Abstract
Nicotinamide adenine dinucleotide (NAD+) level has been associated with various age‐related diseases and its pharmacological modulation emerges as a potential approach for aging intervention. But human NAD+ landscape exhibits large heterogeneity. The lack of rapid, low‐cost assays limits the establishment of whole‐blood NAD+ baseline and the development of personalized therapies, especially for those with poor responses towards conventional NAD+ supplementations. Here, we developed an automated NAD+ analyzer for the rapid measurement of NAD+ with 5 μL of capillary blood using recombinant bioluminescent sensor protein and automated optical reader. The minimal invasiveness of the assay allowed a frequent and decentralized mapping of real‐world NAD+ dynamics. We showed that aerobic sport and NMN supplementation increased whole‐blood NAD+ and that male on average has higher NAD+ than female before the age of 50. We further revealed the long‐term stability of human NAD+ baseline over 100 days and identified major real‐world NAD+‐modulating behaviors.
Keywords: aging, NAD+ , nicotinamide mononucleotide, point‐of care
To facilitate the mapping of heterogeneous NAD+ landscape in human, we developed an automated NAD+ analyzer using bioluminescent sensor proteins for quantifying whole blood NAD+ levels from 5 μL of capillary blood. The comparison with LC–MS for measuring clinical samples established the analyzer as a reliable quantification method. The minimally invasive assay mapped the real‐world NAD+ dynamics associated with gender and age and revealed the real‐world NAD+‐modulating behaviors.
Abbreviations
- BRET
Bioluminescence resonance energy transfer
- ESI
Electrospray ionization
- E. coli
Escherichia coli
- HRR
Heart rate reserve
- HPLC
High‐performance liquid chromatography
- IPTG
Isopropyl β‐D‐thiogalactopyranoside
- LOD
Limit of detection
- LOQ
Limit of quantification
- LC‐MS
Liquid chromatography‐mass spectrometry
- NA
Niacin
- NAM
Nicotinamide
- NAD+
Nicotinamide adenine dinucleotide
- NMN
Nicotinamide mononucleotide
- NR
Nicotinamide riboside
- NMR
Nuclear magnetic resonance
- PBMCs
Peripheral blood mononuclear cells
- PPC
Phosphatidylcholine
- PARPs
Poly(ADP‐ribose) polymerase
- RFP
Red fluorescent protein
1. INTRODUCTION
NAD+ is an essential substrate for numerous redox reactions and regulatory proteins including poly(ADP‐ribose) polymerase (PARPs), Sirtuins, CD38/157 and SARMs, which play important roles in DNA repair, protein deacetylation, immune response, and other fundamental cellular processes (Chini et al., 2021; Covarrubias et al., 2021; Imai & Guarente, 2014; Katsyuba et al., 2020; Rajman, Chwalek, & Sinclair, 2018). In blood, NAD+ is enriched in erythrocytes compared to serum and white blood cells (Chaleckis et al., 2014; Chaleckis, Murakami, Takada, Kondoh, & Yanagida, 2016; Clement, Wong, Poljak, Sachdev, & Braidy, 2019). The decline of NAD+ content in blood cells (Chaleckis et al., 2016), muscle (Zhang et al., 2016), and saliva (Teruya, Goga, & Yanagida, 2021) has been associated with aging and age‐related symptoms such as frailty (Covarrubias et al., 2021; Kumar et al., 2014), rheumatoid arthritis (Busso et al., 2008; Weiqian, Caihong, & Jin, 2018), and heart failure (Carpenter & Dierickx, 2022; Yuan et al., 2022) at old age. In addition, oral administration of NAD+ precursors as a potential intervention strategy of age‐related symptoms has been demonstrated in clinical trials to improve body NAD+ levels (Chini et al., 2018; Imai et al., 2000; Khan et al., 2014; Pirinen et al., 2020; Rajman et al., 2018; Yoshino, Baur, & Imai, 2018), muscle functions, and skeletal muscle insulin sensitivity and signaling (Goody & Henry, 2018; Yoshino et al., 2021), establishing NAD+ level as an emerging indicator of healthy aging.
However, as considerable disparity exists in both the geno‐ and phenotype of human aging, the blood NAD+ landscapes of different people and aging processes are yet to be fully characterized (Balashova et al., 2022; Yang et al., 2022). One major limitation for mapping the NAD+ landscape at the point of care is its quantification method. Conventional NAD+ measurement relies on High‐Performance Liquid Chromatography (HPLC) (Klaidman, Leung, & Adams, 1995; Yoshino & Imai, 2013), Liquid Chromatography‐Mass Spectrometry (LC–MS) (Bustamante et al., 2017; Giner et al., 2021; Trammell & Brenner, 2013), enzyme cycling assays (Graeff & Lee, 2002) or Nuclear Magnetic Resonance (NMR) (de Graaf & Behar, 2014; Lu, Zhu, & Chen, 2016; Shabalin et al., 2018), which requires centralized laboratories and multi‐step sample preparations. Recently developed semisynthetic bioluminescent sensors offered a solution for measuring NAD+ at point‐of‐care (Sallin et al., 2018; Yu et al., 2019). But this sensor relies on a synthetic chemical tether that requires multi‐step synthesis with relatively low yield, limiting its mass production. Hence, more cost‐effective and easy‐to‐produce protein sensors should make the assay much more available and affordable for the large‐scale, real‐world mapping of NAD+ dynamics.
Here we developed an NAD+ assay using an Escherichia coli (E.coli)‐produced (Chen et al., 2023), fully genetically encoded NAD+ sensor and a simple, automated desk‐top reader for measuring NAD+ levels in venous and capillary blood, as well as in saliva with 5 μL of the sample. We used this assay in a placebo‐controlled, double‐blind clinical trial to assess the effect of NMN and aerobic sport. We showed that oral NMN supplementation and aerobic sport can significantly increase whole‐blood NAD+ levels. In addition, the high‐level agreement between the sensor and HPLC–MS established the sensor as a viable NAD+ quantification method. The assay further mapped the NAD+ levels in capillary blood and revealed a marked NAD+ disparity across gender and age. Moreover, the sensor made long‐term and frequent NAD+ monitoring easily available due to the minimal invasiveness of the capillary sampling, with which we mapped the speculated NAD+ circadian rhythm, short‐term pharmacodynamics of oral NMN administration, and the long‐term real‐world NAD+ baseline in human.
2. RESULTS
2.1. Measuring blood NAD + using recombinant bioluminescent sensor protein
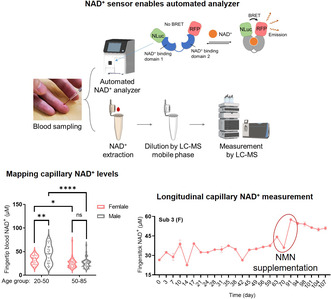
For achieving low‐cost and rapid NAD+ measurement in clinical samples, we developed an NAD+ assay using a simple, automated reader and a fully genetically encoded bioluminescent NAD+ sensor protein named NS‐Goji 1.3. The recombinantly expressed sensor contains an engineered NAD+‐binding domain, a circularly permuted luciferase cpNLuc and a red fluorescent protein (RFP) mScarlet‐I (Figure 1a). NS‐Goji 1.3 undergoes considerable conformational changes upon NAD+ binding and shortens the distance between cpNLuc and mScarlet‐I, thus affecting the Bioluminescence Resonance Energy Transfer (BRET) efficiency between the pair (Figure 1b). The highly specific NAD+‐dependent shift of the sensor's emission intensity at 460 nm and 580 nm is used for quantifying the NAD+ levels in the samples (Figure 1c, Figure S1). To automatize the assay, we developed a reader that integrates a single‐channel pipette robot, a photon‐detector, and a reagent‐containing test strip. As the E. coli expression system makes the sensor production cost‐effective and scalable, the NAD+ sensor is easily available for large‐scale sample analysis. In parallel, an HPLC–MS quantification procedure for NAD+ has been developed as the reference method in which NAD+ was identified by ion pair of 662.0/539.7 and 80.0 m/z and quantified by the intensity of ion 662.0 m/z (Figure 1d–f).
FIGURE 1.
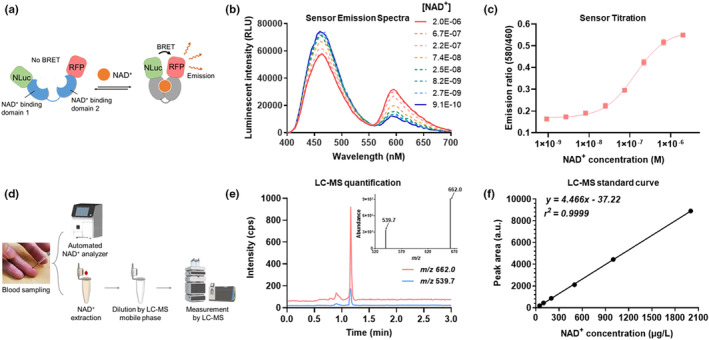
Quantification of whole blood NAD+ by sensor and LC‐MS. (a) Sensing mechanism of the recombinant bioluminescent NAD+ sensor protein NS‐Goji 1.3: The sensor changes conformation as a function of NAD+ concentration, leading to changes in the BRET efficiency between luciferase NLuc and RFP m‐Scarlet I. (b) Sensor's emission spectra at various NAD+ concentrations. (c) Ratios between sensor's emission intensities at 580 nm and 460 nm plotted against various NAD+ concentrations. Values are given as mean ± SD of three independent measurements. (d) Sample preparation procedure for NAD+ analyzer and HPLC‐MS quantification, partially created with BioRender.com. (e) HPLC‐MS retention time and ionization intensity of ions pairs for NAD+ identification and quantification. (f) Calibration curve for NAD+ measurement by HPLC‐MS.
2.2. Aerobic sports and oral NMN significantly increase venous NAD +
Using the analyzer, we quantified the NAD+ levels in venous blood obtained from clinical studies to evaluate the effect of nicotinamide mononucleotide (NMN) supplementation on subjects between 55 and 70 years of age (Table S1). The double‐blinded clinical test compared one placebo group and two treatment groups using different dosages of NMN (500 mg/day and 1000 mg/day) (Figure 2a, Figure S2). After 30 days of daily oral administration, venous blood was collected for assessing whole‐blood NAD+ content using both the sensor and HPLC‐MS. In parallel, whole‐blood NMN and 2/4‐PY were also analyzed by HPLC‐MS. Whole blood samples were measured because NAD+ predominantly exists in blood cells instead of serum or plasma (Clement et al., 2019; Seyedsadjadi et al., 2018).
FIGURE 2.
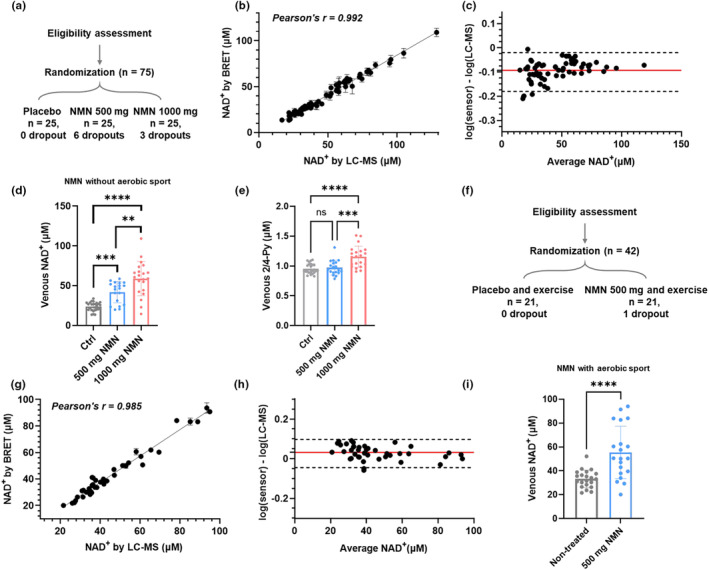
Measurement of venous NAD+ levels in clinical studies of NMN supplementation and aerobic sport. (a) Study design for evaluating effects of oral NMN supplementation. (b) Comparison of NAD+ levels measured by sensor protein and HPLC‐MS. (c) Bland–Altman analysis for venous NAD+ measured by sensor and HPLC‐MS. (d) Effect of oral NMN supplementation on venous NAD+ level. Daily supplementation of 500 mg (n = 19) and 1000 mg NMN (n = 22) for 1 month significantly increased the venous NAD+ concentration compared to the placebo group (n = 25). (e) Venous 2/4‐Py concentrations measured from blood samples with daily supplementation of placebo (n = 25), 500 mg (n = 19) and 1000 mg NMN (n = 22) for 1 month. (f) Study design for evaluating effects of oral NMN supplementation in combination with moderate level of sport. (g) Comparison of NAD+ levels measured by sensor protein and HPLC‐MS. (h) Bland–Altman analysis for venous NAD+ measured by sensor and HPLC‐MS. (i) Effect of aerobic sport with oral NMN supplementation (n = 20) and aerobic sport alone (n = 21) on venous NAD+ level. In (b) and (g), values are given as mean ± SD of three independent measurements. In (d), (e) and (i), error bars represent SD of the respective group. Significance was determined using one‐way ANOVA analysis for (d) and (e), and t‐test for (i), * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
The comparison between NS‐Goji 1.3 and HPLC‐MS demonstrated a good agreement between the two methods with Pearson's r = 0.992 (Figure 2b,c). The measurement further revealed that both dosages of NMN significantly increased the venous NAD+ in a dose‐dependent manner. The recorded average whole blood NAD+ level was 23.8 ± 5.5 μM in the placebo group, 41.7 ± 13.0 μM in the 500 mg/day group, and 58.8 ± 21.1 μM in the 1000 mg/day group (Figure 2d). Notably, the variation of NAD+ level in the placebo group is smaller compared to that of the two treatment groups. The group treated with 1000 mg/day oral NMN featured a wide distribution of the whole blood NAD+ levels (CV = 35.9%), indicating the possible non‐responses towards the NMN administration. We then performed bulk RNA sequencing for the peripheral blood mononuclear cells (PBMCs) of four responders and non‐responders from the 1000 mg/day group and mapped the expression levels of genes involved in NAD+ metabolism (Figure S3). Responders featured a higher expression of NAD+‐synthesizing enzymes such as IDO2, NAPRT, NMNAT2 and NAMPT, as well as a nucleoside transporter SLC29A3; while non‐responders have more expressions of NAD+‐consuming enzymes such as SIRT4, PARP14, CD38 and PARP2 etc. Interestingly, PAPR9, which regulates anti‐viral activities and type I interferon production (Luo et al., 2021; Xing et al., 2021), was highly expressed in the PBMCs of NMN‐responders. We further showed that the whole blood 2/4‐PY level increased significantly for the group administrated 1000 mg/day NMN (Figure 2e) and that whole blood NAD+ correlated well with whole blood 2/4‐PY but not with NMN (Figure S4), indicating an up‐regulated NAD+ catabolism and nicotinamide elimination. Subsequently, we evaluated the effect of aerobic sports on the venous NAD+ levels of the placebo‐ and NMN‐treated (500 mg/day) subjects for 30 days (Figure 2f, Figure S5, Table S2). NS‐Goji 1.3 achieved a similar level of quantification compared to HPLC‐MS for measuring venous NAD+ with Pearson's r = 0.985 (Figure 2g,h). The group with aerobic sport and placebo featured an averaged whole blood NAD+ level of 33.18 ± 7.2 μM, while the group with aerobic sport and NMN showed an significantly increased whole blood NAD+ level of 55.48 ± 21.4 μM (Figure 2i).
In addition to measuring NAD+ in blood, we explored the possibility of NAD+ measurement in saliva. Recent work for identifying salivary aging biomarkers revealed that salivary NAD+ decreases with age (Teruya et al., 2021). We hence tested if the bioluminescent sensor could quantify NAD+ in salivary samples. The analysis performed in parallel with HPLC‐MS demonstrated that the bioluminescent sensor is capable of testing salivary NAD+ with Pearson's r = 0.999 (Figure S6).
Overall, we showed that NS‐Goji 1.3 sensor and the automated analyzer are viable tools for scalable and low‐cost measurements of NAD+. The cost comparison between bioluminescent sensor, NAD+/NADH assay kit and HPLC‐MS is summarized in Table S3. The clinical study further demonstrated that regular aerobic sports enhanced the NAD+ in both placebo‐ and NMN‐treated subjects.
2.3. Fingerstick blood NAD + survey reveals gender‐associated differences in NAD + levels
For conventional NAD+ quantifications, venipuncture limits the frequency of NAD+ sampling due to invasiveness. By contrast, collecting fingerstick capillary blood by disposable lancet (Figure 3a) would largely facilitate the dissemination of NAD+ measurement, which is now made possible by the bioluminescent sensor. To evaluate if fingertip blood is a representative sample source for quantifying NAD+, we compared the venous and fingerstick capillary blood obtained in parallel from 13 volunteers within 30 min. The NAD+ measured from the two sample types showed a good correlation (Pearson's r = 0.987) and are within ± 15% error (Figure 3b and Figure S7). In this test, NS‐Goji 1.3 measurement typically requires 5 μL of fingertip blood, while by contrast, a single venipuncture collection requires several milliliters of sample.
FIGURE 3.
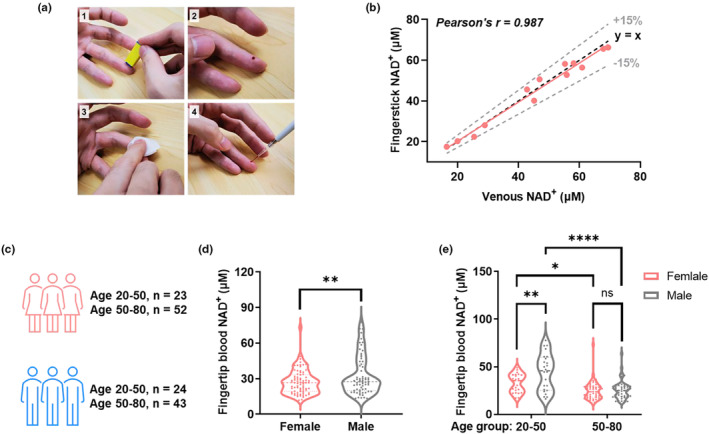
Fingertip blood NAD+ measurement for mapping NAD+ disparity. (a) Procedures of taking fingerstick capillary blood for NAD+ measurement. (b) Comparison between NAD+ levels measured from fingertip and venous blood. The Black dashed line indicates y = x, and the gray dashed line indicates ±15% deviation from y = x. (c) Study design for mapping fingertip blood NAD+ levels for healthy volunteers with different age and gender. (d) Female participants (n = 75) showed a significantly lower NAD+ level than males (n = 67) in this study. (e) 50–85 age group has significantly lower NAD+ levels than 18–50 age group for both male and female. The male has higher NAD+ level than female for 18–50 age group but not for the 50–85 age group. Significance was determined using t‐test for (d) and two‐way ANOVA analysis for (e), * p < 0.05, ** p < 0.01, **** p < 0.0001.
Using this fingerstick blood NAD+ assay, we further mapped the whole blood NAD+ levels of NAD+ supplement‐naïve people including 75 females and 67 males across different age groups (Figure 3c and Table S4). The cross‐gender comparison indicated that females have on average a lower whole blood NAD+ level (27.2 ± 10.3 μM) compared to males (32.5 ± 16.3 μM, Figure 3d). The survey further revealed that participants between 20 and 50 years of age have significantly higher NAD+ content than those aged between 50 and 85 for both genders (Figure 3e). Notably, the age‐related NAD+ decline is more severe for males with the average NAD+ level decreased from 44.2 ± 18.9 μM to 25.9 ± 9.8 μM (p < 0.0001). While for females, the NAD+ content is decreased by a lesser extent from 32.7 ± 9.6 μM to 24.8 ± 9.6 μM with p < 0.05. Moreover, the gender‐related NAD+ disparity is more pronounced for participants younger than 50 years of age, but not significant for those aged over 50 (Figure 3e, Table S4). Given that NAD+ is enriched in blood cells, the gender differences in the whole‐blood NAD+ level could be partially due to the different numbers and compositions of blood cells associated with sex. Interestingly, a few participants have particularly high NAD+ levels for the respective age group, indicating the existence of outliers whose NAD+ metabolism is markedly different. Further studies to identify the genetic and/or acquired factors for this high whole‐blood NAD+ level should provide more insight for developing new NAD+ modulating strategies.
2.4. Measuring real‐world NAD + dynamics using minimal capillary blood samples
NAD+ metabolism plays a central role in circadian rhythm. However, the hypothetical human NAD+ differences between day and night have not been recorded due to the difficulties of collecting venous blood after midnight. As fingertip blood assay is easily applicable at the bedside, we collected and measured the capillary blood NAD+ at 4 am and 10 am from n = 7 subjects (Figure 4a). The measurement showed that the capillary NAD+ levels did not change significantly between 4 am at 10 am (from 36.0 ± 11.0 μM to 38.70 ± 11.1 μM, with p = 0.0735, Figure 4b). To evaluate the potential fluctuations of whole blood NAD+ during the day, we further accessed the capillary NAD+ levels after oral administration of 300 mg of NMN during the day starting from 10 am. The NAD+ level significantly increased 60 min after the NMN administration and returned quickly to the basal level at 120 min (Figure 4c). Apart from the short NMN‐induced perturbation, no significant changes were observed during the day, demonstrating the fast metabolism of NMN and the strong homeostasis of the NAD+ level. Circadian rhythm is regulated by NAD+‐related pathways such as extra‐ and intracellular NAMPT, as well as by other attributes such as energy metabolism and obesity (Park et al., 2023). To capture the subtle diurnal NAD+ changes, measurement in separated blood components such PBMCs, erythrocytes and plasma would be necessary as cells with and without nucleus may be regulated differently. More stringent selection criteria would also be of interest to include subjects with similar metabolic profiles.
FIGURE 4.
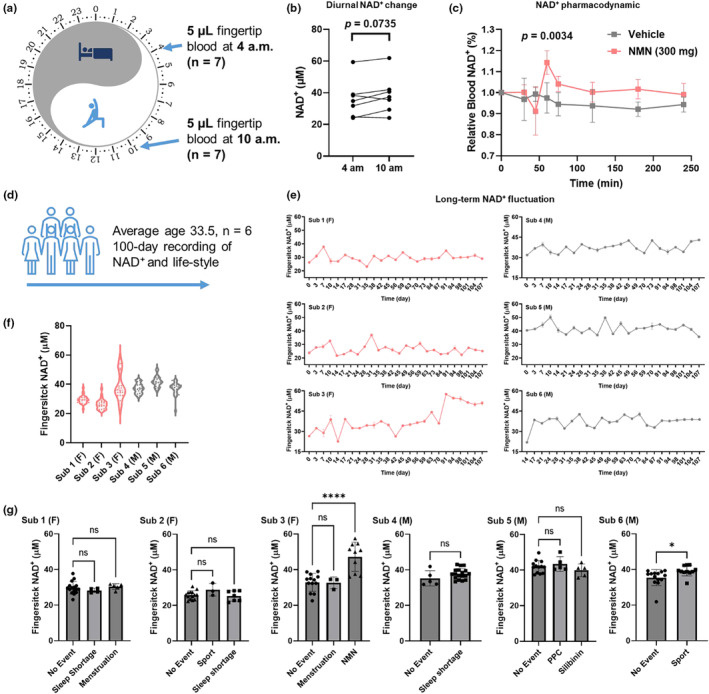
Monitoring real‐world NAD+ dynamics using capillary blood. (a) Study design for comparing capillary NAD+ levels at 4 and 10 am. (b) No significant difference in NAD+ level was observed for capillary blood samples obtained at 4 and 10 am. (n = 7, p = 0.0735), though the average NAD+ level increased from 36.00 μM to 38.69 μM. (c) Oral administration of 300 mg NMN induced short‐term capillary NAD+ spike with significant increases recorded at 60 min (n = 5, p = 0.0034). (d) Scheme for the long‐term monitoring of NAD+ levels. Events including sport, sleep shortage, medication, menstruation, and dietary supplementation were recorded through questionnaire. (e) NAD+ dynamics recorded for n = 6 participants over three months. F and M indicates female and male subjects respectively. (f) Distribution of fingerstick NAD+ levels during the long‐term monitoring. (g) Effects of events on NAD+ levels for each subject. NMN and sport significantly increased NAD+ levels for sub 3 and 6 respectively. Only days with single recorded event were considered, while days with more than one recorded event were excluded from the analysis. Significance was determined using t‐test for (c) and sub 4 & 6 in (g). Two‐way ANOVA analysis was used for significance determination for sub 1, 2, 3 and 5 in (g), * p < 0.05, **** p < 0.0001.
To further map the NAD+ fluctuations in the real world over long terms, we measured the NAD+ levels of n = 6 subjects twice a week over 100 days and recorded major changes in dietary, sportive, and sleeping habits (Figure 4d). The result showed that NAD+ levels were mostly stable during the 100‐day monitoring (Figure 4e,f), except for one participant who spontaneously started NMN administration which induced a notable increase in the capillary NAD+ level (from 32.9 μM to 47.3 μM with p < 0.0001). One subject exercised regularly (11 out of 25 days on which NAD+ was measured), and the recorded NAD+ levels on days with sport were significantly higher than days without. However, the sport‐induced NAD+ increase was not observed for the subject with a low sport frequency (3 out of 24 days of NAD+ measurement). Other recorded events such as sleep deprivation, menstruation, oral administration of phosphatidylcholine (PPC) and silibinin did not significantly affect the capillary NAD+ level (Figure 4g).
Taken together, the capillary blood NAD+ measurement revealed significant NAD+ disparity across gender and age. The sensor also facilitated the real‐world observation of NAD+ modulation by dietary supplements and aerobic sport. We further demonstrated that individual NAD+ levels are relatively stable over a long period of time and continuous oral NMN administration and frequent physical exercise can boost NAD+ levels. This easy‐to‐implement NAD+ test for blood and saliva provided a useful tool for monitoring the effect of various NAD+ modulating strategies at the point‐of‐care.
3. DISCUSSION
Whole‐blood NAD+ level is becoming an important physiological parameter for evaluating NAD+ boosting strategies. Several previously reported clinical studies of NAD+ supplementation established that blood NAD+ content decreases with the progression of age‐related diseases such as mitochondrial myopathy and rheumatoid arthritis (Lautrup et al., 2019; Weiqian et al., 2018). In addition, previous studies showed that oral supplementation of Niacin (NA) (Pirinen et al., 2020), nicotinamide riboside (NR) (Airhart et al., 2017; Conze et al., 2019); Vreones et al., 2023 or NMN (Okabe et al., 2022; Yoshino et al., 2021) can increase the blood NAD+ level and lead to beneficial outcomes. However, the conventional quantification based on HPLC‐MS or enzymatic cycling assays are time‐ and labor‐intensive due to a relatively low automation level, hence limiting the up‐scale and generalization of the NAD+ blood test. Here, we demonstrate that the low‐cost and recombinantly expressed bioluminescent NAD+ sensor protein enables easy‐to‐implement assays for analyzing microliters of clinical samples with a good coherence compared to HPLC‐MS.
The two doses (500 mg and 1000 mg per day) of oral NMN supplementation evaluated in this work can induce a significant increase in blood NAD+ level. However, considerable variation existed for the group supplemented with 1000 mg NMN per day, indicating the heterogeneity of NMN responses among the subjects. Recent work on the NAD+ flux indicated an age‐related increase of CD38 and the resulting NAD+ consumption, contributing to the NAD+ decline during aging (McReynolds et al., 2021). Here, the RNA sequencing of PBMCs from NMN non‐responders indeed indicated a higher expression of NAD+ consuming enzymes including CD38 and PARPs. The existence of the non‐responders may be due to the hyper‐activated NAD+ consumption pathways that degrade NMN and NAD+ as substrates. More detailed studies would be necessary to better characterize these subgroups and to enhance the response rate of NAD+ supplementation. To better achieve supplementation outcomes for the non‐responders, NAD+ precursors and modulators of its consumption pathways may be used in combination, and the convenient NAD+ blood monitoring can help to evaluate the effect of the combo therapies. Using the NAD+ sensor, we also demonstrated that aerobic sports increased the NAD+ basal level from 23.8 ± 5.5 μM to 33.18 ± 7.2 μM among the participants, providing precursor‐independent alternatives for NAD+ modulation.
As considerable disparity exists during human aging, mapping, and characterizing various subgroups of the aging phenotype is key for achieving personalized aging intervention. However, collecting large numbers of clinical samples has been challenging for mapping the NAD+ metabolism among different populations. Here, the bioluminescent sensor enabled an easy‐to‐implement assay for quantifying NAD+ using fingertip blood at a much‐reduced cost compared to HPLC‐MS. The NAD+ survey revealed a gender‐related NAD+ disparity which is especially pronounced before the age of 50. The survey also identified NAD+ supplement‐naïve individuals with particularly high NAD+ levels compared to their peers. As this assay is minimally invasive and cost‐effective, future surveys at larger scales covering different geographic and ethnic groups may identify more naturally occurring cases with high NAD+ levels. Further studies to elucidate the underlying mechanisms should provide new exploitable strategies for NAD+ modulation and aging intervention.
Furthermore, we established that human NAD+ is generally stable over long periods of time, meanwhile NMN administration can induce notable spikes in NAD+ and may shift the original NAD+ homeostasis. The elevated NAD+ level can be maintained by the NMN administration, but a safe and effective NAD+ window induced by such intervention is yet to be determined. In addition, we observed that regular aerobic sport readily increases whole‐blood NAD+ levels though by a lesser extent than the NMN administration. Despite that no short‐term adverse events were observed in neither our study nor other reported clinical studies of NMN administration (Irie et al., 2020; Okabe et al., 2022; Pencina et al., 2023; Yi et al., 2023), there was a clear increase of nicotinamide catabolites including 2/4‐PY, a uremic toxin, and methylnicotinamide (Irie et al., 2020; Okabe et al., 2022), indicating a potential overload of nicotinamide after high doses of NMN supplementation. The long‐term downstream effects of these catabolites should be better characterized to avoid potential toxicity (Poljšak et al., 2022; Rajman et al., 2018) as 2/4‐PY were reported to have inhibitory effects on key enzymes such as PARP‐1 (Rutkowski et al., 2003). Hence, establishing therapeutic windows for NAD+ supplementation should be important for the long‐term management of aging‐related symptoms.
Several limitations should be considered in our study. First, we recruited 76 females and 67 males for assessing the age‐related NAD+ disparity. Even though the sample size is sufficient for evaluating the age‐related decline of NAD+, having more participants would be beneficial for detecting the potential NAD+ differences between more refined age groups. As NAD+ is blood cell‐enriched, additional assays for blood cell compositions and counts would help to interpret the age‐ and gender‐associated difference in the whole‐blood NAD+ level. Furthermore, NAD+ quantification in different blood cell types would provide a more comprehensive mapping of the NAD+ metabolism associated with age, gender, and diurnal metabolic fluctuations. Second, our longitudinal analysis showed that aerobic sport and NMN increases NAD+ for the tested individuals, but more data points and larger sample sizes will help to identify other NAD+‐modulating behaviors and establish new sportive or dietary regimes to enhance NAD+ during physiological aging. The analysis excluded the data points with more than one event recorded on the same day and did not analyze the combined effects of multiple events on the NAD+ level due to the limited data set. Combining NAD+ levels with more comprehensive and continuous data obtained by health‐monitoring wearables such as smartwatches will provide deeper insights into how different daily behaviors and habits can influence NAD+ metabolism and provide new approaches for its active modulation. Third, the NAD+ survey indicated the existence of individuals with notably high NAD+ levels. However, we could not yet characterize the genetic or metabolic features of the individuals within the scope of this study. But the cost‐effective NAD+ survey will enable the mapping of NAD+ at larger scales and lead to future investigations into the underlying mechanisms.
Overall, we demonstrated a low‐cost and easy‐to‐use assay using recombinant sensor protein and an automated optical reader for measuring fingertip blood NAD+ and revealed how parameters such as NAD+ supplementation, aerobic sport, gender, age, circadian rhythm etc., may contribute to the NAD+ disparity amongst people. The widely observed differences in human aging underline the necessity of developing easily available, minimally invasive, and low‐cost sensing tools for measuring aging biomarkers to identify meaningful subgroups of the aging phenotype and to empower personalized aging interventions through the quantitative monitoring of NAD+‐modulating regimes.
4. METHODS
4.1. Clinical subjects and samples
Study protocol for evaluating the effect of oral NMN supplementation and aerobic sports on human subjects have been reviewed and approved by the ethics committee of Guangzhou Sport University (ethics approval number: 2020 LCLL‐009, Registry Number ChiCTR2000040222) in accordance with the Declaration of Helsinki. First, the study evaluates how different doses of NMN affect the whole blood NAD+ level. Then, the study compares the effect of aerobic sports with and without NMN supplementation on the whole blood NAD+. The study recruited participants between 55 and 70 years of age. The exclusion criteria are (1) having hepatic and/or renal dysfunction, infectious diseases, cardiac dysfunction, and/or spinal dysfunction, (2) having chest pain or shortness of breath during sport, (3) having more than 3% changes of body weight within 2 months before the study, (4) having previously taken NA, NMN, nicotinamide (NAM), or other vitamins B3‐related dietary supplements, (5) taking coffee or caffeine‐containing drinks, (6) during pregnancy or lactation, (7) being mentally unfit to participate in the study. For the aerobic sport intervention, subjects participated in centralized aerobic sport for 40–60 min, three times per week. For the first 2 weeks, the aerobic sport intensity was maintained between 40% to 59% of the heart rate reserve (HRR), then aerobic sport intensity was increased to 60% to 85% of the HRR. In addition, subjects participated in resistance training three times per week. For the dietary supplementation, subjects were grouped and tested in a double‐blinded, placebo‐controlled manner with three groups taking the placebo, 500 mg/day NMN, and 1000 mg/day NMN. The randomization was performed by the random number table method. The intervention of NMN administration is double blinded for both the subjects and investigators. The placebo used was mannitol powder contained in opaque capsules with the same weight and appearance. The administration of NMN was performed and registered between 9 and10 am. The venous blood samples were collected one day after the last NMN administration between 8 and 10 am without breakfast via phlebotomy and were collected in plastic blood collection tubes with EDTA as anticoagulant for analysis. The study sets the primary outcome as whole blood NAD+ measurement and secondary outcome as lifestyle assessment scale SF‐36, the result of which is not revealed in this work due to scope reasons. The sample size per group was determined to be 16 with significance level = 0.05, power = 0.8, treatment group = 4, Mean under H0 = 25, Standard deviation = 5, minimal detectable effect = 4, superiority margin = 0.1. Considering the potential dropout, no less than 20 subjects were recruited for each group. The dropout and their reasons were indicated in the CONSORT flow chat.
The study protocol for evaluating fingertip blood NAD+ levels of human subjects have been reviewed and approved by the Institutional Review Board of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences (ethics approval number: SIAT‐IRB‐210915‐H0575) in accordance with the Declaration of Helsinki. Fingerstick sampling was performed to collect 5 μL of capillary blood using disposable fingerstick lancets (STERiLANCE Medical Inc.) for NAD+ measurement. The blood sampling time, administration of medication and/or dietary supplements, aerobic sport, sleep quality and menstruation have been recorded for data analysis with written consent of participants. The exclusion criteria are (1) being younger than 18 years of age, and (2) having hemophobia or other symptoms hindering the fingerstick blood sampling.
4.2. Sensor preparation
The gene of the sensor NS‐Goji 1.3 was constructed in a pET51b (+) vector for recombinant expression in E. coli (DE3). The E. coli (DE3) was cultured 1 L LB medium containing 50 μg/mL ampicillin at 37°C with a shaking rate of 220 rpm to reach an optical density of 0.6–0.8 at 600 nm. Then 0.5 mM isopropyl β‐D‐thiogalactopyranoside (IPTG) was added into the medium to induce sensor expression at 16°C for 16 h. The E. coli (DE3) was collected by centrifugation at 8000 rpm for 15 min and the resulting pellet was suspended in 25 mM Tris–HCl buffer containing 500 mM NaCl and 20 mM imidazole at pH 8.0 for lysis using high‐pressure homogenizer at 4°C. The lysate was centrifuged at 13,000 g for 30 min at 4°C and the resulting supernatant was filtrated by 0.22 μm syringe filters for purification by Ni‐NTA (Smart Lifesciences Lnc., China) and Strep‐Tactin (Smart Lifesciences Lnc., China) columns. The purified protein was desalted and exchanged into 50 mM HEPES buffer containing 50 mM NaCl (pH 7.2) with an Amicon Ultra‐15 centrifugal filter (10 kDa MWCO, Merk Millipore Inc.). Protein concentration was measured by Bradford assay. The obtained protein solution was added into an equal volume of glycerol for long‐term storage at −80°C.
4.3. Blood sample measurement by NAD + sensor
Phlebotomy was performed to collect venous blood samples in plastic blood collection tubes with EDTA as anticoagulant. Fingerstick sampling was performed to collect capillary blood samples using disposable fingerstick lancets (STERiLANCE Medical Inc.). The blood samples were processed by the analyzer's automated sample handling program in which 5 μL of sample were lysed by mixing thoroughly with 4 volumes of 0.5 N perchloric acid. The lysate was then diluted by 10‐fold in a buffer containing 500 mM HEPES, 500 mM NaCl at pH 7.2. 10 μL of the resulting solution was then added into 90 μL of measurement buffer containing 0.5 nM sensor, 100‐fold diluted furimazine, 50 mM HEPES and 50 mM NaCl at pH 7.2, which was previously lyophilized and automatically reconstituted in the analyzer's mixing well by the liquid handler. The sensor's bioluminescent signal was measured using the integrated photon‐detector (Hamamatsu Photonics) of the analyzer with NLuc emission measured at 460 nm (bandwidth 30 nm) and RFP emission was measured at 580 nm (bandwidth 20 nm). The sensors' emission ratio (R) between 580 nm and 460 nm was used to calculate the NAD+ concentrations according to the titration curve.
To titrate the senor, standard aqueous NAD+ solutions with known concentrations were prepared and measured by the above‐mentioned method. The sensors' emission ratio (R) was plotted against the NAD+ concentrations and the resulting curve was fitted to the Hill‐Langmuir equation to obtain the sensor's maximum ratio (Rmax), minimum ratio (Rmin), c50 and Hill coefficient (h)
NAD + concentrations in unknown samples were calculated with the rearranged the equation:
4.4. Blood sample measurement by HPLC–MS
5 μL of whole blood was taken from blood collection tubes and added into 20 μL of 0.5 N perchloric acid with thorough mixing. Cell debris and aggregates were separated by centrifugation at 12000 g for 5 min at 4°C. The supernatant was transferred into sample tubes and stored at −80°C until analysis. Upon analysis, the supernatant was diluted by 10‐fold in aqueous solution with 50% methanol and 50% water and then filtered with a PTFE syringe filter (0.45 μm * 13 mm) before injection into LC–MS.
Agilent Infinity Lab LC/MSD iQ G6160A was used to perform the LC–MS quantification of the blood samples. 10 μL of each prepared sample were injected into LC and separated by a ZORBAX SB‐Aq Column (Agilent, 4.6 × 100 mm, 3.5 μm). The autosampler temperature was set to 4°C and the column temperature was set to 20°C. The following elution program was used: A = aqueous 0.1% formic acid‐water, B = acetonitrile, 0 min 20% A, 0.5 min 20% A, 1.2 min 60% A, 2.5 min 60% A, 2.6 min 20% A, 3 min 20% A, with total run time of 3.0 min and a flow rate of 1.00 mL/min. NAD+, 2/4‐PY and NMN featured a retention time of 1.09 min, 1.15 min and 1.06 min respectively. For MS quantification, MS was equipped with electrospray ionization (ESI) ion source and operates in both positive and negative ion mode. Samples were ionized under high‐flow conditions with curtain gas = 35 psi, collision gas = “high”, cataclastic voltage = 100 V, capillary voltage = 3500 V, and dry temperature = 325°C. The following ion pairs were used for analyte identification: NAD+1: 662 → 539.7 m/z; NAD+2: 662 → 80 m/z; 2/4‐PY1: 152.9 → 136 m/z; 2/4‐PY2: 152.9 → 108 m/z; NMN1: 335 → 122.8 m/z; NMN2: 335 → 97 m/z. Standard solutions with known concentrations of NAD+ (0.07537, 0.1507, 0.3015, 0.7537, 1.507 μmol/L), 2/4‐PY (0.3286, 0.6572, 1.314, 3.286, 6.572 μmol/L), and NMN (0.1496, 0.2992, 0.5984, 1.496, 2.992 μmol/L) have been prepared and automatically injected with single injections for LC–MS analysis to optimize the quantification conditions. A linear regression was then applied (weighting 1/x, rNAD+ = 0.9998, r2/4‐PY = 0.9964, rNMN = 0.9994) to obtain the respective standard curves. The limit of detection (LOD) of NAD+, 2/4‐PY and NMN was 0.0301 μmol/L, 0.131 μmol/L and 0.0598 μmol/L (Signal/Noise ≥3) and the limit of quantification (LOQ) was 0.0754 μmol/L, 0.329 μmol/L, 0.150 μmol/L (Signal/Noise ≥10). All calculation was performed with Agilent OpenLab Data Analysis 2.205.7.2 software.
4.5. Determination of recovery rate and sample stability
100 μL of solution was taken from lysed blood samples with known NAD+ concentrations. The samples were then added with 100, 400, and 700 μg/L standard NAD+. The samples were further diluted in aqueous solution with 50% methanol and 50% water for six parallel LC–MS measurements.
Blood samples lysed with 0.5 N perchloric acid were stored at room temperature and at 4°C for 0 h, 1 h, 2 h, 4 h, 6 h, 12 h, 24 h, and 48 h before LC‐MS measurement using the above‐mentioned method (Figure S8).
4.6. Salivary sample measurement
Salivary samples were collected in 10 mL conical tubes in the morning before breakfast after at least 8 h of fasting, which were then added with various concentrations of NAD+ solutions to prepare spiked salivary samples. The resulting samples were loaded into the NAD+ analyzer and were automatically added with 4 volumes of 0.5 N perchloric acid. The analyzer then dilutes the resulting mixture by 10‐fold in a buffer containing 500 mM HEPES, 500 mM NaCl at pH 7.2, and transfers 10 μL of the resulting solution into 90 μL of measurement buffer containing 0.5 nM sensor, 100‐fold diluted furimazine, 50 mM HEPES, 50 mM NaCl at pH 7.2 for bioluminescent measurement. The sensor's bioluminescent signal was measured using the analyzer's integrated photon‐detector (Hamamatsu Photonics) with NLuc emission measured at 460 nm (bandwidth 30 nm) and RFP emission was measured at 580 nm (bandwidth 20 nm). The sensors' emission ratio (R) between 580 nm and 460 nm was used to calculate the NAD+ concentrations according to the titration curve performed in parallel. HPLC‐MS measurement of the supernatant was performed in parallel according to the above‐mentioned method as reference.
4.7. RNA sequencing of PBMCs
Around 10 mL of intravenous blood was collected in EDTA tubes at 10 am from four subjects with the highest and lowest whole blood NAD+ levels after 1000 mg/day NMN supplementation. The PBMCs layer was collected after centrifugation with Ficoll media at 400 g for 30 min and then washed twice with PBS at 300 g for 10 min at 25°C. The pellet was resuspended with 5 volumes of ACK lysis buffer to lyse red blood cells and then centrifuged at 500 g for 5 min. The PBMCs were then resuspended in RPM1640 medium containing 10% FBS for cell counting and centrifuged and flash‐frozen for RNA extraction and sequencing.
4.8. Statistics
All values were expressed as mean ± standard deviation. Statistics were performed using Prism 8 (Graphpad). All quantification tests were independently performed for at least three times. Data were represented as the mean ± SD. Correlations between two variables were tested with Pearson correlation coefficient. Levels of agreement were evaluated using Bland–Altman analysis. Significance was determined using t‐test, one‐way ANOVA analysis, or two‐way ANOVA analysis. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 throughout the manuscript.
AUTHOR CONTRIBUTIONS
Q.Y., M.H., and L.C. conceived the study. L.C. and P.W. prepared the sensor. P.W., L.C., and Y.H. performed sensor qualifications. M.C. performed HPLC–MS quantifications. M.H. and J.L. provided clinical samples. R.L. contributed to the sample collection. All authors contributed to data analysis or manuscript writing.
CONFLICT OF INTEREST STATEMENT
All authors declare no competing interests.
Supporting information
Data S1.
ACKNOWLEDGMENTS
This work is supported by National Key R&D Program of China (2021YFF1200302, 2020YFC2002904), National Natural Science Foundation of China (22207118), China Postdoctoral Science Foundation (2022 M713282), Research Projects on Key Areas of General Colleges and Universities, Department of Education of Guangdong Province (2022ZDZX2071), R&D plan in key areas of Guangdong Province (2023B1111030002), Shenzhen Science and Technology Program (JCYJ20220818100804009, ZDSYS20210623091810032), Natural Science Foundation of Guangdong Province (2023A1515010715), Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, and Celfull Hebe Fund for the Biological Study of Aging.
Wang, P. , Chen, M. , Hou, Y. , Luan, J. , Liu, R. , Chen, L. , Hu, M. , & Yu, Q. (2023). Fingerstick blood assay maps real‐world NAD + disparity across gender and age. Aging Cell, 22, e13965. 10.1111/acel.13965
Pei Wang, Meiting Chen and Yaying Hou are authors contributed equally.
Qiuliyang Yu: Lead contact
Contributor Information
Liuqing Chen, Email: lq.chen@siat.ac.cn.
Min Hu, Email: whoomin@aliyun.com.
Qiuliyang Yu, Email: q.yu@siat.ac.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
References
- Airhart, S. E. , Shireman, L. M. , Risler, L. J. , Anderson, G. D. , Nagana Gowda, G. A. , Raftery, D. , Tian, R. , Shen, D. D. , & O'Brien, K. D. (2017). An open‐label, non‐randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One, 12(12), e0186459. 10.1371/journal.pone.0186459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashova, N. V. , Zavileyskiy, L. G. , Artiukhov, A. V. , Shaposhnikov, L. A. , Sidorova, O. P. , Tishkov, V. I. , Tramonti, A. , Pomentun, A. A. , & Bunik, V. I. (2022). Efficient assay and marker significance of NAD+ in human blood. Frontiers in Medicine, 9, 886485. 10.3389/fmed.2022.886485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso, N. , Karababa, M. , Nobile, M. , Rolaz, A. , Gool, F. V. , Galli, M. , Leo, O. , So, A. , & Smedt, T. D. (2008). Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS One, 3(5), e2267. 10.1371/journal.pone.0002267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante, S. , Jayasena, T. , Richani, D. , Gilchrist, R. B. , Wu, L. E. , Sinclair, D. A. , Sachdev, P. S. , & Braidy, N. (2017). Quantifying the cellular NAD+ metabolome using a tandem liquid chromatography mass spectrometry approach. Metabolomics, 14(1), 15. 10.1007/s11306-017-1310-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, B. J. , & Dierickx, P. (2022). Circadian cardiac NAD+ metabolism, from transcriptional regulation to healthy aging. American journal of physiology‐cell physiology, 324(4), C1168–C1176. 10.1152/ajpcell.00239.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaleckis, R. , Ebe, M. , Pluskal, T. , Murakami, I. , Kondoh, H. , & Yanagida, M. (2014). Unexpected similarities between the schizosaccharomyces and human blood metabolomes, and novel human metabolites. Molecular BioSystems, 10(10), 2538–2551. 10.1039/C4MB00346B [DOI] [PubMed] [Google Scholar]
- Chaleckis, R. , Murakami, I. , Takada, J. , Kondoh, H. , & Yanagida, M. (2016). Individual variability in human blood metabolites identifies age‐related differences. Proceedings of the National Academy of Sciences of the United States of America, 113(16), 4252–4259. 10.1073/pnas.1603023113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Chen, M. , Luo, M. , Li, Y. , Liao, B. , Hu, M. , & Yu, Q. (2023). Ratiometric NAD+ sensors reveal subcellular NAD+ modulators. ACS Sensors., 8, 1518–1528. 10.1021/acssensors.2c02565 [DOI] [PubMed] [Google Scholar]
- Chini, C. C. S. , Zeidler, J. D. , Kashyap, S. , Warner, G. , & Chini, E. N. (2021). Evolving concepts in NAD+ metabolism. Cell Metabolism, 33(6), 1076–1087. 10.1016/j.cmet.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini, E. N. , Chini, C. C. S. , Netto, J. M. E. , de Oliveira, G. C. , & van Schooten, W. (2018). The pharmacology of CD38/NADase: An emerging target in cancer and diseases of aging. Trends in Pharmacological Sciences, 39(4), 424–436. 10.1016/j.tips.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement, J. , Wong, M. , Poljak, A. , Sachdev, P. , & Braidy, N. (2019). The plasma NAD+ metabolome is dysregulated in “Normal” aging. Rejuvenation Research, 22(2), 121–130. 10.1089/rej.2018.2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conze, D. , Brenner, C. , & Kruger, C. L. (2019). Safety and metabolism of long‐term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double‐blind, placebo‐controlled clinical trial of healthy overweight adults. Scientific Reports, 9(1), 9772. 10.1038/s41598-019-46120-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias, A. J. , Perrone, R. , Grozio, A. , & Verdin, E. (2021). NAD+ metabolism and its roles in cellular processes during ageing. Nature Reviews Molecular Cell Biology, 22(2), 119–141. 10.1038/s41580-020-00313-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf, R. A. , & Behar, K. L. (2014). Detection of cerebral NAD+ by in vivo 1H NMR spectroscopy. NMR in Biomedicine, 27(7), 802–809. 10.1002/nbm.3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giner, M. P. , Christen, S. , Bartova, S. , Makarov, M. V. , Migaud, M. E. , Canto, C. , & Moco, S. (2021). A method to monitor the NAD+ metabolome—from mechanistic to clinical applications. International Journal of Molecular Sciences, 22(19), 10598. 10.3390/ijms221910598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody, M. F. , & Henry, C. A. (2018). A need for NAD+ in muscle development, homeostasis, and aging. Skeletal Muscle, 8(1), 9. 10.1186/s13395-018-0154-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff, R. , & Lee, H. C. (2002). A novel cycling assay for cellular cADP‐ribose with nanomolar sensitivity. Biochemical Journal, 361(2), 379–384. 10.1042/bj3610379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, S. , Armstrong, C. M. , Kaeberlein, M. , & Guarente, L. (2000). Transcriptional silencing and longevity protein Sir2 is an NAD‐dependent histone deacetylase. Nature, 403(6771), 795–800. 10.1038/35001622 [DOI] [PubMed] [Google Scholar]
- Imai, S. , & Guarente, L. (2014). NAD+ and sirtuins in aging and disease. Trends in Cell Biology, 24(8), 464–471. 10.1016/j.tcb.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie, J. , Inagaki, E. , Fujita, M. , Nakaya, H. , Mitsuishi, M. , Yamaguchi, S. , Yamashita, K. , Shigaki, S. , Ono, T. , Yukioka, H. , Okano, H. , Nabeshima, Y. I. , Imai, S. I. , Yasui, M. , Tsubota, K. , & Itoh, H. (2020). Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocrine Journal, 67(2), 153–160. 10.1507/endocrj.EJ19-0313 [DOI] [PubMed] [Google Scholar]
- Katsyuba, E. , Romani, M. , Hofer, D. , & Auwerx, J. (2020). NAD+ homeostasis in health and disease. Nature Metabolism, 2(1), 9–31. 10.1038/s42255-019-0161-5 [DOI] [PubMed] [Google Scholar]
- Khan, N. A. , Auranen, M. , Paetau, I. , Pirinen, E. , Euro, L. , Forsström, S. , Pasila, L. , Velagapudi, V. , Carroll, C. J. , Auwerx, J. , & Suomalainen, A. (2014). Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Molecular Medicine, 6(6), 721–731. 10.1002/emmm.201403943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaidman, L. K. , Leung, A. C. , & Adams, J. D. (1995). High‐performance liquid chromatography analysis of oxidized and reduced pyridine dinucleotides in specific brain regions. Analytical Biochemistry, 228(2), 312–317. 10.1006/abio.1995.1356 [DOI] [PubMed] [Google Scholar]
- Kumar, R. , Mohan, N. , Upadhyay, A. D. , Singh, A. P. , Sahu, V. , Dwivedi, S. , Dey, A. B. , & Dey, S. (2014). Identification of serum sirtuins as novel noninvasive protein markers for frailty. Aging Cell, 13(6), 975–980. 10.1111/acel.12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautrup, S. , Sinclair, D. A. , Mattson, M. P. , & Fang, E. F. (2019). NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metabolism, 30(4), 630–655. 10.1016/j.cmet.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, M. , Zhu, X.‐H. , & Chen, W. (2016). In vivo 31P MRS assessment of intracellular NAD metabolites and NAD+/NADH redox state in human brain at 4 T. NMR in Biomedicine, 29(7), 1010–1017. 10.1002/nbm.3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, X. , Peng, Y. , Chen, Y.‐Y. , Wang, A.‐Q. , Deng, C.‐W. , Peng, L.‐Y. , Wu, Q. J. , Zhao, Y. , Fei, Y. Y. , & Zhang, W. (2021). Genome‐wide DNA methylation patterns in monocytes derived from patients with primary Sjogren syndrome. Chinese Medical Journal, 134(11), 1310–1316. 10.1097/CM9.0000000000001451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds, M. R. , Chellappa, K. , Chiles, E. , Jankowski, C. , Shen, Y. , Chen, L. , Descamps, H. C. , Mukherjee, S. , Bhat, R. Y. , Lingala, S. R. , Chu, Q. , Botolin, P. , Hayat, F. , Doke, T. , Susztak, K. , Thaiss, C. A. , Lu, W. , Migaud, M. E. , Su, X. , … Baur, J. A. (2021). NAD+ flux is maintained in aged mice despite lower tissue concentrations. Cell Systems, 12(12), 1160–1172.e4. 10.1016/j.cels.2021.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe, K. , Yaku, K. , Uchida, Y. , Fukamizu, Y. , Sato, T. , Sakurai, T. , Tobe, K. , & Nakagawa, T. (2022). Oral administration of nicotinamide mononucleotide is safe and efficiently increases blood nicotinamide adenine dinucleotide levels in healthy subjects. Frontiers in Nutrition, 9, 868640. 10.3389/fnut.2022.868640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. W. , Roh, E. , Kang, G. M. , Gil, S. Y. , Kim, H. K. , Lee, C. H. , … Kim, M.‐S. (2023). Circulating blood eNAMPT drives the circadian rhythms in locomotor activity and energy expenditure. Nature Communications, 14(1), 1994. 10.1038/s41467-023-37517-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencina, K. M. , Lavu, S. , Dos Santos, M. , Beleva, Y. M. , Cheng, M. , Livingston, D. , & Bhasin, S. (2023). MIB‐626, an Oral formulation of a microcrystalline unique polymorph of β‐nicotinamide mononucleotide, increases circulating nicotinamide adenine dinucleotide and its metabolome in middle‐aged and older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 78(1), 90–96. 10.1093/gerona/glac049 [DOI] [PubMed] [Google Scholar]
- Pirinen, E. , Auranen, M. , Khan, N. A. , Brilhante, V. , Urho, N. , Pessia, A. , … Suomalainen, A. (2020). Niacin cures systemic NAD+ deficiency and improves muscle performance in adult‐onset mitochondrial myopathy. Cell Metabolism, 31(6), 1078–1090.e5. 10.1016/j.cmet.2020.04.008 [DOI] [PubMed] [Google Scholar]
- Poljšak, B. , Kovač, V. , & Milisav, I. (2022). Current uncertainties and future challenges regarding NAD+ boosting strategies. Antioxidants, 11(9), 1637. 10.3390/antiox11091637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajman, L. , Chwalek, K. , & Sinclair, D. A. (2018). Therapeutic potential of NAD‐boosting molecules: The In vivo evidence. Cell Metabolism, 27(3), 529–547. 10.1016/j.cmet.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski, B. , Slominska, E. , Szolkiewicz, M. , Smolenski, R. T. , Striley, C. , Rutkowski, P. , & Swierczynski, J. (2003). N‐methyl‐2‐pyridone‐5‐carboxamide: A novel uremic toxin? Kidney International, 63, S19–S21. 10.1046/j.1523-1755.63.s84.36.x [DOI] [PubMed] [Google Scholar]
- Sallin, O. , Reymond, L. , Gondrand, C. , Raith, F. , Koch, B. , & Johnsson, K. (2018). Semisynthetic biosensors for mapping cellular concentrations of nicotinamide adenine dinucleotides. eLife, 7, e32638. 10.7554/eLife.32638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedsadjadi, N. , Berg, J. , Bilgin, A. A. , Braidy, N. , Salonikas, C. , & Grant, R. (2018). High protein intake is associated with low plasma NAD+ levels in a healthy human cohort. PLoS One, 13(8), e0201968. 10.1371/journal.pone.0201968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalin, K. , Nerinovski, K. , Yakimov, A. , Kulikova, V. , Svetlova, M. , Solovjeva, L. , Khodorkovskiy, M. , Gambaryan, S. , Cunningham, R. , Migaud, M. E. , Ziegler, M. , & Nikiforov, A. (2018). NAD metabolome analysis in human cells using 1H NMR spectroscopy. International Journal of Molecular Sciences, 19(12), 3906. 10.3390/ijms19123906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruya, T. , Goga, H. , & Yanagida, M. (2021). Human age‐declined saliva metabolic markers determined by LC–MS. Scientific Reports, 11(1), 18135. 10.1038/s41598-021-97623-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trammell, S. A. , & Brenner, C. (2013). Targeted, lcms‐based metabolomics for quantitative measurement of Nad+ metabolites. Computational and Structural Biotechnology Journal, 4(5), e201301012. 10.5936/csbj.201301012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreones, M. , Mustapic, M. , Moaddel, R. , Pucha, K. A. , Lovett, J. , Seals, D. R. , Kapogiannis, D. , & Martens, C. R. (2023). Oral nicotinamide riboside raises NAD+ and lowers biomarkers of neurodegenerative pathology in plasma extracellular vesicles enriched for neuronal origin. Aging Cell, 22), e13754. 10.1111/acel.13754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiqian, C. , Caihong, Y. , & Jin, L. (2018). The role of nicotinamide adenine dinucleotide in the pathogenesis of rheumatoid arthritis: Potential implications for treatment. European Medical Journal, 3(3), 90–97. https://emj.emg‐health.com/rheumatology/article/the‐role‐of‐nicotinamide‐adenine‐dinucleotide‐in‐the‐pathogenesis‐of‐rheumatoid‐arthritis‐potential‐implications‐for‐treatment/ [Google Scholar]
- Xing, J. , Zhang, A. , Du, Y. , Fang, M. , Minze, L. J. , Liu, Y.‐J. , Li, X. C. , & Zhang, Z. (2021). Identification of poly(ADP‐ribose) polymerase 9 (PARP9) as a noncanonical sensor for RNA virus in dendritic cells. Nature Communications, 12(1), 2681. 10.1038/s41467-021-23003-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F. , Deng, X. , Yu, Y. , Luo, L. , Chen, X. , Zheng, J. , Qiu, Y. , Xiao, F. , Xie, X. , Zhao, Y. , Guo, J. , Hu, F. , Zhang, X. , Ju, Z. , & Zhou, Y. (2022). Association of Human Whole Blood NAD+ contents with aging. Frontiers in Endocrinology, 13, 829658. 10.3389/fendo.2022.829658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, L. , Maier, A. B. , Tao, R. , Lin, Z. , Vaidya, A. , Pendse, S. , Thamsa, S. , Andhalkar, N. , Avhad, G. , & Kumbhar, V. (2023). The efficacy and safety of β‐nicotinamide mononucleotide (NMN) supplementation in healthy middle‐aged adults: A randomized, multicenter, double‐blind, placebo‐controlled, parallel‐group, dose‐dependent clinical trial. GeroScience, 45(1), 29–43. 10.1007/s11357-022-00705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino, J. , Baur, J. A. , & Imai, S. (2018). NAD+ intermediates: The biology and therapeutic potential of NMN and NR. Cell Metabolism, 27(3), 513–528. 10.1016/j.cmet.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino, J. , & Imai, S. (2013). Accurate measurement of nicotinamide adenine dinucleotide (NAD+) with high‐performance liquid chromatography. In Hirschey M. D. (Ed.), Sirtuins: Methods and protocols (pp. 203–215). Humana Press. 10.1007/978-1-62703-637-5_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino, M. , Yoshino, J. , Kayser, B. D. , Patti, G. J. , Franczyk, M. P. , Mills, K. F. , Sindelar, M. , Pietka, T. , Patterson, B. W. , Imai, S. I. , & Klein, S. (2021). Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science, 372(6547), 1224–1229. 10.1126/science.abe9985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Q. , Pourmandi, N. , Xue, L. , Gondrand, C. , Fabritz, S. , Bardy, D. , Patiny, L. , Katsyuba, E. , Auwerx, J. , & Johnsson, K. (2019). A biosensor for measuring NAD+ levels at the point of care. Nature Metabolism, 1(12), 1219–1225. 10.1038/s42255-019-0151-7 [DOI] [PubMed] [Google Scholar]
- Yuan, Y. , Liang, B. , Liu, X.‐L. , Liu, W.‐J. , Huang, B.‐H. , Yang, S.‐B. , Gao, Y. Z. , Meng, J. S. , Li, M. J. , Ye, T. , Wang, C. Z. , Hu, X. K. , & Xing, D.‐M. (2022). Targeting NAD+: Is it a common strategy to delay heart aging? Cell Death Discovery, 8(1), 230. 10.1038/s41420-022-01031-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Ryu, D. , Wu, Y. , Gariani, K. , Wang, X. , Luan, P. , D Amico, D. , Ropelle, E. R. , Lutolf, M. P. , Aebersold, R. , Schoonjans, K. , Menzies, K. J. , & Auwerx, J. (2016). NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. (World)., 352, 1436–1443. 10.1126/science.aaf2693 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


