Abstract
Diet is an important determinant of health and consequently is often implicated in the development of disease, particularly gastrointestinal (GI) diseases, given the high prevalence of meal-related symptoms. The mechanisms underlying diet-driven pathophysiology are not well understood, but recent studies suggest that gut microbiota may mediate the effect of diet on GI physiology. In this review, we focus primarily on two distinct GI diseases where the role of diet has been best studied: irritable bowel syndrome and inflammatory bowel disease. We discuss how the concurrent and sequential utilization of dietary nutrients by the host and gut microbiota determines the eventual bioactive metabolite profiles in the gut and the biological effect of these metabolites on GI physiology. We highlight several concepts that can be gleaned from these findings, such as how distinct effects of an individual metabolite can influence diverse GI diseases, the effect of similar dietary interventions on multiple disease states, and the need for extensive phenotyping and data collection to help make personalized diet recommendations.
Keywords: gut–brain axis, microbiota, inflammation, metabolomics, fermentation
1. INTRODUCTION
Diet is an important determinant of an individual’s state of health. The role of diet as a driver of gastrointestinal (GI) symptoms is well understood in conditions such as malabsorption (e.g., lactose intolerance) and specific immune-mediated disorders (e.g., celiac disease, eosinophilic esophagitis, and food allergy). However, people with these disorders constitute a small part of the population who present with food-related symptoms. In a recent survey, nearly 52% of respondents reported meal-related abdominal pain, with 11% acknowledging that more than half of the abdominal pain episodes were directly related to diet (28). Interestingly, these participants were more likely to have disorders Of The gut–brain axis (DGBAs) such as irritable bowel syndrome (IBS) (28). The mechanism(s) underlying food-related symptoms in most of these individuals remains poorly understood. As a result, dietary interventions often entail empiric restriction of various food groups.
The recent recognition of the gut microbiome as an important determinant of GI diseases has prompted investigations of the microbiome as a pivotal link between diet and host physiology. This seems logical, as both the host and the gut microbiome rely on diet for nutrition. Furthermore, both diet and the gut microbiome have been implicated in GI diseases ranging from functional disorders to inflammatory and neoplastic states. In this review, we focus the two best-studied conditions in the context of diet–microbiota interactions: IBS, the prototypical DGBA, and inflammatory bowel disease (IBD), a chronic inflammatory condition of the GI tract.
2. DIET-DERIVED BIOACTIVE METABOLITES ARE DETERMINED BY HOST–MICROBIAL COMETABOLISM AND THE CHEMICAL COMPOSITION OF THE DIET
The conventional wisdom is that most of the digestible components of food are rapidly assimilated via the large absorptive surface of the small intestine. The remaining indigestible components pass distally, where they serve as an energy source for the gut microbiota, leading to the generation of fermentation end products such as short-chain fatty acids (SCFAs). This concept, however, oversimplifies the process. Aside from its dependency on indigestible dietary components, the gut microbiota can derive nutrition from glycoproteins and polysaccharides in the mucus layer lining the host epithelial surfaces, especially in states of deprivation of microbiota-accessible carbohydrates, such as with low fiber intake. The utilization of available nutrients by individual members of the gut microbiota follows their nutritional hierarchy, along with cross-feeding patterns determined by the differential metabolic capacities of individual microbes. The cross-feeding patterns result in cooperative and competitive microbial networks that play an important role in determining the structure of the microbial community in the gut.
Nutrient utilization is not a matter of simple stoichiometry, whereby the total amount of a nutrient gets compartmentalized for the microbes and the host, but rather is a result of the meticulous amalgamation of their respective metabolic machineries. An alteration in microbial community structure can change the dynamics of such metabolic cooperation between the gut microbiome and the host. For instance, during homeostasis, efficient host uptake of amino acids in the small intestine makes them scarce for microbes; however, an overgrowth of amino acid–utilizing bacteria (e.g., Clostridia) can compete with the host, especially if dietary protein is limited. At the same time, increased availability of nutrients that are higher in the nutritional hierarchy (often mono- and disaccharides) reduces the utilization of amino acids by the same bacteria. In addition to bioavailability, regulatory signaling, such as by SCFAs or peptide YY, can affect the use of specific nutrients by the host. Therefore, metabolic end products often cannot be accurately inferred from the composition of a microbial community. In the following subsections, we highlight two examples (tryptophan and dietary fiber) of how differential nutrient utilization by the host and gut microbiota can affect host physiology.
2.1. Tryptophan-Derived Metabolites Differ in Host and Microbial Metabolism
Tryptophan, an essential amino acid, is the precursor of the host neurotransmitter serotonin (5-HT; an important regulator of GI physiology) (Figure 1), as well as of microbial metabolites such as tryptamine and indole derivatives. The overall tryptophan pool depends largely on diet, with a small contribution from gut bacteria (120). Tryptophan is incorporated into proteins and utilized by the host to produce 5-HT (1–2%) and kynurenine (~95%) via distinct pathways (77) (Figure 1). Gut microbial members such as Ruminococcus gnavus and Clostridium sporogenes harbor tryptophan decarboxylase to convert tryptophan to tryptamine, which acts as an agonist of serotonin receptor 4 (5-HT4R) (149) (Figure 1). At the same time, bacteria such as Bacteroides fragilis and Escherichia coli harbor tryptophanase, which facilitates the production of indole and indole derivatives from tryptophan (88). Indole and its downstream products, such as indole acetic acid and indole propionic acid, can exert biological effects on host immune pathways by activating aryl hydrocarbon receptor (AHR) (82) (Figure 1). The levels of these bacterially produced tryptophan-derived bioactive metabolites depend on the gut microbiota composition, the extent and location of tryptophan utilization by gut bacteria, and the activity of genes involved in host tryptophan utilization.
Figure 1.
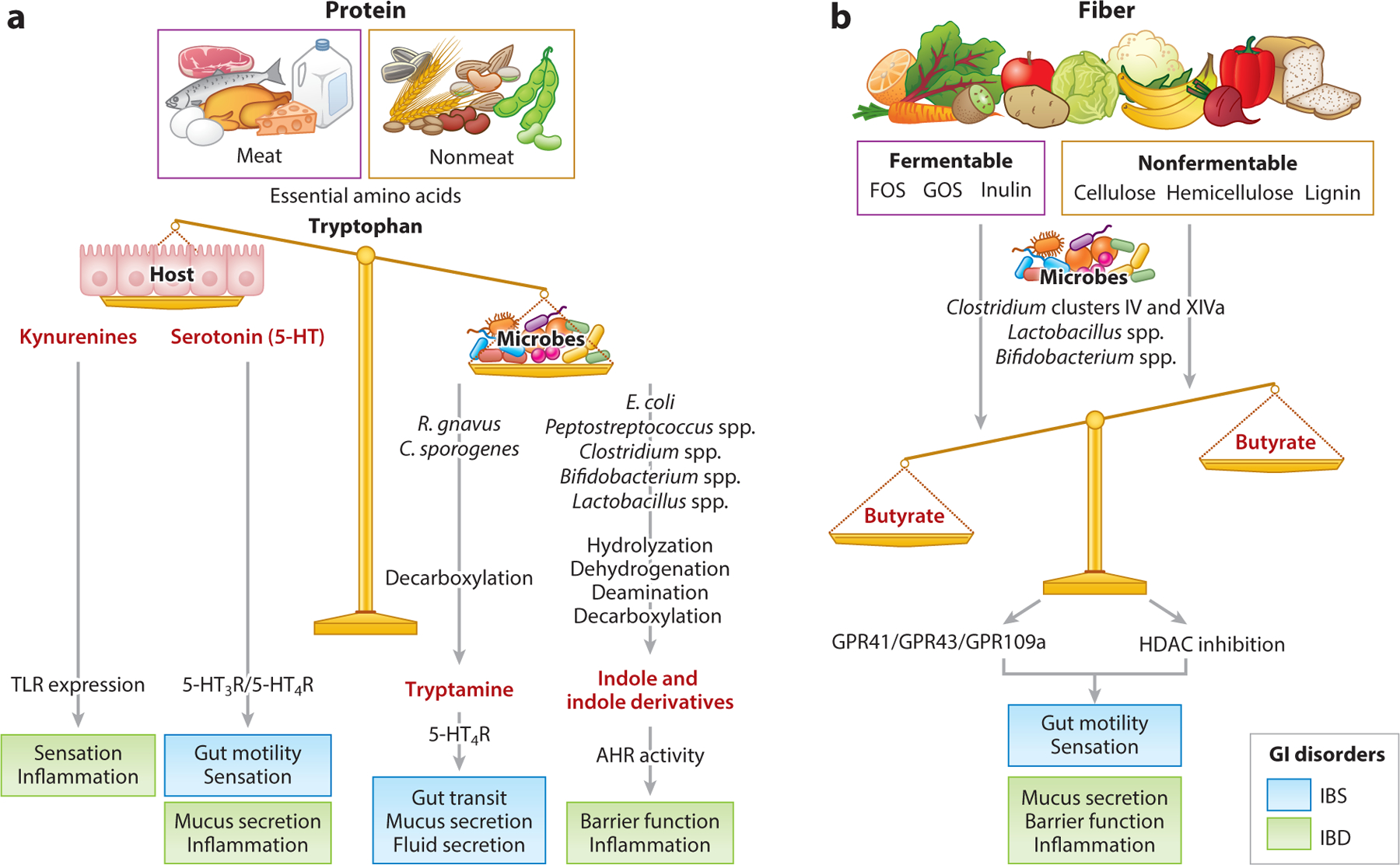
Diet-derived metabolites (red text) can alter multiple biologic pathways underlying diverse gastrointestinal (GI) diseases. (a) Dietary protein, both meat and nonmeat (e.g., grains, seeds, and nuts), includes varying levels of amino acids, such as tryptophan. Tryptophan can be metabolized by the host to produce kynurenines and serotonin (5-HT), which can alter gut physiology through the modulation of Toll-like receptors (TLRs) and serotonin receptors (5-HTRs), respectively. A small amount of tryptophan typically enters the colon, where it is synthesized by gut microbes. Gut microbiota can convert tryptophan to tryptamine or indole and indole derivatives via different metabolic pathways. Tryptamine increases intestinal secretion and mucus release from goblet cells by activating serotonin receptor 4 (5-HT4R), while indole and indole derivatives are ligands for aryl hydrocarbon receptor (AHR) and play an important role in regulating barrier function and immune responses. (b) Dietary fiber includes both fermentable [e.g., fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), and inulin] and nonfermentable (e.g., cellulose, hemicellulose, and lignin) fiber. These are fermented into different short-chain fatty acids, such as butyrate and acetate, on the basis of the type of gut bacteria and the type of fiber. Butyrate acts on epithelial G protein–coupled receptors (GPCRs, e.g., GPR41, GPR43, and GPR109a) and as an epigenetic regulator by inhibiting histone deacetylase (HDAC) activity. Butyrate can increase serotonin synthesis, increase colonic contractility, alleviate visceral hypersensitivity, and augment the barrier. Physiological outcomes associated with irritable bowel syndrome (IBS) are shown in blue, and those associated with inflammatory bowel disease (IBD) are shown in green.
2.2. Fermentation End Products Differ in Composition of Fiber and Gut Microbiota
A fiber-rich diet is considered beneficial because the gut microbiota ferments fiber to produce SCFAs such as butyrate, acetate, and propionate, which affect important aspects of host physiology including metabolism, cell turnover, and the immune system (145). However, human studies show significant interindividual variability in responses to fiber intake as well as differences based on fiber type. This is not surprising, as fiber is an umbrella term that includes diverse groups of carbohydrates with distinct linkages and molecular structures. Individual bacteria harbor genes allowing them to utilize carbohydrates with specific linkages and structures. The biological effects of dietary fiber will depend on the composition of the fiber, the potential of an individual’s gut microbiota to metabolize specific fibers, and the relative amounts of different fermentation end products (86, 117). A recent study (6) found that fructo-oligosaccharide (FOS) can worsen inflammation in patients with IBD, while its metabolism by gut bacteria reduces its inflammatory effects in IBD patients with active inflammation. Interestingly, FOS had an anti-inflammatory effect in healthy individuals. Thus, the inflammatory potential of FOS depends on gut microbial composition as well as on host disease status (6). The levels and types of SCFAs produced can vary according to the composition of fiber and the gut microbiota. The addition of inulin to the diet increased butyrate but decreased acetate production (86). In contrast, fecal butyrate levels were lower in subgroups of patients consuming the same amount of fiber, attributable to lower levels of butyrate-producing bacteria (Figure 1). These observations help explain the interindividual variability in responses to fiber observed in human studies.
2.3. Diet-Derived Metabolites Can Have Distinct Effects in Different Disease States
The concepts described above are relevant to a range of diseases, given that microbial metabolites exert pleiotropic effects on the host. Therefore, the same metabolite can affect multiple host functions, each of which may be relevant in different disease states. Tryptophan metabolites like tryptamine and 5-HT affect GI transit, which is relevant in DGBAs, while tryptamine and indole derivatives can alter mucus and the immune response, which has implications in IBD. Similarly, fermentation end products like butyrate can affect GI motility as well as epithelial barrier function, which are relevant in DGBAs and IBD, respectively (Figure 1).
3. DIET AS AN IMPORTANT DETERMINANT OF THE GUT MICROBIOME
Note that the above-described interactions are not static; instead, they change with changes in an individual’s gut microbiome. An important determinant of the gut microbiome is diet, which can have both long- and short-term effects on the gut microbiome. The long-term effects of a habitual diet are best demonstrated by the marked compositional alterations and reduced gut microbial diversity in individuals from industrialized countries when compared with those from agrarian societies (153). In a set of elegant experiments modeling these microbiomes in germ-free mice, Sonnenburg et al. (137) demonstrated that a low-fiber diet causes an incremental loss of gut microbial diversity in every successive generation, which is reversible in the early stages but results in extinction of specific taxa in subsequent generations that is unrecoverable by dietary intervention alone. This observation provides one explanation for the lower gut microbial diversity observed in Western populations and highlights how small changes can accumulate over generations; therefore, an individual’s microbial community structure likely reflects long-term dietary patterns in the population.
Short-term dietary alterations can also alter the gut microbiome. While these alterations are reversible to varying degrees, depending on the underlying resilience and adaptability of the community, short-term changes may explain in part the varying frequency and severity of symptoms in patients with chronic diseases. These short-term effects also underscore the potential for microbiota-directed dietary interventions as a therapeutic strategy.
4. IN SEARCH OF CULINARY CULPRITS
4.1. Diet Can Influence Symptoms of Irritable Bowel Syndrome via Microbiota-Independent and Microbiota-Driven Mechanisms
IBS is a common DGBA with a global prevalence of approximately 11.2% (49). It is diagnosed according to the presence of abdominal pain at least once a week, in association with defecation or a change in the frequency or form of stool, in the past 3 months with symptom onset within the past 6 months. IBS is categorized into diarrhea-predominant (IBS-D), constipation-predominant (IBS-C), mixed, and unclassified subtypes (53). Physiologic changes such as alterations in GI transit, secretion, sensation, immune activation, intestinal permeability, and the gut–brain axis underlie symptoms in IBS (12). Risk factors associated with IBS include host genetics, stress, psychiatric comorbidities, antibiotics, and early childhood experiences, but diet is most commonly identified by IBS patients as a potential culprit; population-based studies show that nearly 70% of IBS patients report perceived food intolerance (92, 101).
4.2. Mechanisms Linking Direct and Microbiota-Driven Effects of Diet with Irritable Bowel Syndrome Pathophysiology
The mechanisms by which diet can cause symptoms are still under investigation, but recent studies have begun to shed light on both microbiota-independent (Section 4.2.1) and microbiota-dependent mechanisms (Sections 4.2.2–4.2.6) underlying diet-driven symptoms in IBS.
4.2.1. Diet and lipopolysaccharides.
Aguilera-Lizarraga et al. (1) found that direct injection of food antigens, such as gluten, wheat, milk, and soy, into the submucosa can trigger immune responses by activating mast cells in IBS patients but not in healthy subjects. They further showed that mast cell activation causes visceral pain and increases intestinal permeability via histamine-stimulated sensitization of visceral neurons. While this study demonstrated a microbiota-independent mechanism, other studies have found that diets high in fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) can also activate mast cells via the Toll-like receptor 4 (TLR4) pathway, implicating the involvement of gut microbiota (132). Lipopolysaccharides (LPS), a group of heterogeneous cell wall components of gut bacteria which act as ligands for TLR4, are also increased among individuals consuming a high-fat Western diet (115) or a high-FODMAP diet. Apart from its role in mast cell activation, LPS in different forms promotes the survival of enteric neurons (4) and increases smooth muscle contractility (102), suggesting that differences in LPS concentration or structure may drive different host responses. Serum levels of microbial products such as LPS and flagellin, which are affected by diet, have been reported to be significantly elevated in patients with IBS-D (36).
4.2.2. Metabolites derived from microbial fermentation of fiber.
In addition to microbial cell wall components, metabolic end products resulting from host–microbial metabolism of dietary ingredients can drive GI symptoms through their effect on the underlying GI physiology. SCFAs such as acetate, propionate, and butyrate are produced by specific gut microbiota members, and their levels depend on both microbiota composition and dietary fiber intake. Butyrate is a pleiotropic metabolite that can directly signal via G protein–coupled receptors (GPCRs) and alter transcriptional responses via epigenetic modulation (30). Butyrate can alter 5-HT synthesis in enterochromaffin cells in a concentration-dependent manner (122), increase colonic contractility through direct effects on the enteric neuromuscular apparatus, augment the intestinal epithelial barrier, and regulate visceral hypersensitivity via interactions with enteric glia (76). Intracolonic acetate, on the other hand, enhances sensitivity to colorectal distention (150). The specific effects likely depend on host health and the overall metabolite milieu in the gut.
4.2.3. Tryptophan-derived microbial metabolites.
Diet, host mucus, and microbial production are all key sources of amino acids in the gut.A longitudinal study reported that try ptophan and tryptamine levels, but not indole derivatives, were higher in patients with IBS-D than in healthy individuals, despite similar dietary protein intake (95). This difference could be a result of increased tryptophan production and conversion to tryptamine by gut microbiota or, alternatively, decreased utilization by the host. Tryptamine activates 5-HT4R present on the enterocytes and increases intestinal fluid secretion. Another study found no differences among IBS patients and healthy subjects in 5-HT4R expression or response of colonic tissue to tryptamine, suggesting that higher tryptamine levels are likely an important driver of diarrhea (13).
4.2.4. Gluten.
Other diet- and microbiota-driven pathways have been described in IBS-D. Gluten intolerance, which is frequently reported among IBS-D patients in the absence of celiac disease, appears to depend partly on the host genotype (7). IBS-D patients negative for HLA-DQ2/HLA-DQ8 (permissive of but not diagnostic for celiac disease) have been reported to experience a greater reduction in abdominal distention following a gluten-free diet in comparison to their negative counterparts. While this study (7) did not specifically investigate the role of the gut microbiota, other studies have found that the gut microbiota can differentially affect gluten digestion and immunogenicity (e.g., 17). The specific mechanism underlying the effect of gluten in IBS-D still needs to be determined.
4.2.5. Microbial bile acid metabolism.
Bile acids (BAs) are synthesized in the liver, stored in the gallbladder, and used for lipid emulsification for rapid absorption in the small intestine upon release. Dietary fat and turmeric are important stimuli for the release of primary BAs into the small intestine (32). The two primary BAs in humans—chenodeoxycholic acid (CDCA) and cholic acid (CA)—are conjugated with glycine or taurine (at a ratio of three to one). Nearly 95% of the primary BAs are reabsorbed in the distal small intestine. The remaining primary BAs entering the colon are deconjugated, dehydroxylated, and epimerized to secondary BAs—lithocholic acid from CDCA and deoxycholic acid from CA—by gut microbes (100). Primary BAs such as CDCA increase colonic secretion via chloride channels and lower rectal sensory thresholds in healthy individuals (10, 111). In a rodent model, they affected visceral sensitivity through the activation of nuclear receptor farnesoid X receptor, release of nerve growth factor, and downstream expression of transient receptor potential vanilloid 1 in the dorsal root ganglia (89). Patients with IBS-D are likely to have higher levels of fecal BAs, attributable to BA malabsorption and/or a decrease in gut microbiota–driven conversion to secondary BAs (35). Therefore, a high-fat diet can alter GI physiology either directly, by regulating BA release, or indirectly, through microbial metabolism of BAs (154).
4.2.6. Microbial β-glucuronidases.
A recent study found that patients with postinfectious IBS-D have lower levels of bacteria-encoded β-glucuronidases, which can deconjugate bilirubin (39). These patients had higher levels of conjugated bilirubin, which led to decreased inhibition of host proteases and increased intestinal permeability, contributing to the visceral hypersensitivity observed in IBS-D patients. Several additional mechanisms underlying microbiota-driven visceral hypersensitivity have been identified in preclinical models; these include neurotransmitter/peptide-mediated hyperalgesia (e.g., 5-HT, calcitonin gene–related peptide, substance P) (40, 139), altered neuroreceptor signaling [e.g.,5-HT receptors, GABAergic signaling, GPCRs including protease-activated and cannabinoid receptors (reviewed in 3)] (20, 40, 155), and guanylate cyclase C signaling (21).
4.3. Diet Is an Important Determinant of Symptoms and Disease Activity in Inflammatory Bowel Disease
IBD is an idiopathic, chronic, debilitating, inflammatory disorder of the GI tract, encompassing two conditions—Crohn’s disease (CD) and ulcerative colitis (UC). While CD manifests as a patchy transmural inflammation that can be scattered throughout the GI tract, UC is a continuous mucosal inflammation of the colon. Both disorders result from an uncontrolled inflammatory response to gut microbial cues, in the milieu of interacting environmental, genetic, and immunological factors. Epidemiologically, IBD—which used to be a disease of the Western world, with the highest prevalence in European and North American nations (0.5% in the USA)—has made a great shift to the east since the 1990s, with incidences rising rapidly in newly industrialized countries in Africa, Asia (incidence in India of 9.3 cases per 100,000 person years and in China of 3.3 per 100,000 person years), and South America. This shift has been attributed to environmental factors arising from the rapid westernization and industrialization of these societies (73, 75, 107).
These epidemiological transitions have coincided with global shifts in dietary patterns, including the introduction of packaged and processed foods; wide acceptance and usage of food additives, preservatives, and antibiotics; and the promotion of fast-food chains, accompanied by the diminishment of region-specific, local-food diets. The role of diet as one of the key environmental factors shaping IBD risk is demonstrated by studies on migrant epidemiology reporting an enhanced prevalence of IBD in populations migrating from low-incidence areas to high-incidence regions (33). Along with the more pronounced global east–west epidemiological patterning of IBD, a more subtle north–south prevalence disparity is evident in France and Spain. A higher IBD load is observed in the northern parts of these nations, where individuals consume more butter, potatoes, ham, cheese, sausage, and beer, whereas individuals in the southern regions follow a Mediterranean diet (MD), composed mainly of olives, fresh fruits and vegetables, wine, and seafood (22, 116).
4.4. Mechanisms Linking Direct and Microbiota-Driven Effects of Diet with Inflammatory Bowel Disease Pathophysiology
Dietary components can drive pathophysiology of IBD both directly and following their transformation by gut microbiota.
4.4.1. Animal protein and trimethylamine-N-oxide.
The complex interactions among dietary macronutrients, micronutrients, additives, and caloric content; host immunity; genetics; and the gut microbiome are likely important determinants of the risk and clinical course of IBD (Figure 2). A recent large prospective cohort of 125,445 participants found an association between a Western diet, consisting of animal protein such as red meat, poultry, and processed meats, and an increased likelihood of UC development (37). These IBD-exacerbating effects of red meat were also highlighted in two additional study cohorts—the European Prospective Investigation into Cancer and Nutrition cohort (142), demonstrating that an increased linoleic acid intake associated with red meat consumption increases the risk of developing UC more than twofold, and a large French prospective questionnaire study (68). Interestingly, the consumption of processed red meat, but not unprocessed red meat, poultry, or fish, has recently been significantly correlated with increased mortality in patients with CD (23). Red meat is composed mainly of protein, fat, and heme. Elevated protein, fat, and dietary heme alter the gut microbiota composition, which, in turn, negatively affects epithelial cell turnover and gut barrier integrity and elevates intestinal inflammation (Figure 2). Red meat is rich in l-carnitine, phosphatidylcholine, and γ-butyrobetaine, which, through gut microbial metabolism, are converted to trimethylamine, a precursor for the formation of trimethylamine-N-oxide (TMAO) by the host liver flavin-containing monooxygenases (133, 143). Animal data as well as human epidemiological studies show a strong positive association between TMAO and inflammation (5, 45), cardiovascular diseases, colorectal cancer (67), and mortality (69).
Figure 2.
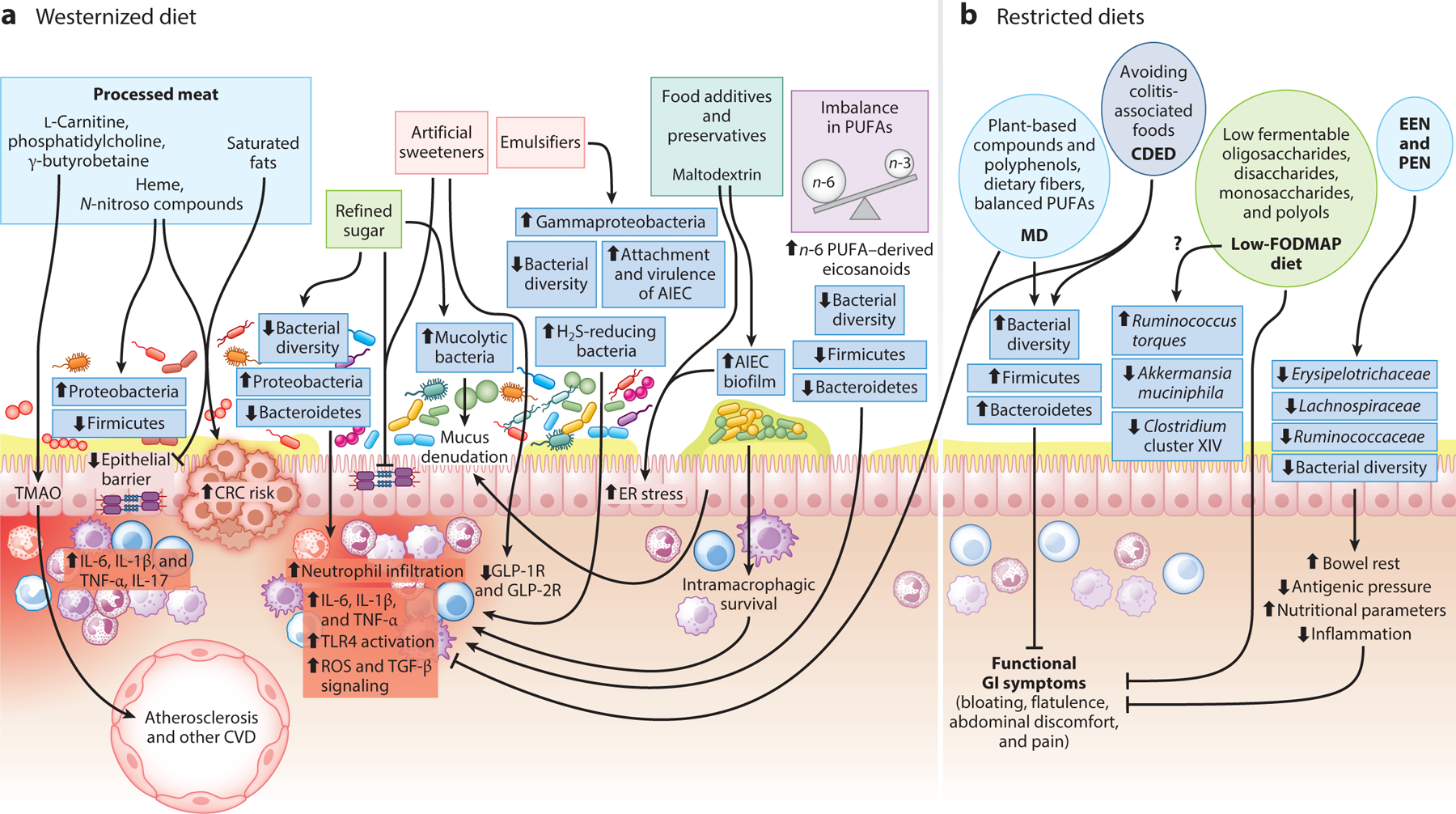
Role of diet in the pathogenesis and prevention of inflammatory bowel disease (IBD). (a) A Westernized diet is rich in ultraprocessed foods, processed red meat, foods with high refined sugar content, artificial sweeteners, food additives, preservatives, and emulsifiers. Processed red meat is rich in l-carnitine, phosphatidylcholine, and γ-butyrobetaine, which are converted to trimethylamine-N-oxide (TMAO) by the action of gut microbial and host liver enzymes. TMAO has been implicated in enhancing the risk of cardiovascular disease (CVD). Additionally, an abundance of heme and N-nitroso compounds, along with saturated fats, promotes gut dysbiosis, hampers epithelial barrier integrity, enhances the release of inflammatory cytokines, and increases the risk of colorectal cancer (CRC). Refined sugars and artificial sweeteners mediate their downstream inflammatory effects by decreasing bacterial diversity, enhancing Proteobacteria and mucolytic bacteria, and reducing beneficial Firmicutes and Bacteroidetes. Artificial sweeteners such as sucralose and acesulfame potassium also mediate their inflammatory effects through inhibition of glucagon-like peptide 1 and 2 receptors (GLP-1R and GLP-2R). Emulsifiers such as polysorbate 80 and carrageenan mediate gut inflammation by promoting gut dysbiosis (enhancement of Gammaproteobacteria, a class of sulfide-reducing bacterial genera) and promote the attachment and virulence of adherent-invasive Escherichia coli (AIEC). Similarly, the common food additive maltodextrin promotes AIEC attachment and biofilm formation. An imbalanced (versus the ideal one-to-one) ratio of n-6 to n-3 polyunsaturated fatty acids (PUFAs), through the elevation of n-6 PUFAs, is also a characteristic feature of sedentary diet regimes and mediates gut inflammation and dysbiosis, characterized by reduced bacterial diversity, and reduces the numbers of beneficial gut bacterial members of the phyla Firmicutes and Bacteroidetes. (b) Dietary regimes that have been actively explored for the alleviation of IBD symptoms include the Mediterranean diet (MD) and the Crohn’s disease–exclusion diet (CDED), both of which exert their anti-inflammatory effects through the enrichment of gut bacterial diversity and beneficial bacterial genera of the phyla Firmicutes and Bacteroidetes. MD is rich in dietary fiber and n-3 PUFAs, which are widely accepted to reduce neutrophil infiltration and expression of inflammatory cytokines in the gut. Low-FODMAP (fermentable oligo-, di-, and monosaccharides and polyols) diets, partial enteral nutrition (PEN), and exclusive enteral nutrition (EEN) alleviate gut inflammation, improve nutritional status, and relieve functional gastrointestinal (GI) symptoms as a result of their simpler constitution, leading to lower antigenic pressure and subsequent bowel rest. However, their administration has been linked to a reduction in both gut microbial diversity and beneficial gut microbial members. Therefore, the impact of their long-term administration, their therapeutic efficiencies, and the underlying mechanisms must be assessed in robust clinical trials.
4.4.2. Processed foods.
The Western diet is rich in (ultra)processed foods, a category encompassing a wide variety of food groups including meat, starchy snacks, dairy, legumes, fruits, and vegetables. Unlike traditional dietary regimes, the Western diet is enriched in simple refined carbohydrates, saturated fats, and processed and industrialized foods, and is lower in fresh fruits and vegetables, legumes, whole cereals, and dietary fiber. Studies have reported detrimental effects of the Western diet on human health and have linked it with obesity, diabetes, IBD, chronic kidney diseases, and other lifestyle-associated disorders. (Ultra)processing of food items aims to enhance their shelf life, palatability, and convenience of storage and distribution, and it involves the incorporation of many nonnatural ingredients and additives such as artificial flavors, stabilizers, preservatives, and emulsifiers. A recent study on a large prospective cohort (116,087 adults) from 21 low-, middle-, and high-income countries across seven geographical regions found that higher intake of ultraprocessed foods was positively correlated with a risk of IBD; however, intake of unprocessed white meat, red meat, dairy, starch, fruits, and vegetables was not associated with the incidence of IBD (105).
4.4.3. Dietary sugars and artificial sweeteners.
Studies have found a significant positive correlation between IBD risk and consumption of nonalcoholic sugary soft drinks. Two recent meta-analyses compiling observational studies on beverage intake and IBD risk mirrored these findings, demonstrating that high intake of soft drinks is positively associated with IBD risk (78, 109). Experiments have found that high dietary sugar is associated with inflammation induction and gut dysbiosis. Interestingly, a questionnaire-based study (121) comparing the dietary pattern of patients with IBD with that of the healthy population showed higher soft drink consumption in patients with IBD (Figure 2).
Artificial sweeteners such as aspartame, saccharine, acesulfame potassium, and sucralose have gained wide acceptance for imparting sweetness without adding extra calories. However, animal studies and trials in healthy human subjects reported that these nonnutritive sweeteners reduce gut microbial diversity (24, 46), perpetuate gut inflammation (54), alter the gut microbiota by enhancing members of Proteobacteria and reducing the representation of beneficial microbial members [Ruminococcaceae, Lachnospiraceae, and Clostridium cluster XIVa (144)], and compromise gut barrier integrity, especially through reduced expression of glucagon-like peptide 1 and 2 receptors (57) (Figure 2). Similarly, synthetic emulsifiers such as polysorbate 80 and carboxymethyl cellulose, which are used as additives to enhance texture and boost shelf life, have been widely implicated in animal studies as causing gut dysbiosis and promoting chronic inflammation (104). In vitro studies utilizing Peyer’s patches from CD patients showed increased translocation of bacteria such as E. coli across M cells and Peyer’s patches, along with enhanced bacterial adherence to the intestinal epithelium and increased translocation and infiltration of these bacteria between intestinal villi (124).
4.4.4. Food additives.
Maltodextrin (E1400), another important food additive that is used as a thickener in processed foods, exacerbated intestinal inflammation in a dose-dependent manner in a murine model of colitis, through induction of endoplasmic reticulum stress and alterations of the mucus layer (83). Reports in mouse models also indicate that maltodextrin favors biofilm formation through CD-associated adherent-invasive E. coli through modulation of bacterial gene expression (108).
The deleterious effects are enhanced by preservatives in processed foods. Sodium benzoate (E211), sodium nitrite (E250), and potassium sorbate (E202), three of the most commonly used preservatives, reduce gut microbial diversity, with increased representation of Proteobacteria and reduced Clostridiales in a human gut microbiota–associated mouse model, at exposure levels typical of European populations (65). Even though human and animal studies have provided mechanistic insights into the negative effects of these nonnutritive dietary additives on gut dysbiosis and intestinal health, randomized controlled trials in humans evaluating the impact of these sweeteners in IBD cohorts are lacking.
4.4.5. Polyunsaturated fatty acids.
A prominent feature of the Western diet is the significantly greater contribution of energy from n-6 polyunsaturated fatty acids (PUFAs) versus n-3 PUFAs. A large, prospective, epidemiological study by Tjonneland et al. (142), based on food frequency questionnaires from more than 200,000 participants across multiple centers, showed a significant association between intake of the n-6 PUFA linoleic acid and increased risk of UC. A systematic literature review by Hou et al. (64) reported an increased risk of developing UC with a high intake of total fat, n-6 PUFAs, and meat, as well as an increased risk of CD with a high intake of saturated fats, n-6 PUFAs, and meat. While the major dietary n-3 PUFAs, namely eicosapentaenoic acid (EPA) and docosahexaenoic acid, and their downstream eicosanoids have anti-inflammatory properties, n-6 PUFAs such as arachidonic acid (AA) and their eicosanoids, such as prostaglandins, thromboxanes, leukotrienes, hydroxyeicosatetraenoic acid, lipoxins, and epoxyeicosatrienoic acid, demonstrate strong proinflammatory activity in IBD. These mediators potentiate neutrophil chemotaxis; enhanced vascular permeability; and production of inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-8 (Figure 2). Interestingly, the metabolism of these fatty acid mediators is itself altered in inflamed mucosa, with higher n-6 PUFA AA, lower n-3 PUFA EPA, and a higher AA-to-EPA ratio, suggesting that fatty acid metabolism is a vicious perpetuator of inflammation in IBD (112).
Recent animal studies and human trials have linked dietary n-6 PUFAs to gut microbial dysbiosis. Miao et al. (97) reported that higher levels of γ-linolenic acid are significantly associated with higher incidence of type 2 diabetes; reduced gut microbial diversity; and reduced beneficial microbial genera such as Prevotella, Odoribacter, Faecalibacterium, Paraprevotella, Blautia, and Butyrivibrio, as well as members of Clostridiales, Rikenellaceae, and Coriobacteriaceae. Mice supplemented with an n-6 high-fat diet at the weaning stage demonstrated an increase in the number of colonic inflammatory and hyperplastic lesions during adulthood, coupled with a marked reduction in members of Firmicutes, Clostridia, and Lachnospiraceae and an enhancement in the representation of proinflammatory Mucispirillum schaedleri and Lactobacillus marinus (128). Similar effects of n-6 supplementation were observed in an aged mouse model, where an n-6 high-fat diet reduced beneficial members of Firmicutes and Bacteroidetes and caused gut inflammation. The observed gut dysbiosis was reversed by fish oil supplementation (51).
5. DIETARY INTERVENTIONS IN GASTROINTESTINAL DISEASE
Patients’ perceived intolerance to dietary components, and the role of these components in the pathophysiology of GI diseases such as IBS and IBD, has made diet a frequent target of therapeutic approaches. However, dietary strategies lack specificity, and similar approaches have been used across GI diseases with distinct pathophysiologies, like IBS and IBD. These strategies fall into several different categories, the most common being restricting, altering, or supplementing nutrients.
5.1. Dietary Restrictions
The most common form of dietary modification is restriction of nutrients that are considered to be important drivers of disease pathophysiology.
5.1.1. Reducing fiber-rich foods.
A common strategy based on the rationale that increased gas production underlies bloating is to reduce foods rich in fermentable fiber. However, this rationale is not supported by current evidence, which suggests that visceral hypersensitivity and decreased movement of gas, rather than increased gas production, are what underlie symptoms of bloating. The benefit of this strategy, if any, is often short-lived. In fact, a systematic review (42) found that long-chain, moderately fermentable soluble dietary fiber, like psyllium, improves symptoms in IBS; therefore, restricting fiber may worsen symptoms over the long term. In contrast, a low-fiber diet is recommended for patients with IBD when there is stricturing disease to prevent episodes of small bowel obstruction. The response to fiber supplementation or restriction is likely dependent on the type of fiber (80), the underlying disease state, and the gut microbiota composition, making it difficult to suggest a one-size-fits-all approach. Therefore, treatment needs to be individualized for every patient.
5.1.2. Low-FODMAP and gluten-free diets.
One of the most common dietary interventions in IBS is reducing FODMAP (typically poorly absorbed short-chain carbohydrates including fructose, lactose, polyols, fructans, and galacto-oligosaccharides) for 12 weeks, followed by slow reintroduction of the food groups. This intervention is partly based on the notion that FODMAP increase osmotic load and generate higher levels of hydrogen, resulting in luminal distention. A pivotal study by Halmos et al. (56) in Australian subjects with IBS showed significant improvement in symptoms in comparison to a Western diet. A recent meta-analysis (34), which included seven randomized controlled studies of 397 patients, showed that a low-FODMAP diet reduced global symptoms compared with control interventions. However, the three randomized controlled trials within this meta-analysis, which compared a low-FODMAP diet with rigorous control diets, had the least heterogeneity among studies and the least magnitude of effect. As a result, the authors concluded that while a low-FODMAP diet can benefit IBS patients, the overall quality of data was very low. This finding suggests that several different dietary interventions improve IBS symptoms, and it would be helpful to find common elements among them. Interestingly, a study in healthy subjects found no reduction in colonic volume with a low-FODMAP diet (135), suggesting that an alternate mechanism may underlie the improvement in symptoms. An important off-target result of a low-FODMAP diet is its deleterious effect on the gut microbiota; the long-term implications of these changes remain unclear. There is also concern that patients may develop avoidant/restrictive food intake disorders, especially because the reintroduction of foods is particularly difficult.
The above-described meta-analysis found no significant benefit of a gluten-free diet in IBS patients. However, as mentioned above, the effect may be dependent on host genotype or other host/environmental factors. Gluten is found mainly in wheat, barley, and rye, which are part of a high-FODMAP diet; therefore, the improvement observed in subsets of patients may also be a result of FODMAP restriction rather than of gluten alone (94). A recent review (62) showed a high prevalence of nonceliac gluten sensitivity in IBD patients; however, there is scant evidence to support a gluten-free diet in these patients. Preclinical studies (96, 156) found an improvement in inflammation and permeability with a gluten-free diet, but there is a lack of high-quality prospective studies in human subjects. The emerging literature on microbial degradation of gluten has implications for both IBD and celiac disease and is an important area for future investigation.
5.1.3. Exclusive or partial enteral nutrition.
Exclusive enteral nutrition (EEN) has been accepted as a first-line dietary intervention for pediatric patients with CD. EEN is based on an exclusively elemental (liquid) diet complete with all essential macronutrients and micronutrients, administered exclusively instead of solids and fluids for 8–12 weeks (146). Many studies have reported that the efficacy of EEN in inducing remission in pediatric patients with mild to moderate CD is comparable to that of corticosteroids (e.g., 63). For instance, in independent Australian and Spanish trials, EEN supplementation for 8 weeks resulted in clinical remission in 84% and 80% of subjects, respectively (106). EEN is also efficacious in perioperative adult patients with CD. A meta-analysis of two prospective cohort studies (151) showed a significant reduction in postoperative complications between patients who received preoperative EEN (22%) versus those who did not. Although limited, other studies have described the benefits of EEN in the management of penetrating CD (152), stricturing CD (61), and extraintestinal CD (103).
Mechanistically, EEN likely exerts its effects through compositional and functional alterations in gut microbiota. Even though it paradoxically reduces gut microbial diversity and abundance of taxa often considered beneficial [members of genera Faecalibacterium, Ruminococcus, and Bifidobacterium and other members of families Erysipelotrichaceae, Lachnospiraceae, and Ruminococcaceae (38)], it enhances the functional capacity of the gut microbiota on the basis of changes in metabolites (93). Due to the simple composition of EEN, a reduction in antigenic pressure and bowel rest may also be important modes of action. Additionally, active ingredients in the EEN formula improve nutritional parameters and may exert anti-inflammatory effects on the intestinal epithelium. EEN is used in adults as a second- or third-line treatment, with corticosteroids as the primary induction therapy, as these are more effective than EEN for induction of clinical remission. However, clinical trials assessing the efficacy of EEN in adult patients with CD have been limited by low sample sizes and higher rates of noncompliance with the diet.
Partial enteral nutrition (PEN), which involves supplementation of half of a patient’s caloric requirement as enteral nutrition along with a whole-food diet, is beneficial for long-term maintenance of remission in patients with CD. Unrestricted PEN in combination with an elemental formula had limited efficacy in a pediatric CD cohort published in 2006 (70); as a result, researchers conceived of the need for a CD- and UC-specific exclusion diet that excludes certain detrimental food items. The Crohn’s disease exclusion diet (CDED), combined with PEN, is a whole-food diet regime designed to reduce exposure to dietary components and foods associated with deleterious changes in gut microbiota (such as expansion of Proteobacteria), compromised barrier integrity, and inflammation in the GI tract. CDED is a multistage, high-protein, low-fat diet consisting of a 12-week induction phase, in which the patient consumes specific foods, followed by a 6-week maintenance phase, in which additional food items are introduced. CDED excludes processed foods and incorporates beneficial fibers, coupled with a liquid formula to meet the patient’s energy needs. A prospective study reported that CDED plus PEN were better tolerated and more effective in a CD cohort when compared with EEN, and that 75% of patients on CDED plus PEN underwent steroid-free clinical remission (85).
5.2. Dietary Modification Toward a Mediterranean Diet
MD is rich in fruits, vegetables, bread, cereals, beans, nuts, and virgin olive oil, along with moderate amounts of dairy, fish, and meat. Table 1 lists human trials assessing the efficacy of MD in IBD. A recent prospective, randomized study including 100 adolescent IBD patients with mild to moderate disease compared the efficacy of MD with that of the regular diet, showing a significant decrease in clinical scores on the Pediatric Crohn’s Disease Activity Index and the Pediatric Ulcerative Colitis Activity Index as well as lower levels of inflammatory markers, such as serum C-reactive protein, calprotectin, TNF-α, IL-17, IL-12, and IL-13 (41). A recent clinical trial by Chicco et al. (26) also observed beneficial effects of MD in IBD. This study involved 142 IBD patients (84 UC and 58 CD) following MD for 6 months. Diet adherence significantly improved body mass index and waist circumference and led to a marked reduction in liver steatosis– and malnutrition-related parameters (26). Similar beneficial effects of MD on IBD were demonstrated by the DINE-CD study (87), in which 40% of patients with mild to moderate CD underwent remission following 6–12 weeks of MD. MD has been associated with beneficial gut microbial profiles, specifically, with enrichment of dietary fiber metabolizers such as Faecalibacterium prausnitzii, Bacteroides cellulosilyticus, and Prevotella, along with other microbes involved in degradation of plant polysaccharide and production of SCFAs and secondary BAs (31).
Table 1.
Human trials investigating the efficacy of MD and LFD in IBD and IBS
| Disease(s) | Participant number | Dietary regimen | Primary outcome | Secondary outcome(s) | Key findings | Reference |
|---|---|---|---|---|---|---|
| MD | ||||||
| CD | 96 | MD and SCD for 6 weeks | Symptomatic remission at week 6 | FC (<250 μ/g) and CRP levels (high-sensitivity CRP < 5 mg/L) | Clinical remission: 46.5% with SCD and 43.5% with MD (p = 0.77) at week 6 FC response: 34.8% with SCD and 30.8% with MD (p = 0.83) CRP response: 5.4% with SCD and 3.6% with MD (p = 0.68) |
Lewis et al. (87) |
| CD and UC | 100 (MD, 50; control diet, 50) | MD with good adherence over 12 weeks with a KIDMED eight-point score | Clinical remission (PCDAI and PUCAI < 10) | CRP, calprotectin, TNF-α, IL-17, IL-12, and IL-13 | PCDAI: MD group, 6.4 ± 8.1; control group, 10.8 ± 7.4 (p = 0.02) PUCAI: MD group, 7.6 ± 11.2; control group, 9.2 ± 7.5 (p = 0.04). Significantly low CRP, TNF-α, IL-17, IL-12, and IL-23 in MD group |
El Amrousy et al. (41) |
| CD and UC | 142 (UC, 84; CD, 58) | MD for 6 months | Anthropometric parameters (BMI, waist circumference, lean and fat body mass, and visceral fat), serum lipid profile, liver function and steatosis, and intestinal disease activity | None | MD improved BMI (p = 0.002), waist circumference (p = 0.041), and liver steatosis (p = 0.0016). Fewer UC and CD patients had active disease. Improvement in QoL in both UC and CD |
Chicco et al. (26) |
| LFD | ||||||
| 89 (LFD, 44; ND, 45) | LFD for 6 weeks | Response rate in IBS-SSS | QoL | Larger proportion of responders in LFD group (81%) than in ND group (46%) (p < 0.01) LFD group showed lower median IBS-SSS than ND group (p = 0.02). Improvement in QoL (greater SIBDQ in LFD group) |
Pedersen et al. (114) | |
| CD and UC | 72 (CD, 52; UC, 20) | LFD for 3 months | FGS | None | Abdominal pain, bloating, wind, and diarrhea improved in CD and UC (p < 0.02). | Gearry et al. (50) |
| IBD | 88 | LFD | FGS by Gastrointestinal Symptoms Rating Scale | Stool output assessed by Bristol Stool Form Scale | Relief from FGS with LFD when compared with baseline (p < 0.001) Improvement in stool frequency (p = 0.001) and form (p = 0.002) |
Prince et al. (119) |
| IBS | Meta-analysis of two RCTs for GFD(n = 111) and seven RCTs for LFD (n = 397) | LFD and GFD | Efficacy of exclusion diets (GFD and LFD) on global IBS symptoms | None | No significant effect of GFD on reduced IBS symptoms LFD associated with reduced IBS symptoms; however, the quality of the data was very low. |
Dionne et al. (34) |
| IBS | Total of 99 (33 per dietary arm) | LFD, GFD, and traditional diet for 4 weeks | Response rate in IBS-SSS | Acceptability of food, stool dysbiosis | Similar improvements in IBS-SSS items regardless of their allocated diet Traditional diet was easier to follow. Stool dysbiosis indices were similar across the diets. |
Rej et al. (123) |
Abbreviations: BMI, body mass index; CD, Crohn’s disease; CRP, C-reactive protein; FC, fecal calprotectin; FGS, functional gastrointestinal symptoms; GFD, gluten-free diet; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; IBS-SSS, IBS Severity Scoring System; IL, interleukin; KIDMED, Mediterranean Diet Quality Index for Children and Teenagers; LFD, low-FODMAP (fermentable oligo-, di-, and monosaccharides and polyols) diet; MD, Mediterranean diet; ND, normal diet; PCDAI, Pediatric Crohn’s Disease Activity Index; PUCAI, Pediatric Ulcerative Colitis Activity Index; QoL, quality of life; RCT, randomized controlled trial; SCD, specific-carbohydrate diet; SIBDQ, Short IBD Quality of Life Questionnaire; TNF, tumor necrosis factor; UC, ulcerative colitis.
MD is rich in n-3 PUFAs, such that the n-3 and n-6 PUFAs strike a perfect balance. Wiese et al. (148) demonstrated the positive effects of EPA and other PUFAs in a prospective UC cohort, where gut inflammatory cytokine levels correlated inversely with PUFA, EPA, and docosapentaenoic acid. A recent study by Scaioli et al. (127) found that 2 g/day of EPA reduced fecal calprotectin levels (100-point reduction) and maintained clinical remission in UC patients (endpoint achieved in 76.6% of the participants on EPA versus 50% in placebo). Costea et al. (29) associated single-nucleotide polymorphisms across three crucial genes (CYP4F3, FADS1, and FADS2) involved in n-3 fatty acid metabolism with an increased risk of CD, implicating an additional, genetic dimension of diet-associated regulation of IBD. n-3 PUFAs likely exert their anti-inflammatory effects through downstream lipid mediators such as resolvins, protectins, and maresins, which can counter IBD-associated inflammation (129). Mechanistically, n-3 PUFAs have been found to (a) decrease chemotaxis of neutrophils and monocytes toward various chemoattractants (52, 140); (b) suppress TLR4 expression and NOD2 signaling by blocking the release of nuclear factor κB from mitogen-activated protein kinase (2); (c) inhibit NLRP3 inflammasome activation and subsequently hamper the release of proinflammatory cytokines (131); and (d) increase the abundance of the butyrate-producing bacterial genera Bifidobacterium, Roseburia, and Lactobacillus, as well as members of the family Lachnospiraceae (147).
MD leads to increased production of SCFAs by the gut microbiota as a result of higher levels of fermentable carbohydrates. Zito et al. (157) found that MD may improve bloating and abdominal pain in IBS patients who adhere to the diet. A recent review comparing the low-FODMAP diet and MD found reciprocal effects on the gut microbiota (74); the long-term implications of such changes still need to be determined.
5.3. Supplementing Diet with Fiber or with Pro-, Pre-, or Synbiotics
This section focuses on the effect of nutrients such as in fiber, prebiotics, and synbiotics that promote bacterial communities with a beneficial effect on health, as well as live biotherapeutic products (probiotics) shown to improve host function.
5.3.1. Fiber, prebiotics, and synbiotics.
A meta-analysis of 14 randomized controlled studies including 906 IBS patients found a significant improvement in symptoms with soluble fiber but not bran (99). Most of these studies used fiber supplementation; few of them modified the diet to increase fiber intake. Although several studies have investigated prebiotics and synbiotics, there are insufficient data to make recommendations (27). A randomized, parallel, double-blind study in IBS patients comparing the effects of an MD-type diet and a prebiotic supplement (β-galacto-oligosaccharide) with a low-FODMAP diet and a placebo xylose supplement found a similar improvement in symptoms in both groups, but a more favorable gut microbiota profile with prebiotic supplementation (66) (Table 2). This finding highlights the potential for dietary modification and supplementation as an alternative to restrictive dietary practices in the management of GI diseases.
Table 2.
Clinical trialsa of prebiotics and synbiotics in IBS and IBD
| Participantsb | Study protocol | Key findings | Reference |
|---|---|---|---|
| Prebiotics | |||
| FGID with flatulence | Prebiotic group: 2.8 g/day Bimuno containing 1.37 g β-galacto-oligosaccharide plus a Mediterranean-type diet (n = 19) Placebo group: 2.8 g xylose plus a low-FODMAP diet (n = 21) Duration: 4 weeks Follow-up: 2 weeks |
Increased Bifidobacterium and decreased Bilophila wadsworthia in patients in prebiotic group; opposite for placebo group before and after treatment Lower symptom scores for pain, distension, and bloating in both groups Symptoms recurred when the low-FODMAP diet was discontinued in the placebo group, but not in the prebiotic group after prebiotic discontinuation. |
Huaman et al. (66) |
| All types of IBS | Prebiotic group: PHGG (n = 49) Placebo group: maltodextrin (n = 59) Dose and duration: 3 g/day for 7 days, then 6 g/day for 11 weeks Follow-up: 4 weeks |
Decreased gas and bloating in IBS patients during and 4 weeks after PHGG treatment, but other symptoms and the severity score were not affected. | Niv et al. (110) |
| All types of IBS with rectal hypersensitivity | Prebiotic group: scFOS (n = 41) Placebo group: maltodextrin (n = 38) Dose and duration: 2.5 g, twice per day, for 4 weeks |
Increased Bifidobacterium in scFOS group; increased Roseburia spp. in placebo group Rectal sensitivity was improved in both groups, and the prebiotic effect was more pronounced in IBS-C patients. IBS severity score (abdominal pain) was improved in both groups, and proportional reduction in patients feeling pain was more pronounced in the scFOS group. Anxiety and depression scores were improved by both treatments, more so in the scFOS group. |
Azpiroz et al. (8) |
| UC | Prebiotic group: 10 g GBF, 3 times per day, with conventional medication (n = 23) Control group: conventional medication only (n = 23) Duration: 2 months |
Decreased serum CRP in GBF group Decreased abdominal pain and cramping in GBF group |
Faghfoori et al. (44) |
| CD (inactive or mild/moderate active) | Prebiotic group: OF-IN (n = 34) Placebo group (nonspecified) (n = 33) Dose and duration: 10 g, twice per day, for 4 weeks |
Decreased Ruminococcus gnavus and increased B. longum in OF-IN group Positive correlation between improvement in disease activity and increase in B. longum abundance |
Joossens et al. (71) |
| CD (active) | Prebiotic group: FOS (n = 54) Placebo group: maltodextrin (n = 49) Dose and duration: 7.5 g, twice per day, for 4 weeks |
Lower IBD questionnaire score in the FOS group No difference in CD activity index, CRP, or fecal concentration of bifidobacteria or F. prausnitzii between treatment groups Increased severity of flatulence, abdominal pain, and borborygmi in FOS group Decreased IL-6+ lamina propria dendritic cells and IL-10 staining on dendritic cells in FOS group |
Benjamin et al. (11) |
| IBS-D | Synbiotic group: mixture of B. lactis, B. longum, B. bifidum, L. acidophilus, and L. rhamnosus, with 947 mg scFOS (n = 35) Placebo group: 978 mg maltodextrin (n = 33) Dose and duration: 5.00 × 109 CFU, twice per day, for 4 + 4 weeks (primary and secondary endpoints) |
Improved global symptoms and severity, as well as flatulence and bowel habit, in synbiotic group at both endpoints | Skrzydło-Radomańska et al. (134) |
| IBS-C | Synbiotic group: 350 mL of sterilized probiotic with L. helveticus and 5.85 g polydextrose (n = 79) Control group: 350 mL of sterilized probiotic with L. helveticus (n = 84) Duration: 7 days |
Faster gut transit and lower fecal pH in both groups Higher fecal weight in synbiotic group but lower in control group Constipation-related symptoms were improved in both groups. |
Bahrudin et al. (9) |
| IBS-C | Synbiotic group: L. acidophilus La-5 (1.8 × 107 CFU/g), B. animalis subsp. lactis BB-12 (2.5 × 107 CFU/g), S. thermophilus, and 90% inulin plus 10% oligofructose (n = 11) Placebo group: heat-treated fermented milk (n = 19) Duration: 4 weeks Follow-up: 1 week |
Transient increase in abundance of used probiotic strains in synbiotic group No functional study |
Bogovič Matijašić et al. (14) |
| All types of IBS | Synbiotic group: Lactol® (including Bacillus coagulans at 15 × 107 spores and FOS at 100 mg) (n = 23) Placebo group: lactose starch and tartrazine (n = 33) Dose and duration: 3 times/day for 12 weeks Follow-up: 9 months |
Decreased frequency of abdominal pain, diarrhea, and constipation in synbiotic group (diarrhea is specific to synbiotic group, improvement of constipation did not differ between groups) Decreased abdominal pain and increased constipation frequency in synbiotic group during follow-up, while both abdominal pain and diarrhea increased in placebo group |
Rogha et al. (125) |
| All types of IBS | Synbiotic group: Probinul (lyophilized bacteria including L. plantarum and S. thermophilus of 5 × 109 CFU each; L. casei subsp. rhamnosus and L. gasseri at 2 × 109 CFU each; B. infantis, B. longum, L. acidophilus, L. salivarus, and L. sporogenes at 1 × 109 CFU each) with 2.2g inulin and 1.3 g tapioca-resistant starch (n = 32) Placebo group (nonspecified) (n = 32) Dose and duration: 5 g, twice per day, for 4 weeks |
Decreased flatulence and increased transit time with higher QoL in synbiotic group | Cappello et al. (18) |
| All types of IBS | Synbiotic group: yogurt containing ≥1011 CFU/150 mL B. animalis subsp. lactis Bb-12 with Bifidobacterium enhancer, acacia dietary fiber, ≥3 × 109 CFU/150 mL S. thermophilus, and ≥109 CFU/150 mL L. acidophilus (n = 58) Control group: traditional yogurt containing ≥1010 CFU/150 mL B. animalis subsp. lactis Bb-12, with ≥3 × 109 CFU/150 mL S. thermophilus and ≥109 CFU/150 mL L. acidophilus; no extrafunctional ingredients (n = 59) Dose and duration: twice per day for 8 weeks |
Overall higher improvement in IBS symptoms and bowel habit satisfaction in synbiotic versus control group, with better alleviation of symptoms in IBS-C patients on synbiotic yogurt and more satisfaction of bowel habit in IBS-D patients on synbiotic yogurt compared with those on control yogurt | Min et al. (98) |
| UC (mild to moderate active) | Synbiotic group: 3 × 109 CFU of E. faecium, L. plantarum, S. thermophilus, B. lactis, L. acidophilus, and B. longum, with 225 mg/dose FOS (n = 18) Placebo group (nonspecified) (n = 18) Dose and duration: twice per day for 8 weeks |
Lower serum CRP in synbiotic group Decreased symptom severity but greater remission in synbiotic group |
Altun et al. (3) |
Includes only studies published between 2010 and 2022, with randomized, double-blind, and placebo-controlled designs.
All IBS patients were selected on the basis of Rome III criteria.
Abbreviations: CD, Crohn’s disease; CFU, colony-forming units; CRP, C-reactive protein; FGID, functional gastrointestinal disorders; FODMAP, fermentable oligo-, di-, and monosaccharides and polyols; FOS, fructo-oligosaccharides; GBF, germinated barley foodstuff; IBS-C, constipation-predominant irritable bowel syndrome; IBS-D, diarrhea-predominant irritable bowel syndrome; IL, interleukin; OF-IN, oligofructose-enriched inulin; PHGG, partially hydrolyzed guar gum; QoL, quality of life; scFOS, short-chain fructo-oligosaccharides; UC, ulcerative colitis.
Preclinical models of IBD have found that a high-fiber (predominantly psyllium), low-protein diet augments intestinal barrier function and reduces inflammation (91). Thus, soluble fiber appears to benefit both patients with IBS and those with IBD. A recent meta-analysis found a linear dose-dependent relationship between dietary fiber intake and CD risk, with a 13% depreciation in CD risk for every 10 g/day increment in fiber intake (90). Food- and supplement-based fiber and prebiotic intervention studies have reported encouraging results with a fiber-enriched semivegetarian diet (25), Plantago ovata seeds (47), oat bran (55), and germinated barley foodstuff (43) in the maintenance of remission and significant improvement in GI symptoms (abdominal pain and reflux). Similar results have been reported in active disease cohorts receiving supplementation of FOS (15 g/day for 3–4 weeks) (11), oligofructose-enriched inulin (10 g twice per day for 4 weeks) (71), whole wheat bran (0.5 cup/day for 4 weeks) (15), inulin-type fructan (7.5 g/day for 9 weeks) (19), and germinated barley foodstuff (72), specifically, a significant reduction in disease activity (according to the Harvey–Bradshaw index) and an improvement in quality of life. A systematic review and meta-analysis assessing the effects of fiber intake on the gut microbiome composition showed that dietary fiber is associated with significantly higher abundances of Bifidobacterium spp. and Lactobacillus spp., as well as with higher levels of fecal butyrate, when compared with a placebo/low-fiber diet (136). These studies were conducted largely in adult IBD patients, and the benefit for the pediatric population remains unclear. Note that fiber is used broadly to include a range of complex carbohydrates (including prebiotics). As mentioned above, the effects of fiber will likely differ on the basis of the carbohydrate structure, health status, and gut microbiota composition.
Some studies have demonstrated a benefit of synbiotics in adult patients with UC. A 4-week supplementation of a combination of Bifidobacterium longum and oligofructose-enriched inulin was associated with sigmoidoscopic and histopathological improvements and reduced the expression of inflammatory cytokines (TNF-α and IL-1β) in comparison to a placebo group (48). Altun et al. (3) reported similar results in a randomized controlled trial involving 8-week supplementation of a symbiotic cocktail composed of Enterococcus faecium, Lactobacillus plantarum, Lactobacillus acidophilus, Streptococcus thermophilus, Bifidobacterium lactis, B. longum, and FOS. A meta-analysis assessing the efficacy of synbiotics in UC revealed encouraging results about the safety and efficacy of synbiotics as a therapeutic option for UC (126). However, the beneficial effects of synbiotics are questionable in pediatric IBD cohorts and in patients with CD, due to reports of poor efficacy and tolerability (58).
5.3.2. Probiotics.
While probiotics have failed to show efficacy in IBS or IBD (118), randomized controlled trials have found that they can mitigate the deleterious effects of dietary restriction on gut microbiota. The potential physiologic implications of such effects remain unclear (138).
6. ROLE OF GUT MICROBIOTA IN GENETICALLY DRIVEN DISEASES
6.1. Lactose Intolerance
Lactose consumed via milk and milk products is digested in the small intestine via the hydrolytic action of a brush border enzyme called lactase phlorizin hydrolase (LPH). The expression of this enzyme declines as an infant shifts from a largely milk-based diet to other sources of nutrition. Most instances of lactose intolerance (e.g., flatulence, bloating, abdominal discomfort/pain, and diarrhea) can be attributed to adult-onset hypolactasia resulting from decreased expression of LPH. Genetic polymorphisms resulting in inactive or dysfunctional LPH and GI disorders that damage the intestinal epithelium can lower LPH production (141). Undigested lactose exerts osmotic pressure in the lumen, triggering diarrhea and abdominal discomfort, and fermentation of lactose by microbial lactase (β-galactosidase) results in increased flatulence (59, 60). Yogurt appears to be an exception, as bacteria commonly used for the production of yogurt (Lactobacillus delbrueckii subsp. bulgaricus and S. thermophilus) produce high levels of β-galactosidase, which depletes lactose both in the yogurt and in the small intestine (79). Therefore, the presence of these bacteria in the small intestine, or their consumption as probiotics or yogurt, may reduce symptoms to varying degrees.
6.2. Celiac Disease
Celiac disease is an autoimmune disease whereby patients mount an immune response to gluten in the small intestine, resulting in diarrhea, abdominal pain, and malabsorption that in turn cause weight loss and malnutrition. Celiac disease has been associated with other autoimmune and genetic disorders, including primary biliary cirrhosis, type 1 diabetes, ataxia, Addison’s disease, and Down syndrome (84, 130). Gluten comprises small peptides such as gliadin and glutenin and is the key component of the dietary grains wheat, barley, and rye. Gluten is deaminated in the lamina propria by tissue transglutaminase and presented as antigens by the major histocompatibility class II molecules encoded by the specific haplotypes HLA-DQ2 and HLA-DQ8, initiating a cascade of proinflammatory Th2 response that in turn causes intestinal enteropathy. The presence of this haplotype is necessary but insufficient to cause disease, and other environmental cues are likely needed (16, 130). The threshold of gluten exposure that can stimulate responses is below 10 mg; therefore, the primary treatment strategy is to eliminate gluten from the diet. Recent studies have highlighted a role for the small intestinal microbiota in modulating gluten-mediated immunopathology. Pseudomonas aeruginosa expresses elastase, which results in peptides that are more easily translocated across the epithelium and are more immunogenic. In addition, Lactobacillus spp. degrade gluten peptides produced by human and other bacteria, reducing their immunogenicity (17). AHR ligands produced through gut microbial metabolism of tryptophan reduce immunopathology in genetically susceptible, gluten-challenged mice (81). Bifidobacterium species like B. lactis and B. longum reduce production of inflammatory cytokines in gluten-challenged cell lines (113).
These two examples—lactose intolerance and celiac disease—highlight that, even though gut bacteria are not the primary drivers of disease in genetic conditions, they can modulate symptoms, disease course, and treatment efficacy in these conditions.
7. PERSPECTIVE
This review has discussed several important concepts and summarized available data on the role of the gut microbiota as a mediator of the effect of diet on host function. Diet-derived metabolites vary according to the activity of different metabolic pathways in the host and in the gut microbiota, which in turn determine the biologic effects of diet. Additional microbial metabolites can have distinct effects on multiple physiologic functions. Thus, the same metabolite can influence the pathophysiology of multiple diseases, which may explain the common finding of decreased levels of certain metabolites such as butyrate across multiple chronic diseases. It also explains why the same diet intervention may show benefits in different diseases.
We are still in the early stages of investigating how bioactive molecules resulting from diet–host–gut microbiota interaction affect pathophysiology and treatment responses in chronic GI diseases. An important consideration from the findings obtained to date is the significant interindividual variability observed when evaluating responses to diet interventions. This variability may arise from differences in bioavailable nutrients in the diet as a result of differences in composition and processing (e.g., cooking), genetic polymorphisms affecting metabolic pathways in the host or underlying host immune status, and differences in metabolic capabilities of the gut microbiota. In addition, other environment and host factors likely contribute to the responses. As we look to the future, we will need to consider all of these factors to be able to provide personalized dietary recommendations to our patients (Figure 3).
Figure 3.
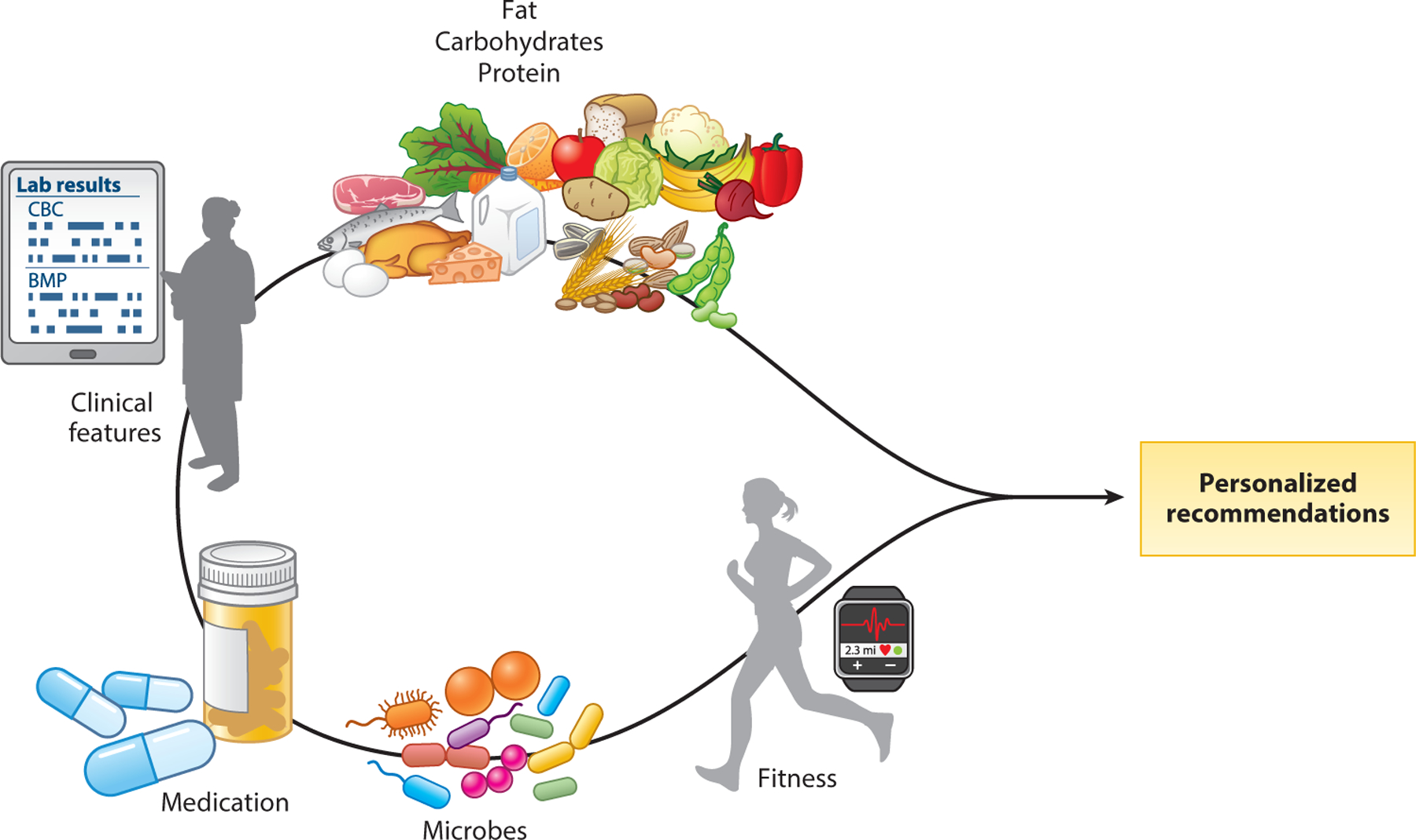
Features of the host, environment, and gut microbiota can help in developing personalized nutrition recommendations. Different types of dietary macro- and micronutrients, together with an individual’s clinical features, exercise, medications/supplements, and gut microbiota, determine the repertoire of microbial bioactive metabolites in the gut, with distinct effects on host physiology. All of these factors and as-yet-unidentified factors need to be considered in order to develop personalized nutrition recommendations.
DISCLOSURE STATEMENT
P.C.K. is an ad hoc consultant for Pendulum Therapeutics and Intrinsic Medicine. The writing of this review was supported by National Institutes of Health grant R01DK114007 (to P.C.K.).
LITERATURE CITED
- 1.Aguilera-Lizarraga J, Florens MV, Viola MF, Jain P, Decraecker L, et al. 2021. Local immune response to food antigens drives meal-induced abdominal pain. Nature 590:151–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allam-Ndoul B, Guénard F, Barbier O, Vohl MC. 2016. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis. 15:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altun HK,Yıldız EA,Akın M. 2019. Effects of synbiotic therapy in mild-to-moderately active ulcerative colitis: a randomized placebo-controlled study. Turk. J. Gastroenterol 30:313–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. 2012. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology 143:1006–16.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arias N,Arboleya S,Allison J,Kaliszewska A,Higarza SG, et al. 2020. The relationship between choline bioavailability from diet, intestinal microbiota composition, and its modulation of human diseases. Nutrients 12:2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong HK, Bording-Jorgensen M, Santer DM, Zhang Z, Valcheva R, et al. 2023. Unfermented β-fructan fibers fuel inflammation in select inflammatory bowel disease patients. Gastroenterology 164:228–40 [DOI] [PubMed] [Google Scholar]
- 7.Aziz I, Trott N, Briggs R, North JR, Hadjivassiliou M, Sanders DS. 2016. Efficacy of a gluten-free diet in subjects with irritable bowel syndrome–diarrhea unaware of their HLA-DQ2/8 genotype. Clin. Gastroenterol. Hepatol 14:696–703.e1 [DOI] [PubMed] [Google Scholar]
- 8.Azpiroz F,Dubray C,Bernalier-Donadille A,Cardot JM,Accarino A, et al. 2017. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: a randomized, double blind, placebo controlled study. Neurogastroenterol. Motil 29:e12911. [DOI] [PubMed] [Google Scholar]
- 9.Bahrudin MF, Rani RA, Tamil AM, Mokhtar NM, Raja Ali RA. 2020. Effectiveness of sterilized symbiotic drink containing Lactobacillus helveticus comparable to probiotic alone in patients with constipation-predominant irritable bowel syndrome. Dig. Dis. Sci 65:541–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. 2002. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am. J. Physiol. Gastrointest. Liver. Physiol 282:G443–49 [DOI] [PubMed] [Google Scholar]
- 11.Benjamin JL,Hedin CR,Koutsoumpas A,Ng SC,McCarthy NE, et al. 2011. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut 60:923–29 [DOI] [PubMed] [Google Scholar]
- 12.Bhattarai Y,Muniz Pedrogo DA,Kashyap PC. 2017. Irritable bowel syndrome: a gut microbiota–related disorder? Am. J. Physiol. Gastrointest. Liver Physiol 312:G52–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattarai Y, Williams BB, Battaglioli EJ, Whitaker WR, Till L, et al. 2018. Gut microbiota–produced tryptamine activates an epithelial G-protein-coupled receptor to increase colonic secretion. Cell Host Microbe 23:775–85.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogovič Matijašić B, Obermajer T, Lipoglavšek L, Sernel T, Locatelli I, et al. 2016. Effects of synbiotić fermented milk containing Lactobacillus acidophilus La-5 and Bifidobacterium animalis ssp. lactis BB-12 on the fecal microbiota of adults with irritable bowel syndrome: a randomized double-blind, placebo-controlled trial. J. Dairy Sci 99:5008–21 [DOI] [PubMed] [Google Scholar]
- 15.Brotherton CS, Taylor AG, Bourguignon C, Anderson JG. 2014. A high-fiber diet may improve bowel function and health-related quality of life in patients with Crohn disease. Gastroenterol. Nurs 37:206–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caio G, Volta U, Sapone A, Leffler DA, De Giorgio R, et al. 2019. Celiac disease: a comprehensive current review. BMC Med. 17:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caminero A, Galipeau HJ, McCarville JL, Johnston CW, Bernier SP, et al. 2016. Duodenal bacteria from patients with celiac disease and healthy subjects distinctly affect gluten breakdown and immunogenicity. Gastroenterology 151:670–83 [DOI] [PubMed] [Google Scholar]
- 18.Cappello C, Tremolaterra F, Pascariello A, Ciacci C, Iovino P. 2013. A randomised clinical trial (RCT) of a symbiotic mixture in patients with irritable bowel syndrome (IBS): effects on symptoms, colonic transit and quality of life. Int. J. Colorect. Dis 28:349–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casellas F, Borruel N, Torrejón A, Varela E, Antolin M, et al. 2007. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment. Pharmacol. Ther 25(9):1061–67 [DOI] [PubMed] [Google Scholar]
- 20.Castro J, Harrington AM, Garcia-Caraballo S, Maddern J, Grundy L, et al. 2017. α-Conotoxin Vc1.1 inhibits human dorsal root ganglion neuroexcitability and mouse colonic nociception via GABAB receptors. Gut 66:1083–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro J, Harrington AM, Hughes PA, Martin CM, Ge P, et al. 2013. Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3′,5′-monophosphate. Gastroenterology 145:1334–46.e1–11 [DOI] [PubMed] [Google Scholar]
- 22.Chaparro M, Garre A, Núñez Ortiz A, Diz-Lois Palomares MT, Rodríguez C, et al. 2021. Incidence, clinical characteristics and management of inflammatory bowel disease in Spain: large-scale epidemiological study. J. Clin. Med 10:2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Fu R, Dan L, Chen X, Sun Y, et al. 2022. Meat consumption and all-cause mortality in 5763 patients with inflammatory bowel disease: a retrospective cohort study. eClinicalMedicine 47:101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi L, Bian X, Gao B, Tu P, Lai Y, et al. 2018. Effects of the artificial sweetener neotame on the gut microbiome and fecal metabolites in mice. Molecules 23:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiba M,Abe T,Tsuda H,Sugawara T,Tsuda S, et al. 2010. Lifestyle-related disease in Crohn’s disease: relapse prevention by a semi-vegetarian diet. World J. Gastroenterol 16:2484–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chicco F, Magrì S, Cingolani A, Paduano D, Pesenti M, et al. 2021. Multidimensional impact of Mediterranean diet on IBD patients. Inflamm. Bowel Dis 27:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chlebicz-Wójcik A, Śliźewska K. 2021. Probiotics, prebiotics, and synbiotics in the irritable bowel syndrome treatment: a review. Biomolecules 11:1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colomier E,Melchior C,Algera JP,Hreinsson JP,Störsrud S, et al. 2022. Global prevalence and burden of meal-related abdominal pain. BMC Med. 20:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costea I, Mack DR, Lemaitre RN, Israel D, Marcil V, et al. 2014. Interactions between the dietary polyunsaturated fatty acid ratio and genetic factors determine susceptibility to pediatric Crohn’s disease. Gastroenterology 146:929–31 [DOI] [PubMed] [Google Scholar]
- 30.D’Souza WN, Douangpanya J, Mu S, Jaeckel P, Zhang M, et al. 2017. Differing roles for short chain fatty acids and GPR43 agonism in the regulation of intestinal barrier function and immune responses. PLOS ONE 12:e0180190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, et al. 2016. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65:1812–21 [DOI] [PubMed] [Google Scholar]
- 32.Dey N, Wagner VE, Blanton LV, Cheng J, Fontana L, et al. 2015. Regulators of gut motility revealed by a gnotobiotic model of diet–microbiome interactions related to travel. Cell 163:95–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhaliwal J, Tuna M, Shah BR, Murthy S, Herrett E, et al. 2021. Incidence of inflammatory bowel disease in South Asian and Chinese people: a population-based cohort study from Ontario, Canada. Clin. Epidemiol 13:1109–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dionne J, Ford AC, Yuan Y, Chey WD, Lacy BE, et al. 2018. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low FODMAPs diet in treating symptoms of irritable bowel syndrome. Am. J. Gastroenterol 113:1290–300 [DOI] [PubMed] [Google Scholar]
- 35.Dior M,Delagrèverie H,Duboc H,Jouet P,Coffin B, et al. 2016. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol. Motil 28:1330–40 [DOI] [PubMed] [Google Scholar]
- 36.Dlugosz A, Nowak P, D’Amato M, Mohammadian Kermani G, Nyström J, et al. 2015. Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil 27:1747–54 [DOI] [PubMed] [Google Scholar]
- 37.Dong C, Chan MS, Jantchou P, Racine A, Oldenburg B, et al. 2022. Meat intake is associated with a higher risk of ulcerative colitis in a large European prospective cohort study. J. Crohn’s Colitis 16:1187–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn KA, Moore-Connors J, Macintyre B, Stadnyk AW, Thomas NA, et al. 2016. Early changes in microbial community structure are associated with sustained remission after nutritional treatment of pediatric Crohn’s disease. Inflamm. Bowel Dis 22:2853–62 [DOI] [PubMed] [Google Scholar]
- 39.Edwinson AL, Yang L, Peters S, Hanning N, Jeraldo P, et al. 2022. Gut microbial β-glucuronidases regulate host luminal proteases and are depleted in irritable bowel syndrome. Nat. Microbiol 7:680–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Ayache N, Galligan JJ. 2019. 5-HT3 receptor signaling in serotonin transporter-knockout rats: a female sex–specific animal model of visceral hypersensitivity. Am. J. Physiol. Gastrointest. Liver Physiol 316:G132–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Amrousy D, Elashry H, Salamah A, Maher S, Abd-Elsalam SM, Hasan M. 2022. Adherence to the Mediterranean diet improved clinical scores and inflammatory markers in children with active inflammatory bowel disease: a randomized trial. J. Inflamm. Res 15:2075–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Salhy M, Otterasen Ystad S, Mazzawi T, Gunderson D. 2017. Dietary fiber in irritable bowel syndrome. Int. J. Mol. Med 40:607–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faghfoori Z, Navai L, Shakerhosseini R, Somi MH, Nikniaz Z, Norouzi MF. 2011. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor α, interleukin-6 and -8 in patients with ulcerative colitis. Ann. Clin. Biochem 48:233–37 [DOI] [PubMed] [Google Scholar]
- 44.Faghfoori Z, Shakerhosseini R, Navai L, Somi MH, Nikniaz Z, Abadi A. 2014. Effects of an oral supplementation of germinated barley foodstuff on serum CRP level and clinical signs in patients with ulcerative colitis. Health Promot. Perspect 4:116–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farhangi MA,Vajdi M. 2020. Novel findings of the association between gut microbiota–derived metabolite trimethylamine N-oxide and inflammation: results from a systematic review and dose-response meta-analysis. Crit. Rev. Food Sci. Nutr 60:2801–23 [DOI] [PubMed] [Google Scholar]
- 46.Farid A, Hesham M, El-Dewak M, Amin A. 2020. The hidden hazardous effects of stevia and sucralose consumption in male and female albino mice in comparison to sucrose. Saudi Pharm. J 28:1290–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernández-Bañares F, Hinojosa J, Sánchez-Lombraña JL, Navarro E, Martínez-Salmerón JF, et al. 1999. Randomized clinical trial of Plantago ovata seeds (dietary fiber) as compared with mesalamine in maintaining remission in ulcerative colitis. Am. J. Gastroenterol 94:427–33 [DOI] [PubMed] [Google Scholar]
- 48.Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, et al. 2005. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut 54:242–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.GBD 2017 Inflamm. Bowel Dis. Collab. 2020. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol 5:17–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR. 2009. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease—a pilot study. J. Crohn’s Colitis 3:8–14 [DOI] [PubMed] [Google Scholar]
- 51.Ghosh S, Molcan E, Decoffe D, Dai C, Gibson DL. 2013. Diets rich in n-6 PUFA induce intestinal microbial dysbiosis in aged mice. Br. J. Nutr 110:515–23 [DOI] [PubMed] [Google Scholar]
- 52.Gorjão R, Verlengia R, Lima TM, Soriano FG, Boaventura MFC, et al. 2006. Effect of docosahexaenoic acid–rich fish oil supplementation on human leukocyte function. Clin. Nutr 25:923–38 [DOI] [PubMed] [Google Scholar]
- 53.Grad S, Dumitrascu DL. 2020. Irritable bowel syndrome subtypes: new names for old medical conditions. Dig. Dis 38:122–27 [DOI] [PubMed] [Google Scholar]
- 54.Guo M,Liu X,Tan Y,Kang F,Zhu X, et al. 2021. Sucralose enhances the susceptibility to dextran sulfate sodium (DSS) induced colitis in mice with changes in gut microbiota. Food Funct. 12:9380–90 [DOI] [PubMed] [Google Scholar]
- 55.Hallert C, Björck I, Nyman M, Pousette A, Grännö C, Svensson H. 2003. Increasing fecal butyrate in ulcerative colitis patients by diet: controlled pilot study. Inflamm. Bowel Dis 9:116–21 [DOI] [PubMed] [Google Scholar]
- 56.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. 2014. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 146:67–75.e5 [DOI] [PubMed] [Google Scholar]
- 57.Hanawa Y, Higashiyama M, Kurihara C, Tanemoto R, Ito S, et al. 2021. Acesulfame potassium induces dysbiosis and intestinal injury with enhanced lymphocyte migration to intestinal mucosa. J. Gastroenterol. Hepatol 36:3140–48 [DOI] [PubMed] [Google Scholar]
- 58.Hansen R, Mahdi G, McIntyre K, Macfarlane GT, Macfarlane S, Wilson DC. 2011. Synbiotics for inflammatory bowel disease: useful in adults but problematic in paediatrics. Arch. Dis. Child 96:A18–19 [Google Scholar]
- 59.He T, Priebe MG, Harmsen HJM, Stellaard F, Sun X, et al. 2006. Colonic fermentation may play a role in lactose intolerance in humans. J. Nutr 136:58–63 [DOI] [PubMed] [Google Scholar]
- 60.He T, Venema K, Priebe MG, Welling GW, Brummer RJM, Vonk RJ. 2008. The role of colonic metabolism in lactose intolerance. Eur. J. Clin. Investig 38:541–47 [DOI] [PubMed] [Google Scholar]
- 61.Heerasing N, Thompson B, Hendy P, Heap GA, Walker G, et al. 2017. Exclusive enteral nutrition provides an effective bridge to safer interval elective surgery for adults with Crohn’s disease. Aliment. Pharmacol. Ther 45:660–69 [DOI] [PubMed] [Google Scholar]
- 62.Herfarth HH, Martin CF, Sandler RS, Kappelman MD, Long MD. 2014. Prevalence of a gluten-free diet and improvement of clinical symptoms in patients with inflammatory bowel diseases. Inflamm. Bowel Dis 20:1194–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heuschkel RB, Menache CC, Megerian JT, Baird AE. 2000. Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J. Pediatr. Gastroenterol. Nutr 31:8–15 [DOI] [PubMed] [Google Scholar]
- 64.Hou JK, Bincy A, El-Serag H. 2011. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am. J. Gastroenterol 106:563–73 [DOI] [PubMed] [Google Scholar]
- 65.Hrncirova L, Machova V, Trckova E, Krejsek J, Hrncir T. 2019. Food preservatives induce proteobacteria dysbiosis in human-microbiota associated Nod2-deficient mice. Microorganisms 7:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huaman JW, Mego M, Manichanh C, Canellas N, Canueto D, et al. 2018. Effects of prebiotics versus a diet low in FODMAPs in patients with functional gut disorders. Gastroenterology 155:1004–7 [DOI] [PubMed] [Google Scholar]
- 67.Jalandra R, Dalal N, Yadav AK, Verma D, Sharma M, et al. 2021. Emerging role of trimethylamine-N-oxide (TMAO) in colorectal cancer. Appl. Microbiol. Biotechnol 105:7651–60 [DOI] [PubMed] [Google Scholar]
- 68.Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault M-C, Carbonnel F. 2010. Animal protein intake and risk of inflammatory bowel disease: the E3N prospective study. Am. J. Gastroenterol 105:2195–201 [DOI] [PubMed] [Google Scholar]
- 69.Jess T, Frisch M, Simonsen J. 2013. Trends in overall and cause-specific mortality among patients with inflammatory bowel disease from 1982 to 2010. Clin. Gastroenterol. Hepatol 11:43–48 [DOI] [PubMed] [Google Scholar]
- 70.Johnson T,Macdonald S,Hill SM,Thomas A,Murphy MS. 2006. Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut 55:356–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joossens M, De Preter V, Ballet V, Verbeke K, Rutgeerts P, Vermeire S. 2012. Effect of oligofructose-enriched inulin (OF-IN) on bacterial composition and disease activity of patients with Crohn’s disease: results from a double-blinded randomised controlled trial. Gut 61:958. [DOI] [PubMed] [Google Scholar]
- 72.Kanauchi O, Oshima T, Andoh A, Shioya M, Mitsuyama K. 2008. Germinated barley foodstuff ameliorates inflammation in mice with colitis through modulation of mucosal immune system. Scand. J. Gastroenterol 43:1346–52 [DOI] [PubMed] [Google Scholar]
- 73.Kaplan GG, Ng SC. 2017. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 152:313–21.e2 [DOI] [PubMed] [Google Scholar]
- 74.Kasti A, Petsis K, Lambrinou S, Katsas K, Nikolaki M, et al. 2022. A combination of Mediterranean and low-FODMAP diets for managing IBS symptoms? Ask your gut! Microorganisms 10:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kedia S, Ahuja V. 2018. Is the emergence of inflammatory bowel disease a prime example of “the third epidemiological transition”? Indian J. Gastroenterol 37:183–85 [DOI] [PubMed] [Google Scholar]
- 76.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, et al. 2015. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17:662–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keszthelyi D, Troost FJ, Masclee AA. 2009. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol. Motil 21:1239–49 [DOI] [PubMed] [Google Scholar]
- 78.Khademi Z, Milajerdi A, Bagher L, Esmaillzadeh A. 2021. Dietary intake of total carbohydrates, sugar and sugar-sweetened beverages, and risk of inflammatory bowel disease: a systematic review and meta-analysis of prospective cohort studies. Front. Nutr 8:707795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kok CR, Hutkins R. 2018. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr. Rev 76(Suppl. 1):4–15 [DOI] [PubMed] [Google Scholar]
- 80.Koochakpoor G, Salari-Moghaddam A, Keshteli AH, Esmaillzadeh A, Adibi P. 2021. Association of coffee and caffeine intake with irritable bowel syndrome in adults. Front. Nutr 8:632469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lamas B, Hernandez-Galan L, Galipeau HJ, Constante M, Clarizio A, et al. 2020. Aryl hydrocarbon receptor ligand production by the gut microbiota is decreased in celiac disease leading to intestinal inflammation. Sci. Transl. Med 12:eaba0624. [DOI] [PubMed] [Google Scholar]
- 82.Lamas B,Richard ML,Leducq V,Pham HP,Michel ML, et al. 2016. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med 22:598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laudisi F, Di Fusco D, Dinallo V, Stolfi C, Di Grazia A, et al. 2019. The food additive maltodextrin promotes endoplasmic reticulum stress–driven mucus depletion and exacerbates intestinal inflammation. Cell. Mol. Gastroenterol. Hepatol 7:457–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lebwohl B, Rubio-Tapia A. 2021. Epidemiology, presentation, and diagnosis of celiac disease. Gastroenterology 160:63–75 [DOI] [PubMed] [Google Scholar]
- 85.Levine A, Wine E, Assa A, Sigall Boneh R, Shaoul R, et al. 2019. Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology 157:440–50.e8 [DOI] [PubMed] [Google Scholar]
- 86.Levrat MA,Remesy C,Demigne C. 1991. High propionic acid fermentations and mineral accumulation in the cecum of rats adapted to different levels of inulin. J. Nutr 121:1730–37 [DOI] [PubMed] [Google Scholar]
- 87.Lewis JD, Sandler RS, Brotherton C, Brensinger C, Li H, et al. 2021. A randomized trial comparing the specific carbohydrate diet to a Mediterranean diet in adults with Crohn’s disease. Gastroenterology 161:837–52.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li G, Young KD. 2013. Indole production by the tryptophanase TnaA in Escherichia coli is determined by the amount of exogenous tryptophan. Microbiology 159:402–10 [DOI] [PubMed] [Google Scholar]
- 89.Li WT, Luo QQ, Wang B, Chen X, Yan XJ, et al. 2019. Bile acids induce visceral hypersensitivity via mucosal mast cell–to–nociceptor signaling that involves the farnesoid X receptor/nerve growth factor/transient receptor potential vanilloid 1 axis. FASEB J. 33:2435–50 [DOI] [PubMed] [Google Scholar]
- 90.Liu X, Wu Y, Li F, Zhang D. 2015. Dietary fiber intake reduces risk of inflammatory bowel disease: result from a meta-analysis. Nutr. Res 35:753–58 [DOI] [PubMed] [Google Scholar]
- 91.Llewellyn SR, Britton GJ, Contijoch EJ, Vennaro OH, Mortha A, et al. 2018. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology 154:1037–46.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Locke GR 3rd, Zinsmeister AR, Talley NJ, Fett SL, Melton LJ. 2000. Risk factors for irritable bowel syndrome: role of analgesics and food sensitivities. Am. J. Gastroenterol 95:157–65 [DOI] [PubMed] [Google Scholar]
- 93.MacLellan A, Connors J, Grant S, Cahill L, Langille MGI, Van Limbergen J. 2017. The impact of exclusive enteral nutrition (EEN) on the gut microbiome in Crohn’s disease: a review. Nutrients 9:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Makharia A, Catassi C, Makharia GK. 2015. The overlap between irritable bowel syndrome and non-celiac gluten sensitivity: a clinical dilemma. Nutrients 7:10417–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mars RAT, Yang Y, Ward T, Houtti M, Priya S, et al. 2020. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell 182:1460–73.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Menta PL, Andrade ME, Leocardio P, Fraga J, Silva M, et al. 2018. Gluten intake increases bacterial translocation and aggravates intestinal inflammation in experimental colitis. Clin. Nutr 37(Suppl. 1):S69 [Google Scholar]
- 97.Miao Z, Lin JS, Mao Y, Chen GD, Zeng FF, et al. 2020. Erythrocyte n-6 polyunsaturated fatty acids, gut microbiota, and incident type 2 diabetes: a prospective cohort study. Diabetes Care 43:2435–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Min YW, Park SU, Jang YS, Kim YH, Rhee PL, et al. 2012. Effect of composite yogurt enriched with acacia fiber and Bifidobacterium lactis. World J. Gastroenterol 18:4563–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moayyedi P,Quigley EM,Lacy BE,Lembo AJ,Saito YA, et al. 2014. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am. J. Gastroenterol 109:1367–74 [DOI] [PubMed] [Google Scholar]
- 100.Molinaro A, Wahlström A, Marschall HU. 2018. Role of bile acids in metabolic control. Trends Endocrinol. Metab 29:31–41 [DOI] [PubMed] [Google Scholar]
- 101.Monsbakken KW, Vandvik PO, Farup PG. 2006. Perceived food intolerance in subjects with irritable bowel syndrome—etiology, prevalence and consequences. Eur. J. Clin. Nutr 60:667–72 [DOI] [PubMed] [Google Scholar]
- 102.Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, et al. 2014. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158:300–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mutalib M, Bezanti K, Elawad M, Kiparissi F. 2016. The role of exclusive enteral nutrition in the management of orofacial granulomatosis in children. World J. Pediatr 12:421–24 [DOI] [PubMed] [Google Scholar]
- 104.Naimi S, Viennois E, Gewirtz AT, Chassaing B. 2021. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Narula N, Wong ECL, Dehghan M, Mente A, Rangarajan S, et al. 2021. Association of ultra-processed food intake with risk of inflammatory bowel disease: prospective cohort study. BMJ 374:n1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Navas-López VM, Blasco-Alonso J, Lacasa Maseri S, Girón Fernández-Crehuet F, Serrano Nieto MJ, et al. 2015. [Exclusive enteral nutrition continues to be first line therapy for pediatric Crohn’s disease in the era of biologics.] Anal. Pediatr 83:47–54 (in Spanish) [DOI] [PubMed] [Google Scholar]
- 107.Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV, et al. 2013. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 62:630–49 [DOI] [PubMed] [Google Scholar]
- 108.Nickerson KP, McDonald C. 2012. Crohn’s disease–associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLOS ONE 7:e52132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nie JY, Zhao Q. 2017. Beverage consumption and risk of ulcerative colitis. Medicine 96:e9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Niv E, Halak A, Tiommny E, Yanai H, Strul H, et al. 2016. Randomized clinical study: partially hydrolyzed guar gum (PHGG) versus placebo in the treatment of patients with irritable bowel syndrome. Nutr. Metab 13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, et al. 2010. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin. Gastroenterol. Hepatol 8:159–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pearl DS, Masoodi M, Eiden M, Brümmer J, Gullick D, et al. 2014. Altered colonic mucosal availability of n-3 and n-6 polyunsaturated fatty acids in ulcerative colitis and the relationship to disease activity. J. Crohn’s Colitis 8:70–79 [DOI] [PubMed] [Google Scholar]
- 113.Pecora F, Persico F, Gismondi P, Fornaroli F, Iuliano S, et al. 2020. Gut microbiota in celiac disease: Is there any role for probiotics? Front. Immunol 11:957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pedersen N, Ankersen DV, Felding M, Wachmann H, Végh Z, et al. 2017. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol 23:3356–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pendyala S, Walker JM, Holt PR. 2012. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 142:1100–1.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Perrin A-E, Dallongeville J, Ducimetière P, Ruidavets J-B, Schlienger J-L, et al. 2005. Interactions between traditional regional determinants and socio-economic status on dietary patterns in a sample of French men. Br. J. Nutr 93:109–14 [DOI] [PubMed] [Google Scholar]
- 117.Perrin P, Pierre F, Patry Y, Champ M, Berreur M, et al. 2001. Only fibres promoting a stable butyrate producing colonic ecosystem decrease the rate of aberrant crypt foci in rats. Gut 48:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Preidis GA, Weizman AV, Kashyap PC, Morgan RL. 2020. AGA technical review on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology 159:708–38.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prince AC,Myers CE,Joyce T,Irving P,Lomer M,Whelan K. 2016. Fermentable carbohydrate restriction (low FODMAP diet) in clinical practice improves functional gastrointestinal symptoms in patients with inflammatory bowel disease. Inflamm. Bowel Dis 22:1129–36 [DOI] [PubMed] [Google Scholar]
- 120.Raboni S, Bettati S, Mozzarelli A. 2009. Tryptophan synthase: a mine for enzymologists. Cell Mol. Life Sci 66:2391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Racine A, Carbonnel F, Chan SSM, Hart AR, Bueno-de-Mesquita HB, et al. 2016. Dietary patterns and risk of inflammatory bowel disease in Europe: results from the EPIC study. Inflamm. Bowel Dis 22:345–54 [DOI] [PubMed] [Google Scholar]
- 122.Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, et al. 2015. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 29:1395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rej A, Sanders DS, Shaw CC, Buckle R, Trott N, et al. 2022. Efficacy and acceptability of dietary therapies in non-constipated irritable bowel syndrome: a randomized trial of traditional dietary advice, the low FODMAP diet, and the gluten-free diet. Clin. Gastroenterol. Hepatol 20:2876–87.e15 [DOI] [PubMed] [Google Scholar]
- 124.Roberts CL, Keita ÅV, Duncan SH, O’Kennedy N, Söderholm JD, et al. 2010. Translocation of Crohn’s disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut 59:1331–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rogha M, Esfahani MZ, Zargarzadeh AH. 2014. The efficacy of a synbiotic containing Bacillus coagulans in treatment of irritable bowel syndrome: a randomized placebo-controlled trial. Gastroenterol. Hepatol. Bed Bench 7:156–63 [PMC free article] [PubMed] [Google Scholar]
- 126.Rufino MN,da Costa AL,Jorge EN,Paiano VF,Camparoto ML, et al. 2022. Synbiotics improve clinical indicators of ulcerative colitis: systematic review with meta-analysis. Nutr. Rev 80:157–64 [DOI] [PubMed] [Google Scholar]
- 127.Scaioli E, Sartini A, Bellanova M, Campieri M, Festi D, et al. 2018. Eicosapentaenoic acid reduces fecal levels of calprotectin and prevents relapse in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol 16:1268–75.e2 [DOI] [PubMed] [Google Scholar]
- 128.Selmin OI, Papoutsis AJ, Hazan S, Smith C, Greenfield N, et al. 2021. n-6 high fat diet induces gut microbiome dysbiosis and colonic inflammation. Int. J. Mol. Sci 22:6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Serhan CN,Yang R,Martinod K,Kasuga K,Pillai PS, et al. 2009. Maresins: novel macrophage mediators with potent anti-inflammatory and proresolving actions. J. Exp. Med 206:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sharma N, Bhatia S, Chunduri V, Kaur S, Sharma S, et al. 2020. Pathogenesis of celiac disease and other gluten related disorders in wheat and strategies for mitigating them. Front. Nutr 7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shen L, Yang Y, Ou T, Key CCC, Tong SH, et al. 2017. Dietary PUFAs attenuate NLRP3 inflammasome activation via enhancing macrophage autophagy. J. Lipid Res 58:1808–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Singh P,Grabauskas G,Zhou SY,Gao J,Zhang Y,Owyang C. 2021. High FODMAP diet causes barrier loss via lipopolysaccharide-mediated mast cell activation. JCI Insight 6:e146529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Skagen K, Trøseid M, Ueland T, Holm S, Abbas A, et al. 2016. The carnitine-butyrobetaine-trimethylamine-N-oxide pathway and its association with cardiovascular mortality in patients with carotid atherosclerosis. Atherosclerosis 247:64–69 [DOI] [PubMed] [Google Scholar]
- 134.Skrzydło-Radomańska B, Prozorow-Król B, Cichoż-Lach H, Majsiak E, Bierla JB, et al. 2020. The effectiveness of synbiotic preparation containing Lactobacillus and Bifidobacterium probiotic strains and short chain fructooligosaccharides in patients with diarrhea predominant irritable bowel syndrome—a randomized double-blind, placebo-controlled study. Nutrients 12:1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sloan TJ, Jalanka J, Major GAD, Krishnasamy S, Pritchard S, et al. 2018. A low FODMAP diet is associated with changes in the microbiota and reduction in breath hydrogen but not colonic volume in healthy subjects. PLOS ONE 13:e0201410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.So D, Whelan K, Rossi M, Morrison M, Holtmann G, et al. 2018. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am. J. Clin. Nutr 107:965–83 [DOI] [PubMed] [Google Scholar]
- 137.Sonnenburg ED,Smits SA,Tikhonov M,Higginbottom SK,Wingreen NS,Sonnenburg JL. 2016. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529:212–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Staudacher HM, Lomer MCE, Farquharson FM, Louis P, Fava F, et al. 2017. A diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and a probiotic restores Bifidobacterium species: a randomized controlled trial. Gastroenterology 153:936–47 [DOI] [PubMed] [Google Scholar]
- 139.Sun H,Ma Y,An S,Wang Z. 2021. Altered gene expression signatures by calcitonin gene–related peptide promoted mast cell activity in the colon of stress-induced visceral hyperalgesia mice. Neurogastroenterol. Motil 33:e14073. [DOI] [PubMed] [Google Scholar]
- 140.Svahn SL, Gutiérrez S, Ulleryd MA, Nookaew I, Osla V, et al. 2019. Dietary polyunsaturated fatty acids promote neutrophil accumulation in the spleen by altering chemotaxis and delaying cell death. Infect. Immun 87:e00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Szilagyi A, Ishayek N. 2018. Lactose intolerance, dairy avoidance, and treatment options. Nutrients 10:1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tjonneland A, Overvad K, Bergmann MM, Nagel G, Linseisen J, et al. 2009. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study. Gut 58:1606–11 [DOI] [PubMed] [Google Scholar]
- 143.Trøseid M, Ueland T, Hov JR, Svardal A, Gregersen I, et al. 2015. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med 277:717–26 [DOI] [PubMed] [Google Scholar]
- 144.Uebanso T, Ohnishi A, Kitayama R, Yoshimoto A, Nakahashi M, et al. 2017. Effects of low-dose non-caloric sweetener consumption on gut microbiota in mice. Nutrients 9:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vernocchi P, Del Chierico F, Putignani L. 2020. Gut microbiota metabolism and interaction with food components. Int. J. Mol. Sci 21:3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wall CL, Day AS, Gearry RB. 2013. Use of exclusive enteral nutrition in adults with Crohn’s disease: a review. World J. Gastroenterol 19:7652–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Watson H, Mitra S, Croden FC, Taylor M, Wood HM, et al. 2018. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 67:1974–83 [DOI] [PubMed] [Google Scholar]
- 148.Wiese DM, Horst SN, Brown CT, Allaman MM, Hodges ME, et al. 2016. Serum fatty acids are correlated with inflammatory cytokines in ulcerative colitis. PLOS ONE 11:e0156387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, et al. 2014. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 16:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Winston J,Shenoy M,Medley D,Naniwadekar A,Pasricha PJ. 2007. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology 132:615–27 [DOI] [PubMed] [Google Scholar]
- 151.Yamamoto T, Shiraki M, Nakahigashi M, Umegae S, Matsumoto K. 2013. Enteral nutrition to suppress postoperative Crohn’s disease recurrence: a five-year prospective cohort study. Int. J. Colorect. Dis 28:335–40 [DOI] [PubMed] [Google Scholar]
- 152.Yang Q, Gao X, Chen H, Li M, Wu X, et al. 2017. Efficacy of exclusive enteral nutrition in complicated Crohn’s disease. Scand. J. Gastroenterol 52:995–1001 [DOI] [PubMed] [Google Scholar]
- 153.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, et al. 2012. Human gut microbiome viewed across age and geography. Nature 486:222–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zheng X, Huang F, Zhao A, Lei S, Zhang Y, et al. 2017. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC Biol. 15:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zielińska M, Fichna J, Bashashati M, Habibi S, Sibaev A, et al. 2017. G protein–coupled estrogen receptor and estrogen receptor ligands regulate colonic motility and visceral pain. Neurogastroenterol. Motil 29:e13025. [DOI] [PubMed] [Google Scholar]
- 156.Zimmermann J,De Fazio L,Kaden-Volynets V,Hitzmann B,Bischoff SC. 2022. Consumption of yeast-fermented wheat and rye breads increases colitis and mortality in a mouse model of colitis. Dig. Dis. Sci 67:4422–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zito FP, Polese B, Vozzella L, Gala A, Genovese D, et al. 2016. Good adherence to Mediterranean diet can prevent gastrointestinal symptoms: a survey from southern Italy. World J. Gastrointest. Pharmacol. Ther 7:564–71 [DOI] [PMC free article] [PubMed] [Google Scholar]


