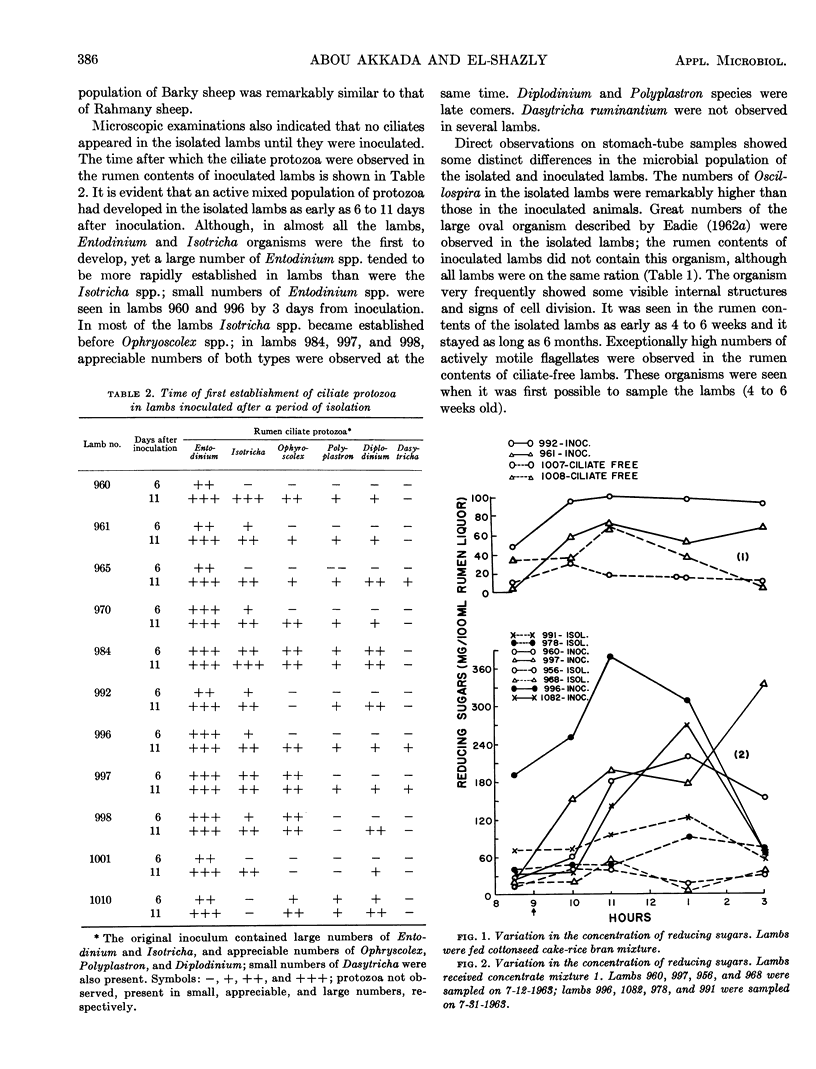
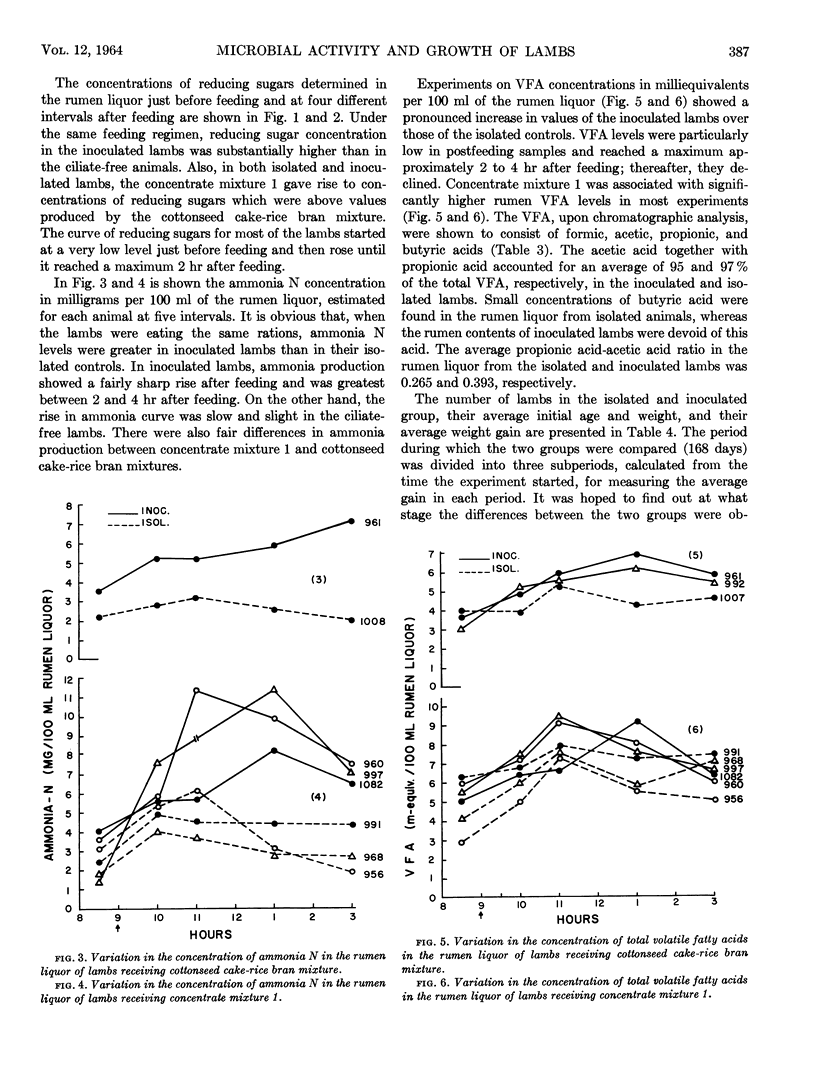
Abstract
A survey of the components of the rumen ciliate population in a series of adult sheep, raised in the Faculty of Agriculture, University of Alexandria, has shown that a mixture of Entodinium, Isotricha, Ophryoscolex, Diplodinium, and Polyplastron species was found in the rumen contents of Egyptian sheep; no Epidinium and a negligible number of Dasytricha ruminantium were also observed. The microbial population, reducing sugars, ammonia, volatile fatty acids (VFA) production, and growth rate of 14 lambs inoculated with whole rumen contents from a mature sheep were compared over a 6-month period with those of 13 lambs maintained under the same conditions, except that they were strictly isolated from other ruminants. Certain large oval organisms and large numbers of flagellates and Oscillospira were frequently observed in the rumen contents of the isolated lambs. The reducing sugars, ammonia, and VFA levels, measured before and at intervals after feeding, in the inoculated lambs showed a pronounced rise above the values found in the ciliate-free animals. The propionic acid-acetic acid ratio in the rumen contents of the faunated lambs was considerably higher than in the nonfaunated controls. The inoculated lambs grew faster than the isolated lambs. Differences in weight gain which ranged from 15 to 17% were statistically significant. The inoculated animals impressed the observers by their good appearance which was superior to that of the ciliate-free lambs. It was, therefore, concluded that the rumen ciliate protozoa are essential for the metabolism and growth of young lambs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABMU AKKADA A. R., HOWARD B. H. The biochemistry of rumen Protozoa, 3. The carbohydrate metabolism of Entodinium. Biochem J. 1960 Sep;76:445–451. doi: 10.1042/bj0760445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABOU AKKADA A. R., BLACKBURN T. H. Some observations on the nitrogen metabolism of rumen proteolytic bacteria. J Gen Microbiol. 1963 Jun;31:461–469. doi: 10.1099/00221287-31-3-461. [DOI] [PubMed] [Google Scholar]
- ABOU AKKADA A. R., HOWARD B. H. The biochemistry of rumen protozoa. 4. Decomposition of pectic substances. Biochem J. 1961 Mar;78:512–517. doi: 10.1042/bj0780512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABOU AKKADA A. R., HOWARD B. H. The biochemistry of rumen protozoa. 5. The nitrogen metabolism of Entodinium. Biochem J. 1962 Feb;82:313–320. doi: 10.1042/bj0820313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKKADA A. R., EADIE J. M., HOWARD B. H. THE BIOCHEMISTRY OF RUMEN PROTOZOA. 7. THE CARBOHYDRASES OF POLYPLASTRON MULTIVESICULATUM (DOGIEL & FEDOROWA). Biochem J. 1963 Nov;89:268–272. doi: 10.1042/bj0890268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY R. W., CLARKE R. T., WRIGHT D. E. Carbohydrases of the rumen ciliate Epidinium ecaudatum (Crawley). Biochem J. 1962 Jun;83:517–523. doi: 10.1042/bj0830517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTIERREZ J. Experiments on the culture and physiology of holotriches from the bovine rumen. Biochem J. 1955 Jul;60(3):516–522. doi: 10.1042/bj0600516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEALD P. J., OXFORD A. E. Fermentation of soluble sugars by anaerobic holotrich ciliate protozoa of the genera Isotricha and Dasytricha. Biochem J. 1953 Feb;53(3):506–512. doi: 10.1042/bj0530506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD B. H. The biochemistry of rumen protozoa. 1. Carbohydrate fermentation by Dasytricha and Isotricha. Biochem J. 1959 Apr;71(4):671–675. doi: 10.1042/bj0710671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD B. H. The biochemistry of rumen protozoa. 2. Some carbohydrases in cell-free extracts of Dasytricha and Isotricha. Biochem J. 1959 Apr;71(4):675–680. doi: 10.1042/bj0710675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. Mutualisms in protozoa. Annu Rev Microbiol. 1950;4:53–66. doi: 10.1146/annurev.mi.04.100150.000413. [DOI] [PubMed] [Google Scholar]
- OXFORD A. E. The rumen ciliate protozoa: their chemical composition, metabolism, requirements for maintenance and culture, and physiological significance for the host. Exp Parasitol. 1955 Nov;4(6):569–605. doi: 10.1016/0014-4894(55)90045-x. [DOI] [PubMed] [Google Scholar]
- WARNER A. C. Criteria for establishing the validity of in vitro studies with rumen micro-organisms in so-called artificial rumen systems. J Gen Microbiol. 1956 Jul;14(3):733–748. doi: 10.1099/00221287-14-3-733. [DOI] [PubMed] [Google Scholar]
- WELLER R. A., GRAY F. V., PILGRIM A. F. The conversion of plant nitrogen to microbial nitrogen in the rumen of the sheep. Br J Nutr. 1958;12(4):421–429. doi: 10.1079/bjn19580056. [DOI] [PubMed] [Google Scholar]
- WILLIAMS P. P., DAVIS R. E., DOETSCH R. N., GUTIERREZ J. Physiological studies of the rumen protozoan Ophryoscolex caudatus Eberlein. Appl Microbiol. 1961 Sep;9:405–409. doi: 10.1128/am.9.5.405-409.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRIGHT D. E. Hydrogenation of lipids by rumen Protozoa. Nature. 1959 Sep 19;184:875–876. doi: 10.1038/184875a0. [DOI] [PubMed] [Google Scholar]


