Abstract
This study proposed to demonstrate the neuroprotective effects of heat-killed Levilactobacillus brevis KU15152. Heat-killed L. brevis KU15152 showed antioxidant activity similar to that of Lacticaseibacillus rhamnosus GG, in terms of radical scavenging activity. To evaluate the neuroprotective effects, conditioned medium (CM) obtained by incubating heat-killed bacteria in intestinal cells (HT-29) was used through gut-brain axis. CM from L. brevis KU15152 protected neuroblastoma cells (SH-SY5Y) against H2O2-induced oxidative stress. Pretreatment with CM significantly alleviated the morphological changes induced by H2O2. Heat-killed L. brevis KU15152 showed an increased brain-derived neurotrophic factor (BDNF) expression in HT-29 cells. L. brevis KU15152–CM remarkably downregulated the Bax/Bcl-2 ratio, while upregulating the expression of BDNF and tyrosine hydroxylase (TH) in SH-SY5Y cells. Furthermore, L. brevis KU15152–CM reduced caspase-3 activity following H2O2 treatment. In conclusion, L. brevis KU15152 can be potentially used as food materials to avoid neurodegenerative diseases.
Keywords: Levilactobacillus brevis, gut–brain axis, oxidative stress, heat-killed probiotics, neuroprotective effect
Introduction
Oxidative stress is triggered when the balance between reactive oxygen species (ROS) generation and the capability to detoxify active intermediates is disrupted [1]. Excessive ROS generation have been associated with diverse neurodegenerative diseases involving Parkinson’s disease (PD), Huntington disease, and Alzheimer’s disease [2]. ROS, including H2O2, hydroxyl radicals, and superoxides, induce protein aggregation, mitochondrial dysfunction, and DNA damage, ultimately result in probable cell death [3, 4]. H2O2 is regarded as a representative ROS contributor that serves as a precursor of extremely reactive hydroxyl radicals [5]. The accumulation of H2O2 in neuronal cells spontaneously leads to apoptosis and neuronal damage, causing changes in function and morphology and resulting in progressive degradation of memory and cognition. Additionally, brain-derived neurotrophic factor (BDNF) and tyrosine hydroxylase (TH) are the critical neuronal biomarker in the neuroprotection. BDNF modulates the survival, differentiation, and proliferation of dopaminergic neurons and TH plays an important role in the dopamine biosynthesis pathway [6, 7].
Interactive signaling between the brain and the gut microbiota is critical for homeostasis [8]. The gut–brain axis (GBA) is an interactive neuroendocrine system comprising immunological factors, direct neurological connections, and endocrine signals [9]. GBA suggests the novel remedies to increase the disorders related to cognitive function and mental health [8]. Probiotics can be used as a potential therapeutic tool through the interlocking of biochemical and hormonal pathways related to GBA [9].
Probiotics are alive microorganisms that provide advantage to the host when treated in appropriate quantities. Probiotics have been used as potential nutritious ingredients for the treatment of diseases, such as colon inflammation and neuronal disorders [10]. Probiotics such as lactobacilli can produce bioactive molecules and antioxidants [11]. Consequently, they have the ability to decrease excessive amounts of free radicals and attenuate several disorders related to oxidative stress, such as PD [12, 13]. In addition, probiotics can generate various neuroactive compounds including gamma-aminobutyric acid, dopamine, acetylcholine, and serotonin [14]. Several PD animal models have suggested that probiotics have neuroprotective effects by decreasing dopaminergic neuronal degeneration [15, 16].
Safety issues regarding the use of live microorganisms remain [17]. In particular, probiotic bacteria can cause infections or autoinflammatory diseases when administered as living organisms [18]. To prevent these risks, many studies have employed heat-killed bacteria, purified components, and their fractions. Among these forms of bacteria, heat-inactivated lactic acid bacteria (LAB) have called as parabiotics and reported on favorable effects, along with antioxidant, anticancer, and anti-inflammatory effects [19]. In addition, the health function of heat-killed bacteria depends on probiotic strain.
Levilactobacillus brevis KU15152 was isolated from kimchi. In our previous study, we reported that L. brevis KU15152 can be utilized as a probiotic strain with anti-inflammatory effects [20]. However, the protective effects of L. brevis KU15152 in neuronal cells against oxidative stress have not yet been described. In terms of neuroprotection related to apoptotic pathway, neuronal cells may show the following indication: increase of cell viability, alleviation of cell morphology, upregulation of BDNF and TH, downregulation of the ratio of Bax/Bcl-2 and inactivation of caspase-3 [21, 22]. Hence, this study was examined the neuroprotective effects of heat-killed L. brevis KU15152 in H2O2-induced SH-SY5Y cells for functional ingredients.
Materials and Methods
Bacterial Strains and Sample Preparations
Levilactobacillus brevis KU15152, KU15159, and KU15176 were isolated from fermented foods. Lacticaseibacillus rhamnosus GG (LGG) was taken from the Korean Collection for Type Cultures (KCTC, Korea) and treated as reference. LAB strains were cultured in MRS broth (Difco Laboratories, USA) at 37°C for 18 h. The strains in MRS broth were mixed with 20% (v/v) glycerol as 1:1 ratio and stored at –80°C until use. Each cultured strain was centrifuged at 14,240 ×g at 4°C for 5 min. The strains were rinsed twice and suspended in PBS (Hyclone, USA). Live bacterial cells were killed at 80°C for 30 min.
Culture Conditions
HT-29 (human colon adenocarcinoma; KCLB 30038) and SH-SY5Y (human neuroblastoma; ATCC CRL-2266) cells were cultured in RPMI 1640 (Hyclone) and DMEM (Hyclone). Each medium included fetal bovine serum (FBS; 10% (v/v); Hyclone) and penicillin–streptomycin (1% (v/v); Hyclone). Each cell was incubated at 37°C in a humidified atmosphere containing 5% CO2.
Antioxidant Activity of Heat-Killed LAB Strains
DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity was measured according to the method described by Song et al. [23], with some modifications. Five hundred microliters of 0.1 mM DPPH solution were dissolved in ethyl alcohol and mixed with the same amount of a heat-killed LAB suspension (1 × 109 CFU/ml). After incubation at 25°C for 30 min, the mixture was centrifuged at 14,240 ×g for 1 min. The absorbance of the supernatant was measured at 517 nm, and the radical scavenging activity was determined using Eq. (1).
| (1) |
where Acontrol and Asample represent the absorbance of the control treated with PBS and the sample treated with heat-killed LAB, respectively.
ABTS (2,2´-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) radical scavenging activity was determined as per the method depicted by Jang et al. [24], with some modifications. Firstly, 14 mM ABTS and 5 mM potassium persulfate were dissolved in distilled water and mixed at a 1:1 ratio. ABTS solution was incubated at 25°C for 16–18 h in the dark. The reacted ABTS solution was weakened using distilled water up to the final absorbance reached 0.7 ± 0.5 at 734 nm. Eight hundred microliters of diluted ABTS solution were blended with 200 μl of heat-killed LAB suspension (1 × 109 CFU/ml), then incubated at 25°C for 15 min. Following centrifugation at 14,240 ×g for 1 min, the absorbance of the supernatant was evaluated at 734 nm. The ABTS radical scavenging activity was calculated using Eq. (1).
Manufacturing of Conditioned Medium (CM) from HT-29 Cells
CM was prepared followed the method of Cheon et al. [25]. HT-29 cells were seeded in 6-well culture plates at a density of 5 × 105 cells/ml. After incubation for 5 d until monolayer formation, heat-killed LAB strains at 1 × 109 CFU/ml or PBS (for control) were treated to the cells. After incubation for 24 h, the supernatant was gathered by centrifugation at 14,000 ×g for 10 min and filtered through a 0.45 μm syringe filter. The CM was stored at –80°C until use.
Cytotoxicity Measurement
MTT assay was conducted to evaluate cytotoxicity of CM using the method by Choi et al. [26]. SH-SY5Y cells were incubated in 96-well culture plates (1 × 105 cells/well) for 24 h. Then, LAB–CM was added. Following incubation for 24 h, the medium was withdrawn and 100 μl of 0.5 mg/ml MTT solution was added. Later 4 h, the MTT solution was removed and DMSO was added to each well. Absorbance was evaluated at 570 nm, and cell viability was determined using Eq. (2).
| (2) |
where Asample and Acontrol represent the absorbance of the cells treated with LAB–CM and the control treated with control–CM, respectively.
H2O2 (Sigma-Aldrich, USA) was used to induce cytotoxicity on SH-SY5Y cells. SH-SY5Y cells were seeded into 96-well culture plates at 1 × 105 cells/well. After incubation for 24 h, the cells were treated with CM for 4 h and then exposed to H2O2 (150 μM) for 20 h. Then, the medium was removed and the cells were treated with MTT solution for 4 h. After removing the solution, DMSO was added to each well. Absorbance was evaluated at 570 nm, and cell viability was calculated using Eq. (2).
Protective effects were also confirmed by morphological observations. SH-SY5Y cells were plated at 1 × 105 cells/well in 96-well culture plates. After treatment with CM and H2O2, the images of the cells were examined using a Nikon Eclipse Ti2-U fluorescence microscope (Nikon Co., Ltd., Japan) and a DS-Ri2 digital camera (Nikon Co., Ltd.).
Assessment of Relative Gene Expression Using RT-PCR
Relative gene expression was measured by Choi et al. [26]. To measure the expression of BDNF in intestinal cells, HT-29 cells were inoculated in 6-well culture plates at a density of 1 × 106 cells/well and incubated for 5 d. The HT-29 cells were incubated with heat-killed LAB (1 × 108 CFU/well) for 24 h.
To detect the expression of Bax, Bcl-2, BDNF, and TH in neuroblastoma cells, SH-SY5Y cells were inoculated in 6-well culture plates at 1 × 106 cells/well and incubated for 24 h. The SH-SY5Y cells pretreated with CM for 4 h, followed by treatment with 150 μM H2O2 for 3 h.
Total RNA was extracted using an RNeasy Mini Kit (Qiagen, Germany). cDNA was synthesized from isolated RNA using a cDNA synthesis kit (Thermo Fisher Scientific, USA). Gene expression was detected using SYBR Green PCR Master mix (Thermo Fisher Scientific) with real-time PCR (QuantStudio 1 Real-Time PCR, Thermo Fisher Scientific). RT-PCR was conducted as follows: initial denaturation at 95°C for 10 min, followed by annealing and extension as 40 cycles at 95°C for 20 s, 60°C for 20 s, and 72°C for 30 s. GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was used as a reference gene. The results were analyzed using the 2-ΔΔCt method. The PCR primers are listed in Table 1.
Table 1.
Primer sequences used in RT-PCR.
| Primer1 | Primer sequence (5’-3’) | Reference | |
|---|---|---|---|
| BDNF | Sense | ATGACCATCCTTTTCCTTACT | [27] |
| Antisenese | GCCACCTTGTCCTCGGAT | ||
| TH | Sense | GAGGAGAAGGAGGGGAAG | [27] |
| Antisenese | ACTCAAACACCTTCACAGCT | ||
| Bax | Sense | GTGGTTGCCCTCTTCTACTTTGC | [25] |
| Antisenese | GAGGACTCCAGCCACAAAGATG | ||
| Bcl-2 | Sense | CGGCTGAAGTCTCCATTAGC | [25] |
| Antisenese | CCAGGGAAGTTCTGGTGTGT | ||
| GAPDH | Sense | GAGTCAACGGATTTGGTCGT | [25] |
| Antisenese | GACAAGCTTCCCGTTCTCAG |
1BDNF, brain-derived neurotrophic factor; TH, tyrosine hydroxylase; Bax, Bcl-2 associated X protein; Bcl-2, B-cell lymphoma-2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Caspase-3 Activity Assay
The activity of caspase-3 was assessed using a caspase-3/cpp32 colorimetric assay kit (BioVision, USA). SH-SY5Y cells were seeded in 6-well culture plates at a density of 1 × 106 cells/well. After 24 h of incubation, the cells were pretreated with CM for 4 h and then with H2O2 for 6 h. Following treatment, the cells were harvested and lysed with a cell lysis buffer. The cell lysates were centrifugated at 10,000 ×g for 1 min at 4°C. Then, 50 μl of cell lysis buffer containing 50 μg of protein was incubated with the same amount of 2 × reaction buffer comprising 10 mM DTT and 5 μl of 4 mM DEVD-pNA substrate at 37°C for 2 h. Absorbance was measured at 405 nm, and caspase-3 activity was decided by comparison with the control group.
Statistical Analysis
All experiments were repeated in triplicate and are presented as the mean ± standard deviation. One-way analysis of variance and Duncan’s multiple-range test were used to compare multiple groups. The results were considered statistically significant at p < 0.05, and all statistical analyses were conducted using SPSS software (IBM, USA).
Results
Antioxidant Activities of Heat-Killed LAB Strains
Table 2 shows the antioxidant activities of the LAB strains. L. brevis KU15152 demonstrated the highest DPPH radical scavenging activity (14.25%) among the four heat-killed LAB strains. LGG indicated an activity close to that of L. brevis KU15152 (12.60%). However, those of L. brevis KU15159 and L. brevis KU15176 were 9.29% and 7.66%, respectively.
Table 2.
Antioxidant activities of the Lactobacillus strains.
| Antioxidant activity | Heat-killed Lactobacillus strains | |||
|---|---|---|---|---|
| LGG1 | L. brevis KU15152 | L. brevis KU15159 | L. brevis KU15176 | |
| DPPH radical scavenging activity (%) | 12.60 ± 1.61a | 14.25 ± 1.86a | 9.29 ± 1.24b | 7.66 ± 2.49b |
| ABTS radical scavenging activity (%) | 50.42 ± 1.54b | 52.85 ± 3.15a | 52.68 ± 1.27a | 46.20 ± 1.42c |
1LGG, L. rhamnosus GG. a–cDifferent letters in the same row indicate significant differences (p < 0.05).
L. brevis KU15152 (52.85%) and L. brevis KU15159 (52.68%) showed the highest ABTS radical scavenging activities. LGG (50.42%) and L. brevis KU15176 (46.20%) showed lower values. Song et al. [28] demonstrated that the ABTS radical scavenging activities of heat-killed LGG and Lactobacillus brevis KCCM 12203P were 37.10%and 22.07%, respectively. Heat-killed Lactobacillus brevis B13-2 showed 47.43% ABTS radical-scavenging activity [23]. It is presumed that strains with antioxidant capabilities could have neuroprotective effects.
Effect of Heat-Killed LAB Strains on H2O2-Stimulated Stress in SH-SY5Y Cells
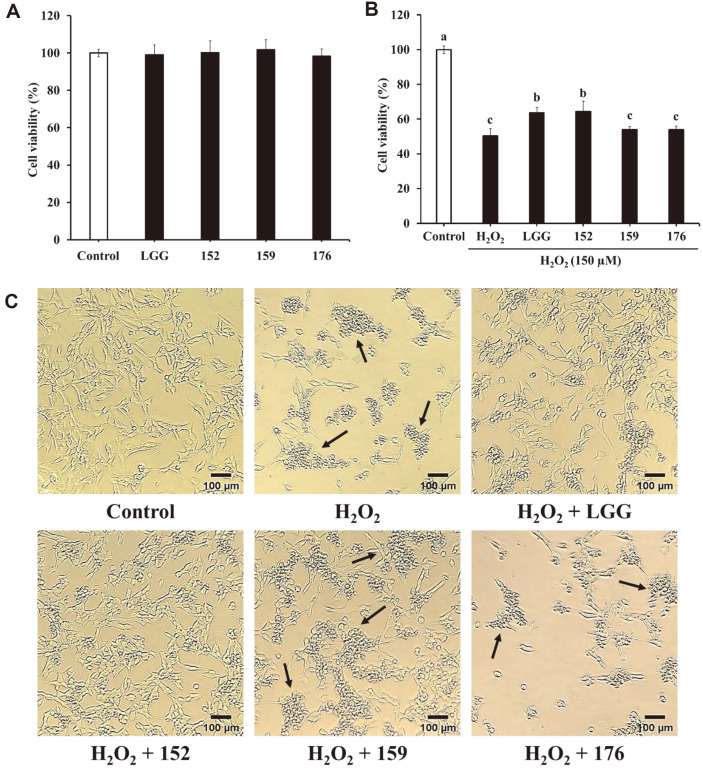
The cytotoxic effects of CM from heat-killed bacteria were assessed using an MTT assay. CM was found to be non-toxic to SH-SY5Y cells (Fig. 1A). As illustrated in Fig. 1B, the cell viability of samples treated with H2O2 only was 50.26%. In contrast, L. brevis KU15152–CM elevated the cell viability by 64.45%. The protective effect of L. brevis KU15152–CM was similar to that of heat-killed LGG (63.64%). L. brevis KU15159–CM (53.90%) and L. brevis KU15176–CM (53.93%) showed similar cell viability as H2O2-treated cells. Lee et al. [29] demonstrated that the CM of Leuconostoc mesenteroides H40 attenuated cell death against H2O2 in SH-SY5Y cells via the MTT assay. These results show that L. brevis KU15152 applies a neuroprotective effect against H2O2-induced toxicity.
Fig. 1. Neuroprotective effects of heat-killed LAB–CM on H2O2-induced toxicity in SH-SY5Y cells.
(A) Effect of LAB–CM on cell viability in SH-SY5Y cells. (B) Effect of LAB–CM on cell viability of H2O2-treated SH-SY5Y cells. (C) Morphological changes in SH-SY5Y cells investigated using microscopy (magnification: 40×). Arrows indicate aggregation and shrinkage of SH-SY5Y cells. LGG, CM of heat-killed L. rhamnosus GG; 152, CM of heat-killed L. brevis KU15152; 159, CM of heat-killed L. brevis KU15159; 176, CM of heat-killed L. brevis KU15176. Data are presented as mean ± standard deviation of triplicate experiments. Different letters on the error bars represent significant differences (p < 0.05).
Apoptotic cells exhibit morphological features, such as degradation of chromosomal DNA, shrinkage of cells, and DNA condensation [30]. The protective effects of CM were also compared using morphological observations (Fig. 1C). H2O2-treated SH-SY5Y cells showed aggregation and shrinkage of cell bodies. In contrast, pretreatment with heat-killed L. brevis KU15152–CM remarkably attenuated the cell damage. These observations implied that CM from heat-killed L. brevis KU15152 protected SH-SY5Y cells from H2O2-mediated toxicity.
Effect of Heat-Killed LAB on mRNA Expression in HT-29 Cells
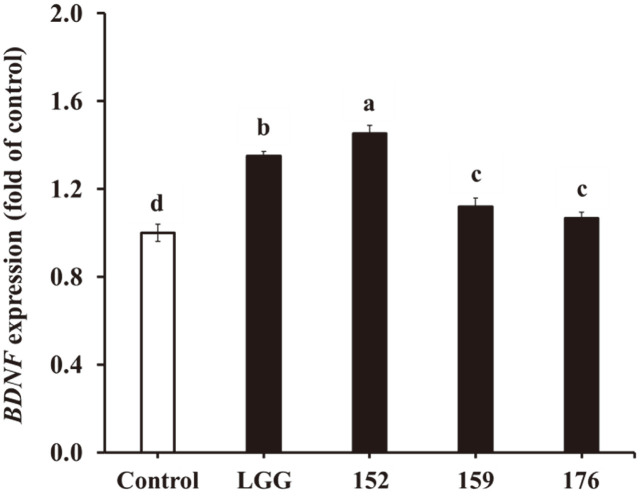
Cells treated with heat-killed LGG and L. brevis KU15152 indicated increased BDNF expression in HT-29 cells by 1.35- and 1.45-fold, respectively (Fig. 2). However, L. brevis KU15159 and L. brevis KU15176 showed relatively low BDNF expression, by 1.12- and 1.07-fold, respectively. Lactobacillus buchneri KU200793 increases BDNF expression in HT-29 cells [25]. L. brevis KU15152 was, thus, expected to have neuroprotective effects.
Fig. 2. Effects of heat-killed LAB on BDNF expression in HT-29 cells, elucidated using RT-PCR.
LGG, heatkilled L. rhamnosus GG; 152, heat-killed L. brevis KU15152; 159, heat-killed L. brevis KU15159; 176, heat-killed L. brevis KU15176. Data are presented as mean ± standard deviation of triplicate experiments. Different letters on the error bars represent significant differences (p < 0.05).
Effect of Heat-Killed LAB–CM on mRNA Expression in SH-SY5Y Cells
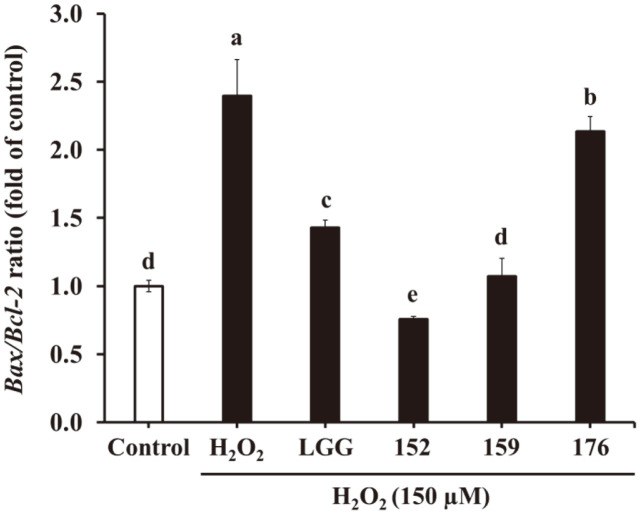
As illustrated in Fig. 3, the Bax/Bcl-2 ratio was considerably increased in the H2O2-treated cells by 2.40-fold compared to that in the H2O2-untreated cells. However, L. brevis KU15152–CM notably lowered this ratio by 0.76-fold. LGG, L. brevis KU15159, and L. brevis KU15176 showed 1.43-, 1.07-, and 2.14-fold increases, respectively. Ruminococcus albus CM decreases the Bax/Bcl-2 ratio in H2O2-induced SH-SY5Y cells [31]. Thus, L. brevis KU15152 may alleviate mRNA expression related to apoptosis.
Fig. 3. Effects of heat-killed LAB–CM on Bax/Bcl-2 ratio related to apoptosis in SH-SY5Y cells by RT-PCR.
LGG, CM of heat-killed L. rhamnosus GG; 152, CM of heat-killed L. brevis KU15152; 159, CM of heat-killed L. brevis KU15159; 176, CM of heat-killed L. brevis KU15176. Data are presented as mean ± standard deviation of triplicate experiments. Different letters on the error bars represent significant differences (p < 0.05).
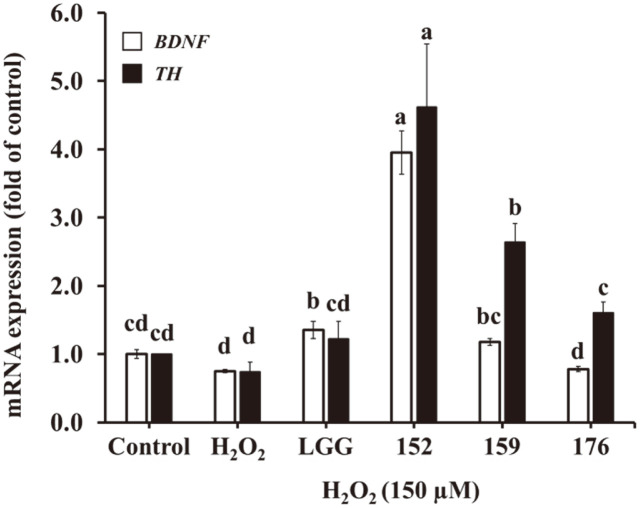
Fig. 4 shows the upregulation of BDNF and TH expression after treatment with CM. Cells treated with only H2O2 showed downregulated expression of BDNF and TH by 0.75- and 0.74-fold, respectively. L. brevis KU15152–CM dramatically increased BDNF and TH expression by 3.96- and 4.62-fold, respectively. The CM of LGG, L. brevis KU15159, and L. brevis KU15176 showed only 1.36-, 1.18-, and 0.78-fold BDNF expression, respectively. In addition, the three strains presented TH expression by 1.22-, 2.64-, and 1.61-fold, respectively. Jang et al. [32] suggested that the Bifidobacterium adolescentis NK98 and Lactobacillus reuteri NK33 enhanced hippocampal BDNF expression in a mouse model. In addition, L. plantarum PS128 ingestion demonstrated neuroprotective effects on dopaminergic neurons in a mouse model by restoring the expression of TH under MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) toxicity [33]. Therefore, L. brevis KU15152 protects SH-SY5Y cells against oxidative stress by enhancing the expression of BDNF and TH.
Fig. 4. Effects of heat-killed LAB–CM on BDNF and TH expression in SH-SY5Y cells using RT-PCR.
LGG, CM of heat-killed L. rhamnosus GG; 152, CM of heat-killed L. brevis KU15152; 159, CM of heat-killed L. brevis KU15159; 176, CM of heat-killed L. brevis KU15176. Data are presented as mean ± standard deviation of triplicate experiments. Different letters on the error bars represent significant differences (p < 0.05).
Effect of Heat-Killed LAB–CM on Caspase-3 Activity
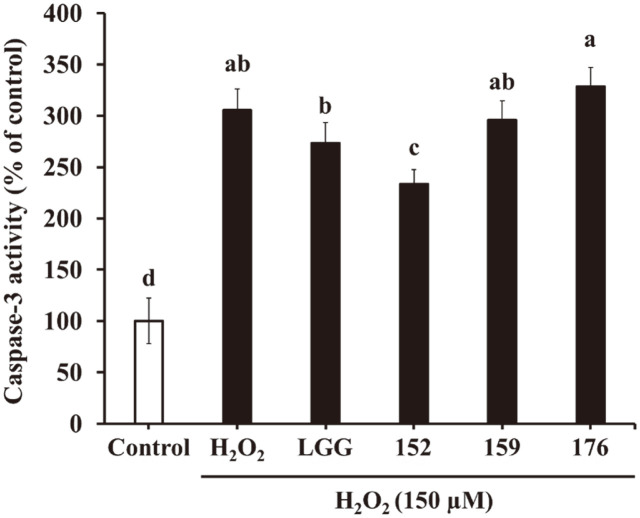
Fig. 5 shows the effects of caspase-3 after treatment with CM in SH-SY5Y cells. Treatment with H2O2 alone showed caspase-3 activity by 305.53% compared to that in untreated cells. However, the CM of L. brevis KU15152 significantly attenuated the enzyme activity by 233.33%. In contrast, LGG, L. brevis KU15159, and L. brevis KU15176 showed an increase in caspase-3 activity of 273.11, 295.57, and 328.26%, respectively. L. plantarum DP189 decreased caspase-3 activity in the substantia nigra of MPTP-induced PD mice [34]. These results confirm that L. brevis KU15152 inhibits H2O2-induced apoptosis in SH-SY5Y cells.
Fig. 5. Effects of heat-killed LAB-CM on caspase-3 activity in the SH-SY5Y cells treated with H2O2 for 6 h.
LGG, CM of heat-killed L. rhamnosus GG; 152, CM of heat-killed L. brevis KU15152; 159, CM of heat-killed L. brevis KU15159; 176, CM of heat-killed L. brevis KU15176. Data are presented as mean ± standard deviation of triplicate experiments. Different letters on the error bars represent significant differences (p < 0.05).
Discussion
In this study, L. brevis KU15152 was used as heat-inactivated form and showed antioxidant capabilities. To measure the neuroprotective effects of heat-killed probiotic strain, the CM was used according to the GBA. The CM of heat-killed L. brevis KU15152 possibly protects SH-SY5Y cells against cytotoxicity. Additionally, treatment with L. brevis KU15152–CM upregulated the expression of neuroprotection-related genes and downregulated the expression of apoptosis-related genes. The CM of heat-killed L. brevis KU15152 also suppressed apoptosis-related enzyme activity. After the heat-inactivation of L. brevis KU15152, the various microbiological components such as exopolysaccharides, peptidoglycans, lipoteichoic acid, and metabolites were composited. It is assumed that the strain showed the neuroprotective effects because of the components [17].
Antioxidant properties are considered to contribute positively to neuroprotective effects [35]. The DPPH assay is subject to reducing the DPPH radical (purple color) to 1,1-diphenyl-2-picryl hydrazine (yellow color), and the ABTS assay is based on reducing the blue/green ABTS radical by antioxidants [36]. LGG, used as a reference strain, possesses strong antioxidant capacity [37]. In DPPH and ABTS assays, heat-killed L. brevis KU15152 showed antioxidant properties comparable to those of heat-killed LGG. These antioxidant activities were expected to contribute to the neuroprotective effects of heat-killed L. brevis KU15152.
Apoptosis, characterized by DNA fragmentation, membrane blebbing, nuclear condensation, and cell shrinkage, is a process of cell death [38]. H2O2 triggers apoptosis in neuronal cells and has generally been used to induce intracellular ROS generation and cell death [39]. It is expected that the neuroprotective effect of the heat-killed probiotic strain was attributed to the cell wall components including EPS as neurotransmitters [17]. The protective effects of LAB were assessed using MTT assay and morphological observations. Pretreatment with L. brevis KU15152–CM increased the suppressed cell viability that was induced by treatment with H2O2. The reduction in cell damage was confirmed by morphological observations.
Bcl-2, an anti-apoptotic Bcl-2 family member, exists in the outer mitochondrial membrane and regulates the release of cytochrome C [40]. Bax (pro-apoptotic factor), exist in the cytosol, which promotes permeabilization of the mitochondrial membrane, and accelerates apoptotic cell death [41]. The Bax/Bcl-2 ratio is allowed to a better indicator of apoptosis than measuring the levels of Bax or Bcl-2, respectively. This ratio indicates the balance between anti- and pro-apoptotic proteins of the Bcl-2 family [42]. Treatment with H2O2 alone upregulated the Bax/Bcl-2 ratio, whereas pretreated L. brevis KU15152–CM downregulated the ratio in SH-SY5Y cells. Therefore, L. brevis KU15152 can protect neuronal cells by controlling the expression of apoptosis-related genes.
BDNF is a crucial neurotrophic factor for the neurogenesis process, and its decreased expression has been considered a damaged motor ability in patients with PD [38]. BDNF plays a significant role in hippocampal long-term potentiation, memory formation, and plasticity [6]. TH is a rate-limiting enzyme in dopamine biosynthesis [38]. TH, which catalyzes the hydroxylation of tyrosine towards L-DOPA, has been considered a molecular agent for determining the level of dopamine [7]. In this study, BDNF expression was confirmed using RT-PCR in HT-29 cells. Among the four heat-killed LAB, L. brevis KU15152-treated cells exhibited the highest BDNF expression in HT-29 cells. According to the GBA, the strain increasing BDNF expression in intestinal cells could have neuroprotective effect. Moreover, the downregulated expression of BDNF and TH was markedly ameliorated by pretreatment with L. brevis KU15152–CM in the H2O2-treated SH-SY5Y cells.
Caspases regulate intracellular apoptotic signals followed by cellular oxidative stress [43]. Apoptosis induced by H2O2 includes caspase-3 activation, which occurs via the apoptotic caspase pathway [44]. H2O2-induced cell damage causes release of mitochondrial cytochrome C. The cytochrome C stimulates caspase-9 and leads to the activation of caspase-3, which in turn occurs DNA damage and cell death [45]. In the present study, caspase-3 activity was amplified after the treatment of H2O2 in SH-SY5Y cells and heat-killed L. brevis KU15152–CM suppressed caspase-3 activity. Despite the treatment of H2O2, L. brevis KU15152–CM decreased the caspase-3 activity by 72.20%. Thus, it can be considered that heat-killed L. brevis KU15152 effectively attenuate apoptosis induced by oxidative stress.
L. brevis KU15152 was isolated from a fermented Korean food. Heat-killed L. brevis KU15152 exhibited antioxidant activity similar to that of LGG. CM prepared from heat-killed L. brevis KU15152 increased cell viability and alleviated morphological damage in SH-SY5Y cells. In addition, heat-killed L. brevis KU15152 demonstrated high BDNF expression in HT-29 cells, and the highest expression of BDNF and TH was observed after treatment with L. brevis KU15152–CM in SH-SY5Y cells. Heat-killed L. brevis KU15152–CM also reduced the expression of apoptosis-related genes and caspase-3 activity. These neuroprotective effects were expected to attribute to the cell components from parabiotic L. brevis KU15152 according to the GBA. Consequently, heat-killed L. brevis KU15152 could be used as food ingredients to prevent neurodegenerative diseases. However, in vivo experiments are required for further study.
Acknowledgments
This work was supported by the Konkuk University Researcher Fund in 2022 and the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (IPET) through the High Value-added Food Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (#321035-5).
Footnotes
Conflict of Interest
The authors have no financial conflicts of interest to declare.
References
- 1.Tian W, Zhao J, Lee JH, Akanda MR, Cho JH, Kim SK, et al. Neuroprotective effects of Cornus officinalis on stress-induced hippocampal deficits in rats and H2O2-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Antioxidants. 2019;9:27. doi: 10.3390/antiox9010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behl C. Alzheimer's disease and oxidative stress: implications for novel therapeutic approaches. Prog. Neurobiol. 1999;57:301–323. doi: 10.1016/S0301-0082(98)00055-0. [DOI] [PubMed] [Google Scholar]
- 3.Kim KJ, Jung YS, You DM, Lee SH, Lee G, Kwon KB, et al. Neuroprotective effects of ethanolic extract from dry Rhodiola rosea L.rhizomes. Food Sci. Biotechnol. 2021;30:287–297. doi: 10.1007/s10068-020-00868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattson MP, Duan W, Chan SL, Cheng A, Haughey N, Gary DS, et al. Neuroprotective and neurorestorative signal transduction mechanisms in brain aging: modification by genes, diet and behavior. Neurobiol. Aging. 2002;23:695–705. doi: 10.1016/S0197-4580(02)00025-8. [DOI] [PubMed] [Google Scholar]
- 5.Suematsu N, Hosoda M, Fujimori K. Protective effects of quercetin against hydrogen peroxide-induced apoptosis in human neuronal SH-SY5Y cells. Neurosci. Lett. 2011;504:223–227. doi: 10.1016/j.neulet.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- 7.Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011;508:1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cryan JF, O'Riordan KJ, Cowan CS, Sandhu KV, Bastiaanssen TF, Boehme M, et al. The microbiota-gut-brain axis. Physiol. Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 9.Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell. Mol. Life. Sci. 2017;74:3769–3787. doi: 10.1007/s00018-017-2550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KT, Yang SJ, Paik HD. Probiotic properties of novel probiotic Levilactobacillus brevis KU15147 isolated from radish kimchi and its antioxidant and immune-enhancing activities. Food Sci. Biotechnol. 2021;30:257–265. doi: 10.1007/s10068-020-00853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazerani P. Probiotics for Parkinson's disease. Int. J. Mol. Sci. 2019;20:4121. doi: 10.3390/ijms20174121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y, Yoon Y, Choi KH. Probiotics-mediated bioconversion and periodontitis. Food Sci. Anim. Resour. 2021;41:905–922. doi: 10.5851/kosfa.2021.e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parashar A, Udayabanu M. Gut microbiota: Implications in Parkinson's disease. Parkinsonism Relat. Disord. 2017;38:1–7. doi: 10.1016/j.parkreldis.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nataraj BH, Shivanna SK, Rao P, Nagpal R, Behare PV. Evolutionary concepts in the functional biotics arena: a minireview. Food Sci. Biotechnol. 2021;30:487–496. doi: 10.1007/s10068-020-00818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastav S, Neupane S, Bhurtel S, Katila N, Maharjan S, Choi H, et al. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J. Nutr. Biochem. 2019;69:73–86. doi: 10.1016/j.jnutbio.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Tan AH, Hor JW, Chong CW, Lim SY. Probiotics for Parkinson's disease: current evidence and future directions. JGH Open. 2021;5:414–419. doi: 10.1002/jgh3.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teame T, Wang A, Xie M, Zhang Z, Yang Y, Ding Q, et al. Paraprobiotics and postbiotics of probiotic lactobacilli, their positive effects on the host and action mechanisms: A review. Front. Nutr. 2020;7:570344. doi: 10.3389/fnut.2020.570344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner RD, Pierson C, Warner T, Dohnalek M, Hilty M, Balish E. Probiotic effects of feeding heat-killed Lactobacillus acidophilus and Lactobacillus casei to Candida albicans-colonized immunodeficient mice. J. Food Prot. 2000;63:638–644. doi: 10.4315/0362-028X-63.5.638. [DOI] [PubMed] [Google Scholar]
- 19.Piqué N, Berlanga M, Miñana-Galbis D. Health benefits of heat-killed (tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019;20:2534. doi: 10.3390/ijms20102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim WJ, Hyun JH, Lee NK, Paik HD. Protective effects of a novel Lactobacillus brevis strain with probiotic characteristics against Staphylococcus aureus lipoteichoic acid-induced intestinal inflammatory response. J. Microbiol. Biotechnol. 2022;32:205–211. doi: 10.4014/jmb.2110.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagci EZ, Vodovotz Y, Billiar TR, Ermentrout GB, Bahar I. Bistability in apoptosis: roles of bax, bcl-2, and mitochondrial permeability transition pores. Biophys. J. 2006;90:1546–1559. doi: 10.1529/biophysj.105.068122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang ZH, Ji Y, Shan W, Zeng B, Raksadawan N, Pastores GM. Therapeutic effects of astrocytes expressing both tyrosine hydroxylase and brain-derived neurotrophic factor on a rat model of Parkinson's disease. Neurosci. 2002;113:629–640. doi: 10.1016/S0306-4522(02)00204-X. [DOI] [PubMed] [Google Scholar]
- 23.Song MW, Chung Y, Kim KT, Hong WS, Chang HJ, Paik HD. Probiotic characteristics of Lactobacillus brevis B13-2 isolated from kimchi and investigation of antioxidant and immune-modulating abilities of its heat-killed cells. LWT-Food Sci. Technol. 2020;128:109452. doi: 10.1016/j.lwt.2020.109452. [DOI] [Google Scholar]
- 24.Jang HJ, Kim JH, Lee HS, Paik HD. Physicochemical analysis of non-fermented probiotic milk with probiotic Lactobacillus plantarum Ln1 isolated from Korea traditional fermented food. Food Sci. Biotechnol. 2022;31:731–737. doi: 10.1007/s10068-022-01076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheon MJ, Lim SM, Lee NK, Paik HD. Probiotic properties and neuroprotective effects of Lactobacillus buchneri KU200793 isolated from Korean fermented foods. Int. J. Mol. Sci. 2020;21:1227. doi: 10.3390/ijms21041227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi GH, Bock HJ, Lee NK, Paik HD. Soy yogurt using Lactobacillus plantarum 200655 and fructooligosaccharides: neuroprotective effects against oxidative stress. J. Food Sci. Technol. 2022;59:4870–4879. doi: 10.1007/s13197-022-05575-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nirmaladevi D, Venkataramana M, Chandranayaka S, Ramesha A, Jameel NM, Srinivas C. Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cell. Mol. Neurobiol. 2014;34:973–985. doi: 10.1007/s10571-014-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song MW, Jang HJ, Kim KT, Paik HD. Probiotic and antioxidant properties of novel Lactobacillus brevis KCCM 12203P isolated from kimchi and evaluation of immune-stimulating activities of its heat-killed cells in RAW 264.7 cells. J. Microbiol. Biotechnol. 2019;29:1894–1903. doi: 10.4014/jmb.1907.07081. [DOI] [PubMed] [Google Scholar]
- 29.Lee NK, Lim SM, Cheon MJ, Paik HD. Physicochemical analysis of yogurt produced by Leuconostoc mesenteroides H40 and its effects on oxidative stress in neuronal cells. Food Sci. Anim. Resour. 2021;41:261. doi: 10.5851/kosfa.2020.e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 31.Park J, Lee J, Yeom Z, Heo D, Lim YH. Neuroprotective effect of Ruminococcus albus on oxidatively stressed SH-SY5Y cells and animals. Sci. Rep. 2017;7:14520. doi: 10.1038/s41598-017-15163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang HM, Lee KE, Kim DH. The preventive and curative effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on immobilization stress-induced anxiety/depression and colitis in mice. Nutrients. 2019;11:819. doi: 10.3390/nu11040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao JF, Cheng YF, You ST, Kuo WC, Huang CW, Chiou JJ, et al. Lactobacillus plantarum PS128 alleviates neurodegenerative progression in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse models of Parkinson's disease. Brain. Behav. Immun. 2020;90:26–46. doi: 10.1016/j.bbi.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Li S, Jiang Y, Zhao Z, Shen Y, Zhang J, et al. Neuroprotective effect of Lactobacillus plantarum DP189 on MPTPinduced Parkinson's disease model mice. J. Funct. Foods. 2021;85:104635. doi: 10.1016/j.jff.2021.104635. [DOI] [Google Scholar]
- 35.Behl C, Moosmann B. Antioxidant neuroprotection in Alzheimer's disease as preventive and therapeutic approach. Free Radic. Biol. Med. 2002;33:182–191. doi: 10.1016/S0891-5849(02)00883-3. [DOI] [PubMed] [Google Scholar]
- 36.Floegel A, Kim DO, Chung SJ, Koo SI, Chun OK. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011;24:1043–1048. doi: 10.1016/j.jfca.2011.01.008. [DOI] [Google Scholar]
- 37.Nie P, Wang M, Zhao Y, Liu S, Chen L, Xu H. Protective effect of Lactobacillus rhamnosus GG on TiO2 nanoparticles-induced oxidative stress damage in the liver of young rats. Nanomaterials. 2021;11:803. doi: 10.3390/nano11030803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi BS, Sapkota K, Kim S, Lee HJ, Choi HS, Kim SJ. Antioxidant activity and protective effects of Tripterygium regelii extract on hydrogen peroxide-induced injury in human dopaminergic cells, SH-SY5Y. Neurochem. Res. 2010;35:1269–1280. doi: 10.1007/s11064-010-0185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi DJ, Kim SL, Choi JW, Park YI. Neuroprotective effects of corn silk maysin via inhibition of H2O2-induced apoptotic cell death in SK-N-MC cells. Life Sci. 2014;109:57–64. doi: 10.1016/j.lfs.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Sun FL, Zhang L, Zhang RY, Li L. Tetrahydroxystilbene glucoside protects human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity. Eur. J. Pharmacol. 2011;660:283–290. doi: 10.1016/j.ejphar.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 41.Mehri S, Abnous K, Mousavi SH, Shariaty VM, Hosseinzadeh H. Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell. Mol. Neurobiol. 2012;32:227–235. doi: 10.1007/s10571-011-9752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao DL, Zou LB, Lin S, Shi JG, Zhu HB. 6,7-Di-O-glucopyranosyl-esculetin protects SH-SY5Y cells from dopamine-induced cytotoxicity. Eur. J. Pharmacol. 2008;580:329–338. doi: 10.1016/j.ejphar.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 43.Bavari M, Tabandeh MR, Najafzadeh Varzi H, Bahramzadeh S. Neuroprotective, antiapoptotic and antioxidant effects of Lcarnitine against caffeine-induced neurotoxicity in SH-SY5Y neuroblastoma cell line. Drug Chem. Toxicol. 2016;39:157–166. doi: 10.3109/01480545.2015.1063062. [DOI] [PubMed] [Google Scholar]
- 44.Ju HY, Chen SC, Wu KJ, Kuo HC, Hseu YC, Ching H, Wu CR. Antioxidant phenolic profile from ethyl acetate fraction of Fructus Ligustri Lucidi with protection against hydrogen peroxide-induced oxidative damage in SH-SY5Y cells. Food Chem. Toxicol. 2012;50:492–502. doi: 10.1016/j.fct.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 45.Kim KB, Lee S, Kim JH. Neuroprotective effects of urolithin A on H2O2-induced oxidative stress-mediated apoptosis in SK-NMC cells. Nutr. Res. Pract. 2020;14:3–11. doi: 10.4162/nrp.2020.14.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]