.B., Kasahara T.M., Barros P.O., Monteiro C., Oliveira A., Tavares F., Vasconcelos C.C., Alvarenga R., et al. Vitamin D modulates different IL-17-secreting T cell subsets in multiple sclerosis patients. J. Neuroimmunol. 2016;299:8–18. doi: 10.1016/j.jneuroim.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 89.Muris A.H., Smolders J., Rolf L., Thewissen M., Hupperts R., Damoiseaux J., SOLARIUM Study Group Immune regulatory effects of high dose vitamin D3 supplementation in a randomized controlled trial in relapsing remitting multiple sclerosis patients receiving IFNbeta; the SOLARIUM study. J. Neuroimmunol. 2016;300:47–56. doi: 10.1016/j.jneuroim.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 90.Langlois J., Denimal D. Clinical and imaging outcomes after vitamin D supplementation in patients with Multiple Sclerosis: A systematic review. Nutrients. 2023;15:1945. doi: 10.3390/nu15081945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.