Abstract
Objective
To assess the benefits and harms of lipid-lowering therapies used to prevent or manage cardiovascular disease including bile acid sequestrants (BAS), ezetimibe, fibrates, niacin, omega-3 supplements, proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors, and statins.
Data sources
MEDLINE, the Cochrane Database of Systematic Reviews, and a grey literature search.
Study selection
Systematic reviews of randomized controlled trials published between January 2017 and March 2022 looking at statins, ezetimibe, PCSK9 inhibitors, fibrates, BAS, niacin, and omega-3 supplements for preventing cardiovascular outcomes were selected. Outcomes of interest included major adverse cardiovascular events (MACE), cardiovascular mortality, all-cause mortality, and adverse events.
Synthesis
A total of 76 systematic reviews were included. Four randomized controlled trials were also included for BAS because no efficacy systematic review was identified. Statins significantly reduced MACE (6 systematic reviews; median risk ratio [RR]=0.74; interquartile range [IQR]=0.71 to 0.76), cardiovascular mortality (7 systematic reviews; median RR=0.85, IQR=0.83 to 0.86), and all-cause mortality (8 systematic reviews; median RR=0.91, IQR=0.88 to 0.92). Major adverse cardiovascular events were also significantly reduced by ezetimibe (3 systematic reviews; median RR=0.93, IQR=0.93 to 0.94), PCSK9 inhibitors (14 systematic reviews; median RR=0.84, IQR=0.83 to 0.87), and fibrates (2 systematic reviews; mean RR=0.86), but these interventions had no effect on cardiovascular or all-cause mortality. Fibrates had no effect on any cardiovascular outcomes when added to a statin. Omega-3 combination supplements had no effect on MACE or all-cause mortality but significantly reduced cardiovascular mortality (5 systematic reviews; median RR=0.93, IQR=0.93 to 0.94). Eicosapentaenoic acid ethyl ester alone significantly reduced MACE (1 systematic review, RR=0.78) and cardiovascular mortality (2 systematic reviews; RRs of 0.82 and 0.82). In primary cardiovascular prevention, only statins showed consistent benefits on MACE (6 systematic reviews; median RR=0.75, IQR=0.73 to 0.78), cardiovascularall-cause mortality (7 systematic reviews, median RR=0.83, IQR=0.81 to 0.90), and all-cause mortality (8 systematic reviews; median RR=0.91, IQR=0.87 to 0.91).
Conclusion
Statins have the most consistent evidence for the prevention of cardiovascular complications with a relative risk reduction of about 25% for MACE and 10% to 15% for mortality. The addition of ezetimibe, a PCSK9 inhibitor, or eicosapentaenoic acid ethyl ester to a statin provides additional MACE risk reduction but has no effect on all-cause mortality.
Résumé
Objectif
Évaluer les bienfaits et les préjudices des thérapies hypolipidémiantes utilisées pour prévenir ou prendre en charge les maladies cardiovasculaires, y compris les chélateurs des acides biliaires (CAB), l’ézétimibe, les fibrates, la niacine, les suppléments d’omega-3, les inhibiteurs de la proprotéine convertase subtilisine-kexine de type 9 (PCSK9) et les statines.
Sources d’information
MEDLINE, la base de données des revues systématiques de Cochrane et une recension dans la littérature grise.
Sélection des études
Les revues systématiques d’essais randomisés contrôlés publiées entre janvier 2017 et mars 2022, portant sur les statines, l’ézétimibe, les inhibiteurs de la PCSK9, les fibrates, les CAB, la niacine et les suppléments d’omega-3 pour la prévention des événements cardiovasculaires (CV) ont été sélectionnées. Parmi les éléments recherchés figuraient les événements CV indésirables majeurs (ECIM), la mortalité CV, la mortalité toutes causes confondues et les effets indésirables.
Synthèse
Au total, 76 revues systématiques ont été incluses. Quatre essais randomisés contrôlés sur les CAB ont aussi été retenus, parce qu’aucune revue systématique sur leur efficacité n’a été recensée. Les statines réduisent les ECIM de manière significative (6 revues systématiques; rapport bénéfice/risque médian [RB/R]=0,74; intervalle interquartile [IIQ]=0,71 à 0,76), la mortalité CV (7 revues systématiques; RB/R médian=0,85, IIQ=0,83 à 0,86) et la mortalité toutes causes confondues (8 revues systématiques; RB/R médian=0,91, IIQ=0,88 à 0,92). Les événements cardiovasculaires indésirables majeurs ont aussi été réduits de manière significative par l’ézétimibe (3 revues systématiques; RB/R médian=0,93, IIQ=0,93 à 0,94), les inhibiteurs de la PCSK9 (14 revues systématiques; RB/R médian=0,84, IIQ=0,83 à 0,87) et les fibrates (2 revues systématiques; RB/R médian=0,86), mais ces interventions n’ont pas eu d’effets sur la mortalité CV ou toutes causes confondues. Les fibrates n’ont pas eu d’effets sur l’une ou l’autre des issues CV lorsqu’ils étaient ajoutés à une statine. Les suppléments combinés d’omega-3 n’ont pas eu d’effets sur les ECIM ou la mortalité toutes causes confondues, mais ont réduit significativement la mortalité CV (5 revues systématiques; RB/R médian=0,93, IIQ=0,93 à 0,94). L’ester éthylique de l’acide eicosapentaénoïque à lui seul a réduit de manière significative les ECIM (1 revue systématique; RB/R=0,78) et la mortalité CV (2 revues systématiques; RB/R de 0,82 et 0,82). Dans la prévention CV primaire, seules les statines ont obtenu des bienfaits constants pour les ECIM (6 revues systématiques; RB/R médian=0,75, IIQ=0,73 à 0,78), la mortalité CV (7 revues systématiques; RB/R médian=0,83, IIQ=0,81 à 0,90) et la mortalité (8 revues systématiques; RB/R médian=0,91, IIQ=0,87 à 0,91).
Conclusion
L’utilisation des statines est étayée par les données probantes les plus constantes pour la prévention des complications CV, obtenant une réduction du risque relatif d’environ 25 % dans le cas des ECIM et de 10 à 15 % sur le plan de la mortalité. L’ajout de l’ézétimibe, d’un inhibiteur de la PCSK9 ou de l’ester éthylique de l’acide eicosapentaénoïque à une statine procure une réduction additionnelle du risque d’ECIM, mais n’influe pas sur la mortalité toutes causes confondues.
Cardiovascular diseases (CVDs) are the leading cause of mortality globally.1 Numerous interventions are available to prevent and manage CVDs, including lipid-lowering agents such as bile acid sequestrants (BAS), ezetimibe, fibrates, niacin, omega-3 supplements, statins, and proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors.2-4
The purpose of this systematic review of systematic reviews is to assess benefits and harms of lipid-lowering pharmacotherapies used for the prevention and management of cardiovascular events. This systematic review will provide evidence for an updated PEER guideline on the management of dyslipidemia in primary care.
METHODS
This review was registered in PROSPERO (protocol number CRD42022333774).5 We performed 7 systematic reviews of systematic reviews following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and systematic review of systematic reviews protocols.6 The following 7 medications or medication classes were reviewed: BAS, ezetimibe, fibrates, niacin, omega-3 supplements, PCSK9 inhibitors, and statins.
We determined a priori to include relevant, systematic reviews of randomized controlled trials (RCTs) with meta-analyses, published in the past 5 years. We limited inclusion to the past 5 years to limit the number of systematic reviews to analyze and the risk of overrepresentation of older trials. In cases where fewer than 5 systematic reviews were identified, inclusion was expanded to publication within the past 8 years. Additionally, the most recent Cochrane systematic review pertaining to each medication class was included regardless of publication date.
Each review focused on a single intervention and included systematic reviews with RCTs that studied an adult patient population receiving pharmacotherapy for primary or secondary prevention of cardiovascular events. Our review also included special populations of interest, namely adults with type 2 diabetes or chronic kidney disease. All medications were compared with placebo, usual care, or no treatment. We included reviews of dual interventions in cases where the additional agent was used in both arms of the trial (eg, niacin and statins vs statins alone).
Systematic reviews were excluded if they did not complete a meta-analysis, included only active comparators (comparing 2 interventions), only reported surrogate outcomes, or focused on pediatric or pregnant patients or those with familial hypercholesterolemia. For interventions that did not have a meta-analysis or efficacy summary statistic, RCTs were included and analyzed.
Search strategy
We performed a comprehensive search strategy using MEDLINE and the Cochrane Database of Systematic Reviews. We limited the search to articles published in English and included articles from study inception (January 2017) to March 2022. The search strategy can be found in Appendix 1, available from CFPlus.* Additionally, a grey literature search was performed using Google Scholar and reference lists from the included systematic reviews. If no systematic reviews with eligible efficacy data were retrieved after expanding the initial search from 5 to 8 years, grey literature sources were used to identify relevant RCTs.
Outcomes
The primary outcome of this review was the proportion of patients who experienced a major adverse cardiovascular event (MACE) in either a primary cardiovascular prevention population or a mixed (primary and secondary cardiovascular prevention) population. We reported on 3-point MACE (composite end point of total cardiovascular death, nonfatal myocardial infarction [MI], or nonfatal stroke) when available, but allowed for other MACE definitions if reported. Secondary outcomes included cardiovascular-related mortality, all-cause mortality, MIs, and strokes.
Further, we collected adverse event data, primarily overall adverse events, serious adverse events, and withdrawals due to adverse events. Each systematic review also collected adverse event data relevant to that specific intervention (eg, muscle-related adverse events for statins).
Data collection and analysis
Selection of trials and data extraction. Title and abstract review, full-text review, and risk-of-bias assessment were completed independently by 2 authors. Included studies were extracted by 2 independent authors following MECIR (Methodological Expectations of Cochrane Intervention Reviews) criteria.7 Disagreements were resolved by consensus or by consulting a third author.
Risk-of-bias assessment. The risk of bias of each systematic review was assessed using a modified version of AMSTAR (A Measurement Tool to Assess Systematic Reviews).8 Randomized controlled trials were assessed using the Cochrane Collaboration’s risk-of-bias tool.9
Data synthesis strategy. For each intervention, we identified the median effect estimate and reported this for each outcome, along with the interquartile range (IQR), for the risk ratio (RR) effect estimates reported across systematic reviews. We chose to report the median as a realistic summary statistic and the IQR to capture any heterogeneity between effect estimates. If fewer than 3 systematic reviews were included for an outcome, individual RRs were reported. When needed, odds ratios (ORs) were converted to RRs, using the formula RR=OR/(1−p+[p×OR]),10 where p is the risk in the control group. When data were unavailable to calculate the RR, the systematic review results were excluded from the summary estimate. To determine if results were statistically significant, we used the statistics from the systematic review. We describe results as positive when at least half the systematic reviews for a specific outcome were statistically significant. Systematic reviews that reported different doses separately (ie, adverse effects of low vs high doses) were treated as 2 individual systematic reviews.
Analysis of subgroups or subsets
When available, in addition to overall results, we analyzed the following subgroups of patients often managed in primary care: those undergoing primary cardiovascular prevention, those undergoing secondary cardiovascular prevention, those with type 2 diabetes, and those with chronic kidney disease.
Quality assessment
We used the GRADE (Grading of Recommendations Assessment, Development and Evaluation) tool to report the certainty of the evidence.11
SYNTHESIS
Our initial search yielded 4768 results (Appendix 1*). Title and abstract review led to the exclusion of 4523 articles, leaving 245 for full-text review. In the end, 76 systematic reviews were included. All interventions but BAS were evaluated in at least 1 high-quality systematic review meeting all AMSTAR criteria (Appendix 2*). Four individual RCTs were included since no efficacy systematic review was identified for BAS. Details on included systematic reviews and RCTs can be found in Appendix 3.*
Full details of the primary outcome and the primary cardiovascular prevention subgroup analysis are available in Tables 1 and 2, respectively. Full details of all other outcomes and the subgroup analysis are available in Appendices 4 and 5.* Serious adverse events, overall adverse events, and withdrawals due to adverse events are presented in Table 3. Full details of adverse events that were retrieved from meta-analyses in systematic reviews are available in Appendix 6.* Certainty of evidence for each outcome with complete details on the GRADE process used can be found in Appendix 7.* Results for efficacy of lipid-lowering treatments for secondary CVD prevention are presented in Appendix 8.* Key findings are summarized below.
Table 1.
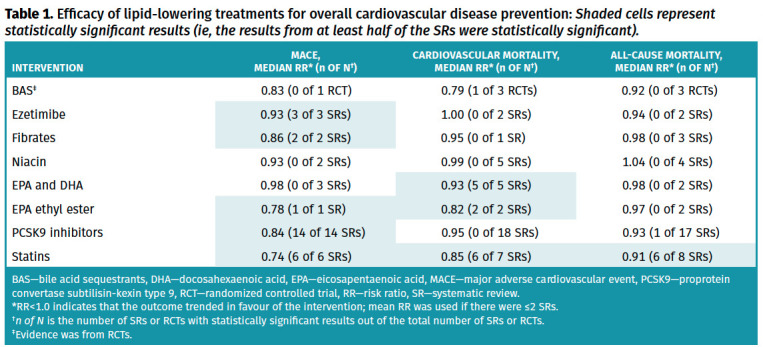
Efficacy of lipid-lowering treatments for overall cardiovascular disease prevention: Shaded cells represent statistically significant results (ie, the results from at least half of the SRs were statistically significant).
Table 2.
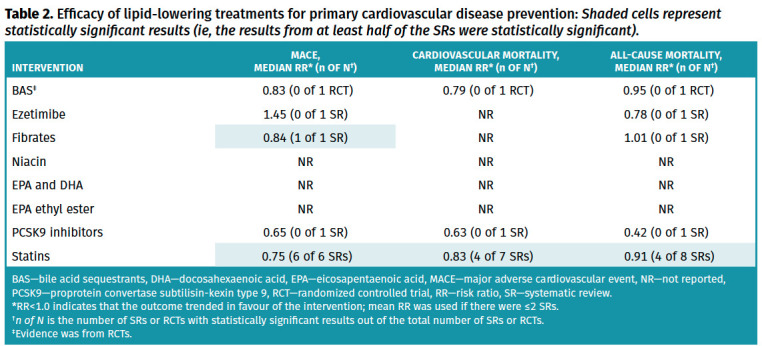
Efficacy of lipid-lowering treatments for primary cardiovascular disease prevention: Shaded cells represent statistically significant results (ie, the results from at least half of the SRs were statistically significant).
Table 3.
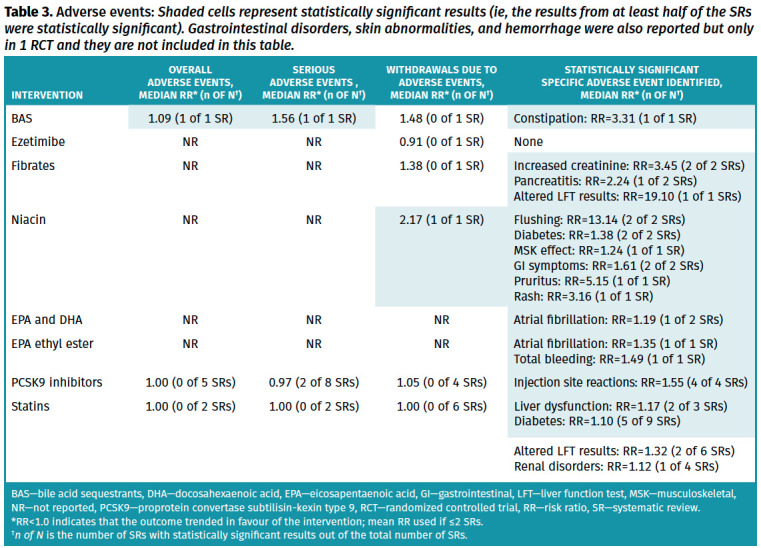
Adverse events: Shaded cells represent statistically significant results (ie, the results from at least half of the SRs were statistically significant). Gastrointestinal disorders, skin abnormalities, and hemorrhage were also reported but only in 1 RCT and they are not included in this table.
Statins
Efficacy. Thirty systematic reviews12-41 (4 to 135 RCTs, 625 to 192,977 participants followed for 5 days to 1040 weeks) looking at statins (various drugs and doses) compared with placebo, usual care, or no statin were included. Overall, statins reduced MACE (6 systematic reviews,23,27,29,30,32,37 median RR=0.74, IQR=0.71 to 0.76; all 6 systematic reviews statistically significant; high certainty of evidence), cardiovascular mortality (7 systematic reviews,13,20,23,27,30,32,37 median RR=0.85, IQR=0.83 to 0.86; 6 systematic reviews statistically significant; high certainty of evidence), and all-cause mortality (8 systematic reviews,12,20,23,27,29,30,32,37 median RR=0.91, IQR=0.88 to 0.92; 6 systematic reviews statistically significant; high certainty of evidence). In primary cardiovascular prevention, statins showed consistent benefits on MACE (6 systematic reviews, median RR=0.75, IQR=0.73 to 0.78), cardiovascular mortality (7 systematic reviews, median RR=0.83, IQR=0.81 to 0.90), and all-cause mortality (8 systematic reviews, median RR=0.91, IQR=0.87 to 0.91).
Safety. Statins did not increase the risk of withdrawals due to adverse events (6 systematic reviews,26,28,30,32,37,41 median RR=1.00, IQR=0.90 to 1.08; no systematic reviews statistically significant; high certainty of evidence), serious adverse events (2 systematic reviews,30,41 RRs of 0.99 and 1.01; no systematic reviews statistically significant; high certainty of evidence), any adverse effect (2 systematic reviews,32,41 RRs of 0.99 and 1.00; no systematic reviews statistically significant; high certainty of evidence), any muscle symptom (5 systematic reviews,13,14,18,41 median RR=1.03, IQR=1.01 to 1.05; 2 systematic reviews statistically significant), myalgia (5 systematic reviews,14,23,30,32 median RR=1.03, IQR=1.02 to 1.11; no systematic reviews statistically significant), myopathy (6 systematic reviews,13,23,30,34,37,41 median RR=1.09, IQR=1.02 to 2.16; 2 systematic reviews statistically significant), rhabdomyolysis (8 systematic reviews,14,23,26,30,32,34,41 median RR=1.15, IQR 0.95 to 2.58; no systematic reviews statistically significant), or creatine kinase level elevation 10 times above the normal upper limit (4 systematic reviews,14,23,30 median RR=1.24, IQR=0.95 to 2.38; no systematic reviews statistically significant).
Statins significantly increase the risk of liver dysfunction (3 systematic reviews,13,23,37 median RR=1.17, IQR=1.15 to 1.33; 2 systematic reviews statistically significant) or elevated liver enzyme levels (6 systematic reviews,23,26,30,32,34,35 median RR=1.32, IQR=1.06 to 2.39; 2 systematic reviews statistically significant) but definitions varied across systematic reviews. The renal risk associated with taking a statin is unclear but likely not clinically important, as most systematic reviews report no statistically significant differences in renal disorders (not defined) (4 systematic reviews,13,23,32,37 median RR=1.12, IQR=1.11 to 1.13; 1 systematic review statistically significant). Statins may increase the incidence of type 2 diabetes (9 systematic reviews,13,15,16,19,23,30,32,36,37 median RR=1.10, IQR=1.07 to 1.14; 5 systematic reviews statistically significant).
Discussion. Statins reduced MACE by about 25% and cardiovascular mortality or all-cause mortality by about 10% in both the overall and the primary cardiovascular prevention populations. Although results for MACE were consistent across systematic reviews, evidence on all-cause mortality in secondary cardiovascular prevention was conflicting, with 3 systematic reviews reporting a statistically significant benefit (RRs of 0.80 to 0.88)21,27,40 and 7 systematic reviews reporting no significant effect (RRs of 0.86 to 1.47).12,17,20,24,31,33,34 Of the systematic reviews reporting no significant effect, 4 were of patients with previous stroke only17,24,31,33 and 1 was of patients with acute coronary syndrome.34 In post hoc analysis, excluding these 5 systematic reviews resulted in an RR of 0.86 (IQR=0.81 to 0.93) with a high certainty of evidence. The 3 largest systematic reviews looking at the effect of statins on all-cause mortality in secondary cardiovascular prevention showed very consistent results, with RRs between 0.85 and 0.88.12,21,40
Pertaining to safety, statins did not cause more overall or serious adverse events but possibly caused more liver or renal disorders and diabetes. Results and definitions used varied between systematic reviews, especially for liver or renal disorders. Adding to that low RRs and a number of reviews reporting no significant effect, any possible increase in liver or renal disorders and diabetes would certainly be outweighed by the expected clinical benefits of statins.
Ezetimibe
Efficacy. Three systematic reviews42-44 with meta-analyses (4 to 26 RCTs, 18,921 to 23,499 participants followed for 52 to 312 weeks) looking at 10 mg of ezetimibe daily were included. Overall, ezetimibe reduced MACE (3 systematic reviews,42-44 median RR=0.93, IQR=0.93 to 0.94; all statistically significant; moderate certainty of evidence) but had no effect on cardiovascular mortality (2 systematic reviews,43,44 RRs of 1.00 and 1.00; no systematic reviews statistically significant; moderate certainty of evidence) or all-cause mortality (2 systematic reviews,43,44 RRs of 0.89 and 0.98; no systematic reviews statistically significant; low certainty of evidence). Variability between both results on all-cause mortality are due to different analyses used (fixed vs random effects analyses).
Safety. Ezetimibe did not increase the risk of withdrawals due to adverse events (1 systematic review,44 RR=0.91; not statistically significant; high certainty of evidence) or other notable adverse events.43
Discussion. Ezetimibe reduced MACE by about 7% (relative), but did not improve cardiovascular mortality or all-cause mortality. It should be noted that 85% to 96% of the participants in all 3 systematic reviews of ezetimibe are from the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial),45 which included 18,144 participants with previous MI taking a statin, and showed a 6% relative risk reduction in MACE (hazard ratio=0.94, 95% CI 0.89 to 0.99). It is therefore difficult to estimate the potential effect of ezetimibe in patients undergoing primary cardiovascular prevention or in those not taking a statin. The evidence for ezetimibe for primary cardiovascular prevention is limited to 1 systematic review44 (1 RCT, 720 participants with familial hypercholesterolemia) and found ezetimibe does not improve MACE.
Since publication of the included systematic reviews, 2 new ezetimibe RCTs have been published. A 2019 open-label RCT compared 10 mg of ezetimibe once a day versus no treatment (no other lipid-lowering therapies were allowed) in 3796 participants 75 years and older without a history of coronary artery disease (CAD) for approximately 4 years.46 Ezetimibe significantly reduced the incidence of the composite outcome of sudden cardiac death, fatal and nonfatal MI, coronary revascularization, or fatal and nonfatal stroke by approximately 35% (ezetimibe 5.2% vs 7.8%) but had no effect on all-cause mortality. However, the trial had multiple major limitations (only 63% of the planned sample size enrolled, the trial had an open-label design, and 28% of participants withdrew or were lost to follow-up).
A 2022 RCT47 compared a combination of 10 mg of ezetimibe and 10 mg of rosuvastatin daily versus 20 mg of rosuvastatin daily in 3780 participants with atherosclerotic CVD for 3 years. The combination was equivalent to rosuvastatin monotherapy on MACE.
Proprotein convertase subtilisin-kexin type 9 inhibitors
Efficacy. Twenty-six systematic reviews with meta-analyses39,43,48-71 (2 to 39 RCTs, 6281 to 97,910 participants followed for 8 to 305 weeks) looking at PCSK9 inhibitors (evolocumab, alirocumab, and bococizumab) were included. Overall, PCSK9 inhibitors reduced MACE (14 systematic reviews,43,49,51,54-56,59,62-64,68-71 median RR=0.84, IQR=0.83 to 0.87; all 14 systematic reviews statistically significant; moderate certainty of evidence) but had no effect on cardiovascular mortality (18 systematic reviews,43,49,51,52,54-60,62,64,65,68-71 median RR=0.95, IQR=0.94 to 0.97; no systematic reviews were statistically significant; high certainty of evidence) or all-cause mortality (17 systematic reviews,43,49,51-60,63,64,68-70 median RR=0.93, IQR=0.88 to 0.95; 1 systematic review statistically significant; high certainty of evidence).
Safety. Proprotein convertase subtilisin-kexin type 9 inhibitors were not associated with significant increases in withdrawals due to adverse events (4 systematic reviews,52,57,64,68 median RR=1.05, IQR=1.00 to 1.08; no systematic review was statistically significant; high certainty of evidence), serious adverse events (8 systematic reviews,49,51,58,64,67,68-70 median RR=0.97, IQR=0.96 to 0.99; 2 systematic reviews statistically significant; high certainty of evidence), or overall adverse events (5 systematic reviews,49,64,68-70 median RR=1.00, IQR=0.99 to 1.01; no systematic review was statistically significant; high certainty of evidence). Injection site reactions were more commonly reported with intervention (2.8% to 3.5%) compared with control (1.8% to 2.1%) (4 systematic reviews,57,64,67,70 median RR=1.55, IQR=1.44 to 1.97; all 4 systematic reviews statistically significant).
Discussion. Proprotein convertase subtilisin-kexin type 9 inhibitors reduced MACE by about 16% (relative) but had no effect on cardiovascular mortality or all-cause mortality. Evidence for PCSK9 inhibitors comes mostly from 2 large RCTs72,73 looking specifically at secondary cardiovascular prevention using a maximum tolerated dose of statin. In primary cardiovascular prevention, trials were smaller and many focused on people with familial hypercholesterolemia. Therefore, the effect of PCSK9 inhibitors in primary cardiovascular prevention or as monotherapy remains unclear.
Fibrates
Efficacy. Three systematic reviews with meta-analyses74-76 (6 to 20 RCTs, 16,112 to 46,099 participants followed for 4.8 to 5.2 years) looking at fibrates compared with placebo, in a general population, were included. Overall, fibrates reduced MACE (2 systematic reviews,74,76 RRs of 0.84 and 0.88; 2 systematic reviews statistically significant; moderate certainty of evidence) but had no effect on cardiovascular mortality (1 systematic review,76 median RR=0.95, IQR not available; no systematic reviews statistically significant; high certainty of evidence) or on all-cause mortality (3 systematic reviews,74-76 median RR=0.98, IQR=0.98 to 1.01; no systematic reviews statistically significant; moderate certainty of evidence).
Safety. Fibrate recipients had an increase in serum creatinine levels (2 systematic reviews,74,76 RRs of 1.88 and 5.01; 2 systematic reviews statistically significant), an increase in pancreatitis (2 systematic reviews,74,76 RRs of 1.74 and 2.74; 1 systematic review statistically significant), and an increase in altered liver function test results (1 systematic review,76 RR=19.1; statistically significant). Withdrawals due to adverse events were numerically higher in the fibrate group, but this was not statistically significant (1 systematic review,74 RR=1.38; not statistically significant; very low certainty of evidence).
Discussion. Fibrates reduced MACE by 12% to 16% (relative) but had no effect on cardiovascular mortality or all-cause mortality. Of note, fibrates showed no benefit when added to a statin.74,75 These results are also supported by a 2022 RCT that compared pemafibrate with placebo in 10,497 participants with diabetes (96% taking a statin) with elevated triglyceride levels.77 At a median follow-up of 3.4 years, no benefit of adding pemafibrate was observed for any cardiovascular outcome.
Bile acid sequestrants
Efficacy. No systematic reviews with meta-analyses reporting efficacy outcomes were identified in the initial search for BAS. A grey literature search identified 1 systematic review reporting on safety of BAS, and 4 RCTs.78-82
The largest RCT was the Lipid Research Clinics trial,81 which followed 3806 participants (mean age 48, 100% male) at high risk of CAD for a mean of 7.4 years. Participants were randomized to high-dose cholestyramine (24 g/day) or placebo. At 7.4 years, the composite end point of CAD death or nonfatal MI occurred in 8.1% in the intervention group compared with 9.8% in the placebo group (RR=0.83, 95% CI 0.67 to 1.01; calculated by the authors as it is not reported in the original publication).
Dorr et al80 randomized 2278 participants (mean age 54, 52% female, 26% had CAD) to 5 g of colestipol hydrochloride daily or placebo. Outcomes were reported by gender only. After 1 to 3 years, for men, death from any cardiovascular cause occurred in 2% of those prescribed colestipol hydrochloride, compared with 4.4% of those taking placebo (P<.02). Death from CAD occurred in 1.6% of men prescribed colestipol hydrochloride, compared with 4.0% prescribed placebo (P<.02). No statistically significant differences in all-cause mortality, death from acute MI, or death from all vascular diseases were found in men. In women, only all-cause mortality and death from CAD were reported, neither of which were statistically different.
Two very small RCTs with 143 and 53 participants79,82 did not find statistically significant differences between BAS and placebo for the primary outcomes of our review and were not included in our summary tables.
Safety. One systematic review78 with meta-analysis (17 RCTs, 2950 participants followed for 8 to 26 weeks) looked at BAS safety in a population with diabetes. Bile acid sequestrants were associated with increases in overall adverse events (1 systematic review,78 RR=1.09; statistically significant; moderate certainty of evidence) and serious adverse events (1 systematic review,78 RR=1.56; statistically significant; moderate certainty of evidence); however, a difference in withdrawals due to adverse events was found (1 systematic review,78 RR=1.48; not statistically significant; low certainty of evidence). Constipation was more common with BAS compared with control (1 systematic review,78 RR=3.31; statistically significant).
Discussion. Bile acid sequestrants do not appear to be beneficial in reducing MACE or mortality. It should be noted that the studies evaluating BAS pre-date the routine use of statins and other contemporary pharmacologic and nonpharmacologic cardiovascular prevention measures.
Niacin
Efficacy. Five systematic reviews83-87 with meta-analyses (4 to 23 RCTs, 34,294 to 39,195 participants followed for 24 to 312 weeks) looking at niacin (0.5 to 7.5 g/day) were included. Overall, niacin had no effect on MACE (2 systematic reviews,83,85 RRs of 0.88 and 0.97; no systematic review statistically significant; high certainty of evidence), cardiovascular mortality (5 systematic reviews,83-87 median RR=0.99, IQR=0.95 to 1.08; no systematic review statistically significant; high certainty of evidence), or all-cause mortality (4 systematic reviews,84-87 median RR=1.04, IQR=1.00 to 1.05; no systematic review statistically significant; high certainty of evidence).
Safety. Niacin caused more withdrawals due to adverse events (1 systematic review,87 RR=2.17; statistically significant; high certainty of evidence). It also increased the risk of flushing (2 systematic reviews,84,87 RRs of 7.69 and 18.59; 2 systematic reviews statistically significant), diabetes (2 systematic reviews,84,87 RRs of 1.32 and 1.44; 2 systematic reviews statistically significant), musculoskeletal effects (1 systematic review,84 RR=1.24; statistically significant), gastrointestinal symptoms (2 systematic reviews,84,87 RRs of 1.69 and 1.53; 2 systematic reviews statistically significant), pruritis (1 systematic review,87 RR=5.15; statistically significant), and rash (1 systematic review,87 RR=3.16; statistically significant).
Discussion. Niacin had no effect on MACE, cardiovascular mortality or all-cause mortality and caused substantial harms. Most trials examined secondary cardiovascular prevention and approximately 70% of all participants were from the HPS2-THRIVE trial (Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events),88 which found no benefit of adding niacin to a statin. The effect of niacin in primary cardiovascular prevention or as monotherapy has not been properly evaluated but is unlikely to be beneficial.
Omega-3 supplements
Eight systematic reviews20,89-95 (7 to 38 RCTs, 65,819 to 149,051 participants followed for 26 to 385 weeks) looking at omega-3 supplements were included. Given the documented heterogeneity among eicosapentaenoic acid (EPA) ethyl ester trials and EPA and docosahexaenoic acid (DHA) combination trials,94 these were analyzed separately.
Eicosapentaenoic acid and DHA
Efficacy: Overall, EPA and DHA supplementation had no effect on MACE (3 systematic reviews,89,91,94 median RR=0.98, IQR=0.97 to 0.99; no systematic review statistically significant; moderate certainty of evidence) or all-cause mortality (2 systematic reviews,20,94 RRs of 0.97 and 0.98; no systematic review statistically significant; high certainty of evidence) but reduced cardiovascular mortality (5 systematic reviews,20,90,93-95 median RR=0.93, IQR=0.93 to 0.94; 5 systematic reviews statistically significant; low certainty of evidence). An increase in nonfatal strokes was also reported (1 systematic review,94 RR=1.16, statistically significant; low certainty of evidence).
Safety: Eicosapentaenoic acid and DHA supplementation increased the risk of atrial fibrillation (2 systematic reviews,92,94 RRs of 1.14 and 1.23, 1 systematic review statistically significant).
Eicosapentaenoic acid ethyl ester
Efficacy: Overall, EPA ethyl ester reduced MACE (1 systematic review,94 RR=0.78; 1 systematic review statistically significant; moderate certainty of evidence) and cardiovascular mortality (2 systematic reviews,20,94 RRs of 0.82 and 0.82; 2 systematic reviews statistically significant; moderate certainty of evidence) but had no effect on all-cause mortality (2 systematic reviews,20,94 RRs of 0.96 and 0.98; no systematic review statistically significant; high certainty of evidence).
Safety: Eicosapentaenoic acid ethyl ester increased the risk of atrial fibrillation (1 systematic review,94 RR=1.35; 1 systematic review statistically significant) and total bleeding (1 systematic review,94 RR=1.49; 1 systematic review statistically significant).
Discussion. Eicosapentaenoic acid and DHA combination supplementation had no effect on MACE or all-cause mortality but had a low certainty of evidence for a modest reduction in cardiovascular mortality (about 7% relative).
Eicosapentaenoic acid ethyl ester alone reduced MACE and cardiovascular mortality by about 20% (relative) but had no effect on all-cause mortality. Results come mainly from 2 RCTs—REDUCE-IT (Reduction of Cardiovascular Events With EPA–Intervention Trial)96 and JELIS (Japan EPA Lipid Intervention Study)97—with results surprisingly different from those for EPA and DHA combination supplements. The JELIS trial was open label, which might have overestimated the effect, and REDUCE-IT used a placebo containing mineral oil, which might have negatively affected the results in the placebo group.98
DISCUSSION
Statins remain the lipid-lowering drugs with the most consistent benefits, with a relative risk reduction of approximately 25% for MACE and approximately 10% for all-cause mortality. Although MACE was also reduced with ezetimibe (about 7% relative), fibrates (about 15% relative), and PCSK9 inhibitors (about 16% relative), these drugs had no effect on cardiovascular mortality or all-cause mortality. Omega-3 supplements might reduce cardiovascular mortality (about 7% relative) but have no effect on MACE or all-cause mortality. Eicosapentaenoic acid ethyl ester supplements might reduce MACE and cardiovascular mortality (about 20% relative) but have no effect on all-cause mortality and increase the risk of atrial fibrillation and bleeding. Niacin and BAS do not appear to provide any cardiovascular benefit.
Unfortunately, except for statins, evidence for primary cardiovascular prevention is very limited. Most evidence arises from secondary cardiovascular prevention trials. Statins appear to have similar benefits in primary and secondary cardiovascular prevention. One systematic review found a 14% relative risk reduction in MACE for fibrates, but no effect when added to a statin.74 Our review did not find statistically significant primary cardiovascular prevention benefits for BAS, ezetimibe, niacin, omega-3 supplements, EPA ethyl ester, or PCSK9 inhibitors.
Strengths and limitations
The principal strength of this systematic review of systematic reviews is the breadth of our review, including the evaluation of many commonly used medications and the provision of a broad perspective on the benefits and harms of lipid-lowering therapies for CVD prevention. We also focused on systematic reviews reporting patient-oriented outcomes, excluding studies reporting only surrogate markers, to ensure our results would help inform a shared decision-making process. This systematic review underwent external peer review (Appendix 9*).
Our review also has limitations. Restricting our search to recent systematic reviews might have limited our results (eg, recent trials, subgroup analysis). In addition, many of the included systematic reviews report on a limited number of trials and regularly include the same trials (eg, for PCSK9s there were 26 systematic reviews, but 2 RCTs provided most of the evidence). This could disproportionately give more weight to older trials, although restricting our search to the past 5 years should have limited this risk. Additionally, the inclusion of lesser-quality systematic reviews may have impacted our median and IQR calculations. Beyond statins, our results show limited data in individuals with no history of CVD. In addition, newer included primary prevention RCTs often focus on subgroups such as people with hypertriglyceridemia or familial hypercholesterolemia, again making it difficult to extrapolate the results to a more typical population seen in primary care.
Another limitation to our review is the exclusion of some drugs. For example, inclisiran, a small interfering RNA molecule inhibiting PCSK9, was not included in our review because it was not available in Canada at the time of our literature search. Finally, we did not perform any systematic analyses to evaluate the difference in benefits and harms of lipid-lowering therapies used either alone or in combination with another medication. Since most large non-statin therapy trials included individuals already taking a statin, our results probably do not allow for a comprehensive shared decision-making discussion for patients who cannot take a statin.
Finally, it should also be noted that most large RCTs looking at lipid-lowering therapies are industry funded, which might influence the results and limit the evaluation of harms.99
Conclusion
Overall, our systematic review of systematic reviews found consistent evidence of benefits for statins on MACE and mortality. Ezetimibe and PCSK9 inhibitors reduce cardiovascular events, but have no benefit on cardiovascular or all-cause mortality. Omega-3 supplements reduce cardiovascular mortality but have no effect on MACE or all-cause mortality, except for EPA ethyl ester, which also reduces MACE. Niacin does not improve cardiovascular outcomes. We found inconsistent benefits for fibrates based on concurrent statin use and for BAS from studies before the statin era.
For primary cardiovascular prevention, our review did not find statistically significant cardiovascular benefits for BAS, ezetimibe, niacin, omega-3 supplements, or PCSK9 inhibitors.
This review helped provide the scientific evidence to inform an updated PEER simplified lipid guideline.
Supplementary Material
Acknowledgment
We thank the College of Family Physicians of Canada, the Ontario College of Family Physicians, the Alberta College of Family Physicians, and the Saskatchewan College of Family Physicians for their financial support. In addition, we thank Drs Karenn Chan and Anthony Train for their assistance with title and abstract review, and Odelia Moses and Deanna Draga for their administrative support.
Editor’s key points
▸ Statins remain the lipid-lowering drugs with the most consistent benefits, with a relative risk reduction of approximately 25% for major adverse cardiovascular events (MACE) and approximately 10% for mortality. Although MACE was also reduced with ezetimibe (about 7% relative), fibrates (about 15% relative), and proprotein convertase subtilisin-kexin type 9 inhibitors (about 15% relative), these drugs had no effect on cardiovascular mortality or all-cause mortality. Omega-3 supplements might reduce cardiovascular mortality (about 7% relative) but have no effect on MACE or all-cause mortality. Eicosapentaenoic acid ethyl ester supplements might reduce MACE and cardiovascular mortality (about 20% relative) but have no effect on all-cause mortality and increase the risk of atrial fibrillation and bleeding. Niacin and bile acid sequestrants do not appear to provide any cardiovascular benefit.
▸ Except for statins, evidence for primary cardiovascular prevention is very limited. Most evidence arises from secondary cardiovascular prevention trials. Statins appear to have similar benefits in primary and secondary cardiovascular prevention.
▸ This systematic review of systematic reviews provided the scientific evidence to inform the updated PEER simplified lipid guideline (page 675).
Points de repère du rédacteur
▸ Les statines demeurent les médicaments hypolipidémiants qui produisent les bienfaits les plus constants; il réduit d’environ 25 % le risque relatif (RR) d’événements cardiovasculaires indésirables majeurs (ECIM) et d’environ 10 % celui de la mortalité. Même si les ECIM ont aussi été réduits avec l’ézétimibe (d’environ 7 % du RR), les fibrates (d’environ 15 % du RR) et les inhibiteurs de la proprotéine convertase subtilisinekexine de type 9 (d’environ 15 % du RR), ces médicaments n’ont pas eu d’effets sur la mortalité cardiovasculaire ou toutes causes confondues. Les suppléments d’omega-3 pourraient réduire la mortalité cardiovasculaire (d’environ 7 % du RR), mais ils n’ont pas d’effets sur les ECIM ou sur la mortalité toutes causes confondues. Les suppléments d’ester éthylique de l’acide eicosapentaénoïque pourraient réduire les ECIM (d’environ 20 % du RR), mais ils n’ont pas d’effets sur la mortalité toutes causes confondues et augmentent le risque de fibrillation auriculaire et de saignements. La niacine et les chélateurs des acides biliaires ne semblent pas procurer de bienfaits cardiovasculaires.
▸ Les données probantes sur la prévention cardiovasculaire primaire sont très limitées, sauf en ce qui concerne les statines. La plupart des données factuelles proviennent d’essais sur la prévention cardiovasculaire secondaire.
▸ Cette revue systématique des revues systématiques a fourni les données probantes scientifiques sur lesquelles se fonde l’actualisation des lignes directrices simplifiées de PEER sur les lipides (page e189).
Footnotes
Appendices 1 to 9 and the disclosure of potential conflicts of interest are available from https://www.cfp.ca. Go to the full text of the article online and click on the CFPlus tab.
Contributors
Dr G. Michael Allan, Dr Émélie Braschi, Dr Nicolas Dugré, Dr Jamie Falk, Liesbeth Froentjes, Dr Scott R. Garrison, Dr Jessica E.M. Kirkwood, Dr Michael R. Kolber, Dr Christina S. Korownyk, Dr Adrienne J. Lindblad, Dr James P. McCormack, Dr Samantha S. Moe, Dr Allison Paige, Danielle Perry, Dr Jen Potter, Betsy S. Thomas, Dr Joey Ton, Dr Justin Weresch, and Dr Jennifer Young completed the evidence reviews and all authors approved the manuscript for submission.
Competing interests
There are no conflicts of interest involving the pharmaceutical industry. All other potential competing interests are available from CFPlus.*
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
References
- 1.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76(25):2982-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140(11):e596-646. Epub 2019 Mar 17. Errata in: Circulation 2019;140(11):e649-50, Circulation 2020;141(4):e60, Circulation 2020;141(16):e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol 2021;37(8):1129-50. Epub 2021 Mar 26. [DOI] [PubMed] [Google Scholar]
- 4.O’Malley PG, Arnold MJ, Kelley C, Spacek L, Buelt A, Natarajan S, et al. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2020 updated U.S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guideline. Ann Intern Med 2020;173(10):822-9. Epub 2020 Sep 22. [DOI] [PubMed] [Google Scholar]
- 5.Perry D, Kolber MR, Lindblad A, Allan GM, Korownyk C, Thomas B.. PEER umbrella review of systematic reviews: lipid-lowering pharmacotherapy for the prevention of major adverse cardiac events. Protocol number: CRD42022333774. Southampton, UK: PROSPERO, National Institute for Health and Care Research; 2022. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022333774. Accessed 2023 Aug 29. [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6(7):e1000097. Epub 2009 Jul 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins JPT, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, et al. Methodological expectations of Cochrane intervention reviews. London, UK: Cochrane; 2022. [Google Scholar]
- 8.Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol 2009;62(10):1013-20. Epub 2009 Feb 20. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ 2014;348:f7450. Erratum in: BMJ 2014;348:g2124. [DOI] [PubMed] [Google Scholar]
- 11.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64(4):401-6. Epub 2011 Jan 5. [DOI] [PubMed] [Google Scholar]
- 12.Byrne P, Demasi M, Jones M, Smith SM, O’Brien KK, DuBroff R.. Evaluating the association between low-density lipoprotein cholesterol reduction and relative and absolute effects of statin treatment: a systematic review and meta-analysis. JAMA Intern Med 2022;182(5):474-81. Erratum in: JAMA Intern Med 2022;182(5):579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai T, Abel L, Langford O, Monaghan G, Aronson JK, Stevens RJ, et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses. BMJ 2021;374:n1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis JW, Weller SC.. Intensity of statin therapy and muscle symptoms: a network meta-analysis of 153 000 patients. BMJ Open 2021;11(6):e043714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domecq JP, Prutsky G, Elraiyah T, Wang Z, Mauck KF, Brito JP, et al. Medications affecting the biochemical conversion to type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab 2019;104(9):3986-95. [DOI] [PubMed] [Google Scholar]
- 16.Engeda JC, Stackhouse A, White M, Rosamond WD, Lhachimi SK, Lund JL, et al. Evidence of heterogeneity in statin-associated type 2 diabetes mellitus risk: a meta-analysis of randomized controlled trials and observational studies. Diabetes Res Clin Pract 2019;151:96-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang JX, Wang EQ, Wang W, Liu Y, Cheng G.. The efficacy and safety of high-dose statins in acute phase of ischemic stroke and transient ischemic attack: a systematic review. Intern Emerg Med 2017;12(5):679-87. Epub 2017 Mar 16. [DOI] [PubMed] [Google Scholar]
- 18.Irwin JC, Khalesi S, Fenning AS, Vella RK.. The effect of lipophilicity and dose on the frequency of statin-associated muscle symptoms: a systematic review and meta-analysis. Pharmacol Res 2018;128:264-73. Epub 2017 Sep 21. [DOI] [PubMed] [Google Scholar]
- 19.Khan SU, Rahman H, Okunrintemi V, Riaz H, Khan MS, Sattur S, et al. Association of lowering low-density lipoprotein cholesterol with contemporary lipid-lowering therapies and risk of diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc 2019;8(7):e011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Hoang T, Kim JM, Bu SY, Choi JH, Park E, et al. All-cause mortality and cardiovascular death between statins and omega-3 supplementation: a meta-analysis and network meta-analysis from 55 randomized controlled trials. Nutrients 2020;12(10):3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koskinas KC, Siontis GCM, Piccolo R, Mavridis D, Raber L, Mach F, et al. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur Heart J 2018;39(14):1172-80. [DOI] [PubMed] [Google Scholar]
- 22.Liang X, He Q, Zhao Q.. Effect of stains on LDL reduction and liver safety: a systematic review and meta-analysis. Biomed Res Int 2018;2018:7092414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M, Wang X, Li X, Chen H, Hu Y, Zhang X, et al. Statins for the primary prevention of coronary heart disease. Biomed Res Int 2019;2019:4870350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manktelow BN, Potter JF.. Interventions in the management of serum lipids for preventing stroke recurrence. Cochrane Database Syst Rev 2009;(3):CD002091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milionis H, Ntaios G, Korompoki E, Vemmos K, Michel P.. Statin-based therapy for primary and secondary prevention of ischemic stroke: a meta-analysis and critical overview. Int J Stroke 2020;15(4):377-84. Epub 2019 Sep 7. [DOI] [PubMed] [Google Scholar]
- 26.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Hegbrant J, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev 2014;(5):CD007784. [DOI] [PubMed] [Google Scholar]
- 27.Ponce OJ, Larrea-Mantilla L, Hemmingsen B, Serrano V, Rodriguez-Gutierrez R, Spencer-Bonilla G, et al. Lipid-lowering agents in older individuals: a systematic review and meta-analysis of randomized clinical trials. J Clin Endocrinol Metab 2019;104(5):1585-94. [DOI] [PubMed] [Google Scholar]
- 28.Riaz H, Khan AR, Khan MS, Rehman KA, Alansari SAR, Gheyath B, et al. Meta-analysis of placebo-controlled randomized controlled trials on the prevalence of statin intolerance. Am J Cardiol 2017;120(5):774-81. Epub 2017 Jun 13. [DOI] [PubMed] [Google Scholar]
- 29.Sandwith L, Forget P.. Statins in healthy adults: a meta-analysis. Medicina (Kaunas) 2021;57(6):585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh BM, Lamichhane HK, Srivatsa SS, Adhikari P, Kshetri BJ, Khatiwada S, et al. Role of statins in the primary prevention of atherosclerotic cardiovascular disease and mortality in the population with mean cholesterol in the near-optimal to borderline high range: a systematic review and meta-analysis. Adv Prev Med 2020;2020:6617905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Squizzato A, Romualdi E, Dentali F, Ageno W.. Statins for acute ischemic stroke. Cochrane Database Syst Rev 2011;(8):CD007551. [DOI] [PubMed] [Google Scholar]
- 32.Taylor F, Huffman MD, Macedo AF, Moore THM, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;(1):CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tramacere I, Boncoraglio GB, Banzi R, Del Giovane C, Kwag KH, Squizzato A, et al. Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: a systematic review and network meta-analysis. BMC Med 2019;17(1):67. Epub 2019 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vale N, Nordmann AJ, Schwartz GG, de Lemos J, Colivicchi F, den Hartog F, et al. Statins for acute coronary syndrome. Cochrane Database Syst Rev 2014;(9):CD006870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villani R, Navarese EP, Cavallone F, Kubica J, Bellanti F, Facciorusso A, et al. Risk of statin-induced hypertransaminasemia: a systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc Innov Qual Outcomes 2019;3(2):131-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Cai R, Yuan Y, Varghese Z, Moorhead J, Ruan XZ.. Association between reductions in low-density lipoprotein cholesterol with statin therapy and the risk of new-onset diabetes: a meta-analysis. Sci Rep 2017;7:39982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yebyo HG, Aschmann HE, Kaufmann M, Puhan MA.. Comparative effectiveness and safety of statins as a class and of specific statins for primary prevention of cardiovascular disease: a systematic review, meta-analysis, and network meta-analysis of randomized trials with 94,283 participants. Am Heart J 2019;210:18-28. Epub 2019 Jan 10. [DOI] [PubMed] [Google Scholar]
- 38.Yin Y, Zhang L, Marshall I, Wolfe C, Wang Y.. Statin therapy for preventing recurrent stroke in patients with ischemic stroke: a systematic review and meta-analysis of randomized controlled trials and observational cohort studies. Neuroepidemiology 2022;56(4):240-9. Epub 2022 Jun 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Z, Du S, Shen S, Luo P, Ding S, Wang G, et al. Comparative efficacy and safety of lipid-lowering agents in patients with hypercholesterolemia: a frequentist network meta-analysis. Medicine (Baltimore) 2019;98(6):e14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong P, Wu D, Ye X, Wu Y, Li T, Tong S, et al. Secondary prevention of major cerebrovascular events with seven different statins: a multi-treatment meta-analysis. Drug Des Devel Ther 2017;11:2517-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Z, Albarqouni L, Curtis AJ, Breslin M, Nelson M.. The safety and tolerability of statin therapy in primary prevention in older adults: a systematic review and meta-analysis. Drugs Aging 2020;37(3):175-85. [DOI] [PubMed] [Google Scholar]
- 42.Hong N, Lee YH, Tsujita K, Gonzalez JA, Kramer CM, Kovarnik T, et al. Comparison of the effects of ezetimibe-statin combination therapy on major adverse cardiovascular events in patients with and without diabetes: a meta-analysis. Endocrinol Metab (Seoul) 2018;33(2):219-27. Epub 2018 May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toyota T, Morimoto T, Yamashita Y, Shiomi H, Kato T, Makiyama T, et al. More- versus less-intensive lipid-lowering therapy. Systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2019;12(8):e005460. Epub 2019 Aug 15. [DOI] [PubMed] [Google Scholar]
- 44.Zhan S, Tang M, Liu F, Xia P, Shu M, Wu X.. Ezetimibe for the prevention of cardiovascular disease and all-cause mortality events. Cochrane Database Syst Rev 2018;11(11):CD012502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372(25):2387-97. Epub 2015 Jun 3. [DOI] [PubMed] [Google Scholar]
- 46.Ouchi Y, Sasaki J, Arai H, Yokote K, Harada K, Katayama Y, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation 2019;140(12):992-1003. Epub 2019 Aug 22. [DOI] [PubMed] [Google Scholar]
- 47.Kim BK, Hong SJ, Lee YJ, Hong SJ, Yun KH, Hong BK, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet 2022;400(10349):380-90. Epub 2022 Jul 18. [DOI] [PubMed] [Google Scholar]
- 48.AlTurki A, Marafi M, Dawas A, Dube MP, Vieira L, Sherman MH, et al. Meta-analysis of randomized controlled trials assessing the impact of proprotein convertase subtilisin/kexin type 9 antibodies on mortality and cardiovascular outcomes. Am J Cardiol 2019;124(12): 1869-75. Epub 2019 Sep 26. [DOI] [PubMed] [Google Scholar]
- 49.Bai J, Gong L, Li QF, Wang Z.. Long-term efficacy and safety of proprotein convertase subtilisin/kexin 9 monoclonal antibodies: a meta-analysis of 11 randomized controlled trials. J Clin Lipidol 2018;12(2):277-91.e3. Epub 2018 Jan 12. [DOI] [PubMed] [Google Scholar]
- 50.Bajaj NS, Patel N, Kalra R, Ahmad A, Venkatraman A, Arora G, et al. Neurological effects of proprotein convertase subtilisin/kexin type 9 inhibitors: direct comparisons. Eur Heart J Qual Care Clin Outcomes 2018;4(2):132-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casula M, Olmastroni E, Boccalari MT, Tragni E, Pirillo A, Catapano AL.. Cardiovascular events with PCSK9 inhibitors: an updated meta-analysis of randomised controlled trials. Pharmacol Res 2019;143:143-50. Epub 2019 Mar 26. [DOI] [PubMed] [Google Scholar]
- 52.Chaiyasothi T, Nathisuwan S, Dilokthornsakul P, Vathesatogkit P, Thakkinstian A, Reid C, et al. Effects of non-statin lipid-modifying agents on cardiovascular morbidity and mortality among statin-treated patients: a systematic review and network meta-analysis. Front Pharmacol 2019;10:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cordero A, Rodríguez-Mañero M, Fácila L, Fernández-Olmo MR, Gómez-Martínez MJ, Valle A, et al. Prevention of myocardial infarction and stroke with PCSK9 inhibitors treatment: a metanalysis of recent randomized clinical trials. J Diabetes Metab Disord 2020;19(2):759-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dicembrini I, Giannini S, Ragghianti B, Mannucci E, Monami M.. Effects of PCSK9 inhibitors on LDL cholesterol, cardiovascular morbidity and all-cause mortality: a systematic review and meta-analysis of randomized controlled trials. J Endocrinol Invest 2019;42(9):1029-39. Epub 2019 Feb 14. [DOI] [PubMed] [Google Scholar]
- 55.Du H, Li X, Su N, Li L, Hao X, Gao H, et al. Proprotein convertase subtilisin/kexin 9 inhibitors in reducing cardiovascular outcomes: a systematic review and meta-analysis. Heart 2019;105(15):1149-59. Epub 2019 Mar 6. [DOI] [PubMed] [Google Scholar]
- 56.Ghadban R, Enezate T, Omran J, Almourani R, Singla A, Balla S.. Clinical outcomes of PCSK9Is: a meta-analysis of randomized clinical trials. Cardiovasc Diagn Ther 2017;7(6):598-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guedeney P, Giustino G, Sorrentino S, Claessen BE, Camaj A, Kalkman DN, et al. Efficacy and safety of alirocumab and evolocumab: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J 2019. Jul 3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 58.Karatasakis A, Danek BA, Karacsonyi J, Rangan BV, Roesle MK, Knickelbine T, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta-analysis of 35 randomized controlled trials. J Am Heart Assoc 2017;6(12):e006910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan SU, Khan MU, Virani SS, Khan MS, Khan MZ, Rashid M, et al. Efficacy and safety for the achievement of guideline-recommended lower low-density lipoprotein cholesterol levels: a systematic review and meta-analysis. Eur J Prev Cardiol 2022;28(18):2001-9. [DOI] [PubMed] [Google Scholar]
- 60.Khan SU, Riaz H, Rahman H, Khan MU, Khan MS, Alkhouli M, et al. Association of baseline LDL-C with total and cardiovascular mortality in patients using proprotein convertase subtilisin-kexin type 9 inhibitors: a systematic review and meta-analysis. J Clin Lipidol 2019;13(4):538-49. Epub 2019 Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee M, Cheng CY, Wu YL, Lee JD, Hsu CY, Ovbiagele B.. Association between intensity of low-density lipoprotein cholesterol reduction with statin-based therapies and secondary stroke prevention: a meta-analysis of randomized clinical trials. JAMA Neurol 2022;79(4):349-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma W, Guo X, Ma Y, Hu Z.. Meta-analysis of randomized clinical trials comparing PCSK9 monoclonal antibody versus ezetimibe/placebo in patients at high cardiovascular risk. Atherosclerosis 2021;326:25-34. Epub 2021 May 7. [DOI] [PubMed] [Google Scholar]
- 63.Monami M, Sesti G, Mannucci E.. PCSK9 inhibitor therapy: a systematic review and meta-analysis of metabolic and cardiovascular outcomes in patients with diabetes. Diabetes Obes Metab 2019;21(4):903-8. Epub 2018 Dec 21. [DOI] [PubMed] [Google Scholar]
- 64.Mu G, Xiang Q, Zhou S, Liu Z, Qi L, Jiang J, et al. Efficacy and safety of PCSK9 monoclonal antibodies in patients at high cardiovascular risk: an updated systematic review and meta-analysis of 32 randomized controlled trials. Adv Ther 2020;37(4):1496-521. Epub 2020 Feb 27. [DOI] [PubMed] [Google Scholar]
- 65.Qin J, Liu L, Su XD, Wang BB, Fu BS, Cui JZ, et al. The effect of PCSK9 inhibitors on brain stroke prevention: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis 2021;31(8):2234-43. [DOI] [PubMed] [Google Scholar]
- 66.Sagris D, Ntaios G, Georgiopoulos G, Konstantinos P, Milionis H.. Proprotein convertase subtilisin-kexin type 9 inhibitors and stroke prevention: a meta-analysis. Eur J Intern Med 2021;85:130-2. Epub 2021 Jan 5. [DOI] [PubMed] [Google Scholar]
- 67.Talasaz AH, Ho ACJ, Bhatty F, Koenig RA, Dixon DL, Baker WL, et al. Meta-analysis of clinical outcomes of PCSK9 modulators in patients with established ASCVD. Pharmacotherapy 2021;41(12):1009-23. Epub 2021 Oct 30. [DOI] [PubMed] [Google Scholar]
- 68.Turgeon RD, Tsuyuki RT, Gyenes GT, Pearson GJ.. Cardiovascular efficacy and safety of PCSK9 inhibitors: systematic review and meta-analysis including the ODYSSEY OUTCOMES trial. Can J Cardiol 2018;34(12):1600-5. [DOI] [PubMed] [Google Scholar]
- 69.Wang W, Feng Z, Bai J.. Effects of alirocumab on cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Rev Cardiovasc Med 2021;22(3):873-81. [DOI] [PubMed] [Google Scholar]
- 70.Zhao Z, Hu X, Zhang Y, Liu D.. Cardiovascular and safety events of PCSK9 inhibitors in statin-treated patients with cardiovascular risk: a systematic review and meta-analysis. J Pharm Pharm Sci 2020;23:422-36. [DOI] [PubMed] [Google Scholar]
- 71.Zhu Y, Shen X, Jiang Q, Wang Z, Wang Z, Dong X, et al. Effects of monoclonal antibodies against PCSK9 on clinical cardiovascular events: a meta-analysis of randomized controlled trials. Herz 2019;44(4):336-46. Epub 2017 Nov 7. [DOI] [PubMed] [Google Scholar]
- 72.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376(18):1713-22. Epub 2017 Mar 17. [DOI] [PubMed] [Google Scholar]
- 73.Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379(22):2097-107. Epub 2018 Nov 7. [DOI] [PubMed] [Google Scholar]
- 74.Jakob T, Nordmann AJ, Schandelmaier S, Ferreira-González I, Briel M.. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev 2016;(11):CD009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keene D, Price C, Shun-Shin MJ, Francis DP.. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ 2014;349:g4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang D, Liu B, Tao W, Hao Z, Liu M.. Fibrates for secondary prevention of cardiovascular disease and stroke. Cochrane Database Syst Rev 2015;(10):CD009580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Das Pradhan A, Glynn RJ, Fruchart JC, MacFadyen JG, Zaharris ES, Everett BM, et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med 2022;387(21):1923-34. Epub 2022 Nov 5. [DOI] [PubMed] [Google Scholar]
- 78.Hansen M, Sonne DP, Mikkelsen KH, Gluud LL, Vilsbøll T, Knop FK.. Bile acid sequestrants for glycemic control in patients with type 2 diabetes: a systematic review with meta-analysis of randomized controlled trials. J Diabetes Complications 2017;31(5):918-27. Epub 2017 Jan 24. [DOI] [PubMed] [Google Scholar]
- 79.Brensike JF, Levy RI, Kelsey SF, Passamani ER, Richardson JM, Loh IK, et al. Effects of therapy with cholestyramine on progression of coronary arteriosclerosis: results of the NHLBI Type II Coronary Intervention Study. Circulation 1984;69(2):313-24. [DOI] [PubMed] [Google Scholar]
- 80.Dorr AE, Gundersen K, Schneider JC Jr, Spencer TW, Martin WB.. Colestipol hydrochloride in hypercholesterolemic patients—effect on serum cholesterol and mortality. J Chronic Dis 1978;31(1):5-14. [DOI] [PubMed] [Google Scholar]
- 81.The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984;251(3):351-64. [DOI] [PubMed] [Google Scholar]
- 82.Watts GF, Lewis B, Lewis ES, Coltart DJ, Smith LDR, Swan AV, et al. Effects on coronary artery disease of lipid-lowering diet, or diet plus cholestyramine, in the St Thomas’ Atherosclerosis Regression Study (STARS). Lancet 1992;339(8793):563-9. [DOI] [PubMed] [Google Scholar]
- 83.D’Andrea E, Hey SP, Ramirez CL, Kesselheim AS.. Assessment of the role of niacin in managing cardiovascular disease outcomes: a systematic review and meta-analysis. JAMA Netw Open 2019;2(4):e192224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garg A, Sharma A, Krishnamoorthy P, Garg J, Virmani D, Sharma T, et al. Role of niacin in current clinical practice: a systematic review. Am J Med 2017;130(2):173-87. Epub 2016 Oct 26. [DOI] [PubMed] [Google Scholar]
- 85.Jenkins DJA, Spence JD, Giovannucci EL, Kim YI, Josse RG, Vieth R, et al. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment: JACC Focus Seminar. J Am Coll Cardiol 2021;77(4):423-36. [DOI] [PubMed] [Google Scholar]
- 86.Riaz H, Khan SU, Rahman H, Shah NP, Kaluski E, Lincoff AM, et al. Effects of high-density lipoprotein targeting treatments on cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol 2019;26(5):533-43. Epub 2018 Dec 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schandelmaier S, Briel M, Saccilotto R, Olu KK, Arpagaus A, Hemkens LG, et al. Niacin for primary and secondary prevention of cardiovascular events. Cochrane Database Syst Rev 2017;(6):CD009744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.HPS2-THRIVE Collaborative Group; Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371(3):203-12. [DOI] [PubMed] [Google Scholar]
- 89.Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol 2018;3(3):225-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cabiddu MF, Russi A, Appolloni L, Mengato D, Chiumente M.. Omega-3 for the prevention of cardiovascular diseases: meta-analysis and trial-sequential analysis. Eur J Hosp Pharm 2020;29(3):134-8. Epub 2020 Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Casula M, Olmastroni E, Gazzotti M, Galimberti F, Zambon A, Catapano AL.. Omega-3 polyunsaturated fatty acids supplementation and cardiovascular outcomes: do formulation, dosage, and baseline cardiovascular risk matter? An updated meta-analysis of randomized controlled trials. Pharmacol Res 2020;160:105060. Epub 2020 Jul 4. [DOI] [PubMed] [Google Scholar]
- 92.Gencer B, Djousse L, Al-Ramady OT, Cook NR, Manson JE, Albert CM.. Effect of long-term marine ω-3 fatty acids supplementation on the risk of atrial fibrillation in randomized controlled trials of cardiovascular outcomes: a systematic review and meta-analysis. Circulation 2021;144(25):1981-90. Epub 2021 Oct 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu Y, Hu FB, Manson JE.. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc 2019;8(19):e013543. Epub 2019 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khan SU, Lone AN, Khan MS, Virani SS, Blumenthal RS, Nasir K, et al. Effect of omega-3 fatty acids on cardiovascular outcomes: a systematic review and meta-analysis. EClinicalMedicine 2021;38:100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xie L, Zhen P, Wei Q, Yu F, Song S, Tong J.. Effects of omega-3 polyunsaturated fatty acids supplementation for patients with cardiovascular disease risks: a dose-response meta-analysis. Am J Transl Res 2021;13(8):8526-39. [PMC free article] [PubMed] [Google Scholar]
- 96.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380(1):11-22. Epub 2018 Nov 10. [DOI] [PubMed] [Google Scholar]
- 97.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369(9567):1090-8. Erratum in: Lancet 2007;370(9583):220. [DOI] [PubMed] [Google Scholar]
- 98.Ridker PM, Rifai N, MacFadyen J, Glynn RJ, Jiao L, Steg PG, et al. Effects of randomized treatment with icosapent ethyl and a mineral oil comparator on interleukin-1β, interleukin-6, C-reactive protein, oxidized low-density lipoprotein cholesterol, homocysteine, lipoprotein(a), and lipoprotein-associated phospholipase A2: a REDUCE-IT biomarker substudy. Circulation 2022;146(5):372-9. Epub 2022 Jun 28. [DOI] [PubMed] [Google Scholar]
- 99.Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L.. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2017;(2):MR000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


