Abstract
There are reports of link of osteoprotegerin (OPG) gene polymorphism to type-2 diabetes (T2D) and hypertension (HTN). The objective of the study was to assess the allele frequency of OPG (rs2073618) gene polymorphism and its association with heart rate variability (HRV) and blood pressure variability profile as CVD risks in diabetes mellitus patients with hypertension undergoing treatment. T2D patients on treatment without hypertension (n = 172), with hypertension (n = 177) and 191 healthy volunteers were recruited for the study. Their blood pressure variability including baroreflex sensitivity (BRS), heart rate variability (HRV), OPG, insulin, lipid profile, receptor-activator for NFkB (RANK), receptor-activator for NFkB-Ligand (RANKL), and tumor necrosis factor-α (TNF-α) were estimated. Allele frequency of OPG (rs2073618) gene polymorphism was assessed from the DNA samples. BRS and HRV indices were decreased, and RANKL/OPG and TNF-α were increased in T2D and T2D + HTN groups, respectively compared to healthy control group. The reduction in BRS was contributed by increased inflammation and reduced SDNN of HRV in GG genotype in T2D + HTN. In GG + GC subgroup, it was additionally contributed by rise in RANKL/OPG level (β − 0.219; p 0.008). Presence of mutant GG genotype contributed to the risk of hypertension among T2D patients (OR 3.004) as well as in general population (OR 2.79). It was concluded that CV risks are more in T2D patients with HTN expressing OPG rs2073618 gene polymorphism.
Subject terms: Physiology, Cardiology, Endocrinology, Health care
Introduction
According to World Health Report 2002, cardiovascular diseases (CVD) will be the largest cause of death and disability by 2020 in India. Nearly half of these deaths are likely to occur in young and middle-aged individuals (30–69 years) especially who have diabetes and or hypertension or are genetically susceptible to develop diabetes and or hypertension1,2. Diabetes mellitus and hypertension are two major established risk factors for CVD3. Further, coexistence of hypertension with insulin resistance increases the risk of target organ damage and clinical cardiovascular accidents4. Nevertheless, not all patients with diabetes mellitus develop hypertension at least in the first 5 years5. However, a quite significant number of patients with diabetes develop hypertension quite early after acquiring the disease6. Genetic susceptibility for hypertension has been proposed to play important role in nearly 50% of insulin resistant individuals to develop hypertension, in the early phase of the disease even after receiving standard antidiabetic treatment7. Till date, no study has been conducted to assess the pathophysiological difference in the CVD risk profile of diabetes patients with or without having hypertension.
Vascular calcification, a factor common to both CVD and hypertension is no longer considered as age related phenomenon8. Irrespective of the site and degree of involvement, the vascular calcification is a strong independent predictor of cardiovascular mortality9. The intimal calcification is associated with atherosclerosis while medial calcification seen in ageing is associated with arterial stiffening leading to reduced vascular compliance8. Arterial stiffening due to atherosclerosis accelerated by calcification is a known pathophysiological mechanism of hypertension10. Osteoprotegerin (OPG) is a biomarker of vascular calcification and associated with CVD. OPG is a decoy receptor for receptor-activator for NFkB-ligand (RANKL), which has been implicated in pathophysiology of CVD. OPG has also been linked to myocardial stiffness11, hypertension and diabetes12. Recently we have reported the link of OPG to CVD risk in diabetes13.
The TNFRSF11B gene at 8q24.12 of chromosome 8 encodes OPG in humans. There are several reports of single nucleotide polymorphisms (SNP) for OPG gene, linked to DM and CVD14. Among these, the 1181 G > C SNP polymorphism has been particularly associated with CVD, left ventricular hypertrophy in essential hypertension15 and abnormal coronary arteries16. The rs2073618 of OPG is an exon variant with G > C transversion at exon 1. This leads to change in the third amino acid of the signal peptide from lysine (AAG), into asparagine (AAC). Polymorphism at exon region can influence splicing by affecting the binding sites of enhancers and silencers thus affecting protein level in circulation. However, studies relating RANK/RANKL/OPG level or associated gene polymorphisms in this pathway, in relation to blood pressure alteration, are scarce in Indian population. We hypothesize that any alteration in the levels of receptors such as RANK and OPG; their major ligands such as RANKL and TNF-alpha; or the corresponding allele polymorphism of OPG could play a pivotal role in insulin resistance associated hypertension and subsequent increase in CV risks in the Southern Indian (Tamil) population.
Sympathetic nervous system (SNS) activation and retrograde inflammation are two major mechanisms in the genesis of hypertension17–19, insulin resistance20,21 as well as CVD22,23. Blood pressure variability (BPV) and heart rate variability (HRV) are two sensitive methods of measurements of CV risks in health and disease24–26. Decreased baroreflex sensitivity (BRS) an important parameter of BPV and decreased HRV as the marker of reduced cardiovagal modulation are the recently established indices of CVD risks in diabetes and hypertension. As BRS is influenced by mechanical properties of vascular wall, high vascular transmural pressure could alter the BRS and influences the autonomic regulation of blood pressure in diabetes with hypertension. Recently we have reported the plausible role of OPG in decreased cardiovagal modulation in diabetes13. Also, we have reported the link OPG with decreased BRS in patients with diabetes receiving oral anti-diabetic drugs27. However, the CVD risks has not been assessed in patients with diabetes with or without hypertension expressing SNP of OPG gene. Therefore, the primary objective of the study was to assess the allele frequency of OPG (rs2073618) gene polymorphism in diabetes mellitus patients under treatment with hypertension compared with similar age, gender and ethnicity matched diabetes patients without hypertension. Also, we have assessed the HRV and BPV profile as CVD risks and their association with gene polymorphism of OPG in these patients.
Materials and methods
Study design
This was a single center cross sectional comparative study in the Southern Indian (Tamil) population consisting of three groups. The study was first approved by an institution research review board and the Ethics Committee (Human studies: JIP/IEC/2018/305) prior to commencement. Verbal approval followed by written informed consent was obtained from the participants before recruitment.
Participants
Participants were divided into three groups: control group, test group 1 and test group 2, based on the following inclusion criteria.
Control group
The healthy volunteers with no history of diabetes mellitus or hypertension were recruited as subjects in control group.
Test groups
For test groups, individuals in the age 20–60 years, diagnosed with diabetes mellitus and under treated with metformin and glimepiride hypoglycemic agents combination therapy for at least for two years, were recruited consecutively from outpatient clinic of medicine dept. Further these patients with T2D were segregated as test group 1 and test group 2 based on absence and presence of hypertension respectively. Thus, Test group 1 consisted of Tamil patients diagnosed as diabetes mellitus without hypertension, and Test group 2 consisted of Tamilian Diabetes mellitus patients diagnosed with hypertension as per JNC guidelines or New ACC/AHA High blood pressure guidelines28, (SBP > 140 Hg or DBP > 90 Hg) and under treatment with amlodipine and enalapril.
Study procedures
The participants were briefed about the study protocol and their written informed consents were obtained (Fig. 1). Basal demographic and anthropometric data were collected, and the personal history was noted using a structured data sheet. The next day morning their fasting blood samples were collected following which the blood pressure, HRV and BRS recordings were taken as per the standard procedures described earlier27.
Figure 1.
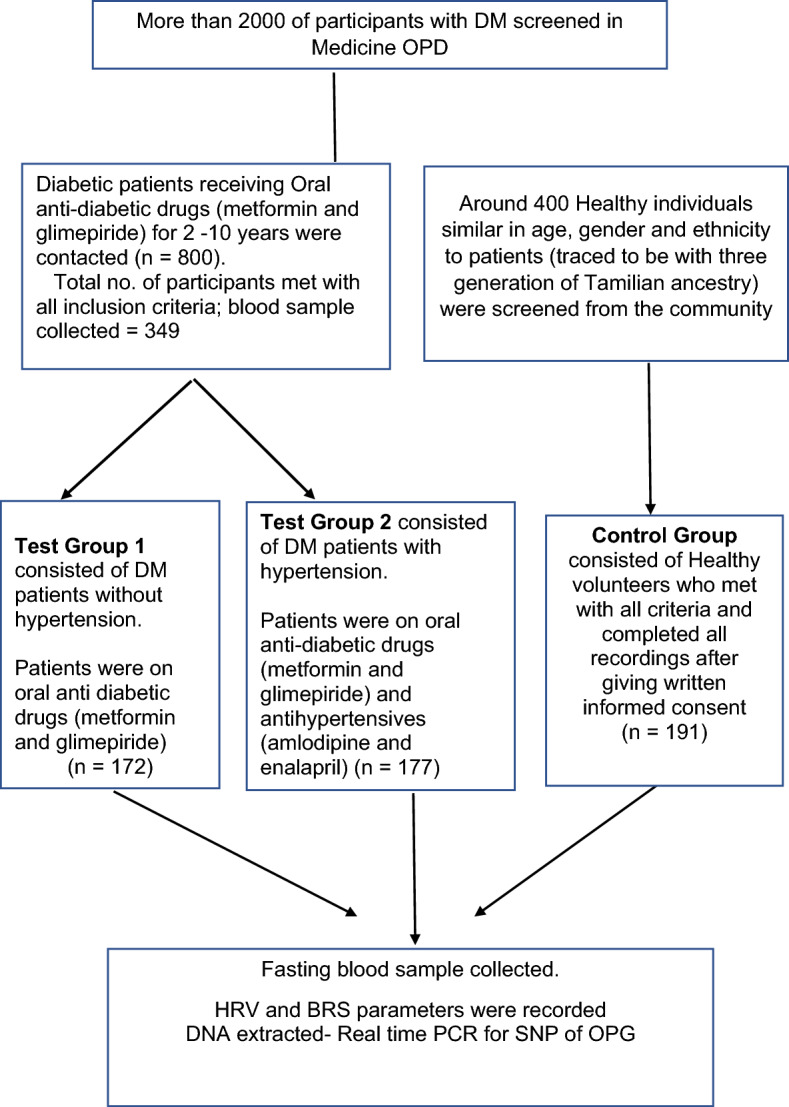
Flow chart of participants’ recruitment.
For the recording of short-term HRV, ECG electrodes were used. The RR tachograms were reconstructed using BIOPAC MP 100 data acquisition system (BIOPAC Inc., USA). The frequency-domain indices of HRV included total power (TP), low frequency power expressed in normalized units (LFnu), high frequency power expressed in normalized units (HFnu), and ratio of low-frequency to high-frequency power (LF-HF ratio); and the time-domain indices included mean standard deviation of RR intervals (SDNN), square root of the mean of the sum of the squares of the differences between adjacent RR interval (RMSSD), adjacent RR interval differing more than 50 ms (NN50), and NN50 counts divided by all RR intervals (pNN50).
For BRS measurement continuous blood pressure variability method by Finapres (Finometer version 1.22a, Finapres Medical Systems, Amsterdam, The Netherlands) was used which analyses the BPV in a non-invasive manner using the principle of finger plethysmography.
Assessment of biochemical parameters
Part of the fasting blood sample was collected in EDTA tube for DNA extraction and rest was collected in serum separator tube. Same day serum was processed for fasting glucose and lipid profile assay and rest was stored in aliquots for ELISA at − 40 °C. Fasting serum glucose (FSG), direct LDL cholesterol and lipid profile were measured by an automated AU5800 chemistry analyzer using commercial kits (Beckman Coulter AU5800, Beckman Coulter Inc, Brea, California, USA).
Genotyping assay
Whole blood samples collected in EDTA tube were used for extraction of genomic DNA by standard phenol–chloroform method. DNA was quantified using a nanodrop. Genotyping was done using predesigned TaqMan probes and primers for OPG rs2073618 (Thermofischer scientific assay ID C_1971047_40), using real time PCR as per manufacturer’s instruction.
Metabolic, inflammatory, and cardiovascular risk markers parameters
TNF-α (Diaclone, France), Insulin (Calbiotech, USA), RANK (Elabscience, USA), RANKL (Elabscience, USA) and OPG (Fine test, China) were estimated by an enzyme-linked immunosorbent assay based on the principle of sandwich ELISA and the mean value was determined from the standard dose–response curves. HOMA-IR was calculated using formula HOMA-IR = Glucose X Insulin/40529. The inter-assay coefficients of variation for ELISA parameters for RANKL, RANK, TNF-α, OPG and insulin were < 6%, < 7%, < 11%, < 5.5% and < 6.1% respectively. The intra-assay of coefficients of variation for ELISA parameters for RANKL, RANK, TNF-α, OPG and insulin were < 6%, < 5.5%, < 3.2%, < 5.5% and < 3.5% respectively.
Statistics
All statistical analyses in the present study were performed using the Statistical Package for Social Sciences Software Version 20.0 (IBM PASW Statistics), Graph Pad Prism version 8.00, Graph Pad Instat Version 3.10 (San Diego, USA) and SNPstat online software. The Shapiro Wilk test tested the normality of the data, and accordingly, appropriate parametric or non-parametric tests were used. The non-Gaussian data is expressed as median with Inter-Quartile Range (IQR). Kruskal–Wallis test ANOVA followed by Dunn’s Bonferroni post hoc test was used to compare the quantitative data among three groups. Spearman correlation was used to test for association among quantitative variables followed by multiple regression analysis was used to assess the independent contribution of variables. Hardy–Weinberg equilibrium (χ2) was tested on genotype frequencies by chi-square test. Direct gene counting was utilized for the calculation of allele frequencies. Differences in genotype distributions and allele frequencies in the groups were compared using the chi-square test. The association of genotypes with T2D with hypertension was analyzed under four genetic models (dominant, recessive, codominant, and homozygotic) using the logistic regression. The results were expressed as a percentage. The association between the disease and genotype was checked by chi-square test and expressed as odds ratio (ORs) with 95% Confidence Intervals (CIs). For all the statistical tests, a p-value < 0.05 was considered significant.
Ethics approval
This study was approved by the JIPMER Institute Ethics Committee [JIP/IEC/2018/305]. All methods in the study were performed in accordance with the Helsinki declarations. All data of the study were anonymised and coded before its use.
Consent to participate
Written informed consent to use the biological sample and case history was obtained from each participant after explaining the purpose, and procedure of the study in their vernacular language.
Results
Anthropometric and demographic findings
The distributions of age, BMI, blood pressure variability parameters, heart rate variability parameter, metabolic-calcification and inflammation markers are shown in Table 1. There were no significant differences found between the groups in their baseline characteristics such as age and BMI.
Table 1.
Comparison of demographic, anthropometric indices, blood pressure and blood pressure variability (BPV) parameters, heart rate variability (HRV) parameters among healthy control, diabetes mellitus (DM) patients on treatment without hypertension and with hypertension (HTN).
| Variables | Control group | Test group (1) | Test group (2) | P values |
|---|---|---|---|---|
| (Healthy control) (n = 191) | (DM without HTN) (n = 172) | (DM with HTN) (n = 177) | ||
| Age (Years) | 51 (43–59) | 50 (46–56) | 52 (47–57) | 0.349 |
| BMI (Kg/m2) | 25.65(23.93–28.24) | 25.80(23.17–28.67) | 26.63(24.33–28.88) | 0.133 |
| BPV parameters | ||||
| BHR (beats per min) | 75 (70–83) | 77 (69.25–84) | 80 (72–88.50)**, # | 0.001 |
| SBP (mmHg) | 122 (112–128) | 121 (112–129) | 133 (122.50–143)***,### | < 0.001 |
| DBP (mmHg) | 79 (72—87) | 78 (72–84) | 83 (78–88 )***,## | < 0.001 |
| RPP (mmHg/min) | 91.63 (80.30–104.58) | 91.67 (80.45–104.49) | 105.57 (93.70–118.30)***,### | < 0.001 |
| BRS (ms/mmHg) | 16.06 (13.55–19.356) | 13.02 (8.79–16.67) | 6.27 (4.31–8.27)***,### | < 0.001 |
| HRV parameters | ||||
| TP(ms2) | 1328 (839—2042 ) | 885(615.50–1185.75)*** | 469 (368–655 )***,### | < 0.001 |
| LFnu | 46.20 (35.92–58.95) | 52.32(43.31–59.91)* | 64.00 (52.00–72.50)***,### | < 0.001 |
| HFnu | 53.799 (41.35–64.07) | 47.67(40.08–56.68)* | 6.00 (27.50–48.00)***,### | < 0.001 |
| LF/HF | 0.85 (0.56–1.41) | 1.09(0.76–1.49)* | 1.79 (1.09–2.70) ***,### | < 0.001 |
| SDNN (ms) | 43.70 (32.70–57.00) | 31.30 (23.77–42.00)*** | 22.70 (15.13–28.00)***,### | < 0.001 |
| RMSSD(ms) | 42.00 (20.00–90.00) | 26.00 (18.85–34.30)*** | 15.80 (10.00–24.05)***,### | < 0.001 |
| NN50 | 20.40 (13.45–25.90) | 12.00 (9.00–19.00)*** | 8.00 (5.00–11.00)***,### | < 0.001 |
| PNN50 | 25.00 (17.00–37.70) | 8.00 (5.00–9.97)*** | 2.20 (2.00–4.13)***,### | < 0.001 |
Data is expressed as Median with interquartile range. Comparison among the groups was done by Kruskalwallis test. *P Value < 0.05; ** P Value < 0.01; *** P Value < 0.001 was considered significant, when comparison is done with control; #P Value < 0.05; ## P Value < 0.01; ### P Value < 0.001 was considered significant, when comparison is done with patients with DM without hypertension. BMI: Body mass index; BHR: Basal heart rate; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; RPP: Rate pressure product; BRS: Baroreflex sensitivity; TP: Total power of HRV, LFnu: Normalized low-frequency power of HRV; HFnu: Normalized high-frequency power of HRV; LF/HF: Ratio of LFnu and HFnu; SDNN: Standard deviation of normal to normal interval; RMSSD: Square root of the mean of the sum of the squares of the differences between adjacent NN intervals. NN50: the number of interval differences of successive NN intervals greater than 50 ms; PNN50; the proportion derived by dividing NN50 by the total number of NN intervals.
BPV, HRV and circulating biomarkers findings
A significant rise was observed in the BHR, SBP and DBP while BRS was reduced in T2D with hypertension patients compared to T2D patients and healthy controls (Table 1). Among the HRV indices TP, SDNN, RMSSD, NN50 and pNN50 were significantly less in T2D patients with hypertension (Table 1) compared to T2D patients and healthy control. LFnu was significantly higher, HFnu was considerably lower, and LF/HF ratio was significantly higher in T2D patients with hypertension (Table 1).
Metabolic and inflammatory markers and atherogenic indices
The serum concentration of glycemic parameters, lipid profile and atherogenic indices among study participants are presented in Table 2. There was no difference in FSG and HbA1c levels between the two patient groups though it was more compared to healthy control. Insulin and HOMA-IR was while TC and HDL cholesterol were low in T2D + HTN group compared to T2D and healthy control group. LDL cholesterol was similar across all groups while triglyceride was high only in T2D + HTN group.
Table 2.
Comparison of metabolic, inflammatory and calcification markers among healthy control, diabetes mellitus (DM) patients on treatment without hypertension and with hypertension (HTN).
| Variables | Control group | Test group (1) | Test group (2) | P values |
|---|---|---|---|---|
| (Healthy control) (n = 191) | (DM without HTN) (n = 172) | (DM with HTN) (n = 177) | ||
| Metabolic parameters for Glycemic profile | ||||
| FSG (mg/dL) | 84 (76–97) | 133 (106.25–165) *** | 133 (117.50–187.50)*** | < 0.001 |
| HbA1c (g%) | 5.4 (5.20–5.60) | 7 (6.44–7.15) *** | 7 (6.41–7.31)*** | < 0.001 |
| Insulin (μU/mL) | 9.71 (6.85–13.16) | 22.56 (9.55–38.64) *** | 26.11 (17.73–49.03)***,### | < 0.001 |
| HOMA-IR | 1.97 (1.36–2.84) | 5.85 (2.71–12.19) *** | 8.29 (5.10–17.06) ***,### | < 0.001 |
| Metabolic parameters for lipid profile | ||||
| TC(mg /dL) | 175 (157 -194) | 157 (135.25–183)*** | 147 (126.50–177) *** | < 0.001 |
| HDL-C(mg /dL) | 44 (40–49) | 37 (33.25–43.75) *** | 37 (32 -40.50) *** | < 0.001 |
| LDL-C(mg /dL) 103.40(88.80–117.20) | 103.40 (88.80-117.20) | 94.50 (73.00–117.00)** | 96.00 (79.50–122.00) | 0.005 |
| TG(mg /dL) | 128 (103 -150) | 135.50 (99.25–180) | 145 (110.50–189.00)** | 0.004 |
| VLDL-C(mg /dL) | 25.60 (20.60–30.0) | 27.10(19.85–36.0) | 29 (22.10–37.80) ** | 0.004 |
| Inflammatory & calcification markers | ||||
| TNF-α (pg/mL) | 4.81 (2.72–7.19) | 14.58 (9.48–20.83)*** | 20.24 (13.00–27.29)***,### | < 0.001 |
| OPG (pg/mL) | 181.76 (134 -300.25) | 215.79 (131.99–306.99) | 260.51 (199.38–349.92)***,### | < 0.001 |
| RANK (ng/mL) | 0.61 (0.50–0.77) | 1.15 (0.61–2.43)*** | 0.97 (0.59–1.77)*** | < 0.001 |
| RANKL (pg/mL) | 7.13(5.69–9.76) | 10.14(6.40–19.77)*** | 11.91(6.10–24.65) *** | < 0.001 |
| RANKL/OPG | 0.03 (0.01–0.04) | 0.06 (0.02–0.12) *** | 0.05 (0.02–0.08) *** | < 0.001 |
Data is expressed as Median with interquartile range. Comparison among the groups was done by Kruskalwallis test. *P Value < 0.05; ** P Value < 0.01; *** P Value < 0.001 was considered significant, when comparison is done with control; #P Value < 0.05; ## P Value < 0.01; ### P Value < 0.001 was considered significant, when comparison is done with patients with DM without hypertension. FSG: Fasting serum glucose; HbA1c: Glycated haemoglobin; HOMA-IR: homeostatic model assessment of insulin resistance; TC: Total cholesterol; TG: Triglyceride; HDL: High density lipoprotein; LDL: Low density lipoprotein; VLDL: Very low-density lipoprotein; Non HDL-C: Non HDL cholesterol; AIP: Atherogenic index of plasma = log10[TG/ HDL- C]; TNF- α: tumor necrosis factor alpha; OPG: Osteoprotegerin; RANK: Receptor activator of NfkB; RANKL: Receptor activator of NfkB ligand.
The median TNF α, OPG and RANKL/OPG levels were higher in T2D with hypertension group (Table 2) compared to T2D as well as control groups. There was no difference in RANK and RANKL levels when compared between two test groups. The correlated of these markers were checked with BRS for both test groups (Table 3).
Table 3.
Spearman correlation of BRS with various parameters in treated T2D patients with and without hypertension.
| Parameters | Test Group (1) | Test Group (2) | ||
|---|---|---|---|---|
| DM without HTN (n = 172) | DM with HTN (n = 177) | |||
| r | P | r | P | |
| BHR | − 0.168 | 0.028 | − 0.312 | 0.000 |
| SBP | − 0.010 | 0.901 | − 0.173 | 0.022 |
| RPP | − 0.127 | 0.098 | − 0.342 | 0.000 |
| HbA1c | − 0.065 | 0.532 | − 0.233 | 0.019 |
| HOMA-IR | 0.032 | 0.700 | 0.019 | 0.807 |
| LF/HF | − 0.080 | 0.294 | − 0.288 | 0.000 |
| TP | 0.050 | 0.514 | 0.309 | 0.000 |
| SDNN | 0.083 | 0.281 | 0.440 | 0.000 |
| RMSSD | 0.064 | 0.407 | 0.333 | 0.000 |
| OPG | − 0.129 | 0.093 | − 0.366 | 0.000 |
| TNF α | − 0.042 | 0.587 | − 0.497 | 0.000 |
| RANK | 0.211 | 0.006 | 0.181 | 0.017 |
| RANKL | 0.049 | 0.527 | − 0.360 | 0.000 |
| RANKL/OPG | 0.143 | 0.063 | − 0.193 | 0.011 |
RPP: rate pressure product; BHR: basal heart rate; SBP: systolic blood pressure, FBG: fasting blood glucose; TG: triglyceride; HDL: high density lipoprotein; AIP: atherogenic index of plasma; SDNN: standard deviation of normal-to-normal interval; RMSSD: square root of the mean squared differences of successive normal to normal intervals; CO: cardiac output; OPG: Osteoprotegerin, *P Value < 0.05 was considered significant.
Genotyping and genetic model analysis
The genotype and allelic frequency distribution of OPG rs2073618 gene polymorphism is shown in Table 4 between T2D patients with hypertension and healthy control and in Table 5 between T2D patients with and without hypertension. For calculation of risks, we used logistic regression analysis using dominant model. The odds for risk of T2D + HTN in general population was 2.79 (1.44–5.21) as the comparison was between T2D + HTN and healthy control. The odds for risk of HTN among diabetic patients was 3.004 (1.55–5.58) as the comparison was between T2D + HTN and T2D without hypertension. Among the T2D + HTN patients, the recessive model was significant when compared with healthy control, and both the recessive and dominant models were significant when compared to T2D patients (Tables 4 and 5 respectively). Therefore, among the T2D and T2D + HTN patients, correlation of decrease in BRS was assessed for various parameters (Table 3). Subsequently contribution of different risk factors towards decreased BRS was analyzed for GG and GG + GC genotype subgroups separately for the T2D + HTN patients (Table 6). In GG genotype subgroup, BRS was contributed by rise in inflammation and reduction in SDNN. In GG + GC genotype subgroup, apart from TNF α and SDNN, the rise in RANKL/OPG level was another independent contributor for BRS (Table 6).
Table 4.
Genotype and allelic frequency distribution of OPG (TNFRSF11B) rs2073618 gene polymorphism between T2D patients with HTN (n = 177) and healthy control (n = 191).
| Genotypes& alleles | T2D with HTN (n = 177) | Healthy control (n = 191) | OR (95% CI) | P value |
|---|---|---|---|---|
| Genotypes | ||||
| CC (2) | 52 (29.4%) | 71 (37.2%) | Ref | –– |
| GC (1) | 81 (45.8%) | 98 (51.3%) | 1.12 (0.70–1.77) | 0.60 |
| GG (0) | 44 (24.9%) | 22 (11.5%) | 2.79 (1.44–5.21) | 0.0014 |
| Alleles | ||||
| C | 185 (52%) | 240 (62%) | Ref | 0.003 |
| G | 169 (48%) | 142 (38%) | 1.54 (1.15–2.07) | |
| Genetic models | ||||
| Dominant | ||||
| CC | 52 (29.4%) | 71 (37.2%) | Ref | 0.11 |
| GC + GG | 125 (70.6%) | 120 (62.8%) | 1.42 (0.92–2.20) | |
| Recessive | ||||
| CC + GC | 135 (79.9%) | 168 (88%) | Ref | 0.0008 |
| GG | 34 (20.1%) | 23 (12%) | 2.54 (1.45–4.45) | |
| Homozygotic | ||||
| CC | 52 (29.4%) | 71 (37.2%) | Ref | 0.0014 |
| GG | 44 (24.9%) | 23 (11%) | 2.79 (1.44–5.21) | |
Genetic model reference; OR: Odds ratio; Ref: Reference; CI: Confidence interval.
Table 5.
Genotype and allelic frequency distribution of OPG (TNFRSF11B) rs2073618 gene polymorphism between T2D patients with HTN and without HTN.
| Genotypes & alleles | T2D with HTN (n = 177) | T2D without HTN(n = 172) | OR (95% CI) | P value |
|---|---|---|---|---|
| Genotypes | ||||
| CC (2) | 52 (29.4%) | 71(41.3%) | Ref | – |
| GC (1) | 81 (45.8%) | 81 (47.1%) | 1.36 (0.86–2.18) | 0.13 |
| GG (0) | 44 (24.9%) | 20 (11.6%) | 3.004 (1.55–5.58) | 0.0006 |
| Alleles | ||||
| C | 185 (52%) | 223 (64%) | Ref | 0.0008 |
| G | 169 (48%) | 121 (36%) | 1.68 (1.24–2.28) | |
| Genetic models | ||||
| Dominant | ||||
| CC | 52 (29.4%) | 71 (41.3%) | Ref | 0.02 |
| GC + GG | 125 (70.6%) | 101 (58.7%) | 1.69 (1.08–2.63) | |
| Recessive | ||||
| CC + GC | 133 (75.1%) | 152 (88.4%) | Ref | |
| GG | 44 (24.9%) | 20 (11.6%) | 2.51 (1.14–4.48) | 0.0001 |
| Homozygotic | ||||
| CC | 52 (29.4%) | 71 (41.3%) | Ref | |
| GG | 44 (24.9%) | 20 (11.6%) | 3.004 (1.55–5.58) | 0.0006 |
Genetic model reference; OR: Odds ratio; Ref: Reference; CI: Confidence interval.
Table 6.
Multiple regression analysis to assess the independent association of BRS (as dependant variable) with metabolic, inflammation and calcification markers (as independent variables) in patients with hypertension in recessive model (GG population) and dominant model (GC + GG population).
| Independent Variable | GG (n = 44) | GC + GG (= 125) | ||||||
|---|---|---|---|---|---|---|---|---|
| Standardized regression coefficient β | 95% CI | P value | Standardized regression coefficient β | 95% CI | P value | |||
| UL | LL | UL | LL | |||||
| BMI | 0.085 | − 0.144 | 0.279 | 0.520 | 0.094 | − 0.301 | 0.079 | 0.250 |
| RANKL/OPG | − 0.179 | − 23.216 | 4.646 | 0.185 | − 0.219 | − 22.327 | − 3.353 | 0.008 |
| TNF α | − 0.414 | − 0.177 | − 0.037 | 0.004 | − 0.367 | − 0.221 | − 0.084 | 0.000 |
| SDNN | 0.356 | 0.021 | 0.151 | 0.011 | 0.218 | 0.014 | 0.095 | 0.009 |
BRS: Baroreflex sensitivity; BMI: Body mass index; RANKL: Receptor activator of NfkB ligand OPG: Osteoprotegerin; TNF α: tumor necrosis factor alpha; SDNN: Standard deviation of normal-to-normal interval. The p value < 0.05 was considered significant.
Discussion
The purpose of a genetic study is mainly to identify genetic risk factors for common and complex diseases30. Cardiovascular disease is one such complex disease and considered a polygenetic disease. The prevalence of CVD and its risk factors such as diabetes and hypertension are high in Indian population. There are previous reports of osteoprotegerin gene polymorphism rs207361831 linked to cardiovascular disease14 in the non-Indian population. However, the reports on single nucleotide gene polymorphism for RANKL-RANK-Osteoprotegerin pathway from Indian subcontinent is lacking. In our previous study, we reported the association of higher serum osteoprotegerin level among patients with diabetes mellitus13. We also reported the association of T950C gene polymorphism for osteoprotegerin among females with gestational diabetes mellitus32. Diabetes mellitus being a major risk factor for CVD, we hypothesize that those with osteoprotegerin gene polymorphism might be at greater risk to develop diabetes and hence CVD. However, among diabetes around 50% of patients develop hypertension during their disease despite treatment, which further elevates their CV risk5. This leads to the original question as to whether there is a genetic association for developing hypertension quite early among patients with insulin resistance and thereby adding to higher CV risk while the others are protected. To the best of our knowledge, there are no reports on the link of polymorphisms in the OPG-RANK-RANKL genes among the diabetic population with and without hypertension. Therefore, the main objective of the present study was to assess the allele frequency of OPG (rs2073618) gene polymorphism in T2D patients under treatment with hypertension compared with T2D patients without hypertension.
In the present study we followed candidate gene approach for genetic association of OPG rs2073618 gene polymorphism (1181G > C) in the exon 1 region, with the cardiovascular risk pattern among patients with T2D and the risk of developing hypertension. We found a positive association of OPG rs2073618 gene polymorphism (1181G > C) in diabetes patients with hypertension as there was a significant over-representation of polymorphic GG genotype in patients when compared with healthy control (OR: 2.79; p = 0.0014; Table 4), and patients with diabetes mellitus without hypertension (T2D + HTN) (OR: 3.004; p = 0.0006; 5). These findings suggest that the 1181G > C variant causing rs2073618 gene polymorphism, could be associated with hypertension. In the model analyses, the recessive model (GG) was significant (p = 0.0008) (Table 4) compared with control while dominant model (GG + GC) was significant when compared to T2D alone (Table 5). This suggests that the risk of developing hypertension is conferred in a recessive model in the general population. However, the risk of hypertension increases several folds among diabetes patients if they carry OPG gene 1181G > C polymorphism even with single G allele. Therefore, G mutant allele for OPG rs2073618 gene polymorphism appears to be associated with risk factors which confer susceptibility to develop hypertension.
Both formation of atherosclerotic plaque and its rupture are associated with adverse cardiovascular events. Among several factors, vascular calcification especially the calcification at intima and media can significantly raise the risk for atherosclerotic plaque formation. When this is associated with a reduced vascular compliance leading to a rise in blood pressure it adds to the risk of plaque rupture and myocardial workload. Therefore, risk factors for vascular calcification significantly increase the CV risks. Baroreflex being the link among heart rate, BP and vascular tone, a reduced BRS is a sensitive noninvasive CV risk marker25. In the present study the basal heart rate, systolic and diastolic blood pressure, and rate pressure product (RPP) were significantly high in test group 2 (T2D with hypertension) compared to control and test group 1 (T2D without hypertension) (Table 1). There was a significant reduction in BRS (Table 1) in patients with T2D + HTN when compared to healthy control (p < 0.001) and compared to patients with T2D (p < 0.001). Reduced BRS is an indicator of central autonomic dysregulation, which in turn is also a marker of loss of elastic properties of arteries in general circulation26,33,34. In other words, the study group 2 patients with T2D + HTN are more prone to CV risks compared to control group as well study group 1 patients with T2D.
The increase in basal heart rate is an index of reduced vagal tone and tachycardia at rest is associated with CV morbidity and mortality35. Significant reduction in TP and time-domain indices of HRV parameters such as, SDNN and RMSSD indicate a significant reduction in the cardiac vagal modulation in both test group 1 and 2. The reduction in SDNN is a gold standard for medical stratification of cardiovascular risks36. In the present study, BRS had an independent association with SDNN, TNF α RANKL/OPG, in patients with T2D + HTN expressing mutant allele of OPG rs2073618 gene polymorphism in GG genotype subgroup and GC + GG genotype subgroup (Table 6). To the best of our knowledge, this is the first report on link of CV risk to OPG (TNFRSF11B) rs2073618 gene polymorphism in Indian population.
The findings of present study demonstrate that patients with diabetes associated hypertension have persistent signs of inflammation and vascular calcification and altered metabolic markers in the form of an atherogenic lipid profile (Table 2). RANKL/OPG significantly contributed to reduced BRS in the population expressing G allele (GG and GC genotype) but not with GG genotype alone suggesting that vascular calcification is not a major link for loss of autonomic control in GG genotype although they are at high risk of hypertension. Nonetheless, it also indicates that the population with GG and GC genotype are at risk of hypertension when one or multiple mechanisms are active such as vascular calcification, inflammation and altered heart rate variability. Therefore, this group (GG + GC) of individuals are at higher cardiometabolic risks.
In the present study, levels of OPG, RANKL and RANKL/OPG were all high in test groups 1 and 2 compared to healthy control. A high RANKL/OPG ratio is associated with arterial calcification as RANKL directly promotes smooth muscle cell calcification37. OPG plays a pivotal role in the RANKL-RANK-Osteoprotegerin pathway. Within physiological range it is protective but at a higher level, it is indicative of high rate of ongoing atherosclerosis. RANKL on the other hand is the ligand in this pathway and a rise in RANKL or the relative ratio of RANKL to OPG is suggestive of ensuing process of atherosclerosis and calcification. It is to be noted that in our study, all patients were under standard antidiabetic and anti-hypertensive treatment for a period of 2–10 years. The metformin treatment has a positive influence on OPG level while both metformin and amplodipin reduce RANKL level38. These effects are associated with reduced CV risk in treated patients with diabetes and/or hypertension. However, despite these beneficial effects, in our study, the median level of RANKL/OPG ratio was found to be higher in test group 2 suggesting a persistence of ongoing atherosclerotic process which was still higher compared to healthy controls (Table 2).
OPG, a member of the tumour necrosis factor α (TNF- α) receptor superfamily. It is secreted from various tissues including endothelium and smooth muscle cells. Its expression is regulated by various cytokines39–41. In the present study, TNF-α was significantly associated with G allele (both GG and GC genotype) and contributed to reduced BRS, which suggests the importance of retrograde inflammation in the onset of hypertension. Reports indicate that the inflammatory mediators like TNF-α can stimulate release of OPG from endothelial cells42. Also, high level of OPG can promote endothelial cell dysfunction and reduce the release of nitric oxide that could possibly be a mechanism of rise in blood pressure43. Thus, a high OPG level with persistent inflammation and reduced vagal tone for prolonged period can tilt the OPG/RANK/RANKL axis and may set the stage for development of hypertension.
There are previous reports indicating the association of rs2073618 polymorphism with T2D10 and morbidities associated with diabetes44,45. In our study, we demonstrated the association of rs2073618 polymorphism with diabetes associated hypertension. To the best of our knowledge, this is the first study to establish an association between SNP rs2073618 of OPG gene in South Indian Tamilians with T2D and hypertension. The mutant G allele occurred more frequently in patients with T2D + HTN. Also, both the GG and GC genotypes were both carrier of high risk for hypertension with an association with several other cardiometabolic and autonomic risk factors (Table 6). Thus, it is likely that hypertension is associated with inflammation, vascular calcification and reduced cardiovagal modulation in population expressing OPG rs2073618 gene polymorphism (1181 G > C) and the G allele is associated with increased risk of hypertension.
Limitations of the study
The major limitation of the study is that the only one SNP for OPG gene was assessed in the present study, which was mainly due to the time and fund constrains of this PhD thesis work. Though the sample size for analysis of OPG gene polymorphism was adequate in the present study, the correlation of CV risk assessment would have still yielded a better level of significance with a higher sample size.
Future scope
Our study provides a new insight into the risk of developing hypertension in diabetes associated with OPG gene polymorphism and the difference in the risk profile in terms of altered level of RANKL, osteoprotegerin, inflammation and autonomic control. This provides a direction towards future studies to be conducted in large cohorts and for the effective strategy of management of hypertension in diabetic subjects in the population with OPG 1181 G > C polymorphism as primary target as part of personalized medicine.
Acknowledgements
We acknowledge the financial support received from JIPMER Authority as part of the PhD thesis work for the first author.
Author contributions
A.N.S.: Carried out the wet lab works of the study. N.N.: Designed the study protocol, organized the conduct of the study, analysed data and drafted the manuscript. B.S.S.: Helped in designing the study and patient recruitment. G.K.P.: Helped in the assessment of H.R.V., B.R.S., helped in writing of the manuscript and selecting the journal. All authors have read and approved the manuscript in the present format.
Funding
The procurement of kits was funded by institute as intramural funding to encourage research.
Data availability
The datasets analysed in this study are available with the corresponding author, which can be obtained on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Report. Reducing risks, promoting healthy life. World Health Organization, Geneva, 7–14 (2002).
- 2.Wilmot E, Idris I. Early onset type 2 diabetes: risk factors, clinical impact and management. Ther. Adv. Chronic. Dis. 2014;5:234–244. doi: 10.1177/2040622314548679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes. 2014;5:444–470. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yen FS, Wei JC, Chiu LT, Hsu CC, Hwu CM. Diabetes, hypertension, and cardiovascular disease development. J. Transl. Med. 2022;20:9. doi: 10.1186/s12967-021-03217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lastra G, Syed S, Kurukulasuriya LR, Manrique C, Sowers JR. Type 2 diabetes mellitus and hypertension: An update. Endocrinol. Metab. Clin. North Am. 2014;43:103–122. doi: 10.1016/j.ecl.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsimihodimos V, Gonzalez-Villalpando C, Meigs JB, Ferrannini E. Hypertension and diabetes mellitus: Coprediction and time trajectories. Hypertension. 2018;71:422–428. doi: 10.1161/HYPERTENSIONAHA.117.10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi Q, et al. Genetic predisposition to high blood pressure associates with cardiovascular complications among patients with type 2 diabetes: Two independent studies. Diabetes. 2012;61:3026–3032. doi: 10.2337/db12-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalra SS, Shanahan CM. Vascular calcification and hypertension: Cause and effect. Ann. Med. 2012;44:S85–S92. doi: 10.3109/07853890.2012.660498. [DOI] [PubMed] [Google Scholar]
- 9.Ndip A, et al. The RANKL/RANK/OPG signaling pathway mediates medial arterial calcification in diabetic charcot neuroarthropathy. Diabetes. 2011;60:2187–2196. doi: 10.2337/db10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan P, et al. Gene polymorphisms in the RANKL/RANK/OPG pathway are associated with type 2 diabetes mellitus in Southern Han Chinese women. Genetic Test. Mol. Biomark. 2016;20:285–290. doi: 10.1089/gtmb.2015.0306. [DOI] [PubMed] [Google Scholar]
- 11.Kamimura D. Elevated serum osteoprotegerin is associated with increased left ventricular mass index and myocardial stiffness. J. Cardiovasc. Med. 2017;18:954–961. doi: 10.2459/JCM.0000000000000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blázquez-Medela AM. Osteoprotegerin is associated with cardiovascular risk in hypertension and/or diabetes. Eur. J. Clin. Invest. 2012;42:548–556. doi: 10.1111/j.1365-2362.2011.02619.x. [DOI] [PubMed] [Google Scholar]
- 13.Jasmine MR, Nanda N, Sahoo J, Velkumary S, Pal GK. Increased osteoprotegerin level is associated with impaired cardiovagal modulation in type 2 diabetic patients treated with oral antidiabetic drugs. BMC Cardiovasc. Disord. 2020;20:453. doi: 10.1186/s12872-020-01729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brändström H, et al. A single nucleotide polymorphism in the promoter region of the human gene for osteoprotegerin is related to vascular morphology and function. Biochem. Biophys. Res. Commun. 2002;293:13–17. doi: 10.1016/S0006-291X(02)00137-7. [DOI] [PubMed] [Google Scholar]
- 15.Shen A, et al. Role of osteoprotegerin and its gene polymorphisms in the occurrence of left ventricular hypertrophy in essential hypertensive patients. Medicine. 2014;93:e154. doi: 10.1097/MD.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celczynska Bajew L. The effects of osteoprotegerin (OPG) gene polymorphism in patients with ischaemic heart disease on the morphology of coronary arteries and bone mineral density. Kardiol. Pol. 2011;69:573–578. [PubMed] [Google Scholar]
- 17.Pal GK, et al. Association of hypertension status and cardiovascular risks with sympathovagal imbalance in first degree relatives of type 2 diabetics. J. Diabetes Investig. 2014;5:449–455. doi: 10.1111/jdi.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seravalle G, Grassi G. Sympathetic nervous system and hypertension: New evidences. Auton. Neurosci. 2022;238:102954. doi: 10.1016/j.autneu.2022.102954. [DOI] [PubMed] [Google Scholar]
- 19.Xiao L, Harrison DG. Inflammation in hypertension. Can. J. Cardiol. 2020;36:635–647. doi: 10.1016/j.cjca.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perin PC, Maule S, Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clin. Exp. Hypertens. 2001;23:45–55. doi: 10.1081/CEH-100001196. [DOI] [PubMed] [Google Scholar]
- 21.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J. Clin. Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grassi G, Seravalle G, Mancia G. Sympathetic activation in cardiovascular disease: Evidence, clinical impact and therapeutic implications. Eur. J. Clin. Invest. 2015;45:1367–1375. doi: 10.1111/eci.12553. [DOI] [PubMed] [Google Scholar]
- 23.Sorriento D, Iaccarino G. Inflammation and cardiovascular diseases: The most recent findings. Int. J. Mol. Sci. 2019;20:3879. doi: 10.3390/ijms20163879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayakrishnan G, et al. Association of prehypertension status with cardiovascular risks in subclinical hypothyroidism. Int. J. Clin. Exp. Physiol. 2017;4:182–189. [Google Scholar]
- 25.Syamsunder AN, et al. Decreased baroreflex sensitivity is linked to the atherogenic index, retrograde inflammation, and oxidative stress in subclinical hypothyroidism. Endocr. Res. 2017;42:49–58. doi: 10.1080/07435800.2016.1181648. [DOI] [PubMed] [Google Scholar]
- 26.Indumathy J, et al. Decreased baroreflex sensitivity is linked to sympathovagal imbalance, body fat mass and altered cardiometabolic profile in pre-obesity and obesity Decreased baroreflex sensitivity is linked to sympathovagal imbalance, body fat mass and altered cardiometabolic profile in pre-obesity and obesity. Metabolism. 2015;64:1704–1714. doi: 10.1016/j.metabol.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Sailaja AN, Nanda N, Suryanarayana BS, Pal GK. Hypertension attenuates the link of osteoprotegerin to reduced baroreflex sensitivity in type 2 diabetes mellitus patients on oral antidiabetic and antihypertensive therapy-a cross sectional study. BMC Endocr. Disord. 2022;9:226. doi: 10.1186/s12902-022-01137-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez-Vila E. A review of the JNC 8 blood pressure guideline. Tex. Heart Inst. J. 2015;42:226–228. doi: 10.14503/THIJ-15-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majid H, Masood Q, Khan AH. Homeostatic model assessment for insulin resistance (HOMA-IR): A better marker for evaluating insulin resistance than fasting insulin in women with polycystic ovarian syndrome. J. Coll. Physicians Surg. Pak. 2017;27:123–126. [PubMed] [Google Scholar]
- 30.Patron J, Serra-Cayuela A, Habn B, Li C, Wishart DS. Assessing the performance of genome wide association studies for predicting disease risk. Plos ONE. 2019;14:0220215. doi: 10.1371/journal.pone.0220215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tschiderer L, Willeit J, Schett G, Kiechl S, Willeit P. Osteoprotegerin concentration and risk of cardiovascular outcomes in nine general population studies: Literature based metaanalysis involving 26,442 participants. PLos One. 2017;12:e0183910. doi: 10.1371/journal.pone.0183910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rakchna M, Nanda N, Sagili H, Rani JM, Naga SA. Asscociation of osteoprotegerin gene T950C polymorphism with cardiometabolic risk factors in gestational diabetes mellitus in south Indian Tamilian women. Diabetes Metab. Syndr. 2021;15:102157. doi: 10.1016/j.dsx.2021.05.030. [DOI] [PubMed] [Google Scholar]
- 33.Keerthi GS, et al. Attenuated baroreflex sensitivity in normotensive prediabetes and diabetes in Indian adults. Endocr. Res. 2016;41:89–97. doi: 10.3109/07435800.2015.1076454. [DOI] [PubMed] [Google Scholar]
- 34.La Rovere MT, Specchia G, Mortara A, Schwartz PJ. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. A prospective study. Circulation. 1988;78:816–824. doi: 10.1161/01.CIR.78.4.816. [DOI] [PubMed] [Google Scholar]
- 35.Custodis F, Reil JC, Laufs U, Böhm M. Heart rate: A global target for cardiovascular disease and therapy along the cardiovascular disease continuum. J. Cardiol. 2013;62:183–187. doi: 10.1016/j.jjcc.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Task force report. Heart rate variability: Standards of measurement, physiological interpretation, and clinival use. Circulation. 93, 1043–1065 (1996). [PubMed]
- 37.Panizo S, et al. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ. Res. 2009;104:1041–1048. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Si D, Zhao Y, He C, Yang PS. Amlodipine improves endothelial dysfunction via RANK/RANKL/OPG system by regulating microRNA 155 in hypertension. Biomed. Pharmacothera. 2019;114:108799. doi: 10.1016/j.biopha.2019.108799. [DOI] [PubMed] [Google Scholar]
- 39.An JJ, et al. Expression and regulation of osteoprotegerin in adipose tissue. Yonsei. Med. J. 2007;48:765–772. doi: 10.3349/ymj.2007.48.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dufresne SS. Osteoprotegerin protects against muscular dystrophy. Am. J. Pathol. 2015;185:920–926. doi: 10.1016/j.ajpath.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Olesen P, Ledet T, Rasmussen LM. Arterial osteoprotegerin: Increased amounts in diabetes and modifiable synthesis from vascular smooth muscle cells by insulin and TNF-alpha. Diabetologia. 2005;48:561–568. doi: 10.1007/s00125-004-1652-8. [DOI] [PubMed] [Google Scholar]
- 42.Rochette L, et al. The role of osteoprotegerin and its ligands in vascular function. Int. J. Mol. Sci. 2019;20:705. doi: 10.3390/ijms20030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alves-Lopes R, et al. Osteoprotegerin regulates vascular function through syndecan-1 and NADPH oxidase derived reactive oxygen species. Clin. Sci. 2021;135:2429–2444. doi: 10.1042/CS20210643. [DOI] [PubMed] [Google Scholar]
- 44.Nehring P, Mrozikiewicz-Rakowska B, Sobczyk-Kopcio A. Osteoprotegerin gene 2073617 and rs3134069 polymorphisms in type 2 diabetes patients and sex specific rs2073618 polymorphism as a risk factor for diabetic foot. Pol. Arch. Med. Wewn. 2013;123:176–182. doi: 10.20452/pamw.1684. [DOI] [PubMed] [Google Scholar]
- 45.Manoc RS, Kumese T, Globocnik PM, Petrovic D, Cilensek I. SNP rs2073618 of the osteoprotegerin gene is associated with diabetic retinopathy in Slovenian patients with type 2 diabetes. BioMed. Res. Int. 2013;2013:364073. doi: 10.1155/2013/364073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed in this study are available with the corresponding author, which can be obtained on reasonable request.


