Abstract
Distraction spinal cord injury is caused by some degree of distraction or longitudinal tension on the spinal cord and commonly occurs in patients who undergo corrective operation for severe spinal deformity. With the increased degree and duration of distraction, spinal cord injuries become more serious in terms of their neurophysiology, histology, and behavior. Very few studies have been published on the specific characteristics of distraction spinal cord injury. In this study, we systematically review 22 related studies involving animal models of distraction spinal cord injury, focusing particularly on the neurophysiological, histological, and behavioral characteristics of this disease. In addition, we summarize the mechanisms underlying primary and secondary injuries caused by distraction spinal cord injury and clarify the effects of different degrees and durations of distraction on the primary injuries associated with spinal cord injury. We provide new concepts for the establishment of a model of distraction spinal cord injury and related basic research, and provide reference guidelines for the clinical diagnosis and treatment of this disease.
Keywords: animal models, behavior, distraction, heterogeneity, histology, mechanism, neurophysiology, spinal cord injury, systematic review, tension
Introduction
Spinal cord injury (SCI) is caused by spinal cord contusion, dislocation, or distraction, often as a result of the sequential combination of primary trauma and secondary injury and is associated with severe disability and high mortality rates (Anjum et al., 2020; Fouad et al., 2021; Ji et al., 2020). Distraction SCI (DSCI) is caused by some degree of longitudinal tension on the spinal cord. As early as the 1970s, studies reported that DSCIs could occur during spontaneous delivery or skeletal traction procedures (Martin et al., 1971; Fried, 1974). Severe spinal deformity is a challenging task to treat from a surgical perspective. The neurological complication rate of spinal deformity surgery reported in previous studies was 2–2.6%, despite improvements and developments in surgical techniques, spinal instrumentation, and intraoperative monitoring (Reames et al., 2011; Kim et al., 2012). The incidence of DSCIs in patients with severe spinal deformities has been reported to be 0.8% (Schwartz et al., 2007); furthermore, distraction injuries continue to be the main cause of SCI during the correction of spinal deformity. Understanding the mechanisms underlying DSCI is a critical prerequisite if we are to reduce the related complication rate.
Numerous in vivo studies have been conducted to investigate the major pathophysiological process of SCI. The pathophysiological process underlying SCI consists of a primary injury caused by mechanical factors, such as distraction and trauma, along with a secondary injury (Ahuja et al., 2017a; Zhou et al., 2018b; Alizadeh et al., 2019; Wang et al., 2022). The primary injury is irreversible and directly causes axonal injury and disruption of the blood-spinal cord barrier within the first few hours (Ahuja et al., 2017b). Secondary injuries include spinal cord ischemia, cellular swelling, free radical-mediated peroxidation, inflammation, and apoptosis, which can occur as early as a few minutes after the initial injury (Pinchi et al., 2019). Tator (2006) performed a detailed analysis of all randomized and prospective controlled trials that had been published since the 1960s, and emphasized that the main obstacle facing clinically effective SCI therapy is ignoring the heterogeneity of this condition. The mechanism underlying SCI (for example, apoptosis, inflammatory response, and axonal degeneration) is known to vary with different types of SCIs under different mechanical forces; therefore, conducting in-depth research on the injury itself is essential (Choo et al., 2008).
To ascertain the appropriate treatment strategies based on the heterogeneity of SCIs, we performed a concise systematic review of DSCIs, a poorly studied topic, focusing particularly on animal models, relevant research methods, and specific neurophysiological, histological, and behavioral characterization.
Methods
This review was conducted using the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines for systematic literature reviews (Page et al., 2021; Additional file 1).
PRISMA 2020 Checklist
| Sectionand Topic | Item # | Checklist item | Location where item is reported |
|---|---|---|---|
| TITLE | P1L1 | ||
| Title | 1 | Identify the report as a systematic review. | |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | P2-P3 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | P3L12-P4L11 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | P4L12 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | P5L5 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | P4L20 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | P4L20 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | P5L3 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automationtools used in the process. | P6L12 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g. for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | P6L12-P6L14 |
| 10b | List and define all other variables for which data were sought (e.g. participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | P6L16-P6L18 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | P5L23 |
| Effectmeasures | 12 | Specify for each outcome the effect measure(s) (e.g. risk ratio, mean difference) used in the synthesis or presentation of results. | / |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g. tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | Table 1 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | P5L23 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | / | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | / | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g. subgroup analysis, meta-regression). | / | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | / | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | P5L23 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | / |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | P6L16 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | P6L24 FIU1 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | P7L1-P7L16 TABLE 1 |
| Risk of bias instudies | 18 | Present assessments of risk of bias for each included study. | P12L10-P12L18 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g. confidence/credible interval), ideally using structured tables or plots. | P7-P12 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | P7-P12 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g. confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction ofthe effect. | P7-P12 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | / | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | / | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | P12L10-P12L18 TABLE 5 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | P7L1-P7L16 TABLE 1 |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | P12-P13 |
| 23b | Discuss any limitations of the evidence included in the review. | P18 | |
| 23c | Discuss any limitations of the review processes used. | P18 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | P16-P17 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | P4L16-L19 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | P4L16-L19 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | P4L16-L19 | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | P1L24 |
| Competing interests | 26 | Declare any competing interests of review authors. | P2L4 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | submission system |
Search strategy
In March 2022, we performed serial literature searches for relevant studies in accordance with guidelines from the Cochrane Collaboration. We searched three databases, including PubMed, Embase, and the Cochrane library, without time restriction. The keywords and MeSH terms used to identify relevant articles were as follows: distraction, traction, SCI, spinal cord lacerations, spinal cord trauma, distraction osteogeneses, callotasis, neurophysiology, biology, histology, behavior, and mechanisms. The method used to search papers was based on the search formula given in Additional Table 1. Search results were exported into NoteExpress software v3.7.0 (https://noteexpress.apponic.com/) for processing. The reference lists of studies included in the systematic review were also checked for studies that were also relevant.
Additional Table 1.
Search terms used for the systematic review
| Database | Search term |
|---|---|
| PubMed | (((((((("distraction spinal cord injury") OR (distractor)) OR (distraction apparatus)) OR ("global osteotomy with continuous distraction")) OR ("spinal cord lacerations")) OR ("distraction osteogeneses")) OR (callotasis)) AND ("animal models")) AND ((((neurophysiology) OR (histology)) OR (behavior)) OR (mechanisms)) |
| Embase | [’neurophysiology’ OR ’histology’ OR ’behavior’ OR ’mechanisms’] AND [’distraction spinal cord injury’ OR ’distractor’ OR ’distraction apparatus’ OR ’global osteotomy with continuous distraction’ OR ’spinal cord lacerations’ OR ’distraction osteogeneses’ OR ’callotasis’ OR ’spinal cord trauma’] AND [’animal models’ OR ’rat’ OR ’mouse’ OR ’rabbit’ OR ’cat’ OR ’guinea pig’ OR ’dog’ OR ’animal’] |
Eligibility criteria
The following inclusion criteria were used to select articles: (1) target population: experimental animals with no species restrictions, including rats, rabbits, cats, pigs, and others; (2) intervention: DSCI surgery with no restriction in terms of surgery, including distractor, distraction apparatus, global osteotomy with continuous distraction, and others; (3) outcomes: neurophysiological, histological, and behavioral characterizations of DSCI; eligible studies included at least one of the aforementioned characterization outcomes; (4) article types: in vivo animal studies relating to DSCI, including controlled studies and observational studies; (5) language restriction: articles written in English language or published with English translations.
We excluded duplicate or multiple publications, such as the same study in different databases and the same study published in different languages. In addition, we also excluded reviews, case reports, commentaries, cadaveric research, and clinical studies.
Risk of bias assessment
The first authors of this study independently applied the risk of bias tool developed by the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) (Hooijmans et al., 2014) to confirm the validity of the included studies. Differences were resolved by consensus between the two reviewers or with assistance from an independent third party. This risk of bias tool included ten entries affiliated to six types: selection bias (sequence generation, baseline characteristics, and allocation concealment), performance bias (random housing and blinding), detection bias (random outcome assessment and blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and other forms of bias. The authors needed to answer a signaling question for each entry, which had to be answered with: “yes” (low risk of bias), “no” (high risk of bias), or “unclear” (unclear risk of bias). The qualification of each risk of bias was categorized as low, high, or unclear.
Data extraction
Two reviewers independently extracted the following data using a pre-standardized data extraction form: the title, authors, publication year, species, sample size, animal morphological models, research methods, and neurophysiological, histological, and behavioral characterizations, as well as other changes. Any disagreements were resolved by discussion between the reviewers. The extracted data were then rechecked by an independent third party. Our primary interests were neurophysiological, histological and behavioral findings, and not interventions tested after DSCIs. Therefore, data from the distraction group were included in studies involving more than one group.
Results
Identification of relevant studies
Our systematic search yielded 927 articles. Following the removal of 15 duplicates, 912 studies were identified by screening titles and abstracts. In this primary screen, 794 studies were excluded because they were clinical studies/case series, unrelated, did not refer to DSCI, or referred to peripheral nerve injury. Finally, after reviewing the full text of these articles, 22 studies were included (Dolan et al., 1980; Kling et al., 1985; Maiman et al., 1989; Jarzem et al., 1992; Liu et al., 2004, 2005; Choo et al., 2008, 2009; Skinner and Transfeldt, 2009; Seifert et al., 2011; Yang et al., 2013; Qiu et al., 2015; Chen et al., 2016; Hong et al., 2016; Wu et al., 2016, 2017; Bell et al., 2017; Shimizu et al., 2018; Guo et al., 2019; Wang et al., 2019; Han et al., 2022; Liang et al., 2022; Figure 1).
Figure 1.
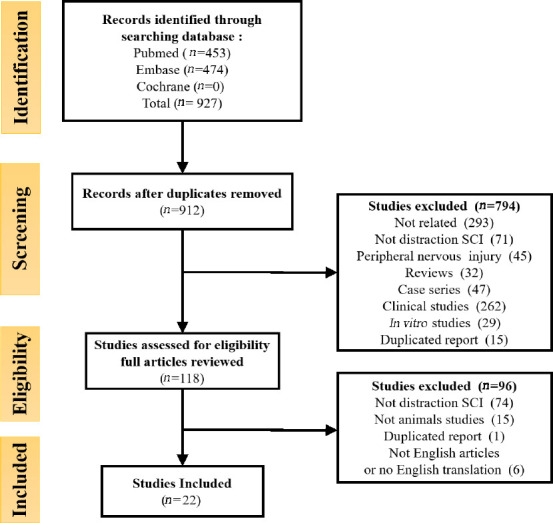
PRISMA (preferred reporting items for systematic reviews) flow diagram of the systematic review.
The systematic search yielded 927 articles. Finally, 22 studies were included.
Characteristics of the articles
The final dataset for analysis included 17 controlled studies and 5 observational studies, including included 537 animals with DSCIs: 323 rats, 72 rabbits, 59 cats, 47 pigs, 25 dogs, and 11 goats. There were 178 males (33.2%), 201 females (37.4%), and 158 animals (29.4%) with unspecified gender.
Overview of outcome measures
Due to the large differences in outcome measures and descriptive results, it was not possible to perform meta-analysis. Outcome assessments were grouped into four categories: neurophysiological, histological, behavioral evaluations, and others (including oxygen pressure, mechanical injury parameters, and imaging parameters). Evaluation methods were categorized into histology, histomorphometry, radiography, fluorescence microscopy, behavioral testing, and magnetic resonance imaging (MRI). Furthermore, the observation time after DSCIs reported by the included studies ranged from 5 minutes (Choo et al., 2009) to 8 weeks (Wang et al., 2019). Table 1 summarizes the objectives and results of the 22 animal studies.
Table 1.
Characteristics of the 22 animal model studies of distraction SCI included in this systematic review
| Studies | Study design | Animal details | Number of animals | Characteristics | Outcome methods | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Neurophysiology | Biology | Behavior | Other | |||||
| Shimizu et al., 2018 | Controlled study | Long-Evans rats, adult, female | 108 | TcMEPs | Protein carbonyl content, VMN perikaryal size & VMN nuclear size | Gait analysis | Intraparenchymal pO2 | h, hm, r, FM, N, Bt |
| Wu et al., 2017 | Controlled study | Japanese white rabbits, male, 4–5 mon | 40 | Ink perfusion, corrosion casts, MDA, SOD | h, hm | |||
| Wu et al., 2016 | Controlled study | Japanese white rabbits, male, 4–6 mon | 32 | CSEPs | Light microscopy, electron microscopy, MDA, SOD | Rivlin inclined plane test A, modified Tarlov scale | h, hm, N, Bt | |
| Chen et al., 2016 | Controlled study | Sprague-Dawley rats, male, 46 d | 10 | Spinal cord and spared tissue areas, myelinated axons density, surviving cells number | Martinez locomotor rating scale, FLAS, grooming test, grip strength test, Montoya staircase | Kinematics measurement | h, hm, FM, Bt, Bc | |
| Hong et al., 2016 | Observational study | Landrace and Yorkshire mixed pigs, 52.3 kg, young | 13 | TcMEPs | Structural changes, nerve sheath | Sensory evaluator kit, muscle strength, wake-up test | Vertebral height, disc height, segmental vertebral height length, thoracolumbar spinal length | h, r, Bt, N |
| Seifert et al., 2011 | Controlled study | Long-Evans rats, female, 300 g | 23 | TcMEPs | Neuronal bodies, extent of tissue damage (astrocytes and macrophages) | BBB | h, hm, FM, N, Bt | |
| Bell et al., 2017 | Controlled study | Long-Evans rats, female, 300 g | 60 | TcMEPs | Evaluate tissue loss, number of neurons & VMN perikaryal size & VMN nuclear, protein carbonyl content | Dynamic plantar aesthesiometer (mechanoception), BBB | Intraparenchymal pO2 | h, hm, FM, N, Bt |
| Choo et al., 2008 | Controlled study | Sprague-Dawley rats, female, 347 g | 33 | Membrane permeability, oxidative stress in neuronal somata, inflammatory responses, axonal degeneration, axonal transport dysfunction, apoptosis | h, hm, FM | |||
| Wang et al., 2019 | Controlled study | Sprague-Dawley rats, male, 280 g | 23 | Morphological characteristics, nucleus/cell body features, number of surviving cell | h, hm, FM | |||
| Qiu et al., 2015 | Observational study | Goats, adult, 28 kg | 11 | SSEP | Tarlov grading score | Spinal cord volume, T10 height, disk height, osteotomy segment height, spinal segment height | r, MRI, N, Bt | |
| Dolan et al., 1980 | Controlled study | Mongrel cats, 3.4 kg | 9 | SER | Spinal cord blood flow | h, hm, r, N | ||
| Choo et al., 2009 | Controlled study | Sprague-Dawley rats, 300 g, male | 43 | Hemorrhage, general morphology, large caliber axons, fine caliber axons, length between juxtaparanodal regions at the nodes of Ranvier | Mechanical injury parameters | h, hm, FM, Bc | ||
| Liu et al., 2004 | Controlled study | Sprague-Dawley rats, adult, 205 g, either sex | 40 | CSEPs | Neuron section area, density of Nissl body, neuron count | Gale combined behavior score | h, hm, N, Bt | |
| Liu et al., 2005 | Controlled study | Sprague-Dawley rats, adult, 235 g | 44 | CSEPs | GFAP-positive cells | Gale combined behavior score | h, hm, N, Bt | |
| Yang et al., 2013 | Controlled study | Landrace and Yorkshire mixed breed, 3.3 mon, 51.6 kg | 20 | TcMEPs | Morphological changes in the axons and morphological changes in myelin sheath, general condition of the spinal cord (hemorrhage and inflammation) | Sensory evaluator kit, muscle strength, wake-up test | Vertebral height, disc height, segmental vertebral height length, thoracolumbar spinal length | h, r, Bt, N |
| Guo et al., 2019 | Controlled study | Sprague-Dawley rats, 16 d, male | 12 | Spinal cord and spared tissue areas; myelinated axons density; surviving cells number | CatWalk system, ladder rung walking test | h, Bt | ||
| Skinner and Transfeldt, 2009 | Observational study | Pigs, young adult | 3 | TcMEPs, EMG, ScMEPs | N | |||
| Jarzem et al., 1992 | Observational study | Mix-breed dogs, 35 kg | 5 | SSEP | Cord tissue pressure, mean arterial pressure, spinal cord blood flow | h, N, | ||
| Kling et al., 1985 | Observational study | Mongrel dogs, adult, 24.5 kg | 13 | Mean arterial pressure, spinal cord blood flow | R | |||
| Maiman et al., 1989 | Controlled study | Conditioned cat | 50 | Evoked potentials | Histologic changes in gray and white matter | Modified Tarlov scale | h, N, Bt, r | |
| Han et al., 2022 | Controlled study | Experimental Bama pigs, 3 mon | 9 | ScMEPs | Histologic structure changes in gray and white matter, nerve fiber bundles structures | Modified Tarlov scale, muscle strength | h, hm, r, MRI, FM, Bt | |
| Liang et al., 2022 | Controlled study | Bama miniature pigs, 3 mon | 9 | ScMEPs | Histologic structure changes in gray and white matter, survival neuron count | Tarlov score, ILMS score | h, hm, Bt, FM | |
BBB: Basso, Beattie, Bresnahan locomotor rating scale; Bc: biomechanics; Bt: behavior test; CSEP: cortical somatosensory evoked potential; CSF: cerebrospinal fluid; CT: computed tomography; DSCI: distraction spinal cord injury; EMG: electromyography; FLAS: forelimb locomotor assessment scale; FM: fluorescence microscopy; GFAP: glial fibrillary acidic protein; h: histology; hm: histomorphometry; Iba-1: allograft inflammatory factor 1; IL: interleukin; ILMS: individual limb motor scale; MDA: malondialdehyde; MRI: magnetic esonance imaging; N: Neurophysiology; NeuN: neuronal nuclei; NF-κB P65: protein 65 of nuclear factor κB; p53: tumor protein P53; p-ERK: phosphorylated extracellular signal-regulated kinase; p-IκBα: phosphorylated nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; p-JNK: phosphorylated c-Jun N-terminal kinase; p-P38: phosphorylated p38 mitogen-activated protein kinases; r: radiography; SCI: spinal cord injury; ScMEP: spinal cord motor evoked potential; SER: spinal evoked response; SOD: superoxide dismutase; SSEP: somatosensory evoked potential; TcMEP: transcranical motor evoked potential; TLR4: Toll-like receptor 4; TNF-α: tumor necrosis factor alpha; VMN: ventral motor neurons.
Neurophysiological analysis
In addition to the studies by Maiman et al. (1989) and Dolan et al. (1980), which only reported evoked potentials and spinal evoked response (SER) without specific instructions, 15 studies applied neurophysiological assessment methods. Eight of these studies used controlled electrophysiological parameters as the criteria for grouping, as well as a criterion to evaluate whether the modeling was successful (Dolan et al., 1980; Maiman et al., 1989; Liu et al., 2004; Yang et al., 2013; Qiu et al., 2015; Hong et al., 2016; Han et al., 2022; Liang et al., 2022). Furthermore, neurological function was evaluated by evoked potentials recorded in seven of these studies (Jarzem et al., 1992; Liu et al., 2005; Skinner and Transfeldt, 2009; Seifert et al., 2011; Wu et al., 2016; Bell et al., 2017; Shimizu et al., 2018). Dolan et al. (1980) compared blood flow in the spinal cord of altered SER and abolished SER groups and concluded that distraction causes severe cord ischemia before causing the loss of SER. In another study, Skinner and Transfeldt suggested that electromyographic injury of distraction discharges elicited ipsilateral hind limb motor evoked potentials and the loss of transcranial motor evoked potentials (TcMEPs) on electromyography (Skinner and Transfeldt, 2009). When osteotomized vertebra were distracted until the TcMEPs signals disappeared or reduced to > 80%, Yang et al. (2013) found that 0%, 68.8%, and 31.2% of animals exhibited complete, incomplete, and no recovery of TcMEPs, respectively.
Biological evaluation
The most commonly used techniques described in the included studies included histological structure, observation by light microscopy or electron microscopy, immunohistochemistry, western blotting, immunofluorescence and histomorphometry.
Histopathological assessment
Table 2 shows the histopathological evaluation used in 22 included studies, as based on viewing area, specific staining, and reference frequency. Wu et al. (2016) reported that the main changes following DSCIs were hemorrhage, edema, neural cell body, and axon degeneration. Two studies (Choo et al., 2009; Chen et al., 2016) conducted morphometric analyses of the nodes of Ranvier in the juxtaparanodal region and observed elongated nodes of Ranvier caused by distraction injuries approximately 4 mm rostral to the lesion at 8 weeks after DSCIs. Three previous studies used hematoxylin and eosin staining, and Luxol fast blue staining, for histological evaluation (Seifert et al., 2011; Han et al., 2022; Liang et al., 2022) and found that tissue loss in the spinal cord was proportional to the degree of spinal distraction. In another study, Shimizu et al. argued that the cell nuclei of animals that were pre-treated with riluzole after DSCI were significantly larger than those of vehicle-treated distraction animals (P < 0.0001), thus indicating that pre-treatment with riluzole reduced damage to the ventral motor neurons during the acute stage of DSCI (Shimizu et al., 2018). Using the ink perfusion and corrosion casting technique, Wu et al. (2017) observed that micro-vessels in the spinal cord were only partially filled and appeared spastic until they eventually ruptured and hemorrhaged when experiencing increasing distraction, thus causing immediate functional and structural damage to the spinal cord tract.
Table 2.
Histopathological assessment of 22 animal model studies of spinal cord injury, as sorted by frequency
| Viewing area | Staining | Number of sample | References |
|---|---|---|---|
| VMN perikaryal size | Nissl staining | 108 | Shimizu et al., 2018 |
| 60 | Bell et al., 2017 | ||
| 40 | Liu et al., 2004 | ||
| VMN nuclear size | DAPI staining | 108 | Shimizu et al., 2018 |
| 60 | Bell et al., 2017 | ||
| Density of myelinated axons | NF/Tubulin/MBP co-immunofluorescence staining | 10 | Chen et al., 2016 |
| 43 | Choo et al., 2009 | ||
| Number of surviving cells | NeuN/Nissl staining | 10 | Chen et al., 2016 |
| 23 | Wang et al., 2019 | ||
| 23 | Seifert et al., 2011 | ||
| 9 | Han et al., 2022 | ||
| Voltage gated potassium channels | Kv1.2 | 43 | Choo et al., 2009 |
| Histologic changes in gray and white matter | Ink perfusion | 40 | Wu et al., 2017 |
| Corrosion casts | 40 | Wu et al., 2017 | |
| H&E | 32 | Wu et al., 2016 | |
| 43 | Choo et al., 2009 | ||
| 13 | Hong et al., 2016 | ||
| 23 | Seifert et al., 2011 | ||
| 60 | Bell et al., 2017 | ||
| 40 | Liu et al., 2004 | ||
| 50 | Maiman et al., 1989 | ||
| 20 | Yang et al., 2013 | ||
| 9 | Liang et al., 2022 | ||
| 9 | Han et al., 2022 | ||
| Weil’s staining | 50 | Maiman et al., 1989 | |
| Kluver-Barrera myelin | 32 | Wu et al., 2016 | |
| Bodian-Bielschowsky | |||
| Toluidine blue | |||
| LFB staining | 10 | Chen et al., 2016 | |
| 20 | Yang et al., 2013 | ||
| 9 | Liang et al., 2022 |
H&E: Hematoxylin and eosin; LFB: Luxol fast blue; MBP: myelin basic protein; VMN: ventral motor neurons.
Mechanistic evaluation
Following histological and chemical assessment, Bell et al. (2017) observed pyknosis in ventral motor neurons which typically showed nuclear contraction and hyperchromasia. Therefore, further evaluation is required to further identify the specific mechanisms responsible for the complex heterogeneity of SCI. Table 3 shows the mechanisms and histological assessment of 22 different animal model studies of SCI, as sorted by frequency. Nine of the included studies evaluated expression of related markers and regulation from six mechanistic dimensions of DSCIs. These studies identified four main aspects underlying the mechanisms responsible for DSCIs.
Table 3.
Mechanism and biological assessment of 22 studies involving animal distraction spinal cord injury models
| Injury mechanisms | Significance and function | Detection techniques | Number of samples | References |
|---|---|---|---|---|
| Oxidative stress damage | Protein carbonyl content | Protein carbonyl assay kit | 108 | Shimizu et al., 2018 |
| 60 | Bell et al., 2017 | |||
| MDA/SOD/3NT | Chromatography & colorimetric method | 40 | Wu et al., 2017 | |
| 32 | Wu et al., 2016 | |||
| 33 | Choo et al., 2008 | |||
| Immune-inflammatory | Reactive astrocytes | GFAP | 23 | Seifert et al., 2011 |
| 60 | Bell et al., 2017 | |||
| 33 | Choo et al., 2008 | |||
| 40 | Liu et al., 2004 | |||
| 9 | Liang et al., 2022 | |||
| 9 | Han et al., 2022 | |||
| IL-1β, IL-6, TNF-α | 9 | Han et al., 2022 | ||
| Activated macrophage | ED1, Iba-1, TLR4, p-IκBα, NF-κB P65, p-JNK, p-ERK, p-P38 | 23 | Seifert et al., 2011 | |
| 60 | Bell et al., 2017 | |||
| 33 | Choo et al., 2008 | |||
| 9 | Liang et al., 2022 | |||
| 9 | Han et al., 2022 | |||
| Apoptosis | Early indicators of apoptosis | Cytochrome c | 33 | Choo et al., 2008 |
| Iba-1, P53, Bax, caspase-3 | 9 | Han et al., 2022 | ||
| Transport | Axonal transport dysfunction | βAPP | 33 | Choo et al., 2008 |
| Membrane permeability | Changes in membrane permeability | Fluorescein-dextran/Cascade blue-dextran | 33 | Choo et al., 2008 |
| Degeneration | Axonal degeneration | (Non)phosphorylated neurofilament epitopes | 33 | Choo et al., 2008 |
3NT: 3-Nitrotyrosine; GFAP: glial fibrillary acidic protein; Iba-1: allograft inflammatory factor 1; IL: interleukin; MDA: malondialdehyde; NF-κB P65: protein 65 of nuclear factor κB; p53: tumor protein P53; p-ERK: phosphorylated extracellular signal-regulated kinase; p-IκBα: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; p-JNK: phosphorylated c-Jun N-terminal kinase; p-P38: phosphorylated p38 mitogen-activated protein kinases; SOD: superoxide dismutase; TLR4: Toll-like receptor 4; TNF-α: tumor necrosis factor alpha; βAPP: beta-amyloid precursor protein.
Oxidative stress damage: Shimizu et al. (2018) observed oxidative stress and metabolic impairments in animal models of SCI and found that in vehicle-treated animals, distraction induced an immediate and significant (P = 0.005) increase in the levels of protein carbonyls (8.3 ± 1.4 nmol/mL); there was a similar change two hours post-injury but there was no change in the carbonyls levels in sham animals. Moreover, previous studies reported increased malondialdehyde levels and reduced superoxide dismutase levels in tissues experiencing an increased degree and duration of distraction during the acute and subacute stages (Wu et al., 2016, 2017; Bell et al., 2017). Choo et al. (2008) detected reduced a reduced extent of 3-nitrotyrosine immunostaining two hours after distraction injury than that following contusion and dislocation in cells exhibiting neuronal morphology and positivity for 3-nitrotyrosine above a fixed background threshold.
Immune-inflammatory damage: Seifert et al. (2011) suggested that the number of reactive astrocytes and activated macrophages/microglia increased with distraction distance when labeled with antibodies against glial fibrillary acidic protein and ectodermal dysplasia 1, respectively. This indicated that the occurrence of reactive gliosis and inflammation was directly proportional to the degree of DSCIs during the subacute stage.
Apoptotic damage: Choo et al. (2008) reported that the immunostaining patterns of cytochrome c were indicative of early apoptosis during the first three hours after distraction injury. In addition, these authors were unable to identify a condition that could initiate the release of cytochrome c in the cytosol of neurons during early post-DSCIs.
Other mechanisms: Chemical techniques, such as the use of beta-amyloid precursor protein, fluorescein-dextran/cascade blue-dextran, and (non-)phosphorylated neurofilament epitopes, can be used to analyze axonal transport dysfunction, changes in membrane permeability, and axonal degeneration, respectively. Three hours after distraction injuries, Choo et al. (2008) tested the intracellular penetration of dextran-conjugated fluorophores and reported that the axolemma had extended rostrally in the gray matter. In the white matter, diffuse punctate staining of beta-amyloid precursor protein was observed in distraction injuries; from a quantitative point-of-view, the levels of beta-amyloid precursor protein were significantly lower than those observed in dislocation injuries (P < 0.001).
Behavioral assessment
Seifert et al. (2011) reported results from the Basso, Beattie and Bresnahan locomotor rating scale as a characteristic of a graded injury, which were in complete agreement with intraoperative neural monitoring data. In another study, Maiman et al. (1989) reported that measures of the Rivlin inclined plane defect of the hind limb and Tarlov score defect increased significantly (P < 0.001) with increasing neurological defects caused by distraction. Liu et al. (2004) demonstrated that groups inthere were highly significant differences in Gale combined behavior scores (P < 0.001) in the group of cortical somatosensory evoked potentials decreased by 50% and 70% compared with sham group. Other studies evaluated walking function associated with contusion and DSCI by applying the CatWalk system and the ladder rung walking test; this research found that step sequence duration, diagonal support, forelimb intensity, forelimb duty cycle, and forelimb paw angle were affected more significantly after distraction than after contusion when evaluated two weeks post-injury (Guo et al., 2019; Wang et al., 2019). However, these researchers reported a similar trend with regards to behavioral analysis irrespective of the scales and tests they applied, including the sensory evaluator kit (Hong et al., 2016), muscle strength (Yang et al., 2013), Martinez locomotor rating scale, and grooming test (Chen et al., 2016).
Other evaluations
Following distraction injury, Bell et al. (2017) and Shimizu et al. (2018) both reported direct measurements of significant reductions in the partial pressure of intraparenchymal oxygen at the epicenter of injury; oxygen pressure levels fluctuated in distraction animals during the prolonged hold phase and did not return to baseline for 15 minutes after distraction. The peak distraction forces have been reported to range from 24.1–43.9 N as distraction proceeds, as determined by mechanical injury measurements (Choo et al., 2009; Chen et al., 2016). Furthermore, Chen et al. (2016) noted that the higher peak forces that occur as a result of distraction and dislocation are the factors that cause ruptured discs and damage to other soft tissues. In another study, Yang et al. (2013) used plain radiography to reveal that DSCIs occurred at a distraction distance of 20.2 ± 4.7 mm (3.6% of thoracolumbar spine length) in farm pigs, thus correlating with thoracolumbar spine length (r = 0.632, P = 0.009). MRI showed a relative enhancement at the center of DSCI lesions in Bama pigs (Han et al., 2022). The relative intensity of T2-weighted MRI was higher in a complete DSCI group with a higher degree of DSCI; this may indicate that edema, inflammation, demyelination, axonal loss, and astrogliosis all occurred following DSCI. However, Qiu et al. (2015) determined that the safe limit of distraction distance was 11.8 ± 3.65 mm in adult goats.
Characteristics of DSCI surgery
Table 4 depicts the characteristics of DSCI surgery. The most common DSCI region studied thus far is the thoracic region (185, 34.4%), followed by the cervical (98, 18.2%), thoracolumbar (115, 21.4%), lumbar (84, 15.5%), cervicothoracic (50, 9.3%), and other (unspecified) regions (8, 1.4 %). In seven studies (165, 30.7%), DSCIs were induced in multiple regions (Maiman et al., 1989; Liu et al., 2004, 2005; Qiu et al., 2015; Hong et al., 2016; Han et al., 2022; Liang et al., 2022). For small animals, such as rats, rabbits, and cats, the distractor device is the most common distraction surgery method. The distractor device, which can be either manual or electric, must have a controllable speed because the speed of distraction has a critical impact on the degree of DSCI. The mean distraction speed recorded in these studies was between 0.083 mm/s and 1 mm/s. However, several studies simulated clinical DSCI by setting the peak velocity at 1–1.3 m/s with a linear electrical actuator (Chen et al., 2016; Guo et al., 2019; Wang et al., 2019). For large animals, local osteotomy and continuous distraction are the most common methods as these allow the optimal evaluation of the “safe” amount of distraction distance. The included studies mainly focused on the acute phase (12, 54.55%) and sub-acute phase (7, 31.82%) of DSCI injury.
Table 4.
Characteristics of DSCI surgery
| Studies | Animal species | Distraction methods | Surgical levels | Distraction distances | Distraction speeds | Periods after DSCIss |
|---|---|---|---|---|---|---|
| Shimizu et al., 2018 | Long Evans rats | Bidirectional distractor device | T10 | 5 mm | 0.5 mm/s | Acute phases (0, 2, 24 h), subacute phases (3 and 7 d) |
| Wu et al., 2017 | Japanese white rabbits | Self-designed spine distractor | L1–L3 | 0% (control), 10%, 20%, and 30% of the length between the L1 and the L3 vertebral segments | 0.083 mm/s | Acute phases (0 min) |
| Wu et al., 2016 | Japanese white rabbits | Distractor to vary the percentage of spine distraction | L1–L3 | 0% (control), 10%, 20%, and 30% of the length between the L1 and the L3 vertebral segments | 0.083 mm/s | Acute phases (6 h) |
| Chen et al., 2016 | Sprague-Dawley rats | UBC multi-mechanism SCI apparatus | C5–C6 | 5.6 mm | Peak velocity 1 m/s | Subchronic phases (8 wk) |
| Hong et al., 2016 | Landrace and Yorkshire mixed pigs | Global osteotomy + continuous distraction | T13–L1 | > 3.6% of the TLSL for significant distraction; < 3.6% of the TLSL for the continuous spinal column distraction | Acute phases (0 min) | |
| Seifert et al., 2011 | Long-Evans rats | UTA spine distractor (distractor clamps and linear actuators) | T9–T11 | 0, 3, 5, 7 mm | 1 mm/s | Subacute phases (7 d) |
| Bell et al., 2017 | Long-Evans rats | Bidirectional spine distraction | C3–C6 | 5 mm | 0.5 mm/s | Acute phases (0, 0.5 h), Subacute phases (7 d) |
| Choo et al., 2008 | Sprague-Dawley rats | SCI multi-mechanism system | T9–T11 | 4.1 ± 0.03 mm | Acute phases (2 h) | |
| Wang et al., 2019 | Sprague-Dawley rats | Linear actuator | C5–C6 | 5.6 mm | Peak velocity 1.3 m/s | Subchronic phases (8 wk) |
| Qiu et al., 2015 | Goats | Osteotome + click-type stopper | T8–T12 | 7.4–18.2 mm | Subacute phases (3 d) | |
| Dolan et al., 1980 | Mongrel cats | Distraction apparatus | L2–L3 | 1.7 ± 0.2 cm in short stretch group; 2.7 ± 0.4 cm in long stretch group | 0.083 mm/s | Acute phases (2 min) |
| Choo et al., 2009 | Sprague-Dawley rats | Multi-mechanism injury system | C4–C5 | 5.1–6.1 mm (without flexion); 4.1–4.6 mm (with flexion) | Acute phases (5 min) | |
| Liu et al., 2004 | Sprague-Dawley adult rats | Special spinal retractor | T12–L3 | 0.1 mm/s | Acute phases (1, 6, 24 h) | |
| Liu et al., 2005 | Sprague-Dawley rats | A specially-designed spinal stretching device | T12–L3 | Acute phases (24 h), Subacute phases (3, 7, 14, and 21 d) | ||
| Yang et al., 2013 | Landrace and Yorkshire mixed pigs | Global osteotomy + distracter with stopper | T9 | 20.2 ± 4.7 mm | Acute phases (2 d) | |
| Guo et al., 2019 | Sprague-Dawley rats | Linear actuator | C5–C6 | 5.6 mm | Peak velocity 1.3 m/s | Subchronic phases (2, 4, 6, 8 wk) |
| Skinner and Transfeldt, 2009 | Pigs, young adult | Hook and rod distraction | Acute phases (0 min) | |||
| Jarzem et al., 1992 | Mix-breed dogs | Specially designed distraction apparatus | ||||
| Kling et al., 1985 | Mongrel dogs | Outrigger distraction unit | T7–T8 | 1–2 cm | ||
| Maiman et al., 1989 | Conditioned cat | Kistler distraction gauge | C2–T10 | Subacute and subchronic phases (2, 4, 6, 8 wk) | ||
| Han et al., 2022 | Experimental Bama pigs | Global osteotomy + gradually distraction with a spinal spreader | T14–L1 | 2 mm/min | Subacute phase (7 d) | |
| Liang et al., 2022 | Bama miniature pigs | Global osteotomy + gradually distraction with a spinal spreader | T14–L1 | 2 mm/min | Subacute phases (7 d) |
DSCI: Distraction spinal cord injury; SCI: spinal cord injury; TLSL: thoracolumbar spinal length; UBC: University of British Columbia; UTA: University of Texas at Arlington.
Quality control of the studies
Table 5 shows the SYRCLE’s risk of bias tool (Hooijmans et al., 2014) when applied to bias information extracted from the studies included in the present study. We found that the highest risks were associated with allocation concealment (selection bias) and blinding (performance bias) in studies that did not declare the concealment method or provide evidence to demonstrate blinding. We also found the lowest risks were associated with incomplete data (attrition bias) and selective reporting (reporting bias). Moreover, we discovered that the highest risks were associated with certain studies (Maiman et al., 1989; Jarzem et al., 1992; Skinner and Transfeldt, 2009; Yang et al., 2013; Shimizu et al., 2018); collectively, these studies were associated with seven high risks of bias in random sequence analysis, baseline characteristics, allocation concealment, random housing, blinding, random outcome assessment, binding of outcome assessment. No other forms of bias were detected.
Table 5.
Summary of the SYRCLE risk of bias
| Study | Random sequence analysis | Baseline characteristics | Allocation concealment | Random housing | Blinding | Random outcome assessment | Binding of outcome assessment | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|---|---|---|
| Shimizu et al., 2018 | High | High | High | High | High | High | High | Low | Low |
| Wu et al., 2017 | Low | Low | High | Low | High | Low | Low | Low | Low |
| Wu et al., 2016 | Low | Low | High | Low | High | High | Low | Low | Low |
| Chen et al., 2016 | High | Low | High | Low | High | High | Low | Low | Low |
| Hong et al., 2016 | High | Low | High | Low | High | High | High | Low | Low |
| Seifert et al., 2011 | High | Low | High | High | High | High | High | Low | Low |
| Bell et al., 2017 | High | High | High | High | High | Low | Low | Low | Low |
| Choo et al., 2008 | High | Low | High | Low | High | Low | High | Low | Low |
| Wang et al., 2019 | High | Low | High | High | High | High | High | Low | Low |
| Qiu et al., 2015 | High | Low | High | Low | High | High | High | Low | Low |
| Dolan et al., 1980 | High | Low | High | High | High | High | High | Low | Low |
| Choo et al., 2009 | High | Low | High | High | High | High | High | Low | Low |
| Liu et al., 2004 | Low | Low | High | Low | High | High | High | Low | Low |
| Liu et al., 2005 | Low | High | High | High | High | Low | High | Low | Low |
| Yang et al., 2013 | High | High | High | High | High | High | High | Low | Low |
| Guo et al., 2019 | High | Low | High | Low | High | High | High | Low | Low |
| Skinner and Transfeld, 2009 | High | High | High | High | High | High | High | Low | Low |
| Jarzem et al., 1992 | High | High | High | High | High | High | High | Low | Low |
| Kling et al., 1985 | High | Low | High | High | High | High | High | Low | Low |
| Maiman et al., 1989 | High | High | High | High | High | High | High | Low | Low |
| Han et al., 2022 | Low | Low | High | Low | High | Low | High | Low | Low |
| Liang et al., 2022 | Low | Low | High | Low | High | Low | High | Low | Low |
Author’s judgments relating to types of bias for each publication involved the review of selection bias (sequence generation, baseline characteristics and allocation concealment), performance bias (random housing and blinding), detection bias (random outcome assessment and blinding of outcome assessment), attrition bias (incomplete outcome data) and reporting bias (selective outcome reporting). SYRCLE: Systematic Review Centre for Laboratory Animal Experimentation.
Discussion
This review summarizes animal morphological models, research methods, and more specific characterizations of DSCIs in 22 specially selected studies. Primarily, the severity of SCI, in terms of neurophysiology, histology, and behavior, increased with the degree and duration of distraction. Moreover, during distraction, the stress and strain produced in any transverse plane direction of the cord would be less than that associated with contusion or dislocation, while longitudinal or axial stress and strain were larger in distraction injuries (Chen et al., 2016). Nonetheless, DSCIs result in the greatest rostral-to-caudal extension when compared to that generated by dislocation and contusion DSCIs (Choo et al., 2008, 2009; Chen et al., 2016; Wang et al., 2019).
Animal models of DSCI
The rat was identified as the most common (60.1%) animal model used in the 22 studies included in this review, thus corroborating the findings of a previous review (72.40%) of 2209 studies of SCI (Iwanami et al., 2005). Generally, DSCI models are controlled by stretching the spinal cord to simulate the tension forces experienced by the spinal cord during SCI (Cheriyan et al., 2014). The animal models described in the studies included in this review were mainly divided into computer-controlled stepping motor, distraction apparatus, and global osteotomy with continuous distraction. Global osteotomy with continuous distraction better approximates human SCI in a variety of different aspects. From bench to bedside, severe spinal deformities can be treated by vertebral column resection techniques using an anterior, posterior, or posterior only approach (Zhou et al., 2011). Global osteotomy with continuous distraction mimics the process of osteotomy in spinal deformity correction surgery, which most likely leads to DSCI (Han et al., 2022; Liang et al., 2022).
Heterogeneity of DSCI
To investigate the complex heterogeneity of DSCI, several studies (Choo et al., 2008, 2009; Chen et al., 2016; Guo et al., 2019; Wang et al., 2019) compared histological and behavioral outcomes after contusion, dislocation, and DSCIs. Chen et al. (2016) reported that although most myelinated axons were generally spared, extracellular spaces were enlarged with structural alterations in the white matter and no grip strength recovery was observed in a DSCI group at the sub-chronic stage. DSCIs result in the greatest rostral-to-caudal extension to the dorsal horn neurons and the least white matter damage and gray matter hemorrhage (Choo et al., 2009; Wang et al., 2019). The biological characteristics reported by our systematic analysis concur perfectly with the findings of an imaging report published in 2019 (Yung et al., 2019) in which fewer focal areas showed more distraction injuries that were distributed in a rostro-caudal manner on diffusion tensor imaging. With regards to clinical SCI, although it is difficult to observe histological changes (Mattucci et al., 2019), traction has been reported to show less peak stress and strain values in the cord than those in dislocation and contusion, as demonstrated by the analysis of finite-element models (Khuyagbaatar et al., 2016).
Primary injury of DSCI
DSCI is thought to be caused by spinal cord ischemia due to vascular compromise and direct traction-induced spinal cord tract disturbances (Seyal and Mull, 2002). The distance and speed of distraction are physical factors that can affect the primary injury caused by DSCI (Seifert et al., 2011; Wu et al., 2017). Previous research has shown that 10% of the length between the relevant vertebral segments may be safe for distraction in small animals. In a previous study, Wu et al. (2016) distracted 0% (control), 10%, 20%, and 30% of the length between L1–L3 vertebral segments and found that in the 10% distraction group, the spinal cord micro-vessels exhibited a spasm-like appearance, although there was no influence on normal spinal cord circulation. The surface vessels of the spinal cord lacked a sufficient volume of blood, and the radicular artery became slender and ruptured, thus suggesting that the extent of microvascular injury was much more severe in the 30% distraction group than in the 20% distraction group (Wu et al., 2017).
Hong et al. (2016) investigated the “safe” amount of distraction that could be applied to goats and pigs; histological data showed that a continuous 74.3% segmental vertebral height distraction over an average of 10.7 min was sufficient to cause delayed SCI in pigs (Hong et al., 2016). In another study, the most clinically significant safe limit for distraction distance was identified as 11.8 ± 3.65 mm in goats (Qiu et al., 2015); the “safe” amount of distraction strongly correlated with the difference between the pre- and post-operative measurements (d value) of the spinal cord volume per 1 mm of the osteotomy segment height.
In the present study, we grouped ten studies (Dolan et al., 1980; Maiman et al., 1989; Liu et al., 2004; Seifert et al., 2011; Yang et al., 2013; Wu et al., 2016, 2017; Bell et al., 2017; Han et al., 2022; Liang et al., 2022) to investigate the different degrees of DSCIs; this analysis indicated that behavioral motor deficits and tissue loss increased with the severity of DSCIs. In addition to evaluating each feature individually, we also evaluated correlations between behavioral and histological parameters. Many significant correlations were observed from Chen et al.’s scatterplots between the various histological outcomes and behavioral scores (Chen et al., 2016). In addition, Guo et al. (2019) reported a more comprehensive evaluation of walking function, with a significant linear correlation of postoperative behavioral and histological parameters. In another study, Wilcox et al. (2017) interpreted a phenomenon wherein an injured group with similar classic hind limb behavioral results presented with similar tissue loss as that observed upon motor dysfunction; this also coincided with the loss of α-motor neurons. Furthermore, the locomotor system was described as being controlled by central pattern generators, descending pathways, and sensory feedback (Fouad and Pearson, 2004). To better understand the microscopic changes and symptomatic development of DSCIs, this focus should not be ignored as it can help to accurately clarify specific relationships between structure and function. Only by adopting this strategy will it be possible to solve the clinical challenges created by DSCIs.
Secondary injuries associated with DSCIs
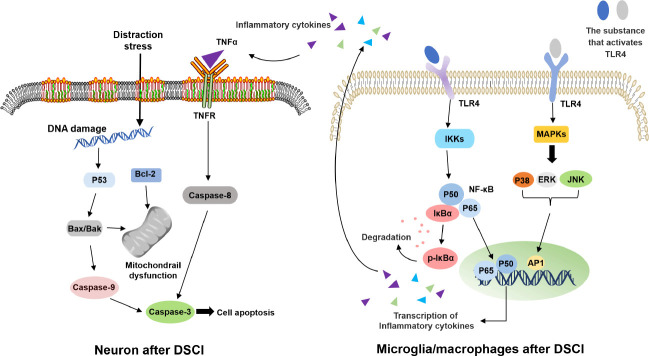
Due to the specialist nature and complexity of modeling surgery, few studies have investigated the potential mechanisms underlying the secondary injuries associated with DSCI (Choo et al., 2008; Hong et al., 2016). Two of our previous studies described potential mechanisms during the subacute stage of DSCIs; a corresponding schematic diagram is shown in Figure 2 (Han et al., 2022; Liang et al., 2022). These studies reported that two phenotypes of microglia/macrophages (M1 and M2) play a highly significant role in inflammation during the subacute stage of SCI; the M1 phenotype is exposed to T helper 1 cytokines and produces proinflammatory factors, whereas the M2 phenotype exerts anti-inflammatory effects (Kigerl et al., 2009; Zhang et al., 2012; Li et al., 2022). One previous study investigated the inflammatory mechanism induced by microglia/macrophages over the first seven days after DSCI (Liang et al., 2022). The stimulation of microglia/macrophages by DSCI led to activation of the classical inflammatory Toll-like receptor 4/nuclear factor κB/mitogen-activated protein kinase pathway; related proteins were found to be increasingly expressed (Liang et al., 2022). The process used to activate the Toll-like receptor 4/nuclear factor κB/ mitogen-activated protein kinase pathway was the same as that required to activate the classical pathway. One critical link is the phosphorylation of IκBα by inhibitory-κB kinase; this is required to transport nuclear factor κB dimers (including p65/p50 dimers) to the nucleus (Oeckinghaus and Ghosh, 2009). The activation of P38 mitogen-activated protein kinase, Jun N-terminal kinase, and extracellular signal-regulated kinase, and the further activation of activator protein-1 can promote the production of proinflammatory cytokines (Roux and Blenis, 2004; Shultz and Zhong, 2017).
Figure 2.
The inflammation mechanism induced by microglia/macrophages and the potential mechanism of apoptosis in neurons during the subacute stage of distraction spinal cord injury (DSCI).
AP1: Activating protein 1; ERK: extracellular signal-regulated kinase; IκBα: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor; IKK: inhibitor of kappa B kinase; JNK: c-Jun N-terminal kinase; MAPK: mitogen-activated protein kinase; NF-κB: nuclear factor κB; p-IκBα: phosphorylated nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; TLR4: Toll-like receptor 4; TNF: tumor necrosis factor; TNFR: tumor necrosis factor receptor.
Another study investigated the potential mechanisms of apoptosis associated with DSCI in neurons seven days after DSCI. Analysis demonstrated increased expression levels of apoptosis-related proteins (P53, Bax, and Caspase-3) in spinal cord tissues, along with reduced levels of anti-apoptosis B-cell lymphoma 2 protein expression (Han et al., 2022). Neuronal apoptosis may involve DNA damage, mitochondrial dysfunction, and may be initiated ligand tumor necrosis factor alpha and the apoptotic proteins Caspase-8 and Caspase-9 (Kigerl et al., 2009; Sui et al., 2017; Nguyen et al., 2021).
Future Perspectives
In a previous study, Shimizu et al. (2018) demonstrated that prophylactic riluzole could alleviate oxidative stress damage associated with DSCIs. Riluzole, a benzothiazole sodium channel blocker (Azbill et al., 2000) has been shown to protect against excitotoxic cell death by inhibiting sodium influx in injured neurons and by limiting the release of glutamate at synapses to reduce neuronal loss and improve clinical outcomes (Simard et al., 2012). In another study, Liu et al. (2005) reported that post-injury treatment with fibroblast growth factor could promote recovery by inducing the massive expression of glial fibrillary acidic protein after DSCIs in rats. This treatment was also shown to be effective for traumatic SCI in rats (Zhang et al., 2013). Fibroblast growth factors are known to stimulate neuronal cell fate determination, migration, and differentiation (Zhou et al., 2018b).
Although there are few studies on DSCI therapeutics, an increasing number of studies are investigating innovative therapeutics for SCI. The effect of methylprednisolone (MP) for the treatment of SCI is still under debate although Fehlings et al. recommend offering patients a 24-hour infusion of MP as a treatment option within 8 hours of acute SCI (Fehlings et al., 2017). The transplantation of Schwann cells, neural stem cells or progenitor cells, olfactory ensheathing cells (OECs), oligodendrocyte precursor cells, and mesenchymal stem cells have all been investigated in animal experiments (Assinck et al., 2017; Mukhamedshina et al., 2019) but have yet to be translated to the clinic. In addition to being used as a biological scaffold to guide the growth of nerve cells, hydrogels can also be directly used as drug delivery carriers (Liu et al., 2022). Given that pharmacological therapies (Kamiya et al., 2015; Zhu et al., 2020), cell-based regenerative therapies (Salewski et al., 2015; Silvestro et al., 2020), and biomaterials (Zhou et al., 2018a; Ma et al., 2020) have become significant research hotspots in SCI, relevant therapeutic interventions for DSCIs should be conducted based on the known heterogeneity of SCIs.
At present, a significant amount of DSCI research is focusing on the early diagnosis and prevention of SCI in clinical practice. For example, Liu et al. (2022) reported that surgeon-directed TcMEP monitoring has a 100% negative predictive value and allows the early identification of physiological cord distress. Surgeons are also increasingly using novel MRI sequences for the early observation and detection of SCI. Magnetic resonance-diffusion tensor imaging was previously shown to reflect the post-SCI pathological status of the spinal cord parenchyma which is known to be associated with locomotor performance (Zhao et al., 2018). Zhao et al. (2022) also used magnetic resonance-diffusion tensor imaging methods to monitor spatial and temporal changes in lesion areas and associated these changes with histological changes. However, a prediction model of neurophysiological monitoring and imaging sequences has yet to be applied for basic DSCI experiments; this has limited the translation of DSCI. Such deficiencies in DSCI research provides a significant opportunity for future research.
Limitations
One limitation of this review was the absence of relevant gene expression and general regulatory mechanisms of SCI (Ryge et al., 2010; Tica et al., 2018); therefore, the mechanisms underlying gene expression following DSCIs has yet to be fully explored. Due to inconsistencies associated with the animal models used in the studies included in this review, it was not possible to compare the relative survival times; this factor could have influenced certain histological parameters such as membrane permeability, hemorrhage, edema, cell death and axon degeneration. Furthermore, the various study designs and quality control aspects of the included studies showed that most were associated with a high risk of bias, especially in termns of sample selection and performance. However, dealing with these limitations is the main focus of our next research.
Conclusion
Our analysis identified several detailed and instructive results. We also highlight the heterogeneity of SCI, especially for different injury types, degrees, interventions, post-surgical durations, and research methods. These characteristics and changes provide some key concepts for basic research and facilitating the clinical diagnosis and treatment of DSCIs.
Additional files:
Additional file 1: PRISMA checklist.
Additional Table 1: Search terms used for the systematic review.
Acknowledgments:
This systematic review is scheduled for 2022, at a time when humans are fighting COVID-19. Heartfelt thanks and high respect to people around the world who have fulfilled their duties with loyalty and made significant contributions to containing the epidemic. We believe humans will victory over coronavirus in the near future certainly.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 81772421 (to YH).
Conflicts of interest: The authors declare that there are no conflicts of interest.
Data availability statement: All relevant data are within the paper and its Additional files.
Editor’s evaluation: The review focused on animal models of distraction spinal cord injury, summarized the modeling methods, injury characteristics, and evaluation methods of DSCI in different species to provide a basis for DSCI animal modeling and analyzed and discussed the topic of the paper from multiple perspectives, which is logical. The conclusion also provides a sort and summary for the treatment of spinal cord injury.
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
References
- 1.Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017a;3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017b;80:S9–S22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 3.Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury:an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK, Lokanathan Y. Spinal cord injury:pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020;21:7533. doi: 10.3390/ijms21207533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20:637–647. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 6.Azbill RD, Mu X, Springer JE. Riluzole increases high-affinity glutamate uptake in rat spinal cord synaptosomes. Brain Res. 2000;871:175–180. doi: 10.1016/s0006-8993(00)02430-6. [DOI] [PubMed] [Google Scholar]
- 7.Bell JES, Seifert JL, Shimizu EN, Sucato DJ, Romero-Ortega MI. Atraumatic spine distraction induces metabolic distress in spinal motor neurons. J Neurotrauma. 2017;34:2034–2044. doi: 10.1089/neu.2016.4779. [DOI] [PubMed] [Google Scholar]
- 8.Chen K, Liu J, Assinck P, Bhatnagar T, Streijger F, Zhu Q, Dvorak MF, Kwon BK, Tetzlaff W, Oxland TR. Differential histopathological and behavioral outcomes eight weeks after rat spinal cord injury by contusion, dislocation, and distraction mechanisms. J Neurotrauma. 2016;33:1667–1684. doi: 10.1089/neu.2015.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheriyan T, Ryan DJ, Weinreb JH, Cheriyan J, Paul JC, Lafage V, Kirsch T, Errico TJ. Spinal cord injury models:a review. Spinal Cord. 2014;52:588–595. doi: 10.1038/sc.2014.91. [DOI] [PubMed] [Google Scholar]
- 10.Choo AM, Liu J, Dvorak M, Tetzlaff W, Oxland TR. Secondary pathology following contusion, dislocation, and distraction spinal cord injuries. Exp Neurol. 2008;212:490–506. doi: 10.1016/j.expneurol.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 11.Choo AM, Liu J, Liu Z, Dvorak M, Tetzlaff W, Oxland TR. Modeling spinal cord contusion, dislocation, and distraction:characterization of vertebral clamps, injury severities, and node of Ranvier deformations. J Neurosci Methods. 2009;181:6–17. doi: 10.1016/j.jneumeth.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Dolan EJ, Transfeldt EE, Tator CH, Simmons EH, Hughes KF. The effect of spinal distraction on regional spinal cord blood flow in cats. J Neurosurg. 1980;53:756–764. doi: 10.3171/jns.1980.53.6.0756. [DOI] [PubMed] [Google Scholar]
- 13.Fehlings MG, Tetreault LA, Wilson JR, Kwon BK, Burns AS, Martin AR, Hawryluk G, Harrop JS. A clinical practice guideline for the management of acute spinal cord injury:introduction, rationale, and scope. Global Spine J. 2017;7:84s–94s. doi: 10.1177/2192568217703387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fouad K, Pearson K. Restoring walking after spinal cord injury. Prog Neurobiol. 2004;73:107–126. doi: 10.1016/j.pneurobio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Fouad K, Popovich PG, Kopp MA, Schwab JM. The neuroanatomical-functional paradox in spinal cord injury. Nat Rev Neurol. 2021;17:53–62. doi: 10.1038/s41582-020-00436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried LC. Cervical spinal cord injury during skeletal traction. JAMA. 1974;229:181–183. [PubMed] [Google Scholar]
- 17.Guo Y, Hu H, Wang J, Zhang M, Chen K. Walking function after cervical contusion and distraction spinal cord injuries in rats. J Exp Neurosci. 2019;13:1179069519869615. doi: 10.1177/1179069519869615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han B, Liang W, Hai Y, Liu Y, Chen Y, Ding H, Yang J, Yin P. Elucidating the potential mechanisms underlying distraction spinal cord injury-associated neuroinflammation and apoptosis. Front Cell Dev Biol. 2022;10:839313. doi: 10.3389/fcell.2022.839313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong JY, Suh SW, Lee SH, Park JH, Park SY, Rhyu IJ, Yang JH. Continuous distraction-induced delayed spinal cord injury on motor-evoked potentials and histological changes of spinal cord in a porcine model. Spinal Cord. 2016;54:649–655. doi: 10.1038/sc.2015.231. [DOI] [PubMed] [Google Scholar]
- 20.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwanami A, Yamane J, Katoh H, Nakamura M, Momoshima S, Ishii H, Tanioka Y, Tamaoki N, Nomura T, Toyama Y, Okano H. Establishment of graded spinal cord injury model in a nonhuman primate:the common marmoset. J Neurosci Res. 2005;80:172–181. doi: 10.1002/jnr.20435. [DOI] [PubMed] [Google Scholar]
- 22.Jarzem PF, Quance DR, Doyle DJ, Begin LR, Kostuik JP. Spinal cord tissue pressure during spinal cord distraction in dogs. Spine (Phila Pa 1976) 1992;17:S227–234. doi: 10.1097/00007632-199208001-00003. [DOI] [PubMed] [Google Scholar]
- 23.Ji HY, Gu J, Xie LH, Wu XT. Application of stem cells, tissue engineering scaffolds and neurotrophic factors in the treatment of spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2020;24:4088–4093. [Google Scholar]
- 24.Kamiya K, Koda M, Furuya T, Kato K, Takahashi H, Sakuma T, Inada T, Ota M, Maki S, Okawa A, Ito Y, Takahashi K, Yamazaki M. Neuroprotective therapy with granulocyte colony-stimulating factor in acute spinal cord injury:a comparison with high-dose methylprednisolone as a historical control. Eur Spine J. 2015;24:963–967. doi: 10.1007/s00586-014-3373-0. [DOI] [PubMed] [Google Scholar]
- 25.Khuyagbaatar B, Kim K, Man Park W, Hyuk Kim Y. Biomechanical behaviors in three types of spinal cord injury mechanisms. J Biomech Eng. 2016;138:081003. doi: 10.1115/1.4033794. [DOI] [PubMed] [Google Scholar]
- 26.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SS, Cho BC, Kim JH, Lim DJ, Park JY, Lee BJ, Suk SI. Complications of posterior vertebral resection for spinal deformity. Asian Spine J. 2012;6:257–265. doi: 10.4184/asj.2012.6.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kling TF, Jr, Fergusson NV, Leach AB, Hensinger RN, Lane GA, Knight PR. The influence of induced hypotension and spine distraction on canine spinal cord blood flow. Spine (Phila Pa 1976) 1985;10:878–883. doi: 10.1097/00007632-198512000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Wu Z, Zhou L, Shao J, Hu X, Xu W, Ren Y, Zhu X, Ge W, Zhang K, Liu J, Huang R, Yu J, Luo D, Yang X, Zhu W, Zhu R, Zheng C, Sun YE, Cheng L. Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Signal Transduct Target Ther. 2022;7:65. doi: 10.1038/s41392-022-00885-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang W, Han B, Hai Y, Liu Y, Liu X, Yang J, Sun D, Yin P. The role of microglia/macrophages activation and TLR4/NF-κB/MAPK pathway in distraction spinal cord injury-induced inflammation. Front Cell Neurosci. 2022;16:926453. doi: 10.3389/fncel.2022.926453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu K, Dong X, Wang Y, Wu X, Dai H. Dopamine-modified chitosan hydrogel for spinal cord injury. Carbohydr Polym. 2022;298:120047. doi: 10.1016/j.carbpol.2022.120047. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Chi LT, Tu ZQ, Sheng B, Zhou ZK, Pei FX. Observation and establishment of an animal model of tractive spinal cord injury in rats. Chin J Traumatol. 2004;7:372–377. [PubMed] [Google Scholar]
- 33.Liu L, Lü B, Tu CQ, Chi LT, Wang GL, Pei FX. Effect of basic fibroblast growth factor on the expression of glial fibrillary acidic protein after tractive spinal cord injury in rats. Chin J Traumatol. 2005;8:117–120. [PubMed] [Google Scholar]
- 34.Ma D, Zhao Y, Huang L, Xiao Z, Chen B, Shi Y, Shen H, Dai J. A novel hydrogel-based treatment for complete transection spinal cord injury repair is driven by microglia/macrophages repopulation. Biomaterials. 2020;237:119830. doi: 10.1016/j.biomaterials.2020.119830. [DOI] [PubMed] [Google Scholar]
- 35.Maiman DJ, Myklebust JB, Ho KC, Coats J. Experimental spinal cord injury produced by axial tension. J Spinal Disord. 1989;2:6–13. [PubMed] [Google Scholar]
- 36.Martin C, Babin JP, Martinez M, Camier P, Lorin JC, Cazauran JM. Spinal cord (and brain stem) injury at birth (4 cases) Arch Fr Pediatr. 1971;28:989–990. [PubMed] [Google Scholar]
- 37.Mattucci S, Speidel J, Liu J, Kwon BK, Tetzlaff W, Oxland TR. Basic biomechanics of spinal cord injury - How injuries happen in people and how animal models have informed our understanding. Clin Biomech (Bristol, Avon) 2019;64:58–68. doi: 10.1016/j.clinbiomech.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Mukhamedshina YO, Gracheva OA, Mukhutdinova DM, Chelyshev YA, Rizvanov AA. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen Res. 2019;14:227–237. doi: 10.4103/1673-5374.244778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen TTM, Gillet G, Popgeorgiev N. Caspases in the developing central nervous system:apoptosis and beyond. Front Cell Dev Biol. 2021;9:702404. doi: 10.3389/fcell.2021.702404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, et al. The PRISMA 2020 statement:an updated guideline for reporting systematic reviews. BMJ. 2021:372–n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pinchi E, Frati A, Cantatore S, D'Errico S, Russa R, Maiese A, Palmieri M, Pesce A, Viola RV, Frati P, Fineschi V. Acute spinal cord injury:a systematic review investigating miRNA families involved. Int J Mol Sci. 2019;20:1841. doi: 10.3390/ijms20081841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu F, Yang JC, Ma XY, Xu JJ, Yang QL, Zhou X, Xiao YS, Hu HS, Xia LH. Influence of vertebral column distraction on spinal cord volume:an experimental study in a goat model. Arch Orthop Trauma Surg. 2015;135:1201–1210. doi: 10.1007/s00402-015-2264-0. [DOI] [PubMed] [Google Scholar]
- 44.Reames DL, Smith JS, Fu KM, Polly DW, Jr, Ames CP, Berven SH, Perra JH, Glassman SD, McCarthy RE, Knapp RD, Jr, Heary R, Shaffrey CI Scoliosis Research Society Morbidity and Mortality Committee. Complications in the surgical treatment of 19,360 cases of pediatric scoliosis:a review of the Scoliosis Research Society Morbidity and Mortality database. Spine (Phila Pa 1976) 2011;36:1484–1491. doi: 10.1097/BRS.0b013e3181f3a326. [DOI] [PubMed] [Google Scholar]
- 45.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases:a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryge J, Winther O, Wienecke J, Sandelin A, Westerdahl AC, Hultborn H, Kiehn O. Transcriptional regulation of gene expression clusters in motor neurons following spinal cord injury. BMC Genomics. 2010;11:365. doi: 10.1186/1471-2164-11-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salewski RP, Mitchell RA, Li L, Shen C, Milekovskaia M, Nagy A, Fehlings MG. Transplantation of induced pluripotent stem cell-derived neural stem cells mediate functional recovery following thoracic spinal cord injury through remyelination of axons. Stem Cells Transl Med. 2015;4:743–754. doi: 10.5966/sctm.2014-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz DM, Auerbach JD, Dormans JP, Flynn J, Drummond DS, Bowe JA, Laufer S, Shah SA, Bowen JR, Pizzutillo PD, Jones KJ, Drummond DS. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89:2440–2449. doi: 10.2106/JBJS.F.01476. [DOI] [PubMed] [Google Scholar]
- 49.Seifert JL, Bell JE, Elmer BB, Sucato DJ, Romero MI. Characterization of a novel bidirectional distraction spinal cord injury animal model. J Neurosci Methods. 2011;197:97–103. doi: 10.1016/j.jneumeth.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Seyal M, Mull B. Mechanisms of signal change during intraoperative somatosensory evoked potential monitoring of the spinal cord. J Clin Neurophysiol. 2002;19:409–415. doi: 10.1097/00004691-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Shimizu EN, Seifert JL, Johnson KJ, Romero-Ortega MI. Prophylactic riluzole attenuates oxidative stress damage in spinal cord distraction. J Neurotrauma. 2018;35:1319–1328. doi: 10.1089/neu.2017.5494. [DOI] [PubMed] [Google Scholar]
- 52.Shultz RB, Zhong Y. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen Res. 2017;12:702–713. doi: 10.4103/1673-5374.206633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silvestro S, Bramanti P, Trubiani O, Mazzon E. Stem cells therapy for spinal cord injury:an overview of clinical trials. Int J Mol Sci. 2020;21:659. doi: 10.3390/ijms21020659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simard JM, Tsymbalyuk O, Keledjian K, Ivanov A, Ivanova S, Gerzanich V. Comparative effects of glibenclamide and riluzole in a rat model of severe cervical spinal cord injury. Exp Neurol. 2012;233:566–574. doi: 10.1016/j.expneurol.2011.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skinner SA, Transfeldt EE. Electromyography in the detection of mechanically induced spinal motor tract injury:observations in diverse porcine models. J Neurosurg Spine. 2009;11:369–374. doi: 10.3171/2009.3.SPINE08881. [DOI] [PubMed] [Google Scholar]
- 56.Sui T, Ge DW, Yang L, Tang J, Cao XJ, Ge YB. Mitomycin C induces apoptosis in human epidural scar fibroblasts after surgical decompression for spinal cord injury. Neural Regen Res. 2017;12:644–653. doi: 10.4103/1673-5374.205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tator CH. Review of treatment trials in human spinal cord injury:issues, difficulties, and recommendations. Neurosurgery. 2006;59:957–982. doi: 10.1227/01.NEU.0000245591.16087.89. discussion 982-987. [DOI] [PubMed] [Google Scholar]
- 58.Tica J, Bradbury EJ, Didangelos A. Combined transcriptomics, proteomics and bioinformatics identify drug targets in spinal cord injury. Int J Mol Sci. 2018;19:1461. doi: 10.3390/ijms19051461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang HD, Wei ZJ, Li JJ, Feng SQ. Application value of biofluid-based biomarkers for the diagnosis and treatment of spinal cord injury. Neural Regen Res. 2022;17:963–971. doi: 10.4103/1673-5374.324823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Zhang M, Guo Y, Hu H, Chen K. Quantification of surviving neurons after contusion, dislocation, and distraction spinal cord injuries using automated methods. J Exp Neurosci. 2019;13:1179069519869617. doi: 10.1177/1179069519869617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilcox JT, Satkunendrarajah K, Nasirzadeh Y, Laliberte AM, Lip A, Cadotte DW, Foltz WD, Fehlings MG. Generating level-dependent models of cervical and thoracic spinal cord injury:Exploring the interplay of neuroanatomy, physiology, and function. Neurobiol Dis. 2017;105:194–212. doi: 10.1016/j.nbd.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 62.Wu D, Zheng C, Wu J, Xue J, Huang R, Wu D, Song Y. The pathologic mechanisms underlying lumbar distraction spinal cord injury in rabbits. Spine J. 2017;17:1665–1673. doi: 10.1016/j.spinee.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 63.Wu J, Xue J, Huang R, Zheng C, Cui Y, Rao S. A rabbit model of lumbar distraction spinal cord injury. Spine J. 2016;16:643–658. doi: 10.1016/j.spinee.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 64.Yang JH, Suh SW, Modi HN, Ramani ET, Hong JY, Hwang JH, Jung WY. Effects of vertebral column distraction on transcranial electrical stimulation-motor evoked potential and histology of the spinal cord in a porcine model. J Bone Joint Surg Am. 2013;95:835–842, S1-2. doi: 10.2106/JBJS.K.00575. [DOI] [PubMed] [Google Scholar]
- 65.Yung A, Mattucci S, Bohnet B, Liu J, Fournier C, Tetzlaff W, Kozlowski P, Oxland T. Diffusion tensor imaging shows mechanism-specific differences in injury pattern and progression in rat models of acute spinal cord injury. Neuroimage. 2019;186:43–55. doi: 10.1016/j.neuroimage.2018.10.067. [DOI] [PubMed] [Google Scholar]
- 66.Zhang HY, Zhang X, Wang ZG, Shi HX, Wu FZ, Lin BB, Xu XL, Wang XJ, Fu XB, Li ZY, Shen CJ, Li XK, Xiao J. Exogenous basic fibroblast growth factor inhibits ER stress-induced apoptosis and improves recovery from spinal cord injury. CNS Neurosci Ther. 2013;19:20–29. doi: 10.1111/cns.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang N, Yin Y, Xu SJ, Wu YP, Chen WS. Inflammation &apoptosis in spinal cord injury. Indian J Med Res. 2012;135:287–296. [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao C, Rao JS, Pei XJ, Lei JF, Wang ZJ, Zhao W, Wei RH, Yang ZY, Li XG. Diffusion tensor imaging of spinal cord parenchyma lesion in rat with chronic spinal cord injury. Magn Reson Imaging. 2018;47:25–32. doi: 10.1016/j.mri.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Zhao C, Rao JS, Duan H, Hao P, Shang J, Fan Y, Zhao W, Gao Y, Yang Z, Sun YE, Li X. Chronic spinal cord injury repair by NT3-chitosan only occurs after clearance of the lesion scar. Signal Transduct Target Ther. 2022;7:184. doi: 10.1038/s41392-022-01010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou C, Liu L, Song Y, Liu H, Li T, Gong Q, Zeng J, Kong Q. Anterior and posterior vertebral column resection for severe and rigid idiopathic scoliosis. Eur Spine J. 2011;20:1728–1734. doi: 10.1007/s00586-011-1861-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou X, Shi G, Fan B, Cheng X, Zhang X, Wang X, Liu S, Hao Y, Wei Z, Wang L, Feng S. Polycaprolactone electrospun fiber scaffold loaded with iPSCs-NSCs and ASCs as a novel tissue engineering scaffold for the treatment of spinal cord injury. Int J Nanomedicine. 2018a;13:6265–6277. doi: 10.2147/IJN.S175914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Y, Wang Z, Li J, Li X, Xiao J. Fibroblast growth factors in the management of spinal cord injury. J Cell Mol Med. 2018b;22:25–37. doi: 10.1111/jcmm.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu S, Chen M, Chen M, Ye J, Ying Y, Wu Q, Dou H, Bai L, Mao F, Ni W, Yu K. Fibroblast growth factor 22 inhibits ER stress-induced apoptosis and improves recovery of spinal cord injury. Front Pharmacol. 2020;11:18. doi: 10.3389/fphar.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]