Abstract
Exposure to phthalates disrupts ovarian function. However, limited studies have investigated the effects of phthalate mixtures on ovulation, especially in women. Human granulosa cells were used to test the hypothesis that exposure to a phthalate mixture (PHTmix) disrupts progesterone (P4)/progesterone receptor (PGR) signaling, which is a crucial pathway for ovulation. In addition, progestin and cyclic adenosine 3′, 5′-monophosphate (cAMP) supplementation were tested as methods to circumvent phthalate toxicity. Granulosa cells from women undergoing in vitro fertilization were acclimated in culture to regain responsiveness to human chorionic gonadotropin (hCG; clinical luteinizing hormone analogue). Granulosa cells were treated with or without hCG, and with or without PHTmix (1–500 μg/ml; dimethylsulfoxide = vehicle control) for 0.5–36 h. In the supplementation experiments, cells were treated with or without R5020 (stable progestin), and with or without 8-Br-cAMP (stable cAMP analogue). Exposure to hCG + PHTmix decreased P4 levels and mRNA levels of steroidogenic factors when compared to hCG. This was accompanied by decreased mRNA levels of PGR and downstream P4/PGR ovulatory mediators (ADAM metallopeptidase with thrombospondin type 1 motif 1 (ADAMTS1), C-X-C motif chemokine receptor 4 (CXCR4), pentraxin 3 (PTX3), and regulator of G protein signaling 2 (RGS2)) in the hCG + PHTmix groups compared to hCG. Exposure to hCG + PHTmix 500 μg/ml decreased cAMP levels and protein kinase A activity compared to hCG. Supplementation with progestin in the hCG + PHTmix 500 μg/ml group did not rescue toxicity, while supplementation with cAMP restored PGR levels and downstream P4/PGR mediator levels to hCG levels. These findings suggest that phthalate mixture exposure inhibits P4/PGR signaling in human granulosa cells via decreased steroidogenesis, cAMP levels, and protein kinase A activity. Restored P4/PGR signaling with cAMP supplementation provides a potential cellular target for intervention of phthalate-induced ovulatory dysfunction in women.
Keywords: phthalates, ovulation, ovary, fertility, mixture, progesterone, progesterone receptor, granulosa cells, rescue
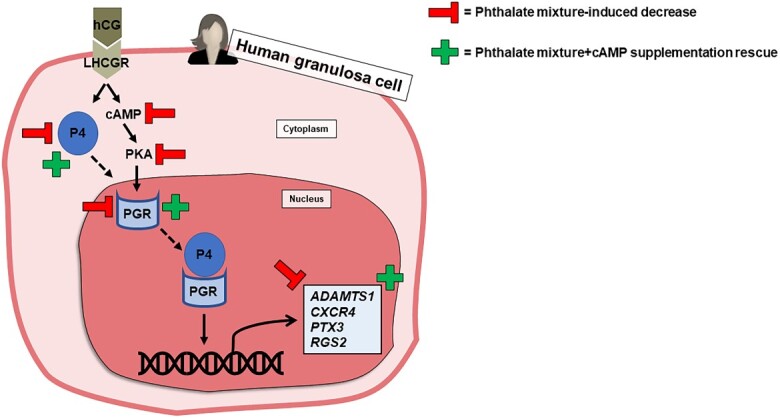
Exposure to a phthalate mixture decreased progesterone production and inhibited PGR signaling in human granulosa cells, and PGR signaling was restored with cAMP supplementation.
Graphical Abstract
Graphical Abstract.
Introduction
Phthalates are a class of chemicals used as plasticizers, solvents, and excipients in a myriad of common consumer products including food and beverage packaging, car upholstery, cosmetics, personal care products, floorings, roofing, and carpeting [1–5]. Due to their widespread production and their use in these items, humans are exposed to a mixture of phthalates daily via oral ingestion, inhalation, and dermal contact. As such, measurable levels of phthalates are detected in human urine, blood, amniotic fluid, breast milk, and even ovarian follicular fluid, which suggests that phthalates can act directly on the human ovary [6–10]. This exposure represents a public health concern because phthalates are endocrine-disrupting chemicals that impair the female reproductive system [1, 5]. Because some phthalates are incorporated in cosmetics and personal care products such nail polish, perfume, and hairspray, adult women have an increased exposure to certain phthalates compared to men [1, 11]. This underscores the importance of further investigating the effects of phthalates on ovarian function.
The primary functions of the ovary include development and maturation of the egg (oocyte), release of the oocyte from the ovary (ovulation) for fertilization, and production of sex steroid hormones to regulate the reproductive system and the initiation and maintenance of pregnancy [1, 5]. These functions are conducted within the follicle, which is the functional endocrine unit of the ovary containing the oocyte surrounded by granulosa cells and theca cells [1, 5]. Previous research using murine models suggests that exposure to certain individual phthalates disrupts ovarian function by accelerating follicle development and decreasing sex steroid hormone levels, which potentially can lead to reproductive disorders including infertility and premature menopause/primary ovarian insufficiency [1, 5, 12–15].
Unfortunately, the extent of knowledge regarding the impacts of phthalates on ovarian function is predominantly from studies conducted in rodents using single phthalate exposures. This is not a physiologically relevant approach as humans are exposed to a mixture of phthalates on a daily basis [8, 16, 17]. To circumvent these limitations, the present study exposed primary human granulosa cells to a phthalate mixture (PHTmix) containing the most commonly used phthalates [18–21]. The PHTmix was derived from urinary phthalate metabolite levels in pregnant women enrolled in the Children’s Environmental Health Research Center study at the University of Illinois at Urbana-Champaign, and it contained diethyl phthalate (DEP), di(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), diisononyl phthalate (DiNP), diisobutyl phthalate (DiBP), and butyl benzyl phthalate (BBzP) [19–21].
Although phthalate exposures have been shown to impair ovarian function, including steroid hormone production [1, 5], much less is known about the direct effects on the crucial ovulatory process. Limited studies suggest that ovulation rates were suppressed in rats following exposure to supraphysiological doses of DEHP [22, 23]. In conjunction, increased urinary phthalate metabolite levels have been shown to be negatively associated with total number of oocytes retrieved in in vitro fertilization (IVF) patients [24, 25]. Our group has recently shown that this PHTmix directly inhibits ovulation in mouse follicles in vitro [26]; however, the direct effects of combined phthalate exposure on ovulation in women is poorly understood. Therefore, this study investigated the effects of the PHTmix on progesterone (P4) and P4 receptor (PGR) action, which are crucial for ovulation and fertility, in granulosa cells obtained from women undergoing IVF.
Ovulation is initiated by the mid-cycle luteinizing hormone (LH) surge or clinical treatment with human chorionic gonadotropin (hCG; a potent LH analogue). LH/hCG stimulate the upregulation of numerous hormones and proteins that facilitate follicle wall breakdown and oocyte release [27]. Arguably the most established LH/hCG-induced ovulatory mediators are P4 and PGR [27–29]. During the periovulatory period, a >36-h process from LH surge/hCG treatment to oocyte release, granulosa cells produce abundant levels of P4 and PGR. As a nuclear transcription factor, P4/PGR signaling results in further upregulation of additional ovulatory mediators, including ADAM metallopeptidase with thrombospondin type 1 motif 1 (ADAMTS1), C-X-C motif chemokine receptor 4 (CXCR4), pentraxin 3 (PTX3), and regulator of G protein signaling 2 (RGS2) [29–32]. P4 and PGR are essential for ovulation and fertility because human and rodent studies demonstrated that inhibition of P4 synthesis, antagonists of PGR, and knocking out Pgr results in anovulation and infertility [28, 29, 33]. Thus, any disruption in this crucial pathway by the PHTmix may have negative effects on female reproductive health.
The present study therefore tested the hypothesis that PHTmix exposure would decrease ovulatory P4 production and PGR levels, leading to hindered P4/PGR signaling in human granulosa cells. Furthermore, the present study sought to elucidate the mechanism by which PHTmix exposure decreased P4/PGR, which would potentially provide a therapeutic target for intervention of phthalate action at the cellular level. As defects in ovulation are the leading cause of infertility in women [34], this study lays the foundation for understanding how exposure to a mixture of ubiquitous toxicants potentially contributes to the prevalence of infertility in women.
Materials and methods
Chemicals
The composition of the PHTmix was as follows: 35% DEP, 21% DEHP, 15% DBP, 15% DiNP, 8% DiBP, and 5% BBzP. The mixture was derived from urinary phthalate metabolite levels from pregnant women enrolled in the Children’s Environmental Health Research Center at the University of Illinois, thus representing human exposure to a mixture of phthalates [19–21]. All phthalates were >98% purity and were purchased from Sigma-Aldrich (St. Louis, MO). Stock solutions of the mixture were prepared using dimethylsulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO) as the vehicle to prepare various concentrations (1.33, 13.3, 133, and 655 mg/ml). This allowed for an equal volume of each stock to be added to the culture to control for vehicle concentration (75 μg/ml). The final concentrations of the PHTmix in culture were 1, 10, 100, and 500 μg/ml.
These concentrations were selected based on their ability to reduce antral follicle growth, decrease sex steroid production, cause oocyte fragmentation, and directly inhibit ovulation in studies using the same PHTmix in mouse antral follicles in vitro [19, 26]. Concentrations of individual phthalates found in the PHTmix have also been shown to disrupt ovarian folliculogenesis and steroidogenesis in mouse models [12, 14]. Previous studies have shown that phthalate metabolites are also readily measured in follicular fluid from women undergoing in vitro fertilization, with nearly 100% of samples containing the primary metabolite of the majority of the phthalates included in the PHTmix [6, 7, 35]. When considering the concentrations of each individual phthalate as a percentage of the total mixture, the concentrations of DBP and DEHP at the lowest concentration of the PHTmix (1 μg/ml) are below the highest measurements of each of the respective metabolites (MBP and MEHP) in human follicular fluid [35]. Specifically, in the 1 μg/ml PHTmix concentration, the concentration of DBP and DEHP are 150 and 210 ng/ml, respectively. These concentrations are below the highest recorded levels measured in these patients for MBP (415 ng/ml) and MEHP (239 ng/ml) [35]. Certain populations are also exposed to much higher levels of DEHP, DBP, and DEP than the general population due to the prevalence of these phthalates in certain medications and medical equipment. The concentrations of these individual phthalates of the PHTmix may encompass medical exposure levels that are seen in humans undergoing certain medical procedures and taking certain medications, especially intravenously [36–39].
In the supplementation experiments, promegestone (R5020) was selected as a non-metabolizable PGR agonist (Perkin-Elmer Inc., Boston, MA), and 8-bromoadenosine 3′, 5′-cyclic monophosphate (8-Br-cAMP; hereafter abbreviated to cAMP for simplicity) was selected as a stable cAMP analogue (Sigma-Aldrich, St. Louis, MO). Three concentrations of R5020 (100, 300, and 1000 ng/ml) were selected. The 100 ng/ml concentration was selected because it was used in previous experiments to mimic high levels of P4 that are produced by cultured primate follicles [40, 41]. The 300 ng/ml concentration was selected because it is roughly equivalent to the mean difference in P4 levels when comparing the hCG control group to the highest PHTmix concentration (highest P4 levels minus lowest P4 levels following treatment), as evident in the present study. The 1000 ng/ml concentration was selected as a supraphysiological dose. The concentration of cAMP that was selected was 1.5 mM because this concentration has been shown to induce steroidogenesis in a similar primary human granulosa cell model as used in the present study [42, 43].
Human granulosa cell collection and in vitro culture
The present study utilized an in vitro culture system using granulosa cells from patients undergoing IVF that recapitulates periovulatory outcomes as observed in the human in vivo [44–47]. The Institutional Review Board of the University of Kentucky Office of Research Integrity approved the protocol for granulosa cell collection. Women undergoing IVF at the Bluegrass Fertility Center (Lexington, KY) underwent a standardized ovarian stimulation protocol by administration of recombinant human FSH for 9–11 days. Following this FSH treatment regimen, the patients were given 10 000 U of hCG to induce the ovulatory cascade, and follicular aspiration was conducted 36 h post-hCG treatment to collect the contents of the follicles, which is the time-point just prior to ovulation. Oocytes were isolated in the fertility clinic and the remaining granulosa cells were subjected to a Percoll gradient to separate the granulosa cells from red blood cells in the aspirate providing a purified population of cells for culture. The isolated granulosa cells were cultured in OptiMEM media containing 10% fetal bovine serum and 1% antibiotic-antimycotic (2.5 × 105 cells/ml). The granulosa cells were cultured for 6 days and the media were changed every 24 h. Following this 6-day acclimation period, the granulosa cells regain responsiveness to hCG and are able to recapitulate periovulatory outcomes. Specifically, these revitalized granulosa cells now possess functional LH/hCG receptors, produce P4 in response to hCG, and have increased mRNA levels of EGF-like peptides, PGR, and prostaglandin synthases/transporters in response to hCG [45, 46]. Thus, the acclimation period allows the granulosa cells to revert back to a preovulatory phenotype, and re-treatment with hCG is capable of inducing ovulatory outcomes as observed in women in vivo [45, 46].
Prior to hCG treatment, the cells were pretreated with DMSO (vehicle control) or the PHTmix (1, 10, 100, and 500 μg/ml) for 48 h. Following 48 h, the cells were first cultured in media without serum for 1 h and were then treated with or without hCG (1 IU/ml) and with or without the PHTmix. Cell and media samples were collected prior to hCG treatment (following 24- and 48-h exposure to vehicle and the PHTmix) and following hCG + PHTmix treatment (0, 0.5, 6, 12, 24, and 36 h) for the assays described below. In the supplementation experiments, cells were also treated with or without R5020 and with or without cAMP.
Cell viability assay
Cell viability was measured using the colorimetric CellTiter 96 Aqueous One Solution cell proliferation assay (MTS) according to the manufacturer’s protocol (Promega, Madison, WI). Briefly, the cells were cultured on 96-well plates in triplicates and were treated with or without the PHTmix and with or without hCG, as described above. The MTS assay was performed on samples collected prior to hCG treatment (following PHTmix exposure for 24 and 48 h) and 24 h following hCG + PHTmix treatment. At each time-point, 20 μl of CellTiter reagent was pipetted into each well and was incubated for 1.5 h. Absorbance was measured at 490 nm in an Infinite F200 plate reader (Tecan USA) to determine the formazan concentration, which is proportional to the number of live cells.
Progesterone measurements
P4 concentrations were measured in media treated immediately before (0 h) and after (6, 12, 24, 36 h) hCG treatment via an Immulite kit [45]. The assay sensitivity was 0.02 ng/ml and intraassay and interassay coefficients of variation were 7% and 12%, respectively. Due to patient-to-patient variability, data are expressed as percent fold-change relative to the hCG alone control group at each time-point.
Gene expression analysis
Following each culture time-point, cells were lysed and stored at −80°C for quantitative real-time polymerase chain reaction (qPCR) analysis. Total RNA (200 ng) was extracted from the cells using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s protocol and was then reverse transcribed to cDNA using the iScript RT kit (Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer’s protocol. The cDNA samples were further diluted using nuclease-free water. The AriaMX Real-Time PCR System (Agilent Technologies, Santa Clara, CA) and accompanying Agilent Aria software was used for analysis of qPCR according to the manufacturer’s protocol. Specific qPCR primer information/sequences (Integrated DNA Technologies, Inc., Coralville, IA; Invitrogen Life Technologies, Inc., Waltham, MA) can be found in Table 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was selected as the reference gene, as statistical analysis confirmed its expression did not significantly differ across time-point or treatment group.
Table 1.
Laboratory-designed primer sequences and TaqMan primer information.
| Laboratory-designed primer sequences | |||
|---|---|---|---|
| Gene name | Gene symbol | 5′-Forward-3′ | 5′-Reverse-3′ |
| ADAM metallopeptidase with thrombospondin type 1 motif 1 | ADAMTS1 | CAGCCCAAGGTTGTAGATGGT | GATCCATTTCCCCCGCAAAC |
| C-X-C motif chemokine receptor 4 | CXCR4 | ACTGAGAAGCATGACGGACA | GATGAAGGCCAGGATGAGGA |
| Cytochrome P450 family 11 subfamily A member 1 | CYP11A1 | AGTCCTCGCCCCTTCAA | AGCTTCTCCCTGTAAATCGGG |
| Hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 2 | HSD3B2 | TGAGGCAGTAAGGACTTGGAC | GTGACCCAGAAGAGGGCGTAA |
| Pentraxin 3 | PTX3 | TGCGATTCTGTTTTGTGCTC | TGAAGAGCTTGTCCCATTCC |
| Regulator of G protein signaling 2 | RGS2 | CGAGGAGAAGCGAGAAAAGA | CCTCAGGAGAAGGCTTGATG |
| Steroidogenic acute regulatory protein | STAR | GAGGTCGATGCTGAGTAGCC | CCTGCAGAAGATCGGAAAAG |
| TaqMan primer information | |||
| Gene name | Gene symbol | Assay ID | |
| Progesterone receptor | PGR | Hs01556702_m1 | |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | Hs99999905_m1 | |
The AriaMX Real-Time PCR System quantifies the amount of PCR product generated by measuring the fluorescence from the SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories, Inc.) or TaqMan Gene Expression Master Mix (Invitrogen Life Technologies, Inc., Waltham, MA) for reactions performed with laboratory-designed primers or TaqMan primers, respectively. The SsoAdvanced program consisted of an enzyme activation step (95°C for 30 s), an amplification and quantification program (45 cycles of 95°C for 10 s, 60°C for 10 s, single fluorescence reading), and a melt curve (65–95°C heating 0.5°C per second with continuous fluorescence readings), as per the manufacturer’s protocol. The TaqMan program included the following steps: 2 min at 50°C to permit AmpErase uracil-N-glycosylase optimal activity, denaturation step for 10 min at 95°C, 15 s at 95°C, and 1 min at 60°C for 50 cycles, followed by 1 min at 95°C, 30 s at 58°C, and 30 s at 95°C for ramp dissociation. Expression data were generated using the mathematical standard comparative (ΔΔCt) method. The ΔCt was calculated by subtracting the reference gene Ct value from the gene of interest Ct value. The ΔΔCt was calculated as the difference between the ΔCt between the treatment groups and the 0-h DMSO groups (immediately prior to hCG treatment). The relative fold-change of expression was then equaled to 2−ΔΔCT for each sample. Due to patient-to-patient variability, data are expressed as percent fold-change relative to the hCG alone control group at each time-point.
Cyclic adenosine monophosphate quantitative assay
Levels of cAMP were quantified using the cAMP enzyme-linked immunosorbent assay (ELISA) (Abcam, Boston, MA) according to the manufacturer’s protocol. Briefly, cellular protein was extracted following 30 min of hCG treatment. Protein lysates were plated on a recombinant protein G-coated plate that facilitates binding of the cAMP antibody to the plate. The amount of cAMP-HRP bound to the plate (competitive ELISA) was measured by absorbance readings at 450 nm from an Infinite F200 plate reader. The assay sensitivity was 0.02 μM. Due to patient-to-patient variability, data are expressed as percent change relative to the hCG alone control group.
Protein kinase A activity assay
Protein kinase A (PKA) activity was measured using the PKA Kinase Activity Assay Kit (Abcam, Boston, MA) according to the manufacturer’s protocol. Briefly, cellular protein was extracted following 30 min of hCG treatment. Protein lysates were used in an ELISA using a peptide as the PKA substrate and an antibody recognizing each phosphorylated substrate. Relative kinase activity was measured by absorbance readings at 450 nm from an Infinite F200 plate reader. The assay intraassay and interassay coefficients of variation were less than 10% and less than 12%, respectively. Due to patient-to-patient variability, data are expressed as percent change relative to the hCG alone control group.
Statistical analysis
Data analysis was conducted using SPSS statistical software (SPSS, Inc., Chicago, IL). Data were expressed as means ± standard error of the mean (SEM). Multiple comparisons between normally distributed experimental groups were made using one-way analysis of variance followed by Tukey post hoc comparison. Multiple comparisons between non-normally distributed experimental groups were made using Kruskal–Wallis tests when appropriate. Statistical significance was assigned at p ≤0.05.
Results
Effect of PHTmix exposure on granulosa cell viability
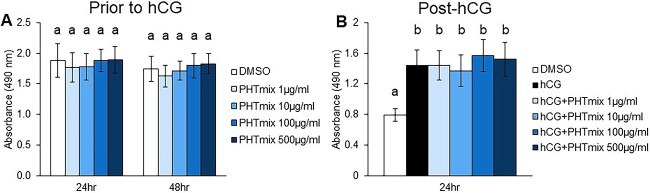
Cell viability was first measured in human granulosa cells collected before and after hCG treatment as a potential mechanism of PHTmix toxicity. Prior to hCG treatment, PHTmix exposure for 24 and 48 h did not alter cell numbers/viability when compared to the DMSO group (Figure 1A). Following hCG treatment for 24 h, there is an expected increase in cell viability output in the hCG alone control group when compared to DMSO (Figure 1B), due to the effect of hCG on cellular metabolism [31]. However, hCG + PHTmix exposure did not alter cell viability when compared to the hCG alone control group (Figure 1B). These data suggest that the concentrations of PHTmix used in the present study are not cytotoxic and any alterations in cellular function are not due to cell death.
Figure 1.
Effect of phthalate mixture exposure on human granulosa cell viability/metabolism. Human granulosa cells were collected at the time of oocyte retrieval from women undergoing in vitro fertilization. The cells were processed as described in Materials and methods to regain responsiveness to hCG. The cells were treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 1–500 μg/ml) for 48 h prior to hCG treatment. Following 48 h, the cells were treated with or without hCG (1 IU/ml) and with or without PHTmix. Cell viability/metabolism was measured via a colorimetric MTS assay following 24- and 48-h PHTmix exposure prior to hCG treatment (A) and following 24 h of hCG + PHTmix treatment (B). Values are presented as absorbance read at 490 nm, which positively correlates with cell viability/metabolism. Graphs represent mean ± SEM. Bars that do not share a letter designation are significantly different (n = 4 patients/group, p ≤ 0.05).
Effect of PHTmix exposure on P4 and PGR levels
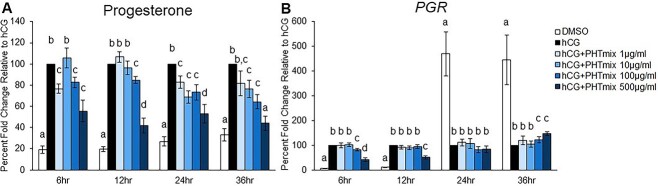
The LH/hCG-induced increases in P4 and PGR in granulosa cells are well characterized, and these increases are required for fertility as disruption or absence of P4/PGR signaling during the ovulatory period results in anovulation [30, 45, 48, 49]. Then, P4 was measured in media samples collected across the ovulatory period (6, 12, 24, and 36 h) in the human granulosa cell model. Treatment with hCG at each time-point significantly increased P4 levels when compared to DMSO (Figure 2A). However, this increase was inhibited by exposure to hCG + PHTmix at multiple time-points and concentrations. The most consistent findings were in the 100 and 500 μg/ml hCG + PHTmix groups, where these concentrations had decreased P4 levels at each time-point when compared to hCG alone (Figure 2A). At 36 h, the levels in the 500 μg/ml group were comparable to the DMSO group that did not receive hCG treatment. P4 levels were also significantly decreased in the 1 μg/ml group at 6 and 24 h, and in the 10 μg/ml group at 24 and 36 h when compared to hCG (Figure 2A).
Figure 2.
Effect of phthalate mixture exposure on P4 levels and PGR mRNA levels. Human granulosa cells from in vitro fertilization patients were treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 1–500 μg/ml) for 48 h prior to hCG treatment. Following 48 h, the cells were treated with or without hCG and with or without PHTmix. Media were collected at multiple time-points following hCG treatment and were subjected to progesterone measurements via an Immulite kit (A). Cells were collected at multiple time-points following hCG treatment and were subjected to qPCR to measure PGR mRNA levels that were normalized to GAPDH (B). Values are presented as a percent fold-change relative to the hCG alone control group for each time-point collected. Graphs represent mean ± SEM. Bars that do not share a letter designation are significantly different (n = 9–12 patients/group, p ≤ 0.05).
Cellular mRNA levels of PGR were also measured across the ovulatory period in the human granulosa cells. The mRNA levels of PGR increased early (6 and 12 h) and decreased late (24 and 36 h) during the ovulatory period in the hCG control group when compared to DMSO (Figure 2B). This transient pattern of PGR expression by hCG is expected in human granulosa cells, as seen in our previous publication [45]. However, this early increase was inhibited by hCG + PHTmix at the 100 and 500 μg/ml concentrations when compared to hCG (Figure 2B). Furthermore, the mRNA levels of PGR were increased at these concentrations at the 36-h time-point when compared to hCG (Figure 2B).
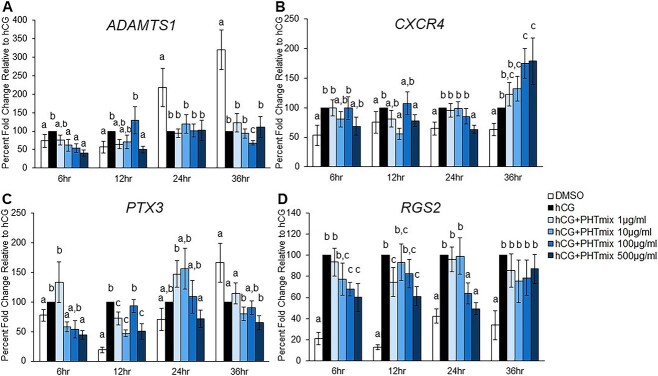
Effect of PHTmix exposure on downstream P4/PGR ovulatory mediators
As a nuclear transcription factor, the increase in P4/PGR signaling during the ovulatory period induces the upregulation of several downstream mediators of ovulation in granulosa cells. A few of these factors that are downstream of P4/PGR and have been shown to mediate biological processes required for ovulation are ADAMTS1, CXCR4, PTX3, and RGS2 [29–32]. Since P4 and PGR levels were decreased in human granulosa cells following exposure to the PHTmix, the mRNA levels of these downstream mediators were measured. Expected increases in each gene were observed in the hCG groups when compared to DMSO (Figure 3). These increases were early during the periovulatory period (6 and 12 h) for ADAMTS1 and PTX3 and at all time-points tested for CXCR4 and RGS2. The early increases in ADAMTS1 were inhibited by hCG + PHTmix exposure at the 10, 100, and 500 μg/ml concentrations when compared to hCG (Figure 3A). Treatment with hCG + PHTmix at the 10 and 500 μg/ml concentrations decreased the levels of CXCR4 at the 12- and 24-h time-points; however, the 100 and 500 μg/ml concentrations further increased CXCR4 levels at the 36-h time-point when compared to hCG (Figure 3B). The early induction of PTX3 at 6 and 12 h was inhibited by hCG + PHTmix at the 1, 10, and 500 μg/ml concentrations when compared to hCG (Figure 3C). Similar decreases in RGS2 at 6, 12, and 24 h were observed at the 1, 100, and 500 μg/ml concentrations when compared to hCG (Figure 3D).
Figure 3.
Effect of phthalate mixture exposure on the mRNA levels of downstream P4/PGR ovulatory mediators. Human granulosa cells from in vitro fertilization patients were treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 1–500 μg/ml) for 48 h prior to hCG treatment. Following 48 h, the cells were treated with or without hCG and with or without PHTmix. Cells were collected at multiple time-points following hCG treatment and were subjected to qPCR to measure the mRNA levels of ADAMTS1 (A), CXCR4 (B), PTX3 (C), and RGS2 (D). All values were normalized to GAPDH and are presented as a percent fold-change relative to the hCG alone control group for each time-point collected. Graphs represent mean ± SEM. Bars that do not share a letter designation are significantly different (n = 9–12 patients/group, p ≤ 0.05).
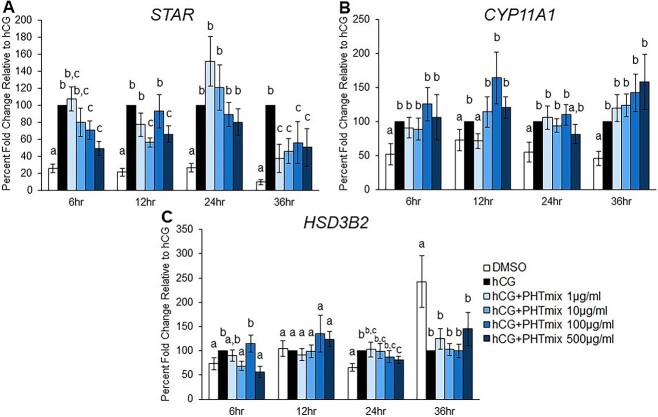
Effect of PHTmix exposure on steroidogenic genes
The increase in P4 levels during the periovulatory period is largely mediated by LH/hCG-induced increases in the levels of steroidogenic proteins/enzymes, namely steroidogenic acute regulatory protein (STAR), cytochrome P450 family 11 subfamily A member 1 (CYP11A1), and hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 2 (HSD3B2) [29, 32, 50]. To elucidate the mechanism by which exposure to the PHTmix decreased P4 levels, the levels of these steroidogenic genes were measured. Expected increases in the levels of STAR (Figure 4A) and CYP11A1 (Figure 4B) by hCG were observed at each time-point tested compared to DMSO. However, the increase in STAR was inhibited by hCG + PHTmix at the 10, 100, and 500 μg/ml concentrations at 6 and 12 h and at all concentrations tested at 36 h when compared to hCG (Figure 4A). Contrary to the many changes in STAR, exposure to hCG + PHTmix decreased the levels of CYP11A1 only at the 12-h time-point by the 1 μg/ml concentration compared to hCG (Figure 4B). HSD3B2 was induced by hCG at the 6 and 24 h time-points when compared to DMSO, but these increases were inhibited by hCG + PHTmix at the 10 (6 h) and 500 μg/ml (6 and 24 h) concentrations when compared to hCG (Figure 4C).
Figure 4.
Effect of phthalate mixture exposure on the mRNA levels of steroidogenic proteins/enzymes. Human granulosa cells from in vitro fertilization patients were treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 1–500 μg/ml) for 48 h prior to hCG treatment. Following 48 h, the cells were treated with or without hCG and with or without PHTmix. Cells were collected at multiple time-points following hCG treatment and were subjected to qPCR to measure the mRNA levels of STAR (A), CYP11A1 (B), and HSD3B2 (C). All values were normalized to GAPDH and are presented as a percent fold-change relative to the hCG alone control group for each time-point collected. Graphs represent mean ± SEM. Bars that do not share a letter designation are significantly different (n = 9–12 patients/group, p ≤ 0.05).
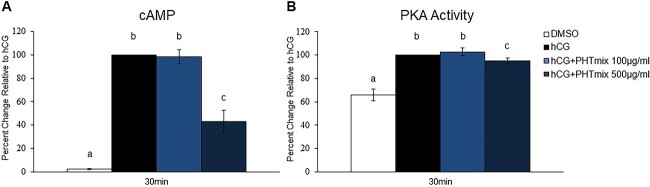
Effect of PHTmix exposure on cAMP levels and PKA activity
Activation of the LH/hCG receptor (LHCGR; a G-protein coupled receptor) by the ovulatory stimulus results in an immediate and abundant increase in intracellular cAMP leading to PKA activation and ultimately induction of PGR [28]. To elucidate the mechanism by which exposure to the PHTmix decreased PGR levels, cAMP levels and PKA activity were measured. The 100 and 500 μg/ml concentrations were selected for analysis because these were the concentrations with decreased PGR levels (Figure 2B). In both assays, hCG increased the levels of cAMP and PKA activity following 30 min of treatment when compared to DMSO (Figure 5A and B). The increases in cAMP and PKA activity were inhibited by hCG + PHTmix at the 500 μg/ml concentration compared to hCG (Figure 5A and B).
Figure 5.
Effect of phthalate mixture exposure on cAMP levels and PKA activity. Human granulosa cells from in vitro fertilization patients were treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 100–500 μg/ml) for 48 h prior to hCG treatment. Following 48 h, the cells were treated with or without hCG and with or without PHTmix. Cells were collected 30 min following hCG treatment to measure cAMP levels (A) and PKA activity (B) via ELISAs. Values are presented as a percent change relative to the hCG alone control group. Graphs represent mean ± SEM. Bars that do not share a letter designation are significantly different (n = 5–8 patients/group, p ≤ 0.05).
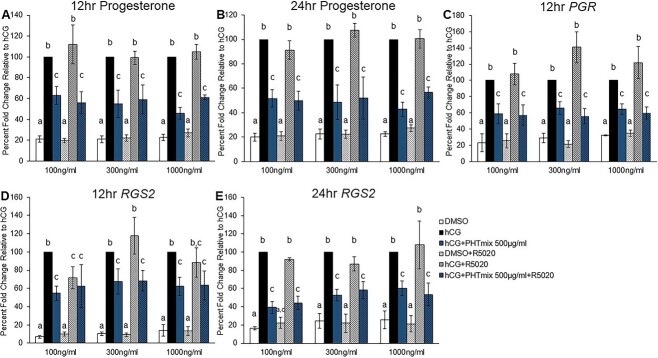
Effect of progestin supplementation on the rescue of PHTmix toxicity
Exposure to hCG + PHTmix resulted in inhibition of the hCG-induced increases in P4 production during the periovulatory period (Figure 2A). Therefore, progestin supplementation to the cells was conducted to investigate whether a PGR agonist could rescue PHTmix toxicity. Promegestone (R5020) was selected because it is a stable PGR agonist that cannot be aromatized or metabolized. The 500 μg/ml concentration was selected for these experiments because this concentration consistently decreased P4 levels across the periovulatory period. Preliminary analysis of the effects of R5020 supplementation on RGS2 levels was selected because RGS2 was the most abundantly induced gene by hCG in the present study and was consistently decreased by the 500 μg/ml concentration (Figure 3D). As expected, hCG increased P4 levels compared to DMSO, and hCG + PHTmix decreased P4 levels compared to hCG (Figure 6A and B), as observed in previous experiments (Figure 2A). Supplementation with R5020 did not alter the levels of P4 at 12 and 24 h in any treatment group or at any concentration of R5020 selected (Figure 6A and B), and the levels of P4 are comparable to the respective treatment groups that did not receive R5020. Thus, R5020 treatment does not cross-react with P4 in the Immulite assay. In a similar fashion, supplementation with R5020 did not alter the levels of PGR at 12 h in any treatment group or at any concentration of R5020 selected when compared to the respective treatment groups that did not receive R5020 (Figure 6C). The expected changes of increased PGR levels in the hCG group compared to DMSO and the decreased PGR levels in the hCG + PHTmix group compared to hCG was observed, as was the case in previous experiments (Figure 2B). The only observed change in R5020 treatment was in the levels of RGS2 at 12 h where treatment with hCG + R5020 at 100 ng/ml decreased the levels of RGS2 when compared to the hCG alone group (Figure 6D). In all other cases, supplementation with R5020 did not alter the levels of RGS2 at 12 and 24 h in any treatment group or at any concentration of R5020 selected when compared to the respective treatment groups that did not receive R5020 (Figure 6D and E). The anticipated changes of increased RGS2 levels in the hCG group compared to DMSO and the decreased RGS2 levels in the hCG + PHTmix group compared to hCG was observed (Figure 6D and E), as was noted in previous experiments (Figure 3D).
Figure 6.
Effect of progestin supplementation on phthalate mixture-induced toxicity. Human granulosa cells from in vitro fertilization patients were treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 500 μg/ml) for 48 h prior to hCG and progestin (R5020) treatment. Following 48 h, the cells were treated with or without hCG, with or without PHTmix, and with or without R5020 (100–1000 ng/ml). Media were collected at multiple time-points following hCG treatment and were subjected to progesterone measurements via an Immulite kit (A, B). Cells were collected at multiple time-points following hCG treatment and were subjected to qPCR to measure the mRNA levels of PGR (C) and RGS2 (D, E) that were normalized to GAPDH. Values are presented as a percent fold-change relative to the hCG alone control group for each time-point collected. Graphs represent mean ± SEM. Bars that do not share a letter designation are significantly different (n = 4–8 patients/group, p ≤ 0.05).
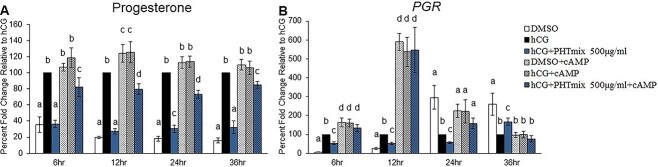
Effect of cAMP supplementation on the rescue of PHTmix-induced decreases in P4 and PGR levels
Exposure to hCG + PHTmix at the 500 μg/ml concentration inhibited the hCG-induced increases in PGR levels (Figure 2B), cAMP levels (Figure 5A), and PKA activity (Figure 5B). Thus, a stable cAMP analogue was supplemented to the cells to investigate if this treatment could rescue PHTmix toxicity at the 500 μg/ml concentration. As shown previously, hCG increased P4 levels at each time-point tested (Figure 7A) and PGR levels at the 6- and 12-h time-points (Figure 7B) when compared to DMSO. Likewise, these increases where inhibited by hCG + PHTmix when compared to hCG. Supplementation with cAMP increased the levels of P4 in each treatment group at each time-point when compared to the respective treatment groups that did not receive cAMP (Figure 7A). Although cAMP supplementation in the PHTmix group did not completely restore P4 levels to DMSO + cAMP, hCG + cAMP, and hCG alone levels, it did increase P4 levels when compared to the hCG + PHTmix group (Figure 7A).
Figure 7.
Effect of cAMP supplementation on phthalate mixture-induced decreases in P4 levels and PGR mRNA levels. Human granulosa cells from in vitro fertilization patients were treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 500 μg/ml) for 48 h prior to hCG and 8-Br-cAMP (cAMP) treatment. Following 48 h, the cells were treated with or without hCG, with or without PHTmix, and with or without cAMP (1.5 mM). Media were collected at multiple time-points following hCG treatment and were subjected to progesterone measurements via an Immulite kit (A). Cells were collected at multiple time-points following hCG treatment and were subjected to qPCR to measure PGR mRNA levels that were normalized to GAPDH (B). Values are presented as a percent fold-change relative to the hCG alone control group for each time-point collected. Graphs represent mean ± SEM. Bars that do not share a letter designation are significantly different (n = 4–8 patients/group, p ≤ 0.05).
Supplementation with cAMP increased PGR levels in each treatment group at the 6- and 12-h time-points when compared to the respective treatment groups that did not receive cAMP, and the levels in the PHTmix + cAMP groups at these time-points were comparable to the DMSO + cAMP and hCG + cAMP groups (Figure 7B). As expected, hCG decreased PGR levels late during the periovulatory period (24 and 36 h) when compared to DMSO, but cAMP supplementation in each treatment group had comparable PGR levels to that of the DMSO alone group at 24 h and that of the hCG alone group at 36 h (Figure 7B).
Supplementation with cAMP had minimal effects on the PHTmix-induced alterations in steroidogenic mRNA levels. Briefly, cAMP supplementation in the PHTmix group increased the mRNA levels of STAR at each time-point relative to the hCG + PHTmix group, and levels were comparable (12 h) or increased (6, 24, and 36 h) relative to hCG alone (Supplementary Figure S1A). Interestingly, cAMP supplementation in the PHTmix group increased CYP11A1 levels at 6 h relative to hCG; however, levels were comparable to the hCG + PHTmix group, which did not differ significantly compared to hCG alone (Supplementary Figure S1B). Supplementation with cAMP in the PHTmix group restored the levels of HSD3B2 to hCG levels at the 6-h time-point, but it decreased levels relative to hCG alone and the hCG + PHTmix group at the 12-h time-point (Supplementary Figure S1C).
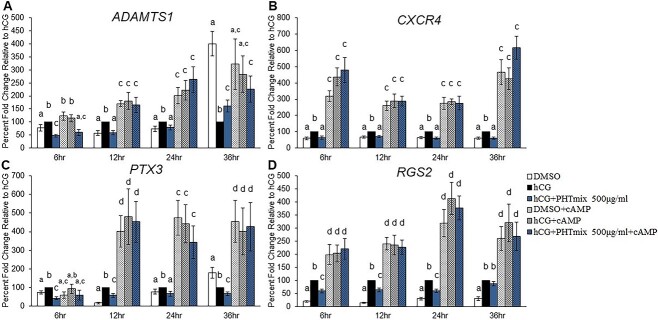
Effect of cAMP supplementation on the rescue of PHTmix-induced decreases in downstream P4/PGR ovulatory mediators
Supplementation with cAMP appeared to partially restore P4 levels (Figure 7A) and fully restore PGR levels in the PHTmix group (Figure 7B). Therefore, downstream P4/PGR ovulatory mediators were measured to assess if cAMP supplementation rescued the defects in P4/PGR signaling caused by PHTmix exposure. As previously shown, hCG increased the levels of ADAMTS1 and PTX3 at the 6-, 12-, and 24-h time-points (Figure 8A and C) and CXCR4 and RGS2 at all time-points tested (Figure 8B and D) when compared to DMSO, and exposure to PHTmix at the 500 μg/ml concentration inhibited these increases. Supplementation with cAMP predominantly increased the levels of each gene in each treatment group at each time-point when compared to the respective treatment groups that did not receive cAMP, except with ADAMTS1 at 6 and 36 h where the levels were comparable to the hCG alone and DMSO alone groups, respectively (Figure 8A). Supplementation with cAMP also did not have an effect of increasing PTX3 levels at the early 6-h time-point (Figure 8C). Aside from these findings, cAMP supplementation in the 500 μg/ml group increased the levels of each gene when compared to PHTmix alone, and the levels were comparable to the DMSO + cAMP and hCG + cAMP groups (Figure 8A–D).
Figure 8.
Effect of cAMP supplementation on phthalate mixture-induced decreases in the mRNA levels of downstream P4/PGR ovulatory mediators. Human granulosa cells from in vitro fertilization patients were treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 500 μg/ml) for 48 h prior to hCG and cAMP treatment. Following 48 h, the cells were treated with or without hCG, with or without PHTmix, and with or without cAMP. Cells were collected at multiple time-points following hCG treatment and were subjected to qPCR to measure the mRNA levels of ADAMTS1 (A), CXCR4 (B), PTX3 (C), and RGS2 (D). All values were normalized to GAPDH and are presented as a percent fold-change relative to the hCG alone control group for each time-point collected. Graphs represent mean ± SEM. Bars that do not share a letter designation are significantly different (n = 5–12 patients/group, p ≤ 0.05).
Discussion
The present study characterized the effects of a phthalate mixture on ovulatory P4/PGR action using primary human granulosa cells. The main findings suggest that exposure to the PHTmix decreased the production of P4, decreased PGR levels, and dysregulated P4/PGR action by decreasing ADAMTS1, CXCR4, PTX3, and RGS2 levels, all of which are essential for successful ovulation and fertility [27–32]. Furthermore, these defects appeared to be driven by the decrease in PGR levels, and less by the decrease in P4 levels. Specifically, supplementing the granulosa cells treated with the highest concentration of the PHTmix with a stable cAMP analogue, shown to increase PGR levels in this model, restored the levels of the downstream P4/PGR ovulatory mediators, while supplementation with a stable progestin failed to rescue phthalate-induced toxicity. As such, these findings suggest that the mechanism by which the PHTmix inhibits ovulatory P4/PGR signaling is via disruptions in the cAMP/PKA pathway, and that this pathway may be a targetable approach at the cellular level for intervention on phthalate toxicity in the ovary during the ovulatory period.
By using human granulosa cells and a phthalate mixture, this study was a novel approach for investigating phthalate action in the ovary. The majority of phthalate ovarian toxicology studies are conducted in rodent species [1, 5]; therefore, it is presently unknown if phthalates directly impair the functions of the human ovary, an organ known to be directly exposed to phthalates [6, 7]. Epidemiological evidence suggests that increased phthalate exposure is associated with poor in vitro fertilization outcomes, including decreased numbers of oocytes retrieved, decreased number of mature/fertilizable oocytes retrieved, and decreased incidence of clinical pregnancy and live births [5, 24, 25]. These data suggest that phthalate exposure may hinder ovarian function and the ovulatory process, even in the presence of exogenous hormones used in the IVF procedure. Therefore, this study utilized an in vitro primary human granulosa cell model that recapitulates ovulatory outcomes observed in normally cycling women in vivo [44–47] to investigate the direct effects of phthalates on human periovulatory granulosa cells.
While the use of primary human samples is a strength of these studies, the source of the samples being from patients undergoing IVF complicates interpretation. The present study used granulosa cell samples collected from women that are fertile (male factor infertility, oocyte preservation, and oocyte donors), have non-ovarian infertility diagnoses (endometriosis and tubal factor infertility), and have ovarian disorders (polycystic ovary syndrome). Presently, we are not able to stratify samples based on their fertility/infertility status, in part due to the limited patient availability and cell number collected from each patient. Correlating these diagnoses with the measurable outcomes in our model is an area of future investigation. Furthermore, measurements of phthalates in the follicular fluid aspirates at the time of oocyte collection will allow us to determine if higher baseline phthalate exposure predisposes the granulosa cells to greater phthalate-induced ovulatory toxicities in our model, which will also be the focus of future work.
An additional limitation to the literature investigating the effects of phthalates on the ovary is that the majority of studies focus on single phthalate exposures [1]. To combat this, the present study utilized a phthalate mixture that was derived from urinary phthalate levels in pregnant women [19–21]. However, a limitation to this study is that parent phthalates were used in the PHTmix instead of phthalate metabolites. Phthalates are rapidly metabolized to monoester metabolites and oxidative derivatives, and it is these metabolites that are thought to be bioactive and act on human tissues [16, 51]. To better increase environmental relevance and mimic human exposure to phthalates, future studies should incorporate phthalate metabolite mixtures at concentrations found in women’s follicular fluid in their in vitro toxicology studies. These are currently active areas of investigation in our laboratory with manuscripts on human and mouse ovulatory models forthcoming.
While use of parent phthalates in the PHTmix is considered a limitation to the present in vitro study, our findings still provide novel mechanistic evidence of the impact of phthalate exposure on human health. High exposure levels of phthalates, such as those during certain intravenous medical procedures, can introduce parent phthalates into the ovarian unit, and mature follicles within the ovary can metabolize parent phthalates rendering them bioactive [52–55]. Significant findings were also observed at the 1 μg/ml concentration, which includes levels of individual phthalates below the highest levels found in women’s follicular fluid [35]. At this lower concentration, the PHTmix decreased ovulatory P4 levels and the mRNA levels of PTX3, RGS2, STAR, and CYP11A1. Thus, our findings are the first, to our knowledge, to characterize the effects and to elucidate a direct mechanism by which phthalate mixture exposure inhibits ovulatory outcomes in human granulosa cells obtained from a primary source.
In order to observe if the PHTmix or the concentrations selected caused cytotoxicity, we first measured cell viability/metabolism prior to and after hCG treatment using a commercial MTS assay. At all time-points tested, exposure to the PHTmix did not alter cell viability/metabolism when compared to the DMSO group (prior to hCG administration) and the hCG alone group (after hCG administration). These results are similar to those from a previous study using the same PHTmix and concentrations, where PHTmix exposure did not cause ovarian follicle death or induce apoptosis in cultured mouse antral follicles [19]. Thus, the observed effects on P4/PGR action in this study are not due to PHTmix-induced granulosa cell death, but are rather due to toxicity downstream of hCG action.
We selected to investigate the effects of the PHTmix on ovulatory P4/PGR action because it has been widely established that P4 and PGR are critical for ovulation and fertility across species [27–29]. During the ovulatory period, LH and hCG bind to their receptor (LHCGR) on granulosa cells, which increases P4 levels via increases in steroidogenic proteins and enzymes [29, 32, 50]. Activation of LHCGR, a G-protein coupled receptor, simultaneously increases PGR levels in granulosa cells via upregulated cAMP/PKA signaling [28]. The increases in P4, PGR, and subsequent P4/PGR signaling in periovulatory granulosa cells across species, including in women, is paramount for ovulation and fertility because inhibiting P4 synthesis, blocking PGR with an antagonist, or knocking out Pgr results in complete ovulatory failure [28, 29, 33]. PGR is a nuclear transcription factor that when activated by P4 binding results in the upregulation of additional key ovulatory mediators, including ADAMTS1, CXCR4, PTX3, and RGS2 [29–32]. These P4/PGR-induced ovulatory mediators are involved in the proteolytic degradation of the follicle wall, infiltration of leukocytes to the periovulatory follicle, expansion of the cumulus oocyte complex, and continued intracellular signaling during the periovulatory period, all of which are processes required for successful ovulation [27–32]. Even knocking out several of these P4/PGR downstream mediators in mice (Adamts1, Ptx3, Rgs2) results in reduced ovulation and subfertility [56–58], thus outlining their importance in the ovulatory process.
In support of phthalate-induced disrupted P4/PGR signaling in human granulosa cells, we first observed decreases in P4 and PGR levels following exposure to the PHTmix. Decreases in P4 levels were largely attributed to the PHTmix-induced decreases in STAR and, to a lesser extent, CYP11A1 and HSD3B2. This is evident as STAR levels were decreased by each concentration of the PHTmix depending on time-point, and these decreases predominantly correspond to the time-point-dependent decreases in P4 levels. The decreases in PGR were only observed at the highest concentrations tested, and the decrease at the 500 μg/ml concentration was via a mechanism involving decreased cAMP levels and PKA activity. Interestingly, we did not observe decreases in cAMP levels and PKA activity in the 100 μg/ml group. Therefore, additional LHCGR-dependent signaling molecules, including extracellular signal-regulated kinase 1/2 (ERK1/2), p38 mitogen-activated protein kinase (p38 MAPK), protein kinase B (AKT), and protein kinase C [27, 59, 60], may be responsible for the 100 μg/ml-induced decreases in PGR levels, and this will be explored in future studies. These pathways will also be further explored at the 500 μg/ml concentration as a potential combined toxic mechanism because a slight but significant decrease in PKA activity was reported in the current study (Figure 5B).
The decreases in ligand (P4) and receptor (PGR), either individually or in combination, led to impaired ovulatory P4/PGR signaling, as demonstrated by the PHTmix-induced decreases in the mRNA levels of ADAMTS1, CXCR4, PTX3, and RGS2. Decreases in these P4/PGR downstream ovulatory mediators can potentially lead to phthalate-induced ovulatory dysfunction given their known induction and potential roles in the ovulatory process [29–32]. Decreases in these downstream mediators at the 1 and 10 μg/ml concentrations appear to be due to decreased P4 production, as PGR levels are not affected at these concentrations. Meanwhile, the combined decreases in P4 production and PGR levels at the 100 and 500 μg/ml concentrations may contribute to the disrupted P4/PGR signaling. To further elucidate the mechanism of P4/PGR signaling disruption and to identify potential therapeutic targets for phthalate toxicity, rescue experiments were conducted using a stable ligand (R5020, non-metabolizable progestin and PGR agonist) and a stable LHCGR activated downstream signaling molecule (8-Br-cAMP, cAMP is known to increase PGR levels [28]). These rescue experiments were conducted at the 500 μg/ml concentration as it consistently decreased the levels of P4, PGR, cAMP/PKA, and downstream P4/PGR ovulatory mediators.
R5020 supplementation at multiple concentrations failed to rescue the phthalate toxicity on downstream P4/PGR signaling. This was evident by the levels of RGS2 in the hCG + PHTmix group and the hCG + PHTmix + R5020 group, which were both comparable to each other and decreased relative to hCG alone. Meanwhile, R5020 supplementation did not cross-react with the P4 measurement assay and did not restore PGR levels to hCG controls. These data suggest that the PHTmix-induced decreases in P4 are not solely responsible for the disrupted P4/PGR signaling at the highest concentration, because a ligand mimicking P4 is unable to rescue phthalate toxicity. An alternative explanation is that the granulosa cells are still producing sufficient functional levels of P4 in this PHTmix group. Specifically, P4 levels are the highest at the 36-h time-point where the hCG alone group produced 750 ng/ml and the hCG + PHTmix 500 μg/ml group produced 372 ng/ml. Although this represents a 50% decrease in P4 levels (Figure 2A), perhaps sufficient P4 is still being produced to be functional, and therefore further supplementation may not be able to override the toxicity at the decreased PGR level.
In support of the hypothesis that PHTmix actions are at the level of PGR, cAMP supplementation restored PHTmix toxicity. Specifically, cAMP supplementation in the PHTmix 500 μg/ml group completely restored PGR levels beyond hCG controls and comparable to hCG + cAMP levels. Supplementation in the PHTmix group even partially restored P4 levels beyond hCG + PHTmix levels, albeit still at levels below the hCG controls. This is partially explained by the modest rescue of STAR levels in the hCG + PHTmix + cAMP group, without significant increases to the other P4 synthetic enzymes, CYP11A1 and HSD3B2. With functional levels of PGR restored, cAMP supplementation in the PHTmix group further restored the levels of ADAMTS1, CXCR4, PTX3, and RGS2 at multiple time-points beyond hCG alone levels and comparable to levels in cells treated with hCG + cAMP. While the partially restored P4 levels cannot be entirely discounted, the cAMP supplementation data, in combination with the lack of rescue in the R5020 experiments, suggest that the decreases in PGR levels are likely responsible for the impaired P4/PGR signaling by the PHTmix at the highest concentration. In addition to decreasing PGR levels, DEHP and its metabolites have been shown to directly bind to PGR, which can be an additional mechanism of disrupted PGR signaling [61].
A concerted effort has been made recently to investigate exposure to phthalate mixtures in mouse models to determine the impact on ovarian function and fertility, and several of these studies used the same PHTmix utilized in this study [5, 62]. These findings in mice suggest that phthalate mixtures disrupt folliculogenesis and antral follicle growth, follicle health, steroid hormone production, estrous cyclicity, and fertility and breeding indices in in vivo dosing and in vitro follicle culture models [19–21, 26, 63]. Several of these impacted parameters suggest that oocyte release may be a direct target of phthalate toxicity. In support of this hypothesis, our previous published study identified that the same PHTmix at the same concentrations tested in the present study directly decreased ovulation rates in mouse antral follicles in vitro via a mechanism involving alterations in P4 levels, Pgr levels, and downstream P4/PGR signaling [26].
In summary, findings from the present study demonstrate for the first time that PHTmix exposure directly impairs the crucial P4/PGR signaling pathway for ovulation in a primary human ovarian cell model known to recapitulate ovulatory outcomes [44–47]. These findings support observations in the mouse and provide a shared mechanism of phthalate-induced ovulatory dysfunction between human granulosa cells and the mouse follicles exposed to the PHTmix. Importantly, the current observations in granulosa cells from women contribute to the growing literature suggesting that exposure to phthalates may impair women’s fertility and reproductive health.
Supplementary Material
Acknowledgments
The authors thank the members of the Hannon Laboratory and Curry Laboratory for technical assistance and critical review of the manuscript. The authors thank the staff at the Bluegrass Fertility Center for assistance in providing the human granulosa cells.
Footnotes
† Grant Support: This work was supported by the National Institute of Environmental Health Sciences grants R01ES033767 (PRH), R00ES028748 (PRH), and P30ES026529 Career Development Award (PRH).
Contributor Information
Patrick R Hannon, Department of Obstetrics & Gynecology, College of Medicine, University of Kentucky, Lexington, KY, USA.
James W Akin, Bluegrass Fertility Center, Lexington, KY, USA.
Thomas E Curry Jr, Department of Obstetrics & Gynecology, College of Medicine, University of Kentucky, Lexington, KY, USA.
Conflict of Interest
The authors declare they have nothing to disclose.
Author Contributions
PRH contributed to experimental conception, design, and execution. TEC contributed intellectual assistance with experimental design. PRH drafted and edited the manuscript. PRH, JWA, and TEC reviewed and approved the final manuscript.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front Endocrinol (Lausanne) 2015; 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koniecki D, Wang R, Moody RP, Zhu J. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ Res 2011; 111:329–336. [DOI] [PubMed] [Google Scholar]
- 3. Xu Y, Cohen Hubal EA, Little JC. Predicting residential exposure to phthalate plasticizer emitted from vinyl flooring: sensitivity, uncertainty, and implications for biomonitoring. Environ Health Perspect 2010; 118:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health 2014; 13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Land KL, Miller FG, Fugate AC, Hannon PR. The effects of endocrine-disrupting chemicals on ovarian- and ovulation-related fertility outcomes. Mol Reprod Dev 2022; 89:608–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Du Y, Guo N, Wang Y, Teng X, Hua X, Deng T, Yao Y, Yuan X, Li Y. Follicular fluid concentrations of phthalate metabolites are associated with altered intrafollicular reproductive hormones in women undergoing in vitro fertilization. Fertil Steril 2019; 111:953–961. [DOI] [PubMed] [Google Scholar]
- 7. Krotz SP, Carson SA, Tomey C, Buster JE. Phthalates and bisphenol do not accumulate in human follicular fluid. J Assist Reprod Genet 2012; 29:773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health 2007; 210:623–634. [DOI] [PubMed] [Google Scholar]
- 9. Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ Health Perspect 2004; 112:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Högberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, Filipsson AF, Jansson B, Johansson N, Appelgren M, Håkansson H. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect 2008; 116:334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. James-Todd T, Stahlhut R, Meeker JD, Powell SG, Hauser R, Huang T, Rich-Edwards J. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ Health Perspect 2012; 120:1307–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hannon PR, Brannick KE, Wang W, Gupta RK, Flaws JA. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol Appl Pharmacol 2015; 284:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hannon PR, Peretz J, Flaws JA. Daily exposure to di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod 2014; 90:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Craig ZR, Hannon PR, Wang W, Ziv-Gal A, Flaws JA. Di-n-butyl phthalate disrupts the expression of genes involved in cell cycle and apoptotic pathways in mouse ovarian antral follicles. Biol Reprod 2013; 88:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hannon PR, Niermann S, Flaws JA. Acute exposure to di(2-ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicol Sci 2016; 150:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci 2009; 364:2063–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kay VR, Chambers C, Foster WG. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol 2013; 43:200–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure assessment issues in epidemiology studies of phthalates. Environ Int 2015; 85:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou C, Flaws JA. Effects of an environmentally relevant phthalate mixture on cultured mouse antral follicles. Toxicol Sci 2016; 156:kfw245–kfw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou C, Gao L, Flaws JA. Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol Appl Pharmacol 2017; 318:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou C, Gao L, Flaws JA. Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology 2017; 158:1739–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davis BJ, Maronpot RR, Heindel JJ. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol 1994; 128:216–223. [DOI] [PubMed] [Google Scholar]
- 23. Sekiguchi S, Ito S, Honma T. Experimental model to study reproductive toxicity of chemicals using induced ovulation in immature F344 rats. Ind Health 2003; 41:287–290. [DOI] [PubMed] [Google Scholar]
- 24. Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, Meeker JD, Calafat AM, Williams PL, for the EARTH Study Team . Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: results from the EARTH study. Environ Health Perspect 2016; 124:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Machtinger R, Gaskins AJ, Racowsky C, Mansur A, Adir M, Baccarelli AA, Calafat AM, Hauser R. Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environ Int 2018; 111:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Land KL, Lane ME, Fugate AC, Hannon PR. Ovulation is inhibited by an environmentally relevant phthalate mixture in mouse antral follicles in vitro. Toxicol Sci 2020; 179:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duffy DM, Ko CM, Jo M, Brannstrom M, Curry TE Jr. Ovulation: parallels with inflammatory processes. Endocr Rev 2019; 40:369–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robker RL, Akison LK, Russell DL. Control of oocyte release by progesterone receptor-regulated gene expression. Nucl Recept Signal 2009; 7:e012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim J, Bagchi IC, Bagchi MK. Control of ovulation in mice by progesterone receptor-regulated gene networks. Mol Hum Reprod 2009; 15:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci U S A 2000; 97:4689–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Choi Y, Park JY, Wilson K, Rosewell KL, Brännström M, Akin JW, Curry TE Jr, Jo M. The expression of CXCR4 is induced by the luteinizing hormone surge and mediated by progesterone receptors in human preovulatory granulosa cells. Biol Reprod 2017; 96:1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sriraman V, Sinha M, Richards JS. Progesterone receptor-induced gene expression in primary mouse granulosa cell cultures. Biol Reprod 2010; 82:402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park CJ, Lin PC, Zhou S, Barakat R, Bashir ST, Choi JM, Cacioppo JA, Oakley OR, Duffy DM, Lydon JP, Ko CMJ. Progesterone receptor serves the ovary as a trigger of ovulation and a terminator of inflammation. Cell Rep 2020; 31:107496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Collaborating Centre for, W.s. and H . Children's, National Institute for Health and Clinical Excellence: Guidance, in Fertility: Assessment and Treatment for People with Fertility Problems. London: Royal College of Obstetricians & Gynaecologists (UK) National Collaborating Centre for Women’s and Children’s Health; 2013. [PubMed] [Google Scholar]
- 35. Du YY FYL, Wang YX, Zeng Q, Guo N, Zhao H, Li YF. Follicular fluid and urinary concentrations of phthalate metabolites among infertile women and associations with in vitro fertilization parameters. Reprod Toxicol 2016; 61:142–150. [DOI] [PubMed] [Google Scholar]
- 36. Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, et al. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol 2002; 16:529–653. [DOI] [PubMed] [Google Scholar]
- 37. Weuve J, Sánchez BN, Calafat AM, Schettler T, Green RA, Hu H, Hauser R. Exposure to phthalates in neonatal intensive care unit infants: urinary concentrations of monoesters and oxidative metabolites. Environ Health Perspect 2006; 114:1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hernández-Díaz S, Mitchell AA, Kelley KE, Calafat AM, Hauser R. Medications as a potential source of exposure to phthalates in the U.S. population. Environ Health Perspect 2009; 117:185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hernandez-Diaz S, Su YC, Mitchell AA, Kelley KE, Calafat AM, Hauser R. Medications as a potential source of exposure to phthalates among women of childbearing age. Reprod Toxicol 2013; 37:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ting AY, Xu J, Stouffer RL. Differential effects of estrogen and progesterone on development of primate secondary follicles in a steroid-depleted milieu in vitro. Hum Reprod 2015; 30:1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction 2010; 140:685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soto E, Silavin SL, Tureck RW, Strauss JF 3rd. Stimulation of progesterone synthesis in luteinized human granulosa cells by human chorionic gonadotropin and 8-bromo-adenosine 3′,5′-monophosphate: the effect of low density lipoprotein. J Clin Endocrinol Metab 1984; 58:831–837. [DOI] [PubMed] [Google Scholar]
- 43. Golos TG, Miller WL, Strauss JF. Human chorionic gonadotropin and 8-bromo-adenosine 3′,5′-monophosphate stimulate [125I]low density lipoprotein uptake and metabolism by luteinized human granulosa cells in culture. J Clin Endocrinol Metab 1985; 61:633–638. [DOI] [PubMed] [Google Scholar]
- 44. Hannon PR, Duffy DM, Rosewell KL, Brännström M, Akin JW, Curry TE Jr. Ovulatory induction of SCG2 in human, nonhuman primate, and rodent granulosa cells stimulates ovarian angiogenesis. Endocrinology 2018; 159:2447–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choi Y, Wilson K, Hannon PR, Rosewell KL, Brännström M, Akin JW, Curry TE Jr, Jo M. Coordinated regulation among progesterone, prostaglandins, and EGF-like factors in human ovulatory follicles. J Clin Endocrinol Metab 2017; 102:1971–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Al-Alem L, Puttabyatappa M, Rosewell K, Brännström M, Akin J, Boldt J, Muse K, Curry TE Jr. Chemokine ligand 20: a signal for leukocyte recruitment during human ovulation? Endocrinology 2015; 156:3358–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosewell KL, al-Alem L, Zakerkish F, McCord L, Akin JW, Chaffin CL, Brännström M, Curry TE Jr. Induction of proteinases in the human preovulatory follicle of the menstrual cycle by human chorionic gonadotropin. Fertil Steril 2015; 103:826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Loutradis D, Bletsa R, Aravantinos L, Kallianidis K, Michalas S, Psychoyos A. Preovulatory effects of the progesterone antagonist mifepristone (RU486) in mice. Hum Reprod 1991; 6:1238–1240. [DOI] [PubMed] [Google Scholar]
- 49. Espey LL, Adams RF, Tanaka N, Okamura H. Effects of epostane on ovarian levels of progesterone, 17 beta-estradiol, prostaglandin E2, and prostaglandin F2 alpha during ovulation in the gonadotropin-primed immature rat. Endocrinology 1990; 127:259–263. [DOI] [PubMed] [Google Scholar]
- 50. Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 2004; 25:947–970. [DOI] [PubMed] [Google Scholar]
- 51. Wittassek M, Koch HM, Angerer J, Brüning T. Assessing exposure to phthalates—the human biomonitoring approach. Mol Nutr Food Res 2011; 55:7–31. [DOI] [PubMed] [Google Scholar]
- 52. Warner GR, Li Z, Houde ML, Atkinson CE, Meling DD, Chiang C, Flaws JA. Ovarian metabolism of an environmentally relevant phthalate mixture. Toxicol Sci 2019; 169:246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Albro PW. Absorption, metabolism, and excretion of di(2-ethylhexyl) phthalate by rats and mice. Environ Health Perspect 1986; 65:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Albro PW, Thomas RO. Enzymatic hydrolysis of di-(2-ethylhexyl) phthalate by lipases. Biochim Biophys Acta 1973; 306:380–390. [DOI] [PubMed] [Google Scholar]
- 55. Genuis SJ, Beesoon S, Lobo RA, Birkholz D. Human elimination of phthalate compounds: blood, urine, and sweat (BUS) study. Sci World J 2012; 2012:615068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol 2002; 16:1154–1167. [DOI] [PubMed] [Google Scholar]
- 57. Bernhardt ML, Lowther KM, Padilla-Banks E, McDonough C, Lee KN, Evsikov AV, Uliasz TF, Chidiac P, Williams CJ, Mehlmann LM. Regulator of G-protein signaling 2 (RGS2) suppresses premature calcium release in mouse eggs. Development 2015; 142:2633–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, Hertzog PJ, Pritchard MA. Adamts-1 is essential for the development and function of the urogenital system. Biol Reprod 2004; 70:1096–1105. [DOI] [PubMed] [Google Scholar]
- 59. Choi Y, Jeon H, Akin JW, Curry TE Jr, Jo M. The FOS/AP-1 regulates metabolic changes and cholesterol synthesis in human periovulatory granulosa cells. Endocrinology 2021; 162:bqab127. 10.1210/endocr/bqab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Richards JS, Ascoli M. Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trends Endocrinol Metab 2018; 29:313–325. [DOI] [PubMed] [Google Scholar]
- 61. Sheikh IA, Abu-Elmagd M, Turki RF, Damanhouri GA, Beg MA, al-Qahtani M. Endocrine disruption: in silico perspectives of interactions of di-(2-ethylhexyl)phthalate and its five major metabolites with progesterone receptor. BMC Struct Biol 2016; 16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fletcher EJ, Santacruz-Márquez R, Mourikes V, Neff A, Laws M, Flaws J. Effects of phthalate mixtures on ovarian folliculogenesis and steroidogenesis. Toxics 2022; 10:251. 10.3390/toxics10050251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Meling DD, Warner GR, Szumski JR, Gao L, Gonsioroski AV, Rattan S, Flaws JA. The effects of a phthalate metabolite mixture on antral follicle growth and sex steroid synthesis in mice. Toxicol Appl Pharmacol 2020; 388:114875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.