Significance
Life on Earth has long been suggested to have originated in submarine hydrothermal systems. Although this hypothesis has been investigated by numerous prebiotic experiments, it remains a conundrum what geochemical mechanism led to accumulation of ammonia, an essential nitrogen species for abiotic synthesis of life’s building blocks. Here, we show that mackinawite, an iron sulfide mineral common in submarine hydrothermal systems, drastically enhances its adsorption capability for ammonia through electroreduction to zero-valent iron, enabling over 90% accumulation of 1 mM ammonia in 1 M NaCl at neutral pH. Given spontaneous generation of electricity widespread in present ocean hydrothermal vent environments, our demonstrated electrochemical mechanism of ammonia accumulation is likely to have been a ubiquitous geochemical phenomenon on the early seafloor.
Keywords: astrobiology, life emergence, prebiotic chemistry, geochemistry
Abstract
An increasing amount of evidence suggests that early ocean hydrothermal systems were sustained sources of ammonia, an essential nitrogen species for prebiotic synthesis of life’s building blocks. However, it remains a riddle how the abiotically generated ammonia was retained at the vent–ocean interface for the subsequent chemical evolution. Here, we demonstrate that, under simulated geoelectrochemical conditions in early ocean hydrothermal systems ( V versus the standard hydrogen electrode), mackinawite gradually reduces to zero-valent iron (), generating interlayer sites. This reductive conversion leads to an up to 55-fold increase in the solid/liquid partition coefficient for ammonia, enabling over 90% adsorption of 1 mM ammonia in 1 M NaCl at neutral pH. A coordinative binding of ammonia on the interlayer sites was computed to be the major mechanism of selective ammonia adsorption. Mackinawite is a ubiquitous sulfide precipitate in submarine hydrothermal systems. Given its reported catalytic function in amination, the extreme accumulation of ammonia on electroreduced mackinawite should have been a crucial initial step for prebiotic nitrogen assimilation, paving the way to the origin of life.
It has long been suggested that submarine hydrothermal systems were among the most suitable settings for the origin and early evolution of life (1–3). Supporting this hypothesis are laboratory simulations demonstrating abiotic synthesis of ammonia/ammonium ion (/; hereafter referred to simply as ammonia), an essential nitrogen species for the production of the building blocks of life. To date, a variety of iron-bearing minerals seen in hydrothermal vent environments, such as iron sulfides (4–6), magnetite (5), komatiite (7), green rust (8), and iron–nickel alloys (9), has been shown to facilitate ammonia formation via the reduction of nitrate () and nitrite () in water. Efficient nitrate and nitrite reductions to ammonia have also been demonstrated on molybdenum sulfide () as an electrocatalyst (10, 11). Based on these results, along with experimental and computational estimates on the production rates of nitrate and nitrite through atmospheric/oceanic processes (12), micro- to submillimolar concentrations of ammonia have been suggested to be supplied sustainably at the vent–ocean interface of early ocean hydrothermal systems (7).
However, it remains a question as to how the abiotically generated ammonia was retained and accumulated within vent chimneys for the subsequent chemical evolution of life. Laboratory simulations of prebiotic organic synthesis typically use 100 mM or higher concentrations of ammonia (13). To the best of our knowledge, 5 mM is the reported lowest concentration of ammonia used for the synthesis of amino acids (14); however, in the presence of 5 mM ammonia, the yields of glycine and alanine from corresponding keto acids (2.5 mM) were as low as 1.1% and 0.36%, respectively (28 and 9 M, respectively). In the early ocean, the steady-state concentration of ammonia has been estimated to be 3.6–70 M (15), which is four to five orders of magnitude lower than that of the major seawater cation, (1 M) (16). Given the neutral or monocationic nature of ammonia, its weak electrostatic interaction with negatively charged mineral surfaces is unlikely to suppress the diffusive loss of ammonia into seawater. Certain clay minerals (e.g., kaolinite, montmorillonite, and vermiculite) and zeolites are known to selectively adsorb ammonia in their interlayer and/or nanopore structures (17–20). However, their availability on the early ocean floor is unclear (21, 22).
Here, we report the exceptional capability of mackinawite (), an iron sulfide mineral ubiquitous in submarine hydrothermal systems, for ammonia accumulation triggered by electrochemical reduction. A recent in situ electrochemical survey of the Okinawa Trough hydrothermal fields revealed that spontaneous generation of electricity is widespread in deep-sea vent chimneys and mineral deposits (23, 24). Subsequent laboratory simulations of the geoelectrochemical system have demonstrated (electro)catalytic functions of metal sulfides for organic/inorganic reactions crucial for the origin of life (25–29). Furthermore, it was found that under a negative electric potential realizable in early ocean hydrothermal systems, certain metal sulfides, including mackinawite, undergo hour- to day-scale electroreduction to corresponding metals, thereby drastically enhancing their capabilities of promoting prebiotically important reactions (30, 31).
Mackinawite has a layered crystalline structure with an interlayer distance of 0.5 nm (32). Because the intralayer Fe–Fe distance (0.260 nm)(32) is close to the molecular diameter of (0.26 nm)(33), the detachment of sulfur atoms through mackinawite electroreduction to zero-valent iron () is expected to form angstrom-size pores on the interlayer surface, within which ammonia may stably coordinate to the exposed site. Our experiment shown below demonstrates a drastic increase in the ammonia adsorption onto mackinawite through its electroreduction.
Notice that our unveiled electrochemical mechanism leading to ammonia accumulation is different from that developed for ammonia removal from wastewaters (34, 35). In the wastewater treatment, an electrochemically generated pH gap between two compartments, separated by a hydrophobic membrane, has been utilized to induce a spontaneous ammonia transfer from the anode to the cathode. Importantly, mackinawite is not a mere ammonia adsorbent. Considering the catalytic function of pure and electroreduced mackinawite in the amination reaction (30, 36, 37), mackinawite is likely to have served as an entry point for nitrogen into prebiotic chemistry, leading to the formation of N-containing building blocks of life.
Results
Ammonia Adsorption onto Electroreduced Mackinawite.
Mackinawite was prepared by simply mixing an aqueous solution of iron chloride () and sodium sulfide (). The obtained mackinawite (300 mg) was immersed in 1 M containing 1 mM (60 mL) and exposed to a constant electric potential for 48 h at room temperature (25 2 °C) using an H-type cell (SI Appendix, Fig. S1). The solution pH was maintained at slightly acidic to neutral (6.3 1.2; SI Appendix, Fig. S2) by continuous bubbling (20 ). In the course of mackinawite electrolysis, the supernatant solution was sampled periodically (1 mL for each) and measured for the dissolved ammonia concentration through high-performance liquid chromatography (HPLC). The quantified concentration was used to calculate the amount of ammonia adsorption onto mackinawite, assuming ammonia partitioning between the liquid and adsorbed phases. After the electrolysis, mackinawite was separated from the supernatant solution, dried under vacuum, and characterized with the techniques described below and in SI Appendix.
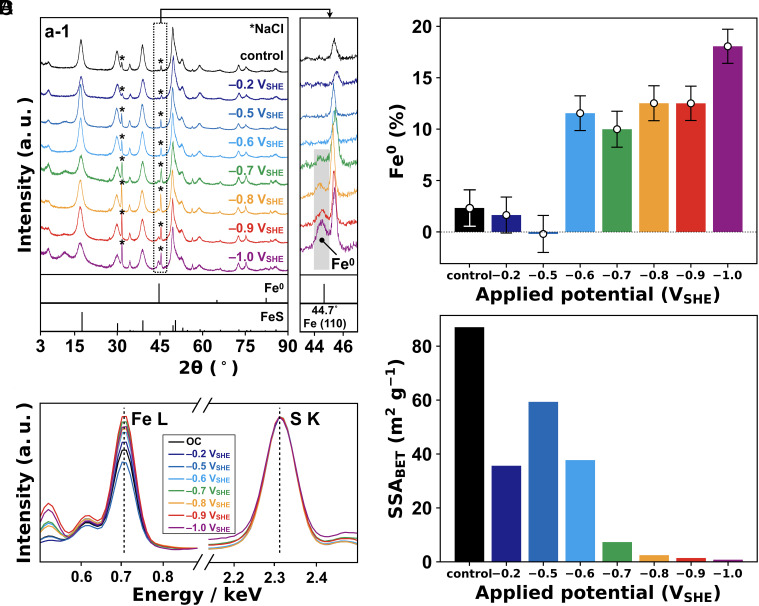
Fig. 1A shows the X-ray diffraction (XRD) patterns of mackinawite after the 48-h experiment. Consistent with our previous observation (30, 31), mackinawite electrolyzed at (versus the standard hydrogen electrode) exhibited a broad but recognizable XRD signal at 44.7°, which is assigned to the -form of with a body-centered cubic structure (Fig. 1A). The formation of was also indicated by energy-dispersive X-ray spectroscopy (EDS) on the electrolyzed mackinawite, in which a clear decrease in the sulfur signal intensity relative to iron was observed in comparison with nonelectrolyzed mackinawite (Fig. 1B and SI Appendix, Fig. S3; EDS data in Fig. 1B are normalized by S K line signal at 2.31 keV.). The Fe/S intensity ratio reflects the degree of electroreduction (Fig. 1C and SI Appendix, Table S1). It was estimated that the percentage of in electrolyzed mackinawite remained approximately zero at and , turned positive at , and increased at lower potentials to 18 1.7% at , in agreement with thermodynamic calculations (30, 31). The occurrence and growth of was furthermore suggested by ex-situ measurements of the iron K-edge extended X-ray absorption fine structure (EXAFS) (SI Appendix, Fig. S4). At , longer reaction duration led to greater spectral distortion particularly in the wavenumber range from 9 to 11 Å. A least-squares fitting of the sample EXAFS spectra with those of pure and pure revealed an increase in the percentage of for the initial 24 h up to around 5% (SI Appendix, Figs. S4D and S5). The estimated percentage after the 48-h electrolysis is lower than that determined by EDS (14.5 1.7%; Fig. 1C) possibly because of the attenuated amplitude of EXAFS oscillation in k space due to the particle size of ; smaller particles in several-nm scale tend to exhibit lower EXAFS oscillation in the k space spectra (38, 39). Thus, although the EXAFS data support the conversion of to , the EDS-determined percentages of (Fig. 1C) are more reliable and hence, will be used in the following discussion. Considering the observation that the concentration of in supernatant solution was suppressed by applying a potential of (SI Appendix, Fig. S6), the electroreduction likely proceeded via the dissolution of and its subsequent electrodeposition. The dissolution/precipitation mechanism is supported by the decrease in the Brunauer–Emmett–Teller specific surface area () of mackinawite, particularly at (Fig. 1D and SI Appendix, Figs. S7 and S8). No appearance of an XRD signal for at the threshold potential (; Fig. 1A) suggests that atoms were atomically dispersed in the mackinawite structure. Such dispersing may serve as seeds for the crystal growth of - at lower potentials.
Fig. 1.
Characteristics of electrolyzed mackinawite. (A) XRD patterns of mackinawite. Asterisks show the residual NaCl, and spectra at 43–47° are magnified on the right. The condition without applying voltage is shown as control. (B) Normalized EDS spectra. EDS profiles in a wider energy range (0–4 keV) are shown in SI Appendix, Fig. S3. (C) Percentages of in electrolyzed mackinawite determined from the EDS signal intensity of Fe (0.7 keV) relative to S (2.3 keV). Error bars are the SD of EDS measurements. (D) BET-specific surface area. We did not carry out the electrochemical experiment at potentials between and because the potential monitored during the control experiment ranged between and (SI Appendix, Fig. S10).
At , the concentration of in supernatant solution was also suppressed (SI Appendix, Fig. S6). Although is insufficient for the electroreduction (Fig. 1A–C), a weak current was observed during the experiment due to evolution (; Eh = at pH 6) (SI Appendix, Fig. S9). This -to- conversion is expected to increase the surface pH of mackinawite, suppressing the dissolution of ().
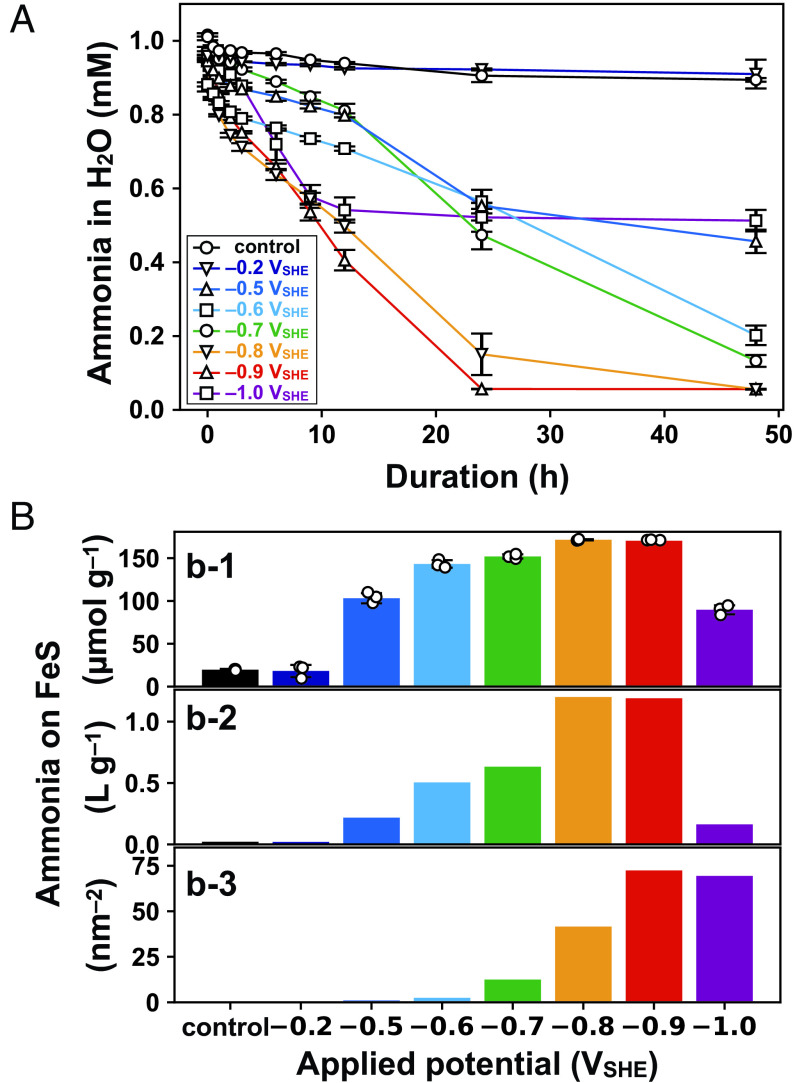
The concentration of dissolved ammonia also varied significantly with applied potential (Fig. 2A). Compared with the result in the absence of an applied potential, which showed a 11 0.24% decrease after the 48-h experiment, no appreciable influence of the exposure was discerned, whereas greater and steeper decreases occurred at lower potentials. The lowest concentration of 0.056 mM was observed at and after the 48-h experiment, which corresponds to 171 mol g of ammonia adsorption onto mackinawite (Fig. 2, b-1) and a solid/liquid partition coefficient of 1.2 (Fig. 2, b-2). As the of mackinawite decreased substantially at (Fig. 1D), the -based adsorption amount of ammonia reached 72.4 molecules at , which was approximately 600 times higher than that in the absence of an applied potential (0.12 molecules ) (Fig. 2, b-3). In units of “molecules ,” the surface density of ammonia adsorption at was similar to that at (Fig. 2, b-3), although the mass-based adsorption value (mol g) at was 48% lower than that at (Fig. 2, b-1). At , the of mackinawite decreased to 0.79 , whereas that after the electrolysis was 1.42 (Fig. 1D and SI Appendix, Table S2). This greater decrease in at lower potential is the cause of the apparent discrepancy. The adsorbed ammonia was also characterized with an elemental analyzer/isotope ratio mass spectrometer (EA/IRMS). The longer the duration of FeS electrolysis at , the more nitrogen (N) was released from the electrolyzed FeS through combustion (SI Appendix, Fig. S11). The amounts of N per gram of FeS samples (mol g) were slightly lower than the adsorption amounts of ammonia determined from the dissolved ammonia concentrations (Fig. 2 A and B) probably because of partial desorption of ammonia through the vacuum drying of FeS samples (Materials and Methods).
Fig. 2.
Ammonia adsorption onto electrolyzed mackinawite. (A) Changes in the dissolved concentration of ammonia during mackinawite electrolysis. Error bars represent the SE of three independent experiments. (B) Mass- and -based adsorption value of ammonia on mackinawite after the 48-h electrolysis (b-1 and b-3, respectively). The solid/liquid partition coefficient is also shown in b-2.
After the electrolysis, the ammonia accumulated on electrolyzed mackinawite was mostly retained over several hours (SI Appendix, Fig. S12). Subsequent monitoring without an externally imposed electric potential found a gradual desorption of ammonia, together with an increase in the open-circuit potential (OCP). The increase in OCP is ascribed to the oxidation of to siderite (), as indicated by the XRD pattern presented in SI Appendix, Fig. S13.
Importance of the Interlayer Sites for Ammonia Accumulation.
What surface mechanism was responsible for the drastic increase in ammonia adsorption onto mackinawite at ? -based adsorption attained at (72.4 molecules ; Fig. 2, b-3) is far greater than the density of surface sites for ion adsorption onto mackinawite (FeSH; 4.0 sites ) (40). Thus, a well-known electrostatic ion–surface interaction is not a major mechanism leading to ammonia accumulation. During the electrolysis, the vicinity of the mackinawite surface is expected to become alkaline owing to evolution () (27). However, a batch adsorption experiment without an applied potential showed, at most, a twofold increase in the ammonia adsorption by solution alkalization (pH 10 was the optimum condition; SI Appendix, Fig. S14), indicating a minor contribution of surface alkaline pH.
In light of the reported high affinity of to pure (41, 42), an alternative interpretation is that the electroreduced mackinawite accumulated ammonia on the interlayer sites through the coordinative binding of . The Fe atoms in mackinawite are oriented in a square planar geometry with an Fe–Fe distance of 0.26 nm, constituting the (001) plane (Fig. 3A) (32, 40). Our first-principles calculations (SI Appendix, Tables S3–S14) revealed that the removal of one sulfur atom from the 441 supercell (corresponding to a 6.25% conversion of to ) resulted in the shrinkage of intra- and intersheet Fe-Fe distances from 2.53/5.02 Å to 2.40/4.98 Å, respectively. When structure optimization was performed with an intercalated molecule, the energetically most favorable position of was confirmed to be on the exposed Fe site (Fig. 3B and SI Appendix, Table S15). The intercalation of distorted the crystal symmetry around the active Fe site from tetragonal to triclinic, elongating the intra- and intersheet Fe–Fe distances toward the original values (2.48–2.60 and 5.05 Å, respectively). The calculated shortest distance between Fe and N (2.00 Å) corresponded well with the sum of their covalent radii (2.03 Å, where Fe is in the low spin state) (43), indicating the covalent character of the Fe–N bond. The overall energy associated with intercalation was , whereas that in pure mackinawite was . Thus, spontaneous intercalation of requires electroreduction.
Fig. 3.

Supercell (221) structures of (A) pure and (B) electrolyzed mackinawite computed by first-principles calculations. In B, the electroreduction is expressed by the removal of one S atom from the interlayer surface. The resultant surface-exposed Fe was found to be the energetically most favorable site for the intercalation (see text).
Consistent with our interpretation, other iron-containing minerals, such as hematite, magnetite, and ferrihydrite, adsorbed greater amounts of ammonia when they were partially electroreduced to (SI Appendix, Figs. S15–S17). Note, however, that on these minerals, only surface-exposing atoms are available for the binding. In contrast, each atom in mackinawite belongs to the interlayer and, thus, potentially serves as the binding site for . In the measurement, the interlayer surface is invisible, owing to the persistence of intercalated , even after vacuum drying (32). As a consequence, electrolyzed mackinawite adsorbed even greater amounts of ammonia than did pure under identical electrochemical conditions. At , for example, pure nanoparticles (95–105 nm, 99.5% purity) adsorbed ammonia at the -based adsorption amount of 7.4 molecules (SI Appendix, Fig. S15). Given the molecular size of (0.26 nm in diameter) (33), the observed surface density is close to the saturation level of monolayer adsorption. In contrast, the 72.4 molecules attained by mackinawite at (Fig. 2, b-3) corresponds to 1.4 atom% of Fe in its structure, which is lower than the amount of generated at this potential (15 1.7%; Fig. 1C). Thus, even denser ammonia adsorption is expected to be achieved if the experiment is performed with higher initial concentrations of ammonia.
Interestingly, in the case when mackinawite was electrolyzed at for 48 h in the absence of dissolved ammonia, after which ammonia was added in the supernatant solution to a concentration of 1 mM, ammonia was found to be largely retained in the liquid phase over the subsequent 48 h under the imposed potential (SI Appendix, Fig. S18). Mackinawite electroreduction possibly causes the aggregation of Fe sheets through formation and crystallization, after which intercalation may be structurally difficult. This inhibitive effect of excessive electroreduction is also suggested in the evolution of dissolved ammonia concentration presented in Fig. 2A. At the lowest examined potential (), the decrease in dissolved ammonia concentration ceased in the first 12 h, possibly because of a rapid aggregation of mackinawite sheets; meanwhile, a slow formation at moderately negative potentials ( and ) allowed continuous ammonia accumulation over 48 h. Thus, extremely low electric potential (e.g., ) is not necessarily preferable; the potentials slightly below the / equilibrium (e.g., ) are rather advantageous to maintaining the ammonia accumulation on mackinawite for a long duration.
Discussion
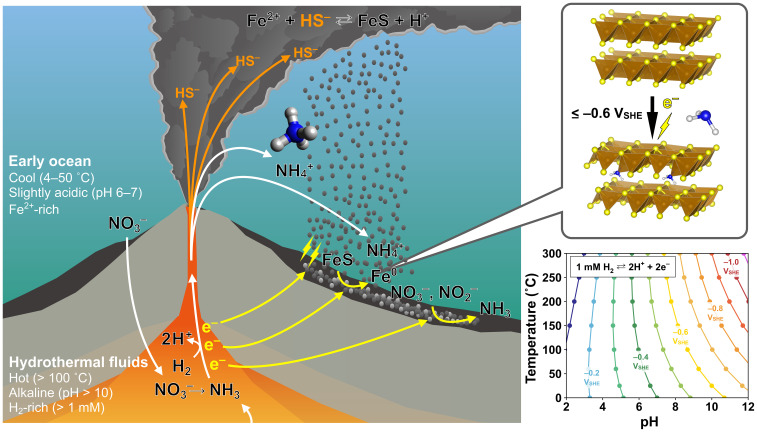
The origin of life in deep-sea hydrothermal systems is a long-standing hypothesis supported by diverse scientific disciplines, including geology (44), biology (45), and astronomy (46). However, its chemical plausibility remains a question because of the apparent difficulty in the retention and accumulation of key prebiotic components. Here, we demonstrated that partial electroreduction of mackinawite drastically enhances its ammonia accumulation capability, by up to 55-fold for the solid/liquid partition coefficient, enabling over 90% adsorption of 1 mM ammonia in 1 M NaCl at neutral pH (Fig. 2B). The electrochemical conditions favorable for this process are fully compatible with those suitable for prebiotic carbon and nitrogen fixations in the formation of the building blocks of life (10, 11, 25–31) (Fig. 4). The threshold potential () corresponds to the / redox potential in a moderately alkaline, high-temperature hydrothermal environment [e.g., pH 8.7 at 100 °C in the presence of 1 mM (27)], which is available even in present-day alkaline hydrothermal systems (47, 48). Although modern ocean alkaline hydrothermal vents are mainly composed of carbonate minerals that are electrochemically nonconductive (49), the early counterparts were sulfide-rich, owing to the presence of metal-rich seawater (50).
Fig. 4.
Geoelectrochemical mechanism of ammonia accumulation in the early ocean hydrothermal system. The schematic was depicted based on our experimental results together with the field and laboratory findings on the geoelectrochemical processes in submarine hydrothermal systems reported in the literature (10, 11, 23, 24, 29, 30). Thermodynamic calculations for the / redox potential as a function of pH and temperature (31) (Right) indicate that the electric potential necessary for the reduction and the subsequent accumulation () is readily realizable in -rich, alkaline hydrothermal systems that were ubiquitous on the early ocean floor (51).
Iron sulfide must have been a predominant sulfide component of the ancient vent chimneys (51). Its catalytic surface with metal impurities (e.g., ) facilitates both the oxidation of () and the reduction of oxidative chemicals (e.g., ) (29); hence, a sustained electron flow across the potential gap between the reductive hydrothermal fluid and seawater has been envisioned (Fig. 4). Pure and electroreduced mackinawite is also capable of facilitating ammonia formation (30) and promotes the amination reaction when a sufficient concentration of ammonia is available (30, 37). These facts, along with our demonstrated extreme accumulation of ammonia on electroreduced mackinawite, suggest that mackinawite played a pivotal role in the formation, accumulation, and assimilation of organic compounds of ammonia in the early ocean hydrothermal vent environments. Submarine hydrothermal systems are currently the sole environment in which spontaneous electricity generation has been observed (24), thus possessing a crucial advantage in abiotic nitrogen assimilation, a requisite step for the originating of life.
Materials and Methods
Preparation of Mineral Adsorbents.
All chemicals were reagent grade, purchased from either Nacalai Tesque Inc., Kanto Chemical Co., Ltd., or Fujifilm Wako Pure Chemical Co., Ltd., except for pure nanoparticle (95–105 nm, 99.5% purity), which was obtained from EM Japan. Deaerated Milli-Q water (18.2 megohms) was used as the solvent. Mineral adsorbents were prepared, in an anaerobic chamber filled with and gases (volume ratio = 96:4).
Mackinawite was prepared by adding 100 mM dropwise into 100 mM under vigorous stirring to a final volume ratio of 1:1. The obtained precipitate was separated from the supernatant solution by centrifugation (8,000 rpm, 10 min) and dried under vacuum.
For the preparation of hematite, 50 mL of 0.2 M was added dropwise into 450 mL of a boiled HCl solution (2 mM) under vigorous stirring (52, 53). The obtained suspension was inserted into a polytetrafluoroethylene (PTFE) bottle, enclosed in a screw-capped stainless-steel outer vessel, and heated at 98 °C for 14 d. After the vessel was cooled to room temperature, the resultant precipitate was rinsed with Milli-Q water, centrifuged (6,000 rpm, 10 min), and dried under vacuum.
For magnetite preparation, 19 mmol of and 10 mmol of were dissolved in 25.85 mL of 0.39 M HCl. The acid mixture was added dropwise into 250 mL of 1.5 M NaOH under vigorous stirring (54). The obtained black precipitate was separated from the supernatant solution by centrifugation (6,000 rpm, 10 min), dried under vacuum, and grounded with an agate mill.
For ferrihydrite preparation, 50 mM was basified to pH 8.0 with 0.1 or 1 M NaOH and stirred over 3 h at a pH maintained at 8 (55). The obtained precipitate was separated from the supernatant solution by centrifugation (6,000 rpm, 10 min), rinsed with Milli-Q water, and dried under vacuum.
Electrochemical Experiments.
Electrochemical experiments were conducted under simulated early ocean conditions in accordance with the procedure reported previously (27, 30, 31). Briefly, 300 mg of solid adsorbent was deposited on a carbon working electrode (5.7 ) in an H-type cell (SI Appendix, Fig. S1), immersed in a deaerated 1 M NaCl containing 1 mM (60 mL), and exposed to a flow of (20 mL , purity 99.995%) to maintain the solution pH at neutral (6.3 1.2; SI Appendix, Fig. S2). Although the experimental pressure (1 atm) is lower than the pressure in deep-sea environments, this parameter has no significant influence on the / redox potential (30).
As the gas flow that was started at least 1 h before each experiment was maintained, a constant potential was applied on the carbon electrode for 48 h by using a multipotentiostat (PS-08; Toho Technical Research). The control experiment was performed in an electrochemical cell without an externally imposed electric potential. All potentials were measured against an Ag/AgCl reference electrode in saturated KCl and were converted to the SHE scale by the following equation:
| [1] |
In the course of the electrolysis of solid adsorbent, the supernatant solution was sampled periodically (1 mL for each) with a stainless needle connected with a PTFE membrane filter (pore size, 0.22 m). The filtrate was measured for pH by a portable pH meter (Seven2Go Pro, Mettler Toledo). The sample solution was also analyzed for the dissolved concentrations of ammonia and using an HPLC and an inductively coupled plasma optical emission spectrometer (ICP-OES), respectively (see below). To prevent the precipitation of via oxidation by atmospheric , the solution for the ICP-OES measurement was acidified with 100 mM HCl immediately after the sampling.
After the electrolysis, the electrochemical cell was transferred immediately into an anaerobic chamber. The solid adsorbent was then separated from the supernatant solution, dried under vacuum, and stored inside the anaerobic chamber.
Analysis of Sample Solutions.
The dissolved ammonium concentration was determined by postcolumn derivatization with -phthalaldehyde (OPA) using a Shimadzu HPLC-fluorescence detector (FD) system. The FD was operated at 365 and 438 nm for excitation and emission, respectively. A Shim-pack WCX-1 column (450 mm, particle diameter = 5 m, pore size = 300 Å) was used at 40.0 °C. A 5 mmol citrate buffer solution (pH 6.2) was flowed at 1.0 mL for elution, and a 20 vol% methanol solution dissolving 20 mM OPA, 200 mM sodium borate, and 2 mM sodium sulfite (pH 9.2) was flowed at 0.5 mL for derivatization.
The dissolved Fe concentration was determined by an ICP-OES (Spectro ARCOS; AMETEK, Berwyn, PA, USA), with 1 ppm Lu as an internal standard (56). Measurements of Fe were performed using a high-matrix setup with a cross-flow nebulizer (nebulizer gas = 0.9 L ), Scott-type standard spray chamber, and Elegra Argon humidifier. The auxiliary gas was set at 1.6 L .
Solid Characterization.
XRD patterns of the solid adsorbents were obtained by an X-ray diffractometer (MiniFlex II, Rigaku) with Cu K radiation. All runs were conducted with 2 ranging from 3° to 90°, using a 0.02° 2 step with a scan rate of 0.01° for mackinawite and 0.1° for the other adsorbents. To prevent oxidation by atmospheric during the measurement, the samples were shielded in an air-sensitive sample holder (Rigaku) inside the anaerobic chamber. Peak identifications were made on the basis of the reference patterns reported in the Powder Diffraction File published by the International Centre for Diffraction Data.
The of solid samples were determined by a BET surface area analyzer (BELSORP mini II; MicrotracBEL, Osaka, Japan) after vacuum drying for 24 h at room temperature (23 2 °C). The obtained adsorption isotherms and BET plots are shown in SI Appendix, Figs. S7 and S8, respectively.
Scanning electron microscopy (SEM) imaging and EDS analysis was performed on a Helios G4 UX (Thermo Fisher Scientific), equipped with an Octane Elite Super (C5) EDS detector (AMETEK) and a cryogenic stage with a preparation chamber (PP3010T, Quorum). The carbon electrode with solid sample deposited was trimmed to approximately 33 mm and mounted on an aluminum stub by double-sided carbon tape in an anaerobic chamber. The stub was then carried in a liquid -filled plastic bag to the SEM instrument, mounted on a transfer shuttle, and inserted into the SEM stage after vacuum evacuation. To acquire surface-sensitive high-resolution images and elemental maps, an acceleration voltage of 1 kV was applied for morphological imaging, and an acceleration voltage of 5 or 20 kV for EDS analysis under a reduced pressure of Pa at room temperature (23 2 °C). The acceleration voltage of 20 kV was applied to average the spatial elemental distribution of mackinawite, as the characteristic X-ray is generated several m from the point irradiated at this voltage (57).
The obtained EDS spectra were analyzed with TEAM EDS software (AMETEK). The peak intensity was converted to an atomic ratio after correction by atomic number (Z), absorption (A), and fluorescence (F). The fraction of against total Fe was calculated as:
| [2] |
where [S K] and [Fe L] denote the atomic concentrations calculated from the characteristic X-ray of the K line of S and that of the L line of Fe, respectively.
Nitrogen contents of solid-phase samples were measured according to the previously reported method with slight modification (58). Adsorbed nitrogen measurement was performed by an ultrasensitive elemental analyzer (FlashEA1112 series, Thermo Fisher Scientific) equipped with a narrower diameter combustion reactor (8- inner diameter quartz tubing, Koshin Rikagaku Seisakusho Co., Ltd., 1,050 °C), reduction reactor (8- inner diameter quartz tubing, Koshin Rikagaku Seisakusho Co., Ltd., 650 °C), zero-blank autosampler (Costech Analytical Technologies Inc.), and 5- oxygen loop (GL Science) coupled with ConFlo IV interface and Delta plus advantage stable isotope mass spectrometer (Thermo Fisher Scientific).
X-ray absorption fine structure (XAFS) spectra at Fe K-edge were measured at BL-12C in a synchrotron radiation facility (Photon Factory) in High Energy Accelerator Research Organization (KEK). In the beamline, X-ray from a synchrotron operated at 2.5 (current: 450 ) was monochromatized with a Si(111) double-crystal monochromator, and focused to an area of 0.50.5 with a bent cylindrical mirror, which also reduced the higher order. XAFS spectra were obtained in transmission mode using two ion chambers to measure intensities of incident () and transmitted () X-rays. The energy step within the X-ray absorption near-edge structure (XANES) region was 0.25 , and the absorbance by the sample (t) was obtained as t = ln(/). FeS samples were diluted with boron nitride (BN) to yield the metal/BN molar ratio of 1:25, and shielded by an -impermeable polyethylene film. Measurement was conducted for a part of the sample with uniform thickness within the area of the X-ray beam.
The percentages of in the electrolyzed FeS were determined by a least-squares fitting of the EXAFS spectra of samples after background subtraction and normalization with XAFS data processing software ATHENA (59). The linear combination of pure and pure was used for the fitting between 8 and 11 Å. The best fit was obtained by calculating the lowest factor, which was defined as:
| [3] |
In this equation, and are the experimental and the calculated absorbances at a given wavenumber , respectively.
First Principles Calculations.
First-principles calculations were performed on a Vienna Ab-initio Simulation Package (VASP v. 5.4.4) (60), run on the Data Analyzer system in JAMSTEC. A Perdew–Burke–Ernzerhof (PBE) (61) generalized gradient approximation (GGA) exchange–correlation functional with Grimme’s DFT-D3 correlation (62) was applied as a density functional, and projector augmented wave (PAW) (63) pseudopotentials were applied without consideration of spin on Fe atoms, following the literature regarding FeS calculation (64, 65). The energy cutoff was set to 450 eV. The threshold for self-consistency of the electron density was set to 10 eV, and the structural convergence threshold was set, as the forces on each ion were below 0.01 eV Å. After the structural optimization of a unit cell taken from a crystallographic database (66) under Brillouin zone sampling using the Monkhorst–Pack scheme (67) with a 222 k-point mesh, supercells periodic to three dimensions were created. The Brillouin zone sampling for the supercell was a 555 k-point mesh for the (222) FeS supercell and a 333 k-point mesh for the (333) and (444) FeS supercells. The electroreduction of mackinawite to was expressed by the removal of one sulfur atom from the FeS supercells. was placed at the removed sulfur position, and the atom positions and cell dimension were optimized. As for intercalation in the pristine mackinawite, was placed on either Fe or S, and the structure optimization was performed as described above. The intercalation energy of () was determined from the electron energy released through the mackinawite– complexation,
| [4] |
where denotes the total electron energies of species X. The simulated cell dimensions are in Å, and the fractional coordinates of all the atoms involved in energy calculations are summarized in the atom coordinates section (SI Appendix, Tables S3–S14). Structures were displayed using VESTA v. 3.5.2 (68).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This work was supported by JSPS KAKENHI (Grant Nos. 19K04048, 19K15379, 20H00209, 21H04527, 22H05149, 22K03801, and 23K13211). We thank Shinsuke Kawagucci (JAMSTEC), Teruhiko Kashiwabara (JAMSTEC), Sakiko Kikuchi (JAMSTEC), Eiji Tasumi (JAMSTEC), Masayuki Miyazaki (JAMSTEC), and Akiko Makabe (JAMSTEC) for their technical assistance in the sample analysis. W.T. also thanks Teppei Yamada (U Tokyo), Ryuhei Nakamura (RIKEN), Hideshi Ooka (RIKEN), and Masahiro Yamamoto (JAMSTEC) for helpful discussions and insight.
Author contributions
W.T., S. Okada, and N.K. designed research; W.T. performed research; S. Okada, Y.M., S. Ono, K.T., Y.T., and N.K. contributed new reagents/analytic tools; W.T., S. Okada, Y.M., S. Ono, Y.T., and N.K. analyzed data; and N.K. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. M.J.R. is a guest editor invited by the Editorial Board.
Contributor Information
Wataru Takahagi, Email: watarut@g.ecc.u-tokyo.ac.jp.
Satoshi Okada, Email: okadasa@jamstec.go.jp.
Norio Kitadai, Email: nkitadai@jamstec.go.jp.
Data, Materials, and Software Availability
The authors declare that the data supporting the findings of this study are available within the paper and its SI Appendix.
Supporting Information
References
- 1.Russell M. J., Daniel R. M., Hall A. J., Sherringham J. A., A hydrothermally precipitated catalytic iron sulphide membrane as a first step toward life. J. Mol. Evol. 39, 231–243 (1994). [Google Scholar]
- 2.Takai K., et al. , Ultramafics-Hydrothermalism-Hydrogenesis-HyperSLiME (UltraH3) linkage: A key insight into early microbial ecosystem in the Archean deep-sea hydrothermal systems. Paleontol. Res. 10, 269–282 (2006). [Google Scholar]
- 3.Lane N., Allen J. F., Martin W., How did LUCA make a living? Chemiosmosis in the origin of life. Bioessays 32, 271–280 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Summers D. P., Ammonia formation by the reduction of nitrite/nitrate by FeS: Ammonia formation under acidic conditions. Orig. Life Evol. Biosph. 35, 299–312 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Brandes J. A., Hazen R. M., Yoder H. S. Jr., Inorganic nitrogen reduction and stability under simulated hydrothermal conditions. Astrobiology 8, 1113–1126 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Singireddy S., et al. , Reduction of nitrite and nitrate to ammonium on pyrite. Orig. Life Evol. Biosph. 42, 275–294 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Nishizawa M., et al. , Stable abiotic production of ammonia from nitrate in Komatiite-Hosted hydrothermal systems in the Hadean and Archean oceans. Minerals 11, 321 (2021). [Google Scholar]
- 8.Hansen H. C. B., Guldberg S., Erbs M., Bender Koch C., Kinetics of nitrate reduction by green rusts—Effects of interlayer anion and Fe(II):Fe(III) ratio. Appl. Clay Sci. 18, 81–91 (2001). [Google Scholar]
- 9.Smirnov A., Hausner D., Laffers R., Strongin D. R., Schoonen M. A. A., Abiotic ammonium formation in the presence of Ni–Fe metals and alloys and its implications for the Hadean nitrogen cycle. Geochem. Trans. 9, 5 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y., Yamaguchi A., Yamamoto M., Takai K., Nakamura R., Molybdenum sulfide: A bioinspired electrocatalyst for dissimilatory ammonia synthesis with geoelectrical current. J. Phys. Chem. C 121, 2154–2164 (2017). [Google Scholar]
- 11.Li Y., et al. , Enzyme mimetic active intermediates for nitrate reduction in neutral aqueous media. Angew. Chem. Int. Ed. 59, 9744–9750 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Wong M. L., Charnay B. D., Gao P., Yung Y. L., Russell M. J., Nitrogen oxides in early earth’s atmosphere as electron acceptors for life’s emergence. Astrobiology 17, 975–983 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Kitadai N., Maruyama S., Origins of building blocks of life: A review. Geosci. Front. 9, 1117–1153 (2018). [Google Scholar]
- 14.Barge L. M., et al. , Effects of geochemical and environmental parameters on abiotic organic chemistry driven by iron hydroxide minerals. J. Geophys. Res. Planets 125, e2020JE006423 (2020). [Google Scholar]
- 15.Summers D. P., Chang S., Prebiotic ammonia from reduction of nitrite by iron (II) on the early earth. Nature 365, 630–633 (1993). [DOI] [PubMed] [Google Scholar]
- 16.Knauth L. P., Temperature and salinity history of the Precambrian ocean: Implications for the course of microbial evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 219, 53–69 (2005). [Google Scholar]
- 17.Bernal M. P., Lopez-Real J. M., Natural zeolites and sepiolite as ammonium and ammonia adsorbent materials. Bioresour. Technol. 43, 27–33 (1993). [Google Scholar]
- 18.Lin L., et al. , Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites. Sep. Purif. Technol. 103, 15–20 (2013). [Google Scholar]
- 19.Alshameri A., et al. , The investigation into the ammonium removal performance of Yemeni natural zeolite: Modification, ion exchange mechanism, and thermodynamics. Powder Technol. 258, 20–31 (2014). [Google Scholar]
- 20.Alshameri A., et al. , Adsorption of ammonium by different natural clay minerals: Characterization, kinetics and adsorption isotherms. Appl. Clay Sci. 159, 83–93 (2018). [Google Scholar]
- 21.James Cleaves H., et al. , Mineral-organic interfacial processes: Potential roles in the origins of life. Chem. Soc. Rev. 41, 5502–5525 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Schoonen M., Smirnov A., Cohn C., A perspective on the role of minerals in prebiotic synthesis. Ambio 33, 539–551 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Nakamura R., et al. , Electrical current generation across a black smoker chimney. Angew. Chem. Int. Ed. 49, 7692–7694 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto M., et al. , Spontaneous and widespread electricity generation in natural Deep-Sea hydrothermal fields. Angew. Chem. Int. Ed. 56, 5725–5728 (2017). [DOI] [PubMed] [Google Scholar]
- 25.A. Yamaguchi et al., Electrochemical CO reduction by Ni-containing iron sulfides: How is CO electrochemically reduced at bisulfide-bearing deep-sea hydrothermal precipitates? Electrochim. Acta141, 311–318 (2014).
- 26.Yamaguchi A., et al. , Multi-regression analysis of CO electroreduction activities on metal sulfides. J. Phys. Chem. C 126, 2772–2779 (2022). [Google Scholar]
- 27.Kitadai N., et al. , Geoelectrochemical CO production: Implications for the autotrophic origin of life. Sci. Adv. 4, eaao7265 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J. E., et al. , In situ FTIR study of CO reduction on inorganic analogues of carbon monoxide dehydrogenase. Chem. Commun. 57, 3267–3270 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Hudson R., et al. , CO reduction driven by a pH gradient. Proc. Natl. Acad. Sci. U.S.A. 117, 22873–22879 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitadai N., et al. , Metals likely promoted protometabolism in early ocean alkaline hydrothermal systems. Sci. Adv. 5, eaav7848 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitadai N., et al. , Thioester synthesis through geoelectrochemical CO fixation on Ni sulfides. Commun. Chem. 4, 37 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolthers M., Van Der Gaast S. J., Rickard D., The structure of disordered mackinawite. Am. Mineral. 88, 2007–2015 (2003). [Google Scholar]
- 33.Breck D. W., Zeolite Molecular Sieves: Structure, Chemistry, and Use (John Wiley& Sons, 1973). [Google Scholar]
- 34.Kuntke P., et al. , Energy-efficient ammonia recovery in an up-scaled hydrogen gas recycling electrochemical system. ACS Sustain. Chem. Eng. 6, 7638–7644 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim K. Y., Moreno-Jimenez D. A., Efstathiadis H., Electrochemical ammonia recovery from anaerobic centrate using a nickel-functionalized activated carbon membrane electrode. Environ. Sci. Technol. 55, 7674–7680 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Hafenbradl D., Keller M., Wächtershäuser G., Stetter K. O., Primordial amino acids by reductive amination of -oxo acids in conjunction with the oxidative formation of pyrite. Tetrahedron Lett. 36, 5179–5182 (1995). [Google Scholar]
- 37.Huber C., Wächtershäuser G., Primordial reductive amination revisited. Tetrahedron Lett. 44, 1695–1697 (2003). [Google Scholar]
- 38.Fonseca G. S., et al. , Synthesis and characterization of catalytic iridium nanoparticles in imidazolium ionic liquids. J. Colloid Interface Sci. 301, 193–204 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Beale A. M., Weckhuysen B. M., EXAFS as a tool to interrogate the size and shape of mono and bimetallic catalyst nanoparticles. Phys. Chem. Chem. Phys. 12, 5562–5574 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Wolthers M., Charlet L., van Der Linde P. R., Rickard D., van Der Weijden C. H., Surface chemistry of disordered mackinawite (FeS). Geochim. Cosmochim. Acta 69, 3469–3481 (2005). [Google Scholar]
- 41.Otero G. S., Pascucci B., Branda M. M., Miotto R., Belelli P. G., Evaluating the size of Fe nanoparticles for ammonia adsorption and dehydrogenation. Comput. Mater. Sci. 124, 220–227 (2016). [Google Scholar]
- 42.Yeo S. C., Han S. S., Lee H. M., Mechanistic investigation of the catalytic decomposition of ammonia (NH) on an Fe(100) surface: A DFT study. J. Phys. Chem. C 118, 5309–5316 (2014). [Google Scholar]
- 43.Cordero B., et al. , Covalent radii revisited. Dalton Trans. 21, 2832–2838 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Ueda H., et al. , Reactions between komatiite and CO-rich seawater at 250 and 350°C, 500 bars: Implications for hydrogen generation in the hadean seafloor hydrothermal system. Prog. Earth Planet. Sci. 3, 35 (2016). [Google Scholar]
- 45.Nunoura T., et al. , A primordial and reversible TCA cycle in a facultatively chemolithoautotrophic thermophile. Science 359, 559–563 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Sekine Y., et al. , High-temperature water-rock interactions and hydrothermal environments in the chondrite-like core of Enceladus. Nat. Commun. 6, 8604 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrill P. L., et al. , Geochemistry and geobiology of a present-day serpentinization site in California: The cedars. Geochim. Cosmochim. Acta 109, 222–240 (2013). [Google Scholar]
- 48.Boyd E. S., Amenabar M. J., Poudel S., Templeton A. S., Bioenergetic constraints on the origin of autotrophic metabolism. Philos. Trans. A Math. Phys. Eng. Sci. 378, 20190151 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelley D. S., et al. , An off-axis hydrothermal vent field near the mid-Atlantic ridge at 30°N. Nature 412, 145–149 (2001). [DOI] [PubMed] [Google Scholar]
- 50.Song H., et al. , The onset of widespread marine red beds and the evolution of ferruginous oceans. Nat. Commun. 8, 399 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shibuya T., Russell M. J., Takai K., Free energy distribution and hydrothermal mineral precipitation in Hadean submarine alkaline vent systems: Importance of iron redox reactions under anoxic conditions. Geochim. Cosmochim. Acta 175, 1–19 (2016). [Google Scholar]
- 52.Lounsbury A. W., et al. , Preferential adsorption of selenium oxyanions onto 110 and 012 nano-hematite facets. J. Colloid Interface Sci. 537, 465–474 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Zhu M., Wang Y., Meng D., Qin X., Diao G., Hydrothermal synthesis of hematite nanoparticles and their electrochemical properties. J. Phys. Chem. C 116, 16276–16285 (2012). [Google Scholar]
- 54.Kang Y. S., Risbud S., Rabolt J. F., Stroeve P., Synthesis and characterization of nanometer-size Fe3O4 and γ-Fe2O3 particles. Chem. Mater. 8, 2209–2211 (1996). [Google Scholar]
- 55.Kitadai N., Nishiuchi K., Takahagi W., Thermodynamic impact of mineral surfaces on amino acid polymerization: Aspartate dimerization on two-line ferrihydrite, anatase, and -alumina. Minerals 11, 234 (2021). [DOI] [PubMed] [Google Scholar]
- 56.Grotti M., Magi E., Leardi R., Selection of internal standards in inductively coupled plasma atomic emission spectrometry by principal component analysis. J. Anal. At. Spectrom. 18, 274–281 (2003). [Google Scholar]
- 57.Sakurada T., et al. , Lateral resolution of EDX analysis with ultra low acceleration voltage SEM. J. Surf. Anal. 12, 118–121 (2005). [Google Scholar]
- 58.N. Ogawa, T. Nagata, H. Kitazato, N. Ohkouchi, “Ultra-sensitive elemental analyzer/isotope ratio mass spectrometer for stable nitrogen and carbon isotope analyses” in Earth, Life and Isotopes, N. Ohkouch, I. Tayasu, K. Koba, Eds. (Kyoto University Press, 2010), pp. 339–353.
- 59.Ravel B., Newville M., ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005). [DOI] [PubMed] [Google Scholar]
- 60.Kresse G., Furthmüller J., Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- 61.Perdew J. P., Burke K., Ernzerhof M., Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- 62.Grimme S., Antony J., Ehrlich S., Krieg H., A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Jollet F., Torrent M., Holzwarth N., Generation of projector augmented-Wave atomic data: A 71 element validated table in the XML format. Comput. Phys. Commun. 185, 1246–1254 (2014). [Google Scholar]
- 64.Dzade N. Y., Roldan A., de Leeuw N. H., Adsorption of methylamine on mackinawite (FeS) surfaces: A density functional theory study. J. Chem. Phys. 139, 124708 (2013). [DOI] [PubMed] [Google Scholar]
- 65.Dzade N. Y., de Leeuw N. H., Adsorption and desulfurization mechanism of thiophene on layered FeS(001), (011), and (111) surfaces: A dispersion-corrected density functional theory study. J. Phys. Chem. C, Nanomater. Interfaces 122, 359–370 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lennie A. R., Redfern S. A. T., Schofield P. F., Vaughan D. J., Synthesis and Rietveld crystal structure refinement of mackinawite, tetragonal FeS. Mineral. Mag. 59, 677–683 (1995). [Google Scholar]
- 67.Monkhorst H. J., Pack J. D., Special points for Brillouin-zone integrations. Phys. Rev. B, Condens. Matter 13, 5188–5192 (1976). [Google Scholar]
- 68.Momma K., Izumi F., VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its SI Appendix.