Abstract
Both repression and induction of substrate utilization have been the subject of many basic research investigations employing pure cultures. In this investigation these effects were studied using heterogeneous microbial populations prevalent in such biological treatment processes as activated sludge systems.
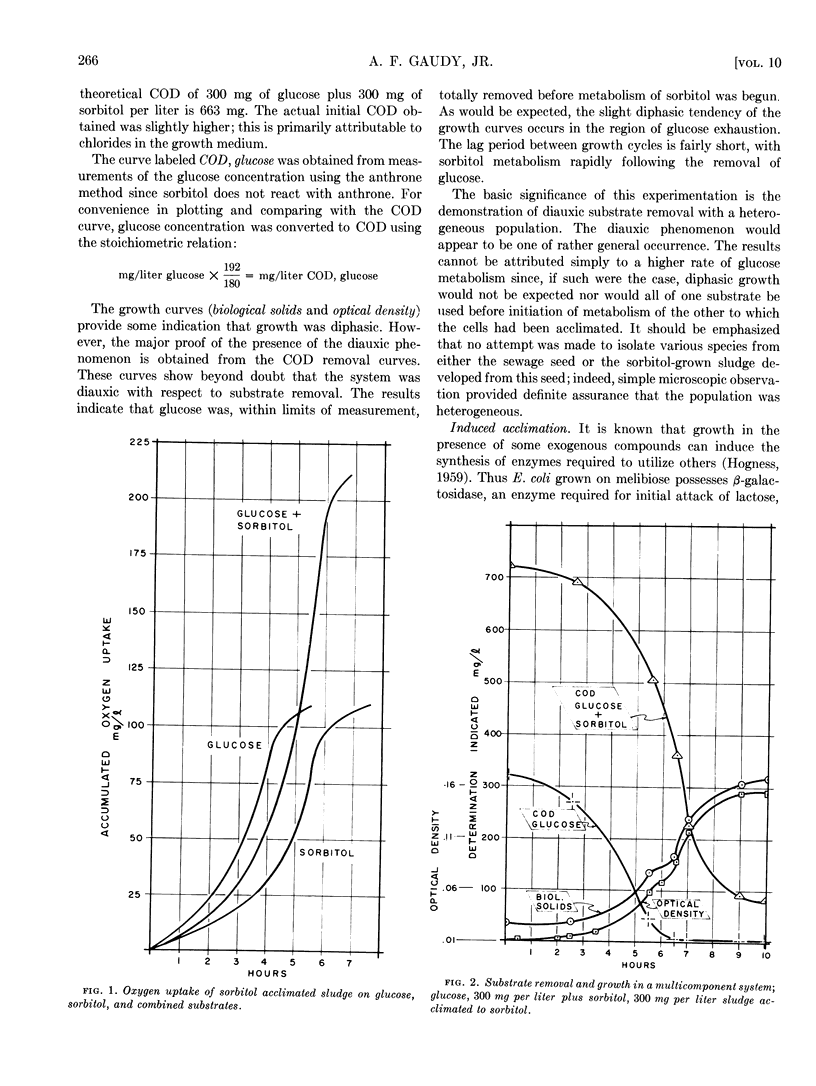
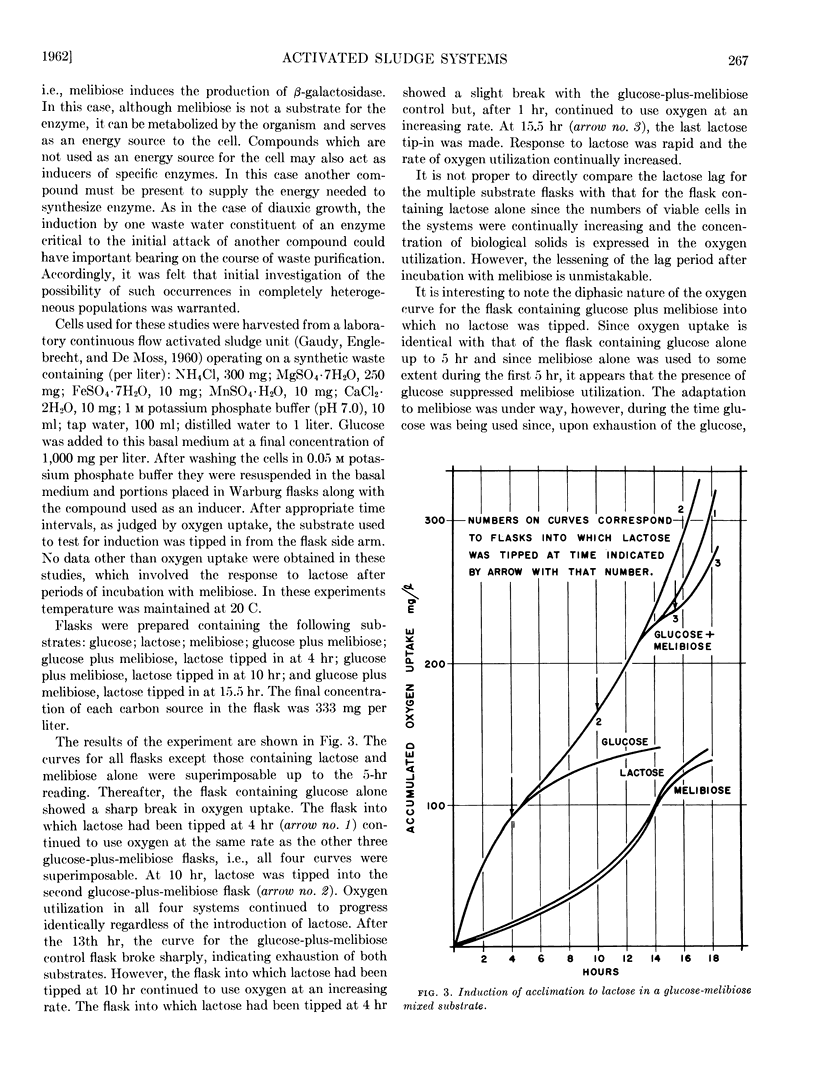
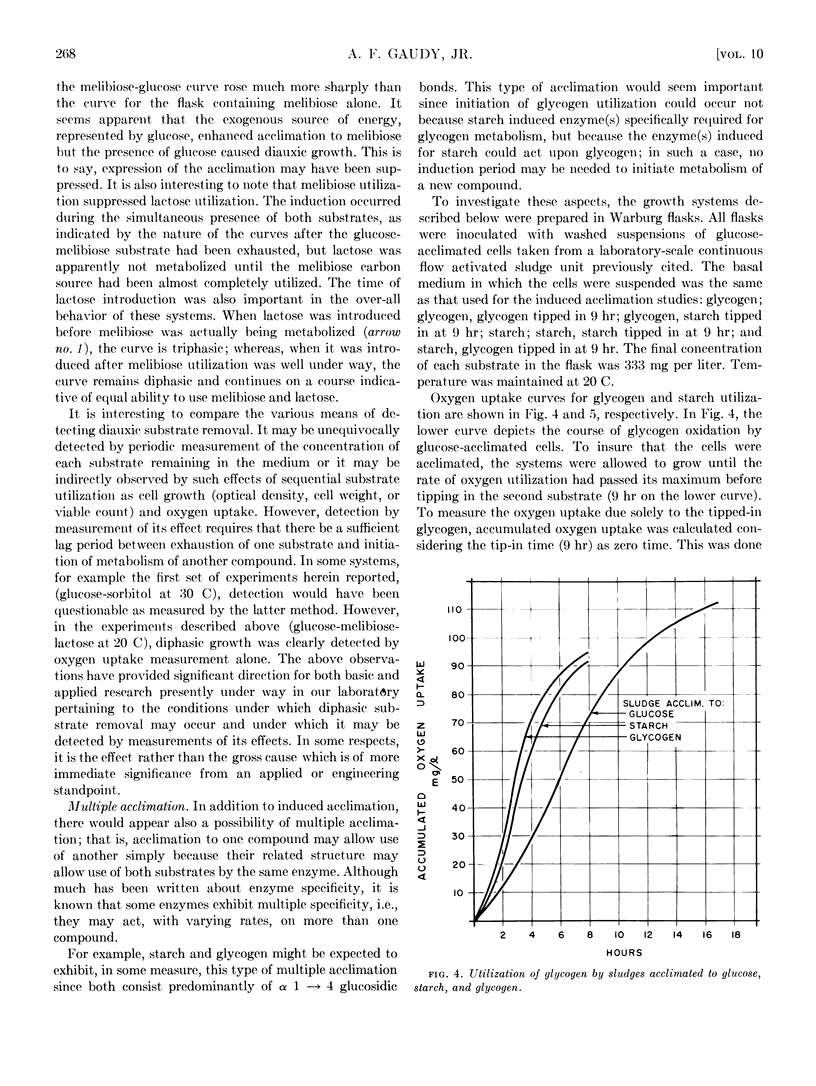
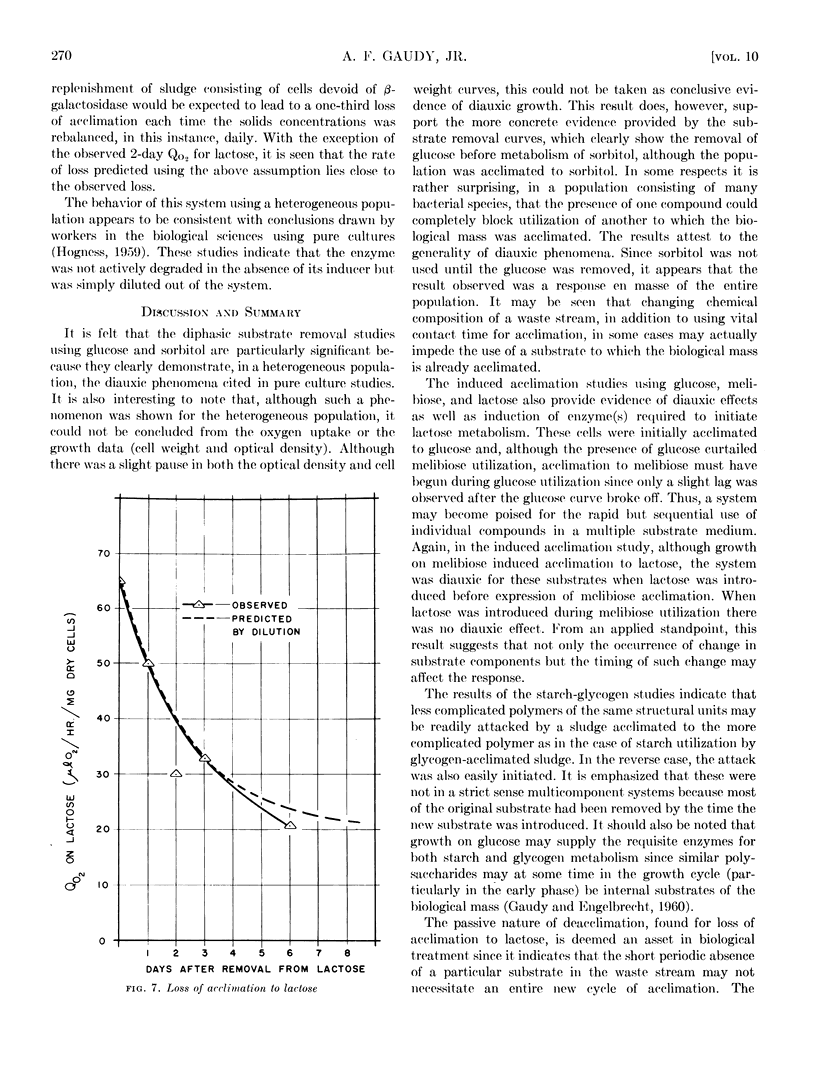
Diauxic substrate removal by activated sludge was observed in a multicomponent medium consisting of glucose and sorbitol. The sludge was acclimated solely to sorbitol; however, the presence of glucose blocked sorbitol removal until glucose was completely utilized. Both diphasic and triphasic oxygen utilization was shown for activated sludges metabolizing multicomponent synthetic wastes consisting of glucose, melibiose, and lactose. It appears from these studies that melibiose utilization was suppressed by the presence of glucose and, although melibiose induced acclimation to lactose, the presence of melibose suppressed lactose utilization. Studies were also conducted using glycogen and starch systems in which it was found that acclimation to either compound conferred immediate acclimation to the other. It was also found that loss of acclimation to lactose was a passive phenomenon and its kinetics could be predicted on the basis of simple diluting out of the enzyme(s) responsible for such acclimation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAGLEY S., DAWES E. A. Critic acid metabolism of Aerobacter aerogenes. J Bacteriol. 1953 Sep;66(3):259–265. doi: 10.1128/jb.66.3.259-265.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUDY A. F., Jr, ENGELBRECHT R. S., DE MOSS R. D. Laboratory scale activated sludge unit. Appl Microbiol. 1960 Sep;8:298–304. doi: 10.1128/am.8.5.298-304.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. Induction and repression of beta-galactosidase in non-growing Escherichia coli. Biochem J. 1961 Jun;79:489–496. doi: 10.1042/bj0790489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- RAVIN A. W. Heritable and non-heritable loss of ability by Aerobacter aerogenes to grow adaptively on single carbon sources. J Gen Microbiol. 1952 May;6(3-4):211–232. doi: 10.1099/00221287-6-3-4-211. [DOI] [PubMed] [Google Scholar]


