Abstract
Background
Use of a central venous catheter (CVC) in neonates is associated with an increase in nosocomial infection. Numerous strategies exist to prevent catheter‐related bloodstream infection (CRBSI); however, CRBSI continues to be a major problem. Antibiotic locking catheters is a new and promising treatment that potentially prevents this severe condition.
Objectives
To assess the effectiveness of antibiotic lock versus no antibiotic lock or alternative antibiotic lock in the prevention of catheter‐related infections in newborn infants of any gestational age during their initial stay in the neonatal unit and to study any relevant adverse effects from antibiotic lock therapy.
Search methods
Methods followed those of the Cochrane Neonatal Review Group (CNRG). We searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2014, Issue 5); MEDLINE (via PubMed); EMBASE (hosted by EBCHOST); CINAHL; abstracts from Pediatric Academic Societies, European Society for Paediatric Research and trials registries; and references cited in our short listed articles using keywords and MeSH headings, up to April 2015.
Selection criteria
We considered all trials utilising random or quasi‐random participant allocation. Participants included all newborn infants of any postmenstrual age who required any type of CVC. We compared an antibiotic lock technique with no antibiotic lock or placebo, such as heparinised saline, for any duration of time.
Data collection and analysis
We extracted data using the standard methods of the CNRG. Two review authors independently assessed the relevance and risk of bias of the retrieved records. We expressed our dichotomous results using risk ratio (RR) with their 95% confidence intervals (CIs). We assessed for heterogeneity using the I2 statistic.
Main results
We included three trials (271 infants) in this review. Two of the three included studies had an overall low risk of bias and the remaining study had high risk of selection and performance biases. The use of an antibiotic lock decreased the incidence of confirmed catheter‐related infection (typical RR 0.15, 95% CI 0.06 to 0.40; 3 studies, 271 infants) (high‐quality evidence). The typical absolute risk reduction (ARR) was 18.5% and the number needed to treat for an additional beneficial outcome (NNTB) was 5. The effect of use of an antibiotic lock on suspected catheter infection was imprecise (typical RR 0.65, 95% CI 0.22 to 1.92) (moderate quality evidence). Confirmed and suspect infection rates combined were lower in the antibiotic lock group (absolute rates, RR 0.25, 95% CI 0.12 to 0.49; rate per 1000 catheter days, RR 0.17, 95% CI 0.07 to 0.40). The ARR was 20.5% and the NNTB was 5. None of the studies report resistance to the antibiotic used during the lock treatment. There was no significant difference in the detectable serum levels of antibiotic. When the data from two studies were pooled, there were significantly fewer episodes of hypoglycaemia in the treatment arm (typical RR 0.51, 95% CI 0.28 to 0.92). There was no statistically significant difference for mortality due to sepsis between the control and intervention group.
Authors' conclusions
Based on a small number of trials and neonates, antibiotic lock solution appeared to be effective in preventing CRBSI in the neonatal population. However, as each included study used a different antibiotics and antibiotic resistance could not be reliably assessed, the evidence to‐date is insufficient to determine the effects of antibiotic lock on infections in neonates.
Plain language summary
Antibiotic lock to prevent catheter infection in infants
Background
Babies in the neonatal intensive care unit require medicines and fluids through their veins. To do this, a small tube (described as a central venous catheter, CVC) is inserted into the infant's vein through the umbilical cord or through the skin. This tube is placed just outside the heart. This tube is then used to give medicines and fluid without causing any discomfort. However, this tube does lead to an increased risk of infection, which can be life threatening. There are many measures taken to try to prevent this, but infection still occurs. This review looks at one way to prevent this infection by putting an antibiotic solution into the tube and leaving it to stay there for a certain length of time (called antibiotic lock) compared with a solution containing no antibiotic.
Study characteristics
We included three studies enrolling 271 infants in this review.
Key findings
These studies showed that infants who's tubes contained an antibiotic solution were less likely to develop an infection. One side effect of this treatment could be the development of 'super' bugs. Super bugs cause a type of infection that some antibiotics may not be able to fight. Our included studies did not show any evidence that antibiotic lock was more or less likely to produce super bugs compared with no antibiotic lock, but to show this convincingly the studies would need to be much larger. The rates of death from an infection caused by the tubes were not reduced by the antibiotic lock.
Quality of the evidence
Relatively few infants were enrolled in the three included studies. Two of the three included studies had overall low risk of bias, and the remaining study had high risk of bias from two sources: i). Selection bias, namely, the manner in which group allocation took place (based on the room infants were nursed in) posed a major concern as to whether the allocation was truly random, and ii). Performance bias, namely, non‐blinding of the people who were involved in the care of the infants might have contributed to differential care and/or expectations which might have affected the results.
Conclusions
Based on a small number of trials and infants, antibiotic lock solution appears to be effective in preventing catheter‐related blood infections in infants. However, as each included study used a different antibiotic and antibiotic resistance could not be reliably assessed, the evidence to‐date is insufficient to determine the effects of antibiotic lock on infections in infants.
Summary of findings
Summary of findings for the main comparison. Antibiotic lock for the prevention of catheter‐related sepsis in neonates.
| Antibiotic lock for the prevention of catheter‐related infection in neonates | ||||||
|
Patient or population: newborn infants who require a central venous catheter Settings: neonatal intensive care unit Intervention: central venous catheters with antibiotic lock | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic lock | |||||
| CRBSI (confirmed) Clinical and microbiological assessments | Study population | RR 0.15 (0.06 to 0.40) | 271 (3 studies) | ⊕⊕⊕⊕ high1 | ‐ | |
| 217 per 1000 | 33 per 1000 (13 to 87) | |||||
| Moderate | ||||||
| 208 per 1000 | 31 per 1000 (12 to 83) | |||||
| CRBSI (suspected) Clinical assessment | Study population | RR 0.65 (0.22 to 1.92) | 271 (3 studies) | ⊕⊕⊕⊝ moderate2 | ‐ | |
| 58 per 1000 | 38 per 1000 (13 to 111) | |||||
| Moderate | ||||||
| 38 per 1000 | 25 per 1000 (8 to 73) | |||||
| Total CRBSI (confirmed and suspected) Clinical and microbiological assessments | Study population | RR 0.25 (0.12 to 0.49) | 271 (3 studies) | ⊕⊕⊕⊕ high3 | ‐ | |
| 275 per 1000 | 69 per 1000 (33 to 135) | |||||
| Moderate | ||||||
| 286 per 1000 | 72 per 1000 (34 to 140) | |||||
| Mortality Clinical assessment | Study population | RR 0.12 (0.01 to 2.13) | 103 (1 study) | ⊕⊝⊝⊝ very low4,5 | ‐ | |
| 75 per 1000 | 9 per 1000 (1 to 161) | |||||
| Moderate | ||||||
| 76 per 1000 | 9 per 1000 (1 to 162) | |||||
| Number of infants with hypoglycaemia Blood sugar level: glucose meter or laboratory‐based plasma glucose level | Study population | RR 0.51 (0.28 to 0.92) | 168 (2 studies) | ⊕⊕⊕⊕ high | ‐ | |
| 306 per 1000 | 156 per 1000 (86 to 281) | |||||
| Moderate | ||||||
| 305 per 1000 | 156 per 1000 (85 to 281) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CRBSI: catheter‐related bloodstream infection; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Confirmed CRBSI was reduced by 85% from the pooled estimate (typical RR 0.15, 95% CI 0.06 to 0.40). 2 There was a wide 95% CI in the estimate of suspected CRBSI (typical RR 0.65, 95% CI 0.22 to 1.92). 3 Total CRBSI (confirmed and suspected) was reduced by 75% (typical RR 0.25, 95% CI 0.12 to 0.49). 4 The only study that provided data for mortality from CRBSI was Filippi 2007. We judged the study at high risk of bias for random sequence generation, allocation concealment, and blinding of participants and personnel (refer to Characteristics of included studies table for details). 5 There was a wide 95% CI in the estimate of mortality from CRBSI (RR 0.12, 95% CI 0.01 to 2.13).
Background
Description of the condition
Central venous catheters (CVCs), including umbilical venous catheters (UVCs), peripherally inserted central catheters (PICCs, percutaneous central venous catheters, PCVCs or 'long‐lines'), femoral lines and subcutaneously tunnelled catheters are commonly used in the neonatal intensive care unit (NICU) for intravenous nutrition, administration of medications and monitoring (Borghesi 2008).
UVCs are usually inserted on the first day of life and should be removed by day 14 (Loisel 1996). PICCs are 'un‐tunnelled' catheters, inserted into the vein close to the site where it enters the skin. For those infants who require long‐term central venous catheterisation or if intravenous access cannot be achieved by any other means, 'tunnelled' catheters (usually with a cuff separating the intravenous and subcutaneous portions) can be surgically implanted by venous dissection (de Brito 2010). CVCs are life‐saving devices, especially for sick and extremely preterm infants with little or no peripheral venous access; however, these catheters have complications, bloodstream infection (BSI) being the most prevalent (O'Grady 2011).
The terms used to describe intravascular infections are often used interchangeably and inaccurately. Catheter‐related bloodstream infection (CRBSI) is the clinical term used to describe formally confirmed BSI associated to the central catheter. Central line‐associated bloodstream infection (CLABSI) is the term used to describe BSI where the catheter is the most likely source (Horan 2008). CLABSI is used for surveillance purposes and may overestimate actual CRBSI (O'Grady 2011).
The reported incidences vary with case definition and with the demographic characteristics of the population studied. The National Healthcare Safety Network (NHSN) classifies CLABSI for infants as laboratory‐confirmed bloodstream infection (LCBI) or clinical sepsis where a central line was in situ at the time of, or within 48 hours before, the onset of the event (NHSN 2011). CLABSI reported to the NHSN demonstrated a higher rate of infection in extremely low birthweight infants. CLABSI for infants weighing 750 g or less were 3.1/1000 catheter‐days decreasing to 1.4/1000 catheter‐days for infants weighing more than 1501 g (Dudeck 2011). Other studies showed infection rates ranging from 0% to 29% of catheters placed, and from 2/1000 catheter‐days to 49/1000 catheter‐days (Cartwright 2004; Chien 2002; Garland 2008; Hoang 2008; Ohki 2008; Olsen 2009; Van de Zwet 2005). Infants, particularly very low birthweight with CLABSI, have a higher risk of mortality with attributable mortality ranging from 4% to 20% (Saint 2000), and a range of important morbidities including the need for intensive care, mechanical ventilation, bronchopulmonary dysplasia, necrotising enterocolitis, retinopathy of prematurity and prolonged hospitalisation (Adams‐Chapman 2006; Bassler 2009; Chapman 2003; Saint 2000).
CLABSI occurs when micro‐organisms adhere to the intraluminal or extraluminal surface of the catheter. Micro‐organisms can enter the catheter from colonisation of the catheter ports (hubs) and insertion sites, contaminated intravenous fluids and injection devices, and from hematogenous dissemination from other sources of infection (Mermel 2001). Once they have entered the catheter, they can adhere and become incorporated into a biofilm made up of extracellular polymers. In this state, micro‐organisms are highly resistant to antimicrobial treatment and are tenaciously bound to the surface catheter enabling sustained colonisation, ultimately leading to hematogenous dispersal. This biofilm makes treatment with antibiotics challenging (Ramirez de Arellano 1994), and often leads to the catheter being removed (Vanholder 2010). Biofilms on indwelling catheters may be composed of Gram‐positive or Gram‐negative bacteria or yeasts. Bacteria commonly isolated from these devices include the Gram‐positive Enterococcus faecalis, Staphylococcus aureus, Staphylococcus epidermidis and Streptococcus viridans; and the Gram‐negative Escherichia coli, Klebsiella pneumonia, Proteus mirabilis and Pseudomonas aeruginosa (Ryder 2005). Biofilm formation can depend on the time duration the catheter has been in situ and have been found both on the internal and external surface in catheters used for more than 48 hours (Machado 2009). Short‐term (fewer than 10 days) catheters have greater biofilm formation on the external surface whereas long‐term catheters have more biofilm formation on the catheter inner lumen (Raad 1993). One study using molecular epidemiology to examine the pathogenesis of neonatal CRBSI found that 67% were intraluminally acquired and 20% were extraluminally acquired (Garland 2008). The most common causative pathogens for late‐onset sepsis are coagulase‐negative staphylococci (accounting for approximately 40%), Staphylococcus aureus (approximately 24%) and Gram‐negative bacilli (approximately 19%) (de Brito 2010; O'Grady 2002).
Numerous strategies and recommendations to reduce and prevent catheter‐related infection have been published. These include strict aseptic techniques during insertion, accessing the catheter lumen for blood sampling administration of medications or fluids and dressing changes (O'Grady 2002; O'Grady 2011; Sannoh 2010; Vanholder 2010), appropriate preparation of the skin (O'Grady 2011), and the use of needleless intravascular catheter systems (Yebenes 2004). Furthermore, in‐line filters are commonly used both to prevent infection and for other equally important reasons, such as removal of air and chemical precipitate (Ball 2003).
These quality improvements are often introduced as 'care bundles' and numerous studies have demonstrated a reduction in CLABSI. Bundles commonly describe insertion bundles and maintenance bundles. These include maximum sterile barrier precautions, namely hat, mask, sterile gown, gloves and full‐sized drapes (O'Grady 2011), hand hygiene standards and appropriate skin disinfectant during insertion. Strategies for catheter maintenance include appropriate hand hygiene, catheter site evaluation, aseptic techniques when accessing the line including 'scrub the hub' and prompt removal when no longer necessary (Kaplan 2011; Miller 2010; Pronovost 2006; Schulman 2011; Wirtschafter 2010). The success of the care bundles are measured by infection rates before and after implementation, there are no randomised controlled trials (RCTs) on the effectiveness of these bundles, although there are cluster‐randomised studies where centres may be allocated to the 'care bundle' arm and to standard care (Lee 2009). Care bundles in adult studies show a reduction post implementation with rates reducing by two‐thirds (Pronovost 2006). Similar reductions have been shown in paediatric intensive care units (PICU) with a multi‐institutional study incorporating 27 PICUs showing a decrease in CLABSI from 5.1/1000 catheter‐days to 3.1/1000 catheter‐days; it was also reported that it was the maintenance bundle that had the greatest impact (Miller 2010). Other quality improvement collaborations in NICUs demonstrate a reduction of CLABSI from 4.32/1000 catheter‐days to 3.22/1000 catheter‐days (Wirtschafter 2010) and from 6.4 catheter‐days to 2.1/1000 catheter‐days (Schulman 2011). Other multicentre neonatal studies have also demonstrated positive results following care‐bundle implementation showing signification reduction in line infection when the bundles were adhered to for ≥ 90% (Kaplan 2011). Another prospective neonatal study that standardised catheter hub care and implemented an education programme reduced CRBSI from 23/1000 catheter‐days to 10/1000 catheter‐days in PICC lines (Sannoh 2010). Despite these strategies, catheter‐related infections remain a major problem in critically ill people, including newborn infants and other methods are required to reduce rates further.
Description of the intervention
The antibiotic lock technique consists of a high‐concentration antibiotic solution instilled into the catheter lumen, filling the dead space for a pre‐specified dwell‐time, usually a few hours (generally 12 hours). The volume of dead space is typically provided by the catheter manufacturer. Other solutions used to instil the lumen of the catheter include 70% ethanol, which is both antimicrobial and fibrinolytic (Wales 2011), and heparinised saline, which reduces essential nutrients for bacterial growth (Rosett 1980). The antibiotics chosen are those that would be empirically effective against the most common types of organisms causing CRBSI, including vancomycin, gentamicin, ciprofloxacin, minocycline, amikacin, cefazolin, cefotaxime and ceftazidime (Cicalini 2004), and are usually used in combination with an anticoagulant, such as heparin. For the antibiotics to be able to penetrate the biofilm, high drug concentrations, 100 to 1000 times higher than for treatment, are required for an extended period of time (Carratalà 2002). Therefore, it is imperative that the stability and compatibility are taken into consideration when selecting antibiotics. The antibiotic needs to be stable for the determined dwell‐time as well as being compatible with other medication combinations, such as heparin.
Current recommendations are to use only an antibiotic lock solution, to prevent central line infection, in people with long‐term catheters and a history of multiple CRBSI despite adherence to maximal aseptic techniques (O'Grady 2011). The use of the antibiotic lock therapy for 'salvage therapy' for CRBSI is generally recommended for uncomplicated infections with Staphylococcus epidermidis (Messing 1988; O'Grady 2002).
How the intervention might work
Biofilm formation is most frequently found on the intraluminal surface of the catheter, and, if this can be prevented from forming, it may be possible to prevent CLABSI. By instilling the central catheter with high‐concentration antibiotics in its lumen over a pre‐determined time period, it is hypothesised that the antibiotic will diffuse down the concentration gradient into any biofilm produced by colonising micro‐organisms in the wall of the line to sterilise it. This may prevent colonisation, subsequent line infections, and related mortality and morbidities, and increase the duration of catheter use (Cicalini 2004). Using this method alongside the other confirmed techniques may assist in further reducing infection rates.
Why it is important to do this review
Antibiotic locking catheters are a new and promising treatment that potentially prevent catheter‐related infections. There are no recommendations for dwell time or dose regimens for antibiotic lock solution in central catheters in infants.
Newborn infants, especially preterm infants, are vulnerable to interruptions in intravenous fluid infusions, and may experience adverse effects such as hypoglycaemia during the time when infusions have to be stopped for the antibiotic lock. Furthermore, as the concentration of antibiotic used in the lock is high, it is possible that preterm infants may experience antibiotic overdose or toxicity. There is also the concern that antibiotic lock solutions will increase antibiotic resistance and potential increase resistant organisms (O'Grady 2002). Finally, the primary type of CVC used in the NICU is the un‐tunnelled peripherally inserted catheter whereas most of the experiences with antibiotic lock technique are in tunnelled CVCs.
Therefore, we performed a systematic review to evaluate the evidence on the use of lock treatments for the prevention of CRBSI in infants and address the safety concerns discussed to obtain an accurate estimate of the benefits and risks of the intervention.
Objectives
To assess the effectiveness of antibiotic lock versus no antibiotic lock or alternative antibiotic lock in the prevention of catheter‐related infections in newborn infants of any gestational age during their initial stay in the neonatal unit and to study any relevant adverse effects from antibiotic lock therapy.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised, quasi‐randomised and cluster randomised trials for this review.
Types of participants
All newborn infants of any postmenstrual age who required CVCs. The CVCs included single‐lumen catheters such as UVCs and percutaneously inserted catheters (long‐lines) or multi‐lumen catheters such as femoral catheters and surgically implanted catheters.
Types of interventions
Antibiotic lock treatment including any type of antibiotics using any type of diluent solution (saline, heparinised saline), of any duration, in any type of central catheter compared with no antibiotic lock or alternative antibiotic lock.
Possible comparisons:
antibiotic lock (using any type of antibiotics of any duration, in any type of central catheter) versus no antibiotic lock;
antibiotic lock therapy versus another antibiotic lock therapy;
antibiotic lock therapy versus alcohol lock solution.
We excluded studies that used antibiotic lock therapy to treat confirmed central catheter infection.
We placed no limits on the minimum and maximum catheter indwelling duration.
Types of outcome measures
Primary outcomes
Rates of confirmed catheter‐related infection.
Rates of suspected catheter‐related infection.
Combined rates of confirmed and suspected catheter‐related infection.
Confirmed catheter‐related infection is defined as:
CRBSI as defined by the Centers for Disease Control and Prevention (CDC) criteria for CRBSI that were relevant to infants, as listed in Appendix 1 (O'Grady 2002);
as there is no consensus on the minimal number of factors required to satisfy a diagnosis of catheter‐related infection, for the purpose of this review, we accepted various definitions adopted by the author of each study, as long as the items included in their definitions were those contained in this set of diagnostic criteria. We accepted definitions that were not consistent with these diagnostic criteria, as long as the study authors justified their definitions with validated sources.
Rates of suspected catheter‐related infections defined as:
CLABSI utilising LCBI or clinical sepsis, as listed in Appendix 1 (NHSN 2011; O'Grady 2011), or diagnosed at the discretion of the physician in‐charge as long as the diagnosis made could be justified with validated sources.
Secondary outcomes
We assessed the following outcomes, where available, throughout the study period, namely, during the catheter in‐dwelling time.
Number of catheters removed before they were no longer clinically required.
Mortality.
Number of catheters occluded.
Episodes of thrombosis.
Episodes of thrombophlebitis.
Skin irritation.
Effect of treatment on blood glucose levels, for example, hypoglycaemia defined as plasma glucose level of less than 45 mg/dL (less than 2.5 mmol/L).
Number of systemic adverse events with medication, such as toxicity or allergic reactions.
Number of catheters with resistant organism cultured at removal.
Length of stay in the NICU and overall hospital stay (days).
* Mortality due to sepsis added post hoc.
Search methods for identification of studies
See: Cochrane Neonatal Review Group (CNRG) search strategy.
Electronic searches
We used the strategy for the CNRG specialised register. The review authors undertook a comprehensive search including the Cochrane Central Registry of Trials (CENTRAL, The Cochrane Library, April 2015, issue 4), MEDLINE (1966 to April 2015), EMBASE (1980 to April 2015) and CINAHL (1982 to April 2015) using the following MeSH terms or text words: "indwelling catheters" OR "catheterization, central venous" OR "venous or vein and catheter" OR "CVC" OR "central venous catheter" OR "CVL" OR "Central vein line" OR "central venous line" OR "PICC" OR "peripherally inserted central catheters" AND "antibacterial agents" OR "antibiotics" OR "antimicrobial" OR "antibiotic lock" AND "infant, newborn" OR "neonat*" AND "controlled clinical trial" OR "randomised controlled trial" OR "quasi‐randomised controlled trial". We used no language restrictions, and made all efforts to have reports in a foreign language translated.
Searching other resources
We searched for unpublished trials from the clinical trials registries (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp). We searched the abstracts and proceedings of major international paediatric and neonatal meetings such as the Pediatric Academic Societies (PAS) and European Society for Paediatric Research annual meeting, available at Abstracts2view. We also searched the proceedings and abstracts of the Perinatal Society of Australia and New Zealand.
Data collection and analysis
Selection of studies
We used the standard methods of The Cochrane Collaboration and its CNRG. The review authors independently assessed the methodological quality of each trial. We then screened these studies for inclusion in the analysis, using pre‐defined criteria, which included study design, relevant intervention, neonates and outcomes. Although not needed, we planned to utilise a referee (Australasian Regional Co‐ordinator for the CNRG) for any unresolved differences.
Data extraction and management
Two review authors independently assessed the methodological quality of each trial and extracted data. Each review author used the same, specifically designed data sheet. We compared results and resolved differences by discussion.
Assessment of risk of bias in included studies
We assessed all included studies for risk of bias, using the standard approach described in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). This included random sequence generation; allocation concealment; blinding of participants, personnel and assessors; incomplete outcome data; selective outcome reporting; and any other issues (e.g. extreme baseline imbalance). The assessors assigned a judgement of 'low risk of bias', 'high risk of bias' or 'unclear risk of bias' for each item.
Random sequence generation
We considered random sequence generation 'low risk' if the participant had an equal chance of being randomised to a group. Low risk methods include the use of a random number table, utilisation of a computer random number generator, tossing a coin, shuffling cards of envelopes or throwing of a dice. We made a 'high risk' judgement when sequence generation occurred using a non‐random component such as by date of birth, hospital number, date of admission, availability of the intervention or allocation by judgement of the clinician. When insufficient information was given to permit judgement of 'high' or 'low' risk of bias, we describe the study as being at an 'unclear risk' of bias attributable to sequence allocation.
Allocation concealment
We considered allocation concealment to be 'low risk' when the investigators enrolling participants could not foresee a participants assignment. 'Low risk' methods include central allocation using a telephone, web‐based or pharmacy‐controlled randomisation, or sequentially numbered opaque, sealed envelopes. We made a 'high risk' judgement if allocation was based on using an open random allocation schedule, assignment envelopes without using the appropriate safeguards, alternate rotation, date of birth or hospital number. When insufficient information was given to permit judgement of 'low' or 'high' risk, we described the study as 'unclear risk' of bias attributable to allocation concealment.
Blinding of participants, personnel and assessors
Performance or detection bias can occur when there is knowledge of the allocated intervention. We deemed a 'low risk' of bias when blinding occurred for the participant, personnel and outcome assessors and it was unlikely that this blinding could have been broken or when there was no blinding or it was incomplete, but we judged that this would not have influenced the outcome. We considered a judgement of 'high risk' if there was no blinding or incomplete blinding and the outcome would have been influenced by the lack of blinding. If the study did not address this issue or insufficient information was given to permit a 'low' or 'high' risk judgement, we described the study as 'unclear risk' of bias attributable to blinding.
Incomplete outcome data
We judged incomplete data to have been handled appropriately when reported completely, including attrition rates and exclusions. We judged a 'low risk' of bias when there were no missing data, missing outcome data balanced in numbers across the intervention groups, reasons for missing outcome data were unlikely to be related to true outcome and the missing outcome data were unlikely to have a clinically relevant impact on the results. Methods considered posing a 'high risk' of bias included reasons for missing data likely to be related to true outcome. We made a judgement of 'unclear risk' if the study did not address this outcome or if there was insufficient reporting of attrition to permit judgement of 'low' or 'high' risk.
Selective reporting
We judged a study to be 'low risk' if a protocol was available and all pre‐specified outcomes were reported. If there was no available protocol, the study was assigned 'low risk' if it was clear that the published report included all expected outcomes, including those that were pre‐specified. We made a 'high risk' judgement if not all the pre‐specified primary outcomes were reported, the primary outcomes were reported using measurements that were not pre‐specified, primary outcomes reported were not pre‐specified and no clear justification was provided, the outcomes were reported incompletely or the study did not report a key outcome that would be expected. We considered insufficient information to permit judgement of 'low' or 'high' risk as 'unclear risk' for selective reporting.
Other bias
We noted any other potential threats to validity and judged them to be 'high' or 'low' risk of bias. We judge the study to be an 'unclear risk' when there may have been risk of bias but there was insufficient information to assess whether an important risk of bias existed.
Appendix 2 provides a detailed description of the criteria on which the judgements were based.
In addition, we selected five clinically important outcomes for assessment in 'Summary of findings' tables following the GRADE approach described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the GRADE profiler (GRADEPro) to create tables by each comparison and for the general population. See 'Summary of findings' table.
Measures of treatment effect
We used the standard methods of the CNRG to synthesise the data. We reported the risk ratio (RR) and the risk difference (RD) with their 95% confidence intervals (CIs) for dichotomous outcomes. For statistically significant results, we calculated the corresponding number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH). For continuous outcomes, we planned to report mean differences (MD) and 95% CIs. For continuous outcomes measured using different scales, we planned to report the standardised mean difference (SMD) and 95% CI.
Unit of analysis issues
We planned to assess the unit of analysis issues in the included studies in two possible ways by which they may have arisen
multiple enrolments of the same infants from either individually randomised trial or cluster randomised trials; AND
NICU clustering in cluster randomised trials.
Multiple enrolments
We assessed for multiple enrolments in the included studies. We found no evidence of multiple enrolments of the same infant.
Cluster‐randomised trials
We had planned to include cluster‐randomised trials, although none was identified by the searches to date. In future updates of the review, we will follow the guidance of the Cochrane Handbook for systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We obtained a drop‐out rate for each study. We considered a drop‐out rate that is equal to or greater than the event rate of the control group as significant. We did not identify a significant drop‐out rate in the included studies.
We examined whether the study authors had performed an intention‐to‐treat (ITT) analysis by assessing whether this is stated in the methods section of the paper, and confirmed this by cross‐checking the number of participants initially randomised and the total number analysed. We included a description on whether ITT was followed in the 'Characteristics of included studies' table, and incorporated this into our overall judgement of the risk of bias under the heading of 'Were incomplete outcome data adequately addressed?'
Assessment of heterogeneity
We examine heterogeneity among the trials in each analysis by inspecting forest plots and used the Chi2 test and the I2 statistic (Higgins 2011). We considered a P value of less than 0.10 in the Chi2 test as suggestive evidence that significant heterogeneity may be present. In addition, we quantified heterogeneity using the I2 statistic as follows: less than 25% (no heterogeneity), 25% to 49% (low heterogeneity), 50% to 74% (moderate heterogeneity) and greater than 75% (high heterogeneity).
Assessment of reporting biases
We would have screened for publication bias by using a funnel plot if there were sufficient number of studies (at least 10) included in the analysis. If publication bias was suspected (i.e. significant asymmetry was found after a visual inspection of the funnel plot), we would have included a statement in our results with a corresponding note of caution in our discussion. Since our review did not include 10 studies, this was not applicable.
Data synthesis
We used a fixed‐effect model for the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We proposed the following subgroup analyses:
antibiotic lock therapy for preterm infants (less than 28 weeks' gestation) with CVCs versus no antibiotic lock therapy;
antibiotic lock therapy for newborn infants with gastrointestinal conditions (e.g. complicated necrotising enterocolitis and gastroschisis) and CVCs versus no antibiotic lock therapy;
antibiotic lock therapy in peripherally un‐tunnelled CVCs versus tunnelled catheters.
We were unable to perform any subgroup analysis.
Sensitivity analysis
Data permitting, we planned a sensitivity analysis to see if results differed by quality of included studies. This was not required.
'Summary of findings' table (added post hoc)
Although not stated in the original protocol, we assessed the quality of evidence for selected outcomes using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. This methodology considers RCTs to be high‐quality evidence that may be 'downgraded' due to limitations in any of five areas: design (risk of bias), inconsistency, imprecision, indirectness and publication bias (Guyatt 2011a; Guyatt 2011b).
We evaluated inconsistency by assessing similarity of point estimates, extent of overlap of CIs and statistical criteria including test for heterogeneity (I2 statistic). We downgraded the quality of evidence when inconsistency was large and unexplained (i.e. some studies suggested important benefit and others no effect or harm without a clinical explanation) (Guyatt 2011c). Imprecision was assessed according with the 95% CI around the pooled estimation (Guyatt 2011d). When trials were conducted in populations other than the target population, the GRADE framework suggests downgrading the quality of evidence because of indirectness (Guyatt 2011e). Information on publication bias was taken from data reported on trials in each included systematic review.
We selected the following outcomes for inclusion in the 'Summary of findings' table: CRBSI (confirmed based on clinical and microbiological assessments); CRBSI (suspected based on clinical assessment; total CRBSI (confirmed and suspected based on clinical and microbiological assessments); mortality from CRBSI (based on clinical assessment) and number of infants with hypoglycaemia (blood sugar level: glucose meter or laboratory‐based plasma glucose level).
We used GRADE profiler to produce tables by each comparison and for the general population (GRADEpro 2008). A summary of the risk estimates and the grading of the evidence are provided in Table 1.
Results
Description of studies
See: Characteristics of included studies; Characteristics of ongoing studies; Characteristics of studies awaiting classification.
Results of the search
Our preliminary search yielded 31 studies. After removing duplicates and excluding studies based on titles and abstract, six studies remained for subsequent review. We assessed these six studies in detail and selected three studies for final inclusion. While of the three studies were not included in the final list, one is an ongoing study and two are awaiting classification. Figure 1 shows the process of screening and selection of the studies.
1.
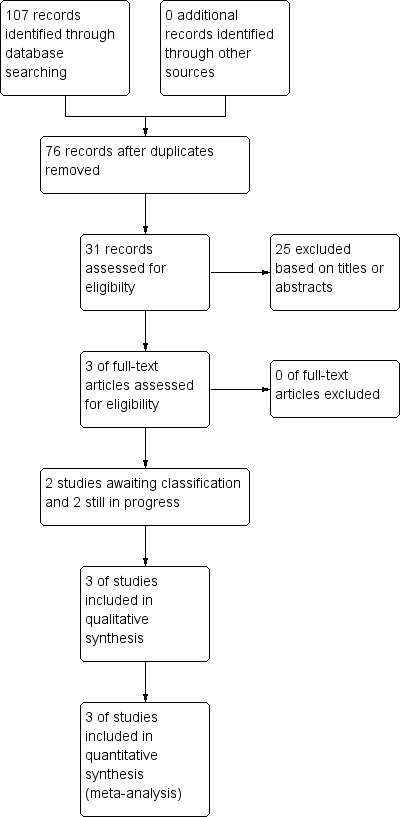
Study flow diagram.
Included studies
We included three studies in this review involving 271 infants (Filippi 2007; Garland 2005; Seliem 2010). Clinical details concerning the neonates, interventions and outcomes are given in the Characteristics of included studies table.
One study was conducted in the US (Garland 2005), one study in Italy (Filippi 2007), and one study in Egypt (Seliem 2010).
Garland 2005 randomised 90 neonates who were admitted to the NICU and required a PICC to one of two lock protocols. The control group's catheter was locked twice daily with 0.4 mL of heparinised normal saline (10 international units (IU)/mL). The intervention group's catheter was locked twice daily with 0.4 mL of heparinised normal saline (10 IU/mL) containing vancomycin (25 μg/mL). Catheters were locked for either 20 minutes for infants primarily fed via parental hyperalimentation or 60 minutes when enteral feeds exceeded 20 mL/kg/day. The same conditions existed for both groups. Catheters were inserted using maximal sterile precautions, including a sterile mask, cap, gloves, gown and a large sterile drape. Insertion sites were disinfected with 10% povidone‐iodine and catheters were dressed with a polyurethane film dressing. Catheter sites were cleansed and re‐dressed on a weekly basis or as needed. Intravenous tubing was changed every three days when used for hyperalimentation and every 24 hours when used for intralipid therapy. Needle‐less access ports were not used during the trial. Catheter hubs were cleansed with alcohol whenever the hub was accessed.
Participant baseline characteristics were similar in both groups and both groups had similar severity of illness, catheter location site and ease of insertion, mean number of catheter manipulations per day, lipid and hyperalimentation days, and duration of catheter placement.
The primary outcome measures were definite, probable and definite plus probable CRBSI and nosocomial colonisation by vancomycin resistant Gram‐positive bacteria during the study. Safety and tolerance were a primary outcome and any adverse effects potentially ascribable to the catheter lock regimen were to be reported. Infection was defined as definite CRBSI by signs of sepsis and positive peripheral blood culture and concordant colonisation of catheter hub or tip and the infant was treated for seven days and no other source of infection was identified (coagulase‐negative staphylococci clonal concordance was confirmed by restriction‐fragment deoxyribonucleic acid (DNA) subtyping). Probable CRBSI was defined using signs of sepsis and either positive peripheral blood cultures for coagulase‐negative staphylococci, with concordant colonisation of the catheter hub (but DNA subtype was not done) or a blood culture through the catheter positive (peripheral sterile or not done) for the same organism from the catheter hub or tip (coagulase negative staphylococci clonal concordance was confirmed by restriction‐fragment DNA subtyping) and no other source of infection was identified and the infants were treated for seven days.
Secondary outcomes included BSI without a source and all nosocomial BSIs.
Filippi 2007 enrolled 103 neonates with a non‐medicated CVC in situ for more than 24 hours. Neonates who were admitted to room one received antibiotic lock treatment and neonates admitted to room two formed the control group. The antibiotic lock group's catheter was locked with 0.3 mL of heparinised normal saline (10 IU/mL) containing fusidic acid 4 mg/mL. The control group's catheter was locked with 0.3 mL of heparinised normal saline (10 IU/mL). The solution was infused once a day when parental nutrition or lines were changed to reduce the number of line manipulations. The solution was maintained in the catheter for 30 to 60 minutes, based on individual clinical conditions. Mean duration of the dwell time was 33 minutes. The same conditions existed for both groups, all neonates received amoxicillin and gentamycin for 10 days and fluconazole for the first month of life. Catheters were inserted using a sterile technique. The skin surface surrounding the insertion point was disinfected with 10% povidone‐iodine. UVCs used were Argyle TM (Kendall, Tullamore, Australia) and PICCs were Premicath or Nutriline (Vygon Medical Products, Aachen, Germany). A transparent dressing was used to cover the insertion site. Intravenous tubings were changed daily and catheter hubs were cleansed with 2% chlorhexidine every time they were accessed. A UVC was inserted on admission for 101 neonates, the remaining two had a PICC. After UVC removal, a PICC was placed in 39 infants. No infant had both catheters in place at the same time.
Participant demographic data were statistically similar in both groups. However, infants in the antibiotic lock group had lower gestation and higher incidence of PICC placement. Both groups had similar clinical conditions, such as intraventricular haemorrhage, necrotising enterocolitis, patent ductus arteriosus and respiratory distress syndrome. Catheter insertion sites and number of catheter manipulations were not discussed.
Removal of the lock solution was attempted each time; however, it was only recovered 29% of the time. When removal was unsuccessful, the catheter was flushed with normal saline and infusions were recommenced.
Outcomes included the number of colonisations; and definite, suspected and definite plus suspected CRBSI. Infection was defined as: definite CRBSI by one positive blood culture with concordant colonisation of the catheter hub or tip, clinical signs of sepsis and no other apparent source of infection; suspected CRBSI by a positive culture of the catheter hub or tip, clinical signs of sepsis, no other source for BSI, with negative or not concordant blood culture, colonisation by a positive culture of catheter hub or tip with neither concordant blood culture nor clinical signs of sepsis or non‐catheter related sepsis by positive blood culture with signs of infection but negative culture of catheter hub or tip.
Seliem 2010 randomised 97 term and preterm neonates, who were admitted to the NICU and were expected to require a UVC for at least 48 hours, to either lock A or lock B protocols. In the Lock A group, UVC was flushed with 0.4 mL of heparinised normal saline (10 IU/mL) twice daily for 20 minutes. In the Lock B group, UVC was flushed with 0.4 mL of heparinised saline that contained amikacin (1.5 mg/mL) twice daily for 20 minutes. The same conditions existed for both groups. UVCs used were single lumen 5.0 French gauge polyvinyl chloride end hole catheters. Catheters were inserted using maximal sterile barriers, including the use of sterile gloves, gown, mask and large drape. The site was disinfected with 10% povidone‐iodine and inserted to keep the tip just above the diaphragm, catheters higher than this were pulled back following x‐ray. Catheters lower than this were removed and re‐inserted. The umbilical stump was cleansed on a daily basis with 70% alcohol. Intravenous tubing was changed every 24 hours using strict sterile technique by two nurses, one wearing sterile gloves, cap, gown and mask to handle all the sterile equipment. Catheter hubs were cleansed with 70% alcohol whenever the hubs were accessed. Catheters were removed when no longer required or on day 14.
Participant demographics were similar in both groups, and both group had similar mean number of catheter dwells and catheter duration. The data did describe neonate illness severity score.
Outcomes included definite, probable and definite plus probable CRBSI; BSI infection without a source and all nosocomial BSIs. Definite CRBSI was defined by positive peripheral blood culture concomitant with positive blood culture from catheter or catheter tip grew the same species AND clinical sign of sepsis AND no other apparent source of infection. Probable CRBSI was defined by a positive peripheral blood culture and positive catheter blood culture that grew a different species OR positive blood cultures from the catheter or catheter tip and the peripheral sample was sterile in the presence of clinical signs of infection.
Excluded studies
We did not exclude any of the studies identified.
Risk of bias in included studies
Data on the 'risk of bias' assessment of the three included trials are described in the 'Risk of bias' section of the Characteristics of included studies table and presented in Figure 2. We found two studies to have a low risk of bias and one study had a high risk of bias. Figure 3 provided a graphical summary of the overall risk of bias.
2.
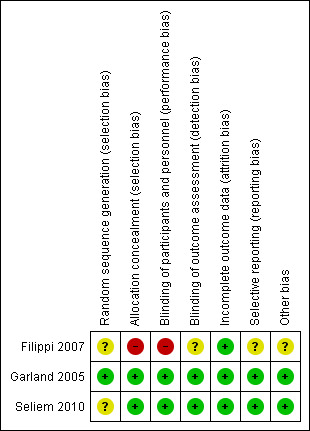
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
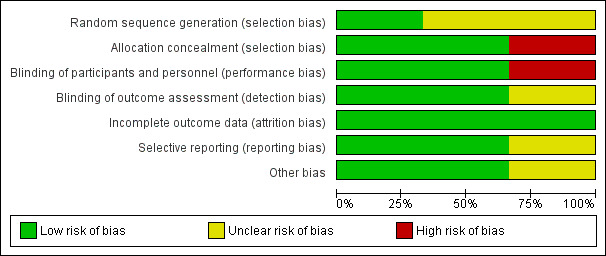
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
To evaluate selection bias, we assessed random sequence generation and allocation concealment. Only one of the studies described the process of randomisation (Garland 2005). This was performed using a computer‐generated randomisation sequence, therefore we deemed this low risk. The other studies did not provide an explanation for the procedure for randomisation, and, therefore, it remains unclear whether sequence generation was random. Therefore, we rated the risk for selection bias in these studies as unclear. One of the studies allocated treatment based on the room the infants were admitted to, therefore allocation concealment was not possible (Filippi 2007). Infants admitted to room one received antibiotic lock and neonates in room two formed the control group and catheters were locked with heparinised saline. We deemed the risk for allocation concealment in this study as high. The other two studies described their method of allocation concealment in detail (Garland 2005; Seliem 2010). In Garland 2005, randomisation was performed by the pharmacist and was kept in a locked pharmacy cabinet, thus maintaining allocation concealment. Infants were allocated to lock one or lock two. Seliem 2010 randomised infants prior to insertion using opaque sealed envelopes containing the randomisation sequence, which was kept in a locked cabinet. We judged both of these studies as having a low risk of bias for allocation concealment.
Blinding
To evaluate performance bias, we assessed the blinding of participants and personnel. One study described in detail that the clinician and participants were blinded to the treatment received (Seliem 2010). The study nurse prepared the lock solution, following the protocol and labelled it A or B. The nature of the lock solution was not known by any of the health professionals. Therefore, we deemed the study as low risk of bias. One study allocated neonates to one of two lock procedures and stated that it was 'double blind' but did not specifically who was blinded. The lock solutions were prepared and labelled lock one or two. We judged both of these studies at low risk of bias. One study was unable to blind participants or clinicians due to the method of allocation (Filippi 2007). Therefore, we judged the study as having a high risk if bias.
To evaluate detection bias, we have checked the blinding of outcome assessors for all separate outcomes. All outcome assessment could have been blinded. In Garland 2005 and Seliem 2010, outcome assessors were blinded for all outcomes, resulting in a low risk of detection bias. In Filippi 2007, there was no discussion on whether the outcomes assessors were blinded, giving an unclear risk of detection bias.
Incomplete outcome data
To evaluate attrition bias, we assessed incomplete outcome data for all outcomes. In all three studies, appropriate explanations were given for the participants not included in the analysis, giving a low risk for attrition bias.
The Seliem 2010 study had 105 infants who required a UVC, four parents refused consent, two were not randomised as the study personnel was unavailable and two neonates required systemic antibiotic treatment. Therefore, 97 neonates were randomised to the study. Following randomisation, three infants were excluded as they died before 48 hours of age, and 11 neonates had the UVC removed with the first 48 hours. Therefore, these infant did not meet the eligibility criteria. Thus, the analysis included 83 neonates.
The Garland 2005 study included 90 randomised infants. However, five were not included on the analysis, three as the PICC line was in situ less than 48 hours, one was transferred to another hospital and one parent withdrew consent. Therefore, analysis included 85 infants.
The Filippi 2007 study included 103 infants who were randomised and included in the analysis. The study excluded eight infants prior to randomisation, six died in the first few hours and two used medicated UVCs. Five infants were transferred to another hospital; however, the data until transfer were included in the analysis.
Selective reporting
To evaluate reporting bias, we assessed selective reporting of outcomes. None of the studies had published protocols available, therefore, it was impossible to know whether pre‐specified outcomes were reported. All of the studies adequately reported all outcomes outlined within the Methods section of the trial report. In all three studies, the risk of reporting bias was judge to be low.
Other potential sources of bias
The Filippi 2007 study reported that the lock solution was administered 376 times and only retrieved at the end of the procedure in 109 cases (29%). Therefore, some infants might have received some level of systemic antibiotic from the lock solution. The authors discussed this in relation to participant safety and deemed the dose to be lower than neonatal doses and, therefore, safe. However, there was no discussion on how this may have potentially treated CRBSI and impacted on the results.
Effects of interventions
See: Table 1
Antibiotic lock versus no antibiotic lock
Confirmed catheter‐related infections
We included all three of the studies in this meta‐analysis. Individually, two of the studies found a statistical difference for confirmed infection rates of catheter locked with an antibiotic compared with heparinised saline alone (Filippi 2007; Seliem 2010). A meta‐analysis of the pooled studies included 271 infants and demonstrated significantly lower infection rates when the catheter was locked with an antimicrobial solution (typical RR 0.15, 95% CI 0.06 to 0.40) (Analysis 1.1; Figure 4). The absolute risk reduction (ARR) was 18.5% and the NNTB was 5. Despite each study using a different antimicrobial and having slightly different classifications of confirmed CRBSI, there was no statistical heterogeneity (I2 = 0%).
1.1. Analysis.
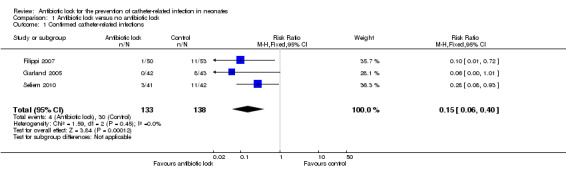
Comparison 1 Antibiotic lock versus no antibiotic lock, Outcome 1 Confirmed catheter‐related infections.
4.
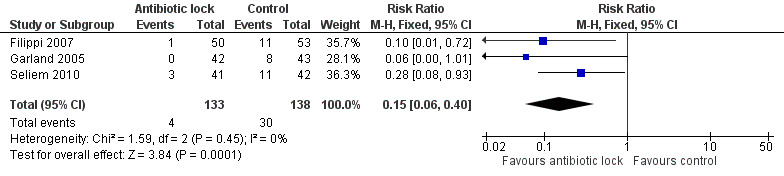
Forest plot of comparison: 1 Antibiotic lock versus no antibiotic lock, outcome: 1.1 Confirmed catheter‐related infections.
Suspected catheter‐related infections
We included all three studies in this comparison. Individually, the studies found no statistically significant difference for suspected infection rates between antibiotic lock and heparinised saline. When the data were pooled (271 infants), the results showed no statistically significant difference (typical RR 0.65, 95% CI 0.22 to 1.92) (Analysis 1.2; Figure 5).
1.2. Analysis.
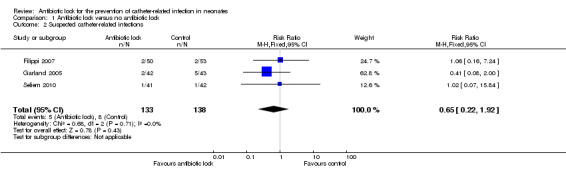
Comparison 1 Antibiotic lock versus no antibiotic lock, Outcome 2 Suspected catheter‐related infections.
5.
Forest plot of comparison: 1 Antibiotic lock versus no antibiotic lock, outcome: 1.2 Suspected catheter‐related infections.
We were unable to analyse any secondary outcomes in relation to this comparison.
Combined rates of confirmed and suspected catheter‐related infection (absolute rates)
All three studies analysed total CRBSI and all individually found statistically significant lower infection rates for combined confirmed and suspected catheter‐related infection with antibiotic lock compared with heparinised saline. A meta‐analysis of the pooled studies (271 infants) demonstrated a significantly lower infection rate in the antibiotic lock group (typical RR 0.25, 95% CI 0.12 to 0.49) (Analysis 1.3; Figure 6 ). The ARR was 20.7% with an NNTB of 5. Despite differences in the studies, there was no heterogeneity (I2 = 0%).
1.3. Analysis.
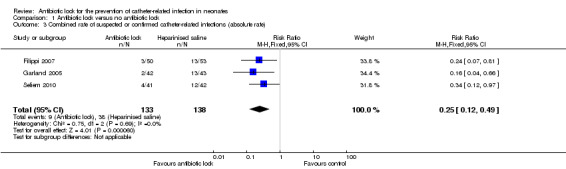
Comparison 1 Antibiotic lock versus no antibiotic lock, Outcome 3 Combined rate of suspected or confirmed catheter‐related infections (absolute rate).
6.
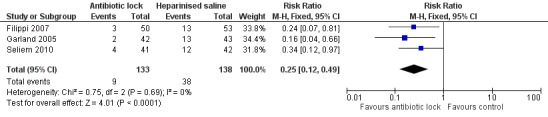
Forest plot of comparison: 1 Antibiotic lock versus no antibiotic lock, outcome: 1.3 Combined rate of suspected or confirmed catheter‐related infections (absolute rate).
Combined rates of confirmed and suspected catheter‐related infection (per 1000 catheter‐days)
Two studies provided data on the incidence density of total CRBSI (confirmed plus suspected) (Garland 2005; Seliem 2010). The pooled data, including 168 infants, demonstrated a significant reduction in infection rates per 1000 catheter‐days with antibiotic lock (typical RR 0.17, 95% CI 0.07 to 0.40) (Analysis 1.4; Figure 7).
1.4. Analysis.
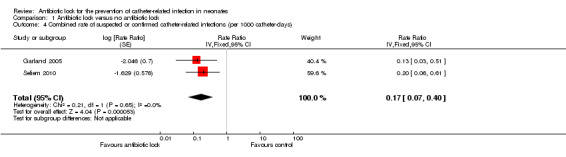
Comparison 1 Antibiotic lock versus no antibiotic lock, Outcome 4 Combined rate of suspected or confirmed catheter‐related infections (per 1000 catheter‐days).
7.

Forest plot of comparison: 1 Antibiotic lock versus no antibiotic lock, outcome: 1.4 Combined rate of suspected or confirmed catheter‐related infections (per 1000 catheter‐days).
Mortality
Two studies provided data on mortality from sepsis including 186 infants. In the Seliem 2010 study, the cause of death was due to overwhelming Gram‐negative bacilli sepsis but it was not clear if these were catheter related. The pooled data did not show a statistically significant difference (typical RR 0.37, 95% CI 0.13 to 1.04) (Analysis 1.5; Figure 8).
1.5. Analysis.
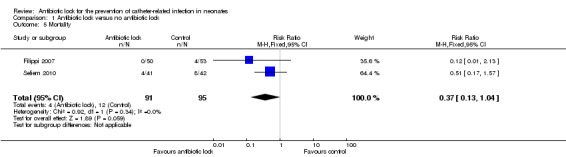
Comparison 1 Antibiotic lock versus no antibiotic lock, Outcome 5 Mortality.
8.
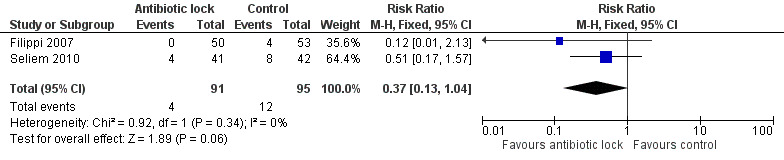
Forest plot of comparison: 1 Antibiotic lock versus no antibiotic lock, outcome: 1.5 Mortality.
Hypoglycaemic episodes
Two studies reported the rates of hypoglycaemia but they used a different definition for hypoglycaemia. Garland 2005 defined hypoglycaemia as bedside whole blood of 40 mg/dL or less, whereas Seliem 2010 defined hypoglycaemia as bedside whole blood glucose concentration less than 45 mg/dL. When comparing hypoglycaemic episodes, one study demonstrated statistically less hypoglycaemia with antibiotic lock (RR 0.46, 95% CI 0.22 to 0.93) (Garland 2005), in comparison to the other study where there was no difference between the two group (RR 0.64, 95% CI 0.23 to 1.80) (Seliem 2010). When the data were pooled, there was statistically significant fewer episodes of hypoglycaemia in the treatment arm (typical RR 0.51, 95% CI 0.28 to 0.92) (Analysis 1.6; Figure 9). Despite the differences in the definition of hypoglycaemia, there was no heterogeneity (I2 = 0%).
1.6. Analysis.
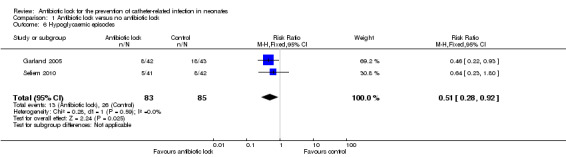
Comparison 1 Antibiotic lock versus no antibiotic lock, Outcome 6 Hypoglycaemic episodes.
9.
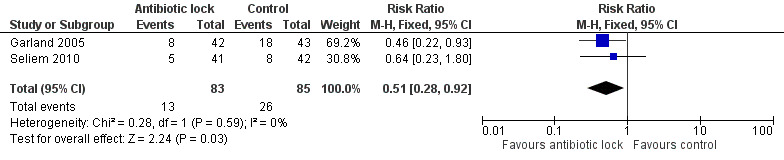
Forest plot of comparison: 1 Antibiotic lock versus no antibiotic lock, outcome: 1.6 Hypoglycaemic episodes.
Adverse events
Two studies reported detectable antibiotic in the neonates' blood (Garland 2005; Seliem 2010). Both studies showed no significant difference in the amount of detectable serum levels and, when the data were pooled (169 infants), there was no statistically significant difference (typical RR 4.25, 95% CI 0.47 to 38.77) (Analysis 1.7; Figure 10).
1.7. Analysis.
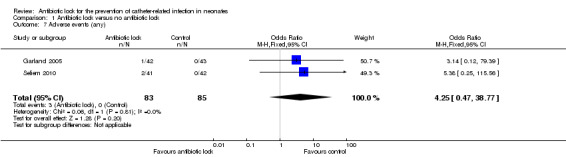
Comparison 1 Antibiotic lock versus no antibiotic lock, Outcome 7 Adverse events (any).
10.
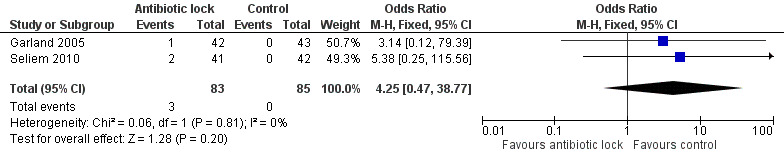
Forest plot of comparison: 1 Antibiotic lock versus no antibiotic lock, outcome: 1.7 Adverse events (any).
Number of catheters with resistant organism cultured at removal
We were unable to perform a meta‐analysis, and we, therefore, provide descriptive results for this outcome measure. All of the studies reported no occurrence of micro‐organism resistance to the antibiotic used in lock during or at removal of the catheter.
There were no data for the following secondary outcomes: number of catheters removed before they were no longer clinically required, number of catheters occluded, episodes of thrombosis, episodes of thrombophlebitis, skin irritation, length of stay in the NICU and overall hospital stay.
Discussion
Summary of main results
Central venous catheter‐related infection causes significant morbidity and mortality in neonates. The objective of this review was to access the efficacy of antibiotic locks versus no antibiotic lock in the prevention of catheter‐related infections in neonates. An antibiotic lock is a novel technique of local prophylaxis where the solution is instilled into the catheter lumen for a prescribed period of time, then removed and discarded. As the majority of neonatal catheter infections are intraluminally derived, the lock technique, in theory, may significantly reduce catheter‐related infection.
A comprehensive search of studies yielded six studies, three of which could be included (271 neonates) in the review. The three studies all used different antibiotics (vancomycin, fusidic acid and amikacin), therefore, it was not possible to perform any subgroup analyses. A meta‐analysis of the three studies shows that antibiotic lock significantly reduced CRBSI, but did not reduce mortality. There were no increased adverse events in the infants who received antibiotic lock, instead, the rate of hypoglycaemia seemed to be lower in that group. There was also no evidence of micro‐organism resistance.
Antibiotic lock solutions are instilled into the dead space of the catheter lumen for usually a few hours. In all the studies found for this review, the dwell time was significantly less than a few hours. The main purpose for CVCs in neonates is for the provision of intravenous fluids and without these the infant would become hypoglycaemic. Therefore, it would be unethical to withhold fluid for a few hours in this population.
The duration the catheter was in situ varied between the studies, from a mean of 5.2 days to 20.3 days. One retrospective cohort study of infants in a NICU demonstrated the incidence rate of CLABSI increased by 14% per day during the first 18 days and, after day 36, there was an increase of 33% (Sengupta 2010). One of our included studies that exclusively used PICCs in the trial (Garland 2005), had longer mean catheter duration and higher baseline rate of suspected or confirmed CLABSI (30%) compared with the studies that used both UVCs and PICCs (24%) (Filippi 2007) or UVCs alone (28%) (Seliem 2010). This potentially demonstrates that the longer a catheter is in situ the greater the risk of catheter‐related infection.
We placed no limitations on the type of CVC and we pooled the data that included both PICC and UVC. Therefore, it is not possible to determine if the antibiotic lock therapy would be more beneficial for longer‐term catheters. However, the apparent clinical heterogeneity on the type of catheter and antibiotic lock used in each of the three studies has limited our certainty in the estimate of the pooled results, despite the absence of substantial statistical heterogeneity, which was most probably due to the small number of included studies. The differing baseline risks of the study population in the outcome of CLABSI, although not substantial, has further placed limitations on the certainty of our pooled estimates, and this should be explored further in future research.
Despite the review demonstrating statistically lower hypoglycaemia rates in the antibiotic lock group, we cannot explain this phenomenon. The hypoglycaemic events were postulated to occur as a direct response from withholding intravenous fluids while the lock was in situ, although none of the studies specifically evaluated the dextrose concentrations of the fluids that was temporally withheld to accommodate for the antibiotic lock solution.
Current recommendations from the CDC are to use a prophylactic antimicrobial lock solution in people with long‐term catheters who have a history of multiple CRBSI despite optimal maximal adherence to aseptic technique (O'Grady 2011). Although this review showed that an antibiotic lock reduced catheter‐related infection, there was not enough evidence gathered to change the current recommendations from the CDC.
See Table 1.
Overall completeness and applicability of evidence
The included trials are from a variety of countries with a variety of CRBSI incidence densities and all individually showed a reduction of CRBSI. In this review, we combined the results of the studies using various antibiotics with activity against Gram‐positive organisms and Gram‐negative organisms. Infections in different units are inherently different and, therefore, one antibiotic may not be suitable for all.
The risk of infection significantly increases during the first 18 days, however, only one of the studies estimated the protection the antibiotic lock had each day the catheter was in situ (Garland 2005). The baseline infection rate was different between the studies in this review, as is the case across different neonatal units. It is important to assess the baseline infection rates prior to introducing antibiotic lock.
There is a need to determine the risk of the development of resistant organisms from using an antibiotic lock solution. However, RCTs to assess this outcome would require a much larger study than identified by this review. None of the studies was adequately powered to detect antimicrobial resistance caused by antibiotic lock. In theory, it is unlikely that the using an antibiotic lock will result in the development of antimicrobial resistance due to the lock not reaching the systemic circulation. Nevertheless, the Filippi 2007 study demonstrated that the lock was not always retrievable. Moreover, the remaining studies identified detectable levels of antibiotic in the serum, albeit only on three occasions in total. Due to these low numbers, studies may never achieve adequate power to evaluate this potential adverse event. However, this concern needs to be assessed rigorously and surveillance for the prevalence of antimicrobial resistance should continue in institutions that use antibiotic locks.
Many studies have shown a reduction in CRBSI by introducing 'care bundles' yet few have reached and maintained zero in this vulnerable population indicating further interventions are required to reduce infection. However, before introducing methods such as antibiotic locks, practitioners need to ensure that other basic methods of preventing infection are being adhered to.
Quality of the evidence
This review included three studies with 271 neonates. Two of the three included studies had overall low risk of bias and the remaining study had high risk of selection and performance biases. There was no substantial statistical heterogeneity in the pooled estimates where the results could be pooled, although the apparent clinical heterogeneity on the type of catheter and antibiotic lock used warrants caution when interpreting the results while awaiting further studies evaluating each specific intervention. Apart from the outcome of mortality from CRBSI, which was contributed to by a single study, there was overall moderate‐ to high‐quality evidence for all other outcomes, which allows a confident interpretation of the results from the available data on the effectiveness and safety of the antibiotic lock for the prevention of catheter‐related infections in neonates despite the small amount of evidence included.
Potential biases in the review process
No potential biases have been declared.
Agreements and disagreements with other studies or reviews
One systematic review (16 RCTs) looked at the effectiveness of antibiotic‐based catheter lock solutions in prevention CRBSI in people with CVC in situ (Snaterse 2010). They performed a meta‐analysis of nine RCTs in adults receiving haemodialysis and found significant benefit in favour of using an antibiotic lock solution with tunnelled and cuffed CVCs (incidence density difference (IDD) ‐1.96, 95% CI ‐2.63 to ‐1.30). They also performed a meta‐analysis of five RCTs in children with cancer and found a small yet statistically significant benefit in reducing BSI (not CRBSI) (IDD ‐0.52, 95% CI ‐1.07 to ‐0.02). The authors noted that the included trials were at high risk of bias, with only two out of the 16 trials having low risk of bias. Nevertheless they concluded by stating, "in haemodialysis patients antibiotic catheter lock solutions are effective in preventing CRBSI. Negative side‐effects on patients, micro‐organism susceptibility and costs are to be considered."
One meta‐analysis of RCTs compared vancomycin‐heparin lock with flush solutions with heparin alone for prevention of BSI associated with CVCs (Safdar 2006). The review included a neonatal population. They include seven trials, and found significantly less BSI when an antibiotic lock of flush solution was used (typical RR 0.49, 95% CI 0.26 to 0.95). They also noted that vancomycin lock solutions (instilling it for a pre‐determined specified time) were superior to vancomycin flushes (typical RR 0.34, 95% CI 0.12 to 0.98). They concluded stating that the "use of a vancomycin lock solution in high‐risk patient populations being treated with long‐term central IVDs [intravascular devices] reduces the risk of BSI."
Three systematic reviews of adults and children requiring haemodialysis concluded that the use of antibiotic lock solution significantly reduced CRBSI in this population. Jaffer 2008 included studies published up to 2005, Labriola 2008 included studies up to March 2007 (one more than in the systematic review of Jaffer 2008) and Yahav 2008 included studies up to November 2007.
Yahav 2008 analysed 11 trials and compared any antibiotic lock solution with heparin and found statistically less CRBSI in the antibiotic lock group (typical RR 0.44, 95% CI 0.38 to 0.50).
The Cochrane review by van de Wetering 2007 included five studies using vancomycin plus heparin compared with heparin alone to flush the CVCs in all people with cancer (mainly children). The meta‐analysis found there was a significant reduction of Gram‐positive catheter‐related bacteraemia in the vancomycin plus heparin group compared with heparin alone (typical odds ratio (OR) 0.43, 95% CI 0.21 to 0.87). They concluded that, "it is justified to flush the catheter with a combination of an antibiotic and heparin, if the catheter related infection‐rate is high."
Authors' conclusions
Implications for practice.
Based on a small number of trials and neonates, this review showed that an antibiotic lock solution appeared to be effective in preventing catheter‐related bloodstream infection in the neonatal population. However, the results should be interpreted with caution due to the small number of trials included and the fact that each trial assessed different antibiotics. There was no evidence that this therapy causes antibiotic‐resistant organisms; however, this remains a potential adverse event as the trials were too small to detect this outcome reliably. Currently, we have no information on the effect this reduction in catheter‐related bloodstream infection impacts on length of neonatal intensive care unit or hospital stay, or the efficacy of this treatment on infants aged less than 28 weeks' gestation and infants with gastrointestinal conditions. Due to a lack of more precise evidence, we were unable to determine the effects of antibiotic lock on infections in neonates.
Implications for research.
Further randomised controlled trials are required to determine the efficacy in infants aged less than 28 weeks' gestation and infants with gastrointestinal conditions. Future studies should also determine the relative efficacy of different anti‐infective lock solutions, including those with broad‐spectrum antibacterial or antifungal activities (or both) and continue to assess the risks of antibiotic resistance to ensure this treatment is safe. Studies also need to address the appropriate duration of lock solution to reduce catheter‐related bloodstream infection with minimal hypoglycaemic effects.
Acknowledgements
We gratefully acknowledge Drs Roger Soll, Arne Ohlsson and Jeffrey Horbar for their comments on the draft review. We thank Ms. Yolanda Brosseau, Managing Editor of the Neonatal Review Group for her assistance leading to the publication of this review.
Appendices
Appendix 1. Definitions of primary outcomes using Centers for Disease Control and Prevention (CDC) criteria
Primary outcomes (CDC definitions, NHSN 2011; O'Grady 2002; O'Grady 2011)
Catheter‐related bloodstream infection (CRBSI)
Bacteraemia or fungaemia in a person with an intravascular catheter with at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infections (i.e. fever, chills, hypotension or a combination)* and no apparent source for the bloodstream infection except the catheter. One of the following should be present: a positive semi‐quantitative (15 colony‐forming units (CFU)/catheter segment) or quantitative (103 CFU/catheter segment catheter) culture whereby the same organism (species and antibiogram) is isolated from the catheter segment and peripheral blood; simultaneous quantitative blood cultures with a 5 : 1 ratio CVC : peripheral; differential period of CVC culture versus peripheral blood culture positivity of two hours.
*The above definition covers CRBSI in all age groups, and some of the symptomatology may not apply to the neonatal population.
Laboratory‐confirmed bloodstream infection (LCBI)
Person has a recognised pathogen (not including skin containments) from one or more blood cultures
AND
Organism cultured from blood is not related to an infection at another site
OR
Infant less than one year of age has at least one of the following signs or symptoms: fever (greater than 38 ºC core), hypothermia (less than 36 ºC core), apnoea or bradycardia
AND
Signs and symptoms and positive laboratory results are not related to an infected at another site
AND
Common skin contaminant (i.e. diphtheroids (Corynebacterium spp.), Bacillus (not B. anthracis) spp., Propionbacterium spp., coagulase‐negative staphylococci (including S. epidermidis), viridians group streptococci, Aerococcus spp., Micrococcus spp.) is cultured from two or more blood cultures drawn on separate occasions (collected within two days of each other).
Clinical sepsis (CSEP)
Infant less than one year of age has at least one of the following signs or symptoms: fever (greater than 38 ºC core), hypothermia (less than 36 ºC core), apnoea or bradycardia AND Blood culture not done or no organism detected AND No apparent infection at another site AND Physicians institutes treatment for sepsis.
Appendix 2. Criteria for a judgement on the sources of bias in the included studies
Was the allocation sequence randomly generated?
Yes, low risk of bias
A random (unpredictable) assignment sequence.
Examples of adequate methods of sequence generation are computer‐generated random sequence, coin toss (for studies with two groups), rolling a dice (for studies with two or more groups), drawing of balls of different colours, dealing previously shuffled cards.
No, high risk of bias
Quasi‐randomised approach: examples of inadequate methods are: alternation, birth date, social insurance/security number, date in which they are invited to participate in the study and hospital registration number
Non‐random approaches: allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests.
Unclear
Insufficient information about the sequence generation process to permit judgement
Was the treatment allocation adequately concealed?
Yes, low risk of bias
Assignment must be generated independently by a person not responsible for determining the eligibility of the participants. This person has no information about the people included in the trial and has no influence on the assignment sequence or on the decision about whether the person is eligible to enter the trial. Examples of adequate methods of allocation concealment are: central allocation, including telephone, web‐based, and pharmacy‐controlled randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
No, high risk of bias
Examples of inadequate methods of allocation concealment are: alternate medical record numbers, unsealed envelopes; date of birth; case record number; alternation or rotation; an open list of random numbers any information in the study that indicated that investigators or participants could influence the intervention group.
Unclear
Randomisation stated but no information on method of allocation used is available.
Blinding was knowledge of the allocated interventions adequately prevented during the study?
Was the participant blinded to the intervention?
Yes, low risk of bias
The treatment and control groups are indistinguishable for the participants or if the participant was described as blinded and the method of blinding was described.
No, high risk of bias
Blinding of study participants attempted, but likely that the blinding could have been broken; participants were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Was the care provider blinded to the intervention?
Yes, low risk of bias
The treatment and control groups were indistinguishable for the care/treatment providers or if the care provider was described as blinded and the method of blinding was described.
No, high risk of bias
Blinding of care/treatment providers attempted, but likely that the blinding could have been broken; care/treatment providers were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Was the outcome assessor blinded to the intervention?
Yes, low risk of bias
Adequacy of blinding should be assessed for the primary outcomes. The outcome assessor was described as blinded and the method of blinding was described.
No, high risk of bias
No blinding or incomplete blinding, and the outcome or outcome measurement was likely to be influenced by lack of blinding.
Unclear
Were incomplete outcome data adequately addressed?
Was the drop‐out rate described and acceptable?
The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must have been be described and reasons given.
Yes, low risk of bias
If the percentage of withdrawals and drop‐outs did not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and did not lead to substantial bias (note: these percentages are arbitrary, not supported by literature);
No missing outcome data;
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias);
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups;
Missing data have been imputed using appropriate methods.
No, high risk of bias
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
Unclear
Were all randomised participants analysed in the group to which they were allocated? (intention‐to‐treat (ITT) analysis)
Yes, low risk of bias
Specifically reported by authors that ITT was undertaken and this was confirmed on study assessment, or not stated but evident from study assessment that all randomised participants were reported/analysed in the group they were allocated to for the most important time point of outcome measurement (minus missing values) irrespective of non‐compliance and co‐interventions.
No, high risk of bias
Lack of ITT confirmed on study assessment (participants who were randomised were not included in the analysis because they did not receive the study intervention, they withdrew from the study or were not included because of protocol violation) regardless of whether ITT reported or not
'As‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation.
Unclear
Described as ITT analysis, but unable to confirm on study assessment, or not reported and unable to confirm by study assessment.
Were reports of the study free of suggestion of selective outcome reporting?
Yes, low risk of bias
If all the results from all pre‐specified outcomes have been adequately reported in the published report of the trial. This information is either obtained by comparing the protocol and the final trial report, or in the absence of the protocol, assessing that the published report included enough information to make this judgement. Alternatively, a judgement could be made if the trial report listed the outcomes of interest in the methods of the trial and then reported all these outcomes in the results section of the trial report.
No, high risk of bias
Not all of the study's pre‐specified primary outcomes were reported;
One or more primary outcomes was reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified;
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting was provided, such as an unexpected adverse effect);
One or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis;
The study report did not include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Other sources of potential bias
Were the groups similar at baseline regarding the most important prognostic indicators?
Groups have to be similar at baseline regarding demographic factors, duration and severity of complaints (e.g. size and duration of ulcer). Alternatively, if there were imbalances at baseline, these have been accounted for in the analysis of the study.
Were co‐interventions avoided or similar?
There were no co‐interventions or there were co‐interventions but they were similar between the treatment and control groups.
Was the compliance acceptable in all groups?
The review author determined if the compliance with the interventions was acceptable, based on the reported intensity, duration, number and frequency of sessions for both the treatment intervention and control intervention(s). For example, ultrasound treatment was usually administered over several sessions; therefore, it was necessary to assess how many sessions each participant attended or if participants completed the course of an oral drug therapy. For single‐session interventions (e.g. surgery), this item is irrelevant.
Were the trials or trialists in receipt of financial support from agencies or organisations with a financial interest in the outcome of the trial?
Appendix 3. CENTRAL search strategy
lock (1593)
flush (2750)
#1 or #2 (4282)
MeSH descriptor: [Catheterization, Central Venous] explode all trees (728)
CVC or "central line" or "central catheter" or PICC or "peripherally inserted central venous catheters" or UVC or "umbilical venous catheter" or "Longline" or "long line" or PCVC or "percutaneous central venous catheters" or "central venous catheter" or "central venous line" (24272)
#4 or #5 (24358)
#3 and #6 (835)
infant or newborn or neonate (35521)
#7 and #8 (151)
infection or sepsis (53022)
#9 and #10 (103) (19 trials)
Appendix 4. MEDLINE search strategy
exp Catheterization, Central Venous/ (11982)
CVC.mp. (2703)
central venous catheter.mp. (5172)
PICC.mp. (508)
umbilical venous catheter.mp. (106)
UVC.mp. (1296)
PCVC.mp. (29)
peripheral central venous catheter (0)
peripheral inserted central catheter.mp. (4)
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (16994)
sepsis.mp. or Sepsis/ (90004)
Infection/ or infection.mp. (893009)
bacteremia.mp. or Bacteremia/ (29327)
CRBSI.mp. or Catheter‐Related Infections/ (2121)
CLABSI.mp. (211)
11 or 12 or 13 or 14 or 15 (968944)
10 and 16 (5577)
lock.mp. (5378)
Anti‐Bacterial Agents/ or antimicrobial‐lock.mp. or Anti‐Infective Agents/ (281877)
antibiotic‐lock.mp. (196)
antibiotic flush.mp. (3)
antibiotic‐lock*.mp. (214)
flush*.mp. (15707)
lock*.mp. (28826)
18 or 19 or 20 or 21 or 22 or 23 or 24 (325721)
17 and 25 (1028)
limit 26 to "all infant (birth to 23 months)" (197)
limit 27 to (clinical trial, all or clinical trial or controlled clinical trial or randomized controlled trial) (30)
Appendix 5. EMBASE search strategy
exp central venous catheter/ (11281)
exp central venous catheterization/ (7533)
cvc.mp. (3716)
PICC.mp. or peripherally inserted central venous catheter/ (1416)
UVC.mp. (1466)
umbilical venous catheter.mp. (166)
pcvc.mp. (39)
1 or 2 or 3 or 4 or 5 or 6 or 7 (21089)
newborn sepsis/ or sepsis.mp. or sepsis/ (135028)
infection/ or infection.mp. (1668128)
bacteremia/ or bacteremia.mp. (36255)
catheter infection/ or CRBSI.mp. (10216)
CLABSI.mp. (424)
9 or 10 or 11 or 12 or 13 (1758301)
antiinfective agent/ or lock*.mp. or antibiotic agent/ (377996)
flush*.mp. (39855)
antiinfective agent/ or antibiotic lock.mp. or antibiotic agent/ (348819)
antibiotic‐lock.mp. (253)
antibiotic flush.mp. (3)
15 or 16 or 17 or 18 or 19 (416885)
8 and 14 and 20 (1997)
8 and 20 (2467)
infant/ (540242)
newborn/ (497987)
23 or 24 (902652)
21 and 25 (231)
from 26 keep 38, 44, 110 (3)
limit 26 to (clinical trial or randomized controlled trial or controlled clinical trial or multicenter study or phase 1 clinical trial or phase 2 clinical trial or phase 3 clinical trial or phase 4 clinical trial) (27)
Appendix 6. CINAHL search strategy
Catheterization, central venous (2219)
central line OR central venous line OR central venous catheter OR longline OR CVC (1993)
PICC OR Perpheral inserted central catheters OR PCVC or UVC or umbilical venous catheter (430)
S1 OR S2 O2 S3 (3784)
"Antibiotic OR antibiotics OR antibiotic lock" OR (MH "Antibiotics, Combined") (231)
antimicrobial OR antimicrobial lock (5362)
flush* (1296)
lock* (2567)
S5 or S6 or S7 or S8 (9344)
S4 and S9 (198)
4 and 9 Limiters ‐ Age Groups: Infant, Newborn: birth‐1 month, Infant: 1‐23 months (31)
Data and analyses
Comparison 1. Antibiotic lock versus no antibiotic lock.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Confirmed catheter‐related infections | 3 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.06, 0.40] |
| 2 Suspected catheter‐related infections | 3 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.22, 1.92] |
| 3 Combined rate of suspected or confirmed catheter‐related infections (absolute rate) | 3 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.12, 0.49] |
| 4 Combined rate of suspected or confirmed catheter‐related infections (per 1000 catheter‐days) | 2 | Rate Ratio (Fixed, 95% CI) | 0.17 [0.07, 0.40] | |
| 5 Mortality | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.13, 1.04] |
| 6 Hypoglycaemic episodes | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.28, 0.92] |
| 7 Adverse events (any) | 2 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.25 [0.47, 38.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Filippi 2007.
| Methods | Quasi‐randomised controlled trial | |
| Participants | 103 neonates recruited from July 2004 to November 2005 Mean birthweight: 1378 g and 1037 g Gestation age: 213 days and 192.7 days Setting: NICU, Careggi University Hospital, Florence, Italy Inclusion criteria: infants requiring a non‐medicated CVC for ≥ 24 hours Exclusion criteria: neonates with a medicated CVC, CVC removed within 24 hours and infants transferred to other hospitals or died within the first 24 hours of life |
|
| Interventions | Intervention: catheter lumen locked with 0.3 mL heparinised normal saline (10 IU/mL) containing fusidic acid (4 mg/mL) Control: catheter lumen locked with 0.3 mL heparinised normal saline (10 IU/mL) for 30‐60 minutes based on clinical condition and tolerance to suspension of IV fluids, as assessed by the rates of hypoglycaemia and hypotension (once per day) Catheter inserted using a sterile technique. Skin surface surrounding the insertion point was disinfected with 10% povidone‐iodine. Inserted UVCs were Argyle™ and PICCs were Premicath or Nutriline. A transparent dressing was used to cover the insertion site. IV catheters were changed daily and catheter hubs were cleansed with 2% chlorhexidine every time they were accessed All locks were performed when parental nutrition and tubing were changed to reduce the number of line manipulations |
|
| Outcomes |
Definite CRBSI: 1 positive BC with concordant colonisation of the catheter hub or tip, clinical signs of sepsis and no other apparent source of infection Suspected CRBSI: positive culture of the catheter hub or tip, clinical signs of sepsis, no other source for BSI, with negative or not concordant BC Colonisation: positive culture of catheter hub or tip with neither concordant BC nor clinical signs of sepsis Non‐catheter related sepsis: positive BC with signs of infection but negative culture of catheter hub or tip |
|
| Notes | 2 room, 8 bed, level 3 NICU Designed as a pilot study UVC was inserted at admission in 101 neonates (98.1%) and PICC in 2 (1.9%). After UVC removal, a PICC was placed in 39 infants (37.9%). No infant had both catheters in place at the same time Total catheter days were 978 (562 of UVC and 416 of PICC); median duration in place was 6 days (range 1 to 13) for UVCs and 8 days (range 2 to 39) for PICCs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no discussion on how the infants were randomised to the admitting room |
| Allocation concealment (selection bias) | High risk | Neonates who were admitted to room 1 received antibiotic lock treatment and neonates admitted to room 2 formed the control group. Therefore, there was no concealment to allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible due to the nature of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis with 331 eligible infants 220 did not require a CVC, 6 not included due to death, 2 not included due to UVC requiring medication |
| Selective reporting (reporting bias) | Unclear risk | Hypoglycaemia results not available Quote: "Intravenous glucose interruption did not induce significant secondary hypoglycemias;" however, this was not an outcome |
| Other bias | Unclear risk | The lock was only retrieved at the end of the procedure in 109 cases (29%); therefore, the infants potentially received some level of systemic treatment Potential source of bias related to study design |
Garland 2005.
| Methods | Randomised controlled, double‐blind comparative trial | |
| Participants | 90 neonates enrolled between May 2000 and 2001, 85 analysed Mean birthweight: 1296 g and 1055 g Gestation age: 28.3 weeks and 27.5 weeks Setting: NICU in St Joseph Regional Medical Centre, Milwaukee, WI Inclusion criteria: all neonates who required a peripheral central line for ≥ 48 hours Exclusion criteria: none reported |
|
| Interventions | Intervention: catheter locked with 0.4 mL heparinised saline containing vancomycin (25 μg/mL) Control: catheter locked with 0.4 mL heparinised saline (10 IU/mL) for either 20 minute for infants primarily fed via parental hyperalimentation or 60 minutes when enteral feeds exceeded 20 mL/kg/day Catheters were inserted using maximal sterile precautions, including a sterile mask, cap, gloves, gown and a large drape. Insertion sites were disinfected with 10% povidone‐iodine and catheters were dressed with a polyurethane film dressing. Catheter sites were cleansed and re‐dressed on a weekly basis or as needed. IV tubing was changed every 3 days when used for hyperalimentation and every 24 hours when used for intralipid therapy. Needle‐less access ports were not used during the trial. Catheter hubs were cleansed with alcohol whenever the hub was accessed |
|
| Outcomes |
Definite CRBSI: signs of sepsis and positive peripheral BC and concordant colonisation of catheter hub or tip. Infant was treated for 7 days and no other source of infection was identified (coagulase‐negative staphylococci clonal concordance was confirmed by restriction‐fragment DNA subtyping) Probable CRBSI: signs of sepsis and either positive peripheral BC for coagulase‐negative staphylococci with concordant colonisation of the catheter hub (but DNA subtype was not done) OR a BC through the catheter positive (peripheral sterile or not done) for the same organism from the catheter hub or tip (coagulase‐negative staphylococci clonal concordance was confirmed by restriction‐fragment DNA subtyping) and no other source of infection was identified. Infants were treated for 7 days BSI without a source: signs of sepsis, positive BC from peripheral or catheter, no other source of infection. Catheter cultures negative or different to those in the BC Safety/tolerance Vancomycin resistance: measured by rectal and axillary swab on insertion and removal of catheter; and from positive blood, catheter and hub cultures. Resistance was measured by growth of organism on a vancomycin‐containing agar Vancomycin toxicity: serum vancomycin levels measured on days 7 and 14 in the first 73 infants before a lock Hypoglycaemia: blood glucose ≤ 40 mg/dL (bedside whole blood concentration). Measured at the end of every 20 minute dwell, and at 20 and 40 minutes in the 60‐minute dwell. |
|
| Notes | 50 bed, level 3 NICU Study designed as a pilot After first 40 infants enrolled, the frequency of the lock was increased from 2 to 3 times per day. However, after an increase in nosocomial bacteria rate in these 21 infants the lock was reduced back to twice a day Mean (± SD) catheter days: control 19.6 (12.3); intervention 20.3 (11.4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a computer‐generated block sequence |
| Allocation concealment (selection bias) | Low risk | Randomisation was controlled by the pharmacist using a computer‐generated block sequence that was kept in a locked pharmacy cabinet Neonates were allocated to lock 1 protocol or lock 2 protocol |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All analysis were performed by investigators who were blind to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis with 568 infants admitted to NICU 40 consent refused, 3 study personnel unavailable to obtain consent, 1 neonate with congenital hyperinsulinaemia, 434 PICC line not required 90 neonates randomised, 85 neonates analysed 5 neonates excluded after randomisation was appropriately explained PICC line removed < 48 hours: 2 in intervention, 1 in control; parent removed infant from study: 1 in control; 1 transferred: 1 in control |
| Selective reporting (reporting bias) | Low risk | Results available for all outcomes |
| Other bias | Low risk | |
Seliem 2010.
| Methods | Randomised, blinded, controlled trial | |
| Participants | 97 neonates, enrolled between February 2008 and February 2009; 83 included in the analysis Mean birthweight: 2434 g and 2005 g Gestation age: 33.5 weeks and 33.1 weeks Setting: NICU at Mansoura University Children's Hospital, Mansoura, Egypt Inclusion criteria: all neonates who were expected to require a UVC for ≥ 48 hours Exclusion criteria: neonates with an indwelling UVC for > 24 hours without a lock technique, and infants who received systemic antibiotic therapy or who were transferred to other hospitals in the first day of life |
|
| Interventions | Intervention: 0.4 mL heparinised saline containing amikacin (1.5 mg/mL) (Protocol B) for 20 minutes twice a day Control: 0.4 mL heparinised saline (10 IU/mL) (Protocol A) for 20 minutes twice a day UVCs used were single lumen 5.0 French gauge polyvinyl chloride end hole catheters. Catheters were inserted using maximal sterile barriers, including the use of sterile gloves, gown, mask and large drape. The site was disinfected with 10% povidone‐iodine and inserted to keep the tip just above the diaphragm, catheters higher than this were pulled back following x‐ray, catheter lower than this were removed and re‐inserted. The umbilical stump was cleansed on a daily basis with 70% alcohol. IV tubing was changed every 24 hours using strict sterile technique by 2 nurses, 1 wearing sterile gloves, cap, gown and mask to handle all the sterile equipment. Catheter hubs were cleansed with 70% alcohol whenever the hubs were accessed. Catheters were removed when no longer required or on day 14 |
|
| Outcomes |
Definite CRBSI: positive peripheral BC concomitant with positive BC from catheter or catheter tip grew the same species AND clinical sign of sepsis AND no other apparent source of infection Probable CRBSI: positive peripheral BC and positive catheter BC grew a different species OR positive BC from the catheter or catheter tip and the peripheral sample was sterile in the presence of clinical signs of infection Definite and probable CRBSI: definite and probable combined BSI without a source: positive peripheral BC with clinical signs of infection and a negative BC withdrawn from the catheter or catheter tip culture All BSI Hypoglycaemia (< 45 mg/dL): measured at the end of the dwell time Amikacin resistance: axillary and rectal swabs and entry and end of the study, growth on amikacin‐containing agar considered resistant |
|
| Notes | 30 bed, level 3 NICU Mean (± SD) catheter‐days: control 10.3 (3.6); intervention 11.6 (2.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no explanation to how the sequence was generated |
| Allocation concealment (selection bias) | Low risk | Quote: "Neonates randomised using opaque sealed envelope containing the randomisation sequence" Opaque sealed envelopes kept in a locked cabinet |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and clinicians were blind to the nature of the locking solution Quote: "Only the research nurse was aware of the nature of the locking solution and responsible for its preparation according to the protocol" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All analyses were performed by investigators who were blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis with 626 neonates admitted to NICU 105 required a UVC, 4 parents refused consent, 2 study personnel not available, 2 infants received systemic antibiotic, 97 neonates randomised Participants excluded after randomisation were appropriately explained 11 UVCs were removed within 48 hours, 3 neonates died before 48 hours, therefore, did not meet eligibility criteria |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | |
BC: blood culture; BSI: bloodstream infection; CRBSI: catheter‐related bloodstream infection; CVC: central venous catheter; DNA: deoxyribonucleic acid; IU: international unit; IV: intravenous; NICU: neonatal intensive care unit; PICC: peripherally inserted central catheter; SD: standard deviation; UVC: umbilical venous catheter.
Characteristics of studies awaiting assessment [ordered by study ID]
Bertini 2008.
| Methods | Randomised controlled trial |
| Participants | Infants < 1500 g with a CVC |
| Interventions | Fusidic‐heparin lock group (37 infants) Control group (41 infants) Fusidic‐heparin lock with silver coated CVC (44 infants) |
| Outcomes | Prevention of staphylococci CRBSI |
| Notes | Currently in abstract only |
Graham 2003.
| Methods | Randomised controlled trial |
| Participants | Infants < 1500 g at birth, > 48 hours of age with a CVC |
| Interventions | Intervention: catheter flushed with vancomycin (25 μg) and heparin (9.5 IU/mL) Control: catheter flushed with heparin (9.5 IU/mL) catheters flushed each line change (24 hourly for lipids and 48 hourly for other fluids) |
| Outcomes | Reduction in CRBSI |
| Notes | Currently in abstract only |
CRBSI: catheter‐related bloodstream infection; CVC: central venous catheter; IU: international unit.
Characteristics of ongoing studies [ordered by study ID]
Fort 2011.
| Trial name or title | Safety and Efficacy Study of Ethanol Locking to Prevent Central Line Infection in Premature Neonates |
| Methods | Randomised, parallel assignment, double blind |
| Participants | 150 infants < 32 weeks' gestation at birth who required a PICC |
| Interventions | Intervention: placement of 0.5 mL 70% ethanol, every 72 hours, for 15 minutes, into PICC lines Control: placement of 0.5 mL heparinised saline, every 72 hours, for 15 minutes, into PICC lines |
| Outcomes | Primary endpoint of the study is to compare the incidence of PICC‐related sepsis in infants with treated with ethanol vs. control Evaluation of PICC colonisation following ethanol locking To determine the adverse effects of flushing ethanol locks into premature infants following lock therapy To determine how ethanol locking affects central line function and integrity in vivo Birthweight stratification will use a 3‐tiered subset (< 1000 g, 1000‐1250 g and > 1250 g) to compare the primary and secondary endpoints |
| Starting date | February 2010 |
| Contact information | Amber E Fort; forta@ecu.edu James J Cummings; cummingsj@ecu.edu |
| Notes | ClinicalTrials.gov identifier Number: NCT01365312 |
PREVAIL study.
| Trial name or title | PREVAIL study ‐ PREVenting infection using Antimicrobial Impregnated Long lines |
| Methods | Unblinded, 2‐arm randomised controlled trial to determine the effectiveness and cost‐effectiveness of antimicrobial impregnated (with rifampicin and miconazole) long lines (termed peripherally inserted central venous catheters, or AM‐PICC) compared with standard PICC (S‐PICC) for reducing BSI |
| Participants | Infants receiving a PICC |
| Interventions | Antimicrobial impregnated (with rifampicin and miconazole) peripherally inserted central venous catheters (AM‐PICC) compared with a standard PICC (S‐PICC) |
| Outcomes | Time to first BSI based on a positive blood culture (including fungal BSI) taken between 24 hours after randomisation and 48 hours after removal As part of the primary endpoint there will be 2 sensitivity analyses:
|
| Starting date | The study will run from December 2014 to August 2017 |
| Contact information | The study will run from 18 neonatal units in the UK. The lead centre will be Bradford Teaching Hospitals NHS Foundation Trust. The study will be co‐ordinated through the Medicines for Children Clinical Trials Unit, University of Liverpool Contact details; Prof. Ruth Gilbert, MRC Centre of Epidemiology for Child Health, UCL Institute of Child Health, 30 Guilford Street, London, WC1N 1EH, UK; email: r.gilbert@ucl.ac.uk |
| Notes | Funded by National Institute for Health Research Health Technology Assessment Programme ‐ Health Technology Assessment (UK). ISRCTN81931394 |
BSI: bloodstream infection; PICC: peripherally inserted central catheter.
Differences between protocol and review
We changed the title from 'Antibiotic lock for the prevention of catheter‐related sepsis in neonates' to 'Antibiotic lock for the prevention of catheter‐related infection in neonates'.
In the review, we have re‐worded the primary outcomes from the protocol to improve the reporting in the final review. We changed 1. rates of confirmed sepsis per 1000 catheter‐days to rates of confirmed catheter‐related infection; 2. rates of suspected catheter‐related infection per 1000 catheter‐days to rates of suspected catheter‐related infection; 3. absolute rates of infection in both intervention and control groups to combined rates of confirmed and suspected catheter‐related infection; and 4. we moved all‐cause mortality during the study period from a primary outcome to a secondary outcome.
We changed the assessment of heterogeneity in line with Cochrane Neonatal Review Group guidelines.
Contributions of authors
KTAN, NML and JET wrote the protocol.
JET and KTAN searched for studies.
JT, KTAN and NML extracted the data and entered it into Review Manager 5 (RevMan 2012).
JT and NML assessed the studies for risk of bias.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
All the review authors declare to have no competing financial conflict of interest.
New
References
References to studies included in this review
Filippi 2007 {published data only}
- Filippi L, Pezzati M, Di Amario S, Poggi C, Pecile P. Fusidic acid and heparin lock solution for the prevention of catheter‐related bloodstream infections in critically ill neonates: a retrospective study and a prospective, randomized trial. Pediatric Critical Care Medicine 2007;8(6):556‐62. [DOI] [PubMed] [Google Scholar]
Garland 2005 {published data only}
- Garland JS, Alex CP, Henrickson KJ, McAuliffe TL, Maki DG. A vancomycin‐heparin lock solution for prevention of nosocomial bloodstream infection in critically ill neonates with peripherally inserted central venous catheters: a prospective, randomized trial. Pediatrics 2005;116(2):e198‐205. [DOI] [PubMed] [Google Scholar]
Seliem 2010 {published data only}
- Seliem W, Abdel‐Hady H, El‐Nady G. Amikacin‐heparin lock for prevention of catheter‐related bloodstream infection in neonates with extended umbilical venous catheters use: a randomized controlled trial. Journal of Neonatal‐Perinatal Medicine 2010;3(1):33‐41. [Google Scholar]
References to studies awaiting assessment
Bertini 2008 {published data only}
- Bertini G, Ceciarini F, Benuzzi A, Elia S, Dani C, Rubaltelli FF. A fusidic‐heparin lock solution and silver coated central venous catheters in preventing staphylococcus catheter‐related bloodstream infections in VLBW infants. Proceedings of the Society of Pediatric Research Annual Meeting; 2008 2‐6 May 2008; Honolulu, Hawaii. 2008.
Graham 2003 {published data only}
- Graham A, Finer NN. Vancomycin flush technique to prevent coagulase negative staphylococcus infection in VLBW infants: a prospective placebo‐controlled randomized trial. Proceedings of the Society of Pediatric Research Annual Meeting; Seattle, WA. 3‐6 May, 2003.
References to ongoing studies
Fort 2011 {published data only}
- Fort AE, Cummings JJ. A prospective, randomized, blinded, placebo‐controlled trial of periodic, brief ethanol locks to prevent peripherally inserted central catheter (PICC) infections in preterm infants in the neonatal intensive care unit. Journal of Investigative Medicine 2011;59:421‐2. [Google Scholar]
PREVAIL study {published data only}
- PREVAIL study ‐ PREVenting infection using Antimicrobial Impregnated Long lines. Ongoing study The study will run from December 2014 to August 2017.
Additional references
Adams‐Chapman 2006
- Adams‐Chapman I, Stoll BJ. Neonatal infection and long‐term neurodevelopmental outcome in the preterm infant. Current Opinion in Infectious Diseases 2006;19(3):290‐7. [DOI] [PubMed] [Google Scholar]
Ball 2003
- Ball PA. Intravenous in‐line filters: filtering the evidence. Current Opinion in Clinical Nutrition and Metabolic Care 2003;6(3):319‐25. [DOI] [PubMed] [Google Scholar]
Bassler 2009
- Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CM, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics 2009;123(1):313‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borghesi 2008
- Borghesi A, Stronati M. Strategies for the prevention of hospital‐acquired infections in the neonatal intensive care unit. Journal of Hospital Infection 2008;68(4):293‐300. [DOI] [PubMed] [Google Scholar]
Carratalà 2002
- Carratalà J. The antibiotic‐lock technique for therapy of 'highly needed' infected catheters. Clinical Microbiology and Infection 2002;8(5):282‐9. [DOI] [PubMed] [Google Scholar]
Cartwright 2004
- Cartwright DW. Central venous lines in neonates: a study of 2186 catheters. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89(6):F504‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 2003
- Chapman RL, Faix RG. Persistent bacteremia and outcome in late onset infection among infants in a neonatal intensive care unit. Pediatric Infectious Disease Journal 2003;22(1):17‐21. [DOI] [PubMed] [Google Scholar]
Chien 2002
- Chien LY, Macnab Y, Aziz K, Andrews W, McMillan DD, Lee SK. Variations in central venous catheter‐related infection risks among Canadian neonatal intensive care units. Pediatric Infectious Disease Journal 2002;21(6):505‐11. [DOI] [PubMed] [Google Scholar]
Cicalini 2004
- Cicalini S, Palmieri F, Petrosillo N. Clinical review: new technologies for prevention of intravascular catheter‐related infections. Critical Care 2004;8(3):157‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Brito 2010
- Brito CS, Brito DV, Abdallah, VO, Gontijo Filho PP. Occurrence of bloodstream infection with different types of central catheter in critically neonates. Journal of Infection 2010;60(2):128‐32. [DOI] [PubMed] [Google Scholar]
Dudeck 2011
- Dudeck MA, Horan TC, Peterson KD, Allen‐Bridson K, Morrell G, Pollock DA, et al. National Healthcare Safety Network (NHSN) report, data summary for 2010, device‐associated module. American Journal of Infection Control 2011;39(10):798‐816. [DOI] [PubMed] [Google Scholar]
Garland 2008
- Garland JS, Alex CP, Sevallius JM, Murphy DM, Good MJ, Volberding AM, et al. Cohort study of the pathogenesis and molecular epidemiology of catheter‐related bloodstream infection in neonates with peripherally inserted central venous catheters. Infection Control and Hospital Epidemiology 2008;29(3):243‐9. [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro [Version 3.2 for Windows]. The GRADE Working Group, 2008.
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester: John Wiley & Sons Ltd.
Hoang 2008
- Hoang V, Sills J, Chandler M, Busalani E, Clifton‐Koeppel R, Modanlou HD. Percutaneously inserted central catheter for total parenteral nutrition in neonates: complications rates related to upper versus lower extremity insertion. Pediatrics 2008;121(5):e1152‐9. [DOI] [PubMed] [Google Scholar]
Horan 2008
- Horan TC, Andrus M, Dudeck, MA. CDC/NHSN surveillance definition of health care‐associated infection and criteria for specific types of infections in the acute care setting. American Journal of Infection Control 2008;36(5):309‐32. [DOI] [PubMed] [Google Scholar]
Jaffer 2008
- Jaffer Y, Selby NM, Taal MW, Fluck RJ, McIntyre CW. A meta‐analysis of hemodialysis catheter locking solutions in the prevention of catheter‐related infection. American Journal of Kidney Diseases 2008;51(2):233‐41. [DOI] [PubMed] [Google Scholar]
Kaplan 2011
- Kaplan HC, Lannon C, Walsh MC, Donovan EF, Ohio Perinatal Quality Collaborative. Ohio statewide quality‐improvement collaborative to reduce late‐onset sepsis in preterm infants. Pediatrics 2011;127(3):427‐35. [DOI] [PubMed] [Google Scholar]
Labriola 2008
- Labriola L, Crott R, Jadoul M. Preventing haemodialysis catheter‐related bacteraemia with an antimicrobial lock solution: a meta‐analysis of prospective randomized trials. Nephrology, Dialysis, Transplantation 2008;23(5):1666‐72. [DOI] [PubMed] [Google Scholar]
Lee 2009
- Lee SK, Aziz K, Singhal N, Cronin CM, James A, Lee DS, et al. Improving the quality of care for infants: a cluster, randomized controlled trial. CMAJ 2009;181(8):469‐76. [PUBMED: 19667033] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loisel 1996
- Loisel DB, Smith MM, MacDonald MG, Martin GR. Intravenous access in newborn infants: impact of extending umbilical venous catheter use on requirement for peripheral venous line. Journal of Perinatology 1996;16(6):461‐6. [PubMed] [Google Scholar]
Machado 2009
- Machado JD, Suen VM, Figueiredo JF, Marchini JS. Biofilms, infection, and parenteral nutrition therapy. Journal Parenteral and Enteral Nutrition 2009;33(4):397‐403. [DOI] [PubMed] [Google Scholar]
Mermel 2001
- Mermel LA, Farr BM, Sherertz RJ, Raad II, O'Grady N, Harris JS, et al. Guidelines for the management of intravascular catheter‐related infections. Clinical Infectious Diseases 2001;32(9):1249‐72. [DOI] [PubMed] [Google Scholar]
Messing 1988
- Messing B, Peitra‐Cohen S, Debure A, Beliah M, Bernier JJ. Antibiotic‐lock technique: a new approach to optimal therapy for catheter‐related sepsis in home‐parenteral nutrition patients. Journal of Parenteral and Enteral Nutrition 1988;12(2):185‐9. [DOI] [PubMed] [Google Scholar]
Miller 2010
- Miller MR, Griswold M, Harris JM 2nd, Yenokyan G, Huskins WC, Moss M, et al. Decreasing PICU catheter‐associated bloodstream infections: NACHRI's quality transformation efforts. Pediatrics 2010;125(2):206‐13. [DOI] [PubMed] [Google Scholar]
NHSN 2011
- National Healthcare Safety Network (NHSN). Central Line‐Associated Bloodstream Infection (CLABSI), 2011. www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf. (accessed 4 December 2012).
O'Grady 2002
- O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter‐related infections. The Hospital Infection Control Practices Advisory Committee, Center for Disease Control and Prevention, U.S. Pediatrics 2002;110(5):e51. [DOI] [PubMed] [Google Scholar]
O'Grady 2011
- O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Centre for Disease Control and Prevention (CDC). Guidelines for the prevention of Intravascular catheter‐related infections, 2011. www.cdc.gov/hicpac/bsi/bsi‐guidelines‐2011.html. (accessed 4 December 2012).
Ohki 2008
- Ohki Y, Yoshizawa Y, Watanabe M, Kuwashima M, Morikawa A. Complications of percutaneously inserted central venous catheters in Japanese neonates. Pediatrics International 2008;50(5):636‐9. [DOI] [PubMed] [Google Scholar]
Olsen 2009
- Olsen AL, Reinholdt J, Jensen AM, Andersen LP, Jensen ET. Nosocomial infection in a Danish neonatal intensive care unit: a prospective study. Acta Paediatrica 2009;98(8):1294‐9. [DOI] [PubMed] [Google Scholar]
Pronovost 2006
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. New England Journal of Medicine 2006;355(26):2725‐32. [DOI] [PubMed] [Google Scholar]
Raad 1993
- Raad I, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, Bodey GP. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. Journal of Infectious Diseases 1993;168(2):400‐7. [DOI] [PubMed] [Google Scholar]
Ramirez de Arellano 1994
- Ramirez de Arellano E, Pascual A, Martinez‐Martinez L, Perea EJ. Activity of eight antibacterial agents on staphylococcus epidermidis attached to Teflon catheters. Journal of Medical Microbiology 1994;40(1):43‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rosett 1980
- Rosett W, Hodges GR. Antimicrobial activity of heparin. Journal of Clinical Microbiology 1980;11(1):30‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryder 2005
- Ryder, MA. Catheter‐related infections: it's all about biofilm. Topics in Advanced Practice Nursing eJournal 2005; Vol. 5.
Safdar 2006
- Safdar N, Maki DG. Use of vancomycin‐containing lock or flush solutions for prevention of bloodstream infection associated with central venous access devices: a meta‐analysis of prospective, randomized trials. Clinical Infectious Diseases 2006;43(4):474‐84. [DOI] [PubMed] [Google Scholar]
Saint 2000
- Saint S, Veenstra DL, Lipsky BA. The clinical and economic consequences of nosocomial central venous catheter‐related infection: are antimicrobial catheters useful?. Infection Control and Hospital Epidemiology 2000;21(6):375‐80. [DOI] [PubMed] [Google Scholar]
Sannoh 2010
- Sannoh S, Clones B, Munoz J, Montecalvo M, Parvez B. A multimodal approach to central venous catheter hub care can decrease catheter‐related bloodstream infection. American Journal Infection Control 2010;38(6):424‐9. [DOI] [PubMed] [Google Scholar]
Schulman 2011
- Schulman J, Stricof R, Stevens TP, Horgan M, Gase K, Holzman IR, et al. Statewide NICU central‐line‐associated bloodstream infection rates decline after bundles and checklists. Pediatrics 2011;127(3):436‐44. [DOI] [PubMed] [Google Scholar]
Sengupta 2010
- Sengupta A, Lehmann C, Diener‐West M, Perl T, Milstone A. Catheter duration and risk of CLA‐BSI in neonates with PICCs. Pediatrics 2010;125(4):648‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snaterse 2010
- Snaterse M, Ruger W, Scholte op Reimer WJM, Lucas C. Antibiotic‐based catheter lock solutions for prevention of catheter‐related bloodstream infection: a systematic review of randomised controlled trials. Journal of Hospital Infection 2010;75(1):1‐11. [DOI] [PubMed] [Google Scholar]
van de Wetering 2007
- Wetering MD, van Woensel JB. Prophylactic antibiotics for preventing early central venous catheter Gram positive infections in oncology patients. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD003295.pub2] [DOI] [PubMed] [Google Scholar]
Van de Zwet 2005
- Zwet WC, Kaiser AM, Elburg RM, Berkhof J, Fetter WP, Parlevliet GA, et al. Nosocomial infections in a Dutch neonatal intensive care unit: surveillance study with definitions for infection specifically adapted for neonates. Journal of Hospital Infection 2005;61(4):300‐11. [DOI] [PubMed] [Google Scholar]
Vanholder 2010
- Vanholder R, Canaud B, Fluck R, Jadoul M, Labriola L, Marti‐Monros A, et al. Catheter‐related blood stream infections (CRBSI): a European view. Nephrology, Dialysis, Transplantation 2010;25(6):1753‐6. [DOI] [PubMed] [Google Scholar]
Wales 2011
- Wales PW, Kosar C, Carricato M, Silva N, Lang K, Avitzur Y. Ethanol lock therapy to reduce the incidence of catheter‐related bloodstream infections in home parenteral nutrition patients with intestinal failure: preliminary experience. Journal of Pediatric Surgery 2011;46(5):951‐6. [PUBMED: 21616259] [DOI] [PubMed] [Google Scholar]
Wirtschafter 2010
- Wirtschafter DD, Pettit J, Kurtin P, Dalsey M, Chance K, Morrow HW, et al. A statewide quality improvement collaborative to reduce neonatal central line‐associated blood stream infections. Journal of Perinatology 2010;30(3):170‐81. [DOI] [PubMed] [Google Scholar]
Yahav 2008
- Yahav D, Rozen‐Zvi B, Gafter‐Gvili A, Leibovici L, Gafter U, Paul M. Antimicrobial lock solutions for the prevention of infections associated with intravascular catheters in patients undergoing hemodialysis: systematic review and meta‐analysis of randomized, controlled trials. Clinical Infectious Diseases 2008;47:83‐93. [DOI] [PubMed] [Google Scholar]
Yebenes 2004
- Yebenes JC, Vidaur L, Serra‐Prat M, Sirvent JM, Batlle J, Motje M, et al. Prevention of catheter related blood stream infection in critically ill patients using a disinfectable, needle free connector: a randomised controlled trial. American Journal of Infection Control 2004;32(5):291‐5. [DOI] [PubMed] [Google Scholar]


