Significance
The motor system has been recognized as a fundamental neural machinery for spatial and social cognition, making the study of the interplay between architecture and social behavior worthwhile. Here, we tested how a virtual architectural experience alters the subsequent processing of body expressions, showing that the motor system participates at two distinct stages: the earliest influenced by the dynamic architectural experience and the latter modulated by the actual physical characteristics. These findings highlight the existence of an overlapping motor neural substrate devoted to spatial and social cognition, with the architectural space exerting an early and possibly adapting effect on the later social experience. Ultimately, spatial design may impact the processing of human emotions.
Keywords: premotor cortex, architectural cognition, emotional body expressions, EEG, virtual reality
Abstract
The interplay between space and cognition is a crucial issue in Neuroscience leading to the development of multiple research fields. However, the relationship between architectural space and the movement of the inhabitants and their interactions has been too often neglected, failing to provide a unifying view of architecture's capacity to modulate social cognition broadly. We bridge this gap by requesting participants to judge avatars’ emotional expression (high vs. low arousal) at the end of their promenade inside high- or low-arousing architectures. Stimuli were presented in virtual reality to ensure a dynamic, naturalistic experience. High-density electroencephalography (EEG) was recorded to assess the neural responses to the avatar’s presentation. Observing highly aroused avatars increased Late Positive Potentials (LPP), in line with previous evidence. Strikingly, 250 ms before the occurrence of the LPP, P200 amplitude increased due to the experience of low-arousing architectures, reflecting an early greater attention during the processing of body expressions. In addition, participants stared longer at the avatar’s head and judged the observed posture as more arousing. Source localization highlighted a contribution of the dorsal premotor cortex to both P200 and LPP. In conclusion, the immersive and dynamic architectural experience modulates human social cognition. In addition, the motor system plays a role in processing architecture and body expressions suggesting that the space and social cognition interplay is rooted in overlapping neural substrates. This study demonstrates that the manipulation of mere architectural space is sufficient to influence human social cognition.
The interplay between spatial and social environment is a fundamental aspect of daily life (1, 2). The awareness that the amount of time we spend indoors could significantly influence human behavior moved neuroscientists to explore human responses to the built environment, which can be considered the prototypic field for studying the interaction between space and social cognition (3–5). Previous studies demonstrated that the brain contains multiple, plastic, and dynamic space mappings accomplished by fronto-parietal networks characterized by visuomotor properties, mainly described in nonhuman primate and neglect patients’ studies (6–10). These cortical regions engaged in space coding partially overlap with networks devoted to action and intention understanding, possibly indicating a functional binding between spatial and social processing (11, 12).
Several studies demonstrated that static architectural features modulate cerebral regions devoted to emotion perception (13–15) and that the motor system is involved in processing affordable architectural transitions (16, 17). From a theoretical point of view, Djebbara et al. provided a psychobiological framework describing the role of the pulvinar in integrating sensory processes, further affecting the higher visual cortex and the related cortico-cortical connections leading to sensorimotor responses integrating environmental features with attention and behavior (18). In addition, Jelic et al. proposed an enactive approach to studying architectural experience, emphasizing the motor system’s role and motivational factors as constituents of the body architecture interactions (19). Overall, it is recognized that the built environment fundamentally impacts human well-being at multiple temporal and spatial scales (20).
However, despite the increasing number of works in the field (21, 22), the presence and interactions among individuals and their movement within the architectural space have been neglected so far, failing to provide a unified view of architecture’s capacity to broadly modulate social cognition, such as the perception of other’s body expressions.
In this regard, a large body of evidence has shown that body expressions convey affective information, playing a fundamental role in social interactions (23, 24). For instance, cortical correlates of the processing of emotional body expressions show increased P200 when observing emotional rather than neutral body postures, pointing to greater attention to socially relevant cues (25). The observation of high-arousing body postures also generates higher Late Positive Potential (LPP) amplitude than low-arousing ones (26, 27). The modulation of such event-related potentials (ERPs) reflects a change in the level of exogenous attention captured by the stimulus (28) and greater attention allocation to motivationally relevant stimuli (29, 30) at an earlier and later stage, respectively.
In natural viewing conditions, body expressions are presented within different contexts carrying affective information, which may all be relevant and processed together, thus biasing the perception of the body expression. However, only a few studies focused on the effect of the environment in shaping the mechanisms underlying the processing of body expressions, and none of these consider architectural spaces. Specifically, Van den Stock and colleagues described neural evidence showing that the affective information provided by the environment modulates the processing of body stimuli due to the changing activity of cerebral regions endowed with visual functions and others involved in space and body processing (31).
The present study bridges this gap by linking the judgment of emotional body expressions to the dynamic experience of architecture. We exploited virtual reality to ensure a naturalistic experience. In our study, participants made a virtual promenade inside high- or low-arousing architecture and then judged avatars’ emotional expression (high vs. low arousal). The use of virtual reality is pivotal since it permits subjects to experience the architectural space in a dynamic and immersive way (32, 33), ensuring the same neurophysiological response as in a real scenario (34). Because the processing of emotional body expressions is typically reflected in the modulation of brain components at different latencies, high-density EEG was recorded to test the hypothesis that the dynamic experience of architectural spaces modulates neural responses to the avatar’s presentation, thus affecting the early or late stage of attention. Considering that low-arousing states spare attentional resources (35), we expected that a low-arousing architectural experience would produce an increase of attention to body expression. Since spatial attention derives from the activation of brain maps transforming spatial information into motor representations (36, 37), we expect to observe a different involvement of motor regions devoted to attention mechanisms and sensorimotor integration depending on spatial and social conditions. In fact, motor-related areas are more active while processing body postures with socially relevant cues than neutral ones, reflecting motor resonance mechanisms for preparing an adaptative social response (25). Moreover, space coding relies on parieto-frontal cortical networks processing visuomotor information (6, 8, 10). Hence, we expect the motor system to be essential in processing body expressions within architectural spaces.
Our study demonstrates that the immersive and dynamic architectural experience influences human social cognition. Behavioral and electrophysiological evidence converge toward the hypothesis that the dynamic architectural experience may modulate attentional mechanisms at an early stage of body expression processing. In addition, the motor system plays a fundamental role in processing architecture and body expressions, proving how space and social cognition interplay is rooted in overlapping neural substrates.
Results
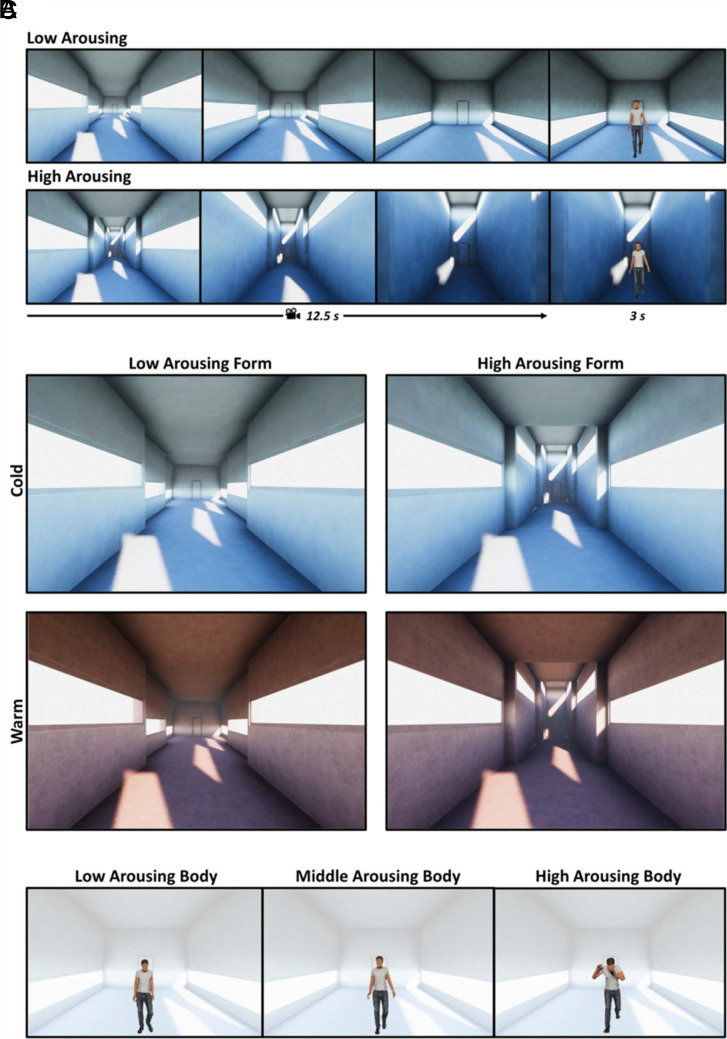
Participants dynamically experienced a virtual architecture before judging an emotional body posture. Specifically, they perceived the virtual environment through a first-person moving camera, realizing a virtual promenade. As the camera stopped (the promenade lasted 12.5 s), an avatar was presented for 3 s, and finally, participants judged the arousal level of the virtual avatar’s posture (Fig. 1A). This procedure allowed us to create a social context to investigate the influence that a dynamic experience of architecture plays on the processing of emotional body postures. Four virtual architectures were selected from a set of 54 models based on their level (low, high) of perceived arousal, in a cold and warm color, as described in a previous study (32) (Fig. 1B). Materials and Methods and SI Appendix provide further details concerning stimuli characterization. Emotional body postures were selected from a set of 45 stimuli based on their level (low, medium, and high) of perceived arousal as described in a previous study (38) (Fig. 1C).
Fig. 1.
Representation of the experimental trials and virtual stimuli. (A) Schematic representation of two experimental trials. The upper (Lower) panels, from left to right, show three first-person perspectives of the low (high) arousing architecture, corresponding to the participants’ view at the start of the promenade, at the end of the first nucleus, and at the end of the second one. The last frame corresponds to the presentation of the avatar in the third nucleus. (B) Virtual environments with low/high arousing forms (columns) in the cold/warm colored version (rows). (C) Example of avatars with low-, middle-, and high-arousing body posture, respectively. The transparent background is to highlight the body posture and represent the final nucleus of the low-arousing architecture.
EEG Study.
The EEG study compares ERPs and the corresponding pattern of cortical current density at an early and late stage of the emotional body posture presentation, appearing at the end of the virtual promenade. If the processing of dynamical architectural features affects the attention to the avatar at a late stage, we would observe LPP modulations depending on the arousal level of the architecture. Alternatively, if the processing of the dynamical architectural features affects the attention to the avatar at an early stage, we would expect a modulation of the P200 specifically mediated by architectural forms.
Increased arousal ratings correspond to the observation of body postures in low-arousing architectures.
The repeated-measures ANOVA revealed that participants coherently judged the emotional body postures according to the corresponding arousal level (main factor Body: F(2, 48) = 115.13, P < 0.001, ηp2 = 0.833). Arousal ratings were significantly different among the three levels of avatar’s bodily arousal (low < middle, P < 0.001; low < high, P < 0.001; middle < high, P < 0.001; Bonferroni corrected). These subjective arousal scores were higher within the architectures characterized by low-arousing forms [main factor Form: (F(1, 23) = 6.76, P = 0.016, ηp2 = 0.227)]. Instead, the main factor Color [F(1, 23) = 0.66, P = 0.425, ηp2 = 0.027] and the interaction Form × Body [F(2, 46) = 0.144, P = 0.866, ηp2 = 0.006] were not significant. For this reason, the factor Color will not be considered in the following EEG analysis.
Distinct neural temporal dynamics process architecture and body characteristics.
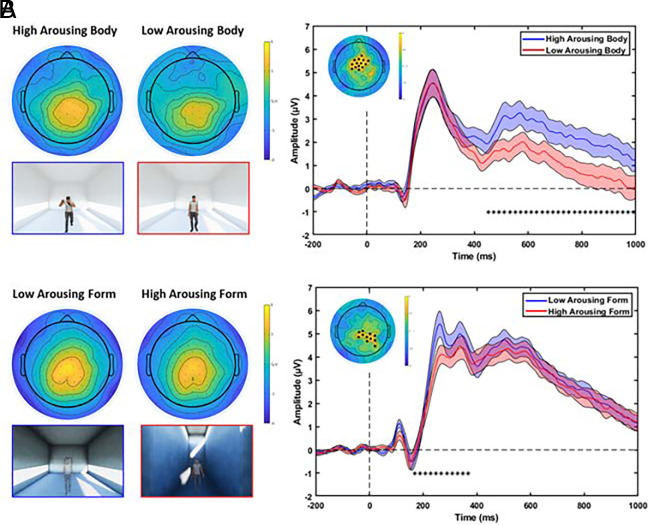
Fig. 2 shows the topographic maps and ERPs related to significant neural activations for the body and form characteristics. We performed a factorial mass univariate analysis in a late and early ERP window. In the late window, the analysis revealed a significant modulation of the LPP amplitude related to the arousal level represented by body characteristics. In fact, we report a significant cluster of electrodes for the factor Body (P = 0.006) within the late time interval of 452 to 1,000 ms from the avatar presentation. Then, pairwise comparisons within the main effect Body were conducted through cluster mass permutation tests on the mean ERP amplitudes in this late time window. Specifically, we found a cluster of fronto-central electrodes with higher LPP amplitude elicited by avatars with high-arousing body postures compared to avatars with low-arousing characteristics (P = 0.005). Fig. 2A presents the topographic maps of voltage distribution averaged in the late interval for the high- and low-arousing body conditions, showing that the LPP amplitude was mainly located at centro-parietal electrodes. On the right, the grand average ERP of centro-frontal electrodes of the significant cluster is presented, comparing the LPP elicited by the presentation of avatars with high- (blue) vs. low-arousing (red) body postures. Also, a different cluster of fronto-central electrodes showed a significantly higher LPP amplitude (P = 0.014) when avatars had high-arousing body postures rather than middle ones. No significant differences were found comparing avatars with low- and middle-arousing body postures (all P-values > 0.154). No significant clusters were found for the main effect Form (all P-values > 0.67) and interaction Form × Body (all P-values > 0.156) in the late window.
Fig. 2.
Topographic and ERP activations related to the distinct neural temporal dynamics processing architecture and body characteristics. (A) The left pictures represent the topographic voltage distributions of the LPP (452 to 1,000 ms) to the presentation of avatars with high- and low-arousing body postures. The right pictures represent the grand average ERPs for the high- (blue) and low-arousing (red) body posture conditions. (B) The left pictures represent the topographic voltage distribution of the P200 (168 to 384 ms) to the presentation of avatars within low- and high-arousing form. The right pictures represent the grand average ERPs for low- (blue) and high-arousing (red) architecture conditions. Figures within the blue and red frames below the scalp maps highlight the corresponding experimental conditions. The ERPs were averaged across the electrodes defining the significant cluster, highlighted with black dots on the topographic map in the figure Inset (colormap codes the t-statistic, cluster-based corrected). The SE is presented as light shadows of the corresponding color. The significant time interval is defined by back asterisks.
Strikingly, in the early analysis window, the factorial analysis revealed a significant modulation of the P200 amplitude during the observation of avatars related to the differences in the architectural forms. In fact, we report one significant cluster for the factor Form (P = 0.023) spanning the early time range between 168 and 384 ms after the presentation of the avatar. Fig. 2B shows the topographic maps of voltage distribution and the grand average ERPs of significant electrodes in this early time interval. Specifically, we found a cluster of electrodes in centroparietal areas with greater activity elicited by the presentation of the avatar within the low-arousing architecture compared to the high-arousing condition. The largest difference between the two conditions was reached around 250 ms after the avatar onset. No significant clusters of electrodes were found for the main effect Body (all P-values > 0.481) as well as for the interaction Form × Body (all P-values > 0.559).
Overlapping cortical motor activations process architecture and body characteristics.
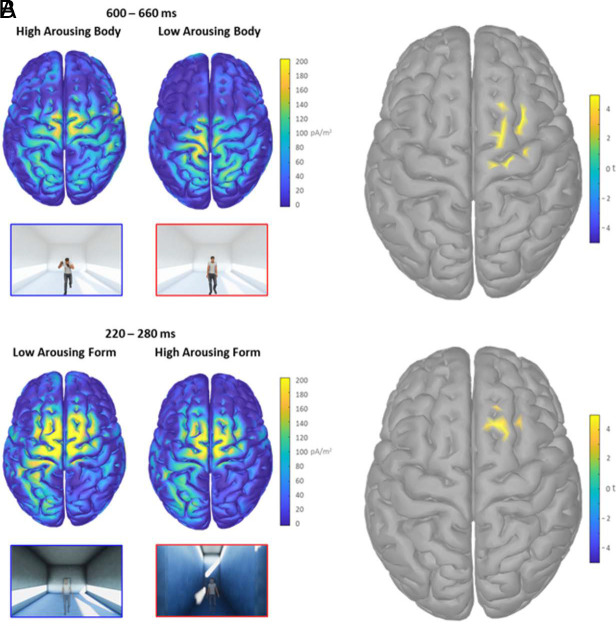
Fig. 3A shows the cortical currents density maps elicited by the presentation of high-arousing body postures and the corresponding statistical cortical maps. These maps are significantly higher than those elicited by the low arousing body postures in the time window between 600 and 660 ms. The dipole with the current density peak within the right dorsal premotor cortex (PMC) corresponds to a tp = 5.038 (P = 4.24*10−5, lower than the false discovery rate (FDR) corrected alpha threshold 4.29*10−4; Montreal Neurologic Institute (MNI) coordinates: X = 30.6, Y = 7.3, Z = 65). Fig. 3B shows the cortical generators of the P200 peak and the significant statistical difference in the right dorsal PMC corresponding to the observation of body postures presented in low-arousing architectures compared to the high-arousing condition. Specifically, in the 220 to 280 ms time window centered on the P200 peak, we found a significant cluster of activation with tp = 4.861 (P = 6.58*10−5, lower than the FDR-corrected alpha threshold 2.77*10−3; MNI coordinates: X = 18.4, Y = 21.5, Z = 67.1). Overall, findings returned from this EEG study indicate an early-stage modulation of attention mechanisms to the observation of body postures due to the dynamic experience of low-arousing architecture. The activation of premotor areas drives this process.
Fig. 3.
Cortical maps related to the overlapping motor activation for architecture and body characteristics. (A) The left pictures represent the two cortical maps of current density averaged in the 600 to 660 ms interval elicited by the presentation of avatars with high- and low-arousing body postures. The right picture shows the significant dipoles revealed by the corresponding statistical comparison within the cortical map. (B) The left figures represent the two cortical maps of the current density averaged in the 220 to 280 ms interval elicited by the presentation of avatars within the low- and high-arousing architecture. The right picture shows the significant dipoles revealed by the corresponding statistical comparison. The colormaps code the distribution of current density and the corresponding t statistic.
Eye-Tracking Study.
To corroborate these results with covert behavioral correlates of attention, we performed an eye-tracking study to investigate how the fixation times (FTs) to emotional body postures change according to the different dynamic architectural experiences. If the cerebral activations due to architecture characteristics depend on a modulation of attention mechanisms, we would observe increased FTs on salient avatar’s body parts after the promenade within low-arousing architectures.
Increased arousal ratings and FTs correspond to observation of body postures in low-arousing architectures.
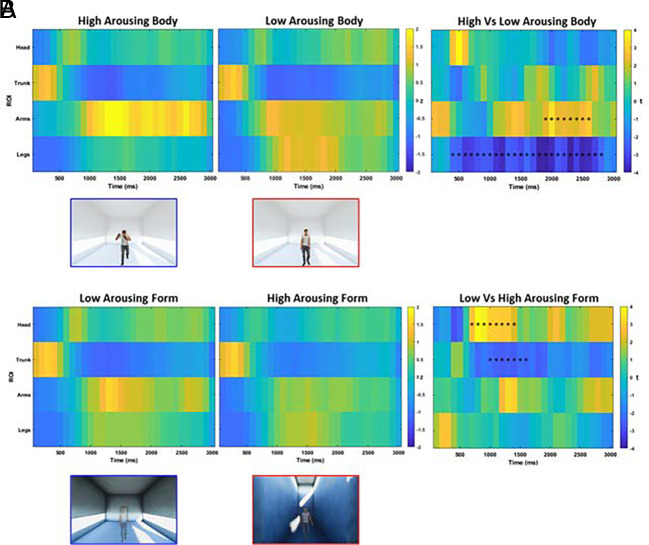
Results on arousal ratings reflected the trend observed in the EEG study (SI Appendix). Fig. 4A presents the results of a time-varying analysis comparing FTs between low- and high-arousing body postures across different regions of interest (ROIs) and time bins. The Montecarlo analysis returned two significant clusters, one for the legs region between 400 and 2,800 ms (P < 0.001, cluster corrected) and one for the arms from 1,900 to 2,600 ms (P = 0.011, cluster corrected) after the presentation of the avatar. Specifically, when looking at high arousing body postures, participants spent more time gazing at avatars' arms and shorter time to the avatar’s legs. Fig. 4B shows the results of the Montecarlo analysis comparing the FTs according to the different architectural experiences. We found two significant clusters, one for the head region between 700 and 1,400 ms (P = 0.002, cluster corrected) and one for the trunk from 1,000 to 1,600 ms (P = 0.018, cluster corrected) after the presentation of the avatar, revealing that participants spent more time looking at avatars’ head (and less at the trunk) after the virtual promenade within low-arousing architectures.
Fig. 4.
Increased FTs to body postures after the dynamic experience of low-arousing architecture. Panel A shows the time course of FTs on avatar’s ROIs with high- (Left) and low-arousing (Middle) body postures. Panel B shows the time course of FTs on avatar’s ROIs within the low- (Left) and high-arousing (Middle) architecture. For the left and central panels, the color of each time bin codes the time spent staring at the ROI, z-scored with respect to the empty control condition. In the right panel, the statistical comparison between the two conditions is presented: The color of each time bin represents the t-statistic, and black asterisks identify the significant clusters within each ROI.
Discussion
The present work explored the interplay between spatial and social cognition by investigating electrophysiological and behavioral reactions to expressive avatars within an immersive and dynamic architectural experience. Reported findings reveal that the involvement of late and early attentional mechanisms was differently triggered by emotional body expressions and architectural spaces. As widely reported in the literature, the observation of arousing body postures elicited increased LPP amplitude. Strikingly, we also found a modulation of the P200 amplitude in response to the avatar presentation. This modulation depended on the dynamic experience of different arousing architecture: Low-arousing architectural experiences generated higher P200 potential than high-arousing ones. The source localization highlighted a contribution of the dorsal PMC to both LPP and P200 generation, pointing to overlapping neural substrates within the motor system processing spatial and body characteristics. As further evidence of the involvement of attentional mechanisms due to the architectural experience, the eye-tracking study revealed increased FTs over the avatar’s head after the promenade within low-arousing architectures. Finally, subjective judgments revealed that the avatar's body was scored as more arousing after the dynamic experience of low-arousing architectures. These findings show that the dynamic experience of architecture modulates the processing of others’ body expressions.
The source analysis revealed that the dorsal PMC is the neural generator of both LPP and P200, thus being involved in the early and late stages of emotional body expression processing. Previous studies suggested the role of the dorsal PMC in supporting several cognitive functions, such as motor preparation and attention (39). Specifically, the activity of the PMC reflects the preparation of a motor program to respond to an external stimulus presented in the space, independently from its actual execution. In the present study, the PMC is more activated by the high arousal level expressed by the avatar’s body than the low-arousing one. This result aligns with previous findings showing that high-arousing body postures with socially relevant cues elicit a greater PMC activity than postures with low arousal levels (25). Strikingly, our results revealed a stronger PMC activity after the dynamic experience of low-arousing architectures at an early stage of the other’s body processing. Hence, we argue that the low-arousing state generated by the architectural experience may foster the preparation of an adaptative social response to the avatar’s body expression, thus eliciting a greater motor excitability in the PMC as a signature of greater availability of attentional resources.
The LPP and P200 are the ERP components sensitive to the late and early processing of the emotional stimulus. On the one hand, the LPP indexes sustained attention on arousing stimuli (29, 40), reflecting the evaluative process of the stimulus significance that may initiate an approaching or aversive response. Our results revealed that the observation of arousing body postures increased LPP amplitude compared with low- and middle-arousing postures, in line with previous research (26, 27). On the other hand, the P200 indexes an early capture of attention, facilitating fast detection of biologically relevant stimuli (28, 41). Strikingly, the dynamic experience of architecture elicited a higher P200 amplitude when observing the avatars within the low-arousing condition compared with the high-arousing one. Previous research found that low-arousing positive states broaden attentional resources (35), while high-arousing negative ones narrow the scope of attention (42) and impair attentional control by disrupting the balance between the goal-oriented and stimulus-driven attentional system (43, 44). Also, the P200 amplitude is reduced in anxious participants (45, 46) and positively correlates with the availability of attentional resources (47). Hence, we argue that the modulation of the P200 amplitude reflects a different attentional shift due to the greater availability of attentional resources generated by the low-arousing architectural experience.
The involvement of the PMC reflects the preparation of an adaptative motor response, indexing the attentional redirection on the avatars’ body within architectural spaces. In fact, Rizzolatti and colleagues originally proposed the role of the PMC in attention mechanisms, arguing that attention systems are not separated from those for sensorimotor integration (36, 37). In this view, the modulation of PMC would reflect a higher attentional demand requested by specific architectures and body expressions. In parallel, the motor system activity represents a neural signature of embodied cognition, subserving the understanding of spatial and body characteristics (48). Thus, the modulation of PMC in our findings could reflect not the source of attentional demand, rather its target. In other words, higher-order attentional centers could attune the PMC, requesting its higher or lower activity according to the spatial and social experience of the participant.
Notably, the difference in the neural activity—depending on both body and architectural conditions—also corresponds to a different pattern of eye movements on the avatar’s body districts as convergence toward the role played by the motor system in integrating attention mechanisms and sensorimotor information. Indeed, highly expressive regions of the body such as the arms (49) were stared longer than other less informative in a late observation window depending on the avatar's arousal level. Instead, after the experience of low-arousing architectures, we found an early increase of fixations on the head of the avatar, reflecting the higher initial attention paid to such an informative cue for emotion recognition (50) and the lower anxiety state of participants generated by the low-arousing architecture. Indeed, people tend to look at other’s faces when they feel comfortable during social interactions (51).
Finally, subjective arousal ratings revealed that participants judged the avatars’ body posture as more arousing after the dynamic experience within the low-arousing architecture compared to the high-arousing condition. This is because the strength of the alert response depends mainly on the level of arousal. If it is initially high, it can increase only by a small amount, while when it is initially low, a larger amount of attentional resources is available for the response to stimulus presentation, thus revealing an adaptation effect due to the dynamic experience of architecture (52, 53). Such an arousing state then influences the subsequent processing of the avatars’ body postures in terms of eye movement pattern and subjective arousal judgment. Since the P200 is elicited after the adaptation stimulus, it appears that adaptation mechanisms could influence the availability of attentional resources. This argument could be further developed by investigating the cerebral data with higher spatial resolution and administering behavioral tasks linking adaptation effects and attentional functions.
Although in the present study we used a task in the domain of social cognition, we cannot disregard the possibility that the architectural effect we illustrate is specific to the human body. The reactions to different target stimuli, such as inanimate objects, would shed light on this. Also, further studies are needed to transfer this knowledge from virtual to real scenarios to consider the whole multisensory experience the architecture provides.
Over the last few years, researchers have already described the architectural experience in terms of sensorimotor integration, pointing to the modulation of sensorimotor brain areas depending on architectural affordances (16, 17), as well as reflecting the involvement of the motor system during the processing of architectural elements within the surrounding space (13, 54). Here, the presence of an emotional body expression adds a social component to the architectural experience, which has been neglected so far. Our findings showed that the involvement of motor-related brain areas depends on spatial experience. Such evidence describes how the architectural space influences the processing of others’ affective states.
Materials and Methods
Participants.
We recruited 24 participants (26.66 ± 4.02 y, 12 female) for the EEG study and 29 (26.96 ± 3.91 y, 14 female) for the eye-tracking study, satisfying the sample size returned by the power analysis. All participants were naive to the purpose of the experiment and had a normal or corrected-to-normal vision, with no history of psychiatric and neurological disorders. The local ethical committee approved the study (Comitato Etico AVEN), which was conducted according to the principles expressed in the Declaration of Helsinki. Each participant provided written informed consent before the experiment.
Stimuli.
Virtual architectures were selected from a database developed and characterized in a previous study (32) in which participants dynamically experienced 54 different architectural spaces and then rated such experience in terms of arousal. Here, we chose the two architectural forms (in a cold and warm colored texture) that maximally generated either a low- or a high-arousing state in the participants. Specifically, spaces were characterized by varying three architectural features (side walls distance, ceiling height, and windows sill height) between consecutive nuclei. The high-arousing architecture had decreasing side walls distance and increasing ceiling and windows’ sill height. Conversely, the low-arousing form had increasing side walls, constant ceiling, and decreasing windows’ sill height. Also, an empty environment was designed as a control condition. Avatars’ body postures were selected from a validated database (38), choosing 10 different postures for each level of arousal (low, middle, and high).
Experimental Setup and Procedure.
After reading the written instructions, the HTC Vive Pro Eye head-mounted display (HMD) was comfortably arranged over the participant’s head. Each experimental trial started with 500 ms of static observation of the architectural space from the entrance. Afterward, participants made a straight virtual promenade of 12.5 s crossing the first two nuclei of the architecture (32). Then, participants remained steady for 750 ± 250 ms, and finally, a virtual avatar appeared in the middle of the scene for 3 s. Then, participants judged the arousal level expressed by the avatar’s body posture. To this aim, a gray panel was presented reporting the following sentence: “this person looks in a … state” ranging from “Deactivated” to “Activated.” Participants judged the avatar’s arousal by using the Vive Controller. The experiment consisted in 150 trials divided into six blocks. The first and the last blocks comprised 15 trials each, where five body postures with low-, middle-, and high-arousal were presented within the empty environment. Conversely, 30 body postures (10 for each arousal level) were randomly presented within the central blocks' low- and high-arousing architectures. At the end of each block, participants were allowed to take the HMD off and have some rest. (Movies S1–S5 in SI Appendix show examples of experimental trials).
Behavioral Data Collection and Analysis.
Participants judged the avatar’s arousal by pressing the joypad trackpad button. The cursor of the corresponding panel moved by steps of 0.0083, ensuring a continuous-like movement within the scale ranging between [0, 1], i.e., from deactivated to activated. Then, participants confirmed their choice by clicking the joypad button. Before any statistical data analysis, we discarded trials with possible dips of attention (2.39% ± 3.24 for the EEG study and 2.40% ± 2.27 for the eye-tracking study). Arousal ratings were z-score normalized considering the mean and SD of the scores provided in the empty scene. These normalized scores were analyzed via a 2 × 2 × 3 repeated measures (rm) ANOVA, where the within factors were Form (Low-, High-Arousing), Color (Cold, Warm), and Body (Low-, Middle-, High-Arousing).
EEG Data Collection and Preprocessing.
The EEG was continuously recorded at a sampling rate of 500 Hz (vertex reference) using the 128-channel Geodesic EEG System (Electrical Geodesics Inc.) and the HydroCel Geodesic Sensor Net, which arrays 19 electrode sensors (AgCl-coated electrodes) in a geodesic pattern over the surface of the head at the equivalent 10 to 20 system locations. Consistent positioning was achieved by aligning the Sensor Net with skull landmarks (nasion, vertex, and preauricular points). We used the Net Amps300 high-input impedance amplifier. Low-noise EEG data were obtained, guaranteeing sensor-skin impedances below 50 kΩ except for the reference one, which was kept below 10 kΩ.
EEG data were imported into MATLAB to perform the following analysis with EEGLAB v2021.0 (55). We excluded the outermost belt of electrodes of the sensor net, prone to show residual muscular artifacts, thus discarding 19 peripheral channels located on the cheeks and nape (56). Data were subsampled at 250 Hz, and the PREP pipeline was performed for line noise removal, identification and interpolation of bad channels, and data rereferencing to the common reference (57). To identify ocular, muscular, and remaining channel noise, we computed the Independent Component Analysis (ICA) on the EEG principal components that explained 99% of the data variance (55.50 ± 11.21). To this aim, data were first band-pass filtered ([2, 100] Hz) (58), segmented in epochs around the avatar presentation ([−1,500, 4,000] ms), removing the mean value across the epoch (59), and visually inspected to remove corrupted trials (5.46% ± 8.23). Then, we run the runICA algorithm available in EEGLAB v2021.0 (55). Bad independent components (ICs) (16% ± 7.98) were identified using the ICLabel toolbox (60).
ERP analysis.
The EEG dataset resulting from the Prep pipeline was band-pass filtered ([0.1, 30] Hz) (61) and segmented in epochs around the avatar presentation ([−1,500, 1,000] ms). Then, ICA weights were applied to the data and pruned previously identified bad components. A final bad trial rejection (5.43% ± 6.57) was performed by visual inspection. The ERP analysis was performed using the Factorial Mass Univariate ERP Toolbox (FMUT) (62). First, trials were baseline corrected ([−200, 0] ms). Then, two factorial analyses were performed with within-factors Form and Body in two different time windows (0 to 400 ms and 300 to 1,000 ms) to investigate ERPs at both early and late processing stages. Corrections for multiple comparisons were performed through a cluster-based permutation approach. Specifically, the significance threshold was set to 0.05, the number of permutations to 10,000, and the electrode neighbor distance to 4 cm. The FMUT analysis revealed significant spatiotemporal clusters identifying ERP components. Hence, we finally performed pairwise cluster mass permutation tests on the mean ERP amplitudes resulting from the significant time windows.
ERP source analysis.
We localized ERP sources by solving the inverse problem with the Tikhonov-regularized minimum norm (63). Statistical analysis was conducted at the source level to unveil the cortical generators of the ERPs that emerged at the scalp level. Specifically, for the P200, we averaged the cortical activity within a 60 ms time window centered on the P200 peak and then compared the conditions low- vs. high-arousing Form by computing paired t test (two-tailed) between each dipole. Instead, considering that the LPP is a slow tonic component, we averaged the cortical activity elicited within sliding windows of 60 ms each, from 420 to 960 ms. For each time window, we then computed the paired t test (two-tailed) between each dipole, comparing the activity elicited by high- vs. low-arousing Body. The significance threshold (alpha = 0.05) was adjusted using a FDR approach implemented in Brainstorm to correct for multiple comparisons.
Eye-gaze data collection and analysis.
Data from one participant were discarded due to technical reasons during the eye-tracking recording, thus we analyzed data from 28 participants. We computed FTs during the observation of the emotional body postures over four different ROIs identifying the head, the trunk, the arms, and the legs of the avatar, respectively (49, 64). Fixations that lasted less than 200 ms were discarded (65–67), and then, FTs were z-scored considering the corresponding ROIs in the empty control scene. To analyze the spatiotemporal dynamics of the participants’ gaze behavior, we computed the time spent looking at each ROI within time slices of 100 ms each (68) and compared FT between low- vs. high-arousing body postures and low- vs. high-arousing architectures through Montecarlo statistics (5,000 iterations, significance threshold 0.05). Considering the strongly correlated spatiotemporal structure of FTs, we adopted a cluster correction method to control for multiple comparisons. Finally, a metapermutation was performed, running the Montecarlo permutation 20 more times and eventually computing the averaged P-value (69).
SI Appendix provides details about Power Analysis, Stimuli Characterization, Virtual Environment, Preliminary Data Clean Up, Source Localization Parameters for the EEG study, and Power Analysis and Eye-Gaze Data Collection and Analysis for the eye-tracking study.
Data and scripts used for the present work are available at the following public repository: https://zenodo.org/record/8341102.
Supplementary Material
Appendix 01 (PDF)
Representation of one experimental trial showing the promenade within the low-arousing architecture (cold texture) and the presentation of an avatar with a high-arousing body posture.
Representation of one experimental trial showing the promenade within the low-arousing architecture (warm texture) and the presentation of an avatar with a low-arousing body posture.
Representation of one experimental trial showing the promenade within the high-arousing architecture (cold texture) and the presentation of an avatar with a high-arousing body posture.
Representation of one experimental trial showing the promenade within the high-arousing architecture (warm texture) and the presentation of an avatar with a low-arousing body posture.
Representation of one experimental trial showing the promenade within the empty control environment and the presentation of an avatar with a middle-arousing body posture.
Acknowledgments
A research agreement between Lombardini22 and the Parma unit of the Institute of Neuroscience of the National Research Council of Italy supported the present study.
Author contributions
P.P., D.R., P.A., F.C., and G.V. designed research; P.P. and G.M.G. performed research; P.P. and G.M.G. analyzed data; and P.P., P.A., F.C., G.R., and G.V. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: T.D.A., Salk Institute for Biological Studies; E.R.M., University of California San Diego; and O.V., University of Toronto.
Contributor Information
Giacomo Rizzolatti, Email: giacomo.rizzolatti@unipr.it.
Giovanni Vecchiato, Email: giovanni.vecchiato@in.cnr.it.
Data, Materials, and Software Availability
All data shared in this article appear within the main text or SI Appendix. Data have been deposited at https://zenodo.org/record/8341102 (70).
Supporting Information
References
- 1.Schafer M., Schiller D., Navigating social space. Neuron 100, 476–489 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorfman A., Weiss O., Hagbi Z., Levi A., Eilam D., Social spatial cognition. Neurosci. Biobehav. Rev. 121, 277–290 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Sternberg E. M., Wilson M. A., Neuroscience and architecture: Seeking common ground. Cell 127, 239–242 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Gepshtein S., Snider J., Neuroscience for architecture: The evolving science of perceptual meaning. Proc. Natl. Acad. Sci. U.S.A. 116, 14404–14406 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eberhard J. P., Applying neuroscience to architecture. Neuron 62, 753–756 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Fogassi L., et al. , Space coding by premotor cortex. Exp. Brain Res. 89, 686–690 (1992). [DOI] [PubMed] [Google Scholar]
- 7.Fogassi L., et al. , Coding of peripersonal space in inferior premotor cortex (area F4). J. Neurophysiol. 76, 141–157 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Rizzolatti G., Fadiga L., Fogassi L., Gallese V., The space around us. Science 277, 190–191 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Berti A., Rizzolatti G., Coding Near and Far Space (Oxford University Press, 2002) (July 19, 2021). [Google Scholar]
- 10.Cléry J., Guipponi O., Odouard S., Wardak C., Ben Hamed S., Cortical networks for encoding near and far space in the non-human primate. Neuroimage 176, 164–178 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Rizzolatti G., Sinigaglia C., The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nat. Rev. Neurosci. 11, 264–274 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Arioli M., Cattaneo Z., Ricciardi E., Canessa N., Overlapping and specific neural correlates for empathizing, affective mentalizing, and cognitive mentalizing: A coordinate-based meta-analytic study. Hum. Brain Mapping 42, 4777–4804 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vecchiato G., et al. , Electroencephalographic correlates of sensorimotor integration and embodiment during the appreciation of virtual architectural environments. Front. Psychol. 6, 1944 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bower I., Tucker R., Enticott P. G., Impact of built environment design on emotion measured via neurophysiological correlates and subjective indicators: A systematic review. J. Environ. Psychol. 66, 101344 (2019). [Google Scholar]
- 15.Vartanian O., et al. , Impact of contour on aesthetic judgments and approach-avoidance decisions in architecture. Proc. Natl. Acad. Sci. U.S.A. 110, 10446–10453 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djebbara Z., Fich L. B., Petrini L., Gramann K., Sensorimotor brain dynamics reflect architectural affordances. Proc. Natl. Acad. Sci. U.S.A. 116, 14769–14778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djebbara Z., Fich L. B., Gramann K., The brain dynamics of architectural affordances during transition. Sci. Rep. 11, 2796 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djebbara Z., Jensen O. B., Parada F. J., Gramann K., Neuroscience and architecture: Modulating behavior through sensorimotor responses to the built environment. Neurosci. Biobehav. Rev. 138, 104715 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Jelić A., Tieri G., De Matteis F., Babiloni F., Vecchiato G., The enactive approach to architectural experience: A neurophysiological perspective on embodiment, motivation, and affordances. Front. Psychol. 7, 481 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinter-Wollman N., Jelić A., Wells N. M., The impact of the built environment on health behaviours and disease transmission in social systems. Philos. Trans. R Soc. Lond. B Biol. Sci. 373, 20170245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higuera-Trujillo J. L., Llinares C., Macagno E., The cognitive-emotional design and study of architectural space: A scoping review of neuroarchitecture and its precursor approaches. Sensors 21, 2193 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S., Sanches de Oliveira G., Djebbara Z., Gramann K., The embodiment of architectural experience: A methodological perspective on neuro-architecture. Front. Hum. Neurosci. 16, 833528 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Gelder B., de Borst A. W., Watson R., The perception of emotion in body expressions. Wiley Interdiscip. Rev. Cogn. Sci. 6, 149–158 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Van den Stock J., Righart R., de Gelder B., Body expressions influence recognition of emotions in the face and voice. Emotion 7, 487–494 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Conty L., Dezecache G., Hugueville L., Grèzes J., Early binding of gaze, gesture, and emotion: Neural time course and correlates. J. Neurosci. 32, 4531–4539 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flaisch T., Häcker F., Renner B., Schupp H. T., Emotion and the processing of symbolic gestures: An event-related brain potential study. Soc. Cogn. Affect. Neurosci. 6, 109–118 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D., Wang X., The processing characteristics of bodily expressions under the odor context: An ERP study. Behav. Brain Res. 414, 113494 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Carretié L., Exogenous (automatic) attention to emotional stimuli: A review. Cogn. Affect. Behav. Neurosci. 14, 1228–1258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacNamara A., Joyner K., Klawohn J., Event-related potential studies of emotion regulation: A review of recent progress and future directions. Int. J. Psychophysiol. 176, 73–88 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schupp H. T., Kirmse U. M., Case-by-case: Emotional stimulus significance and the modulation of the EPN and LPP. Psychophysiology 58, e13766 (2021). [DOI] [PubMed] [Google Scholar]
- 31.den Stock J. V., Vandenbulcke M., Sinke C. B. A., de Gelder B., Affective scenes influence fear perception of individual body expressions. Hum. Brain Mapping 35, 492–502 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Presti P., et al. , Measuring arousal and valence generated by the dynamic experience of architectural forms in virtual environments. Sci. Rep. 12, 13376 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slater M., Immersion and the illusion of presence in virtual reality. Br. J. Psychol. 109, 431–433 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Kisker J., Gruber T., Schöne B., Behavioral realism and lifelike psychophysiological responses in virtual reality by the example of a height exposure. Psychol. Res. 85, 68–81 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Gable P., Harmon-Jones E., The blues broaden, but the nasty narrows: attentional consequences of negative affects low and high in motivational intensity. Psychol. Sci. 21, 211–215 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Craighero L., Rizzolatti G., “CHAPTER 31 - The Premotor Theory of Attention” in Neurobiology of Attention, Itti L., Rees G., Tsotsos J. K., Eds. (Academic Press, 2005), pp. 181–186. [Google Scholar]
- 37.Rizzolatti G., Riggio L., Dascola I., Umiltá C., Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia 25, 31–40 (1987). [DOI] [PubMed] [Google Scholar]
- 38.Presti P., et al. , The Avatar’s gist: How to transfer affective components from dynamic walking to static body postures. Front. Neurosci. 16, 842433 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genon S., et al. , The right dorsal premotor mosaic: Organization, functions, and connectivity. Cerebral. Cortex 27, 2095–2110 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hajcak G., Foti D., Significance?… Significance! Empirical, methodological, and theoretical connections between the late positive potential and P300 as neural responses to stimulus significance: An integrative review. Psychophysiology 57, e13570 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Doallo S., Holguín S. R., Cadaveira F., Attentional load affects automatic emotional processing: Evidence from event-related potentials. NeuroReport 17, 1797–1801 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Fredrickson B. L., Branigan C., Positive emotions broaden the scope of attention and thought-action repertoires. Cogn. Emotion 19, 313–332 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eysenck M. W., Derakshan N., Santos R., Calvo M. G., Anxiety and cognitive performance: Attentional control theory. Emotion 7, 336–353 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Judah M. R., Grant D. M., Mills A. C., Lechner W. V., The neural correlates of impaired attentional control in social anxiety: An ERP study of inhibition and shifting. Emotion 13, 1096–1106 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Rossignol M., Fisch S.-A., Maurage P., Joassin F., Philippot P., Reduced processing of facial and postural cues in social anxiety: Insights from electrophysiology. PLOS One 8, e75234 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q., Ran G., Li X., The perception of facial emotional change in social anxiety: An ERP Study. Front. Psychol. 9, 1737 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghani U., Signal N., Niazi I. K., Taylor D., ERP based measures of cognitive workload: A review. Neurosci. Biobehav. Rev. 118, 18–26 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Rizzolatti G., Sinigaglia C., The mirror mechanism: A basic principle of brain function. Nat. Rev. Neurosci. 17, 757–765 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Pollux P. M. J., Craddock M., Guo K., Gaze patterns in viewing static and dynamic body expressions. Acta Psychol. 198, 102862 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Kret M., Stekelenburg J., Roelofs K., De Gelder B., Perception of face and body expressions using electromyography, pupillometry and gaze measures. Front. Psychol. 4, 28 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kret M. E., Stekelenburg J. J., de Gelder B., Roelofs K., From face to hand: Attentional bias towards expressive hands in social anxiety. Biol. Psychol. 122, 42–50 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Halovic S., Kroos C., Stevens C., Adaptation aftereffects influence the perception of specific emotions from walking gait. Acta Psychol. 204, 103026 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Fedorov L. A., Chang D.-S., Giese M. A., Bülthoff H. H., de la Rosa S., Adaptation aftereffects reveal representations for encoding of contingent social actions. Proc. Natl. Acad. Sci. U.S.A. 115, 7515–7520 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bower I. S., et al. , Enlarged interior built environment scale modulates high-frequency EEG oscillations. eNeuro 9, ENEURO.0104-22.2022 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delorme A., Makeig S., EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Michel C. M., et al. , EEG source imaging. Clin. Neurophysiol. 115, 2195–2222 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Bigdely-Shamlo N., Mullen T., Kothe C., Su K.-M., Robbins K. A., The PREP pipeline: Standardized preprocessing for large-scale EEG analysis. Front. Neuroinform. 9, 16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klug M., Gramann K., Identifying key factors for improving ICA-based decomposition of EEG data in mobile and stationary experiments. Eur. J. Neurosci. 54, 8406–8420 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Groppe D. M., Makeig S., Kutas M., Identifying reliable independent components via split-half comparisons. NeuroImage 45, 1199–1211 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pion-Tonachini L., Kreutz-Delgado K., Makeig S., ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. Neuroimage 198, 181–197 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luck S. J., An Introduction to the Event-Related Potential Technique (MIT Press, ed. 2, 2014). [Google Scholar]
- 62.Fields E. C., Kuperberg G. R., Having your cake and eating it too: Flexibility and power with mass univariate statistics for ERP data. Psychophysiology 57, e13468 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baillet S., et al. , Evaluation of inverse methods and head models for EEG source localization using a human skull phantom. Phys. Med. Biol. 46, 77–96 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Kleinsmith A., Semsar A., “Perception of emotion in body expressions from gaze behavior” in Extended Abstracts of the 2019 CHI Conference on Human Factors in Computing Systems, CHI EA '19 (Association for Computing Machinery, New York, NY, 2019), pp. 1–6. [Google Scholar]
- 65.Juarez M. C., et al. , Objectively measuring social attention of thyroid neck scars and transoral surgery using eye tracking. Laryngoscope 129, 2789–2794 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Salthouse T. A., Ellis C. L., Determinants of eye-fixation duration. Am. J. Psychol. 93, 207–234 (1980). [PubMed] [Google Scholar]
- 67.Salvucci D. D., Goldberg J. H., “Identifying fixations and saccades in eye-tracking protocols” in Proceedings of the 2000 Symposium on Eye Tracking Research & Applications, ETRA '00 (Association for Computing Machinery, New York, NY, 2000), pp. 71–78. [Google Scholar]
- 68.Chaby L., Hupont I., Avril M., Luherne-du Boullay V., Chetouani M., Gaze behavior consistency among older and younger adults when looking at emotional faces. Front. Psychol. 8, 548 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen M. X., Analyzing Neural Time Series Data: Theory and Practice (MIT Press, 2014). [Google Scholar]
- 70.Presti P., et al. , Data about architectural experience influence on other's body expression processing. Zenodo. https://zenodo.org/record/8341102. Deposited 13 September 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Representation of one experimental trial showing the promenade within the low-arousing architecture (cold texture) and the presentation of an avatar with a high-arousing body posture.
Representation of one experimental trial showing the promenade within the low-arousing architecture (warm texture) and the presentation of an avatar with a low-arousing body posture.
Representation of one experimental trial showing the promenade within the high-arousing architecture (cold texture) and the presentation of an avatar with a high-arousing body posture.
Representation of one experimental trial showing the promenade within the high-arousing architecture (warm texture) and the presentation of an avatar with a low-arousing body posture.
Representation of one experimental trial showing the promenade within the empty control environment and the presentation of an avatar with a middle-arousing body posture.
Data Availability Statement
All data shared in this article appear within the main text or SI Appendix. Data have been deposited at https://zenodo.org/record/8341102 (70).