Abstract
Toxoplasma gondii belongs to the phylum Apicomplexa and is an important cause of congenital disease and infection in immunocompromised patients. T. gondii shares several characteristics with plants including a non-photosynthetic plastid termed apicoplast and a multi-vesicular organelle that was named the plant-like vacuole (PLV) or vacuolar compartment (VAC). The name plant-like vacuole was selected based on its resemblance in composition and function to plant vacuoles. The name VAC represents its general vacuolar characteristics. We will refer to the organelle as PLVAC in this review. New findings in recent years have revealed that the PLVAC represents the lysosomal compartment of T. gondii which has adapted peculiarities to fulfill specific Toxoplasma needs. In this review, we discuss the composition and functions of the PLVAC highlighting its roles in ion storage and homeostasis, endocytosis, exocytosis, and autophagy.
Keywords: Toxoplasma gondii, apicomplexan, vacuolar compartment, plant-like vacuole, digestive, lysosome, calcium, zinc
TOXOPLASMA gondii infects approximately one-third of the world’s population (Hill & Dubey 2002; Pappas et al. 2009) and is an opportunistic pathogen of immunocompromised patients like HIV-infected individuals, fetuses, and organ transplant recipients (Luft & Remington 1992; Weiss & Dubey 2009). The resulting pathogenesis of T. gondii infection is due primarily to tissue destruction originating from the fast-growing tachyzoite form, which engages in a lytic cycle that consists of multiple rounds of host cell invasion, replication, and egress (Black et al. 2000; Blader et al. 2015). T. gondii secretes proteins from specific apical organelles, micronemes and rhoptries, which mediate host cell attachment and invasion (Carruthers & Sibley 1997). Invasion is facilitated by mechanical extrusion of the conoid, a tubulin-based apical structure (Hu et al. 2002; Dos Santos Pacheco et al. 2020). After parasite replication, active egress of T. gondii ultimately results in lysis of host cells and can cause significant tissue damage (Frenal et al. 2017). Once outside host cells T. gondii tachyzoites are challenged by sharp changes in the concentrations of the surrounding ions and nutrients in addition to their exposure to the host immune response.
Toxoplasma possesses a lysosome-like organelle that was originally termed the plant-like vacuole (PLV) (Miranda et al. 2010) or vacuolar compartment (VAC) (Parussini et al. 2010), which we will rename PLVAC in this article. The PLVAC expresses several pumps and transporters that enable the storage of toxic ions like zinc, calcium, protons, and others. Also, the PLVAC expresses hydrolytic enzymes and functions as a digestive organelle similar to mammalian lysosomes and plant lytic vacuoles (Boller & Kende 1979). Like a plant lytic vacuole, the PLVAC contains the vacuolar-H+-ATPase, the vacuolar-H+-pyrophosphatase (V-H+-PPase) and the tonoplast intrinsic proteins (TIP)-like aquaporins (Martinoia et al. 2007). In addition, the PLVAC is most noticeable as a single entity in extracellular and early intracellular stages of the tachyzoite and fragmented into multiple smaller components in intracellular stages. For these reasons it was proposed that the PLVAC helps parasites survive the sharp change in ionic composition of the surrounding milieu upon egress (Miranda et al. 2010).
Molecular components of the PLVAC (Table 1)
Table 1:
Molecular Components of the PLVAC
| Protein Name/gene ID | Known Function | Localization | Membrane/luminal | Phenotype (Fitness Score) | References |
|---|---|---|---|---|---|
| Cathepsin Protease L (TGME49_321530) | Cysteine Protease | PLVAC | Luminal | Dispensable in tachyzoites (0.68)/Significant in bradyzoites | (Parussini et al. 2010, Dou and Carruthers 2011) |
| Cathepsin Protease B (TGME49_249670) | Cysteine protease | PLVAC | Luminal | Dispensable (0.99) | (Que et al. 2002, Dou and Carruthers 2011) |
| Aspartyl Protease 1 (TGME49_201840) | Aspartic Peptidase family | PLVAC | Luminal | Dispensable (-0.36) | (McDonald et al. 2020) |
| Vacuolar Pyrophosphatase (TGME49_248670) | V-type H+-translocating pyrophosphatase VP1 | PLVAC, PM, LE compartment, acidocalcisomes | Membrane | Dispensable (1.27) | (Miranda et al. 2010) (Liu et al. 2014) |
| Aquaporin1 (TGME49_215450) | Aquaporin water channel | PLVAC, PM | Membrane | Dispensable (0.84) | (Miranda et al. 2010) |
| Chloroquine Resistance Transporter (TGME49_313930) | CRT-like Chloroquine resistance transporter like | PLVAC | Membrane | Mild in tachyzoites/Moderate in bradyzoites (0.96) | (Warring et al. 2014, Kannan et al. 2019) (Thornton et al. 2019) |
| NHE3 (TGME49_305180) | Na+/H+ Exchanger Cation/H+ exchanger | PLVAC, LE | Membrane | Dispensable (0.0) | (Francia et al. 2011) |
| V-H + -ATPase (multiple gene IDs) | Vacuolar-H+-ATPase Multi-subunit | PLVAC, PM, pre-rhoptries | Membrane | Essential | (Stasic et al. 2019) |
| Zinc Transporter (TgZnT) (TGME49_251630) | Vacuolar Zinc Transporter | PLVAC, acidocalcisomes | Membrane | Growth defect of mutants (-3.06) | (Chasen et al. 2019) |
| Pi-Na+ Transporter (TGME49_240210) | Phosphate transporter, PiT family | PLVAC, cytoplasmic vesicles, PM | Membrane | Mild growth defect of mutants. Lower virulence (-1.32) | (Asady et al. 2020) |
| Vacuolar Iron Transporter (TgVIT) (TGME49_266800) | Vacuolar Iron Transporter | PLVAC, other unknown vesicles | Membrane | Mild growth defect of mutants. Lower virulence (-1.22) | (Aghabi 2022) |
Several transporters and pumps that localize to the PLVAC have been characterized. The first description of this vacuolar compartment was based on the localization of the cysteine protease Cathepsin L, the vacuolar H+-pyrophosphatase, and the water channel Aquaporin 1 (Table 1). The presence of several plant-like activities like the vacuolar proton pyrophosphatase (TgVP1), a vacuolar proton ATPase (TgV-ATPase), a cathepsin L-like protease (TgCPL), and an aquaporin (TgAQP1) explain the naming of plant-like vacuole or PLV. The organelle was also termed the vacuolar compartment or VAC reflecting its general vacuole-like characteristics (Fig. 1, inset).
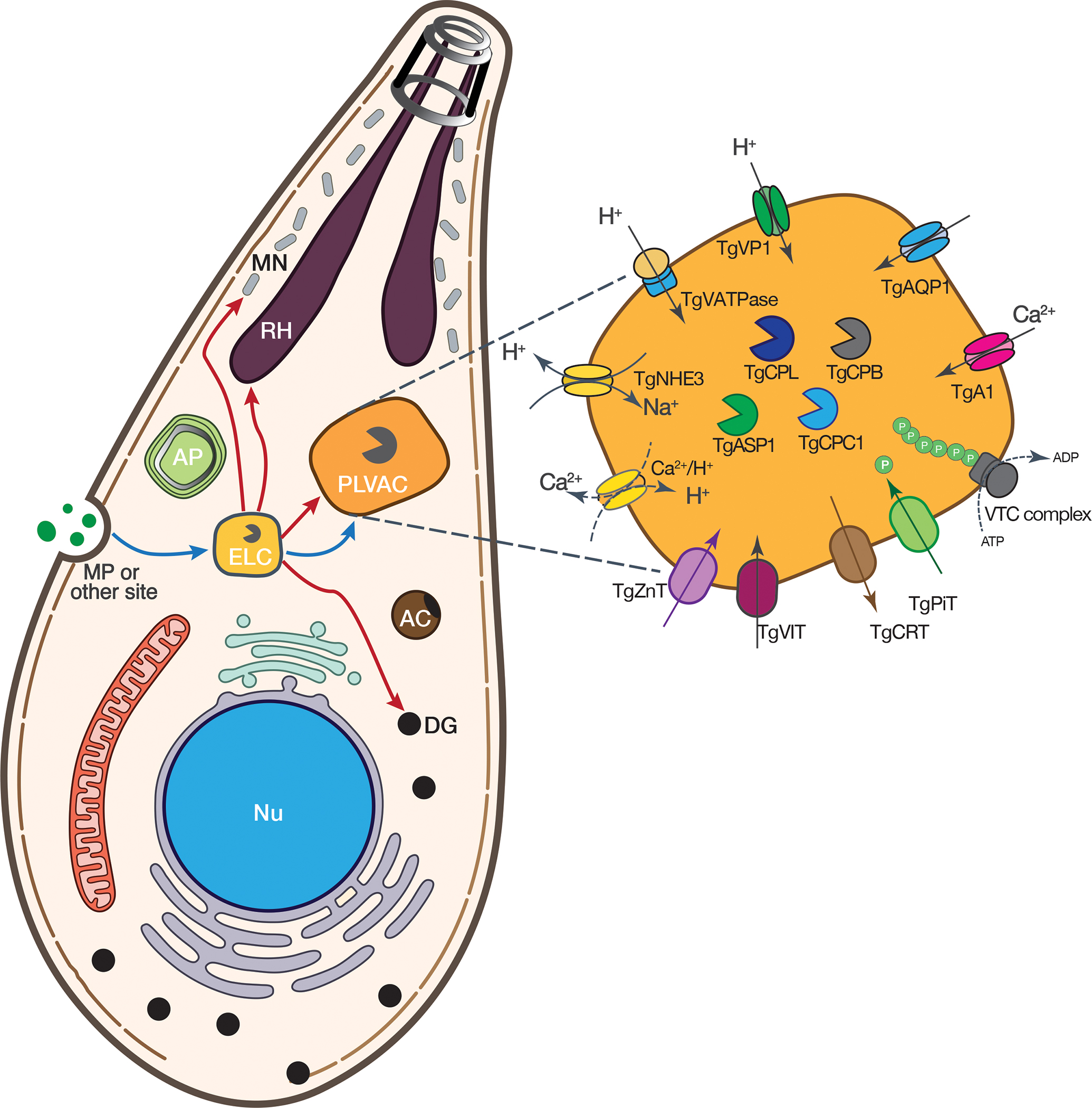
Schematic of a T. gondii tachyzoite highlighting the endolysosomal organelles, ELC and PLVAC and their connection with the secretory organelles (rhoptries, micronemes and dense granules). The inset shows an enlarged PLVAC with the known components. Some activities are shown with dashed lines as they have not been molecularly demonstrated. TgVP1, vacuolar proton pyrophosphatase; TgVATPase, vacuolar proton transport ATPase; NHE3, sodium proton exchanger; TgZnT, zinc transporter, TgCRT, chloroquine resistance transporter; TgPiT, phosphate sodium transporter; TgAQP1, aquaporin; TgA1, Ca2+-ATPase; Ca2+/H+, Ca2+ proton exchanger; TgCPL, cathepsin L; TgCPB, cathepsin B; TgCPC1, cathepsin C1; TgASP1, aspartic protease.
The T. gondii Cathepsin L or TgCPL, is a cysteine protease and represents one of five papain-like cathepsins present in T. gondii (Dou & Carruthers 2011). TgCPL is first synthesized as an immature precursor, which is processed into a mature form that contains the key catalytic residues important for proteolytic activity (Huang et al. 2009; Larson et al. 2009). TgCPL defined the PLVAC compartment, a unique structure distinct from other apical organelles, but clearly associated with the exocytic and the endosomal system. TgCPL displayed a specific localization to a large vacuolar compartment usually found toward the apical end of the tachyzoite. The localization of TgCPL to the PLVAC in extracellular and newly invaded T. gondii tachyzoites was determined using specific antibodies (Parussini et al. 2010). Some co-localization of TgCPL was seen with the micronemal protein TgAMA1 (Parussini et al. 2010), indicating a possible link to the microneme pathway. Potential substrates for TgCPL were determined to be proTgM2AP, proTgMIC3 and proTgMIC6 (Parussini et al. 2010). Deletion of the TgCPL gene in RH T. gondii parasites caused an invasion defect, which was also seen with cysteine protease inhibitors (Teo et al. 2007; Larson et al. 2009; Chaparro et al. 2018). RHΔcpl tachyzoites also showed diminished replication, which is recapitulated with protease inhibitors targeting TgCPL (Dou et al. 2013; Chaparro et al. 2018).
Although the dominant protease of the PLVAC is cathepsin L, other proteases like cathepsin B, C and D have been shown to co-localize with PLVAC markers. TgCPB maturation was dependent on TgCPL, unlike the self-maturation of many cathepsins (Dou et al. 2013). A deletion mutant of TgCPB did not show an evident growth defect and showed a limited role in the digestion of host proteins (McDonald et al. 2020). Concerning TgASP1 (a cathepsin D-like aspartyl protease), the data support that its maturation also involves the activity of TgCPL, although other proteases are also likely involved. TgASP1 was not required for tachyzoite replication or digestion of host protein in the PLVAC (McDonald et al. 2020). We discuss further the role of PLVAC proteolytic activities in the chronic infection section.
The vacuolar-H+- pyrophosphatase (V-H+-PPase) is a membrane-associated proton pump that couples the hydrolysis of pyrophosphate (PPi) to the active transport of protons against the electrochemical gradient (Maeshima 2000). They are termed vacuolar because of their preferential localization to vacuolar compartments. These enzymes, which are absent in mammalian cells, have been mostly studied in plants in which their main role is the transport of protons into the plant vacuole (Drozdowicz & Rea 2001). V-H+-PPases are also present in protists, including apicomplexan parasites like T. gondii and Plasmodium falciparum (McIntosh & Vaidya 2002). In T. gondii, the enzyme (TgVP1) was first localized in intracellular parasites to an endosome-associated compartment (named VP1 compartment), which accumulated the microneme protein MIC2 when the propeptide of its partner protein MIC2-associated protein (M2AP) was deleted (Brydges et al. 2006). This compartment is likely what is now termed the endosomal-like compartment or ELC (Jackson et al. 2013), which is probably a mixture of early and late endosomes (Parussini et al. 2010). However, VP1 can redistribute to the PLVAC in extracellular parasites (Miranda et al. 2010), potentially because of fusion of the ELCs with the PLVAC. This notable redistribution highlights the dynamic nature of the T. gondii endolysosomal system. The proton pumping activity of TgVP1 was inhibited by PPi analogues like imidodiphosphate (IDP) and aminomethylene diphosphonate (AMDP) and was stimulated by K+ ions (Miranda et al. 2010). Both AMDP and other PPi analogues inhibited T. gondii growth (Rodrigues et al. 2000; Drozdowicz et al. 2003). Proton pumping activity was enriched in a subcellular fraction obtained after a subcellular fractionation followed by an iodixanol discontinuous gradient. Enrichment for this PLVAC marker was determined following the AMDP sensitive PPi hydrolysis activity (Miranda et al. 2010). The fraction was also enriched for other PLVAC markers like TgCPL and TgV-ATPase. In plants, the V-H+-PPase and the V-H+-ATPase are located in the same membrane (Rea et al. 1992) and, consistent with this, Miranda et al. (Miranda et al. 2010) observed an additive acidification activity stimulated by PPi and ATP in fractions enriched for the PLVAC.
Deletion of the TgVP1 gene in the Δvp1 mutant caused defects in attachment and invasion of host cells, which correlated with defective microneme secretion and TgCPL maturation (Liu et al. 2014). In addition, the Δvp1 mutant could not regulate osmotic stress and was less virulent to mice. The mutant was viable and able to grow in vitro, and the mild phenotype was attributed to a compensatory role of the TgV-ATPase.
The vacuolar- H+-ATPase (V-ATPase), a protein complex consisting of 14 different subunits, couples the hydrolysis of ATP to the pumping of protons across membranes, often into the lumen of acidic vesicles (Forgac 2007; Saroussi & Nelson 2009). The function and localization of the T. gondii TgV-ATPase were inferred through the characterization of the a1 subunit (vha1), which is a 100-kDa integral membrane that spans both domains of the complex (Vo and V1) (Forgac 2007). The N-terminal domain connects V1 and Vo and stabilizes the complex during rotary catalysis. The C-terminal domain is membrane-embedded and is involved in proton transport (Wang et al. 2008). It had been demonstrated that the V-ATPase pumps protons to the extracellular milieu, which likely contributes to the homeostasis of intracellular pH and to the PM membrane potential (Moreno et al. 1998). Vha1 localized to both the plasma membrane and the PLVAC (Stasic et al. 2019). Conditional control of the expression of the vha1 gene permitted the characterization of this essential gene. Conditional repression of vha1 caused defects in all the major steps of the lytic cycle, including maturation and secretion of micronemes, invasion, motility, and egress. Defects in invasion and H+ pumping activity were evident earlier than the microneme secretion defect. Altered H+ pumping impacted Ca2+ regulation and signaling, which negatively impacted invasion (Stasic et al. 2019; Stasic et al. 2021). Depletion of vha1 negatively impacted the maturation and correct localization of several microneme proteins, most likely due to defective acidification of the compartment where their respective maturases are processed. A model was proposed based on the presence of two acidic endosome compartments expressing different levels of TgVP1. Acidification of both organelles would be important for either the maturation process of maturases and/or for their specific proteolytic activity on their substrates, including essential adhesins secreted for invasion. The presence of more than one of these compartments could represent how lytic activity is regulated by limiting and/or allowing contact with substrates.
The PLVAC also expresses an aquaporin water channel, TgAQP1, which has high similarity to the plant aquaporins known as tonoplast intrinsic proteins (TIPs), found in lytic plant vacuoles (Martinoia et al. 2007). A motif analysis of representative proteins from the major intrinsic protein (MIP) family of aquaporins supports the close relationship between TgAQP1 and plant γ-TIPs (Miranda et al. 2010). Overexpression of the TgAQP1 gene permitted its visualization in the PLVAC using specific anti-AQP antibodies. The IFA signal of the AQP co-localized with TgCPL signal in extracellular parasites. The normal level of expression of the TgAQP1 gene is most likely very low making it difficult to visualize endogenous levels of the protein. Deletion of the TgAQP1 gene did not affect growth of T. gondii. No functional analysis was done of the TgAQP1 protein, but T. gondii overexpressing the TgAQP1 gene were more resistant to HgCl2, an AQP1 inhibitor (Miranda et al. 2010).
A zinc transporter (TgZnT) was localized to vesicles (acidocalcisomes) that fuse with the PLVAC and mutant parasites for the TgZnT showed a retarded growth phenotype, which was exacerbated by increasing zinc concentrations in the extracellular milieu (Chasen et al. 2019), supporting a role for the transporter in zinc tolerance in extracellular tachyzoites.
Zinc is an essential element as it acts as a cofactor for a large number of enzymes (Coleman 1992). However, its cytosolic concentrations need to be tightly regulated as high sustained concentrations of zinc could be deleterious for all cells (Choi & Koh 1998; Kim et al. 2000; Sheline et al. 2000). Although the concentration of zinc in cells ranges from 0.1–0.5 mM, most of the metal is bound to proteins or sequestered into lysosomal compartments, and the cytosolic resting concentration of zinc is reported to be at picomolar levels (Outten & O’Halloran 2001; Woodier et al. 2015). It was proposed that the TgZnT in the PLVAC plays an essential role in the homeostasis of zinc and protection against its toxicity. The function of the transporter was validated by yeast complementation (Chasen et al. 2019).
The chloroquine resistance transporter (CRT) was localized to the digestive vacuole (DV) of Plasmodium parasites (Fidock et al. 2000), a lysosomal organelle and the site of hemoglobin degradation and chloroquine action (Fidock et al. 2000). Mutations in PfCRT resulted in reduced chloroquine accumulation in the DV causing parasites to become resistant to the drug (Martin et al. 2009). Although originally identified as the main basis for chloroquine resistance, later work determined that mutations in the PfCRT were associated with the parasite sensitivity to many antimalarials (Martin 2020). PfCRT belongs to a family of transporters termed the CRT family, which are found in all apicomplexan parasites, other protists, and plants. The Arabidopsis chloroquine-like transporter was localized to the plastid where it mediates glutathione transport (Maughan et al. 2010).
More recent studies characterizing T. gondii Δcrt mutants, showed a severely swollen PLVAC (Kannan et al. 2019; Thornton et al. 2019). The deletion of TgCRT arrested the separation of PLVAC from its precursor, the ELC, during parasite replication (Thornton et al. 2019). Also noted was aberrant localization of markers and down-regulated expression of several proteases that reside in the PLVAC (Thornton et al. 2019). These defects resulted in altered microneme secretion, invasion, and virulence. Additionally, TgCRT is speculated to serve as a transporter for small nutrient molecules as its native function, and accordingly the loss of TgCRT is expected to cause accumulation of solutes in the PLVAC and increased osmotic pressure, which further leads to the enlargement of the PLVAC. The studies supported the central role of TgCRT in controlling the PLVAC size and integrity through the movement of small molecules (osmolytes) from the organelle into the cytosol to maintain the PLVAC osmolarity.
A study characterizing the phosphate transporter TgPiT, determined its localization to the PLVAC, mainly to internal vesicles formed by the inward budding of its limiting membrane. TgPiT also labeled other cytoplasmic vesicles and the plasma membrane (Asady et al. 2020). Gene deletion and complementation determined that TgPiT is important for parasite survival, virulence, and osmoregulation. The Δpit mutant showed reduced cell volume and showed a reduction in the overall levels of polyphosphate (polyP) and phosphate (Pi) content despite an increase in the number of acidocalcisomes, the main site for polyP storage (Rodrigues et al. 2002). The Δpit mutant showed reduced acidic Ca2+ release, which was attributed to reduced levels of polyPs. It is possible that the intraluminal membranes labeled with TgPiT are acidocalcisomes since acidocalcisomes have been shown to interact with the PLVAC (Miranda et al. 2010).
A report on the molecular characterization of the vacuolar type Na+/H+ exchanger, TgNHE3, showed its co-localization with TgVP1 in intracellular parasites, likely associated with the ELC (Francia et al. 2011). Extracellular tachyzoites of the TgNHE3 knockout mutant showed sensitivity to hyperosmotic shock, high sodium, and contained higher intracellular Ca2+ concentration [Ca2+]i, which resulted in a reduced host invasion efficiency, presumably due to the observed reduced secretion of micronemes. The results suggested that the function of ELCs and PLVAC in extracellular parasites is analogous to those of the vacuolar compartments of plants and yeasts, providing the parasite with a mechanism to resist ionic fluctuations and, potentially, regulate protein trafficking.
A plasma membrane type Ca2+-ATPase (TgA1) was characterized and determined to be of the vacuolar type, and it was localized with a homologous antibody to a large vacuolar compartment in extracellular T. gondii (Luo et al. 2001). This work was published prior to the characterization of the PLVAC, and it was suggested to be a large acidocalcisome. Considering that the PLVAC most likely stores Ca2+, it is possible that TgA1 is the Ca2+ pump involved. This has not been clearly shown. A TgA1 knockout mutant showed a mild growth phenotype but had microneme and invasion defects and reduced virulence (Luo et al. 2005). These biological defects were attributed to reduced levels of polyphosphates and increased resting calcium levels.
Polyphosphate (polyP) is a ubiquitous polymer of tens to hundreds of phosphate residues linked by high-energy phosphoanhydride bonds and can reach millimolar levels in protozoan parasites (assuming distribution across the entire cellular volume and expressed as Pi monomer), while the concentration in host cells is at the micromolar level. PolyP plays essential roles in cells as a phosphate reservoir, energy source, chelator of metal ions, and possesses regulatory roles in cell metabolism and stress response, differentiation, and gene expression. PolyP was shown to be enriched in acidocalcisome fractions. Considering that these organelles fuse/interact with the PLVAC as shown in a number of images (Fig. 2B-c from (Miranda et al. 2010)), most likely the PLVAC is a store of polyP as it has been shown for the yeast vacuole (Li & Kane 2009).
Studies in yeast showed that the synthesis of polyP, is catalyzed by a five subunits protein complex also known as the Vacuolar Transporter Chaperones or VTC1–5 (Cohen et al. 1999; Ogawa et al. 2000; Desfougeres et al. 2016). Two genes with homology to the yeast VTC proteins ScVtc2p (VTC2) and ScVtc4p (VTC4) were identified in T. gondii and we have shown that the VTC4 subunit co-localizes with the V-H+-ATPase to acidocalcisomes and the PLVAC (Stasic et al. 2021). As polyP is negatively charged, it is most likely involved in chelating positively charged ions like Ca2+, Zn2+ and others. Alkalinization of acidic compartments containing polyP would activate their hydrolysis causing the release of Ca2+ or other ions (Rodrigues et al. 2002), which implicates polyP in the homeostasis of positively charged ions.
The PLVAC and its relationship to other vacuoles
The PLVAC in Toxoplasma, the digestive vacuole (DV) in Plasmodium, and the yeast vacuole in Saccharomyces cerevisiae share many features of the lysosome, such as storage of acidic hydrolytic enzymes and an acidic environment that facilitates degradation of proteins. Vacuoles in fungi (Klionsky et al. 1990) and plants (Marty 1999) share common features with mammalian lysosomes as they are highly dynamic and constantly changing and re-modeling with the cell cycle stages and growth conditions (Weisman 2006; Bandyopadhyay et al. 2014; Zhang et al. 2014; de Marcos Lousa & Denecke 2016). Mammalian lysosomes are smaller and more numerous (Novikoff et al. 1956; de Duve 2005; de Marcos Lousa & Denecke 2016) while most fungal vacuoles are larger and present in smaller numbers (Weisman 2006). Plant vacuoles, however, are large compartments and they can fill up to 90% of the plant cell volume (Owens & Poole 1979). One important function that is shared by lysosomes and other vacuoles is their lytic degradation activities performed by several specific hydrolases. For this function, these compartments need to maintain an acidic pH, between 4.5–5.5 for lysosomes and 5.5–6.2 for yeast and plant vacuoles (Preston et al. 1989; Martinez-Munoz & Kane 2008; Li & Kane 2009; Mindell 2012; Martiniere et al. 2013). The pH of the DV was estimated at ~5.2 (Kuhn et al. 2007), but the pH of the PLVAC has not been quantitatively determined. The presence of both proton pumps, TgVP1 and the V-H+-ATPase in the PLVAC compartment, represents a similar arrangement to the plant vacuole. Neither yeast nor the mammalian lysosome expresses the V-H+-PPase (Rea & Poole 1993) which is found in plants, bacteria, and some protists and it is absent in the Opisthokonta supergroup of eukaryotes (Rea & Poole 1993). There is no evidence for the expression of the mammalian lysosomal marker LAMP in any apicomplexan parasite (ToxoDB).
The PLVAC in T. gondii was shown to be involved in the digestion of host cytosolic proteins during the acute infection (Dou et al. 2014), mirroring hemoglobin degradation in the malarial DV in blood-stage infections (Francis et al. 1997). The PLVAC also functions in the turnover of parasite organelles via autophagy during chronic infection (Di Cristina et al. 2017). TgCPL was shown to be the major hydrolase for degradation functions (Larson et al. 2009; Parussini et al. 2010; Dou & Carruthers 2011; Dou et al. 2014) and, as noted above, it also proteolytically activates other PLVAC-localized proteases like TgCPB and TgASP1 (Dou et al. 2013; McDonald et al. 2020). In the Plasmodium DV a cascade of hydrolases carries on polypeptide degradation. Three falcipains (cathepsin L-like cysteine proteases) and four plasmepsins (cathepsin D-like aspartic proteases), which are endopeptidases, initialize the cleavage of hemoglobin into polypeptide fragments (Rosenthal 2004; Bonilla et al. 2007), followed by subsequent cleavages via falcilysin (Zn2+-dependent metalloprotease) (Murata & Goldberg 2003), dipeptidyl aminopeptidase I (PfDPAP1, cysteine protease), aminopeptidase P (Mn2+-dependent metalloprotease), and M1-family aminopeptidase (Zn2+-dependent metalloprotease) (Dalal & Klemba 2007; Ragheb et al. 2011; Rosenthal 2011). In addition, plasmepsins are proteolytically activated by falcipains in the malarial DV. Unpublished data from the Dou lab showed that a T. gondii cathepsin C-like protease (TgCPC1), equivalent to PfDPAP1, was localized to the PLVAC. In the yeast vacuole, seven proteases were characterized, including three metalloproteases, three serine proteases, and one aspartyl protease (Hecht et al. 2014; Parzych & Klionsky 2019). Two endopeptidases, PEP4 (aspartyl endoprotease) and PRB1 (serine endopeptidase), act as major proteases that undergo self-cleavage and mature the other vacuolar proteases (Klionsky et al. 1990; Van Den Hazel et al. 1996; Hecht et al. 2014).
It is presently not clear how the hydrolysis products generated in the PLVAC and DV are transported out into the cytosol. Both organelles express a CRT (Fidock et al. 2000; Warring et al. 2014); however, the natural function of the CRT protein in apicomplexan parasites is still not entirely defined. A few in vitro studies reported that recombinant PfCRT transports a variety of substrates, such as 4 to 11-residue peptides derived from hemoglobin or other erythrocyte proteins, L-arginine, L-lysine, L-histidine, glutathione, and iron ions (Patzewitz et al. 2013; Juge et al. 2015; Bakouh et al. 2017; Shafik et al. 2020). PfCRT is an essential gene (Zhang et al. 2018), indicating its important role in malaria parasites. This contrasts with the function of the TgCRT in T. gondii as mutant parasites are viable (Kannan et al. 2019; Thornton et al. 2019).
These lysosome-like organelles are acidified by the V-H+-ATPase complex (Yamashiro et al. 1990; Hayashi et al. 2000; Saliba et al. 2003; Stasic et al. 2019) and in addition in S. cerevisiae an NHE family of Na+/H+ exchanger (Nhx1), which also transports K+, was shown to regulate both luminal and cytoplasmic pH and control vesicle trafficking out of the endosome (Brett et al. 2005). In addition, the PLVAC and DV express a V-H+-PPase, for proton pumping into the vacuole (Luo et al. 1999; Saliba et al. 2003; Liu et al. 2014). The import of negatively charged phosphate to the PLVAC by the Na+-phosphate co-transporter may help neutralize accumulated positive charges derived from proton translocation (Asady et al. 2020).
All three vacuoles, yeast, DV and PLVAC, serve as Ca2+ stores in their respective cells (Biagini et al. 2003; Li & Kane 2009; Miranda et al. 2010). TgA1 in T. gondii would be responsible for the uptake of Ca2+ into the PLVAC (Luo et al. 2001), while the yeast vacuole expresses two transmembrane proteins, PMC1 (Ca2+-ATPase ortholog) and VCX1 (Ca2+/H+ exchanger) for Ca2+ uptake with PMC1 having higher affinity. A Ca2+/H+ exchanger was described in T. gondii (Guttery et al. 2013), but its localization was not definitively determined.
Biogenesis and dynamics
The PLVAC is a dynamic structure and its morphology changes along the parasite’s lytic cycle. In extracellular and newly invaded stages, the organelle exists as a single large vacuole that becomes fragmented during intracellular replication, based on TgCPL staining (Miranda et al. 2010; Parussini et al. 2010). Intracellular fragmentation of the PLVAC was dependent on the cell cycle (Parussini et al. 2010). Parasites that remained extracellular for prolonged times (> 1 h) retain a coalesced PLVAC (Miranda et al. 2010). The signaling molecules or signaling pathways required for the formation and fragmentation of PLV are not yet known.
In Toxoplasma, the organelles that form earlier parts of the endolysosomal system are not well defined. As mentioned earlier, a mixture of endolysosomal organelles and vesicles was named the endosomal-like compartment (ELC) (Jackson et al. 2013), which likely includes early and late endosome, and a series of intermediary vesicles (Fig. 1). The ELC was proposed as a precursor in the biogenesis of the PLVAC, as well as of other secretory organelles like microneme, rhoptries, and dense granules (Jimenez-Ruiz et al. 2016; Sangare et al. 2016; Venugopal & Marion 2018). The classical endocytic trafficking pathway utilizes the class c core vacuole/endosome tethering factor (CORVET) complex and the homotypic fusion and vacuole protein sorting (HOPS) complex that regulates early to late endosome and late endosome to lysosome trafficking, respectively (Mizuno-Yamasaki et al. 2012; Numrich & Ungermann 2014). The CORVET and HOPS complexes are associated with early endosome and late endosome/lysosome/vacuole markers, respectively. BLAST searches of the T. gondii genome reveal the presence of orthologs for 4 core components of the CORVET and HOPS complexes, including Vps11, Vps16, Vps18, and Vps33 (Morlon-Guyot et al. 2015). Genes for the small GTPase proteins associated with early endosome and late endosome, Rab5 and Rab7, and the corresponding guanine nucleotide exchange factor (GEF), Vps9 for Rab5 and Mon1/Ccz1 for Rab7, are also encoded in the T. gondii genome. Based on these observations, it is predicted that T. gondii uses Rab5, Vps9, and the CORVET complex to coordinate the trafficking from the trans-Golgi network to early endosomes, and switch gears to Rab7, Mon1/Ccz1, and HOPS complex to achieve early-to-late endosome/PLVAC transition. Interestingly, T. gondii encodes three Rab5 orthologs (Rab5A, 5B, 5C), and one Rab7. Both Rab5A and Rab5C are well localized to ELCs (Kremer et al. 2013; Sakura et al. 2016), whereas the plant-like Rab5B is only partially co-localized with Rab5A and Rab5C and to the parasite cell membrane (Kremer et al. 2013). TgRab7 is observed in both ELCs and PLVAC (Miranda et al. 2010; Parussini et al. 2010). As a core component of both CORVET and HOPS complexes, Vps11 is associated with the PLVAC and ELC (Morlon-Guyot et al. 2015). Conditional knockdown of Vps11 caused defective biogenesis of multiple organelles in the parasites, including PLVAC, rhoptries, micronemes, and dense granules (Morlon-Guyot et al. 2015). Two proteins that interact with the CORVET and HOPS complexes in the parasites were identified (Morlon-Guyot et al. 2018). One shows low similarity to the known Vps8 subunit, an effector in the CORVET complex, while the other one carries a BEACH (beige and Chediak Higashi) domain, named a BEACH domain-containing protein (TgBDCP). Vps8 and TgBDCP co-localized with Rab5, but not Rab7, suggesting that they are probably within the CORVET complex. Surprisingly, TgBDCP was co-localized with TgCPL, indicating that it may be directly involved in the biogenesis of the PLVAC or the traffic of some proteins to the PLVAC. The conditional knockout of both TgVps8 and TgBDCP impaired the formation of the PLVAC (Morlon-Guyot et al. 2018).
Repression of the V-H+-ATPase lead to a fragmented PLVAC in extracellular tachyzoites (Stasic et al. 2019). Interestingly, the genetic deletion of TgCRT causes enlarged PLVAC, and the swollen organelle contained markers of both PLVAC and ELC (Thornton et al. 2019), indicating that the biogenesis of the PLVAC was arrested in the Δcrt mutant. Due to a significant volume increase in the Δcrt PLVAC, it was speculated that the acidity within the enlarged PLVAC would be reduced. Both findings suggested that the pH inside the PLVAC is critical for the vesicular trafficking within the endolysosomal system in T. gondii.
Functions of the PLVAC
Degradative functions of the PLVAC
The T. gondii PLVAC possesses many characteristics found in typical lysosomes including being populated by cathepsin proteases, which are commonly involved in degrading cellular proteins or proteins originating from endocytic events. T. gondii expresses six cathepsins including five papain-like cysteine proteases (TgCPL, TgCPB, and TgCPC1–3 (Dou & Carruthers 2011)) and one cathepsin D-like protease (TgASP1) (Shea et al. 2007; McDonald et al. 2020). TgCPL and TgCPB, have been localized to the PLVAC (Fig.1) (Miranda et al. 2010; Dou et al. 2013). TgCPC1 and TgCPC2 were reported to be secreted in the parasitophorous vacuoles (PV) and TgCPC3 is reportedly only expressed in the sporozoite stage (Que et al. 2007). In eukaryotes, cathepsin proteases are translated as preprocathepsins where the prepeptide is removed in the ER and the procathepsin is trafficked to the early endosome or lysosome. In the acidic environment of the lysosome, the procathepsin undergoes proteolytic processing. In T. gondii, TgCPL and TgCPB are synthesized as inactive procathepsins, trafficked to the PLVAC, where they are matured at low pH (Parussini et al. 2010; Dou et al. 2013). It was reported that a Δcpl knockout strain accumulated host expressed GFP in the PLVAC (Dou et al. 2014). Recently, it was determined that host-derived proteins are endocytosed in as little as 7 min post-invasion, trafficked to the PLVAC, and digested in under 30 min (McGovern et al. 2018). Ablation of TgCPL in the Prugniaud strain, which is competent for differentiation to bradyzoites, showed that the PLVAC was a major player in proteolytic activity in the chronic stage (Di Cristina et al. 2017). Additionally, the PLVAC was shown to play a role in autophagy as undigested organelles appeared in the PLVAC in the Δcpl mutant (Di Cristina et al. 2017). Collectively, these results showed that the PLVAC serves as a digestive organelle and that TgCPL, and possibly TgCPB, are active proteases able to digest some host-derived proteins.
Protein trafficking and maturation through a hybrid endo/exocytic system
In mammalian and yeast cells, endocytosis begins by delivering cargo to the early endosome (marked by Rab5), followed by traffic to the late endosome (marked by Rab7), and finally fusion with the mature lysosome (also marked by Rab7) (Galvez et al. 2012). In plants, however, endocytosed cargo can transit through the trans-Golgi network (TGN) first before being routed through the early endosome and finally ending in the lysosome (Viotti et al. 2010). More recent work in yeast has also identified the TGN as an early and recycling endosome (Day et al. 2018). However, beyond the TGN the endocytic systems of mammalian or model systems typically do not intersect extensively with their exocytic systems, with a few exceptions in specialized cell types.
A difficulty in understanding the endocytic pathway in T. gondii was the inability for chemical dyes to be endocytosed and a lack of known receptors or trackable ligands usually involved in the endocytic process. There are some reported instances of endocytosed cargo shown to be internalized by T. gondii, but the mechanism(s) remains unknown (Nichols et al. 1994; Botero-Kleiven et al. 2001). More recent work showed that intracellular T. gondii is also able to endocytose host-derived proteins in an intravacuolar network-dependent manner, but the mechanism(s) and site of entry into the parasite remain unclear (Dou et al. 2014). These reports collectively suggested that T. gondii is capable of endocytic events, but much still needs to be studied about this process. T. gondii possesses a trans-Golgi network (TGN), ELCs, and the PLVAC and proteins important for endocytic trafficking like clathrin, dynamin, Rab5 and Rab7 are expressed (Tomavo et al. 2013). In addition, T. gondii expresses the plant-like TgVP1 and TgAQP1 which indicates that endocytic trafficking may resemble that of plants (Pieperhoff et al. 2013).
The PLVAC is a multi-vesicular highly dynamic compartment and its formation and fragmentation suggested constant fusion and budding of internal vesicles. Rab7, usually associated with late endosomes, co-localized with the PLVAC marker TgVP1 in intracellular parasites, supporting the role of the PLVAC in the endocytic pathway (Bucci et al. 2000). Recent reports suggested that the ELCs and the PLVAC are major compartments of the endocytic system (Dou & Carruthers 2011; McGovern et al. 2018). In addition, ingested host proteins made their way through the ELCs (possibly via the TGN) and were finally trafficked to and digested in the PLVAC (McGovern et al. 2018).
Exocytic trafficking destined to the rhoptries and micronemes, proceeds through the TGN and ELCs, and involves clathrin, dynamin and Rab5 (Harper et al. 2006; Sloves et al. 2012; Kremer et al. 2013; Pieperhoff et al. 2013; Sangare et al. 2016; Venugopal & Marion 2018). Ablation of the dynamin-related protein DrpB, the sortilin-like receptor (TgSORTLR), or the clathrin heavy chain, TgCHC1, resulted in loss of micronemes and rhoptries organelles (Breinich et al. 2009; Sloves et al. 2012; Pieperhoff et al. 2013). TgCHC1 was localized to the Golgi where it most likely plays a role in the stabilization of the Golgi as its ablation resulted in abnormal Golgi (Pieperhoff et al. 2013). HOPS and CORVET complexes, which are essential for the early to late endosome transition, lysosome biogenesis, and endolysosomal trafficking (Solinger & Spang 2013) are also critical for the biogenesis of all secretory organelles (rhoptries, micronemes, and dense granules) (Morlon-Guyot et al. 2018). Collectively, these findings suggest that the T. gondii endocytic system is also a conduit for proteins destined for exocytosis.
Additional evidence supports a role for the ELCs and PLVAC in the proteolytic maturation of microneme proteins (McGovern et al. 2018; Stasic et al. 2019). Disruption of the PLVAC and ELC proton pump TgVP1 resulted in defects in microneme secretion, microneme organelle localization and host cell invasion/attachment (Liu et al. 2014). Ablation of TgCPL, which resides primarily in the PLVAC, resulted in maturation defects of two important microneme proteins, TgM2AP and TgMIC3 (Parussini et al. 2010). Disruption of the V-ATPase, which partially localizes to the PLVAC and ELC, resulted in defects in TgMIC3, TgMIC2, and TgM2AP localization, maturation, and secretion (Stasic et al. 2019). Rhoptry protein and organelle maturation were also impacted by disruption of the V-ATPase (Stasic et al. 2019). These findings provide additional evidence that the microneme and rhoptry proteins transit through the parasite endolysosomal system where they undergo proteolytic maturation.
TgCPL in the PLVAC was also identified as the maturase for TgCPB (Dou et al. 2013), and because both localize to the PLVAC, it is likely that both work together in the degradation of polypeptides in the PLVAC. Although TgCPB was reported to be involved in the maturation of ROP2, 3, and 4 (Que et al. 2002), TgCPB null mutant parasites exhibit normal maturation of ROP2 (Dou et al. 2013). Despite being a highly active protease, TgCPL can limit proteolysis at low concentrations and under suboptimal enzymatic conditions (Parussini et al. 2010). That TgCPL is found in low abundance in ELCs, which are likely less acidic, provides a possible explanation for how it contributes to the proteolytic maturation of certain microneme proteins without degrading them further. It should also be noted that a post-Golgi resident protease, TgASP3, is principally responsible for the proteolytic maturation of most microneme and rhoptry proteins (Dogga et al. 2017). These studies support a model in which T. gondii utilizes endolysosomal proteases for the proteolytic maturation of exocytic proteins, thereby ensuring their efficient processing prior to reaching their respective regulated exocytic organelles.
Salt homeostasis
As T. gondii advances through its lytic cycle, it encounters sharp changes in the ion composition of its surrounding milieu. Intracellular tachyzoites, which replicate inside a porous parasitophorous vacuole (PV), are surrounded by an ionic environment that is in equilibrium with the host cytosol, in which the concentration of calcium, zinc, chloride, and sodium is low while potassium is high. The concentration of ATP will likely be high while glucose could be low. Upon exit from host cells this surrounding milieu will change to high calcium, zinc, sodium and chloride and low potassium and ATP. Coping with these ionic and nutrient changes is of paramount importance for the extracellular tachyzoite which needs to glide, find a host cell to invade, secrete adhesins needed for attachment and actively invade a targeted cell (Black & Boothroyd 2000; Blader et al. 2015). A role for the PLVAC in ion homeostasis was postulated as it was noticed that it existed as a single entity shortly after the parasites egressed and fragmented soon after invasion of host cells (Miranda et al. 2010; Parussini et al. 2010). Overexpression of TgVP1 resulted in increased tolerance to 150 mM NaCl and significantly more plaques than parental cells under identical conditions (Miranda et al. 2010). This result supported a role for the PLVAC in accumulation of sodium and protection of parasite fitness when subjected to salt stress. This interpretation was further supported by the demonstration of proton transport stimulated by PPi in enriched PLVAC fractions (Miranda et al. 2010). The proton gradient generated through the membrane of the PLVAC was collapsed by the addition of sodium, indicating the presence of a sodium/proton exchange activity (Miranda et al. 2010). A sodium/proton exchanger TgNHE3 was characterized and localized to the ELC and shown to be important for survival of extracellular T. gondii in hyperosmotic media. The knock-out mutant of the TgNHE3 gene was less tolerant to hyperosmotic conditions (Francia et al. 2011) and showed significantly lower survival in media with hyperosmotic conditions created with high concentrations of sorbitol (0.75 M), or NaCl (0.5 M), sodium gluconate (0.75 M), or KCl (0.6 M) (Francia et al. 2011). A role of the PLVAC in osmotic homeostasis was also shown by the analysis of the TgVP1 knock-out mutant, Δvp1. The volume recovery response resulting from exposing T. gondii extracellular tachyzoites to hypoosmotic buffers was defective in the Δvp1 mutant. This deficient response was interpreted as a defective H+-gradient leading to lower capacity to accumulate osmolytes resulting in a defective cell volume response, which also negatively impacted invasion and other stress responses like high extracellular ionic concentrations and hyperosmotic stress. During the process of invasion there is an observable change in cell volume as the parasite squeezes itself through a tight junction that forms between the parasite and the host cell plasma membrane (Mordue et al. 1999). Inside the host cell cytosol the parasite appears to recover its normal cell volume, highlighting the importance of cell volume regulation during the lytic cycle of T. gondii (Liu et al. 2014). Taken together, the experimental evidence supports a role for the PLVAC in ion homeostasis which is also important for controlling osmotic homeostasis in T. gondii.
Calcium homeostasis
Changes in cytosolic Ca2+ are essential for a variety of cellular processes. It has been demonstrated that the endoplasmic reticulum (ER) is the main Ca2+ store in most cells. However, the evidence demonstrates that Ca2+ can also be stored in acidic compartments. Acidic stores include acidocalcisomes, vacuoles, lysosomes, lysosome-like organelles, secretory granules, and the Golgi apparatus (Patel & Docampo 2010). Ca2+ uptake in these compartments is usually mediated by Ca2+ pumps or exchangers and for release, acidic stores use two-pore channels or members of the transient receptor potential superfamily (Patel & Docampo 2010). In the case of the PLVAC, a calcium pump TgA1 was shown to localize to a large vacuole interpreted at the time as a large acidocalcisome (Luo et al. 2001). A Ca2+/H+ exchange activity was demonstrated in PLVAC-enriched fractions energized with PPi, by the release of protons, taken up by the activity of TgVP1, upon addition of Ca2+. The transport of Ca2+ by the Ca2+/H+ exchanger uses the energy generated by the proton gradient (Miranda et al. 2010). A different experiment with tachyzoites loaded with the Ca2+ indicator Fura2 showed that addition of glycyl-L-phenylalanine-naphthylamide (GPN), resulted in an increase in cytosolic Ca2+ (Miranda et al. 2010). GPN is known to cause osmotic swelling of lysosomes in mammalian cells and Ca2+ release into the cytosol, a phenomenon that has been used to demonstrate the presence of Ca2+ in lysosomes (Haller et al. 1996; Morgan et al. 2020). This result indicated that the PLVAC stores Ca2+ and could play a role in Ca2+ homeostasis or signaling. The V-ATPase would pump protons and contributes to the proton gradient through the PLVAC membrane. Conditional knockdown of the V-ATPase resulted in a poorly formed PLVAC and diminished intracellular levels of Ca2+ (Stasic et al. 2021). Additionally, the release of Ca2+ triggered by GPN was significantly reduced in the V-ATPase knockdown mutant (Stasic et al. 2021). These data linked proton pumping into the PLVAC to Ca2+ storage and cytosolic Ca2+ homeostasis. A recent finding of a predicted Ca2+ binding protein (TgEFP1) localized to the ELC/PLVAC supports a role for the organelle in Ca2+ storage (Dave et al. 2022).
The mechanism of Ca2+ release from the PLVAC has not been molecularly determined since the initially predicted candidate, the T. gondii two-pore channel (TgTPC), was recently localized to the apicoplast (Li et al. 2021).
Storage of toxic components and elements
An essential role of the plant vacuole is to regulate the concentrations of heavy metal ions like iron, zinc, and copper, within the optimal functional range. Compartmentalization of these ions depends on the activity of the V-H+-ATPase and the V-H+-PPase, which create a proton gradient that is used by a set of tonoplast (plant vacuolar membrane) transporters directly driven by a proton motive force (Sharma et al. 2016).
In T. gondii, overexpression of the PLVAC-localized TgAQP1 made parasites more resistant to the toxic effect of mercury (Miranda et al. 2010). In addition, a zinc transporter (TgZnT) was characterized and localized to the membrane of the PLVAC (Chasen et al. 2019). Interestingly both, complete ablation or overexpression of the TgZnT gene caused a growth defect in both mutants (Chasen et al. 2019). Interestingly the ΔTgZnT mutant was hypersensitive to exogenous zinc (Chasen et al. 2019). The function of TgZnT was validated by expressing the gene in a yeast strain mutant for its endogenous zinc transporters (Chasen et al. 2019). Although TgZnT localized to the PLVAC, it was not clear if zinc is actively transported into the PLVAC. However, these results suggested that T. gondii TgZnT is important for the propagation of the parasite and, due to its localization in the PLVAC, it is tempting to speculate that it transports zinc into to the PLVAC. Collectively, these observations demonstrated that the PLVAC may be relevant for protection from environmental ionic stress.
A Vacuolar Iron Transporter (VIT) was recently characterized and found to localize to a vesicular compartment that partially co-localized with TgCPL (Aghabi 2022). The study found that the VIT was important for detoxification of excess iron and for parasite virulence (Aghabi 2022).
Critical roles for the PLVAC during chronic infection
The function of the PLVAC in bradyzoites has been mainly studied with respect to its role in protein digestion. Kannan et al. (Kannan et al. 2021) showed that bradyzoites lacking CPL activity accumulated host-derived mCherry in the PLVAC, indicating that, like tachyzoites, chronic stage T. gondii ingests and digests proteins from the cytosol of infected cells. Ingestion of host cytosolic mCherry occurred in both in vitro differentiated bradyzoites and those derived from brain cysts isolated from infected mice. Although these studies suggest that bradyzoites have an active ingestion pathway, the importance and role of this process during chronic infection remain to be determined.
In addition to digesting host-derived protein, proteolytic digestion in the PLVAC is critical for the degradation of parasite-derived material delivered to the PLVAC via macroautophagy (hereafter referred to as autophagy). Autophagy is a “self-eat” pathway that eukaryotic cells use for several purposes including the turnover of subcellular structures and organelles. Autophagy involves the development and enlargement of double membrane cup termed a phagophore that engulfs cytoplasmic structures in a targeted (selective) or non-specific (bulk) manner. Closure of the phagophore to generate an autophagosome is followed by fusion with an endolysosomal compartment such as lysosomes or a vacuole to create an autolysosome. Earlier work reported autophagosome-like structures in T. gondii tachyzoites exposed to resource limited conditions in vitro, suggesting the parasite has a functional autophagy system (Besteiro et al. 2011; Ghosh et al. 2012). Upon differentiation of bradyzoites lacking TgCPL activity, Di Cristina et al. (Di Cristina et al. 2017) observed the development of large dark inclusions that were visible by phase contrast microscopy in the cytoplasm of parasites. Analysis by transmission electron microscopy revealed that the dark inclusions contain undigested material including remnants of parasite organelles, consistent with them being autolysosomes with attenuated proteolytic activity. Additional experiments showed the accumulation of TgATG8, a canonical marker of autophagosomes, associated with the PLVAC, indicating defective turnover of autophagosomes. Importantly, CPL-deficient bradyzoites showed a marked loss of viability both in vitro and during chronic infection of mice. More recent work showed that bradyzoites lacking the integral membrane protein TgATG9 are defective in autophagy and exhibit a severe loss of viability following in vitro differentiation and during chronic infection of mice (Smith et al. 2021). Together these studies suggested a critical role for autophagy and the turnover of autophagosomes in bradyzoites, possibly to maintain cellular homeostasis during persistence.
As noted above, TgCRT likely functions to export products of proteolytic digestion (peptides, amino acids) from the PLVAC into the parasite cytoplasm. Consistent with this role, bradyzoites lacking TgCRT showed a striking enlargement of the PLVAC (Kannan et al. 2019). The enlargement was partially reversed by inhibition of TgCPL, which is consistent with a proposed role for TgCRT as a transporter of proteolytic products. Interestingly, TgCRT-deficient bradyzoites do not appear to be defective in the turnover of autophagosomal material, suggesting that digestive function is not impaired in such parasites. Other transporters that also function in export of digestive projects probably exist but have yet to be reported.
Summary and future perspectives and unanswered questions
It has been 12 years since the first reports of the presence of the large organelle, termed at the time the PLV or VAC in T. gondii. It is interesting that many images of tachyzoites in previous publications clearly showed the PLVAC prominently displayed, but took the discovery in 2010 of PLVAC markers, mainly TgCPL and TgVP1, to identify this organelle. Subsequent publications showing more markers and their roles highlighted the PLVAC lysosomal-like characteristics. The PLVAC is clearly a compartment with multiple functions for T. gondii as the plant vacuole is for the plant cell. Some of these functions are more essential than others, which could be related to the conditions used for culturing the parasites or the host cells used. Other functions like aiding in the maturation of important secretory proteins while also acting as the site where proteins are digested is an intriguing biological duality. It is interesting to highlight the greater relevance of some proteins for bradyzoites than tachyzoites. There are likely other functions not currently identified that this organelle plays throughout the parasite lifecycle.
Meanwhile during these 12 years the field of T. gondii has expanded significantly due to new genetic tools like CRISPR/Cas9 for gene editing, use of biotin-ligase fusion-proteins to identify proximal and interacting proteins with common subcellular localizations, and the adaptation of tools to regulate expression transcriptionally or translationally, enabling studies of more immediate phenotypic differences without the risk of compensatory changes. The availability of CRISPR/Cas9 tools resulted in a burst of interest in the characterization of the role of molecules in the slow-growing bradyzoite, which has resulted in several important discoveries. We anticipate more proteins of the PLVAC will be identified by these revolutionary technologies in the coming years. Some of these could help understand its interaction with other endolysosomal compartments, its fast biogenesis in the extracellular parasite and its fragmentation during intracellular replication, and why the PLVAC with highly lytic activities aids in the maturation of secretory proteins.
Acknowledgments
Work in our laboratories was funded by the US National Institutes of Health to ZD (AI143707), SNJM (AI128356 and AI096836) and VC (AI120607 and AI160610). AJS was partially funded by a UGA and T32 fellowship (T32AI060546).
References
- 1.Aghabi DS, Megan; ZHicheng Dou; Guerra, Alfredo G; Harding, Clare R (2022) The vacuolar iron transporter mediates iron detoxification in Toxoplasma gondii. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asady B, Dick CF, Ehrenman K, Sahu T, Romano JD & Coppens I (2020) A single Na+-Pi cotransporter in Toxoplasma plays key roles in phosphate import and control of parasite osmoregulation. PLoS Pathog 16, e1009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakouh N, Bellanca S, Nyboer B, Moliner Cubel S, Karim Z, Sanchez CP, Stein WD, Planelles G & Lanzer M (2017) Iron is a substrate of the Plasmodium falciparum chloroquine resistance transporter PfCRT in Xenopus oocytes J Biol Chem 292, 16109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandyopadhyay D, Smith LC, Garcia DR, Yadav RN & Banik BK (2014) An expeditious green route toward 2-aryl-4-phenyl-1H-imidazoles. Org Med Chem Lett 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besteiro S, Brooks CF, Striepen B & Dubremetz JF (2011) Autophagy protein Atg3 is essential for maintaining mitochondrial integrity and for normal intracellular development of Toxoplasma gondii tachyzoites. PLoS Pathog 7, e1002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biagini GA, Bray PG, Spiller DG, White MR & Ward SA (2003) The digestive food vacuole of the malaria parasite is a dynamic intracellular Ca2+ store. J Biol Chem 278, 27910–5. [DOI] [PubMed] [Google Scholar]
- 7.Black MW, Arrizabalaga G & Boothroyd JC (2000) Ionophore-resistant mutants of Toxoplasma gondii reveal host cell permeabilization as an early event in egress. Mol Cell Biol 20, 9399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black MW & Boothroyd JC (2000) Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev 64, 607–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blader IJ, Coleman BI, Chen CT & Gubbels MJ (2015) Lytic Cycle of Toxoplasma gondii: 15 Years Later. Annu Rev Microbiol 69, 463–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boller T & Kende H (1979) Hydrolytic Enzymes in the Central Vacuole of Plant Cells. Plant Physiology 63, 1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonilla JA, Bonilla TD, Yowell CA, Fujioka H & Dame JB (2007) Critical roles for the digestive vacuole plasmepsins of Plasmodium falciparum in vacuolar function. Mol Microbiol 65, 64–75. [DOI] [PubMed] [Google Scholar]
- 12.Botero-Kleiven S, Fernández V, Lindh J, Richter-Dahlfors A, von Euler A & Wahlgren M (2001) Receptor-Mediated Endocytosis in an Apicomplexan Parasite (Toxoplasma gondii). Experimental Parasitology 98, 134–44. [DOI] [PubMed] [Google Scholar]
- 13.Breinich MS, Ferguson DJP, Foth BJ, van Dooren GG, Lebrun M, Quon DV, Striepen B, Bradley PJ, Frischknecht F, Carruthers VB & Meissner M (2009) A Dynamin Is Required for the Biogenesis of Secretory Organelles in Toxoplasma gondii. Current Biology 19, 277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brett CL, Tukaye DN, Mukherjee S & Rao R (2005) The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell 16, 1396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brydges SD, Zhou XW, Huynh MH, Harper JM, Mital J, Adjogble KD, Daubener W, Ward GE & Carruthers VB (2006) Targeted deletion of MIC5 enhances trimming proteolysis of Toxoplasma invasion proteins. Eukaryot Cell 5, 2174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucci C, Thomsen P, Nicoziani P, McCarthy J & van Deurs B (2000) Rab7: A Key to Lysosome Biogenesis. Molecular Biology of the Cell 11, 467–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carruthers VB & Sibley LD (1997) Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol 73, 114–23. [PubMed] [Google Scholar]
- 18.Chaparro JD, Cheng T, Tran UP, Andrade RM, Brenner SBT, Hwang G, Cohn S, Hirata K, McKerrow JH & Reed SL (2018) Two key cathepsins, TgCPB and TgCPL, are targeted by the vinyl sulfone inhibitor K11777 in in vitro and in vivo models of toxoplasmosis. PLoS One 13, e0193982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chasen NM, Stasic AJ, Asady B, Coppens I & Moreno SNJ (2019) The Vacuolar Zinc Transporter TgZnT Protects Toxoplasma gondii from Zinc Toxicity. mSphere 4, e00086–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi DW & Koh JY (1998) Zinc and brain injury. Annu Rev Neurosci 21, 347–75. [DOI] [PubMed] [Google Scholar]
- 21.Cohen A, Perzov N, Nelson H & Nelson N (1999) A novel family of yeast chaperons involved in the distribution of V-ATPase and other membrane proteins. J Biol Chem 274, 26885–93. [DOI] [PubMed] [Google Scholar]
- 22.Coleman JE (1992) Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem 61, 897–946. [DOI] [PubMed] [Google Scholar]
- 23.Dalal S & Klemba M (2007) Roles for two aminopeptidases in vacuolar hemoglobin catabolism in Plasmodium falciparum. J Biol Chem 282, 35978–87. [DOI] [PubMed] [Google Scholar]
- 24.Dave N, LaFavers K & Arrizabalaga G (2022) The Dually Localized EF-Hand Domain-Containing Protein TgEFP1 Regulates the Lytic Cycle of Toxoplasma gondii. Cells 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day KJ, Casler JC & Glick BS (2018) Budding Yeast Has a Minimal Endomembrane System. Dev Cell 44, 56–72 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Duve C (2005) The lysosome turns fifty. Nat Cell Biol 7, 847–9. [DOI] [PubMed] [Google Scholar]
- 27.de Marcos Lousa C & Denecke J (2016) Lysosomal and vacuolar sorting: not so different after all! Biochem Soc Trans 44, 891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desfougeres Y, Gerasimaite RU, Jessen HJ & Mayer A (2016) Vtc5, a Novel Subunit of the Vacuolar Transporter Chaperone Complex, Regulates Polyphosphate Synthesis and Phosphate Homeostasis in Yeast. The Journal of biological chemistry 291, 22262–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Cristina M, Dou Z, Lunghi M, Kannan G, Huynh M-H, McGovern OL, Schultz TL, Schultz AJ, Miller AJ, Hayes BM, van der Linden W, Emiliani C, Bogyo M, Besteiro S, Coppens I & Carruthers VB (2017) Toxoplasma depends on lysosomal consumption of autophagosomes for persistent infection. Nature Microbiology 2, 17096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dogga SK, Mukherjee B, Jacot D, Kockmann T, Molino L, Hammoudi PM, Hartkoorn RC, Hehl AB & Soldati-Favre D (2017) A druggable secretory protein maturase of Toxoplasma essential for invasion and egress. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dos Santos Pacheco N, Tosetti N, Koreny L, Waller RF & Soldati-Favre D (2020) Evolution, Composition, Assembly, and Function of the Conoid in Apicomplexa. Trends Parasitol 36, 688–704. [DOI] [PubMed] [Google Scholar]
- 32.Dou Z & Carruthers VB (2011) Cathepsin proteases in Toxoplasma gondii. Adv Exp Med Biol 712, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dou Z, Coppens I & Carruthers VB (2013) Non-canonical Maturation of Two Papain-family Proteases in Toxoplasma gondii. J Biol Chem 288, 3523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dou Z, McGovern OL, Di Cristina M & Carruthers VB (2014) Toxoplasma gondii Ingests and Digests Host Cytosolic Proteins. mBio 5(4), e01188–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drozdowicz YM & Rea PA (2001) Vacuolar H(+) pyrophosphatases: from the evolutionary backwaters into the mainstream. Trends in plant science 6, 206–11. [DOI] [PubMed] [Google Scholar]
- 36.Drozdowicz YM, Shaw M, Nishi M, Striepen B, Liwinski HA, Roos DS & Rea PA (2003) Isolation and characterization of TgVP1, a type I vacuolar H+-translocating pyrophosphatase from Toxoplasma gondii. The dynamics of its subcellular localization and the cellular effects of a diphosphonate inhibitor. J Biol Chem 278, 1075–85. [DOI] [PubMed] [Google Scholar]
- 37.Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LM, Sidhu AB, Naude B, Deitsch KW, Su XZ, Wootton JC, Roepe PD & Wellems TE (2000) Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell 6, 861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forgac M (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8, 917–29. [DOI] [PubMed] [Google Scholar]
- 39.Francia ME, Wicher S, Pace DA, Sullivan J, Moreno SNJ & Arrizabalaga G (2011) A Toxoplasma gondii protein with homology to intracellular type Na+/H+ exchangers is important for osmoregulation and invasion. Experimental Cell Research 317, 1382–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francis SE, Sullivan DJ Jr. & Goldberg DE (1997) Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu Rev Microbiol 51, 97–123. [DOI] [PubMed] [Google Scholar]
- 41.Frenal K, Dubremetz JF, Lebrun M & Soldati-Favre D (2017) Gliding motility powers invasion and egress in Apicomplexa. Nat Rev Microbiol 15, 645–60. [DOI] [PubMed] [Google Scholar]
- 42.Galvez T, Gilleron J, Zerial M & O’Sullivan GA (2012) SnapShot: Mammalian Rab proteins in endocytic trafficking. Cell 151, 234–.e2. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh D, Walton JL, Roepe PD & Sinai AP (2012) Autophagy is a cell death mechanism in Toxoplasma gondii. Cell Microbiol 14, 589–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guttery DS, Pittman JK, Frenal K, Poulin B, McFarlane LR, Slavic K, Wheatley SP, Soldati-Favre D, Krishna S, Tewari R & Staines HM (2013) The Plasmodium berghei Ca(2+)/H(+) exchanger, PbCAX, is essential for tolerance to environmental Ca(2+) during sexual development. PLoS Pathog 9, e1003191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haller T, Dietl P, Deetjen P & Völkl H (1996) The lysosomal compartment as intracellular calcium store in MDCK cells: a possible involvement in InsP3-mediated Ca2+ release. Cell Calcium 19, 157–65. [DOI] [PubMed] [Google Scholar]
- 46.Harper JM, Huynh MH, Coppens I, Parussini F, Moreno S & Carruthers VB (2006) A cleavable propeptide influences Toxoplasma infection by facilitating the trafficking and secretion of the TgMIC2-M2AP invasion complex. Mol Biol Cell 17, 4551–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi M, Yamada H, Mitamura T, Horii T, Yamamoto A & Moriyama Y (2000) Vacuolar H(+)-ATPase localized in plasma membranes of malaria parasite cells, Plasmodium falciparum, is involved in regional acidification of parasitized erythrocytes. J Biol Chem 275, 34353–8. [DOI] [PubMed] [Google Scholar]
- 48.Hecht KA, O’Donnell AF & Brodsky JL (2014) The proteolytic landscape of the yeast vacuole. Cell Logist 4, e28023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill D & Dubey JP (2002) Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect 8, 634–40. [DOI] [PubMed] [Google Scholar]
- 50.Hu K, Roos DS & Murray JM (2002) A novel polymer of tubulin forms the conoid of Toxoplasma gondii. J Cell Biol 156, 1039–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang R, Que X, Hirata K, Brinen LS, Lee JH, Hansell E, Engel J, Sajid M & Reed S (2009) The cathepsin L of Toxoplasma gondii (TgCPL) and its endogenous macromolecular inhibitor, toxostatin. Mol Biochem Parasitol 164, 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jackson AJ, Clucas C, Mamczur NJ, Ferguson DJ & Meissner M (2013) Toxoplasma gondii Syntaxin 6 is required for vesicular transport between endosomal-like compartments and the Golgi complex. Traffic 14, 1166–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jimenez-Ruiz E, Morlon-Guyot J, Daher W & Meissner M (2016) Vacuolar protein sorting mechanisms in apicomplexan parasites. Molecular and biochemical parasitology 209, 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juge N, Moriyama S, Miyaji T, Kawakami M, Iwai H, Fukui T, Nelson N, Omote H & Moriyama Y (2015) Plasmodium falciparum chloroquine resistance transporter is a H+-coupled polyspecific nutrient and drug exporter. Proc Natl Acad Sci U S A 112, 3356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kannan G, Di Cristina M, Schultz AJ, Huynh MH, Wang F, Schultz TL, Lunghi M, Coppens I & Carruthers VB (2019) Role of Toxoplasma gondii Chloroquine Resistance Transporter in Bradyzoite Viability and Digestive Vacuole Maintenance. mBio 10 (4), e01324–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kannan G, Thaprawat P, Schultz TL & Carruthers VB (2021) Acquisition of Host Cytosolic Protein by Toxoplasma gondii Bradyzoites. mSphere 6(1): e00934–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim AH, Sheline CT, Tian M, Higashi T, McMahon RJ, Cousins RJ & Choi DW (2000) L-type Ca(2+) channel-mediated Zn(2+) toxicity and modulation by ZnT-1 in PC12 cells. Brain Res 886, 99–107. [DOI] [PubMed] [Google Scholar]
- 58.Klionsky DJ, Herman PK & Emr SD (1990) The fungal vacuole: composition, function, and biogenesis. Microbiol Rev 54, 266–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kremer K, Kamin D, Rittweger E, Wilkes J, Flammer H, Mahler S, Heng J, Tonkin CJ, Langsley G, Hell SW, Carruthers VB, Ferguson DJ & Meissner M (2013) An overexpression screen of Toxoplasma gondii Rab-GTPases reveals distinct transport routes to the micronemes. PLoS Pathog 9, e1003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuhn Y, Rohrbach P & Lanzer M (2007) Quantitative pH measurements in Plasmodium falciparum-infected erythrocytes using pHluorin. Cell Microbiol 9, 1004–13. [DOI] [PubMed] [Google Scholar]
- 61.Larson ET, Parussini F, Huynh MH, Giebel JD, Kelley AM, Zhang L, Bogyo M, Merritt EA & Carruthers VB (2009) Toxoplasma gondii cathepsin L is the primary target of the invasion-inhibitory compound morpholinurea-leucyl-homophenyl-vinyl sulfone phenyl. The Journal of biological chemistry 284, 26839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li SC & Kane PM (2009) The yeast lysosome-like vacuole: endpoint and crossroads. Biochim Biophys Acta 1793, 650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li ZH, King TP, Ayong L, Asady B, Cai X, Rahman T, Vella SA, Coppens I, Patel S & Moreno SNJ (2021) A plastid two-pore channel essential for inter-organelle communication and growth of Toxoplasma gondii. Nat Commun 12, 5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu J, Pace D, Dou Z, King TP, Guidot D, Li Z-H, Carruthers VB & Moreno SNJ (2014) A vacuolar-H+-pyrophosphatase (TgVP1) is required for microneme secretion, host cell invasion, and extracellular survival of Toxoplasma gondii. Mol Microbiol 93, 698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luft BJ & Remington JS (1992) Toxoplasmic encephalitis in AIDS. Clin Infect Dis 15, 211–22. [DOI] [PubMed] [Google Scholar]
- 66.Luo S, Marchesini N, Moreno SN & Docampo R (1999) A plant-like vacuolar H(+)-pyrophosphatase in Plasmodium falciparum. FEBS Lett 460, 217–20. [DOI] [PubMed] [Google Scholar]
- 67.Luo S, Ruiz FA & Moreno SN (2005) The acidocalcisome Ca2+-ATPase (TgA1) of Toxoplasma gondii is required for polyphosphate storage, intracellular calcium homeostasis and virulence. Mol Microbiol 55, 1034–45. [DOI] [PubMed] [Google Scholar]
- 68.Luo S, Vieira M, Graves J, Zhong L & Moreno SN (2001) A plasma membrane-type Ca(2+)-ATPase co-localizes with a vacuolar H(+)-pyrophosphatase to acidocalcisomes of Toxoplasma gondii. EMBO J 20, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maeshima M (2000) Vacuolar H(+)-pyrophosphatase. Biochim Biophys Acta 1465, 37–51. [DOI] [PubMed] [Google Scholar]
- 70.Martin RE (2020) The transportome of the malaria parasite. Biol Rev Camb Philos Soc 95, 305–32. [DOI] [PubMed] [Google Scholar]
- 71.Martin RE, Ginsburg H & Kirk K (2009) Membrane transport proteins of the malaria parasite. Mol Microbiol 74, 519–28. [DOI] [PubMed] [Google Scholar]
- 72.Martinez-Munoz GA & Kane P (2008) Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. The Journal of biological chemistry 283, 20309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martiniere A, Bassil E, Jublanc E, Alcon C, Reguera M, Sentenac H, Blumwald E & Paris N (2013) In vivo intracellular pH measurements in tobacco and Arabidopsis reveal an unexpected pH gradient in the endomembrane system. Plant Cell 25, 4028–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinoia E, Maeshima M & Neuhaus HE (2007) Vacuolar transporters and their essential role in plant metabolism. J Exp Bot 58, 83–102. [DOI] [PubMed] [Google Scholar]
- 75.Marty F (1999) Plant vacuoles. Plant Cell 11, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maughan SC, Pasternak M, Cairns N, Kiddle G, Brach T, Jarvis R, Haas F, Nieuwland J, Lim B, Muller C, Salcedo-Sora E, Kruse C, Orsel M, Hell R, Miller AJ, Bray P, Foyer CH, Murray JA, Meyer AJ & Cobbett CS (2010) Plant homologs of the Plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proc Natl Acad Sci U S A 107, 2331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McDonald C, Smith D, Di Cristina M, Kannan G, Dou Z & Carruthers VB (2020) Toxoplasma Cathepsin Protease B and Aspartyl Protease 1 Are Dispensable for Endolysosomal Protein Digestion. mSphere 5 (1): e00869–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGovern OL, Rivera-Cuevas Y, Kannan G, Narwold A & Carruthers VB (2018) Intersection of Endocytic and Exocytic Systems in Toxoplasma gondii. Traffic, 336–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McIntosh MT & Vaidya AB (2002) Vacuolar type H+ pumping pyrophosphatases of parasitic protozoa. Int J Parasitol 32, 1–14. [DOI] [PubMed] [Google Scholar]
- 80.Mindell JA (2012) Lysosomal acidification mechanisms. Annu Rev Physiol 74, 69–86. [DOI] [PubMed] [Google Scholar]
- 81.Miranda K, Pace DA, Cintron R, Rodrigues JC, Fang J, Smith A, Rohloff P, Coelho E, de Haas F, de Souza W, Coppens I, Sibley LD & Moreno SN (2010) Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole. Mol Microbiol 76, 1358–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mizuno-Yamasaki E, Rivera-Molina F & Novick P (2012) GTPase networks in membrane traffic. Annu Rev Biochem 81, 637–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mordue DG, Desai N, Dustin M & Sibley LD (1999) Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J Exp Med 190, 1783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moreno SN, Zhong L, Lu HG, Souza WD & Benchimol M (1998) Vacuolar-type H+-ATPase regulates cytoplasmic pH in Toxoplasma gondii tachyzoites. Biochem J 330 ( Pt 2), 853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morgan AJ, Yuan Y, Patel S & Galione A (2020) Does lysosomal rupture evoke Ca(2+) release? A question of pores and stores. Cell Calcium 86, 102139. [DOI] [PubMed] [Google Scholar]
- 86.Morlon-Guyot J, El Hajj H, Martin K, Fois A, Carrillo A, Berry L, Burchmore R, Meissner M, Lebrun M & Daher W (2018) A proteomic analysis unravels novel CORVET and HOPS proteins involved in Toxoplasma gondii secretory organelles biogenesis. Cell Microbiol 20, e12870. [DOI] [PubMed] [Google Scholar]
- 87.Morlon-Guyot J, Pastore S, Berry L, Lebrun M & Daher W (2015) Toxoplasma gondii Vps11, a subunit of HOPS and CORVET tethering complexes, is essential for the biogenesis of secretory organelles. Cell Microbiol 17, 1157–78. [DOI] [PubMed] [Google Scholar]
- 88.Murata CE & Goldberg DE (2003) Plasmodium falciparum falcilysin: a metalloprotease with dual specificity. J Biol Chem 278, 38022–8. [DOI] [PubMed] [Google Scholar]
- 89.Nichols BA, Chiappino ML & Pavesio CEN (1994) Endocytosis at the micropore of Toxoplasma gondii. Parasitol Res 80, 91–8. [DOI] [PubMed] [Google Scholar]
- 90.Novikoff AB, Beaufay H & De Duve C (1956) Electron microscopy of lysosomerich fractions from rat liver. J Biophys Biochem Cytol 2, 179–84. [PMC free article] [PubMed] [Google Scholar]
- 91.Numrich J & Ungermann C (2014) Endocytic Rabs in membrane trafficking and signaling. Biol Chem 395, 327–33. [DOI] [PubMed] [Google Scholar]
- 92.Ogawa N, DeRisi J & Brown PO (2000) New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol Biol Cell 11, 4309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Outten CE & O’Halloran TV (2001) Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292, 2488–92. [DOI] [PubMed] [Google Scholar]
- 94.Owens T & Poole RJ (1979) Regulation of cytoplasmic and vacuolar volumes by plant cells in suspension culture. Plant Physiol 64, 900–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pappas G, Roussos N & Falagas ME (2009) Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol 39, 1385–94. [DOI] [PubMed] [Google Scholar]
- 96.Parussini F, Coppens I, Shah PP, Diamond SL & Carruthers VB (2010) Cathepsin L occupies a vacuolar compartment and is a protein maturase within the endo/exocytic system of Toxoplasma gondii. Molecular Microbiology 76, 1340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parzych KR & Klionsky DJ (2019) Vacuolar hydrolysis and efflux: current knowledge and unanswered questions. Autophagy 15, 212–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patel S & Docampo R (2010) Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol 20, 277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patzewitz EM, Salcedo-Sora JE, Wong EH, Sethia S, Stocks PA, Maughan SC, Murray JA, Krishna S, Bray PG, Ward SA & Muller S (2013) Glutathione transport: a new role for PfCRT in chloroquine resistance. Antioxid Redox Signal 19, 683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pieperhoff MS, Schmitt M, Ferguson DJP & Meissner M (2013) The Role of Clathrin in Post-Golgi Trafficking in Toxoplasma gondii. PLoS One 8, e77620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Preston RA, Murphy RF & Jones EW (1989) Assay of vacuolar pH in yeast and identification of acidification-defective mutants. Proc Natl Acad Sci U S A 86, 7027–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Que X, Engel JC, Ferguson D, Wunderlich A, Tomavo S & Reed SL (2007) Cathepsin Cs Are Key for the Intracellular Survival of the Protozoan Parasite, Toxoplasma gondii. J Biol Chem 282, 4994–5003. [DOI] [PubMed] [Google Scholar]
- 103.Que X, Ngô H, Lawton J, Gray M, Liu Q, Engel J, Brinen L, Ghosh P, Joiner KA & Reed SL (2002) The Cathepsin B of Toxoplasma gondii,Toxopain-1, Is Critical for Parasite Invasion and Rhoptry Protein Processing. J Biol Chem 277, 25791–7. [DOI] [PubMed] [Google Scholar]
- 104.Ragheb D, Dalal S, Bompiani KM, Ray WK & Klemba M (2011) Distribution and biochemical properties of an M1-family aminopeptidase in Plasmodium falciparum indicate a role in vacuolar hemoglobin catabolism. The Journal of biological chemistry 286, 27255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rea P. A & Poole R. J (1993) Vacuolar H+-translocating pyrophosphatase. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 157–80. [Google Scholar]
- 106.Rea PA, Kim Y, Sarafian V, Poole RJ, Davies JM & Sanders D (1992) Vacuolar H(+)-translocating pyrophosphatases: a new category of ion translocase. Trends Biochem Sci 17, 348–53. [DOI] [PubMed] [Google Scholar]
- 107.Rodrigues CO, Ruiz FA, Rohloff P, Scott DA & Moreno SN (2002) Characterization of isolated acidocalcisomes from Toxoplasma gondii tachyzoites reveals a novel pool of hydrolyzable polyphosphate. The Journal of biological chemistry 277, 48650–6. [DOI] [PubMed] [Google Scholar]
- 108.Rodrigues CO, Scott DA, Bailey BN, De Souza W, Benchimol M, Moreno B, Urbina JA, Oldfield E & Moreno SN (2000) Vacuolar proton pyrophosphatase activity and pyrophosphate (PPi) in Toxoplasma gondii as possible chemotherapeutic targets. Biochem J 349 Pt 3, 737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosenthal PJ (2004) Cysteine proteases of malaria parasites. Int J Parasitol 34, 1489–99. [DOI] [PubMed] [Google Scholar]
- 110.Rosenthal PJ (2011) Falcipains and other cysteine proteases of malaria parasites. Adv Exp Med Biol 712, 30–48. [DOI] [PubMed] [Google Scholar]
- 111.Sakura T, Sindikubwabo F, Oesterlin LK, Bousquet H, Slomianny C, Hakimi MA, Langsley G & Tomavo S (2016) A Critical Role for Toxoplasma gondii Vacuolar Protein Sorting VPS9 in Secretory Organelle Biogenesis and Host Infection. Sci Rep 6, 38842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saliba KJ, Allen RJ, Zissis S, Bray PG, Ward SA & Kirk K (2003) Acidification of the malaria parasite’s digestive vacuole by a H+-ATPase and a H+-pyrophosphatase. J Biol Chem 278, 5605–12. [DOI] [PubMed] [Google Scholar]
- 113.Sangare LO, Alayi TD, Westermann B, Hovasse A, Sindikubwabo F, Callebaut I, Werkmeister E, Lafont F, Slomianny C, Hakimi MA, Van Dorsselaer A, Schaeffer-Reiss C & Tomavo S (2016) Unconventional endosome-like compartment and retromer complex in Toxoplasma gondii govern parasite integrity and host infection. Nat Commun 7, 11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saroussi S & Nelson N (2009) Vacuolar H(+)-ATPase-an enzyme for all seasons. Pflugers Arch 457, 581–7. [DOI] [PubMed] [Google Scholar]
- 115.Shafik SH, Cobbold SA, Barkat K, Richards SN, Lancaster NS, Llinas M, Hogg SJ, Summers RL, McConville MJ & Martin RE (2020) The natural function of the malaria parasite’s chloroquine resistance transporter. Nat Commun 11, 3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sharma SS, Dietz KJ & Mimura T (2016) Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ 39, 1112–26. [DOI] [PubMed] [Google Scholar]
- 117.Shea M, Jakle U, Liu Q, Berry C, Joiner KA & Soldati-Favre D (2007) A family of aspartic proteases and a novel, dynamic and cell-cycle-dependent protease localization in the secretory pathway of Toxoplasma gondii. Traffic 8, 1018–34. [DOI] [PubMed] [Google Scholar]
- 118.Sheline CT, Behrens MM & Choi DW (2000) Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis. J Neurosci 20, 3139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sloves P-J, Delhaye S, Mouveaux T, Werkmeister E, Slomianny C, Hovasse A, Dilezitoko Alayi T, Callebaut I, Gaji Rajshekhar Y, Schaeffer-Reiss C, Van Dorsselear A, Carruthers Vern B & Tomavo S (2012) Toxoplasma Sortilin-like Receptor Regulates Protein Transport and Is Essential for Apical Secretory Organelle Biogenesis and Host Infection. Cell Host & Microbe 11, 515–27. [DOI] [PubMed] [Google Scholar]
- 120.Smith D, Kannan G, Coppens I, Wang F, Nguyen HM, Cerutti A, Olafsson EB, Rimple PA, Schultz TL, Mercado Soto NM, Di Cristina M, Besteiro S & Carruthers VB (2021) Toxoplasma TgATG9 is critical for autophagy and long-term persistence in tissue cysts. Elife 10: 10, e59384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Solinger JA & Spang A (2013) Tethering complexes in the endocytic pathway: CORVET and HOPS. FEBS J 280, 2743–57. [DOI] [PubMed] [Google Scholar]
- 122.Stasic AJ, Chasen NM, Dykes EJ, Vella SA, Asady B, Starai VJ & Moreno SNJ (2019) The Toxoplasma Vacuolar H+-ATPase Regulates Intracellular pH and Impacts the Maturation of Essential Secretory Proteins. Cell Reports 27, 2132–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stasic AJ, Dykes EJ, Cordeiro CD, Vella SA, Fazli MS, Quinn S, Docampo R & Moreno SNJ (2021) Ca 2+ entry at the plasma membrane and uptake by acidic stores is regulated by the activity of the V-H+ -ATPase in Toxoplasma gondii. Mol Microbiol 115(5):1054–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Teo CF, Zhou XW, Bogyo M & Carruthers VB (2007) Cysteine protease inhibitors block Toxoplasma gondii microneme secretion and cell invasion. Antimicrob Agents Chemother 51, 679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thornton LB, Teehan P, Floyd K, Cochrane C, Bergmann A, Riegel B, Stasic AJ, Di Cristina M, Moreno SNJ, Roepe PD & Dou Z (2019) An ortholog of Plasmodium falciparum chloroquine resistance transporter (PfCRT) plays a key role in maintaining the integrity of the endolysosomal system in Toxoplasma gondii to facilitate host invasion. PLoS Pathog 15, e1007775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tomavo S, Slomianny C, Meissner M & Carruthers VB (2013) Protein trafficking through the endosomal system prepares intracellular parasites for a home invasion. PLoS Pathog 9, e1003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Van Den Hazel HB, Kielland-Brandt MC & Winther JR (1996) Review: biosynthesis and function of yeast vacuolar proteases. Yeast 12, 1–16. [DOI] [PubMed] [Google Scholar]
- 128.Venugopal K & Marion S (2018) Secretory organelle trafficking in Toxoplasma gondii: A long story for a short travel. Int J Med Microbiol 308, 751–60. [DOI] [PubMed] [Google Scholar]
- 129.Viotti C, Bubeck J, Stierhof Y-D, Krebs M, Langhans M, van den Berg W, van Dongen W, Richter S, Geldner N, Takano J, Jürgens G, de Vries SC, Robinson DG & Schumacher K (2010) Endocytic and Secretory Traffic in Arabidopsis Merge in the Trans-Golgi Network/Early Endosome, an Independent and Highly Dynamic Organelle. The Plant Cell 22, 1344–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y, Toei M & Forgac M (2008) Analysis of the membrane topology of transmembrane segments in the C-terminal hydrophobic domain of the yeast vacuolar ATPase subunit a (Vph1p) by chemical modification. J Biol Chem 283, 20696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Warring SD, Dou Z, Carruthers VB, McFadden GI & van Dooren GG (2014) Characterization of the Chloroquine Resistance Transporter Homologue in Toxoplasma gondii. Euk Cell 13, 1360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weisman LS (2006) Organelles on the move: insights from yeast vacuole inheritance. Nat Rev Mol Cell Biol 7, 243–52. [DOI] [PubMed] [Google Scholar]
- 133.Weiss LM & Dubey JP (2009) Toxoplasmosis: A history of clinical observations. Int J Parasitol 39, 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Woodier J, Rainbow RD, Stewart AJ & Pitt SJ (2015) Intracellular Zinc Modulates Cardiac Ryanodine Receptor-mediated Calcium Release. J Biol Chem 290, 17599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yamashiro CT, Kane PM, Wolczyk DF, Preston RA & Stevens TH (1990) Role of vacuolar acidification in protein sorting and zymogen activation: a genetic analysis of the yeast vacuolar proton-translocating ATPase. Mol Cell Biol 10, 3737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang C, Hicks GR & Raikhel NV (2014) Plant vacuole morphology and vacuolar trafficking. Front Plant Sci 5, 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang M, Wang C, Otto TD, Oberstaller J, Liao X, Adapa SR, Udenze K, Bronner IF, Casandra D, Mayho M, Brown J, Li S, Swanson J, Rayner JC, Jiang RHY & Adams JH (2018) Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science 360 (6388), eaap784. [DOI] [PMC free article] [PubMed] [Google Scholar]


