Abstract
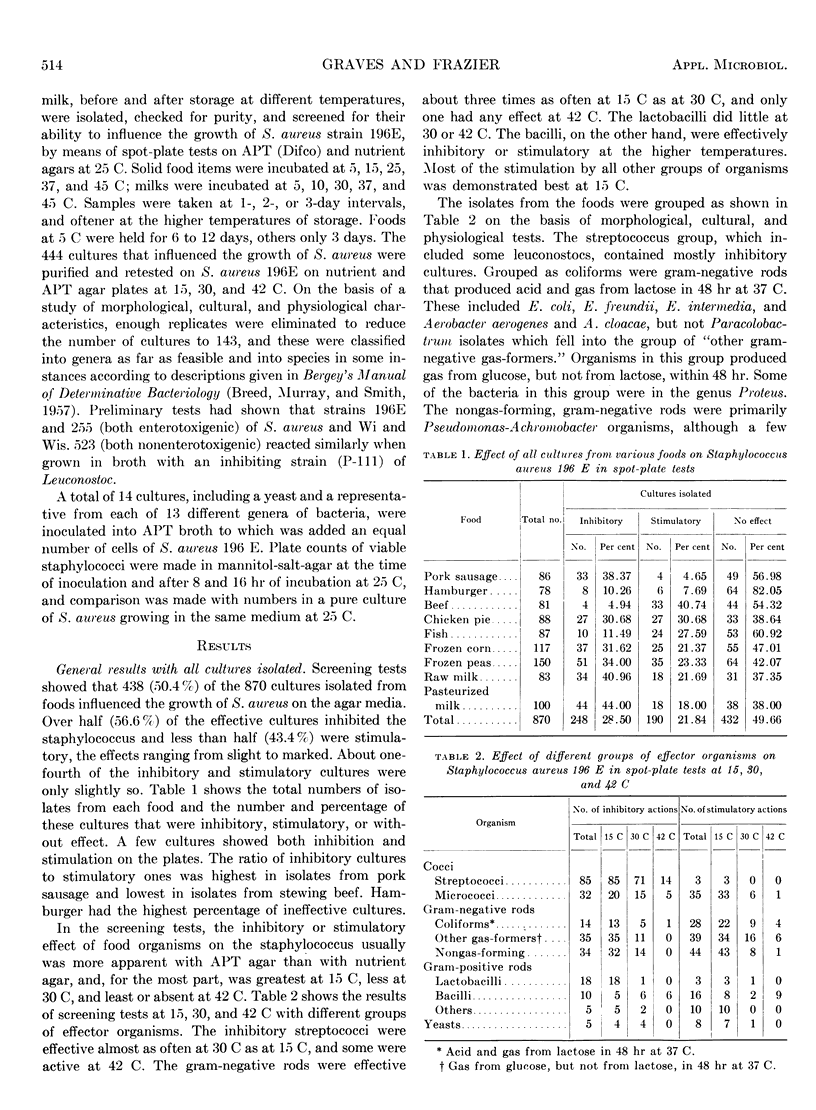
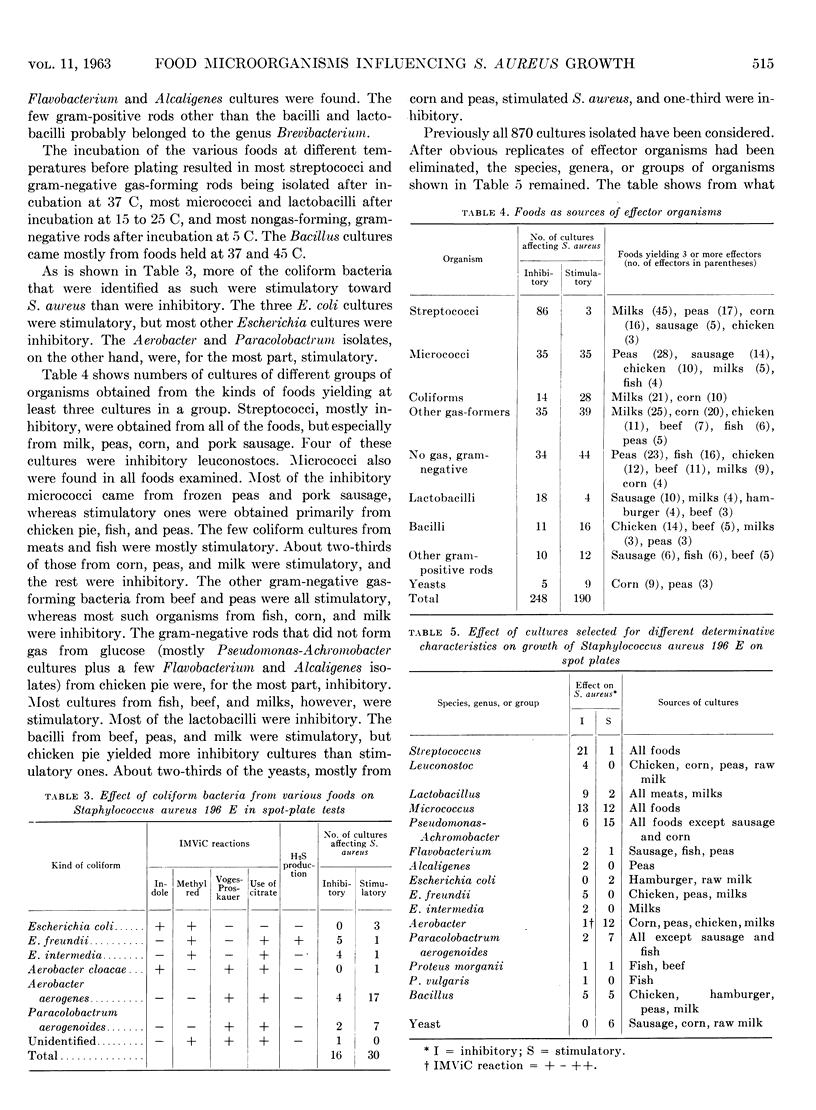
Some 870 cultures of predominating micro-organisms were isolated from market samples of hamburger, fresh pork sausage, fresh fish fillets, stewing beef, frozen chicken pot pie, frozen corn, frozen peas, and pasteurized and raw milk, before and after storage at different temperatures. The isolates were screened for their ability to influence the growth of Staphylococcus aureus strain 196E by means of spot-plate tests on APT and nutrient agars at 25 C. The 438 cultures that influenced the growth of S. aureus were retested on spot plates at 15, 30, and 42 C. After elimination of replicates, the 143 remaining cultures were classified into species, genera, or groups, and 14 different cultures were tested for their influence on the growth of S. aureus in APT broth at 25 C. Over half of the effective cultures inhibited S. aureus and less than half were stimulatory. Pork sausage had the highest proportion of inhibitory cultures, and stewing beef had the lowest. APT agar was better than nutrient agar for screening, and incubation at 15 C gave more effector organisms than at 30 and 42 C. Most of the lactic acid bacteria were inhibitory, but other groups of bacteria contained more stimulatory cultures than inhibitory ones. The three Escherichia coli cultures were stimulatory, but most other Escherichia cultures were inhibitory. Aerobacter and Paracolobactrum isolates were mostly stimulatory. Cultures of other kinds of bacteria were more or less evenly distributed between inhibitory ones and stimulatory ones. Genera containing mostly inhibitory bacteria were Streptococcus, Leuconostoc, and Lactobacillus. Inhibitory species were E. freundii and E. intermedia. Tests with S. aureus in broth indicated that all cultures inhibitory according to spot plates were inhibitory in broth, but stimulation on spot plates did not always indicate the same phenomenon in broth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EMMANOUILIDOU-ARSENI A., SOULTANI D. Antibacterial action of Candida. J Bacteriol. 1960 Jul;80:137–138. doi: 10.1128/jb.80.1.137-138.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOCKHART W. R., POWELSON D. M. Staling in bacterial cultures. J Bacteriol. 1953 Mar;65(3):293–296. doi: 10.1128/jb.65.3.293-296.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis I. M. BACTERIAL ANTAGONISM WITH SPECIAL REFERENCE TO THE EFFECT OF PSEUDOMONAS FLUORESCENS ON SPORE FORMING BACTERIA OF SOILS. J Bacteriol. 1929 Feb;17(2):89–103. doi: 10.1128/jb.17.2.89-103.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford A. E. Diplococcin, an anti-bacterial protein elaborated by certain milk streptococci. Biochem J. 1944;38(2):178–182. doi: 10.1042/bj0380178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON A. C., BLACK J. J., GUNDERSON M. F. Staphylococci in competition. I. Growth of naturally occurring mixed populations in precooked frozen foods during defrost. Appl Microbiol. 1962 Jan;10:16–22. doi: 10.1128/am.10.1.16-22.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEBURY T., GALE D., TAYLOR D. F. An approach to the study of interactive phenomena among microorganisms indigenous to man. J Bacteriol. 1954 Feb;67(2):135–152. doi: 10.1128/jb.67.2.135-152.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON R., JOHNSON A. The inhibitory action of saliva on the diphtheria bacillus: hydrogen peroxide, the inhibitory agent produced by salivary streptococci. J Infect Dis. 1951 Jan-Feb;88(1):81–85. doi: 10.1093/infdis/88.1.81. [DOI] [PubMed] [Google Scholar]
- TROLLER J. A., FRAZIER W. C. Repression of Staphylococcus aureus by food bacteria. I. Effect of environmental factors on inhibition. Appl Microbiol. 1963 Jan;11:11–14. doi: 10.1128/am.11.1.11-14.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROLLER J. A., FRAZIER W. C. Repression of Staphylococcus aureus by food bacteria. II. Causes of inhibition. Appl Microbiol. 1963 Mar;11:163–165. doi: 10.1128/am.11.2.163-165.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINCENT J. G., VEOMETT R. C., RILEY R. F. Relation of the indigenous flora of the small intestine of the rat to post-irradiation bacteremia. J Bacteriol. 1955 Jan;69(1):38–44. doi: 10.1128/jb.69.1.38-44.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne E. S. Antagonism by Aerobacter Strains. J Bacteriol. 1947 Apr;53(4):469–478. doi: 10.1128/jb.53.4.469-478.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]


