Abstract
To evaluate neuropsychiatric symptoms in patients with Alzheimer’s disease (AD) and their association with cognition and functionality during lockdown of the COVID-19’s first wave. We included 91 patients and caregivers of people with AD from a memory clinic. The RUDAS, M@T, and CDR were administered to patients and NPI/ADCS-ADL to caregivers. Baseline and lockdown measurements scales were analyzed to compare the frequencies at baseline versus lockdown and conditional Odds Ratio (ORc) was calculated for the neuropsychiatric symptoms. During the pandemic, significant increase in the number of cases was observed in depression (23%), agitation (36.8%), aberrant motor activity (12%), sleep disorders (26.3%), and appetite change (12.1%). In worsening of pre-existing symptoms, the most frequent were delusions (75%), followed by sleep disorders (71.7%). Lockdown induces a rapid increase of neuropsychiatric symptoms affecting cognitive symptoms and functionality of Peruvian patients with AD.
Keywords: Alzheimer’s disease, caregiver, COVID-19, dementia, quarantine
Introduction
Behavioral and psychological symptoms of dementia (BPSD), also known as neuropsychiatric symptoms, are signs and symptoms of disturbed perception, thought content, altered mood, or behavior. 1 BPSD includes agitation, depression, apathy, anxiety, repetitive behaviors, psychosis, aggression, sleep disturbances, wandering, and various socially inappropriate behaviors. 2
The prevalence of BPSD ranges from 25 to 80%. 3 In general, all people with dementia will experience one of these symptoms throughout their illness; they can be present even in mild cognitive impairment (depression, apathy, and irritability).4,5 Their severity increases as dementia progress, and they tend to fluctuate in the same patient. 4 These symptoms have several contributing factors. One is related to the person with dementia (neurobiological factors of dementia, acute medical illness, unmet needs, and pre-existing personality factors and psychiatric illnesses); another is caregiver-related factors and environmental factors. 1
The COVID-19 pandemic, caused by SARS-CoV-2, was officially declared in March 2020. 6 Since then, the vast majority of countries have to adopt extraordinary measures to limit the spread of the virus. One is the confinement of the people at home except for essential outings. 7 Dementia is associated with a greater risk of death by SARS-CoV-2, 8 not being solely due to their vulnerability to the infection, and may also relate to the cognitive, behavioral, and psychological effects of interruption of routine activities (rapid environmental changes) by confinement due to COVID-19 pandemic. Disruption of routine may lead to the onset/worsening of BPSD that increases distress levels in caregivers, favors contagion, and increases the risk of self-injury, hospitalization, and death. 7
The BPSD in the COVID-19 era span from apathy to severe hyperactivity and impact on the patient and their environment. Anxiety, agitation, and apathy appear to worsen after protracted isolation due to environmental restrictions. 7 In the vulnerable population group, people living with dementia could feel lonely and abandoned, mostly if they do not resort to telecommunication. 9 The current context may lead to increased stress and behavioral problems in patients with dementia; delirium caused by hypoxia, a prominent clinical feature of COVID-19, could complicate the presentation of dementia, increasing patient suffering, the cost of medical care, and the need for dementia support. 9 A variety of adverse psychological effects, including symptoms of post-traumatic stress, confusion, and anger, have been reported as consequences of confinement related to COVID-19. 10 The BPSD are strongly associated with stress and depression in caregivers, reduced employment income, and lower quality of life. 1
Therefore, this study aims to describe the onset or to worsen psychological and behavioral symptoms of patients with Alzheimer’s disease during COVID-19 pandemic confinement.
Methods
Study Design
A prospective longitudinal study was carried out where a cohort of patients with Alzheimer’s disease was followed.
Participants
During the confinement, 91 patients and caregivers of people with dementia due to Alzheimer’s disease (AD) based in supportive biomarkers (imaging and cerebrospinal fluid) who attended the cognitive neurology outpatient clinic and Unit Cognitive Impairment and Dementia Prevention of the Instituto Peruano de Neurociencias in Lima (Peru) were asked to participate in the study. Inclusion criteria were: 1) the patient should have a complete cognitive, behavioral, and functional assessment performed six months prior to the onset of the confinement due to pandemic, 2) being the main informal caregiver of a patient with AD (provide care for patients for a minimum of 6 hours a day for at least 3 times a week), and 3) both must have signed written consent before study enrollment. Exclusion criteria included 1) caregivers who provide care for less than 6 hours a day and less than 3 times a week, 2) current acute/unstable phase of a mental disorder of the caregiver, 3) COVID-19 infection or flu-like self-limiting symptoms of the caregiver or his/her family members or close relatives/friends during the confinement, based in measure of antibodies (Ig G and Ig M), 4) active involvement as a medical or non-medical professional in the management of the COVID-19 pandemic, or 5) being a caregiver of an individual living in assisted living facilities or nursing care facilities. The diagnosis of mild and major neurocognitive disorders was based on the DSM-5 diagnostic criteria, 11 and the diagnosis of Alzheimer’s disease 12 had been established according to international diagnostic criteria by a neurologist after an extensive diagnostic workup in the six months preceding the introduction of the restriction measures. The study was conducted in accordance with the latest revision of the Declaration of Helsinki and ethical approval for this study was obtained from Instituto de Evaluacion de Tecnologias en Salud e Investigacion (IETSI-EsSalud).
Procedures
Questionnaire and Interview to Caregivers
A caregiver-based interview format was employed. A standardized set of questions, regarding both their distress and mental reaction to COVID-19 crisis, as well as patients’ symptoms, was asked to caregivers during the confinement. The confinement questionnaire was applied to caregivers, exploring the physical and human environment of the dementia patient and how the caregiver’s activities have been affected or modified by the confinement measures. We also explored some consequences. To assess the development of neuropsychiatric symptoms, functional impairment, and the caregiver distress pertaining to them, Likert items were employed to compare with similar evaluation on previous months to the onset of the pandemic confinement (Supplementary material). Neuropsychiatric symptoms were assessed with the neuropsychiatric inventory questionnaire (NPI), 13 while items assessing functional impairment were based on the Alzheimer’s Disease Cooperative Study-Activities of Daily Living, ADCS-ADL. 14
Neuropsychiatric Inventory Questionnaire (NPI)
We used NPI-12, a clinical informant interview surveying the following behavioral disturbances: delusions, hallucinations, agitation/aggression, irritability, depression, anxiety, euphoria, disinhibition, aberrant motor behavior, apathy, sleep, and appetite and was administered by two trained professionals. With a maximum of 144 points, the NPI-12 delivers a total symptom score based on frequency and severity of each subdomain. According to the criteria-based rating scheme, the severity of each manifestation was classified into four grades (from 1 to 3; 0 if absent) and the frequency of each manifestation was also classified into five grades (from 1 to 4; 0 if absent). The NPI score (severity x frequency) was calculated for each manifestation (range of possible scores: 0-12). The presence of a symptom was expressed as an NPI subset score >0. 13
Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL)
This is an informant/caregiver administered 23-item scale to measure performance of activities of daily living. 14 The caregivers are asked by a trained professional in an interview format to rate the patient’s level of performance for each activity in the past four weeks. The caregivers are carefully instructed not to base their responses on their own perceptions about the patient’s abilities but on the patient’s actual performance in daily life. The score ranges from 0 to 78 with higher scores indicating better functioning.
Of note, data on neuropsychiatric (NPI), cognitive, and functional (ADCS-ADL) symptoms during the pre-lockdown period were available for all patients who were included in the present study. In particular, we are including data from the last assessment of these 91 patients and caregivers’ dyads in the six months preceding the introduction of the COVID-19 restrictive measures were available and were analyzed to grasp alterations in these symptoms between the pre-lockdown period and the confinement.
Clinical Evaluation of Patients
The cognitive evaluation included brief cognitive tests and Clinical Dementia Rating (CDR). 15 The brief cognitive tests applied were Rowland Universal Dementia Assessment Scale (RUDAS) and Memory Alteration Test (M@T).
Rowland Universal Dementia Assessment Scale (RUDAS) is a simple tool that takes 10 minutes to administer and is comprised of 6 components exploring memory, body orientation, visuospatial praxis, motor praxis, judgment, and language. It has a maximum score of 30, where a lower score denotes poor cognitive performance. The RUDAS has recently been evaluated in patients’ age ≥60 years with a mid-level education 16 and illiterate population 19 in Peru.
Memory alteration test (M@T) is a valid screening test that assesses the temporal orientation and different types of memory (episodic, textual, and semantic) and discriminates between healthy elderly subjects, patients with aMCI, and patients with early AD. This test is oral and does not require reading or writing skills or the use pencil and paper, allowing the evaluation of very low-educated subjects. All questions of M@T have singles correct answers and covering five domains: temporal orientation, 5 short term memory, 10 semantic memory, 13 free recall, and facilitated recall. 10 Thus, the maximum score of this test is 50 points. This is a cognitive test with high internal consistency and validity, short application (5-10 min), easy to perform and to interpret, validated in Peru in mid-level 17 and low educational level. 18
Clinical Dementia Rating (CDR) is used to measure social changes, behaviors, and functions of the patient. The score is designed to stage dementia severity and is based on independently semi-structured interviews of patients and informants as well as clinical judgment from the treating physician. It is calculated based on 6 cognitive and behavioral domains including memory, orientation, judgment and problem solving, community affairs, home and hobbies performance, and personal care. We used the following scale: 0 (normal), 0.5 (questionable), 1 (mild), 2 (moderate), 3 (severe), 4 (profound), and 5 (terminal). A global CDR score (max 5) was calculated using a standard algorithm.
Statistical Analyses
The characteristics of the general population were described in absolute and relative frequencies (percentages); the age variable, being numerical, and after evaluation of normality, was presented as mean and standard deviation.
Baseline and lockdown measurements of the NPI, RUDAS, M@T, and ADCS-ADL scales were presented in graphs; likewise, each component of the NPI was presented in bar graphs. We used the McNemar’s test to compare the frequencies at baseline versus lockdown. Conditional Odds Ratio (ORc) was calculated for the neuropsychiatric symptoms of the NPI, calculated by conditional logistic regression and applying Yates’s correction in case a discordant cell was zero. We considered significant differences with a P-value <.05 as statistically significant.
All analyses were conducted using STATA® v16.0.
Results
We surveyed 91 caregivers and tested 91 patients diagnosed with Alzheimer’s disease (AD). All participants had undergone a previous evaluation months before confinement; they were re-evaluated between 2 and 6 months after the onset of COVID-19 pandemic confinement.
The patients’ mean age was 73.4 years (±7.3 years), ranging from 58 to 90 years, with a predominance of women than men (60.4 vs. 39.6%). Additionally, seven patients had some other comorbidities such as hepatitis B, 3 arachnoid cyst, 1 sequelae of traumatic brain injury (TBI), 2 and left frontal lobe stroke. 1
Confinement Questionnaire for Caregivers of Patients With AD
The median time spent on caregiving work before lockdown was 50%; however, during lockdown, it was 20% (P<.001); conversely, the median time spent on home activities before lockdown was 20%; however, during lockdown, the median time spent on home activities was 50% (P < .001).
The median percentage of time spent caring for the dementia patient before and during lockdown was 15 and 25%, respectively (P < .001).
This cohort involves a small number of patients with moderate stage AD (CDR = 2). Other questions about confinement are detailed in Table 1.
Table 1.
General Characteristics of the Cohort of Patients Diagnosed with AD and Confinement Questionnaire for Caregivers During the First Wave Confinement by COVID-19 in Lima, Peru.
| Characteristics | N | % |
|---|---|---|
| Age (years) a | 73.4 | 7.3 |
| Sex | ||
| Male | 36 | 39.6 |
| Female | 55 | 60.4 |
| Disease severity according to CDR | ||
| Questionable (.5) | 49 | 53.9 |
| Mild 1 | 37 | 40.7 |
| Moderate 2 | 5 | 5.4 |
| Confinement questions | ||
| 1. Who do you live with? | ||
| a. I live with my parents, siblings or other relatives | 60 | 65.9 |
| b. I live with my partner | 29 | 31.9 |
| c. I live alone | 2 | 2.2 |
| 2. The property has b | ||
| a. Study room | 12 | 9.84 |
| b. Library room | 10 | 8.2 |
| c. Garden | 28 | 22.95 |
| d. Terrace or balcony | 25 | 20.49 |
| e. None | 47 | 38.52 |
| 3. How many live-in households? | ||
| 1-3 | 31 | 34.1 |
| 4-7 | 60 | 65.9 |
| 4. Infected with COVID-19 at home (%) | 30 | 33.0 |
| 5. How informed are you about COVID-19? | ||
| a. More or less informed | 35 | 38.5 |
| b. Well informed | 50 | 54.0 |
| c. Very well informed | 6 | 6.5 |
| 6. During your quarantine, how has your relationship with your family changed? | ||
| a. It has gotten worse (e.g., we fight more) | 43 | 47.2 |
| b. It is the same as before | 44 | 48.4 |
| c. It has improved (e.g., we communicate better) | 4 | 4.4 |
| 7. Has the pandemic has affected your mood? | ||
| a. Little | 11 | 12.1 |
| b. More or less | 31 | 34.0 |
| c. A lot | 36 | 39.6 |
| d. Too much | 13 | 14.3 |
| 8. Do you feel that the pandemic has affected your life? | ||
| No | 17 | 18.7 |
| Yes | 74 | 81.3 |
aAverages and standard deviation are presented.
bCan add up to more than 91 as there were multiple responses.
Assessment of cognitive, functional, and neuropsychiatric symptoms of AD before and during the first wave of the COVID-19 pandemic:
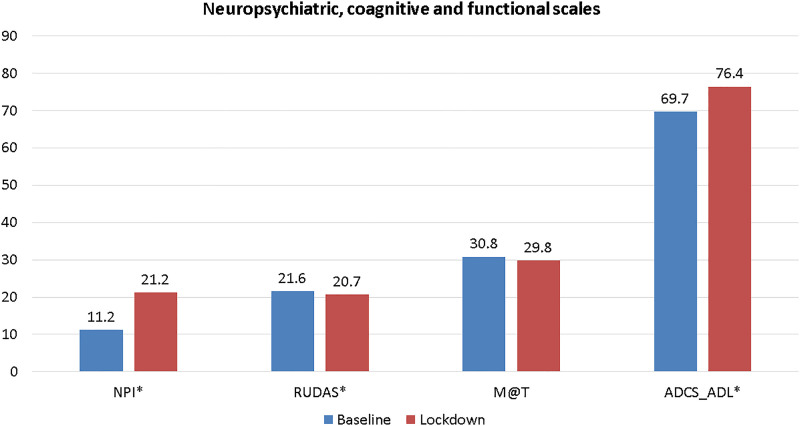
Regarding the assessment of functionality and neuropsychiatric symptoms, ADCS-ADL and NPI scores were significantly increased in lockdown concerning baseline (6.6 and 10 points, respectively, both with P-value < .001). At the same time, brief cognitive assessment measured by M@T and RUDAS scores decreased in lockdown from baseline (P-value < .001). The comparison is seen in Figure 1.
Figure 1.
Brief cognitive test scores (RUDAS and M@T), functionality (ADCS-ADL), and neuropsychiatric symptoms (NPI) in patients with AD diagnosis according to pre-pandemic (baseline) and during lockdown by COVID-19. NPI: Neuropsychiatric inventory; RUDAS: Rowland Universal Dementia Assessment Scale; M@T: test of memory impairment; ADCS-ADL: Alzheimer’s Disease Cooperative Study-Activities of Daily Living. *Statistical differences (P < .001) evaluated with paired t test.
Environmental and biological factor analysis relates to the cognitive, neuropsychiatric symptoms, and functionality of the patients before and during the first wave of the COVID-19 pandemic.
Table 2 shows subgroups analysis based on age, sex, disease severity (CDR), spacious housing, the number of people per household, the impact on mood, and time spent on AD patient care duties, with NPI and ADCS-ADL scores increased significantly during lockdown concerning baseline, while RUDAS and M@T scores decreased considerably during lockdown concerning baseline. However, we did not observe significant differences between the dichotomous categories of each of the variables, so we did not find any factor that explains the variations in the neuropsychiatric, cognitive, and functional symptoms of patients with AD this first wave of the COVID-19 pandemic.
Table 2.
Characteristics and NPI, RUDAS, M@T, and ADCS-ADL of the Cohort of Patients Diagnosed with AD During the First Wave Confinement by COVID-19 in Lima, Peru.
| NPI | RUDAS | M@T | ADCS-ADL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Basal | Lockdown | Δ | P-value | Basal | Lockdown | Δ | P-value | Basal | Lockdown | Δ | P-value | Basal | Lockdown | Δ | P-value | ||||||||
| x | DS | x | DS | x | DS | x | DS | X | DS | X | DS | x | DS | x | DS | |||||||||
| Age | .484± | .173 ± | .143± | .143 ± | ||||||||||||||||||||
| Under 65 | 9.3 | 3 | 20.8 | 8.3 | 11.6 | <.001 * | 22.8 | 2.7 | 21.6 | 2.6 | −1.3 | <.001 * | 32.3 | 3.2 | 30.7 | 3.4 | −1.6 | <.001 * | 72.2 | 3.8 | 79.9 | 6.2 | 7.8 | <.001 * |
| 65 years and over | 11.5 | 4 | 21.3 | 10 | 9.8 | <.001 * | 21.4 | 3.2 | 20.6 | 2.9 | −0.8 | <.001 * | 30.6 | 4.3 | 29.7 | 4.2 | −0.9 | <.001 * | 69.4 | 4.3 | 75.8 | 6.6 | 6.4 | <.001 * |
| Sex | .939± | .776± | .661± | .527± | ||||||||||||||||||||
| Male | 10.8 | 4 | 20.7 | 9 | 9.9 | <.001 * | 22.3 | 2.9 | 21.4 | 2.6 | −0.9 | <.001 * | 31.8 | 3.8 | 30.7 | 4 | −1.1 | <.001 * | 70.1 | 4.9 | 76.2 | 6.4 | 6.1 | <.001 * |
| Female | 11.5 | 4 | 21.5 | 11 | 10.1 | <.001 * | 21.2 | 3.3 | 20.2 | 2.9 | −0.9 | <.001 * | 30.5 | 4.4 | 29.5 | 4.1 | −1 | <.001 * | 69.5 | 4 | 76.4 | 6.9 | 6.9 | <.001 * |
| Functional assessment (CDR) | .162± | <.001 ± | .005± | .005± | ||||||||||||||||||||
| Questionable (.5) | 9.1 | 2 | 17.6 | 7.8 | 8.5 | <.001 * | 24.2 | 1.1 | 22.9 | 1.2 | −1.3 | <.001 * | 33.8 | 2.8 | 32.7 | 2.6 | 1.1 | <.001 * | 71.6 | 3.4 | 77.5 | 5.8 | 5.9 | <.001 * |
| Mild 1 | 12.4 | 3 | 24.3 | 10 | 11.9 | <.001 * | 18.8 | 1.8 | 18.4 | 1.8 | −0.4 | .019* | 27.8 | 2.2 | 26.7 | 2.5 | −1.1 | <.001 * | 68.1 | 4.3 | 75.4 | 7.6 | 7.2 | <.001 * |
| Moderate 2 | 23.9 | 1 | 33.2 | 11 | 9.3 | <.001 * | 16.6 | 2.1 | 16.2 | 2 | −0.4 | .374* | 23.6 | 2.2 | 24.6 | 3.1 | −1 | .298* | 63.8 | 3.3 | 72.8 | 6.5 | 9 | .056 * |
| Housing with wide spaces | .007± | .493± | .143 ± | .220± | ||||||||||||||||||||
| Yes | 10.4 | 3 | 22.8 | 11 | 12.4 | <.001 * | 21.9 | 3 | 20.9 | 2.6 | −1 | <.001 * | 30.6 | 4.3 | 29.7 | 4.2 | −0.9 | <.001 * | 70.1 | 4.3 | 76.4 | 7.3 | 6.4 | <.001 * |
| Not | 11.9 | 5 | 19.7 | 9.2 | 7.7 | <.001 * | 21.3 | 3.4 | 20.5 | 3.1 | −0.8 | <.001 * | 32.3 | 3.2 | 30.7 | 3.4 | −1.6 | <.001 * | 69.4 | 4.5 | 76.3 | 6.1 | 6.8 | <.001 * |
| How many live in the home | .713± | .479± | .141± | .884± | ||||||||||||||||||||
| From 1 to 3 | 11.7 | 4 | 22.1 | 8.1 | 10.5 | <.001 * | 21.1 | 2.7 | 20.3 | 2.3 | −0.8 | <.001 * | 30.2 | 4.2 | 28.8 | 3.9 | −1.3 | <.001 * | 68.8 | 4.6 | 75.3 | 7.1 | 6.5 | <.001 * |
| From 4 to 7 | 11 | 4 | 20.7 | 11 | 9.8 | <.001 * | 21.9 | 3.4 | 20.9 | 3.1 | −0.9 | <.001 * | 31.2 | 4.2 | 30.3 | 4.1 | −0.9 | <.001 * | 70.2 | 4.2 | 76.9 | 6.5 | 6.7 | <.001 * |
| You feel that the pandemic affected your life | .353± | .78± | .973± | .68± | ||||||||||||||||||||
| Not | 10.6 | 5 | 18.9 | 9.9 | 8.3 | <.001 * | 22.4 | 2.5 | 21.4 | 2.2 | −0.9 | <.001 * | 32.4 | 3.7 | 31.4 | 3.3 | −1 | <.001 * | 69.8 | 4.6 | 75.9 | 6.1 | 6.1 | <.001 * |
| Yes | 11.4 | 4 | 21.7 | 10 | 9.9 | <.001 * | 21.4 | 3.3 | 20.6 | 3 | −0.9 | <.001 * | 30.5 | 4.3 | 29.5 | 4.2 | −1 | <.001 * | 69.7 | 4.3 | 76.5 | 6.9 | 6.7 | <.001 * |
| Time spent by the caregiver | .548± | .589± | .010± | .332± | ||||||||||||||||||||
| Decrease | 8.7 | 2 | 16.3 | 8.5 | 7.6 | .014 * | 22 | 3.3 | 21.1 | 2.5 | −0.9 | .041* | 32.3 | 4.7 | 30 | 4.2 | −2.3 | <.001 * | 70.8 | 3.5 | 76 | 5.1 | 5.2 | .026* |
| Remains the same | 10.6 | 3 | 20.3 | 8.3 | 9.8 | <.001 * | 22.8 | 2.7 | 21.8 | 2.5 | −1 | <.001 * | 31.5 | 4.2 | 30.6 | 4.2 | −0.9 | <.001 * | 70.6 | 4.2 | 76.4 | 7.4 | 5.9 | <.001 * |
| Increase | 12.4 | 5 | 23.2 | 11 | 10.8 | <.001 * | 20.4 | 3.2 | 19.7 | 2.9 | −0.8 | <.001 * | 29.9 | 4 | 29.1 | 3.9 | −0.8 | <.001 * | 68.7 | 4.5 | 76.3 | 6.5 | 7.6 | <.001 * |
| Mood | .674± | .461± | .64± | .194± | ||||||||||||||||||||
| Not affected | 9.5 | 3 | 18.5 | 9.8 | 9 | .003* | 23.5 | 2.1 | 22.4 | 2.2 | −1.1 | <.001 * | 33.9 | 3.1 | 33.1 | 2.7 | −0.8 | .068* | 70 | 4.1 | 74.4 | 5 | 4.4 | .048* |
| Affected | 11.4 | 5 | 21.6 | 10 | 10.1 | <.001 * | 21.3 | 3.2 | 20.5 | 2.9 | −0.9 | <.001 * | 30.4 | 4.2 | 29.4 | 4 | −1 | <.001 * | 69.7 | 4.4 | 76.6 | 6.9 | 6.9 | <.001 * |
x: Mean. Δ: Difference between pandemic score and baseline according to the category being evaluated.
±P-value evaluated by linear regression comparing the difference of the score with the marked category.
*P-value evaluated by paired t-test comparing averages of the score evaluated in the basal stage with lockdown according to the marked category.
According to AD severity, the NPI and ADCS-ADL show a significant increase in their scores during lockdown concerning baseline at each stage. While RUDAS and M@T show a similar decrease according to disease severity, only functionality and cognition measured by ADCS-ADL and M@T/RUDAS seem to worsen compared to disease severity stages; however, the small sample size of the group of patients with moderate stage AD prevents us from confirming this trend.
According to NPI, variations in the frequency of psychological and neuropsychiatric symptoms of AD before and during the first wave of the COVID-19 pandemic.
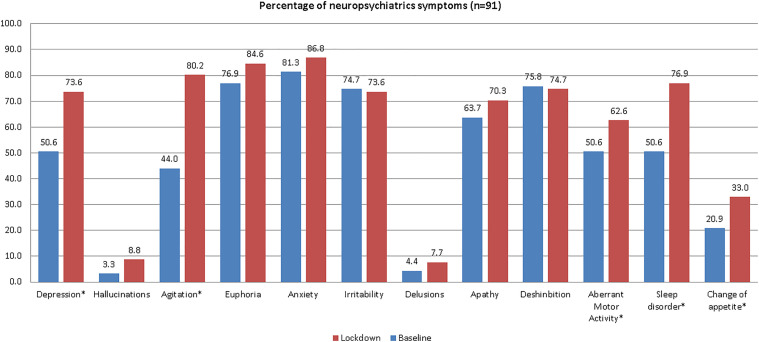
A significant increase in the number of cases was observed in depression (23%), agitation (36.8%), aberrant motor activity (12%), sleep disorders (26.3%), and appetite change (12.1%). The baseline and lockdown percentages are shown in Figure 2.
Figure 2.
Variations in the frequency of neuropsychiatric symptoms based on NPI in a cohort of patients with Alzheimer’s disease before and during the first wave of the COVID-19 pandemic in Lima, Peru.
The onset/worsening of neuropsychiatric symptoms was observed in all domains. The most frequent symptoms presenting for the first time in the pandemic were sleep disorders (71.1%), followed by agitation (68.6%) and depression (53.3%). In worsening of pre-existing symptoms, the most frequent were delusions (75%), followed by sleep disorders (71.7%). The odds ratios of changes in neuropsychiatric symptoms are reported in Table 3, where we can observe that the probability of having depression as a de novo symptom is eight times, compared to those with a previous diagnosis.
Table 3.
Frequency of new cases and worsening of neuropsychiatric symptoms in patients with Alzheimer’s disease before and during the first wave of the COVID-19 pandemic in Lima, Peru.
| New Onset, % | Worsening, % | ORc | P-value | |
|---|---|---|---|---|
| Depression | 53.3 | 47.8 | 8.00 | <.001 |
| Hallucinations | 5.7 | 66.7 | 11.00 | .219* |
| Agitation | 68.6 | 65.0 | 17.50 | <.001 |
| Euphoria | 57.1 | 51.4 | 2.40 | .144 |
| Anxiety | 58.8 | 59.5 | 2.00 | .302 |
| Irritability | 21.7 | 54.4 | .83 | 1.000 |
| Delusions | 3.4 | 75.0 | 7.00 | .250* |
| Apathy | 39.4 | 53.4 | 1.86 | .263 |
| Disinhibition | 31.8 | 39.1 | .88 | 1.000 |
| Aberrant motor activity | 33.3 | 65.2 | 3.75 | .019 |
| Sleep disorder | 71.1 | 71.7 | 4.00 | <.001 |
| Change of appetite | 26.4 | 42.1 | 2.38 | .052 |
ORc: Odds ratio, calculated by conditional logistic regression. * Yates’s correction. Only means that p-values are statistically significant (P < .05).
Discussion
In this study, we compare the neuropsychiatric symptoms before and during lockdown of the first wave of COVID-19 epidemic in patients with AD from Peru. There was a statistically significant increase in levels of agitation, depression, appetite/eating disturbance, nighttime behavior disturbances, and aberrant motor activity, but we failed to find a biological, psychosocial, or environmental factor to explain these changes.
Patients with MCI and dementia due to AD are a particularly vulnerable population.19,20 Most have memory problems that can make it difficult for them to understand what is happening around them and difficulties to follow the recommendations from public health system (physical distancing, frequent hand washing, and use of facial masks).19,21 On the other hand, as for most of the population, the patients’ routines have been altered, and their environment may be more chaotic as a result of the uncertainties caused by the pandemic.21,22 Furthermore, their cognitive stimulation programs have been interrupted or severely modified. All these circumstances might force to social isolation and generate the possibility to high levels of stress, anxiety, and depression that seem to be particularly associated with loneliness. 23 Similarly, with our study, worsening or emergence of new neuropsychiatric symptoms was found in a substantial proportion of patients with cognitive decline as a result of social isolation,7,24 although changes in symptom severity were found to be similar between patients with MCI and dementia. A study from Spain evaluating 40 patients with mild AD dementia MCI reported that their neuropsychiatric symptoms (particularly apathy, anxiety, agitation, and aberrant motor behavior) significantly worsened after 5 weeks of lockdown. 25 Also, a report of 139 patients with dementia subjective cognitive, MCI, and decline from a memory center in Rome showed worsening or onset of neuropsychiatric symptoms in 54.7% (mainly agitation/aggression, apathy, and depression) after 1 month of lockdown. 26 In this sense, worsening of neuropsychiatric symptoms has been found particularly associated with significant lower general cognitive functioning before confinement in a sample of 38 patients with a clinical diagnosis of probable AD. 27 In our region, Argentinian researchers collected 324 participants who responded to the interview by telephone in 109 cases (33.6%), by e-mail in 62 (19.1%), by video conference in 30 (9.3%), and at the emergency department in 23 (7.1%); over 90% of patients presented neuropsychiatric alterations; 63% of caregivers showed signs of burden, with nearly half presenting severe burden. 28 Opposite to our study, in a sample of 93 older adults with MCI or mild dementia, those living alone reported significantly a decrease in their well-being, reporting more levels of anxiety and sleeping problems. 29 In a nation-wide survey performed in Italy after 1 month from the beginning of COVID-19 quarantine, an increased burden of neuropsychiatric symptoms was reported in approximately 60% of community-dwelling persons affected by dementia by their family caregivers and the profiles of neuropsychiatric symptoms changes were influenced by type of dementia, disease severity, and gender. Anxiety and depression were associated with a diagnosis of AD, mild disease severity, and female gender. 30 However, in another study in Milan, Italy, despite a worsening of patients’ functional status, there were no significant changes before and after the COVID-19 lockdown in the mean NPI score and the caregivers’ burden decreased significantly; 31 but, a nation-wide survey that involved 87 Italian Dementia Centers including 4913 patients (2934 women, 1979 men) shows that lockdown for COVID-19 is associated with an acute worsening of clinical symptoms in patients with dementia as well as increase of caregivers’ burden 32 in order to review the evidence regarding the neuropsychiatric and cognitive manifestations of COVID-19 as well as its direct and indirect consequences in survivors, especially in elderly individuals with dementia. 19
We found that patients with AD experienced a worsening of symptoms such as sleep disorder, aberrant motor activity, and agitation–aggression during the lockdown. It is striking that no other study has so far reported sleep disorder as a worsening of a pre-existing neuropsychiatric symptom.25,30 In contrast, agitation–aggression has been reported as a frequent symptom, similar to aberrant motor behavior in patients with AD and frequent neuropsychiatric symptoms when evaluating all dementias in general25,30,33 (Table 4); however, a minor increase was observed in our study, but it was not significant. The new-onset neuropsychiatric symptoms during pandemic lockdown were reported as a higher frequency in AD (26.7%) than other dementias; in this same study from Cagnin A. et al, the most common symptoms reported were sleep disorders, irritability, and agitation–aggression. 30 We found a similar finding in our research; sleep disorders and agitation–aggression were present and frequent, but we also frequent the depression. This last symptom has not been reported in previous studies in patients with AD or other types of dementia. We considered it could be due to protracted isolation and family contact loss due to confinement in homes in a country with close family and friendship ties such as Peru.
Table 4.
Neuropsychiatric Symptoms During Lockdown in Dementia Patients.
| Authors | New Neuropsychiatric Symptoms | Worsening of Pre-existing Neuropsychiatric Symptoms | Dementia Type |
|---|---|---|---|
| Lara et al, 25 Spain, 2020 | -- | Agitation–aggression, apathy, and aberrant motor behavior | AD |
| Cagnin A. et al, 30 Italy, 2020 | Sleep disorders, irritability, and agitation–aggression | Agitation–aggression, irritability, and apathy | AD, FTD, LBD, VD |
| Manini et al, 33 Milan—Italy, 2020 | Anxiety, agitation–aggression, and apathy | Agitation–aggression, irritability, and apathy | AD, FTD, LBD, CBD, VD, MD |
| Barguilla A et al., 39 Barcelona, Spain | - | Agitation–aggression, depression, anxiety, changes in appetite | AD, MCI |
| Custodio N. et al, Lima—Peru, 2020 | Sleep disorders, agitation–aggression, and depression | Sleep disorder, aberrant motor activity, and agitation–aggression | AD |
Alzheimer’s disease (AD), Frontotemporal Dementia (FTD), Lewy Body Dementia (LBD), Corticobasal Degeneration (CBD), Vascular Dementia (VD), Mixed Dementia (MD), Mild Cognitive Impairment (MCI).
This is the first research in LA addressing prevalence and type of increase in neuropsychiatric symptoms as acute consequence of imposed isolation due to COVID-19 quarantine in a population of patients affected by AD. Although the sample is small, it is part of a cohort of patients with a structured diagnosis of Alzheimer’s disease with a standardized assessment and validated brief cognitive tests (RUDAS and M@T) at various levels of education for Peru, and considerations drawn from the results of this study could therefore be extended to community-dwelling subjects affected by AD in LA. Another strength is the evidence of worsening of cognitive and functional evaluation of patients with AD, rarely reported in international research. To our knowledge, in a study with a smaller sample of 32 individuals with frontotemporal lobar dementia from a dementia care center in Tricase (Italy), caregivers were interviewed by telephone using a structured clinical assessment and reported that compared to their last visit (mean of 6.78 months), 53% of patients showed significant worsening in cognitive function, particularly in memory, along with worsening in behavior and language function during COVID-19 confinement. 34 In United States of America, a study of one million population-level electronic health record data recorded as having dementia, the analysis showed that people with dementia had a 2-fold increased risk of contracting COVID-19. The odds were highest in people with vascular dementia (adjusted OR (AOR) 3.17), followed by presenile dementia (AOR 2.62) and AD (AOR 1.86). In addition, dementia was associated with a greater likelihood of hospitalization and death as a consequence of COVID-19. 35 The strong link between COVID-19 and vascular dementia indicates a possible role for pre-existing cerebrovascular pathology in SARS-CoV-2 infection, whereas people with dementia often have comorbidities that are risk factors for COVID-19, such as hypertension, cardiovascular disease, obesity, or type 2 diabetes. 36 We speculate that the absence of environmental and psychosocial factors that explain the exacerbation or worsening of neuropsychiatric symptoms could be biologic factors or evolution of disease in adverse circumstance. We should note that participants with AD reported high levels of depression and agitation both before and during the COVID-19 crisis. Although this may be partly the result of recall bias, these scores reflect the high occurrence of both depression and agitation in AD. 37 Also, 25-71% people with AD experience agitation during the course of the disease. 38 Both before and during the COVID-19 crisis, the depression and agitation scores of our participants were increased before the COVID-19 pandemic. Therefore, the neuropsychiatric symptoms might surge/worsen due to a direct effect of SARS-CoV-2, rather than being an indirect consequence of COVID-19 pandemic–related isolation.
Limitations
One limitation in our study is that our findings lack generalizability as a result of having been conducted in a memory clinic in patients who were high-educated older adults, excluding patients with low level of education, rural population, or whose predominant speech was other than Spanish. Consequently, the results of this study may not be applicable to these population subgroups. Probably, other limitations included sample size and implications for limited generalizability. Another limitation of our study is the assessment of anxiety and depression into domains of NPI, which might result in over expression of diagnosis. Moreover, the interview was delivered to caregivers, and therefore, reports could be influenced by their emotional status and level of distress. Finally, there was absence of information on type of drug prescription modification made in some patients with neuropsychiatric symptoms, so the frequency of symptoms may have been underestimated. This would have been interesting since use of some drugs classes, such as antipsychotics, modifies the risk of stroke and mortality and since an untailored therapeutic plan during an unexpected lockdown could be partially responsible for worsening of neuropsychiatric symptoms
In some cases, we were unable to differentiate between primary caregivers and secondary caregivers, so the frequency of symptoms may have been underestimated.
Conclusions
Quarantine during the pandemic’s first wave in Peru induces a rapid increase of neuropsychiatric symptoms affecting cognitive symptoms and functionality of patients with Alzheimer’s disease.
Supplemental Material
Supplemental Material, sj-pdf-1-aja-10.1177_15333175211039089 for Neuropsychiatric Symptoms in Patients With Alzheimer’s Disease During SARS-COV-2 Pandemic in Peru by Nilton Custodio, Sheila Castro-Suárez, Rosa Montesinos, Virgilio E. Failoc-Rojas, Rossana Cruz del Castillo and Eder Herrera-Perez in American Journal of Alzheimer's Disease & Other Dementias
Acknowledgments
The authors would like to thank the study participants and their caretakers for their time.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplementary Material: Supplementary Material for this article is available online.
ORCID iDs
Nilton Custodio https://orcid.org/0000-0002-8025-3272
Sheila Castro-Suarez https://orcid.org/0000-0003-2992-9342
References
- 1.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, et al. Neuropsychiatric symptoms in Alzheimer's disease. Alzheimers Dement. 2011;7(5):532-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: Findings from the Cache County Study on Memory in Aging. Am J Psychiatr. 2000;157(5):708-714. [DOI] [PubMed] [Google Scholar]
- 4.Vik-Mo AO, Giil LM, Ballard C, Aarsland D. Course of neuropsychiatric symptoms in dementia: 5-year longitudinal study. Int J Geriatr Psychiatr. 2018;33(10):1361-1369. [DOI] [PubMed] [Google Scholar]
- 5.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA. 2002;288(12):1475-1483. [DOI] [PubMed] [Google Scholar]
- 6.Munayco CV, Tariq A, Rothenberg R, Soto-Cabezas GG, Reyes MF, Valle A, et al. Early transmission dynamics of COVID-19 in a southern hemisphere setting: Lima-Peru: February 29(th)-March 30(th), 2020. Infect Dis Model. 2020;5:338-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonetti A, Pais C, Jones M, Cipriani MC, Janiri D, Monti L, et al. Neuropsychiatric symptoms in elderly with dementia during COVID-19 pandemic: Definition, treatment, and future directions. Front Psychiatr. 2020;11:579842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cipriani G, Fiorino MD. Access to care for dementia patients suffering from COVID-19. Am J Geriatr Psychiatr. 2020;28(7):796-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Li T, Barbarino P, Gauthier S, Brodaty H, Molinuevo JL, et al. Dementia care during COVID-19. Lancet. 2020;395(10231):1190-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padala SP, Jendro AM, Orr LC. Facetime to reduce behavioral problems in a nursing home resident with Alzheimer's Dementia during COVID-19. Psychiatr Res. 2020;288:113028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Philadelphia: American Psychiatric Pub; 2013. [Google Scholar]
- 12.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufer DI, Cummings JL, Christine D, Bray T, Castellon S, Masterman D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer's Disease: The Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46(2):210-215. [DOI] [PubMed] [Google Scholar]
- 14.Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease cooperative study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S33-S39. [PubMed] [Google Scholar]
- 15.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566-572. [DOI] [PubMed] [Google Scholar]
- 16.Custodio N, Montesinos R, Lira D, Herrera-Perez E, Chavez K, Hernandez-Córdova G, et al. Validation of the RUDAS in patients with a middle-level education in Lima, Peru. Am J Alzheimers Dis Other Demen. 2019;34(7-8):513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Custodio N, Lira D, Herrera-Perez E, Nuñez Del Prado L, Parodi J, Guevara-Silva E, et al. The memory alteration test discriminates between cognitively healthy status, mild cognitive impairment and Alzheimer's Disease. Dement Geriatr Cogn Dis Extra. 2014;4(2):314-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Custodio N, Lira D, Herrera-Perez E, Montesinos R, Castro-Suarez S, Cuenca-Alfaro J, et al. Memory alteration test to detect amnestic mild cognitive impairment and early Alzheimer's Dementia in population with low educational level. Front Aging Neurosci. 2017;9:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso-Lana S, Marquié M, Ruiz A, Boada M. Cognitive and neuropsychiatric manifestations of COVID-19 and effects on elderly individuals with dementia. Front Aging Neurosci. 2020;12:588872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibanez A, Santamaria-Garcia H, Guerrero Barragan A, Kornhuber A, Ton AMM, Slachevsky A, et al. The impact of SARS-CoV-2 in dementia across Latin America: A call for an urgent regional plan and coordinated response. Alzheimers Dement (N Y). 2020;6(1):e12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki M, Hotta M, Nagase A, Yamamoto Y, Hirakawa N, Satake Y, et al. The behavioral pattern of patients with frontotemporal dementia during the COVID-19 pandemic. Int Psychogeriatr. 2020;32(10):1231-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bavel JJV, Baicker K, Boggio PS, Capraro V, Cichocka A, Cikara M, et al. Using social and behavioural science to support COVID-19 pandemic response. Nat Hum Behav. 2020;4(5):460-471. [DOI] [PubMed] [Google Scholar]
- 23.Bzdok D, Dunbar RIM. The neurobiology of social distance. Trends Cognit Sci. 2020;24(9):717-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manca R, De Marco M, Venneri A. The impact of COVID-19 infection and enforced prolonged social isolation on neuropsychiatric symptoms in older adults with and without Dementia: A review. Front Psychiatr. 2020;11:585540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lara B, Carnes A, Dakterzada F, Benitez I, Piñol-Ripoll G. Neuropsychiatric symptoms and quality of life in Spanish patients with Alzheimer's disease during the COVID-19 lockdown. Eur J Neurol. 2020;27(9):1744-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canevelli M, Valletta M, Toccaceli Blasi M, Remoli G, Sarti G, Nuti F, et al. Facing dementia during the COVID-19 outbreak. J Am Geriatr Soc. 2020;68(8):1673-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boutoleau-Bretonnière C, Pouclet-Courtemanche H, Gillet A, Bernard A, Deruet AL, Gouraud I, et al. The effects of confinement on neuropsychiatric symptoms in Alzheimer's disease during the COVID-19 crisis. J Alzheimers Dis. 2020;76(1):41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorbara M, Graviotto HG, Lage-Ruiz GM, Turizo-Rodriguez CM, Sotelo-López LA, Serra A, et al. COVID-19 and the forgotten pandemic: Follow-up of neurocognitive disorders during lockdown in Argentina. Neurologia. 2021;36(1):9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman-Casanova JM, Dura-Perez E, Guzman-Parra J, Cuesta-Vargas A, Mayoral-Cleries F. Telehealth home support during COVID-19 confinement for community-dwelling older adults with mild cognitive impairment or mild dementia: Survey study. J Med Internet Res. 2020;22(5):e19434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cagnin A, Di Lorenzo R, Marra C, Bonanni L, Cupidi C, Laganà V, et al. Behavioral and psychological effects of coronavirus disease-19 quarantine in patients with dementia. Front Psychiatr. 2020;11:578015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cravello L, Martini E, Viti N, Campanello C, Assogna F, Perotta D. Effectiveness of a family support intervention on caregiving burden in family of elderly patients with cognitive decline after the COVID-19 lockdown. Front Psychiatr. 2021;12:590104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rainero I, Bruni AC, Marra C, Cagnin A, Bonanni L, Cupidi C, et al. The impact of COVID-19 quarantine on patients with dementia and family caregivers: A nation-wide survey. Front Aging Neurosci. 2020;12:625781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manini A, Brambilla M, Maggiore L, Pomati S, Pantoni L. The impact of lockdown during SARS-CoV-2 outbreak on behavioral and psychological symptoms of Dementia. Neurol Sci. 2021;42(3):825-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capozzo R, Zoccolella S, Frisullo ME, Barone R, Dell'Abate MT, Barulli MR, et al. Telemedicine for delivery of care in frontotemporal lobar degeneration during COVID-19 pandemic: Results from Southern Italy. J Alzheimers Dis. 2020;76(2):481-489. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, Davis PB, Gurney ME, Xu R. COVID-19 and dementia: Analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wood H. Elevated risk of COVID-19 in people with dementia. Nat Rev Neurol. 2021:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starkstein SE, Jorge R, Mizrahi R, Robinson RG. The construct of minor and major depression in Alzheimer's disease. Am J Psychiatr. 2005;162(11):2086-2093. [DOI] [PubMed] [Google Scholar]
- 38.Mintzer JE, Brawman-Mintzer O, Mirski DF, Barkin K. Anxiety in the behavioral and psychological symptoms of Dementia. Int Psychogeriatr. 2000;12(S1):139-142. [Google Scholar]
- 39.Barguilla A, Fernández-Lebrero A, Estragués-Gázquez I, García-Escobar G, Navalpotro-Gómez I, Manero RM, et al. Effects of COVID-19 pandemic confinement in patients with cognitive impairment. Front Neurol. 2020;11:589901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-aja-10.1177_15333175211039089 for Neuropsychiatric Symptoms in Patients With Alzheimer’s Disease During SARS-COV-2 Pandemic in Peru by Nilton Custodio, Sheila Castro-Suárez, Rosa Montesinos, Virgilio E. Failoc-Rojas, Rossana Cruz del Castillo and Eder Herrera-Perez in American Journal of Alzheimer's Disease & Other Dementias