Abstract
Osteoarthritis (OA) is a painful joint disease that is common among the middle-aged and elderly populations, with an increasing prevalence. Therapeutic options for OA are limited, and the pathogenic mechanism of OA remains unclear. The roles of cytokines and signaling pathways in the development of OA is a current research hot spot. Interleukin (IL)-17 is a pleiotropic inflammatory cytokine produced mainly by T helper 17 cells that has established roles in host defense, tissue repair, lymphoid tissue metabolism, tumor progression, and pathological processes of immune diseases, and studies in recent years have identified an important role for IL-17 in the progression of OA. This narrative review focuses on the mechanisms by which IL-17 contributes to articular cartilage degeneration and synovial inflammation in OA and discusses how IL-17 and the IL-17 signaling pathway affect the pathological process of OA. Additionally, therapeutic targets that have been proposed in recent years based on IL-17 and its pathway in OA are summarized as well as recent advances in the study of IL-17 pathway inhibitors and the potential challenges of their use for OA treatment.
Keywords: osteoarthritis, interleukin-17, joint cartilage, synovium, target
1. Introduction
Osteoarthritis (OA) is one of the main causes of joint stiffness, pain and disability in the middle-aged and elderly populations, with hip and knee OA ranking 11th on the global list of disabling factors [1]. The pathogenesis of OA is complex, and the etiology is not completely clear. It is currently believed to be the result of a combination of risk factors, mainly including advanced age, obesity, joint misalignment, increased pressure load, genetics, and inflammation, with advancing age and obesity being the most prominent [2]. OA is characterized by progressive cartilage degeneration followed by the gradual development of synovial inflammation, subchondral bone sclerosis, bone redundancy formation, degeneration and tearing of the meniscus, inflammation and fibrosis of the infrapatellar fat pad [3,4], and even involvement of the entire joint [5,6]. Chronic joint pain severely affects the quality of life of OA patients, which can lead to the development of depressive states, increasing the probability of self-harm and suicide [7,8]. With the continued aging of the population and the increase in obesity in recent years globally, the reported number of OA cases increased from 248 million in 1990 to 528 million in 2019, following an increasing trend each year [9]. The current prevalence of OA in China is approximately 15%, and the condition affects up to 50% of people over 60 years of age, with a greater prevalence in women versus men and in rural populations vs urban populations, with geographical variation [10]. This high prevalence results in a huge consumption of medical resources and a huge burden on individuals, the economy, and society [11,12]. However, the current clinical treatments for OA are mainly aimed at relieving pain, protecting joint function, and slowing disease progression, and no non-surgical treatment strategy is available to control or reverse OA [13]. Although, patients with end-stage OA can choose joint replacement surgery, this treatment option is associated with risks of postoperative bleeding, infection, thrombosis, and persistent joint pain, and some patients are unable to tolerate the surgical procedure [14]. Therefore, research to understand the pathogenesis of OA in depth and to identify targets and drugs for effective OA treatment is needed to address urgent clinical problems at present.
OA is the result of an imbalance between cartilage synthesis and degradation that occurs under the combined effects of mechanical and biological factors, such as trauma, inflammation, aging, and various genetic, immune, and metabolic factors [7,15]. Articular cartilage includes chondrocytes and cartilage matrix (mainly containing water, type II collagen, and proteoglycans). Under normal conditions, type II collagen interlinks with other collagens in the extracellcular matrix (ECM) in the form of cross-linked microfibils to maintain the biomechanical skeleton of articular cartilage, and the ECM has a strong water retention capacity (hydrophilic and negatively charged), thus allowing frictionless movement of the articular surfaces and eliminating the crowding forces generated by pressure loads on the articular surfaces [16]. In addition, proteoglycans protect the articular surfaces from compressive deformation by regulating the fluid pressure in the cartilage tissue. With the occurrence of OA, interaction between chondrocytes and ECM is altered, proteoglycans and type II collagen are degraded, chondrocyte catabolism is enhanced, cartilage surfaces are compressed and eroded, and inflammatory factors are increased, leading to synovial inflammation and further aggravating the development of OA [17,18,19].
Chondrocyte differentiation and apoptosis as well as cartilage matrix synthesis and degradation are dynamically balanced to maintain cartilage homeostasis. Disturbance of cytokine homeostasis disrupts this intrachondral homeostasis, and thus, is one of the most important factors in the pathogenesis of OA [20]. Researchers have found elevated levels of tumor necrosis factor alpha (TNF-α), interleukin (IL)-1, and IL-6 in the synovial fluid, synovium, and cartilage ECM of OA patients, which suggests that low-level inflammation is involved in the pathogenesis of OA [21,22,23]. In addition, the infrapatellar fat pad, which is as rich in vascular and neural tissue as the synovium, appears to be an anatomical-functional unit with the synovium, and is thought to be one of the causes of OA joint pain [24,25]. The infrapatellar fat pad also produces pro-inflammatory cytokines and chemokines that induce synovial inflammation and promote OA progression [26]. These cytokines are known to stimulate chondrocytes, disturb the balance of anabolic and catabolic metabolism, induce high expression of matrix metalloproteinase 3 (MMP3), MMP13 and other factors, and contribute to cartilage matrix degradation [27,28]. They also stimulate synovial cells to release proteases, causing synovial inflammation and promoting bone resorption [28], which induces progressive degradation and destruction of articular cartilage. Accordingly, the important roles of cytokines in the pathogenesis of OA have been confirmed.
In recent years, the relationship between the pleiotropic inflammatory cytokine IL-17A (also commonly referred to as IL-17) and OA has attracted considerable attention. Previous studies showed that IL-17 is associated with host defense, tissue repair, lymphoid tissue metabolism, and tumor progression [29], and IL-17 has been most extensively studied in relation to autoimmune diseases, such as rheumatoid arthritis (RA) [30], ankylosing spondylitis [31], and psoriatic arthritis [32]. The circulating IL-17 level was shown to play an important role in the pathogenesis and progression of inflammatory arthritis [33]. Synergy between IL-17 and TNF-α has been demonstrated to activate the production of proinflammatory mediators, such as IL-1β, IL-6, IL-8, prostaglandin E2 (PGE2), and MMPs, to promote the progression of early inflammation to chronic arthritis [34]. In the context of OA, IL-17 affects the inflammatory response, complement production, hypoxia response, angiogenesis, and glycolytic pathways in chondrocytes and synovial fibroblasts [35]. IL-17 may play a crucial role in the pathogenesis of OA and is closely associated with joint pain in OA patients [36]. Significant correlation between IL-17 expression and cartilage defects and bone marrow lesions was observed in the serum of patients with knee osteoarthritis (KOA), and a positive correlation was also observed between IL-17 expression and the severity of KOA [37,38]. Several animal models have also confirmed the role of IL-17 in inflammatory arthritis [39,40,41], and injection of IL-17 into the rabbit knee joint induces a model of OA similar to that induced by the Hulth method [42]. Therefore, This review article summarizes current knowledge regarding the mechanism of action of IL-17 in OA and discusses the potential challenges of using IL-17 inhibitors for the treatment of OA.
2. Introduction of IL-17
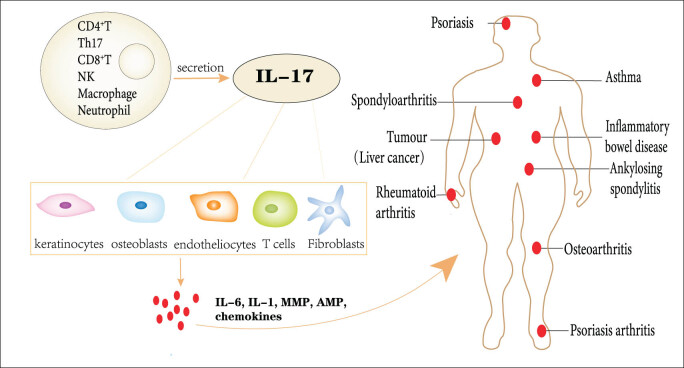
The IL-17 family is a class of structurally similar inflammatory molecules that includes six cytokines, IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (also known as IL-25), and IL-17F. They are mainly derived from T helper 17 (Th17) cells, CD8+ T cells, γδT cells, and natural killer (NK) T cells [43] and can also be produced by neutrophils and macrophages during inflammation (Figure 1) [44,45]. The most well-known one is IL-17A (referred to hereafter as IL-17 unless otherwise stated). To achieve their biological effects, members of the IL-17 family must bind to the corresponding receptor (R) complex on the cell surface to regulate gene transcription. The IL-17 recepter (IL-17R) family has five receptor subunit members, which are labeled IL-17RA, IL-17RB, IL-17RC, IL-17RD, IL-17RE, of which IL-17A and IL-17F exist as homologs or heterodimers and act together in a complex formed with IL-17RA and IL-17RC. Interestingly, IL-17RA exerts pleiotropic functions by binding and interacting with IL-17RB, IL-17RC, IL-17RD, and IL-17RE [46,47]. In the inflammatory environment, IL-17 can be active against various cells, including keratin-forming cells, fibroblasts, osteoblasts, endothelial cells, and immune cells [48], and is involved in the pathological processes of inflammation, autoimmunity, tumorogenesis, and metabolic disorder through the production of various molecules, such as inflammatory factors (IL-6, IL-1, TNF), chemokines, antimicrobial peptides (AMPs), MMPs, and acute phase proteins (Figure 1) [49].
Figure 1.
IL-17 is produced by Th17 cells, CD8+ T cells, γδ T cells, NKT cells, etc. IL-17 acts on keratin-forming cells, fibroblasts, osteoblasts, endothelial cells, and immune cells and participates in the pathological processes of tumor development, immune diseases, and inflammation by inducing production of inflammatory factors, inflammatory mediators, and MMPs.
3. IL-17 signaling pathway
IL-17RA consists of an extra-membrane fibronectin-like structural domain, an intracytoplasmic SEF/IL-17(SEFIR) structural domain and a distal activation structural domain (CBAD). In addition, IL-17RA also has SEFEX, an extension sequence of SEFIR [50]. The signal transduction and negative regulatory pathway of IL-17 consists of four main steps: (1) IL-17 (IL-17A, IL-17A/F, IL-17F) binds to receptor complexes formed by IL-17RA and IL-17RC and induces binding of the receptor SEFIR structural domain to the multifunctional signaling protein Act1 (with E3 ubiquitin ligase activity). Act1 is critical in IL-17 signaling pathway-dependent autoimmune and inflammatory diseases, and IL-17-induced expression of inflammation-related genes is suppressed when Act1 is defective [51]. (2) Act1 rapidly recruits and ubiquitinates TNF receptor-associated factor 6 (TRAF6), a critical step in signaling pathway transduction. TRAF3 and TRAF4 can negatively regulate this pathway by interfering with the coupling between Act1 and TRAF6. Additionally, deubiquitinating enzymes A20 and USP25 can remove the ubiquitination disability of TRAF6 to similarly prevent TRAF6 from binding to Act1. Meanwhile, USP25 also inhibits TRAF5 activity and affects post-transcriptional RNA stability. (3) TRAF6 promotes the activation of mitogen-activated protein kinase (MAPK)/AP-1, C/EBPβ, and δ transcription factor, and through transforming growth factor beta (TGF-β)-activated kinase 1 (TAK1), phosphorylates nuclear factor kappa B (NF-κB), targeting an important transcriptional target NF-κB inhibitor zeta (IBζ), which is involved in psoriasis development. (4) Act1 recruits TRAF2/5 and binds HuR and Arid5a to promote post-transcriptional RNA stabilization (Figure 2) [52,53]. In conclusion, IL-17 signaling is very complex, and the above description summarizes only the main processes. Many specific molecular mechanisms and branches remain to be further explored.
Figure 2.
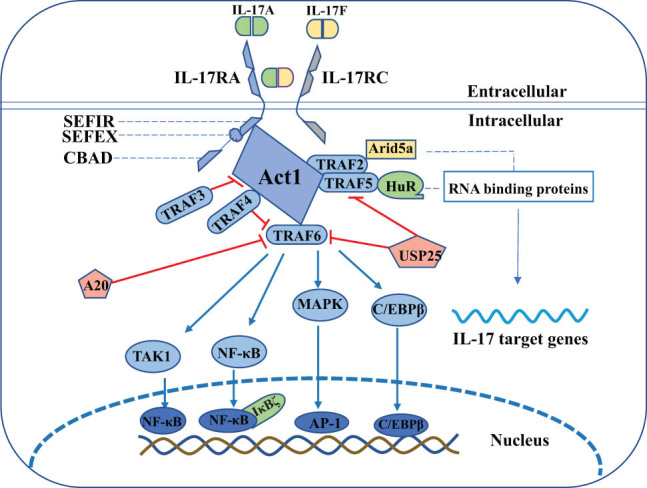
IL-17 signal transduction pathway.
4. Role of IL-17 in the pathogenesis of OA
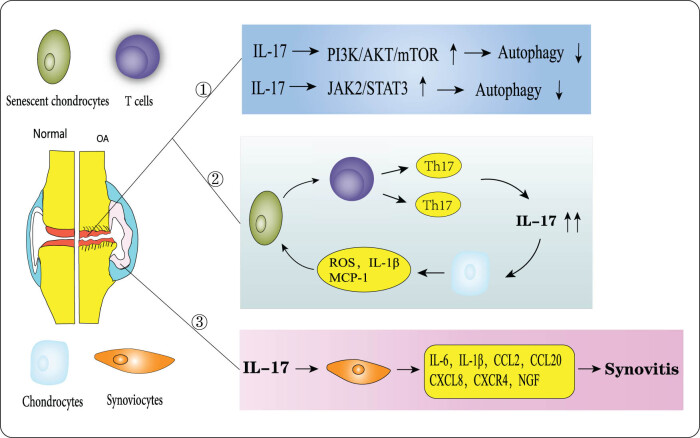
The etiology of osteoarthritis remains incompletely understood, but various molecular mechanisms have been shown to be involved in this pathological process, including the JAK/STAT signaling pathway [54], PI3K/AKT/mTOR signaling pathway [55,56], p38 MAPK/c-Fos/AP-1 pathway [57], and Wnt/β-linked protein pathway [58]. These signaling pathways have been found to mainly affect the OA pathological process by regulating chondrocyte survival, subchondral bone remodeling, and synovial inflammation. Interestingly, IL-17 is also involved in the degeneration and destruction of articular cartilage and synovial inflammatory processes in the pathology of OA (Figure 3).
Figure 3.
Mechanism of action of IL-17 within articular cartilage and synovium in OA. (1) IL-17 reduces autophagy through activation of the PI3K/AKT/mTOR and JAK/STAT3 signaling pathways; (2) IL-17 promotes chondrocyte senescence in OA, and senescent chondrocytes induce differentiation of naive T cells to Th17 cells in a vicious cycle; and (3) IL-17 promotes synovial inflammation.
4.1. Cartilage
The degeneration and destruction of articular cartilage are recognized as central to the progression of OA [59] along with the involvement of IL-17 and its pathways, mainly in the form of effects on chondrocyte autophagy, senescence, and cartilage matrix [41].
Autophagy is a stress response mechanism for cell survival that involves removal of intracellular microorganisms through lysosomes and degradation of dysfunctional or damaged organelles and proteins, and is an important system for energy and nutrient metabolic homeostasis within the body [60,61]. Abnormal cellular autophagy can advance the progression of chondrocyte senescence and apoptosis, with peroxide production and mitochondrial dysfunction being the main mechanisms [62]. Previous studies by our group and others have confirmed that autophagy, as a form of cell death, is closely associated with OA progression [63,64].
Furthermore, autophagy and inflammation are two biological processes important for cells in physiological and pathological states, with autophagy regulating innate and adaptive immune responses and, conversely, immune response-induced cytokine release regulating autophagy levels [65,66,67]. IL-17 is known to influence autophagy in two ways. (1) IL-17 promotes autophagy: IL-17 can induce autophagy in B cells in vitro by increasing Erk1/2 phosphorylation, as evidenced by an increase in the activity of autophagy proteins Beclin-1 and P62 on the ubiquitin proteasome system, and by an increase in the anti-apoptotic capacity of B cells [68]. In contrast, 3-MA, an inhibitor of autophagy, significantly reversed the above effects of IL-17. It was found that IL-17 increased the accumulation of RAW264.7 cellular autophagy protein LC3B-II–induced autophagic activity, significantly increased the number and size of cellular autophagosomes, and promoted the antimicrobial activity of primitive macrophages [69]. (2) IL-17 inhibits autophagy expression: In normal skin fibroblasts, IL-17 stimulation significantly increased the expression of p-STAT3 and hypoxia-inducible factor-1α (HIF-1α) and increased P62 activity, suggesting an accumulation of autophagosomes (P62), leading to autophagy defects. hIF-1α inhibitor reversed the IL-17-induced autophagy downregulation [70]. The IL-17/STAT3/HIF-1α/P62 signaling axis cascade is associated with autophagy inhibition.
However, how IL-17 regulates autophagy in OA remains less well characterized. An study observed significantly elevated IL-17 expression in an IL-1β-induced OA chondrocyte inflammation model, which exacerbated autophagy defects and promoted cartilage degeneration by mediating PI3K/AKT/mTOR autophagy-related pathways [41]. In addition, stimulation of mouse osteoblasts with IL-17 was found to suppress the level of autophagy by activating the JAK2/STAT3 signaling pathway and downregulating Beclin 1, LC3, and Atg7 expression [71]. In summary, in OA, IL-17 promotes OA progression mainly by inhibiting autophagy expression, which is closely related to the PI3K/AKT/mTOR and JAK2/STAT3 signaling pathways, and targeting these two pathways and blocking the action of IL-17 may be the direction of OA treatment. Unfortunately, additional research in this specific area is lacking, and it remains unclear whether IL-17 is an independent upstream target of autophagy.
Cellular senescence also is involved in OA pathogenesis [15,72]. Chondrocyte senescence affects articular cartilage biomechanics, biochemistry, and cellular function, making articular cartilage more susceptible to damage [73]. In addition, one of the hallmarks of aging is mitochondrial dysfunction, which in OA leads to an imbalance in cellular energy metabolism and an increase in reactive oxygen species (ROS) production, exacerbating the progression of OA as evidenced by an increase in oxidative stress in articular cartilage, cytokine production, an increase in inflammation-mediated catabolism of cartilage matrix, and increases in calcification of the cartilage matrix and apoptosis of chondrocytes [74,75]. Experiments in an animal model of post-traumatic OA showed anterior cruciate ligament transection (ACLT) induced a Th17-type immune response with increased IL-17 expression and that senescent OA chondrocytes drove the differentiation of naive T cells to Th17 cells [39]. Furthermore, intra-articular injection of neutralizing antibodies to IL-17 into the OA animal model decreased the expression of the senescence marker P16 (Cdkn1α) [39]. Wang et al. reported that stimulation of chondrocytes with IL-17 induces production of reactive oxygen species, monocyte chemoattractant protein-1 (MCP-1), and IL-1β along with increased senescence-associated β-galactosidase activity, a prolonged stationary/gap phase (G0/G1) in the cell cycle, a shortened S-phase in DNA synthesis, and ultimately premature senescence of chondrocytes [76]. In summary, the Th17/IL-17 axis can accelerate chondrocyte senescence, and a reciprocal promoting relationship exists between IL-17 secretion and cellular senescence-associated protein expression. Moreover, this combined effect may induce more rapid destruction of articular cartilage than each single factor.
Chondrocytes are responsible for the synthesis and secretion of cartilage needed to form the cartilage matrix, and this activity is essential for the maintenance of cartilage homeostasis and is regulated by physical and chemical signaling within the joint that influences the physiological function of chondrocytes [77,78]. IL-17 was found to be involved in cartilage matrix synthesis and catabolic pathways [79]. Elevated IL-1β and IL-6 expression was observed in a sodium iodoacetate-induced IL-1 receptor antagonist (IL-1Ra) knockout mouse model of OA, and cartilage tissue thinning and chondrocyte reduction also were observed [80]. However, silencing of IL-17 significantly inhibited inflammatory mediator release and cartilage damage. The same study also stimulated human chondrocytes with IL-17 and found that MMP1, MMP3, and MMP13 were upregulated while SOX9 (a protein associated with chondrocyte anabolism) was downregulated, which led to increased cartilage matrix degradation and exacerbated cartilage tissue damage. Hu et al. reported that treatment of chondrocytes with recombinant IL-17 results in activation of the NF-κB and MAPK signaling pathways, upregulation of the cartilage catabolic factors IL-6, MMP3, and zinc metalloproteinase 4 (ADAMTS-4) expression, promotion of cartilage matrix degradation, disruption of homeostasis within cartilage, and aggravation of OA progression [81]. In summary, stimulation by IL-17 disrupts the balance in cartilage matrix metabolism, and the resulting feedback affects the physiological function of chondrocytes, leading to cartilage tissue degeneration. Thus, the role of IL-17 in OA is not limited to inflammatory effects, as this factor also plays a key role in the pathological progression of OA by influencing chondrocyte senescence, apoptosis, and cartilage matrix degradation.
4.2. Synovium
The synovium is a connective tissue located in the inner layer of the joint capsule that surrounds tendons and forms the lining of bursae and fat pads, which secrete and regulate the formation of synovial fluid, providing nutrients to the chondrocytes [82]. Injury-related molecules, products of mitochondrial dysfunction, cytokines, and metabolites in joints activate synovial cells to promote the release of large amounts of pro-inflammatory cytokines and inflammatory mediators, inducing OA-related cartilage matrix degradation and osteophyte formation [83,84,85]. Pro-inflammatory cytokines and chemokines are central regulators of synovial inflammation in OA [20,21]. Interestingly, IL-17 amplifies the synovial inflammatory effects in OA by promoting the expression of pro-inflammatory factors (e.g., IL-6 and IL-1β) and chemokines (e.g., CXCL8, CCL20, CXCL3, and CXCR4) [86]. Deligne et al. found that IL-17 and IL-22 are highly expressed in conditioned medium collected from synovial tissues of OA patients and responsible for inducing the combined release of IL-6, IL-23 and TGF-β1; upregulating MMP9 expression; and ultimately driving synovial inflammation and cartilage matrix degradation [87]. Moreover, in a clinical trial of end-stage hip and knee OA, patients with detectable IL-17 levels in synovial fluid had significantly increased levels of adipokines (leptin, resistin), IL-6, C-C motif chemokine ligand 2 (CCL2), CCL17, and nerve growth factor (NGF) [88]. These findings supported those of previous studies regarding the involvement of adipokines in cartilage degeneration, synovitis, subpatellar fat pad changes, and bone formation [89,90]. Again, IL-17 was shown to promote OA progression by increasing adipokine and inflammatory factor production. In conclusion, IL-17 itself has limited pro-inflammatory effects and cannot directly act on chondrocytes, but it exerts intense inflammatory effects by enhancing and synergizing the pro-inflammatory effects of other cytokines and inflammatory mediators, leading to cartilage degradation, matrix degradation, and synovial inflammation in OA.
5. IL-17–based therapeutic targets and drugs for OA
5.1. Targets
Levels of long non-coding (lnc)RNA cancer susceptibility candidate 2 (lncRNA CASC2) were found to be elevated in the blood and synovial fluid of OA patients, and lncRNA CASC2 was shown to regulate chondrocyte proliferation and apoptosis through mediation of the IL-17 signaling pathway [91]. In human OA synovial fibroblasts (OASFs), the CCN family protein connective tissue growth factor (CCN2) inhibits miR-655 synthesis by mediating the ILK and Syk signaling pathways, whereas miR-655 can bind to IL-17 and directly inhibits IL-17 activity; therefore, CCN2 down-regulates miR-655 expression and indirectly promotes IL-17 synthesis, leading to increased inflammation in OA [40]. Low levels of lncRNA growth arrest-specific transcript-5 (GAS-5) were shown to affect IL-17–related immune and cytokine expression and to be a potential marker of OA progression [92]. The cyclic RNA ciRS-7/micro-RNA7 (mi-RNA7) axis is aberrantly expressed in OA and may drive OA progression through upregulation of IL-17–mediated inflammatory responses [43]. IL-17 is the target gene of miR-136, the expression of which is negatively correlated with miR-136 expression, and thus, miR-136 can be used as a potential biomarker of KOA [93]. TRAF3 significantly inhibits IL-17–induced activation of NF-κB and MAPK, as well as the production of downstream MMPs, resulting in a protective effect against OA [49]. miR-34a, miR-146a, and miR-181a are mediators of adipokine-induced oxidative stress and synovial inflammation in humans with OA via NF-κB pathway expresssion in synoviocytes [94]. In conclusion, most therapeutic targets for OA based on IL-17 and its signaling pathway are related to mRNA expression, and targeting of the gene transcription–translation pathway is expected to be a new strategy for OA therapy.
5.2. Drugs
According to the 2019 edition of the Chinese Osteoarthritis Diagnosis and Treatment Guidelines, OA is most commonly treated currently with a combination of treatments, including health education, weight management, symptomatic drug therapy (non-steroidal anti-inflammatory drugs, NSAIDs), intra-articular sodium hyaluronate injection, physical therapy, and surgery, with the aims of relieving pain and improving joint function [13,95,96,97]. The 2019 American College of Rheumatology/Arthritis Foundation guidelines on OA of the hands, hips, and knees emphasize exercise, weight loss, self-management (tai chi), use of canes and hand orthotics and braces, oral and topical NSAIDs, intra-articular glucocorticosteroid injections, acupuncture, and heat treatments, to name a few, but none of them can cure OA [97]. These guidelines are similar to the Chinese guidelines for the diagnosis and treatment of osteoarthritis. However, NSAIDs can cause gastrointestinal, hepatic, renal, and cardiovascular side effects, and thus, their use should be limited [98]. Several new therapeutic approaches have been explored in recent years, including combination injections of nerve growth factor, stem cells, and platelet-rich plasma, but the balance of efficacy and safety for these approaches remains questionable [5,99]. Accordingly, treatment of OA remains a challenge worldwide. Multiple studies in recent years have demonstrated that regulation of Th17 cells, IL-17 expression, IL-17 signaling pathway activation, and mRNA expression of inflammatory mediators may be effective strategies for treating OA, with effects of reducing cartilage tissue damage and improving joint pain, inflammation, and function (Table 1). The most important of these effects is the control of synovial inflammation in OA.
Table 1.
Potential IL-17–related treatments for OA
| Drug name | Target | Research platform | Effect | Refs. |
|---|---|---|---|---|
| RA10-6 | IL-17/IL-6 | Mice | Suppression of OA synovial inflammation | [110] |
| Krocina™ | Th17/IL-17 | Humans (clinical trial) | Suppression of OA synovial inflammation | [111] |
| Resveratrol | IL-17/NF-κB | Rats | Repair of OA soft tissue damage | [111] |
| Platelet-rich- plasma | IL-17 | Rats | Improvement of joint function, pain and inflammatory | [112,113,114] |
| Baccharis | IL-17 | Mice | Suppression of OA immune inflammatory | [115] |
| Pioglitazone | IL-17/NF-κB | Human myeloid cells and tissues | Inhibition of mRNA expression of inflammatory mediators | [116] |
| 1,25-Vit D3 | Th17 | Dendritic cells | Inhibition of pro-inflammatory cytokine | [117] |
| Dexamethas-one | Th17 | Dendritic cells | Inhibition of pro-inflammatory cytokine | [118] |
| Apremilast | IL-17 | ATDC5 chondrocytes | Inhibition of inflammatory factors, cellular senescence, ROS | [76] |
| Salidroside | CD4+/IL-17 | Rats | Regulates inflammation and immune response | [118] |
6. Inhibitors of IL-17
Inhibitors of IL-17 or the IL-17 signaling pathway, including Secukinumab (AIN457), Ixekizumab (LY2439821), and Brodalumab (AMG827), have been approved in recent years for clinical use for the treatment of ankylosing spondylitis [100,101], moderate-to-severe plaque psoriasis and psoriatic arthritis [102,103,104], radiographic and nonradiographic spondyloarthritis [105], and Netherton’s syndrome [106] (Table 2), and have shown excellent efficacy, safety, and tolerability. Additionally, some small molecule inhibitors of the orally available IL-17 pathway continue to be studied in the clinical research phase, such as IMU-935, an inhibitor of retinoic acid-related orphan receptor γt (RORγt), which is a major regulator of Th17 cell secretion and IL-17A/F production in innate and adaptive immunity and is involved in the regulation of bacterial and fungal immune responses [107]. As a treatment for psoriasis, IMU-935 was shown to reduce the release of pro-inflammatory cytokines and inflammatory mediators by inhibiting the RORγt/Th17/IL-17 signaling pathway [108,109], but its drug efficacy and risk of adverse events have yet to be established. Although IL-17 pathway inhibitors are not currently used for the treatment of OA, research evidence that IL-17 plays an important role in the pathological progression of OA and is positively correlated with OA severity suggests that inhibitors of IL-17 and its pathway may become the next clinical research target for OA treatment strategies.
Table 2.
Mechanism and characteristics of drugs that inhibit IL-17 or the IL-17 signaling pathway
| Drug | Target | Molecular mechanism | Refs. |
|---|---|---|---|
| Secukinumab | IL-17A | Human recombinant monoclonal antibody of IgG1/κ isotype; selectively binds to IL-17A and inhibits its combination with IL-17RA; blocks trans- duction of IL-17 signaling pathway. | [119,120] |
| Ixekizumab | IL-17A | Human IgG4/κ monoclonal antibody; binds to IL-17A with high affinity and selectivity to neutralize its activity; inhibits release of proinflamma- tory cytokines and chemokines. | [121] |
| Brodalumab | IL-17RA | Human monoclonal antibody of IgG2/κ isotype; specifically blocks IL-17R and inhibits proinflammatory signaling inducted by multiple IL-17 cytokines, including IL-17A and IL-17F. | [122] |
| IMU-935 | RORγt | Specifically inhibits the RORγt/Th17/IL-17 signaling pathway; affects Th17 cell secretion and IL-17A/F production; inhibits release of relat-ed pro-inflammatory cytokines and inflammatory mediators. | [108,109] |
Although down regulation of IL-17 and inhibition of the IL-17 signaling pathway can be beneficial in OA as mentioned above, inhibitors of IL-17 and its pathway are currently inappropriate for the treatment of OA based on the mechanism of action of IL-17 in the pathological process of OA, and many potential challenges persist. (1) IL-17 cannot act directly on chondrocytes but rather affects chondrocyte survival by indirectly upregulating or downregulating the expression of related pathways and proteins. Inhibiting IL-17 expression can reduce its role in promoting chondrocyte apoptosis and senescence but cannot block the pathological changes that occur in OA itself. (2) The pro-inflammatory effect of IL-17 itself is limited; this effect is instead, mainly achieved by synergizing and amplifying the effects of other pro-inflammatory factors and inflammatory mediators to indirectly promote synovial inflammation in OA. Thus, inhibiting the activity of IL-17 and its receptors only weakens or eliminates this amplifying effect but cannot block the occurrence of synovial inflammation. (3) OA progression is supported by many molecular mechanisms that are connected by subtle links. It is not accurate to consider IL-17 activity as an independent cause of OA. Therefore, an in-depth understanding of the relationship between IL-17 and other signaling pathways in OA is critical. Much basic and clinical research is needed to address questions like whether targeting IL-17 gene expression and inhibiting IL-17 pathway activation will have different effects, and whether the combined application of IL-17 inhibitors and blockers of other pathways will have different efficacies.
7. Conclusions
IL-17 is involved in the maintenance of innate and adaptive immunity, and dysregulated production of IL-17 has been shown to promote OA development from multiple perspectives. On the one hand, it promotes OA progression by regulating chondrocyte autophagy and senescence and cartilage matrix degradation, while on the other hand, it induces synovial inflammation by mediating the IL-17 signaling pathway to promote the release of inflammatory cytokines and adipocytokines. These research findings provide valuable evidence for the application of inhibitors of IL-17 and its signaling pathway in OA treatment. Additionally, IL-17 expression was found to be positively correlated with the severity of OA, and thus, it may become a new immunological indicator for evaluating the efficacy of OA treatment and may be an effective target for OA therapy. However, the following questions persist regarding the role of IL-17 in OA: (1) Does IL-17 affect chondrocyte mitosis and cell cycle progression in OA? (2) Are multiple members of the IL-17 family involved in OA development? (3) What relationships exist between IL-17 and other signaling pathways, and what are their side effects? In the future, it will be important to also investigate the impact of IL-17 on other joint tissues such as the meniscus and infrapatellar fat pad, considering that OA is a whole joint disease. A clear understanding of the specific molecular mechanism of IL-17’s action in OA and a means to target this axis accurately without affecting physiological functions could facilitate effective approaches for the prevention and treatment of OA based on IL-17 and its signaling pathways.
Footnotes
Funding information: This work was supported by the Foundation of Guizhou Science and Technology Department (No. QIANKEHEZHICHEN [2023] YIBAN576) and the Foundation of Zunyi Science and Technology Department (Serial Number. HZ359(2022)).
Author contributions: M.T. designed the framework of the manuscript. J.X., P.Z. and X.P. wrote the manuscript and participated in the revision. F.C., C.L., T.P. checked the related literature. All authors reviewed the final version and agreed to its submission. All authors have read and agreed to the published version of the manuscript.
Conflict of interest: Authors state no conflict of interest.
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Contributor Information
Xiao-Li Pan, Email: xiaojuanmtyy@163.com.
Mei Tian, Email: 570210191@qq.com.
References
- [1].Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–30. [DOI] [PubMed]
- [2].Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. [DOI] [PubMed]
- [3].Battistelli M, Favero M, Burini D, Trisolino G, Dallari D, De Franceschi L, et al. Morphological and ultrastructural analysis of normal, injured and osteoarthritic human knee menisci. Eur J Histochem. 2019;63(1):2998. [DOI] [PMC free article] [PubMed]
- [4].Zhou S, Maleitzke T, Geissler S, Hildebrandt A, Fleckenstein FN, Niemann M, et al. Source and hub of inflammation: The infrapatellar fat pad and its interactions with articular tissues during knee osteoarthritis. J Orthop Res. 2022;40(7):1492–504. [DOI] [PubMed]
- [5].Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–59. [DOI] [PubMed]
- [6].Primorac D, Molnar V, Rod E, Jeleč Ž, Čukelj F, Matišić V, et al. Knee osteoarthritis: A review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes (Basel). 2020;11(8):854. [DOI] [PMC free article] [PubMed]
- [7].Vina ER, Kwoh CK. Epidemiology of osteoarthritis: Literature update. Curr Opin Rheumatol. 2018;30(2):160–7. [DOI] [PMC free article] [PubMed]
- [8].Quicke JG, Conaghan PG, Corp N, Peat G. Osteoarthritis year in review 2021: Epidemiology & therapy. Osteoarthr Cartil. 2022;30(2):196–206. [DOI] [PubMed]
- [9].Long H, Liu Q, Yin H, Wang K, Diao N, Zhang Y, et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022;74(7):1172–83. [DOI] [PMC free article] [PubMed]
- [10].Tang X, Wang S, Zhan S, Niu J, Tao K, Zhang Y, et al. The prevalence of symptomatic knee osteoarthritis in China: Results from the China health and retirement longitudinal study. Arthritis Rheumatol. 2016;68(3):648–53. [DOI] [PubMed]
- [11].Whittaker JL, Runhaar J, Bierma-Zeinstra S, Roos EM. A lifespan approach to osteoarthritis prevention. Osteoarthr Cartil. 2021;29(12):1638–53. [DOI] [PubMed]
- [12].Fu M, Zhou H, Li Y, Jin H, Liu X. Global, regional, and national burdens of hip osteoarthritis from 1990 to 2019: Estimates from the 2019 Global Burden of Disease Study. Arthritis Res Ther. 2022;24(1):8. [DOI] [PMC free article] [PubMed]
- [13].Abramoff B, Caldera FE. Osteoarthritis: Pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104(2):293–311. [DOI] [PubMed]
- [14].Gupta P, Quan T, Patel CJ, Gu A, Campbell JC. Extended length of stay and postoperative complications in octogenarians with hypertension following revision total knee arthroplasty. J Clin Orthop Trauma. 2022;26:101787. [DOI] [PMC free article] [PubMed]
- [15].Xie J, Wang Y, Lu L, Liu L, Yu X, Pei F. Cellular senescence in knee osteoarthritis: Molecular mechanisms and therapeutic implications. Ageing Res Rev. 2021;70:101413. [DOI] [PubMed]
- [16].Buckwalter JA, Mankin HJ. Articular cartilage: Tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477–86. [PubMed]
- [17].Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthr Cartil. 2004;12(Suppl A):S31–3. [DOI] [PubMed]
- [18].Martínez-Moreno D, Jiménez G, Gálvez-Martín P, Rus G, Marchal JA. Cartilage biomechanics: A key factor for osteoarthritis regenerative medicine. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1067–75. [DOI] [PubMed]
- [19].Pettenuzzo S, Arduino A, Belluzzi E, Pozzuoli A, Fontanella CG, Ruggieri P, et al. Biomechanics of Chondrocytes and Chondrons in Healthy Conditions and Osteoarthritis: A Review of the Mechanical Characterisations at the Microscale. Biomedicines. 2023;11(7):1942. [DOI] [PMC free article] [PubMed]
- [20].Molnar V, Matišić V, Kodvanj I, Bjelica R, Jeleč Ž, Hudetz D, et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci. 2021;22(17):9208. [DOI] [PMC free article] [PubMed]
- [21].Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. [DOI] [PubMed]
- [22].Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, et al. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr Cartil. 2010;18(11):1441–7. [DOI] [PubMed]
- [23].Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthr Cartil. 2015;23(11):1966–71. [DOI] [PMC free article] [PubMed]
- [24].Han W, Aitken D, Zhu Z, Halliday A, Wang X, Antony B, et al. Hypointense signals in the infrapatellar fat pad assessed by magnetic resonance imaging are associated with knee symptoms and structure in older adults: A cohort study. Arthritis Res Ther. 2016;18(1):234. [DOI] [PMC free article] [PubMed]
- [25].Belluzzi E, Macchi V, Fontanella CG, Carniel EL, Olivotto E, Filardo G, et al. Infrapatellar fat pad gene expression and protein production in patients with and without osteoarthritis. Int J Mol Sci. 2020;21(17):6016. [DOI] [PMC free article] [PubMed]
- [26].Belluzzi E, Stocco E, Pozzuoli A, Granzotto M, Porzionato A, Vettor R, et al. Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. Biomed Res Int. 2019;2019:6390182. [DOI] [PMC free article] [PubMed]
- [27].Chevalier X. Upregulation of enzymatic activity by interleukin-1 in osteoarthritis. Biomed Pharmacother. 1997;51(2):58–62. [DOI] [PubMed]
- [28].Wang T, He C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. [DOI] [PubMed]
- [29].Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL. IL-17 receptor-based signaling and implications for disease. Nat Immunol. 2019;20(12):1594–602. [DOI] [PMC free article] [PubMed]
- [30].Robert M, Miossec P. IL-17 in rheumatoid arthritis and precision medicine: From synovitis expression to circulating bioactive levels. Front Med (Lausanne). 2018;5:364. [DOI] [PMC free article] [PubMed]
- [31].Tsukazaki H, Kaito T. The Role of the IL-23/IL-17 Pathway in the Pathogenesis of Spondyloarthritis. Int J Mol Sci. 2020;21(17):6401. [DOI] [PMC free article] [PubMed]
- [32].Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55(3):379–90. [DOI] [PMC free article] [PubMed]
- [33].Zhang X, Yuan Y, Pan Z, Ma Y, Wu M, Yang J, et al. Elevated circulating IL-17 level is associated with inflammatory arthritis and disease activity: A meta-analysis. Clin Chim Acta. 2019;496:76–83. [DOI] [PubMed]
- [34].Schinocca C, Rizzo C, Fasano S, Grasso G, La Barbera L, Ciccia F, et al. Role of the IL-23/IL-17 pathway in rheumatic diseases: An overview. Front Immunol. 2021;12:637829. [DOI] [PMC free article] [PubMed]
- [35].Mimpen JY, Baldwin MJ, Cribbs AP, Philpott M, Carr AJ, Dakin SG, et al. Interleukin-17A causes osteoarthritis-like transcriptional changes in human osteoarthritis-derived chondrocytes and synovial fibroblasts in vitro. Front Immunol. 2021;12:676173. [DOI] [PMC free article] [PubMed]
- [36].Liu Y, Peng H, Meng Z, Wei M. Correlation of IL-17 level in synovia and severity of knee osteoarthritis. Med Sci Monit. 2015;21:1732–6. [DOI] [PMC free article] [PubMed]
- [37].Wang K, Xu J, Cai J, Zheng S, Yang X, Ding C. Serum levels of resistin and interleukin-17 are associated with increased cartilage defects and bone marrow lesions in patients with knee osteoarthritis. Mod Rheumatol. 2017;27(2):339–44. [DOI] [PubMed]
- [38].Hu G, Ma Z, Xu M, Lei T. Study of TNF-α, IL-17, COMP, ADAMTS-7 and mRNA expression levels in patients with osteoarthritis. Chin J Front Med (electron version). 2018;10(12):100–3.
- [39].Faust HJ, Zhang H, Han J, Wolf MT, Jeon OH, Sadtler K, et al. IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J Clin Invest. 2020;130(10):5493–507. [DOI] [PMC free article] [PubMed]
- [40].Liu SC, Hsieh HL, Tsai CH, Fong YC, Ko CY, Wu HC, et al. CCN2 facilitates IL-17 production and osteoclastogenesis in human osteoarthritis synovial fibroblasts by inhibiting miR-655 expression. J Bone Miner Res. 2022;37(10):1944–55. [DOI] [PubMed]
- [41].Zhou X, Li J, Zhou Y, Yang Z, Yang H, Li D, et al. Down-regulated ciRS-7/up-regulated miR-7 axis aggravated cartilage degradation and autophagy defection by PI3K/AKT/mTOR activation mediated by IL-17A in osteoarthritis. Aging (Albany NY). 2020;12(20):20163–83. [DOI] [PMC free article] [PubMed]
- [42].Wang Z, Zheng C, Zhong Y, He J, Cao X, Xia H, et al. Interleukin-17 can induce osteoarthritis in rabbit knee joints similar to Hulth’s method. Biomed Res Int. 2017;2017:2091325. [DOI] [PMC free article] [PubMed]
- [43].McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50(4):892–906. [DOI] [PMC free article] [PubMed]
- [44].Liao X, Zhang W, Dai H, Jing R, Ye M, Ge W, et al. Neutrophil-Derived IL-17 Promotes Ventilator-Induced Lung Injury via p38 MAPK/MCP-1 Pathway Activation. Front Immunol. 2021;12:768813. [DOI] [PMC free article] [PubMed]
- [45].Nejman-Gryz P, Paplińska-Goryca M, Proboszcz M, Grabczak M, Hermanowicz-Salamon J, Krenke R. The expression of IL17RA on sputum macrophages in asthma patients. Cytokine. 2021;143:155518. [DOI] [PubMed]
- [46].Amatya N, Garg AV, Gaffen SL. IL-17 signaling: The yin and the yang. Trends Immunol. 2017;38(5):310–22. [DOI] [PMC free article] [PubMed]
- [47].Lorè NI, Chen K, Bulek K. Editorial: The IL-17 Cytokine Family in Tissue Homeostasis and Disease. Front Immunol. 2021;12:641986. [DOI] [PMC free article] [PubMed]
- [48].Abusleme L, Moutsopoulos NM. IL-17: overview and role in oral immunity and microbiome. Oral Dis. 2017;23(7):854–65. [DOI] [PMC free article] [PubMed]
- [49].Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23(7):1069–75. [DOI] [PMC free article] [PubMed]
- [50].Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43(3):402–7. [DOI] [PMC free article] [PubMed]
- [51].Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8(3):247–56. [DOI] [PubMed]
- [52].Majumder S, McGeachy MJ. IL-17 in the pathogenesis of disease: Good intentions gone awry. Annu Rev Immunol. 2021;39:537–56. [DOI] [PMC free article] [PubMed]
- [53].Swaidani S, Liu C, Zhao J, Bulek K, Li X. TRAF regulation of IL-17 cytokine signaling. Front Immunol. 2019;10:1293. [DOI] [PMC free article] [PubMed]
- [54].Qiao Z, Tang J, Wu W, Tang J, Liu M. Acteoside inhibits inflammatory response via JAK/STAT signaling pathway in osteoarthritic rats. BMC Complement Altern Med. 2019;19(1):264. [DOI] [PMC free article] [PubMed]
- [55].Xu K, He Y, Moqbel SAA, Zhou X, Wu L, Bao J. SIRT3 ameliorates osteoarthritis via regulating chondrocyte autophagy and apoptosis through the PI3K/Akt/mTOR pathway. Int J Biol Macromol. 2021;175:351–60. [DOI] [PubMed]
- [56].Sun K, Luo J, Guo J, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr Cartil. 2020;28(4):400–9. [DOI] [PubMed]
- [57].Lim H, Kim HP. Matrix metalloproteinase-13 expression in IL-1β-treated chondrocytes by activation of the p38 MAPK/c-Fos/AP-1 and JAK/STAT pathways. Arch Pharm Res. 2011;34(1):109–17. [DOI] [PubMed]
- [58].Zhou Y, Wang T, Hamilton JL, Chen D. Wnt/β-catenin signaling in osteoarthritis and in other forms of arthritis. Curr Rheumatol Rep. 2017;19(9):53. [DOI] [PMC free article] [PubMed]
- [59].Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465–80. [PubMed]
- [60].Kitada M, Koya D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. 2021;17(11):647–61. [DOI] [PubMed]
- [61].Yu L, Chen Y, Tooze SA. Autophagy pathway: Cellular and molecular mechanisms. Autophagy. 2018;14(2):207–15. [DOI] [PMC free article] [PubMed]
- [62].Roca-Agujetas V, de Dios C, Lestón L, Marí M, Morales A, Colell A. Recent insights into the mitochondrial role in autophagy and its regulation by oxidative stress. Oxid Med Cell Longev. 2019;2019:3809308. [DOI] [PMC free article] [PubMed]
- [63].Chen Y, Pan X, Zhao J, Li C, Lin Y, Wang Y, et al. Icariin alleviates osteoarthritis through PI3K/Akt/mTOR/ULK1 signaling pathway. Eur J Med Res. 2022;27(1):204. [DOI] [PMC free article] [PubMed]
- [64].Tang Y, Li Y, Xin D, Chen L, Xiong Z, Yu X. Icariin alleviates osteoarthritis by regulating autophagy of chondrocytes by mediating PI3K/AKT/mTOR signaling. Bioengineered. 2021;12(1):2984–99. [DOI] [PMC free article] [PubMed]
- [65].Netea-Maier RT, Plantinga TS, van de Veerdonk FL, Smit JW, Netea MG. Modulation of inflammation by autophagy: Consequences for human disease. Autophagy. 2016;12(2):245–60. [DOI] [PMC free article] [PubMed]
- [66].Ge Y, Huang M, Yao YM. Autophagy and proinflammatory cytokines: Interactions and clinical implications. Cytokine Growth Factor Rev. 2018;43:38–46. [DOI] [PubMed]
- [67].Zhong J, Wang Z, Yuan W, Shen Y, Chen L. Interleukin-17 promotes osteoclastogenesis and periodontal damage via autophagy in vitro and in vivo. Int Immunopharmacol. 2022;107:108631. [DOI] [PubMed]
- [68].Yuan J, Yu M, Li HH, Long Q, Liang W, Wen S, et al. Autophagy contributes to IL-17-induced plasma cell differentiation in experimental autoimmune myocarditis. Int Immunopharmacol. 2014;18(1):98–105. [DOI] [PubMed]
- [69].Orosz L, Papanicolaou EG, Seprényi G, Megyeri K. IL-17A and IL-17F induce autophagy in RAW 264.7 macrophages. Biomed Pharmacother. 2016;77:129–34. [DOI] [PubMed]
- [70].Lee SY, Lee AR, Choi JW, Lee CR, Cho KH, Lee JH, et al. IL-17 Induces Autophagy Dysfunction to Promote Inflammatory Cell Death and Fibrosis in Keloid Fibroblasts via the STAT3 and HIF-1α Dependent Signaling Pathways. Front Immunol. 2022;13:888719. [DOI] [PMC free article] [PubMed]
- [71].Wang Z, Wei Y, Lei L, Zhong J, Shen Y, Tan J, et al. RANKL expression of primary osteoblasts is enhanced by an IL-17-mediated JAK2/STAT3 pathway through autophagy suppression. Connect Tissue Res. 2021;62(4):411–26. [DOI] [PubMed]
- [72].Loeser RF. The role of aging in the development of osteoarthritis. Trans Am Clin Climatol Assoc. 2017;128:44–54. [PMC free article] [PubMed]
- [73].Verzijl N, Bank RA, TeKoppele JM, DeGroot J. AGEing and osteoarthritis: A different perspective. Curr Opin Rheumatol. 2003;15(5):616–22. [DOI] [PubMed]
- [74].Loeser RF. Aging and osteoarthritis. Curr Opin Rheumatol. 2011;23(5):492–6. [DOI] [PMC free article] [PubMed]
- [75].Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7(3):161–9. [DOI] [PubMed]
- [76].Wang B, Sun W, Bi K, Li Y, Li F. Apremilast prevents IL‑17‑induced cellular senescence in ATDC5 chondrocytes mediated by SIRT1. Int J Mol Med. 2021;47(3):12. [DOI] [PMC free article] [PubMed]
- [77].Rellmann Y, Eidhof E, Dreier R. Review: ER stress-induced cell death in osteoarthritic cartilage. Cell Signal. 2021;78:109880. [DOI] [PubMed]
- [78].Zhang Z. Chondrons and the pericellular matrix of chondrocytes. Tissue Eng Part B Rev. 2015;21(3):267–77. [DOI] [PubMed]
- [79].Liu S, Deng Z, Chen K, Jian S, Zhou F, Yang Y, et al. Cartilage tissue engineering: From proinflammatory and anti‑inflammatory cytokines to osteoarthritis treatments (Review). Mol Med Rep. 2022;25(3):99. [DOI] [PMC free article] [PubMed]
- [80].Na HS, Park JS, Cho KH, Kwon JY, Choi J, Jhun J, et al. Interleukin-1-interleukin-17 signaling axis induces cartilage destruction and promotes experimental osteoarthritis. Front Immunol. 2020;11:730. [DOI] [PMC free article] [PubMed]
- [81].Hu G, Zhang N, Li J, Wang J, Wu W, Li J, et al. Tumor necrosis factor receptor associated factor 3 modulates cartilage degradation through suppression of interleukin 17 signaling. Am J Pathol. 2020;190(8):1701–12. [DOI] [PubMed]
- [82].Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res Ther. 2017;19(1):18. [DOI] [PMC free article] [PubMed]
- [83].Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18(5):258–75. [DOI] [PMC free article] [PubMed]
- [84].Ingale D, Kulkarni P, Electricwala A, Moghe A, Kamyab S, Jagtap S, et al. Synovium-synovial fluid axis in osteoarthritis pathology: A key regulator of the cartilage degradation process. Genes (Basel). 2021;12(7):989. [DOI] [PMC free article] [PubMed]
- [85].Li Z, Huang Z, Bai L. Cell interplay in osteoarthritis. Front Cell Dev Biol. 2021;9:720477. [DOI] [PMC free article] [PubMed]
- [86].Qiu Z, Mei Y, Zhong W, Fu C, Xie X, Niu X, et al. Bioinformatics analysis of the effects of tumor necrosis factor-alpha and interleukin-17A on fibroblast-like synoviocytes in patients with osteoarthritis. Rheumatol Arthritis. 2020;9(09):7–12.
- [87].Deligne C, Casulli S, Pigenet A, Bougault C, Campillo-Gimenez L, Nourissat G, et al. Differential expression of interleukin-17 and interleukin-22 in inflamed and non-inflamed synovium from osteoarthritis patients. Osteoarthr Cartil. 2015;23(11):1843–52. [DOI] [PubMed]
- [88].Snelling SJ, Bas S, Puskas GJ, Dakin SG, Suva D, Finckh A, et al. Presence of IL-17 in synovial fluid identifies a potential inflammatory osteoarthritic phenotype. PLoS One. 2017;12(4):e0175109. [DOI] [PMC free article] [PubMed]
- [89].Richter M, Trzeciak T, Owecki M, Pucher A, Kaczmarczyk J. The role of adipocytokines in the pathogenesis of knee joint osteoarthritis. Int Orthop. 2015;39(6):1211–7. [DOI] [PubMed]
- [90].Wang K, Xu J, Cai J, Zheng S, Han W, Antony B, et al. Serum levels of interleukin-17 and adiponectin are associated with infrapatellar fat pad volume and signal intensity alteration in patients with knee osteoarthritis. Arthritis Res Ther. 2016;18(1):193. [DOI] [PMC free article] [PubMed]
- [91].Huang T, Wang J, Zhou Y, Zhao Y, Hang D, Cao Y. LncRNA CASC2 is up-regulated in osteoarthritis and participates in the regulation of IL-17 expression and chondrocyte proliferation and apoptosis. Biosci Rep. 2019;39(5):BSR20182454. [DOI] [PMC free article] [PubMed]
- [92].Zhang H, Wu Y, Li W, Chen H. Clinical significance and mechanism of LncRNA GAS-5 in osteoarthritis. Am J Transl Res. 2021;13(7):8465–70. [PMC free article] [PubMed]
- [93].Wan L, Zhao Q, Niu G, Xiang T, Ding C, Wang S. Plasma miR-136 can be used to screen patients with knee osteoarthritis from healthy controls by targeting IL-17. Exp Ther Med. 2018;16(4):3419–24. [DOI] [PMC free article] [PubMed]
- [94].Cheleschi S, Gallo I, Barbarino M, Giannotti S, Mondanelli N, Giordano A, et al. MicroRNA mediate visfatin and resistin induction of oxidative stress in human osteoarthritic synovial fibroblasts via NF-κB pathway. Int J Mol Sci. 2019;20(20):5200. [DOI] [PMC free article] [PubMed]
- [95].Ghouri A, Conaghan PG. Prospects for therapies in osteoarthritis. Calcif Tissue Int. 2021;109(3):339–50. [DOI] [PMC free article] [PubMed]
- [96].Zhang Z, Huang C, Jiang Q, Zheng Y, Liu Y, Liu S, et al. Guidelines for the diagnosis and treatment of osteoarthritis in China (2019 edition). Ann Transl Med. 2020;8(19):1213. [DOI] [PMC free article] [PubMed]
- [97].Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2020;72(2):149–62. [DOI] [PMC free article] [PubMed]
- [98].Gorsline RT, Kaeding CC. The use of NSAIDs and nutritional supplements in athletes with osteoarthritis: Prevalence, benefits, and consequences. Clin Sports Med. 2005;24(1):71–82. [DOI] [PubMed]
- [99].Katz JN. Platelet-rich plasma for osteoarthritis and achilles tendinitis. JAMA. 2021;326(20):2012–4. [DOI] [PubMed]
- [100].van der Heijde D, Cheng-Chung Wei J, Dougados M, Mease P, Deodhar A, Maksymowych WP, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392(10163):2441–51. [DOI] [PubMed]
- [101].Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373(26):2534–48. [DOI] [PubMed]
- [102].Mease PJ, Helliwell PS, Hjuler KF, Raymond K, McInnes I. Brodalumab in psoriatic arthritis: Results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80(2):185–93. [DOI] [PMC free article] [PubMed]
- [103].Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38. [DOI] [PubMed]
- [104].Lebwohl M, Strober B, Menter A, Gordon K, Weglowska J, Puig L, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–28. [DOI] [PubMed]
- [105].Deodhar A, Blanco R, Dokoupilová E, Hall S, Kameda H, Kivitz AJ, et al. Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with secukinumab: Primary results of a randomized, placebo-controlled phase III study. Arthritis Rheumatol. 2021;73(1):110–20. [DOI] [PMC free article] [PubMed]
- [106].Luchsinger I, Knöpfel N, Theiler M, Bonnet des Claustres M, Barbieux C, Schwieger-Briel A, et al. Secukinumab therapy for netherton syndrome. JAMA Dermatol. 2020;156(8):907–11. [DOI] [PMC free article] [PubMed]
- [107].Saenz SA, Local A, Carr T, Shakya A, Koul S, Hu H, et al. Small molecule allosteric inhibitors of RORγt block Th17-dependent inflammation and associated gene expression in vivo. PLoS One. 2021;16(11):e0248034. [DOI] [PMC free article] [PubMed]
- [108].Gege C. Retinoic acid-related orphan receptor gamma t (RORγt) inverse agonists/antagonists for the treatment of inflammatory diseases - where are we presently. Expert Opin Drug Discov. 2021;16(12):1517–35. [DOI] [PubMed]
- [109].Polasek TM, Fliegert F, Betscheider I, Groeppel M, Vitt D, Kohlhof H, et al. 057 First clinical experience with IMU-935, an orally available small molecule inhibitor of IL-17. J Invest Dermatol. 2021;141(10S):S158.
- [110].Chen L, Li DQ, Zhong J, Wu XL, Chen Q, Peng H, et al. IL-17RA aptamer-mediated repression of IL-6 inhibits synovium inflammation in a murine model of osteoarthritis. Osteoarthr Cartil. 2011;19(6):711–8. [DOI] [PubMed]
- [111].Long Z, Xiang W, Li J, Yang T, Yu G. Exploring the mechanism of resveratrol in reducing the soft tissue damage of osteoarthritis based on network pharmacology and experimental pharmacology. Evid Based Complement Altern Med. 2021;2021:9931957. [DOI] [PMC free article] [PubMed]
- [112].Del Amo C, Perez-Valle A, Atilano L, Andia I. Unraveling the signaling secretome of platelet-rich plasma: Towards a better understanding of its therapeutic potential in knee osteoarthritis. J Clin Med. 2022;11(3):473. [DOI] [PMC free article] [PubMed]
- [113].Ragab GH, Halfaya FM, Ahmed OM, Abou El-Kheir W, Mahdi EA, Ali TM, et al. Platelet-rich plasma ameliorates monosodium iodoacetate-induced ankle osteoarthritis in the rat model via suppression of inflammation and oxidative stress. Evid Based Complement Altern Med. 2021;2021:6692432. [DOI] [PMC free article] [PubMed]
- [114].Riewruja K, Phakham S, Sompolpong P, Reantragoon R, Tanavalee A, Ngarmukos S, et al. Cytokine profiling and intra-articular injection of autologous platelet-rich plasma in knee osteoarthritis. Int J Mol Sci. 2022;23(2):890. [DOI] [PMC free article] [PubMed]
- [115].Gutiérrez-Román AS, Trejo-Tapia G, González-Cortazar M, Jiménez-Ferrer E, Trejo-Espino JL, Zamilpa A, et al. Anti-arthritic and anti-inflammatory effects of Baccharis conferta Kunth in a kaolin/carrageenan-induced monoarthritis model. J Ethnopharmacol. 2022;288:114996. [DOI] [PubMed]
- [116].Liu Y, Qu Y, Liu L, Zhao H, Ma H, Si M, et al. PPAR-γ agonist pioglitazone protects against IL-17 induced intervertebral disc inflammation and degeneration via suppression of NF-κB signaling pathway. Int Immunopharmacol. 2019;72:138–47. [DOI] [PubMed]
- [117].Wang G, Zhang J, Fang Y, Cao W, Xu B, Chen X. Stimulation of tolerogenic dendritic cells using dexamethasone and 1,25-dihydroxyvitamin D3 represses autologous T cell activation and chondrocyte inflammation. Exp Ther Med. 2019;17(1):679–88. [DOI] [PMC free article] [PubMed]
- [118].Gao H, Peng L, Li C, Ji Q, Li P. Salidroside alleviates cartilage degeneration through NF-κB pathway in osteoarthritis rats. Drug Des Devel Ther. 2020;14:1445–54. [DOI] [PMC free article] [PubMed]
- [119].Blair HA. Secukinumab: A review in ankylosing spondylitis. Drugs. 2019;79(4):433–43. [DOI] [PMC free article] [PubMed]
- [120].Blair HA. Secukinumab: A review in psoriatic arthritis. Drugs. 2021;81(4):483–94. [DOI] [PMC free article] [PubMed]
- [121].Craig S, Warren RB. Ixekizumab for the treatment of psoriasis: Up-to-date. Expert Opin Biol Ther. 2020;20(6):549–57. [DOI] [PubMed]
- [122].Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201(6):1605–13. [DOI] [PMC free article] [PubMed]