Abstract
Background
Despite the fact that obesity and overweight are serious major health problems worldwide, fighting against them is also considered a challenging issue. Several interventional studies have evaluated the potential weight-reduction effect of nicotinamide adenine dinucleotide (NAD+) precursor. In order to obtain a better viewpoint from them, this study aimed to comprehensively investigate the effects of NAD+ precursor supplementation on weight loss, adiponectin, and leptin.
Methods
Scopus, PubMed/Medline, Web of Science, Cochrane, and Embase databases were searched using standard keywords to identify all controlled trials investigating the weight loss and related hormones effects of NAD+ precursor. Pooled weighted mean difference and 95% confidence intervals were achieved by random-effects model analysis for the best estimation of outcomes.
Results
Twenty two treatment arms with 5,144 participants’ were included in this systematic review and meta-regression analysis. The pooled findings showed that NAD+ precursor supplementation has an effect on lowering BMI (weighted mean difference (WMD): −0.19 kg/m2, 95% confidence interval (CI): −0.29 to −0.09, p < 0.001) and increasing adiponectin (WMD: 1.59 μg/mL, 95% CI: 0.49 to 2.68, p = 0.004) in humans compared with control groups. However, no significant effect was observed on body weight and leptin. There was a significant relationship between doses of intervention with changes in BMI. In addition, subgroup analysis showed that BMI reduction was greater when receiving nicotinic acid (NA) supplementation than nicotinamide (NE) supplementation.
Conclusion
NAD+ precursor had significant effects on weight management with the reduction of BMI and increasing adiponectin.
Keywords: NAD+ precursor, niacin, obesity, obesity hormone, weight loss
Introduction
Overweight and obesity are defined as abnormal or excessive fat accumulation that presents a risk to health. A body mass index (BMI) over 25 is considered overweight, and over 30 is obese. The issue has grown to epidemic proportions, with over 4 million people dying each year as a result of being overweight or obese in 2017 according to the global burden of disease (1). Adiposity, particularly abdominal adiposity, is associated with an increased risk of most chronic diseases such as type 2 diabetes (T2DM), cancer, cardiovascular disease (CVD), atherosclerosis, and metabolic syndrome (2). Moreover, low-grade chronic inflammation linked to adiposity, along with adipocyte development and a change in the pattern of activity of adipokines especially, leptin and adiponectin, leads to adipose tissue (AT) dysfunction (3). Following these changes, serious disorders are generally observed, such as a decrease in mitochondrial biogenesis and an increase in oxidative stress and inflammation (4–6). Therefore, one might hypothesise that elements such as nicotinamide adenine dinucleotide (NAD) + and their related components, which regulate mitochondrial function, metabolism, cellular stress response, carbohydrates and fatty acids synthesis, ATP generation, and ketogenesis, have been significantly involved in the adjustment of metabolic complications associated with obesity (7–9). NAD+ is synthesised in two major pathways in the human body: de novo synthesis and salvage, the latter of which has a more effective role in maintaining levels of NAD+ in the body. The compounds that are created from the salvage pathway are called NAD+ precursors (10). Furthermore, the presence of Sirtuins, NAD + -dependent protein deacetylases (SIRT1–7), is crucial for NAD + 's biological function in humans (11). Studies have shown that NAD+ precursors and Sirtuins gene expression were significantly down-regulated in obese subjects (12–14), whereas their expression increased progressively in subjects with a weight loss programme (15). However, the effects of NAD+ precursor supplementation on weight and other related factors, as well as adipokines, are still not completely clear. Therefore, the objective of this systematic review and meta-analysis of randomized controlled trials is to analyze and evaluate the effects of NAD+ precursor supplementation on weight loss and obesity hormone.
Methods
Search strategy
The Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) criteria were followed for conducting this study (16). Without regard to language or time restrictions, a thorough search was carried out in the PubMed/MEDLINE, Web of Science, SCOPUS, and Embase databases from the beginning to February 2023. Additionally, similar papers and gray literature were considered in the search. Medical subject headings (MeSH) and Emtree (Embase subject headings) were selected to search the online databases, as follow: (“NAD” OR “NAD precursor” OR “Nicotinic Acids” OR “Niacin” OR “Niacinamide” OR “Nicotinamide Mononucleotide”) AND (“weight” OR “Waist Circumference” OR “Body Mass Index” OR “Adiponectin” OR “Leptin”) AND (“Clinical Trials as Topic” OR “Cross-Over Studies” OR “Double-Blind Method” OR “Single-Blind Method” OR “Random Allocation” OR “Clinical Trial”) (The specific search strategy is described in the Supplementary Appendix S1). The reference lists of the publications retrieved and linked review studies were manually searched to identify potentially overlooked qualifying trials.
Eligibility criteria
Using titles, abstracts, or the complete texts of the research, two writers separately removed duplicate articles before finding and reviewing relevant publications. In the end, the papers were separated based on the following standards: (1) Randomized clinical trials studies; (2) NAD+ precursor supplementation (nicotinic acid (NA) or nicotinamide (NE) supplementation) was given as an intervention in individual’s aged 18 and over; and 3) baseline and post measurements in both group (intervention and control) weight, BMI, adiponectin, and leptin were recorded. The most recent or longest follow-up period was used when a research revealed results at more than one follow-up time. Studies with duplicated data, studies with ambiguous information, studies in which NAD+ precursor was used as an intervention alongside other commonly prescribed medications, non-randomized trial designs, animal studies, studies without a control group, reviews, and meta-analysis studies were also excluded. The PICOS criteria for inclusion and exclusion of studies were as follows. Population: individual’s aged 18 and over; Intervention: NAD+ precursor supplementation (nicotinic acid (NA) or nicotinamide (NE) supplementation); Comparator: other intervention or placebo; Outcomes: weight, BMI, adiponectin, and leptin; Study design: randomized clinical trials studies.
Data extraction
The qualifying studies were examined by two authors independently. The first author’s name, the study’s location, the year it was published, the sample size (for the intervention and control groups), the participant characteristics (such as the percentage of men, the participant’s BMI, age, and health status), the type of outcomes, duration of the intervention, the dosage and type of the intervention, and the means and standard deviations (S.D.s) of the intended outcomes at baseline, post-intervention, and/or changes between baseline and post-intervention, were all extracted.
Quality assessment
The details of the evaluation of the study’s quality are presented in Table 1. Using the Cochrane risk-of-bias test for randomized trials (RoB 2), version 2, the quality of the included RCTs was methodologically evaluated (17). Based on the following potential sources of bias: blinding of outcome assessment, allocation concealment, participant and staff blinding, random sequence generation, incomplete outcome data, selective reporting, and other bias, two authors independently rated each study as having a low, high, or unclear risk of bias. A “High risk” rating indicates significant bias that may invalidate the results. These studies have serious errors in design, analysis, or reporting; have large amounts of missing information; or have discrepancies in reporting. Studies are assessed as at unclear risk of bias when too few details are available to make a judgement of ‘high’ or ‘low’ risk; when the risk of bias is genuinely unknown despite sufficient information about the conduct; or when an entry is not relevant to a study (for example because the study did not address any of the outcomes in the group of outcomes to which the entry applies). A “Low risk” study has the least bias, and results are considered valid. Any discrepancies were discussed with a third author in order to come to a consensus. The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) grading method was also used to evaluate the quality of the current analytic research (18). A reliable 10-point assessment system that assesses elements affecting study quality is the GRADE checklist. This scale has seven components: (1) risk of bias, (2) precision, (3) heterogeneity, (4) directness, (5) publishing bias, (6) funding bias, and (7) study design.
Table 1.
Risk of bias assessment according to the Cochrane collaboration’s risk of bias assessment tool.
| Study, Year (reference) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Overall assessment of risk of bias |
|---|---|---|---|---|---|---|---|
| El-Kady et al. (2022) | Low | Low | Low | Low | Unclear | Low | Low |
| Liao et al. (2021) | Low | Unclear | Low | Low | Unclear | Low | Unclear |
| Canner et al. (2003) | Low | Low | Low | High | Unclear | Low | Unclear |
| Linke et al. (2008) | Low | Low | Low | Low | Unclear | Low | Low |
| Fabbrini et al. (2010) | Low | Unclear | Low | Low | Unclear | Low | Unclear |
| Aye et al. (2014) | Low | Low | Low | Low | Unclear | Low | Low |
| Vittone et al. (2007) | Low | High | Low | Low | Unclear | Low | Low |
| Vaccari et al. (2007) | Low | Low | High | Low | Unclear | Low | Unclear |
| Superko et al. (2004) | Low | Low | Unclear | Low | Unclear | Low | Low |
| Savinova et al. (2015) | Low | Unclear | Low | Low | Unclear | Low | Unclear |
| Bays et al. (2010) | Low | Low | Low | Low | Unclear | Low | Low |
| Okabe et al. (2022) | Low | Unclear | Unclear | Low | Unclear | Low | Low |
| Ko et al. (1998) | Low | High | High | Low | High | Low | High |
| Kei et al. (2011) | Low | Low | High | Unclear | Unclear | Low | Unclear |
| Chauhan et al. (2011) | Low | Low | Unclear | Unclear | Unclear | Low | Unclear |
| Osar et al. (2004) | Low | Low | High | Low | Unclear | Low | Unclear |
| Westphal et al. (2007) | Low | Low | Unclear | Low | Unclear | Low | Low |
| Lee et al. (2009) | Low | Low | Low | Low | Unclear | Low | Low |
Data synthesis and statistical analysis
The data were examined using STATA version 12.0 software. Different data types were converted using a predetermined procedure to the mean and standard deviations (S.D.s) (19, 20). For instance, in the absence of standard deviations, we calculated the change using the method below: The definition of standard deviation changes is square root [(S.D. baseline 2 + SD final 2) - (2R S.D. baseline 2 S.D. final)]. The following formula is used to convert the standard error of the mean (SEM) to standard deviation: S.D. is equal to SEM × √n, where n is the total number of participants in each group. The random-effects model was employed in the meta-analysis of research results. The weighting of the research followed the typical inverse variance technique. The data from the longest time point were used for the analysis, which allowed for the handling of many assessments within a single study group. Using Q Statistics and I-squared (I2), the degree of study heterogeneity was evaluated. Insignificant, low, moderate, and high heterogeneity were found with I2 values ranging from 0% to 25, 26 to 50%, 5 to 75%, and 76 to 100%, respectively (21). To identify possible causes of heterogeneity, a pre-defined subgroup analysis based on the dosage, duration, and type of the intervention was conducted. A sensitivity analysis was done to determine the contribution of each research to the overall mean difference. In order to establish if there was publication bias, we utilized the official Egger’s test (22).
Results
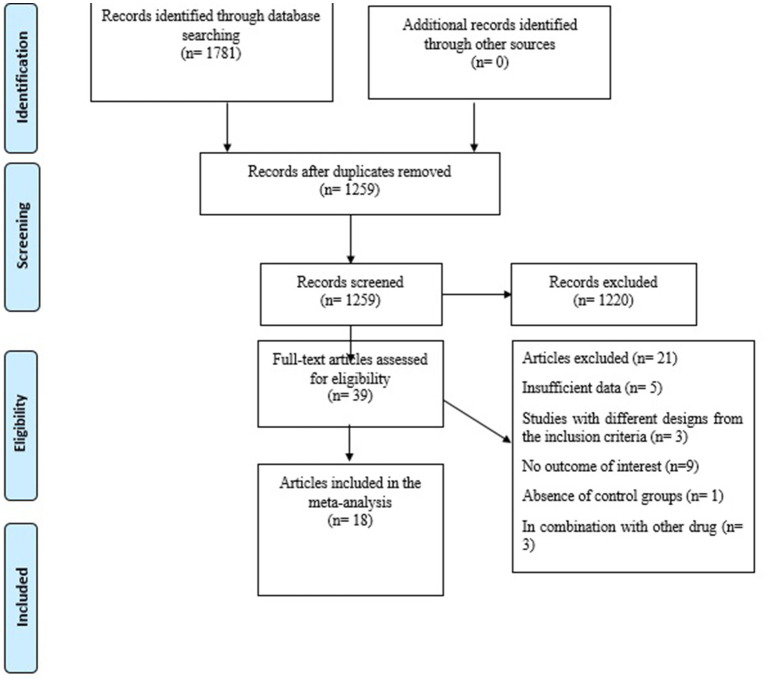
Figure 1 depicts a flowchart of the research selection process with exclusion criteria. This value indicates that the aforementioned electronic databases generated 1781 articles. After removing publications with duplicate research, there were 1,259 total. Following an assessment of the research’s titles and abstracts, 1,220 papers were dropped since they did not meet the inclusion requirements. 39 articles were found utilizing the full-text search during the secondary screening. For the reasons listed above, 21 of the investigations were dropped. Finally, 18 papers with 22 treatments arm were included in the quantitative meta-analysis since they matched the qualifying requirements.
Figure 1.
Flow chart of the study, including identification, screening, eligibility, and the final sample included.
Study characteristics
The features of the pooled articles are shown in Table 2. Our surveys reveal that seven studies have been carried out in USA, 2 articles in the Germany and UK, respectively, and other studies were conducted in Japan, China, India, Egypt, Turkey, Hong Kong, and Greece. All articles were published between 1998 and 2022 and follow up intervention ranged from 4 to 144 weeks. The mean age and percentage of male participants ranged from 31 to 65 years and 0–100%, respectively, at the baseline. The doses prescribed in the studies were between 250 and 3,000 mg per day, and in 4 studies the supplement type was in the form of NE and the rest were in the form of NA. Five studies conducted on patients with metabolic syndrome, five articles on people with a history of cardiovascular disease or dyslipidemia, two studies on each of type 2 diabetes (T2DM) and non-alcoholic fatty liver (NAFLD) as well as healthy samples, and two studies on patients with impaired glucose tolerance and polycystic ovary syndrome.
Table 2.
Characteristics of eligible studies.
| Author (year) | Country | Population | Mean age year | Sex (Male %) | Sample size study (intervention/control) | Follow up of intervention (Weeks) | Type and dose (mg/day) of intervention | Baseline of BMI (kg/m2) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| El-Kady et al. (2022) | Egypt | NAFLD | 45.6 | 41.9 | 31/30 | 12 | 1,000 mg NE | 32.69 | BMI, weight, Adiponectin |
| Liao et al. (2021) | China | Healthy Subjects | 37 | 83.3 | 12/12 | 6 | 300, 600, 1,200 mg NE | 22 | BMI, weight |
| Canner et al. (2003) | USA | Metabolic Syndrome and Healed Myocardial Infarction | NR | NR | 964/2468 | 48 | 2000 mg NA | NR | BMI |
| Linke et al. (2008) | Germany | Patients with impaired glucose tolerance | 45.5 | 70 | 30/30 | 24 | 1,000 mg NA | 37.9 | BMI, weight, Adiponectin, Leptin |
| Fabbrini et al. (2010) | USA | NAFLD | 43 | 30 | 9/9 | 16 | 2000 mg NA | 35.8 | BMI, weight |
| Aye et al. (2014) | UK | Polycystic ovary syndrome | 31 | 0 | 13/12 | 12 | 1,000 mg NA | 35.8 | BMI |
| Vittone et al. (2007) | USA | Patients with metabolic syndrome | 54 | 86.2 | 80/80 | 144 | 2000 mg NA | 29.7 | BMI |
| Vaccari et al. (2007) | USA | Patients with metabolic syndrome | 32 | 56 | 30/15 | 52 | 1,000 mg NA | 29.7 | BMI, Adiponectin |
| Superko et al. (2004) | USA | Hypercholesterolemic subjects | 53 | 71.6 | 60, 59/61 | 14 | 1,500, 3,000 mg NA | 28 | BMI |
| Savinova et al. (2015) | USA | Patients with metabolic syndrome | 47 | 57 | 14/14 | 16 | 2000 mg NA | 32.7 | BMI |
| Bays et al. (2010) | USA | Dyslipidemic patients without metabolic syndrome | 57.7 | 62.4 | 221, 320/110, 160 | 24 | 2000 mg NA | 31.5 | BMI |
| Okabe et al. (2022) | Japan | Healthy Subjects | 42.9 | 26.6 | 15/15 | 16 | 250 mg NE | 21.3 | BMI, weight |
| Ko et al. (1998) | Hong Kong | T2DM | 59.2 | 36.3 | 32/30 | 12 | 750 mg NA | 25.7 | BMI |
| Kei et al. (2011) | Greece | Dyslipidemic patients | 58 | 46.6 | 30/30 | 12 | 2000 mg NA | 29.1 | weight |
| Chauhan et al. (2011) | India | Hyperlipidemic patients | NR | NR | 17/20 | 12 | 2,250 mg NA | NR | weight |
| Osar et al. (2004) | Turkey | Patients with Poorly Controlled Type 2 Diabetes Mellitus | 58 | 46.6 | 15/15 | 4 | 3,000 mg NE | 30 | weight |
| Westphal et al. (2007) | Germany | Patients with metabolic syndrome | 55 | 100 | 20/10 | 6 | 1,500 mg NA | 32.4 | Adiponectin, Leptin |
| Lee et al. (2009) | UK | Patients with coronary artery disease | 65 | 94 | 22/29 | 48 | 2000 mg NA | 31 | Adiponectin |
BMI, body mass index; NAFLD, Nonalcoholic fatty liver disease; TD2M, Type 2 diabetes; NR, not report; NA, Nicotinic acid; NE, Nicotinamide.
The findings of the evaluation of the eligible studies’ quality are shown in Table 1. Additionally, a score of 8.8 (very good quality) was determined after the GRADE score system was used to assess the quality of the current meta-analysis.
Meta-analysis results
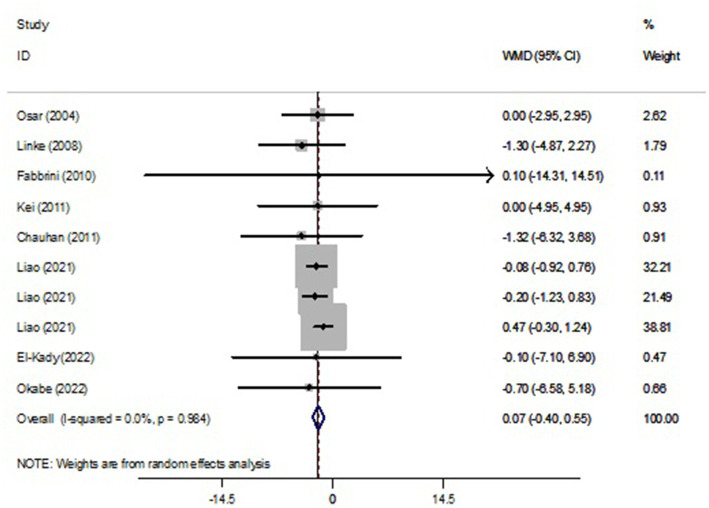
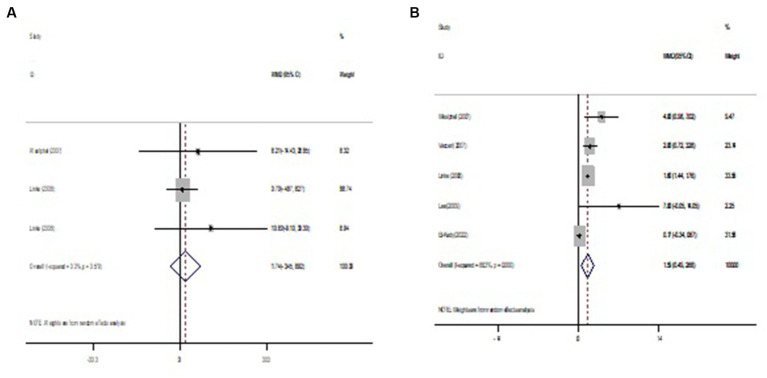
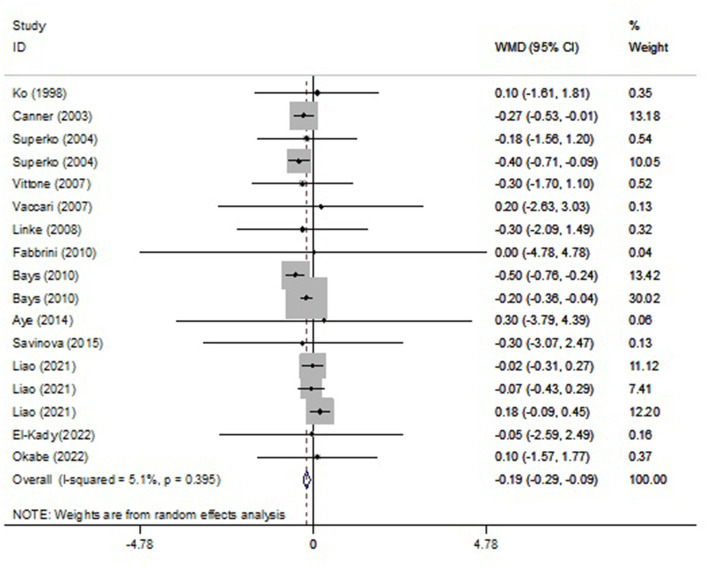
Pooled findings from the random-effects model indicated that body mass index (BMI) (weighted mean difference (WMD): −0.19 kg/m2, 95% confidence interval (CI): −0.29 to −0.09, p < 0.001) were significantly reduced after NAD+ precursor supplementation compared to control group. However, no significant effect was observed on body weight (WMD: 0.07 kg, 95% CI: −0.40 to 0.55, p = 0.766), leptin (WMD: 1.74 ng/mL, 95% CI: −3.45 to 6.92, p = 0.512) compared to the control group. Also, Pooled findings indicated that compared to the control group, adiponectin (WMD: 1.59 μg/mL, 95% CI: 0.49 to 2.68, p = 0.004) was significantly increased after with NAD+ precursor supplementation. Furthermore, significant heterogeneity was found among the studies for adiponectin (Cochran Q test, p < 0.001, I2 = 88.3%). However, low heterogeneity was reported for weight (Cochran Q test, p = 0.984, I2 = 0.0%), BMI (Cochran Q test, p = 0.395, I2 = 5.1%), and leptin (Cochran Q test, p = 0.579, I2 = 0.0%) (Figures 2–4).
Figure 2.
Forest plot of randomized controlled trails investigating the effects of NAD+ precursor supplementation on weight (kg).
Figure 4.
Forest plot of randomized controlled trails investigating the effects of NAD+ precursor supplementation on (A) leptin (ng/ml) and (B) adiponectin (μg/mL).
Figure 3.
Forest plot of randomized controlled trails investigating the effects of NAD+ precursor supplementation on body mass index (BMI) (kg/m2).
Subgroups analysis
The findings of the subgroup also show the greater effect of NAD+ precursor supplementation on BMI decrease in a dose ≥2 g and a duration of intervention >12 weeks. In addition, subgroup analysis showed that BMI reduction was greater when receiving nicotinic acid (NA) supplementation than nicotinamide (NE) supplementation (Supplementary Table).
Meta-regression
Meta-regression between NAD+ precursor and absolute mean differences in body weight and BMI based on dosage and duration of intervention was performed. Only, there was a significant relationship between dose of intervention with changes in BMI (coefficient (Coef) = −0. 0001847, p = 0.033). However, meta-regression analysis did not show a significant linear relationship between dose and duration of intervention with weight changes (Supplementary Figures S1, S2).
Sensitivity analysis
To discover the effect of each article on the pooled effect size for the levels of weight, BMI, adiponectin, and leptin, we step-by-step discarded each trial from the analysis. The leave-one-out sensitivity analysis indicated the robustness of the results (Supplementary Figure S3).
Publication bias
Evaluating the publication bias by visual inspection of the funnel, no evidence for publication bias based on the Egger’s tests was detected for weight (p = 0.531), BMI (p = 0.621), adiponectin (p = 1.00), and leptin (p = 0.602; Supplementary Figure S4).
Discussion
The results of our systematic review and meta-analysis showed that nicotinamide adenine dinucleotide (NAD+) precursor supplementation has an effect on lowering BMI and increasing adiponectin in humans compared with control groups. Furthermore, there was a significant relationship between doses of intervention with changes in BMI. The findings of the subgroup also show the greater effect of NAD+ precursor supplementation on BMI decrease in a dose ≥2 g and a duration of intervention >12 weeks.
Currently, there is no meta-analysis on the effect of NAD+ precursors on obesity in the human body. Earlier meta-analysis investigated and described the effect of NAD+ precursor supplementation on improving TG, TC, LDL, and HDL levels in humans, but resulted in hyperglycemia, compared with placebo or no treatment (23, 24). Animal studies evaluating obese mice have shown an association between NAD+ supplementation and improved indices of obesity as well as molecular regulation of adipocytes (25, 26).
Nicotinamide adenine dinucleotide (NAD+) is an important molecule in energy and signal transduction, besides acting as substrate for enzymes such as sirtuins, poly-ADP ribose polymerases (PARPs), and cyclic ADP ribose synthetases that regulate key cellular processes of energy metabolism, DNA damage repair, and calcium signaling (27). Improving NAD+ availability via NAD+ precursor supplementation has emerged as a potential strategy to augment tissue-specific NAD+ homeostasis and improve physiological function (28). Thus, NAD+ supplementation therapy is a new treatment for obesity in recent years because acts via activation of the NAD + -dependent sirtuin enzyme family, thereby regulating oxidative metabolism (29, 30).
NAD+ precursors such as nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) is a recently discovered vitamin B3, are available as over-the-counter dietary supplements, and oral supplementation with these precursors reduction in fat mass and an increase in lean mass; improved glucose tolerance and alleviated adipose tissue inflammation in pre-clinical models with obesity (26). Remie et al. (31) has demonstrated that NAD+ supplementation therapy with 1,000 mg/d for 6 weeks in healthy overweight or obese men and women increased skeletal muscle NAD+ metabolites, affected skeletal muscle acetylcarnitine metabolism, and induced minor changes in body composition.
Consistent with the effect of NAD+ precursor supplementation on body composition observed by Remie et al. (31), we have shown lowering BMI at 0.19 kg/m2 and BMI decrease was higher in a dose ≥2 g and a duration of intervention >12 weeks. In another study in 2018, which was conducted to investigate dietary nicotinamide riboside (NR) supplementation in a 12-week period on the improvement of insulin sensitivity and other metabolic parameters in obese and insulin-resistant men, the results showed that NR in doses of 2 g/day had no effect on resting energy expenditure, lipolysis, lipid oxidation, or body composition (32). In a study, with the aim of determining the effect of long-term NR supplementation on increasing mitochondrial biogenesis and metabolic health on twenty monozygotic twins discordant with BMI with an increasing dose of NR (250 to 1,000 mg per day) for 5 months, NR did not improve obesity or metabolic health (33). Udin et al. (34) evaluated the effects of NAD+ supplementation in rats fed a high-fat diet. The results showed that NAD+ supplementation reduced the BMI of the rats, suggesting that NAD+ supplementation may be an effective strategy for weight loss and improving metabolic health. In another animal study on diet-induced obese rats, NA decreased visceral adipose tissue and improved adiponectin levels and lipid profile (35). However, a study on developing rats did not show any useful results regarding the effect of NE on factors related to obesity (36).
However, despite the promising result for BMI lowering in the face of supplementation, no significant effect was observed on body weight. Despite previous evidence from preclinical studies on promoting resistance to weight gain no significant effect on body weight in human studies was observed in our meta-analysis. We believe that the discrepancy between the results observed for the BMI and body weight outcomes is related to the smaller number and consequent smaller effect size of studies selected for the body weight outcome in relation to the BMI outcome (37). Moreover, we observed that for the outcome of body weight, the majority of weight of the studies obtained from the effect size was concentrated in the evidence demonstrated by Liao et al. (38), which was responsible for 92.5% of the overall effect (% weight), this can overestimate the total effect.
To investigate the role of the effect of NAD+ precursor supplementation on obesity, we evaluated leptin and adiponectin. Adipose tissue is a metabolic and endocrine organ that secretes a number of adipokines that contribute to the etiology of obesity-related metabolic complications (39, 40). Leptin is a hormone produced by adipose tissue that plays an important role in regulating appetite and energy metabolism. Elevated leptin levels are associated with suppressed appetite and increased energy expenditure, while reduced leptin levels are associated with increased appetite and decreased energy expenditure (41). Adiponectin, on the other hand, is a hormone secreted by fat cells that has been linked to the regulation of metabolism and insulin sensitivity (42). Dysfunction of leptin signaling and reduced adiponectin levels may contribute to the development of obesity. In our review we observed no effect on leptin levels. In contrast, increased levels were observed in patients who used supplementation. Elevated levels of adiponectins in humans may correlate with inflammation or the body’s reaction to exacerbation on contact with new substances, in the case of NAD+ supplementation, so that in the supplemented group there was an increase in its levels, as an attempt to combat the effects of pro-inflammatory cytokines, there is an increase in adiponectins (43). In addition, the fact that a low BMI is associated with an increase in adiponectin can be explained, identifying that the secretion of this adipokine is related to the quality, not the quantity, of adipose tissue, and in addition to BMI, in situations of greater age this also tends to occur (42).
Another interesting finding regarding the effects of NAD+ precursors on BMI was their greater magnitude with the use of NA when compared to NE. However, it is important to highlight that only five RCTs evaluating the effect of NE supplementation on BMI were included in this meta-analysis. In fact, when compared to NA, NE has been less studied regarding its effect on BMI. Thus, more studies evaluating the effect of NE supplementation would be important to better investigate its therapeutic potential in obesity outcomes, allowing a better comparison in relation to other NAD+ precursors. In addition, unlike NE, NA reduces the levels of lipid profiles, including cholesterol and triglycerides, which can explain its greater effect on BMI and be effective on factors related to obesity.
Our study had some limitations that jeopardized the extraction of robust conclusions. Clinically and statistically significant heterogeneities was found for adiponectin. These may be explained by the differences in the intervention-specific factors (e.g., type, dose, administration route, and duration of drugs) and weight-specific factors (e.g., age, sex, physiology, genetics, familial history, race/ethnicity, physical activity, socioeconomic status, dietary intakes, and drug, tobacco, or alcohol consumption) (44). Nonetheless, we attempted to identify some possible sources of heterogeneity in data by performing a subgroup analysis. In addition, lack of registration of the current study in PROSPERO due to time limit was another limitation of this study.
Despite its limitations, the current study has several positive features: a rigorous methodology was used based on the PRISMA guidelines; a comprehensive literature search included different independent databases; search, selection, and data extraction applied to the selected studies were performed separately, and in duplicate, by two researchers; a third party was accessed to solve disagreements. Furthermore, the present study likely included the largest effect size for each outcome assessed at obesity.
Conclusion
The finding of this paper showed that NAD+ precursor supplementation has an effect on lowering BMI and increasing adiponectin in humans compared with control groups. However, no significant effect was observed on body weight and leptin. Given the evidence of decreased BMI in humans and increased adiponectin, it is important to emphasize that NAD+ supplementation should not be seen as a one-time solution for weight loss and should be combined with healthy eating habits and exercise under professional guidance from a dietitian.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the research council and ethics committee Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Author contributions
MS and YB contributed in conception, design, and statistical analysis. MS, YB, ST, MG, and NS contributed in data collection and manuscript drafting. MS supervised the study. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1208734/full#supplementary-material
References
- 1.World Health Organization . Obesity and overweight (2016). Available at: http://wwwwhoint/mediacentre/factsheets/fs311/en
- 2.Williams EP, Mesidor M, Winters K, Dubbert PM, Wyatt SB. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep. (2015) 4:363–70. doi: 10.1007/s13679-015-0169-4 [DOI] [PubMed] [Google Scholar]
- 3.Paniagua JA. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J Diabetes. (2016) 7:483. doi: 10.4239/wjd.v7.i19.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naukkarinen J, Heinonen S, Hakkarainen A, Lundbom J, Vuolteenaho K, Saarinen L, et al. Characterising metabolically healthy obesity in weight-discordant monozygotic twins. Diabetologia. (2014) 57:167–76. doi: 10.1007/s00125-013-3066-y, PMID: [DOI] [PubMed] [Google Scholar]
- 5.Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K, et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes. (2015) 64:3135–45. doi: 10.2337/db14-1937 [DOI] [PubMed] [Google Scholar]
- 6.Huang C-J, McAllister MJ, Slusher AL, Webb HE, Mock JT, Acevedo EO. Obesity-related oxidative stress: the impact of physical activity and diet manipulation. Sports Med.Open. (2015) 1:1–12. doi: 10.1186/s40798-015-0031-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. (2012) 13:225–38. doi: 10.1038/nrm3293, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conze D, Brenner C, Kruger CL. Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci Rep. (2019) 9:1–13. doi: 10.1038/s41598-019-46120-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. (2008) 28:115–30. doi: 10.1146/annurev.nutr.28.061807.155443, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Rajman L, Chwalek K, Sinclair DA. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab. (2018) 27:529–47. doi: 10.1016/j.cmet.2018.02.011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. (2007) 32:12–9. doi: 10.1016/j.tibs.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 12.Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. (2012) 16:180–8. doi: 10.1016/j.cmet.2012.07.003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei X, Jia R, Wang G, Hong S, Song L, Sun B, et al. Depot-specific regulation of NAD+/SIRTs metabolism identified in adipose tissue of mice in response to high-fat diet feeding or calorie restriction. J Nutr Biochem. (2020) 80:108377. doi: 10.1016/j.jnutbio.2020.108377 [DOI] [PubMed] [Google Scholar]
- 14.Jukarainen S, Heinonen S, Rämö JT, Rinnankoski-Tuikka R, Rappou E, Tummers M, et al. Obesity is associated with low NAD+/SIRT pathway expression in adipose tissue of BMI-discordant monozygotic twins. J Clin Endocrinol. (2016) 101:275–83. doi: 10.1210/jc.2015-3095, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Rappou E, Jukarainen S, Rinnankoski-Tuikka R, Kaye S, Heinonen S, Hakkarainen A, et al. Weight loss is associated with increased NAD+/SIRT1 expression but reduced PARP activity in white adipose tissue. J Clin Endocrinol Metabol. (2016) 101:1263–73. doi: 10.1210/jc.2015-3054 [DOI] [PubMed] [Google Scholar]
- 16.Parums DV. Review articles, systematic reviews, meta-analysis, and the updated preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines. Med Sci Mon. (2021) 27:e934475–1. doi: 10.12659/MSM.934475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. Cochrane Handbook Syst Rev Interv. (2019):205–28. doi: 10.1002/9781119536604.ch8 [DOI] [Google Scholar]
- 18.Schwingshackl L, Knüppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, et al. Perspective: NutriGrade: a scoring system to assess and judge the Meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr. (2016) 7:994–1004. doi: 10.3945/an.116.013052, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 The Cochrane Collaboration. Available at: www.cochrane-handbook.org (2011). [Google Scholar]
- 20.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong O, Wang J, Tan Y, Lei X, Tang Z. Effects of NAD+ precursor supplementation on glucose and lipid metabolism in humans: a meta-analysis. Nutr Metab. (2022) 19:20. doi: 10.1186/s12986-022-00653-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahebkar A, Reiner Ž, Simental-Mendia LE, Ferretti G, Cicero AF. Effect of extended-release niacin on plasma lipoprotein (a) levels: a systematic review and meta-analysis of randomized placebo-controlled trials. Metabolism. (2016) 65:1664–78. doi: 10.1016/j.metabol.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 25.Roh E, Myoung Kang G, Young Gil S, Hee Lee C, Kim S, Hong D, et al. Effects of chronic NAD supplementation on energy metabolism and diurnal rhythm in obese mice. Obesity. (2018) 26:1448–56. doi: 10.1002/oby.22263, PMID: [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Zhu W, Wang M, Xi P, Wang H, Tian D. Nicotinamide mononucleotide alters body composition and ameliorates metabolic disorders induced by a high-fat diet. IUBMB Life. (2023) 75:548–62. doi: 10.1002/iub.2707, PMID: [DOI] [PubMed] [Google Scholar]
- 27.Yoshino J, Baur JA, Imai S-i. NAD+ intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. (2018) 27:513–28. doi: 10.1016/j.cmet.2017.11.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elhassan YS, Philp AA, Lavery GG. Targeting NAD+ in metabolic disease: new insights into an old molecule. J Endocr Soc. (2017) 1:816–35. doi: 10.1210/js.2017-00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soma M, Lalam SK. The role of nicotinamide mononucleotide (NMN) in anti-aging, longevity, and its potential for treating chronic conditions. Mol Biol Rep. (2022) 49:9737–48. doi: 10.1007/s11033-022-07459-1 [DOI] [PubMed] [Google Scholar]
- 30.Houtkooper RH, Auwerx J. Exploring the therapeutic space around NAD+. The Rockefeller University Press; (2012), 199, 205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remie CM, Roumans KH, Moonen MP, Connell NJ, Havekes B, Mevenkamp J, et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am J Clin Nutr. (2020) 112:413–26. doi: 10.1093/ajcn/nqaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. (2018) 108:343–53. doi: 10.1093/ajcn/nqy132, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Lapatto HA, Kuusela M, Heikkinen A, Muniandy M, van der Kolk BW, Gopalakrishnan S, et al. Nicotinamide riboside improves muscle mitochondrial biogenesis, satellite cell differentiation, and gut microbiota in a twin study. Sci Adv. (2023) 9:eadd5163. doi: 10.1126/sciadv.add5163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uddin GM, Youngson NA, Chowdhury SS, Hagan C, Sinclair DA, Morris MJ. Administration of nicotinamide mononucleotide (NMN) reduces metabolic impairment in male mouse offspring from obese mothers. Cells. (2020) 9:791. doi: 10.3390/cells9040791, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morakinyo AO, Samuel TA, Adekunbi DA, Adegoke OA. Niacin improves adiponectin secretion, glucose tolerance and insulin sensitivity in diet-induced obese rats. Egypt J Basic Appl Sci. (2015) 2:261–7. doi: 10.1016/j.ejbas.2015.08.003 [DOI] [Google Scholar]
- 36.Li D, Tian Y-J, Guo J, Sun W-P, Lun Y-Z, Guo M, et al. Nicotinamide supplementation induces detrimental metabolic and epigenetic changes in developing rats. Br J Nutr. (2013) 110:2156–64. doi: 10.1017/S0007114513001815 [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care. (2013) 17:R2–9. doi: 10.1186/cc11919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao B, Zhao Y, Wang D, Zhang X, Hao X, Hu M. Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: a randomized, double-blind study. J Int Soc Sports Nutr. (2021) 18:54. doi: 10.1186/s12970-021-00442-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Investig. (2018) 48:e12997. doi: 10.1111/eci.12997 [DOI] [PubMed] [Google Scholar]
- 40.Westphal S, Borucki K, Taneva E, Makarova R, Luley C. Extended-release niacin raises adiponectin and leptin. Atherosclerosis. (2007) 193:361–5. doi: 10.1016/j.atherosclerosis.2006.06.028, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Landecho M, Tuero C, Valentí V, Bilbao I, de la Higuera M, Frühbeck G. Relevance of leptin and other adipokines in obesity-associated cardiovascular risk. Nutrients. (2019) 11:2664. PUBMED. doi: 10.3390/nu11112664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao S, Kusminski CM, Scherer PE. Adiponectin, leptin and cardiovascular disorders. Circ Res. (2021) 128:136–49. doi: 10.1161/CIRCRESAHA.120.314458, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kırdar S, Serter M, Ceylan E, Şener AG, Kavak T, Karadağ F. Adiponectin as a biomarker of systemic inflammatory response in smoker patients with stable and exacerbation phases of chronic obstructive pulmonary disease. Scand J Clin Lab Invest. (2009) 69:219–24. doi: 10.1080/00365510802474400 [DOI] [PubMed] [Google Scholar]
- 44.Jéquier E, Tappy L. Regulation of body weight in humans. Physiol Rev. (1999) 79:451–80. doi: 10.1152/physrev.1999.79.2.451 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.