Abstract
To determine the clinical manifestations and outcomes of the coronavirus disease 2019 (COVID-19) in children who underwent liver transplantation (LT). A retrospective study was conducted at a transplant center in Thailand to include LT recipients aged < 18 years who had been infected with COVID-19. Out of a total of 54 children, there were 31 probable cases (57.4%) diagnosed using an antigen test kit and 23 confirmed cases (42.6%) diagnosed using polymerase chain reaction (14 children) or severe acute respiratory syndrome coronavirus 2 antigen (9 children). Approximately half of the children (25, 46.3%) received the BNT162b2 vaccine before the infection, with 3 and 2 doses in 5 and 18 children, respectively. While some had COVID-19 during the delta pandemic, most (46 children, 85.2%) were infected during the omicron pandemic, of which manifestations included fever (67.4%), cough (50%), and rhinorrhea (47.8%), and symptoms lasted approximately 3 days. None had severe diseases. All patients with mild-to-moderate disease were advised to continue the same immunosuppressive therapy as before the infection. Compared to unvaccinated children or children with one dose of the vaccine, fever was less common in those who received ≥ 2 doses (OR: 0.08; 95%CI: 0.01–0.57, adjusted for age and immunosuppressive types). Favipiravir was prescribed in most patients (90.7%). Only a few children had long COVID-19 or abnormal liver function tests lasting > 1 month (4 children, 7.4%, both). Pediatric LT recipients with COVID-19 during the delta and omicron variant pandemic reported mild symptoms despite undergoing immunosuppressive therapy.
Keywords: children, coronavirus disease 2019, liver transplantation, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread worldwide since 2019, causing the coronavirus disease 2019 (COVID-19) pandemic. The disease has a wide range of clinical manifestations, including asymptomatic infection, upper and lower respiratory tract infections, which contribute to significant morbidities and mortalities.[1] Previous studies reported various incidences and outcomes of COVID-19 depending on the patients’ age groups and the virus variants. In the initial phase of the outbreak, Thailand, which was the first country in Southeast Asia to report patients with COVID-19 outside of China,[2] applied lockdown measures, and the outbreak was controlled.[2,3] Hence, children were initially less susceptible to the Wuhan strain of the SARS-CoV-2.[1] Despite the restriction of public gatherings to manage the alpha variant outbreak,[3] the alpha variant (B.1.1.7) outbreak in Thailand started in January and rapidly increased in April 2021. However, our pediatric liver transplantation (LT) recipients were rarely infected with the Wuhan strain or the alpha variant in these periods. The delta variant (B.1.617) pandemic started in June 2021.[4] Thereafter, the number of Thai children with COVID-19 has risen. Since January 2022, the omicron variant (B.1.1.529) has been the major strain among patients with COVID-19 in the country.[5]
Compared to adults, infected children generally have less severe disease.[1] Most of them are asymptomatic or present with mild symptoms.[6,7] Only certain children are at-risk for severe COVID-19, particularly young children or patients with co-morbidities, such as obesity, chronic lung disease, congenital heart disease.[1,7]Although children undergoing LT are considered immunocompromised, previous data revealed that COVID-19 is not severe in these children.[8–10] Unfortunately, most data are derived from a small number of cases. Moreover, the information in Asia is sparse. For these reasons, we aimed to study the clinical manifestations and outcomes of COVID-19 in pediatric LT recipients.
2. Methods
2.1. Study population
A retrospective study was performed at an active pediatric LT center in Bangkok, Thailand. Enrolled subjects included LT recipients aged 1 to 18 years who were at a regular follow-up at the center and were diagnosed with COVID-19 from December 2019 to October 2022. Since the COVID-19 pandemic, caregivers of LT recipients were strongly advised to contact our center if the patients were suspicious with COVID-19. This approach was strictly implemented during the pandemic to ensure that all LT recipients must receive proper management regarding COVID-19 and immunosuppression, which could be provided directly by our center or remotely from our telecare and network services. A medical record was reviewed in terms of the manifestations, diagnosis, management, and outcomes of COVID-19. During the study period, Thai Food and Drug Administration has approved the use of SARS-CoV-2 BNT162b2 vaccine in children aged > 12 years since June 2021 and in children aged > 5 years since December 2021. The study protocol was approved by the Institutional Review Board from the Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University. It also followed the Helsinki declaration.
2.2. Diagnosis of COVID-19
The diagnosis of COVID-19 was according to the World Health Organization (WHO)’s definition,[11] consisting of confirmed COVID-19 which was diagnosed by detectable SARS-CoV-2 from polymerase chain reaction (PCR) or antigen (Ag)-detection tests, and probable COVID-19 diagnosed by positive antigen test kit (ATK).
2.3. Outcome measures
The primary outcome of the study was the clinical manifestations of COVID-19. The secondary outcome was the severity of COVID-19, which was categorized as mild (symptomatic infection without pneumonia or hypoxia), moderate (presenting with clinical signs of non-severe pneumonia without any signs of severe pneumonia), and severe (demonstrating signs of pneumonia and central cyanosis or tachypnea).[12] Additionally, long COVID (post-COVID-19 conditions) was defined based on the modified Delphi consensus in children,[13] of which symptoms must occur within 12 weeks and last for >2 months after the onset of the COVID-19. The symptoms of the post-COVID-19 conditions must not be caused by other etiologies. We also collected the data regarding abnormal liver function after the infection.
2.4. Statistical analyses
The statistical analysis was done by using STATA program (StataCorp., version 14, College Station, TX). Descriptive statistics were reported as mean (SD), median (IQR), and percentage. We compared the patient characteristics by using Student t-test, Mann–Whitney U test, Chi-square test, and Fisher exact test. Logistic regression analysis was performed to demonstrate the association between variables. P < .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
Among 185 pediatric LT recipients aged 1 to 18 years, 54 patients (29.2%) were diagnosed with COVID-19 with a median age and duration after LT of 8.7 (IQR: 4.6, 12.5) and 5.6 (IQR: 2.3, 10.0) years, respectively. The most common primary liver disease was biliary atresia (44 patients, 81.5%), and some children had other comorbid diseases, including allergic rhinitis (7 patients, 13%), cardiac diseases (4 patients, 7.4%), and obesity (2 patients, 3.7%). None had preexisting chronic lung or reactive airway diseases. Patients received 3 types of immunosuppressive therapy, including monotherapy (31.5%), double therapy (37%), and triple therapy (31.5%) (Table 1).
Table 1.
Baseline characteristics of participants (N = 54).
| Characteristics | Results |
|---|---|
| Age (years), median (IQR) | 8.7 (4.6, 12.5) |
| Age at liver transplantation (years), median (IQR) | 1.9 (1.3, 2.9) |
| Duration after liver transplantation (years), median (IQR) | 5.6 (2.3, 10.0) |
| Gender: female, N (%) | 34 (63.0) |
| Primary liver disease, N (%) | |
| • Biliary atresia | 44 (81.5) |
| • Other chronic liver disease | 6 (11.1) |
| • Acute liver failure | 4 (7.4) |
| Immunosuppressive protocol before the infection, N (%) | |
| • Monotherapy (tacrolimus) | 17 (31.5%) |
| • Double therapy (tacrolimus or cyclosporin with MMF or prednisolone) | 20 (37%) |
| • Triple therapy (tacrolimus, MMF, and prednisolone) | 17 (31.5%) |
| Underlying disease, N (%) | |
| • Allergic rhinitis | 7 (13.0) |
| • Cardiac disease | 4 (7.4) |
| • Obesity | 2 (3.7) |
| Diagnostic test of COVID-19, N (%) | |
| • Nasal swab for SARS-CoV-2 PCR | 14 (25.9) |
| • Nasal swab for SARS-CoV-2 antigen | 9 (16.7) |
| • Nasal swab for SARS-CoV-2 antigen kit test | 41 (75.9) |
COVID-19 = coronavirus disease of 2019, MMF = mycophenolate mofetil, PCR = polymerase chain reaction, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
The patients with COVID-19 consisted of 23 confirmed cases (42.6%) diagnosed by PCR (14 children) or SARS-CoV-2 Ag (9 children) and 31 probable cases (57.4%) diagnosed by ATK. Index cases were identified in 47 patients (87.0%); most were household members (78.7%), followed by school peers (4.9%).
3.2. Clinical manifestations of COVID-19 infection
While 8 patients were diagnosed with COVID-19 during the delta variant pandemic, most children (46 children, 85.2%) were infected during the omicron variant pandemic, with comparable median age in both groups [8.4 (IQR: 6.6, 14.1), and 8.7 (IQR: 4.5, 12.5), respectively, P = .59]. Almost half (25 patients, 46.3%) received BNT162b2 vaccine before the infection, reported with 1 dose in 2 children, 2 doses in 18 children, and 3 doses in 5 children, respectively. Table 2 shows the details of vaccination among the participants. The proportion of patients who received at least 2 doses of the vaccine appeared to be lower during the delta variant pandemic [1 patient (12.5%) and 22 patients (47.8%), respectively, P = .06]. The patients infected during the omicron pandemic with the triple therapy were reported with less access to the vaccine before the infection (OR: 0.11, 95%CI: 0.02–0.67, P = .02). No children received other types of vaccine to prevent SARS-CoV-2 infection.
Table 2.
Characteristics of pediatric liver transplant recipients with coronavirus disease 2019 who had a history of receiving SARS-CoV-2 BNT162b2 vaccine before the infection (N = 25).
| Patient | Age (years) | Body mass index (kg/m2) | Trough level of tacrolimus (ng/mL) | Dose of mycophenolate mofetil (mg/kg/day) | Dose of prednisolone (mg/kg/day) | Total doses of SARS-CoV-2 BNT162b2 vaccine before the infection | Duration from the last dose of the vaccine to the infection (days) | Periods of coronavirus disease 2019 pandemic |
|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 20 | 3.3 | 25 | – | 2 | 60 | Delta variant |
| 2 | 12 | 19 | 3.9 | – | – | 2 | 155 | Omicron variant |
| 3 | 9 | 15 | 5.3 | 25 | – | 1 | 13 | Omicron variant |
| 4 | 11 | 17 | 4.8 | – | – | 2 | 1 | Omicron variant |
| 5 | 15 | 25 | 3.1 | 10 | – | 2 | 159 | Omicron variant |
| 6 | 12 | 24 | 4 | – | – | 1 | 15 | Omicron variant |
| 7 | 11 | 18 | 5.8 | 5 | – | 2 | 5 | Omicron variant |
| 8 | 10 | 19 | 3.7 | – | – | 2 | 1 | Omicron variant |
| 9 | 10 | 15 | 4.2 | – | – | 2 | 12 | Omicron variant |
| 10 | 5 | 14 | 5.7 | 30 | – | 2 | 1 | Omicron variant |
| 11 | 16 | 24 | 2.2 | 30 | – | 2 | 223 | Omicron variant |
| 12 | 8 | 21 | 3.1 | – | – | 2 | 29 | Omicron variant |
| 13 | 14 | 24 | 5.4 | 25 | – | 3 | 52 | Omicron variant |
| 14 | 5 | 14 | 3 | – | – | 2 | 84 | Omicron variant |
| 15 | 11 | 11 | 4.2 | – | – | 2 | 81 | Omicron variant |
| 16 | 13 | 24 | 5.6 | – | – | 2 | 218 | Omicron variant |
| 17 | 15 | 21 | 2.2 | – | – | 3 | 75 | Omicron variant |
| 18 | 13 | 13 | 7.8 | 20 | 0.5 | 2 | 246 | Omicron variant |
| 19 | 5 | 14 | 5.4 | – | 2.5 | 2 | 115 | Omicron variant |
| 20 | 16 | 17 | 3.1 | 10 | – | 3 | 122 | Omicron variant |
| 21 | 9 | 16 | 4.6 | 10 | – | 2 | 256 | Omicron variant |
| 22 | 7 | 27 | 10 | 30 | 0.3 | 2 | 168 | Omicron variant |
| 23 | 14 | 17 | 6.7 | 10 | – | 3 | 130 | Omicron variant |
| 24 | 12 | 16 | 4.2 | – | – | 2 | 149 | Omicron variant |
| 25 | 7 | 16 | 2.8 | – | – | 3 | 68 | Omicron variant |
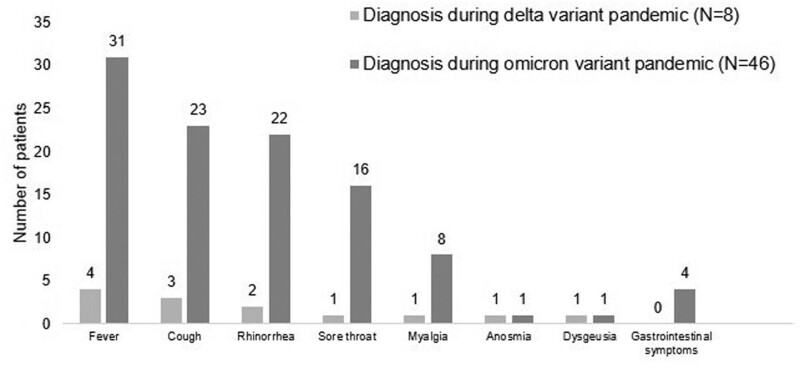
The common manifestations during the omicron variant pandemic included fever (67.4%), cough (50%), and rhinorrhea (47.8%) (Fig. 1). None had breathing difficulty. A small number of patients (4 children, 8.7%) during the omicron variant pandemic had gastrointestinal symptoms. During the omicron variant pandemic, comparisons were made between those who received at least 2 doses of the BNT162b2 vaccine and those who did not. Patients who received at least 2 doses of the vaccine were considered older [11.7 (IQR: 8.3, 14.1) vs 4.5 (IQR: 2.9, 9.8) years, (P < .01)]. The immunosuppressive therapy was also different between the 2 groups, describing with monotherapy (25% vs 50%), double therapy (33.3% vs 40.9%), and triple therapy (41.7% vs 9.1%) (P = .03). However, there were no significant differences in terms of the treatment with favipiravir (P > .99) or comorbidities, such as allergic rhinitis (P = .67), cardiac diseases (P > .99), and obesity (P = .48). We observed that fever was less found in those who received at least 2 doses of the vaccine compared to the unvaccinated children or children received 1 dose of the vaccine (OR: 0.08; 95%CI: 0.01–0.57, adjusted for age and immunosuppressive types, P = .01). Table 3 demonstrates patient characteristics and outcomes during the omicron pandemic according to the vaccination status. The median duration of illness was similar in the delta and omicron pandemics, described with 3 (IQR: 2, 6) and 3 (IQR: 2, 4) days, respectively (P = .64).
Figure 1.
Clinical manifestations of coronavirus disease 2019 among pediatric liver transplant recipients.
Table 3.
Clinical manifestations of pediatric liver transplant recipients with coronavirus disease of 2019 during the omicron variant pandemic according to the SARS-CoV-2 BNT162b2 vaccination status (N = 46).
| Clinical manifestations | No vaccination or receiving only 1 dose of SARS-CoV-2 BNT162b2 vaccine (N = 24, 52.2%) | Receiving at least 2 doses of SARS-CoV-2 BNT162b2 vaccine (N = 22, 47.8%) | OR* | 95%CI* | P * | Adjusted OR** | 95%CI** | P ** |
|---|---|---|---|---|---|---|---|---|
| Fever | 21 (87.5) | 10 (45.6) | 0.12 | 0.03–0.52 | <.01 | 0.08 | 0.01–0.57 | .01 |
| Cough | 12 (50) | 11 (50) | 1 | 0.31–3.18 | >.99 | 1.28 | 0.28–5.90 | .76 |
| Rhinorrhea | 12 (50) | 10 (45.6) | 0.83 | 0.26–2.66 | .8 | 1.02 | 0.21–5.00 | .98 |
| Sore throat | 5 (20.8) | 11 (50) | 3.8 | 1.04–13.83 | 0 | 1.9 | 0.43–8.45 | .4 |
A group of patients with no vaccination or receiving only 1 dose of SARS-CoV-2 BNT162b2 vaccine served as reference.
Adjusted for age and types of immunosuppressive therapy.
3.3. Outcomes of COVID-19 infection
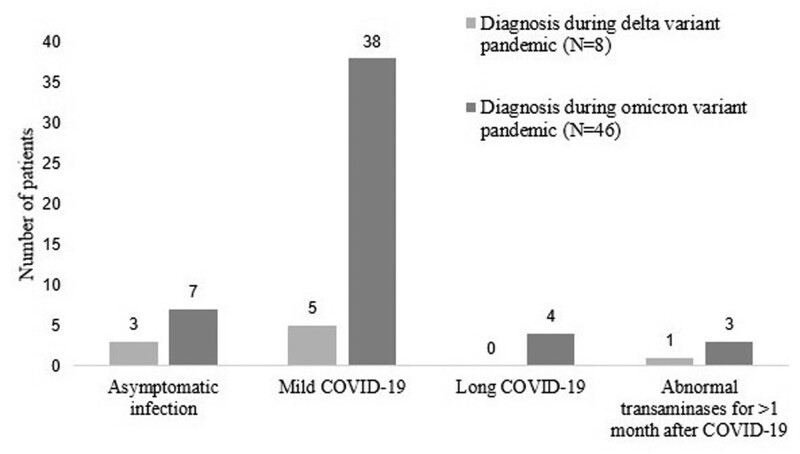
Most patients in both periods had mild infection (44 patients, 81.5%) (Fig. 2). A smaller proportion of patients (10 patients, 18.5%) were asymptomatic and underwent diagnostic tests for SARS-CoV-2 due to their contact with COVID-19 patients. None had severe disease requiring adjustment of the immunosuppressive drugs or additional corticosteroids. Sixteen patients (29.6%) were admitted to the hospital ward or field hospital without the need for respiratory support. Favipiravir was prescribed in most patients (49 patients, 90.7%) without side effects.
Figure 2.
Outcomes of coronavirus disease 2019 in pediatric liver transplant recipients.
After the infection, only 4 children (7.4%) had long COVID-19; 3 of them were unvaccinated. The patient who was vaccinated was obese and received only 2 doses of the vaccine, of which the last dose was 5 months before the infection. In addition, 4 children (7.4%) had abnormal transaminases >1 month. However, the transaminases returned to baseline in all 4 children within 3 months. Two children with COVID-19 during the delta variant pandemic developed acute cellular rejection a few months after the infection, but both had poor compliance to the immunosuppressive therapy. None were vaccinated before the rejection. Moreover, one patient infected during the delta variant pandemic had reinfection in the omicron variant pandemic approximately 13 months after the first infection and 7 months after the second dose of the BNT162b2 vaccine. Additionally, none of our children developed multisystem inflammatory syndrome in children.
4. Discussion
Pediatric LT recipients with COVID-19 were mainly diagnosed during the omicron variant pandemic, of which common manifestations were fever, cough, and rhinorrhea. None had moderate or severe disease. Most were described with good outcomes and rarely developed long COVID-19.
The number of infected patients in this study was higher during the pandemic of the omicron variant, which is more contagious than the delta variant.[14] The increased prevalence of the disease after the omicron variant pandemic was also demonstrated in healthy Thai children.[15] While the patients infected with the omicron variant may be younger than the delta variant,[14] our patients’ ages were comparable in both periods of the pandemic.
While the number of COVID-19 patients has increased over time, the disease caused by the omicron variant is less severe than that caused by earlier strains.[1,14] COVID-19 infections in healthy children during the omicron variant pandemic are mild, with fever and cough being the most common symptoms, similar to our findings.[1] This study found that the majority of pediatric LT recipients also have mild disease with a very low mortality rate, comparable to studies conducted on healthy children during the pre-[8] and post-delta variant pandemic.[14] Only a small number of patients were reported requiring oxygen support.[9,10]
Although children who underwent LT are considered immunocompromised, we hypothesized that COVID-19 in these children was not severe due to several factors. Compared to adults, children were reported to have decreased expression of the angiotensin-converting enzyme 2 receptor,[16] which is crucial for SARS-CoV-2’s cell entry.[1] The prevalence of co-morbidities of COVID-19,[1,7] such as obesity, cardiac disease, and chronic lung disease, was also low in this study. Moreover, the pathophysiology of COVID-19 primarily involves an excessive host-immune response.[17] A previous study proposed that corticosteroids and tacrolimus may have some beneficial effects on the COVID-19 disease,[18] and immunosuppressant agents were not an independent risk factor for severe COVID-19.[16]
The two-dose regimen of the BNT162b2 vaccine has high effectiveness for the prevention of COVID-19 infection, hospitalization, and admission to the intensive care unit among children aged 5 to 18 years.[19] However, the protectiveness tends to decrease over time and is less effective among the omicron variant.[19] To our knowledge, there are limited studies regarding vaccine effectiveness in pediatric LT recipients. Previous reports demonstrated a lower immunogenic response in adolescents LT recipients compared to healthy controls,[20] and a booster vaccine might be required.[21] Although our study did not reveal any differences between severity, duration of symptoms, and incidence of long COVID-19 among patients with and without vaccination, we found that fever was less found in patients who received at least 2 doses of BNT162b2 compared with unvaccinated patients or patients received only 1 dose of the vaccine during the omicron variant pandemic. Unfortunately, the number of patients who received a booster dose was too low to demonstrate statistical significance.
Most children in this study received favipiravir for the treatment of COVID-19, according to the national treatment guidelines,[22] suggesting the consideration of favipiravir therapy among symptomatic children with immunosuppressed state. However, the efficacy of favipiravir is still debatable. While the beneficial effects on the disease duration were previously mentioned,[23] favipiravir efficacy is not well-demonstrated in a large randomized controlled trial in adults.[24] Although Anugulruengkitt et al[7] revealed that 90% of children who received the medication were less likely to have fever >72 hours after the treatment, a randomized controlled trial may be needed to confirm its efficacy in children. In terms of the immunosuppression adjustment during the pandemic, we followed the suggestions from the Beijing Working Party for LT,[25] which stated that patients with mild-to-moderate COVID-19 should continue the same immunosuppressive treatment before the infection unless they develop severe COVID-19.
Only 4 children in this study had abnormal transaminases >1 month, and all spontaneously resolved under close observation. SARS-CoV-2 is known to be one of the causes of acute liver injury in pediatric LT recipients.[26] To our knowledge, the virus rarely causes persistently abnormal liver enzymes. In addition, acute cellular rejection was reported in 2 children (3.7%), similar to a previous multicenter report (5.4%).[26] We hypothesized that acute rejection was due to poor compliance to the immunosuppressive drugs, which was well-documented in both cases, rather than its association with COVID-19 infection. Moreover, the prevalence of long COVID-19 in our children was lower than previous reports in healthy children.[27,28]
This study has some limitations of its retrospective nature that was prone to missing data. We were also unable to identify all pediatric LT recipients with asymptomatic COVID-19. Moreover, the sample size was relatively small, which may reduce the effect of statistical analysis. Additionally, some of the diagnosis was based on ATK, which was not as specific as the Ag- or PCR-based tests. Despite these limitations, this study provides valuable information on clinical manifestations and outcomes of COVID-19 infection among a specific population of pediatric patients who underwent LT. In addition, the evaluation of long-term effects, such as the occurrence of long COVID-19 symptoms and abnormal liver function tests after the infection, provides insights into the potential impact of COVID-19 on pediatric LT recipients beyond the acute phase of the illness.
5. Conclusions
Despite undergoing immunosuppressive therapy, most pediatric LT recipients with COVID-19 had mild symptoms and favorable outcomes during the delta and omicron variant pandemics. Even though favipiravir exhibited no adverse effects in this report, further studies regarding its efficacy in COVID-19 are needed.
Acknowledgments
The authors would like to thank our colleagues from Ramathibodi Hospital, particularly Ramathibodi Excellence Center in Organ Transplantation and Division of Infectious Disease, Department of Pediatrics, for their assistance in the patient care during the pandemic of coronavirus.
Author contributions
Conceptualization: Songpon Getsuwan, Sophida Boonsathorn, Sujittra Chaisavaneeyakorn, Napapat Butsriphum, Pornthep Tanpowpong, Chatmanee Lertudomphonwanit, Suporn Treepongkaruna.
Data curation: Songpon Getsuwan, Napapat Butsriphum.
Formal analysis: Songpon Getsuwan.
Investigation: Songpon Getsuwan.
Methodology: Songpon Getsuwan.
Project administration: Songpon Getsuwan.
Supervision: Sophida Boonsathorn, Pornthep Tanpowpong.
Visualization: Songpon Getsuwan, Sophida Boonsathorn.
Writing – original draft: Songpon Getsuwan.
Writing – review & editing: Sophida Boonsathorn.
Abbreviations:
- Ag
- antigen
- ATK
- antigen test kit
- COVID-19
- coronavirus disease 2019
- LT
- liver transplantation
- PCR
- polymerase chain reaction
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
The study protocol was approved by the Institutional Review Board (IRB, Faculty of Medicine Ramathibodi Hospital, Bangkok, Thailand, COA. No. MURA 2023/93). It also followed the Helsinki declaration.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Getsuwan S, Boonsathorn S, Chaisavaneeyakorn S, Butsriphum N, Tanpowpong P, Lertudomphonwanit C, Treepongkaruna S. Clinical manifestations and outcomes of coronavirus disease 2019 among pediatric liver transplant recipients in the delta and omicron variant pandemic: A retrospective study. Medicine 2023;102:41(e35537).
Contributor Information
Songpon Getsuwan, Email: songpon.get@mahidol.edu.
Sujittra Chaisavaneeyakorn, Email: sujittrachai@hotmail.com.
Napapat Butsriphum, Email: ms.napapat@gmail.com.
Pornthep Tanpowpong, Email: tuinoi1@gmail.com.
Chatmanee Lertudomphonwanit, Email: chatmanee.puk@gmail.com.
Suporn Treepongkaruna, Email: suporntr@gmail.com.
References
- [1].Nathanielsz J, Toh ZQ, Do LAH, et al. SARS-CoV-2 infection in children and implications for vaccination. Pediatr Res. 2022;93:1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dechsupa S, Assawakosri S, Phakham S, et al. Positive impact of lockdown on COVID-19 outbreak in Thailand. Travel Med Infect Dis. 2020;36:101802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Uansri S, Tuangratananon T, Phaiyarom M, et al. Predicted impact of the lockdown measure in response to coronavirus disease 2019 (COVID-19) in greater Bangkok, Thailand, 2021. Int J Environ Res Public Health. 2021;18:12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chaiyakulsil C, Sritipsukho P, Satdhabudha A, et al. An epidemiological study of pediatric COVID-19 in the era of the variant of concern. PLoS One. 2022;17:e0267035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Assawakosri S, Kanokudom S, Suntronwong N, et al. Omicron BA.1, BA.2 and COVID-19 booster vaccination. J Infect Dis. 2022;226:1480–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Butt AA, Dargham SR, Loka S, et al. Coronavirus disease 2019 disease severity in children infected with the omicron variant. Clin Infect Dis. 2022;75:e361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Anugulruengkitt S, Teeraananchai S, Chantasrisawad N, et al. Clinical outcomes of pediatric COVID-19 in a tertiary care center in Bangkok, Thailand. IJID Regions. 2021;1:159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bansal N, Ovchinsky N, Foca M, et al. COVID-19 infection in pediatric solid organ transplant patients. Pediatr Transplant. 2022;26:e14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Buescher G, Sebode M, Marjot T, et al. SARS-CoV-2 in pediatric liver transplant recipients: The European experience. J Pediatr Gastroenterol Nutr. 2022;74:e41–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yuksel M, Akturk H, Mizikoglu O, et al. A single-center report of COVID-19 disease course and management in liver transplanted pediatric patients. Pediatr Transplant. 2021;25:e14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].World Health Organization. Clinical management of COVID-19: living guideline, 15 September 2022: World Health Organization; 2022. Available at: https://who.int/iris/handle/10665/362783. [PubMed]
- [12].World Health Organization. Clinical management of COVID-19: interim guidance, 27 May 2020: World Health Organization; 2020. Available at: https://apps.who.int/iris/bitstream/handle/10665/332196/WHO-2019-nCoV-clinical-2020.5-eng.pdf?sequence=1&isAllowed=y.
- [13].Stephenson T, Allin B, Nugawela MD, et al. Long COVID (post-COVID-19 condition) in children: a modified Delphi process. Arch Dis Child. 2022;107:674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhu X-y, Lu Y-f, Xue F, et al. SARS-CoV-2 BA.2 (Omicron) variant infection in pediatric liver transplanted recipients and cohabitants during 2022 Shanghai outbreak: a prospective cohort. Virol J. 2023;20:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Suntronwong N, Vichaiwattana P, Klinfueng S, et al. SARS-CoV-2 infection- induced seroprevalence among children and associated risk factors during the pre- and omicron-dominant wave, from January 2021 through December 2022, Thailand: a longitudinal study. PLoS One. 2023;18:e0279147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kwak BO, Eun BW. COVID-19 in immunocompromised children and adolescents. Clin Exp Pediatr. 2023;66:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Minotti C, Tirelli F, Barbieri E, et al. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J Infect. 2020;81:e61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Feldman AG, Danziger-Isakov LA. The impact of COVID-19 on the pediatric solid organ transplant population. Semin Pediatr Surg. 2022;31:151178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sabu JM, Zahid I, Jacob N, et al. Effectiveness of the BNT162b2 (Pfizer-BioNTech) vaccine in children and adolescents: a systematic review and meta-analysis. Vaccines. 2022;10:1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sintusek P, Buranapraditkun S, Khunsri S, et al. Safety and humoral and cellular immunogenicity of the BNT162b2 SARS-CoV-2 vaccine in liver-transplanted adolescents compared to healthy adolescents. Vaccines. 2022;10:1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tubjaroen C, Prachuapthunyachart S, Potjalongsilp N, et al. Immunogenicity of an mRNA-Based COVID-19 vaccine among adolescents with obesity or liver transplants. Vaccines. 2022;10:1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ministry of Public Health. Thai Guidelines for COVID-19; 2022. [Cited 2023 June 14]. Available at: https://covid19.dms.go.th/backend///Content//Content_File/Covid_Health/Attach/25650422162203PM_CPG_COVID-19_n_v.22_20220422.pdf.
- [23].Udwadia ZF, Singh P, Barkate H, et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021;103:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Golan Y, Campos JAS, Woolson R, et al. Favipiravir in patients with early mild-to-moderate coronavirus disease 2019 (COVID-19): a randomized controlled trial. Clin Infect Dis. 2023;76:e10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu H, He X, Wang Y, et al. Management of COVID-19 in patients after liver transplantation: Beijing working party for liver transplantation. Hepatol Int. 2020;14:432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sin P, Díaz LA, Martínez M, et al. Acute liver injury among pediatric liver transplantation recipients with coronavirus disease 2019: an international collaborative study. J Pediatr Gastroenterol Nutr. 2021;73:391–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jarupan M, Jantarabenjakul W, Jaruampornpan P, et al. Long COVID and hybrid immunity among children and adolescents post-delta variant infection in Thailand. Vaccines. 2023;11:884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lopez-Leon S, Wegman-Ostrosky T, Ayuzo del Valle NC, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. 2022;12:9950. [DOI] [PMC free article] [PubMed] [Google Scholar]