Summary
Background
Cancer is a leading cause of premature mortality globally. This study estimates premature deaths at ages 30–69 years and distinguishes these as deaths that are preventable (avertable through primary or secondary prevention) or treatable (avertable through curative treatment) in 185 countries worldwide.
Methods
For this population-based study, estimated cancer deaths by country, cancer, sex, and age groups were retrieved from the International Agency for Research on Cancer's GLOBOCAN 2020 database. Crude and age-adjusted cancer-specific years of life lost (YLLs) were calculated for 36 cancer types.
Findings
Of the estimated all-ages cancer burden of 265·6 million YLLs, 182·8 million (68·8%) YLLs were due to premature deaths from cancer globally in 2020, with 124·3 million (68·0%) preventable and 58·5 million (32·0%) treatable. Countries with low, medium, or high human development index (HDI) levels all had greater proportions of YLLs at premature ages than very high HDI countries (68·9%, 77·0%, and 72·2% vs 57·7%, respectively). Lung cancer was the leading contributor to preventable premature YLLs in medium to very high HDI countries (17·4% of all cancers, or 29·7 million of 171·3 million YLLs), whereas cervical cancer led in low HDI countries (26·3% of all preventable cancers, or 1·83 million of 6·93 million YLLs). Colorectal and breast cancers were major treatable cancers across all four tiers of HDI (25·5% of all treatable cancers in combination, or 14·9 million of 58·5 million YLLs).
Interpretation
Alongside tailored programmes of early diagnosis and screening linked to timely and comprehensive treatment, greater investments in risk factor reduction and vaccination are needed to address premature cancer inequalities.
Funding
Erasmus Mundus Exchange Programme and the International Agency for Research on Cancer.
Translations
For the German, French, Spanish and Chinese translations of the abstract see Supplementary Materials section.
Introduction
Cancer has become an increasingly important cause of premature mortality globally,1 and is associated with high societal and economic costs. As an example, productivity losses linked to premature cancer deaths were estimated at €104·6 billion or 0·62% of the national gross domestic product in Europe,2 and at US$46·3 billion or 0·33% of the combined gross domestic product of the BRICS countries (Brazil, Russia, India, China, and South Africa).3 It has been argued that premature deaths from specific cancers should be avoidable in the presence of effective public health interventions and health-care systems.4 However, this approach neglects the nature of cancer as a heterogeneous group of diseases with varying natural histories, aetiologies, and outcomes.
In this study, we discuss premature mortality (preventable and treatable) with more nuance, which distinguishes between deaths that could be averted before the development of the disease, through effective public health measures (preventable mortality), or after the onset of disease through health-care interventions (treatable mortality).5 Using such an approach, the Organisation for Economic Co-operation and Development (OECD) estimated that in 2017 there were 1·9 million premature deaths from diseases and injuries that were preventable and over 1 million premature deaths treatable across the 38 OECD member countries.6 About a third (31%) of those preventable and a quarter (27%) of those treatable were cancers. However, the study only considered a comparatively small number of (high-income) countries. Given the increasing importance of cancer as a cause of death worldwide, a more comprehensive assessment of premature mortality is needed.
This study extends the OECD work to a global estimation and presentation of the burden of premature, preventable, and treatable deaths and years of life lost (YLLs) from 36 cancer types in 2020 in 185 countries, by world region and the Human Development Index (HDI). In identifying the main types of cancers driving premature cancer mortality, and disaggregating this burden by sex, we aim to aid efforts to implement, monitor, and tailor national health policies and cancer control plans to optimally reduce premature mortality in the countries’ communities.
Methods
Data sources
For this population-based study, cancer mortality estimates were retrieved from the GLOBOCAN 2020 database of the International Agency for Research on Cancer.7 We obtained number of deaths by country, sex, and 5-year age groups (starting from 0–4, and going up to 85+) for 36 specific cancer types according to the International Classification of Diseases, tenth revision listed as the primary cause of death: lip, oral cavity (C00–06), salivary glands (C07–C08), oropharynx (C09–C10), nasopharynx (C11), hypopharynx (C12–C13), oesophagus (C15), stomach (C16), colon (C18), rectum (C19–20), anus (C21), liver and intrahepatic bile ducts (C22), gallbladder (C23), pancreas (C25), larynx (C32), trachea, bronchus and lung (C33–34), melanoma of skin (C43), skin, non-melanoma (C44), mesothelioma (C45), Kaposi sarcoma (C46), female breast (C50), vagina (C51), vulva (C52), cervix uteri (C53), corpus uteri (C54), ovary (C56), penis (C60), prostate (C61), testis (C62), kidney and renal pelvis (C64–65), bladder (C67), brain and CNS (C70–72), thyroid (C73), Hodgkin lymphoma (C81), non-Hodgkin lymphoma (C82–86, C96), multiple myeloma and immunoproliferative diseases (C88+90), leukaemia (C91–95), other specified sites (C17, C24, C30–31, C37–38, C40–41, C47–49, C57–58, C63, C66, C68–69, C74–75), and unspecified sites (C76–80, C97). The 36 specific cancer types were selected because they are the most common cancer types, constituting 92% of the total cancer deaths globally.8
Research in context.
Evidence before this study
We searched PubMed for publications from Feb 1, 2010, to Feb 10, 2023, using the search terms (cancer) AND (mortality) AND ((premature) OR (preventable) OR (treatable) OR (amenable) OR (avertable) OR (avoidable)) for global and international studies assessing the burden of premature cancer mortality. Various age thresholds and definitions of premature and avoidable mortality have been developed—eg, premature deaths occurring at ages 30–69 years or before 75 years, and deaths considered amenable through the health system. Previous studies have shown that cancer is a leading cause of both premature and avoidable mortality. Although international variations in premature avoidable mortality from non-communicable diseases (NCDs) have been reported, few studies have undertaken a comprehensive study on cancer and distinguished premature deaths into preventable and treatable components by country and cancer type. When constructing a list of all diseases and injuries considered preventable or treatable, the Organisation for Economic Co-operation and Development (OECD) and Eurostat also included a subgroup of cancers, but did this analysis in a select group of high-income countries only. The study from the Global Burden of Disease, Injuries and Risk Factors 2017 included countries from all regions of the world but, similar to the OECD and Eurostat approach, omitted key infection-attributable cancers from their analysis, such as Kaposi sarcoma and non-Hodgkin lymphoma. To our knowledge, this is the first global analysis of premature, preventable, and treatable age-standardised years of life lost (YLLs) by country, cancer, and sex.
Added value of this study
This study brings new perspectives to existing evidence on premature cancer mortality by estimating YLLs in 185 countries and 36 cancer types, distinguishing them as either preventable or treatable deaths. We estimate that of the 183 million YLLs globally due to premature cancer death in 2020, around two-thirds (68%) were preventable and a third (32%) treatable, with considerable variability between and within world regions. Our study also highlights the diverse effect of premature death from specific cancer types in individual countries, across levels of human development, and by sex. This analysis provides the epidemiological framework for the Lancet Commission on women, power, and cancer.
Implications of all the available evidence
Many countries are lagging in achieving the UN Sustainable Development Goals target 3·4 of a one-third reduction in premature mortality from NCDs by 2030. This study draws attention to the substantial burden of deaths from cancer at ages 30–69 years, all of which we consider potentially avoidable through primary prevention or early detection (ie, early diagnosis or screening), or through curative treatment. In combination with existing evidence of avoidable cancer mortality, our findings aid the work of the Lancet Commission on women, power, and cancer, governments, and health-care planners to identify and implement tailored interventions to accelerate progress in both cancer control and in meeting the global Sustainable Development Goals target for NCDs.
In presenting the results, we grouped specific cancer sites in the oral cavity and the pharynx together (C00–C14) and cancers of the colorectum (C18–21), although both are heterogeneous groups of cancers with different prognoses. We aggregated countries using the UN-defined world regions.8 We presented results by HDI, a composite index based on life expectancy, education, and gross national income in its four-tier classification of countries as: low (HDI<0·55), medium (HDI 0·55–0·70), high (HDI 0·70–0·79), or very high HDI (≥0·80), derived from the 2019 Human Development Report of the UN.8 Population data estimates for 2020 were retrieved from the UN World Population Prospects.8 All data used were from secondary sources and did not require ethical approval.
Defining premature, preventable, and treatable cancer deaths
We defined premature mortality as per the WHO definition of deaths between and including ages 30 to 69 years.9 Preventable or treatable cancer deaths were identified from existing lists of avoidable causes of death,5, 10 which we modified and extended on the basis of the following. Cancer deaths were considered preventable if there was sizeable evidence to show that (1) primary prevention—ie, reduced exposure to risk factors through public health policies, programmes, or interventions (population attributable fraction ≥30%)11, 12, 13, 14, 15, 16—would lead to a reduction in cancer deaths or (2) early detection, including population-based screening, was effective in reducing deaths through either preventing the occurrence of cancer or its progression to malignancy (cervical, colorectal, and breast cancer).17 Cancer deaths were considered treatable if there was sufficient evidence that effective evidence-based treatment with curative intent led to improved 5-year relative survival,18 and a reduction in cancer deaths. We prioritised prevention over treatment for cancers that are both preventable and treatable but have a population attributable fraction of 100%, following the argument that preventing cancer from occurring in the first place eliminates the need for treatment. If evidence suggested that prevention and treatment might be similarly effective in relation to reducing cancer-specific mortality (eg, for female breast cancer and colorectal cancer), we allocated deaths equally to prevention and treatment. In comparison to the OECD and Eurostat list,10 we included an additional 11 cancer types listed and marked in appendix 5 (pp 7–9).
Analytical approach
The number and proportion of premature cancer deaths as the basis of this analysis are in appendix 5 (pp 10–15). YLLs due to premature deaths from cancer were calculated by multiplying the country-specific, cancer-specific, age-specific, and sex-specific number of deaths by the remaining years of life left at each mid-age of the 5-year age groups using the standard life expectancies from WHO.19 The YLLs is considered a robust indicator for the comparison of premature avoidable mortality across different populations,4 and we derived the proportion of YLLs due to premature cancer death by summing YLLs due to cancer between the ages of 30 and 69 years9 and dividing by the total YLLs from cancer death across all ages. Age-standardised rates of YLLs due to premature cancer death per 100 000 person-years were computed using the direct method and the World Standard Population.20 The proportions of premature cancer deaths and YLLs that were preventable or treatable were calculated following the list in appendix 5 (pp 4–9). Uncertainty intervals (95% UI) of cancer deaths and YLLs were estimated using three data quality components of mortality data in each country (appendix 5 p 3). All results expressed as total numbers of YLLs and age-standardised rates were rounded to the nearest hundred. All data manipulation, analyses, and visualisation were done using the statistical software R (version 1.3.1093).
Sensitivity analysis
We did a sensitivity analysis to assess the robustness of the thresholds used to determine the preventability and treatability of a given cancer type (appendix 5 p 6).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
A global total of 5·28 million deaths from cancer occurred prematurely in 2020, of which 3·63 million were preventable and 1·65 million were treatable (appendix 5 pp 10–15). There were 2·90 million premature deaths in men, and 2·30 million premature deaths in women. In terms of premature YLLs, there were 182·8 million from cancer globally, which accounted for 68·8% of the total YLLs (265·6 million) from cancer across all ages (table). Of the total premature YLLs, 124·3 million (68·0%) of 182·8 million were avoidable through primary prevention or early detection, and 58·5 million (32·0%) through treatment with curative intent. Although men had a higher proportion of preventable premature YLLs than women (70·3% [70·4 million/100·2 million] vs 65·2% [53·8 million/82·6 million]), the proportion of treatable premature YLLs was higher for women than for men (34·8% [28·8 million/82·6 million] vs 29·7% [29·7 million/100·2 million]; table).
Table.
Premature, preventable, and treatable YLLs from cancer (ages 30–69 years) by world region, sex, HDI, and income group (Gross National Income), 2020
| Total YLLs, n (UI) |
Premature cancer mortality |
Premature preventable cancer mortality |
Premature treatable cancer mortality |
||||
|---|---|---|---|---|---|---|---|
| n (%; UI) | ASR (UI) | n (%; UI) | ASR (UI) | n (%; UI) | ASR (UI) | ||
| Eastern Africa | |||||||
| Both | 8 592 600 (8 136 000–9 074 700) | 5 848 800 (68·1%; 5 538 000–6 177 000) | 5700 (5400–6000) | 3 796 900 (64·9%; 3 595 100–4 009 900) | 3700 (3500–3900) | 2 052 000 (35·1%; 1 942 900–2 167 100) | 2000 (1900–2100) |
| Male | 3 345 100 (3 067 300–3 648 000) | 1 987 300 (59·4%; 1 822 300–2 167 300) | 4200 (3800–4600) | 1 140 100 (57·4%; 1 045 400–1 243 400) | 2300 (2100–2500) | 847 200 (42·6%; 776 900–924 000) | 1900 (1700–2000) |
| Female | 5 247 500 (4 887 500–5 634 000) | 3 861 500 (73·6%; 3 596 600–4 145 900) | 7100 (6600–7600) | 2 656 700 (68·8%; 2 474 500–2 852 400) | 4900 (4600–5300) | 1 204 700 (31·2%; 1 122 100–1 293 500) | 2200 (2000–2300) |
| Middle Africa | |||||||
| Both | 2 708 500 (2 564 300–2 860 900) | 1 857 900 (68·6%; 1 759 000–1 962 500) | 4700 (4400–4900) | 1 142 300 (61·5%; 1 081 400–1 206 500) | 2900 (2700–3000) | 715 700 (38·5%; 677 600–756 000) | 1800 (1700–1900) |
| Male | 1 136 700 (1 041 200–1 240 800) | 700 300 (61·6%; 641 500–764 400) | 3800 (3500–4100) | 378 700 (54·1%; 346 900–413 400) | 2000 (1800–2100) | 321 500 (45·9%; 294 500–351 000) | 1800 (1700–2000) |
| Female | 1 571 900 (1 465 500–1 686 000) | 1 157 700 (73·6%; 1 079 300–1 241 700) | 5500 (5200–6000) | 763 500 (66·0%; 711 800–818 900) | 3700 (3500–4000) | 394 200 (34·0%; 367 500–422 800) | 1800 (1700–2000) |
| Northern Africa | |||||||
| Both | 5 807 900 (5 646 900–5 973 500) | 4 189 300 (72·1%; 4 073 200–4 308 700) | 4900 (4700–5000) | 2 653 900 (63·3%; 2 580 300–2 729 600) | 3100 (3000–3200) | 1 535 400 (36·7%; 1 492 800–1 579 100) | 1800 (1700–1800) |
| Male | 3 097 700 (2 972 900–3 227 800) | 2 209 100 (71·3%; 2 120 100–2 301 900) | 5300 (5100–5500) | 1 513 300 (68·5%; 1 452 300–1 576 800) | 3600 (3500–3800) | 695 800 (31·5%; 667 800–725 100) | 1600 (1600–1700) |
| Female | 2 710 200 (2 607 800–2 816 500) | 1 980 100 (73·1%; 1 905 400–2 057 800) | 4500 (4300–4600) | 1 140 600 (57·6%; 1 097 500–1 185 400) | 2600 (2500–2700) | 839 500 (42·4%; 807 800–872 500) | 1900 (1800–1900) |
| Southern Africa | |||||||
| Both | 1 993 900 (1 970 900–2 017 000) | 1 520 500 (76·3%; 1 503 000–1 538 200) | 6200 (6100–6300) | 1 012 100 (66·6%; 1 000 400–1 023 800) | 4100 (4100–4200) | 508 500 (33·4%; 502 600–514 400) | 2100 (2100–2100) |
| Male | 913 500 (898 700–928 600) | 680 900 (74·5%; 669 800–692 100) | 6300 (6200–6400) | 429 700 (63·1%; 422 700–436 800) | 3900 (3900–4000) | 251 100 (36·9%; 247 100–255 300) | 2300 (2300–2400) |
| Female | 1 080 300 (1 063 200–1 097 700) | 839 700 (77·7%; 826 300–853 200) | 6300 (6200–6400) | 582 300 (69·4%; 573 100–591 700) | 4400 (4300–4400) | 257 300 (30·6%; 253 200–261 500) | 1900 (1900–2000) |
| Western Africa | |||||||
| Both | 6 177 600 (5 795 700–6 584 600) | 4 397 600 (71·2%; 4 125 800–4 687 400) | 4700 (4400–5000) | 2 559 900 (58·2%; 2 401 600–2 728 500) | 2700 (2500–2900) | 1 837 800 (41·8%; 1 724 200–1 958 900) | 2000 (1800–2100) |
| Male | 2 548 800 (2 305 900–2 817 300) | 1 645 900 (64·6%; 1 489 100–1 819 300) | 3700 (3400–4100) | 849 000 (51·6%; 768 100–938 400) | 1900 (1700–2100) | 796 900 (48·4%; 721 000–880 800) | 1900 (1700–2000) |
| Female | 3 628 800 (3 340 500–3 941 900) | 2 751 700 (75·8%; 2 533 100–2 989 200) | 5600 (5100–6000) | 1 710 800 (62·2%; 1 574 900–1 858 500) | 3500 (3200–3800) | 1 040 900 (37·8%; 958 200–1 130 700) | 2100 (1900–2300) |
| Caribbean | |||||||
| Both | 1 587 300 (1 551 200–1 624 300) | 1 000 800 (63·0%; 978 000–1 024 100) | 4900 (4800–5100) | 605 700 (60·5%; 591 900–619 800) | 3000 (2900–3100) | 395 100 (39·5%; 386 100–404 300) | 2000 (1900–2000) |
| Male | 824 600 (800 000–850 000) | 481 400 (58·4%; 467 000–496 200) | 4900 (4800–5100) | 284 700 (59·1%; 276 200–293 500) | 2900 (2800–3000) | 196 700 (40·9%; 190 800–202 700) | 2000 (2000–2100) |
| Female | 762 700 (736 600–789 700) | 519 400 (68·1%; 501 600–537 800) | 5000 (4800–5100) | 320 900 (61·8%; 309 900–332 300) | 3100 (3000–3200) | 198 500 (38·2%; 191 700–205 500) | 1900 (1800–2000) |
| Central America | |||||||
| Both | 3 621 500 (3 588 400–3 655 000) | 2 349 400 (64·9%; 2 327 900–2 371 100) | 3300 (3300–3400) | 1 369 200 (58·3%; 1 356 600–1 381 800) | 1900 (1900–2000) | 980 200 (41·7%; 971 300–989 300) | 1400 (1400–1400) |
| Male | 1 687 500 (1 665 500–1 709 800) | 978 600 (58·0%; 965 800–991 500) | 3000 (3000–3000) | 529 500 (54·1%; 522 600–536 500) | 1600 (1600–1700) | 449 100 (45·9%; 443 300–455 100) | 1400 (1300–1400) |
| Female | 1 934 000 (1 909 000–1 959 300) | 1 370 800 (70·9%; 1 353 100–1 388 800) | 3600 (3600–3700) | 839 700 (61·3%; 828 800–850 700) | 2200 (2200–2300) | 531 100 (38·7%; 524 200–538 100) | 1400 (1400–1400) |
| South America | |||||||
| Both | 13 590 900 (13 505 700–13 676 600) | 8 958 800 (65·9%; 8 902 700–9 015 300) | 4500 (4500–4600) | 5 367 400 (59·9%; 5 333 700–5 401 200) | 2700 (2700–2700) | 3 591 400 (40·1%; 3 568 900–3 614 100) | 1800 (1800–1800) |
| Male | 6 787 700 (6 729 100–6 846 800) | 4 272 300 (62·9%; 4 235 400–4 309 500) | 4600 (4500–4600) | 2 463 200 (57·7%; 2 441 900–2 484 700) | 2600 (2600–2700) | 1 809 100 (42·3%; 1 793 400–1 824 800) | 1900 (1900–1900) |
| Female | 6 803 100 (6 740 700–6 866 100) | 4 686 600 (68·9%; 4 643 600–4 730 000) | 4500 (4500–4600) | 2 904 200 (62·0%; 2 877 500–2 931 100) | 2800 (2800–2800) | 1 782 400 (38·0%; 1 766 000–1 798 900) | 1700 (1700–1700) |
| Northern America | |||||||
| Both | 15 262 000 (15 213 900–15 310 200) | 8 938 100 (58·6%; 8 909 900–8 966 300) | 4100 (4100–4100) | 5 666 100 (63·4%; 5 648 300–5 684 000) | 2600 (2600–2600) | 3 271 900 (36·6%; 3 261 600–3 282 300) | 1500 (1500–1500) |
| Male | 8 029 300 (7 994 500–8 064 200) | 4 654 800 (58·0%; 4 634 600–4 675 100) | 4300 (4300–4300) | 2 962 700 (63·6%; 2 949 900–2 975 600) | 2700 (2700–2700) | 1 692 100 (36·4%; 1 684 700–1 699 400) | 1600 (1600–1600) |
| Female | 7 232 700 (7 199 200–7 266 300) | 4 283 200 (59·2%; 4 263 400–4 303 200) | 3900 (3900–4000) | 2 703 400 (63·1%; 2 690 900–2 716 000) | 2500 (2400–2500) | 1 579 900 (36·9%; 1 572 500–1 587 200) | 1500 (1500–1500) |
| Eastern Asia | |||||||
| Both | 92 017 700 (91 750 200–92 285 900) | 64 028 500 (69·6%; 63 842 400–64 215 100) | 6200 (6200–6200) | 50 040 300 (78·2%; 49 894 900–50 186 200) | 4800 (4800–4800) | 13 988 100 (21·8%; 13 947 500–14 028 900) | 1400 (1400–1400) |
| Male | 56 124 000 (55 905 100–56 343 800) | 39 611 400 (70·6%; 39 456 900–39 766 600) | 7600 (7600–7700) | 32 589 100 (82·3%; 32 461 900–32 716 700) | 6300 (6200–6300) | 7 022 400 (17·7%; 6 995 000–7 049 900) | 1400 (1400–1400) |
| Female | 35 893 600 (35 735 600–36 052 400) | 24 417 000 (68·0%; 24 309 500–24 525 000) | 4800 (4800–4800) | 17 451 300 (71·5%; 17 374 400–17 528 500) | 3400 (3400–3400) | 6 965 800 (28·5%; 6 935 100–6 996 600) | 1400 (1400–1400) |
| Southeastern Asia | |||||||
| Both | 20 986 500 (20 711 200–21 265 500) | 16 097 700 (76·7%; 15 886 600–16 311 700) | 5400 (5400–5500) | 10 533 400 (65·4%; 10 395 200–10 673 400) | 3600 (3500–3600) | 5 564 400 (34·6%; 5 491 400–5 638 300) | 1900 (1800–1900) |
| Male | 11 378 200 (11 159 300–11 601 300) | 8 735 600 (76·8%; 8 567 600–8 906 900) | 6100 (6000–6200) | 5 918 300 (67·7%; 5 804 400–6 034 300) | 4100 (4100–4200) | 2 817 300 (32·3%; 2 763 200–2 872 600) | 2000 (1900–2000) |
| Female | 9 608 300 (9 436 900–9 782 900) | 7 362 100 (76·6%; 7 230 800–7 495 900) | 4800 (4800–4900) | 4 615 100 (62·7%; 4 532 800–4 699 000) | 3000 (3000–3100) | 2 747 000 (37·3%; 2 698 000–2 796 900) | 1800 (1800–1800) |
| South central Asia | |||||||
| Both | 41 955 200 (41 457 400–42 458 900) | 32 226 700 (76·8%; 31 844 300–32 613 600) | 4100 (4100–4200) | 19 479 600 (60·4%; 19 248 500–19 713 500) | 2500 (2500–2600) | 12 747 000 (39·6%; 12 595 800–12 900 100) | 1600 (1600–1600) |
| Male | 21 771 500 (21 409 300–22 139 800) | 16 374 200 (75·2%; 16 101 800–16 651 200) | 4200 (4100–4300) | 9 584 300 (58·5%; 9 424 900–9 746 500) | 2500 (2400–2500) | 6 789 900 (41·5%; 6 676 900–6 904 800) | 1700 (1700–1700) |
| Female | 20 183 700 (19 844 200–20 529 000) | 15 852 400 (78·5%; 15 585 800–16 123 700) | 4100 (4000–4200) | 9 895 300 (62·4%; 9 728 800–10 064 600) | 2600 (2500–2600) | 5 957 100 (37·6%; 5 856 900–6 059 100) | 1500 (1500–1600) |
| Western Asia | |||||||
| Both | 6 992 600 (6 906 100–7 080 300) | 4 893 400 (70·0%; 4 832 800–4 954 800) | 4800 (4800–4900) | 2 962 100 (60·5%; 2 925 400–2 999 300) | 3000 (3000–3000) | 1 931 300 (39·5%; 1 907 400–1 955 500) | 1800 (1800–1900) |
| Male | 3 923 700 (3 859 100–3 989 300) | 2 690 900 (68·6%; 2 646 700–2 735 900) | 5400 (5300–5400) | 1 756 800 (65·3%; 1 727 900–1 786 200) | 3600 (3500–3700) | 934 100 (34·7%; 918 800–949 800) | 1800 (1700–1800) |
| Female | 3 069 000 (3 011 600–3 127 500) | 2 202 500 (71·8%; 2 161 300–2 244 500) | 4400 (4300–4500) | 1 205 300 (54·7%; 1 182 800–1 228 300) | 2400 (2400–2500) | 997 200 (45·3%; 978 500–1 016 200) | 2000 (1900–2000) |
| Eastern Europe | |||||||
| Both | 17 470 600 (17 369 000–17 572 700) | 12 295 800 (70·4%; 12 224 300–12 367 700) | 6600 (6600–6600) | 7 874 500 (64·0%; 7 828 700–7 920 500) | 4200 (4200–4200) | 4 421 300 (36·0%; 4 395 600–4 447 100) | 2400 (2400–2400) |
| Male | 9 875 100 (9 796 700–9 954 200) | 7 190 100 (72·8%; 7 133 000–7 247 700) | 8500 (8500–8600) | 4 757 000 (66·2%; 4 719 200–4 795 000) | 5600 (5600–5700) | 2 433 200 (33·8%; 2 413 800–2 452 600) | 2900 (2900–2900) |
| Female | 7 595 400 (7 530 800–7 660 600) | 5 105 600 (67·2%; 5 062 200–5 149 400) | 5100 (5100–5200) | 3 117 500 (61·1%; 3 091 000–3 144 300) | 3100 (3100–3200) | 1 988 100 (38·9%; 1 971 200–2 005 200) | 2000 (2000–2000) |
| Northern Europe | |||||||
| Both | 5 414 800 (5 377 000–5 453 000) | 2 604 100 (48·1%; 2 585 900–2 622 500) | 4200 (4100–4200) | 1 626 600 (62·5%; 1 615 200–1 638 100) | 2600 (2600–2600) | 977 500 (37·5%; 970 700–984 400) | 1600 (1600–1600) |
| Male | 2 877 100 (2 850 200–2 904 300) | 1 354 000 (47·1%; 1 341 400–1 366 900) | 4400 (4300–4400) | 847 000 (62·6%; 839 100–855 000) | 2700 (2700–2700) | 507 000 (37·4%; 502 300–511 800) | 1700 (1600–1700) |
| Female | 2 537 700 (2 511 500–2 564 200) | 1 250 100 (49·3%; 1 237 100–1 263 100) | 4000 (4000–4000) | 779 600 (62·4%; 771 500–787 700) | 2500 (2400–2500) | 470 500 (37·6%; 465 600–475 400) | 1500 (1500–1600) |
| Southern Europe | |||||||
| Both | 8 644 800 (8 582 300–8 707 700) | 4 682 300 (54·2%; 4 648 500–4 716 400) | 4700 (4700–4700) | 3 049 500 (65·1%; 3 027 500–3 071 700) | 3000 (3000–3000) | 1 632 800 (34·9%; 1 621 000–1 644 700) | 1700 (1700–1700) |
| Male | 5 032 700 (4 984 600–5 081 200) | 2 722 200 (54·1%; 2 696 200–2 748 400) | 5500 (5500–5600) | 1 837 400 (67·5%; 1 819 900–1 855 100) | 3700 (3700–3700) | 884 800 (32·5%; 876 400–893 300) | 1800 (1800–1900) |
| Female | 3 612 200 (3 571 900–3 652 900) | 1 960 100 (54·3%; 1 938 300–1 982 200) | 3900 (3900–4000) | 1 212 100 (61·8%; 1 198 600–1 225 800) | 2400 (2400–2400) | 748 000 (38·2%; 739 700–756 400) | 1500 (1500–1500) |
| Western Europe | |||||||
| Both | 11 285 800 (11 220 400–11 351 700) | 6 039 500 (53·5%; 6 004 400–6 074 700) | 4800 (4800–4900) | 4 000 200 (66·2%; 3 977 000–4 023 500) | 3200 (3100–3200) | 2 039 300 (33·8%; 2 027 400–2 051 200) | 1700 (1700–1700) |
| Male | 6 376 400 (6 326 800–6 426 400) | 3 453 800 (54·2%; 3 427 000–3 480 900) | 5500 (5500–5600) | 2 325 300 (67·3%; 2 307 200–2 343 500) | 3700 (3700–3700) | 1 128 500 (32·7%; 1 119 800–1 137 400) | 1900 (1800–1900) |
| Female | 4 909 400 (4 866 400–4 952 800) | 2 585 700 (52·7%; 2 563 000–2 608 500) | 4100 (4100–4200) | 1 674 900 (64·8%; 1 660 200–1 689 700) | 2700 (2600–2700) | 910 700 (35·2%; 902 800–918 800) | 1500 (1500–1500) |
| Australia and New Zealand | |||||||
| Both | 1 183 200 (1 169 300–1 197 300) | 616 200 (52·1%; 608 900–623 500) | 3700 (3600–3700) | 374 400 (60·8%; 370 000–378 900) | 2200 (2200–2200) | 241 700 (39·2%; 238 900–244 600) | 1500 (1400–1500) |
| Male | 652 400 (642 400–662 500) | 327 900 (50·3%; 322 900–333 000) | 3900 (3900–4000) | 198 800 (60·6%; 195 700–201 900) | 2400 (2300–2400) | 129 100 (39·4%; 127 200–131 200) | 1600 (1500–1600) |
| Female | 530 800 (521 300–540 500) | 288 300 (54·3%; 283 100–293 500) | 3400 (3400–3500) | 175 700 (60·9%; 172 500–178 900) | 2100 (2000–2100) | 112 600 (39·1%; 110 600–114 600) | 1400 (1300–1400) |
| Oceania excluding Australia and New Zealand | |||||||
| Both | 346 000 (295 300–435 900) | 264 100 (76·3%; 225 300–332 700) | 6600 (5600–8300) | 166 000 (62·9%; 141 600–209 100) | 4200 (3500–5200) | 98 100 (37·1%; 83 700–123 600) | 2500 (2100–3100) |
| Male | 157 500 (126 300–225 800) | 112 700 (71·5%; 90 400–161 600) | 6000 (4800–8600) | 70 300 (62·4%; 56 400–100 700) | 3700 (3000–5300) | 42 400 (37·6%; 34 000–60 800) | 2200 (1800–3200) |
| Female | 188 500 (154 100–268 900) | 151 400 (80·3%; 123 800–216 000) | 7300 (6000–10400) | 95 700 (63·2%; 78 200–136 600) | 4600 (3800–6600) | 55 700 (36·8%; 45 500–79 400) | 2700 (2200–3800) |
| Very high HDI | |||||||
| Both | 74 790 800 (73 200 900–76 415 200) | 43 164 800 (57·7%; 42 247 200–44 102 400) | 4700 (4600–4800) | 27 933 100 (64·7%; 27 339 300–28 539 800) | 3000 (3000–3100) | 15 231 800 (35·3%; 14 908 000–15 562 600) | 1700 (1600–1700) |
| Male | 41 897 900 (40 688 100–43 143 600) | 24 273 900 (57·9%; 23 573 000–24 995 600) | 5400 (5200–5500) | 16 159 300 (66·6%; 15 692 700–16 639 700) | 3500 (3400–3600) | 8 114 600 (33·4%; 7 880 300–8 355 900) | 1800 (1800–1900) |
| Female | 32 892 900 (31 869 900–33 948 800) | 18 890 900 (57·4%; 18 303 400–19 497 300) | 4100 (4000–4200) | 11 773 800 (62·3%; 11 407 600–12 151 700) | 2500 (2500–2600) | 7 117 100 (37·7%; 6 895 800–7 345 600) | 1600 (1500–1600) |
| High HDI | |||||||
| Both | 123 517 100 (121 538 700–125 527 700) | 89 171 300 (72·2%; 87 743 000–90 622 800) | 5900 (5800–6000) | 65 037 000 (72·9%; 63 995 300–66 095 700) | 4300 (4200–4300) | 24 134 300 (27·1%; 23 747 700–24 527 100) | 1600 (1600–1600) |
| Male | 71 569 900 (70 011 300–73 163 300) | 51 821 600 (72·4%; 50 693 000–52 975 300) | 6900 (6700–7000) | 39 767 300 (76·7%; 38 901 300–40 652 700) | 5300 (5200–5400) | 12 054 200 (23·3%; 11 791 700–12 322 600) | 1600 (1600–1600) |
| Female | 51 947 200 (50 727 900–53 195 700) | 37 349 700 (71·9%; 36 473 100–38 247 400) | 4900 (4800–5000) | 25 269 700 (67·7%; 24 676 600–25 877 000) | 3300 (3200–3400) | 12 080 100 (32·3%; 11 796 500–12 370 400) | 1600 (1500–1600) |
| Medium HDI | |||||||
| Both | 50 655 000 (48 658 900–52 733 000) | 38 979 200 (77·0%; 37 443 200–40 578 200) | 4500 (4300–4600) | 24 335 300 (62·4%; 23 376 400–25 333 600) | 2800 (2700–2900) | 14 643 900 (37·6%; 14 066 800–15 244 600) | 1700 (1600–1700) |
| Male | 26 242 100 (24 598 100–27 995 900) | 19 866 900 (75·7%; 18 622 200–21 194 600) | 4600 (4300–4900) | 12 222 900 (61·5%; 11 457 200–13 039 800) | 2800 (2600–3000) | 7 644 000 (38·5%; 7 165 100–8 154 800) | 1700 (1600–1900) |
| Female | 24 412 900 (23 191 600–25 698 500) | 19 112 400 (78·3%; 18 156 200–20 118 800) | 4400 (4100–4600) | 12 112 500 (63·4%; 11 506 500–12 750 300) | 2800 (2600–2900) | 6 999 900 (36·6%; 6 649 700–7 368 500) | 1600 (1500–1700) |
| Low HDI | |||||||
| Both | 16 569 900 (14 102 500–19 469 100) | 11 422 400 (68·9%; 9 721 500–13 420 900) | 5000 (4200–5800) | 6 927 400 (60·6%; 5 895 900–8 139 500) | 3000 (2600–3500) | 4 494 900 (39·4%; 3 825 600–5 281 400) | 2000 (1700–2300) |
| Male | 6 772 100 (5 328 100–8 607 600) | 4 183 900 (61·8%; 3 291 700–5 317 800) | 3900 (3100–5000) | 2 260 700 (54·0%; 1 778 600–2 873 400) | 2100 (1600–2600) | 1 923 200 (46·0%; 1 513 100–2 444 400) | 1800 (1400–2300) |
| Female | 9 797 800 (7 880 200–12 182 000) | 7 238 500 (73·9%; 5 821 800–8 999 900) | 6000 (4800–7400) | 4 666 700 (64·5%; 3 753 400–5 802 400) | 3900 (3100–4800) | 2 571 800 (35·5%; 2 068 400–3 197 600) | 2100 (1700–2600) |
| High income | |||||||
| Both | 55 247 600 (54 813 300–55 690 600) | 29 694 100 (53·7%; 29 460 600–29 932 200) | 4300 (4300–4400) | 19 032 100 (64·1%; 18 882 500–19 184 700) | 2700 (2700–2800) | 10 662 000 (35·9%; 10 578 200–10 747 500) | 1600 (1600–1600) |
| Male | 30 559 000 (30 233 500–30 894 200) | 16 307 000 (53·4%; 16 133 300–16 485 900) | 4700 (4700–4800) | 10 623 800 (65·1%; 10 510 600–10 740 300) | 3100 (3000–3100) | 5 683 200 (34·9%; 5 622 700–5 745 600) | 1700 (1700–1700) |
| Female | 24 688 600 (24 401 900–24 983 500) | 13 387 100 (54·2%; 13 231 600–13 546 900) | 3900 (3900–4000) | 8 408 300 (62·8%; 8 310 700–8 508 700) | 2500 (2400–2500) | 4 978 800 (37·2%; 4 920 900–5 038 200) | 1500 (1500–1500) |
| Upper-middle income | |||||||
| Both | 119 522 800 (118 872 200–120 185 300) | 85 662 100 (71·7%; 85 195 800–86 136 900) | 6100 (6000–6100) | 63 249 200 (73·8%; 62 904 900–63 599 800) | 4500 (4400–4500) | 22 412 900 (26·2%; 22 290 900–22 537 100) | 1600 (1600–1600) |
| Male | 69 921 500 (69 431 100–70 424 200) | 50 466 800 (72·2%; 50 112 900–50 829 600) | 7300 (7200–7300) | 39 274 700 (77·8%; 38 999 300–39 557 100) | 5600 (5600–5700) | 11 192 100 (22·2%; 11 113 600–11 272 500) | 1600 (1600–1600) |
| Female | 49 601 300 (49 176 800–50 037 600) | 35 195 300 (71·0%; 34 894 100–35 504 900) | 4900 (4900–5000) | 23 974 500 (68·1%; 23 769 300–24 185 400) | 3300 (3300–3400) | 11 220 800 (31·9%; 11 124 800–11 319 500) | 1600 (1600–1600) |
| Lower-middle income | |||||||
| Both | 69 549 900 (67 597 400–71 644 700) | 53 241 200 (76·6%; 51 746 500–54 844 800) | 4600 (4500–4800) | 32 814 500 (61·6%; 31 893 300–33 802 900) | 2900 (2800–2900) | 20 426 700 (38·4%; 19 853 200–21 041 900) | 1800 (1700–1800) |
| Male | 35 937 200 (34 487 400–37 547 900) | 27 081 100 (75·4%; 25 988 500–28 294 800) | 4700 (4600–5000) | 16 508 700 (61·0%; 15 842 700–17 248 600) | 2900 (2800–3000) | 10 572 400 (39·0%; 10 145 900–11 046 300) | 1800 (1800–1900) |
| Female | 33 612 700 (32 321 800–35 033 800) | 26 160 100 (77·8%; 25 155 400–27 266 100) | 4500 (4300–4700) | 16 305 800 (62·3%; 15 679 600–16 995 200) | 2800 (2700–2900) | 9 854 300 (37·7%; 9 475 800–10 270 900) | 1700 (1600–1800) |
| Low income | |||||||
| Both | 11 921 300 (10 957 300–13 000 000) | 8 149 000 (68·4%; 7 490 100–8 886 500) | 5200 (4800–5700) | 5 160 300 (63·3%; 4 743 000–5 627 300) | 3300 (3000–3600) | 2 988 700 (36·7%; 2 747 100–3 259 200) | 1900 (1700–2100) |
| Male | 4 834 200 (4 243 100–5 541 100) | 2 952 700 (61·1%; 2 591 600–3 384 400) | 4100 (3600–4700) | 1 725 700 (58·4%; 1 514 700–1 978 100) | 2400 (2100–2700) | 1 226 900 (41·6%; 1 076 900–1 406 300) | 1700 (1500–2000) |
| Female | 7 087 000 (6 349 100–7 941 200) | 5 196 400 (73·3%; 4 655 300–5 822 600) | 6200 (5600–7000) | 3 434 600 (66·1%; 3 076 900–3 848 500) | 4200 (3700–4700) | 1 761 800 (33·9%; 1 578 300–1 974 100) | 2100 (1900–2300) |
| World | |||||||
| Both | 265 639 400 (259 316 700–272 116 400) | 182 809 400 (68·8%; 178 458 200–187 266 800) | 5200 (5100–5300) | 124 280 000 (68·0%; 121 321 900–127 310 200) | 3600 (3500–3600) | 58 529 500 (32·0%; 57 136 400–59 956 600) | 1700 (1600–1700) |
| Male | 146 539 600 (141 844 700–151 389 800) | 100 183 500 (68·4%; 96 973 800–103 499 500) | 5800 (5600–6000) | 70 435 200 (70·3%; 68 178 600–72 766 500) | 4100 (4000–4200) | 29 748 400 (29·7%; 28 795 300–30 733 000) | 1700 (1700–1800) |
| Female | 119 099 900 (114 911 600–123 440 800) | 82 625 900 (69·4%; 79 720 300–85 637 400) | 4700 (4500–4800) | 53 844 800 (65·2%; 51 951 300–55 807 300) | 3000 (2900–3200) | 28 781 100 (34·8%; 27 769 000–29 830 100) | 1600 (1600–1700) |
ASR=age-standardised rate (per 100 000 person-years). HDI=Human Development Index. UI=uncertainty interval. LLs=years of life lost.
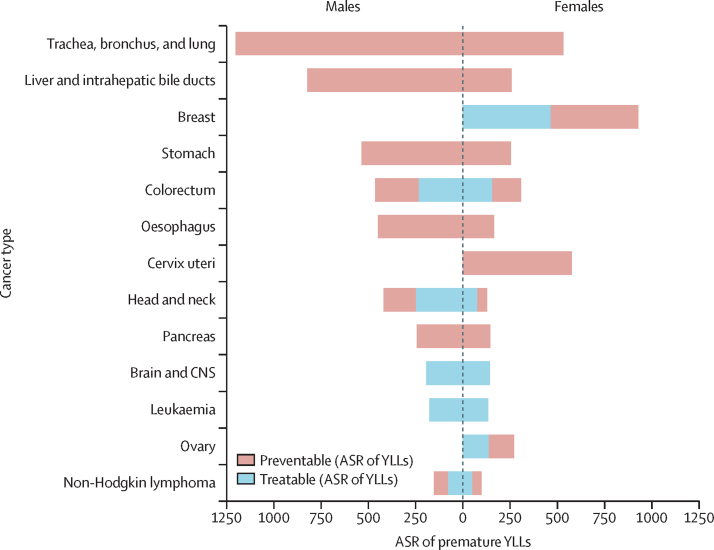
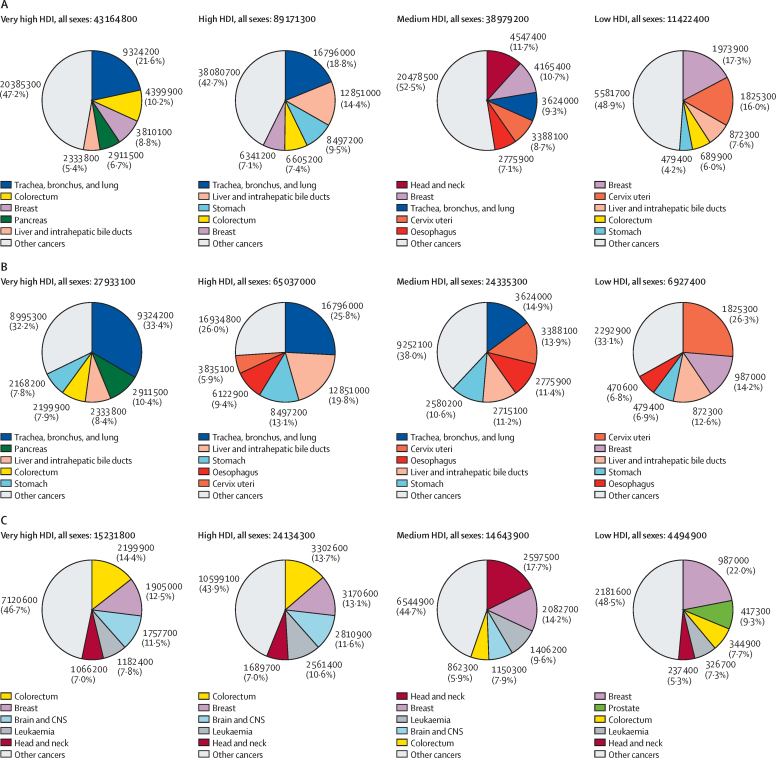
Globally, among all sexes combined, the following cancers ranked highest in age-standardised rates of premature YLLs per 100 000 person-years: lung (860 YLLs), liver (540 YLLs), breast (470 YLLs), stomach (390 YLLs), and colorectal cancer (380 YLLs; presented by sex in figure 1). Lung cancer accounted for the largest proportion of preventable premature cancer deaths in most HDI groups, ranging from 14·9% (3·62 million/24·3 million) in the medium HDI group to 33·4% (9·32 million/27·9 million) in the very high HDI group (figure 2B, all sexes combined). The low HDI group was the only exception to this, in which the leading cause of preventable premature cancer was cervical cancer, at 26·3% (1·83 million/6·93 million) of the total preventable premature YLLs from cancer. For treatable cancers, colorectal cancer was among the largest contributors, contributing 5·9% (862 300/14·6 million) in the medium HDI group, 7·7% (344 900/4·49 million) in the low HDI group, and 13·7% (3·30 million/ 24·1 million) and 14·4% (2·20 million/15·2 million) of the treatable premature YLLs in the high HDI and very high HDI groups, respectively. Other leading treatable causes of premature cancer death included breast cancer, which contributed 13% (3·17 million/24·1 million) in the high HDI group, 13% (1·91 million/15·2 million) in the very high HDI group, 14% (2·08 million/14·6 million) in the medium HDI group, and 22% (987 000/4·49 million) of treatable YLLs in the low HDI group. Head and neck cancers and leukaemia were also among the major cancer types contributing to treatable premature YLLs in all four HDI tiers, and prostate cancer was a major contributor unique to the low HDI group.
Figure 1.
ASR per 100 000 person-years of premature, preventable, and treatable YLLs from cancer (ages 30–69 years) by major cancer types in men and women, 2020
ASR=age-standardised rate. YLLs=years of life lost.
Figure 2.
Years of life lost from premature cancer mortality (ages 30–69 years) according to cancer types by HDI and sex, 2020
(A) Premature (preventable and treatable combined). (B) Preventable. (C) Treatable. HDI=Human Development Index.
When presented by sex (appendix 5 pp 86–91), lung and liver cancer contributed the largest to preventable cancers in men, whereas colorectal cancer, head and neck, and prostate cancer contributed to treatable premature deaths and YLLs. In women, lung cancer ranked first among the major preventable cancers in high and very high HDI countries, cervical cancer led in medium to low HDI countries, and breast cancer was the main contributor to treatable causes of premature YLLs from cancer at all HDI levels.
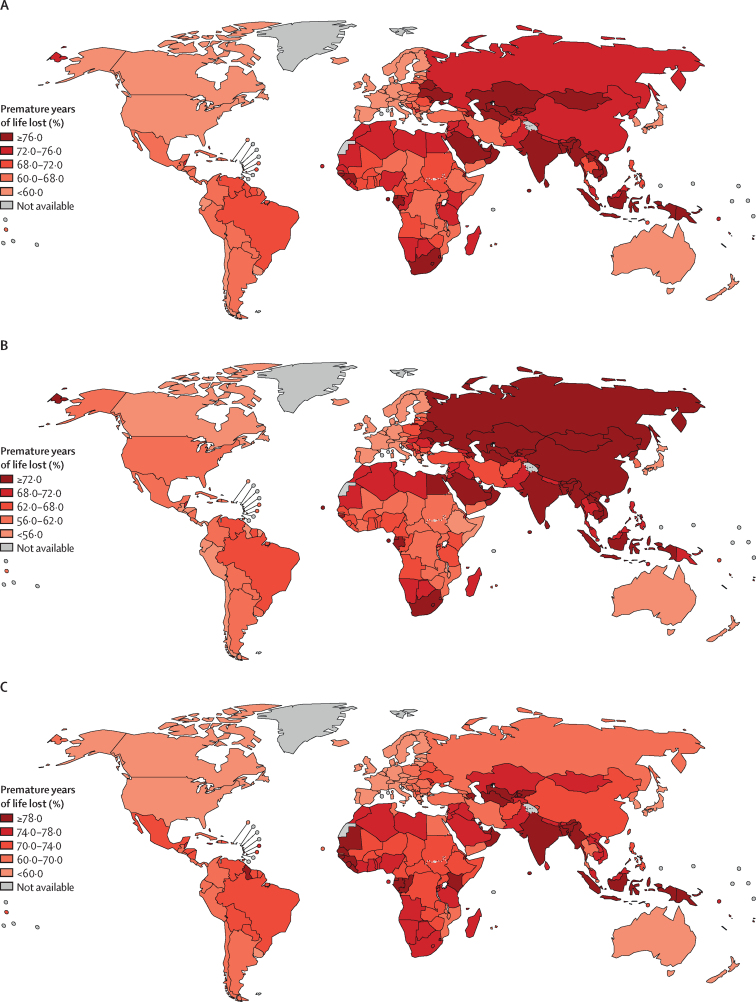
Premature YLLs ranged from 11·4 million in the low HDI group to 89·2 million in the high HDI group (table). The proportion of YLLs attributed to premature cancer death ranged from 57·7% (43·2 million/74·8 million) in the very high HDI group to 77·0% (39·0 million/ 50·7 million) in the medium HDI group, and 68·9% (11·4 million/16·7 million) in the low HDI group. This proportion was lowest in northern Europe (48·1% [2·6 million/5·4 million]), Australia and New Zealand (52·1% [616 200/1·2 million]), and western Europe (53·5% [6·0 million/11·3 million]), although with considerable within-region variation. In northern Europe, proportions of premature YLLs ranged from 44·1% (205 300/465 700) in Sweden to 63·0% (116 600/185 300) in Lithuania (figure 3A). The regions with the highest proportions of premature YLLs were southeastern Asia with 76·7% (16·1 million/21·0 million), south central Asia with 76·8% (32·2 million/42·0 million), and southern Africa with 76·3% (1·5 million/2·0 million; table). Similarly, there was considerable within-region variation; for example, in southeastern Asia, proportions ranged from 69·1% (202 200/292 600) in Singapore to 81·5% (3·2 million/3·9 million) in Viet Nam (figure 3A).
Figure 3.
Premature years of life lost from cancer (ages 30–69 years) as a proportion of all-ages years of life lost from cancer in all sexes (A), men (B), and women (C), 2020
Changing the lower and upper limit to classify a cancer as preventable or treatable did not change our results—ie, two-thirds of all premature cancer deaths were avertable through prevention and another one-third through curative treatment (appendix 5 pp 16–31).
Discussion
Over half of all cancer deaths (5·28 million of 9·96 million) occurred prematurely in 2020, causing 182·8 million life-years lost from this disease worldwide. There were large variations in the proportion of premature YLLs, with 68·8% of YLLs occurring prematurely in countries categorised as low HDI compared with 57·7% in very high HDI countries. We also found marked differences by cancer type and sex, indicating the importance of monitoring premature cancer-specific mortality as a sex-disaggregated indicator of national cancer control. To our knowledge, this is the first global analysis exploring the multidimensional variation of premature (preventable and treatable) cancer mortality.
The preventable proportion of premature YLLs varied by country, possibly due to differences in the types of preventable cancers contributing to premature death, and in the prevalence of cancer risk factors in the population. Men had a higher proportion of preventable premature YLLs than women, most likely shaped by the high lung cancer burden. The high proportion of premature death from lung cancer in men in many countries can be attributed to correspondingly high levels of tobacco use. Although smoking prevalence has fallen over the last decade, one-fifth of the population globally continued to use tobacco in 2020, with the largest group of smokers being men living in middle-income countries (68% of all smokers).21 The dominance of head and neck cancers in medium HDI countries, particularly among men, most likely reflects the high prevalence of smokeless tobacco use in south Asian countries such as India, Nepal, and Bangladesh.21 The importance of lung cancer as a major preventable cancer in women in high and very high HDI countries has also been shown in previous research,22 and points to the recent changes in the underlying determinants, and consequently to the changing cancer profile among women. Where smoking prevalence is low, air pollution (including household), and occupational exposure to asbestos, were among the leading risk factors of attributable cancer death rates.23 Other preventable cancers, such as liver and oesophagus, are associated with alcohol consumption. Globally, 4·1% of new cancer cases in 2020 were attributable to alcohol use.24 In comparative terms, countries in central and eastern Europe, and eastern Asia, had the highest proportion of alcohol-attributable cancer cases.24 Behavioural risks, such as tobacco (any form) and alcohol consumption, and environmental and occupational risks, are most likely substantial in the variability of premature preventable cancer deaths.
The preventable proportion of premature YLLs from cancer was also high among low HDI countries (60·6%), in which women had a notably higher premature cancer burden than men. A larger proportion of preventable premature cancers in low-resource countries is linked to viral infection.11 In women, cervical cancer accounted for a sizeable portion of premature preventable cancer YLLs in low and medium HDI countries, for which the necessary cause is human papillomavirus. Improved human papillomavirus vaccination coverage and screening (linked with treatment) for cervical cancer has the potential to virtually eliminate cervical cancer,25 and is crucial to decreasing disparities in premature infection-related cancer deaths between populations.
According to our estimates, around a third of premature YLLs from cancer would be avoidable by equitable access to effective treatment, with substantially higher proportions in southern Africa and southeast Asia, in comparison to northern Europe and Australia and New Zealand (figure 3A). This points to key differences in national income and development in the quality of the cancer care system and therapeutic capacities, including access to essential services, such as surgery, radiotherapy, chemotherapy, and rehabilitation. Previous research has reported that the percentage of countries with available cancer diagnosis and treatment services is substantially higher among countries with a high income than those classified as low income—for example, around only a quarter of the low-income countries (vs 95% of the high-income countries) reported cancer surgeries to be widely available.26 The large inequalities in the distribution of radiotherapy machines, with an average of 0·06 machines per million inhabitants in low-income countries as compared with more than seven per million in high-income countries,27 most likely also contribute to the substantial variation in premature cancer mortality. Colorectal and breast cancer are major contributors to treatable premature cancer mortality across all countries. Women had a higher proportion of treatable premature YLLs than men, possibly related to the high rate of breast cancer. In high-income settings, implementation of screening programmes for colorectal and breast cancer have been reported to help reduce mortality via the successful treatment of early-stage cancers,28, 29 although ongoing debates remain on the efficacy of screening in other settings. In low-income and middle-income countries, these screening programmes have been ineffective due to a complex interplay of factors, including a scarcity of access to effective treatment, a scarcity of quality control, fragmented or weak referral mechanisms, a scarcity of home-grown adaptions to screening guidelines to improve implementation, and high losses to follow-up.30 As such, interventions strengthening capacity for primary and secondary prevention should be paired with improved access to cost-effective, multimodal quality cancer treatment.
The low HDI countries had greater proportions of YLLs, which occurred prematurely than the very high HDI countries. These differences most likely relate to a lower life expectancy in countries under transition (ie, low and medium HDI countries), which have a larger proportion of the population in premature age groups (ages 30–69 years). Arguing that weaker, fragmented, and less resilient health systems are also linked to lower life expectancy in many lower HDI countries, we might infer these determinants are in large part responsible for the relatively higher proportion of premature cancer deaths (and YLLs) in such settings. Countries that have been able to invest in strong, well funded health systems have arguably been able to mitigate a larger proportion of premature YLLs from cancer, in part due to the cost-effective implementation of public health policies and programmes, such as tobacco control and high-quality cervical screening programmes (with high population coverage), and equitable access to high-quality cancer care, including survivorship care. These disparities in cancer incidence and survival should be addressed by current global initiatives advocating universal health coverage alongside achieving the related targets within the Sustainable Development Goals, including target 3.4.
This study has several strengths. Using a comprehensive and up-to-date list of preventable and treatable cancers, we present estimates of premature mortality by country and sex for 36 cancer types, yielding a unique and comprehensive understanding of premature avoidable mortality from cancer globally. We believe this list should be continuously updated to respond to the increasing body of knowledge on cancer causes and cancer care. Another strength is that we present crude and age-adjusted and sex-adjusted cancer-specific YLLs to provide a more robust measure of estimation of avoidable mortality,4 and which allows for fair comparison between populations. Using this method, we were able to make similar estimates of preventable and treatable cancer deaths among those aged 70 years and older (appendix 5 pp 6, 32–37). These data can therefore advance evidence-informed cancer policy among subpopulations by age, sex, and country.
This study also has limitations in relation to methodology and the underlying assumptions. The cancer survival threshold we used to define amenability to effective treatment limits consideration of comorbidities and other factors that hinder successful treatment. Even though the 5-year relative survival that we used in this study partly predicts long-term survival for some cancer types, for other cancers, such as breast and prostate, excess mortality from the disease might still occur beyond this period.31 Similarly, we assumed all premature deaths from cancer types classified as preventable cancers to be avertable only through primary prevention or early diagnosis, although a proportion of these deaths might be avertable through curative treatment. In contrast, we selected a conservative value for the threshold of the population attributable fraction, which might have resulted in under-reporting of preventable deaths for certain cancer types. As prevalence of risk factors and access to cancer care vary by country, further analyses incorporating country-specific data on these factors are important. Furthermore, there is large variation in the quality and coverage of cancer mortality data between countries (appendix 5 pp 38–73). Cancer mortality data are often suboptimal, particularly in low-income and middle-income countries;8 therefore, these findings need to be interpreted with caution. A validation study32 of the estimation method used showed that under-estimation or over-estimation can vary by country and cancer type, and the accuracy of the method used is highly dependent on representativeness of the scale and pattern of cancer in each country. For example, for some countries with available historical data, we predicted mortality rates for 2020, a method which is considered to be the gold standard. However, the model did not do well when there were recent changes in the trends—eg, implementation of a diagnostic programme or a new treatment with a large impact on survival (and subsequently mortality). The least reliable method, in which rates of neighbouring countries were used (done in three countries), did well if cancer determinants were similar across countries in the same region. As such, to ensure that the estimates were as valid as possible, use of the best method and data sources, complemented with local knowledge of cancer pattern and determinants, are key (appendix 5 pp 3–5). Similar limitations also apply for population estimates in such settings. Global initiatives to improve data coverage and data quality are important to attain better estimates to inform national policy. Finally, our findings are a snapshot of premature mortality in 2020 and, as such, do not necessarily predict progress in cancer control over time. An analysis of 47 countries across five continents reported decreasing mortality rates for eight major cancer types with the exception of lung cancer in women and liver cancer in men, for which mortality rates have increased in the past 10 years.33 Lung cancer, which contributed to the largest mortality in these countries, showed up to a 4·2% annual decline in men in almost all countries, compared with an increase by up to 4·3% in women in half of the countries studied. Future research on sex-specific progress over time is needed to monitor and evaluate public health policy.
Using our defining criteria for premature, preventable, and treatable cancers, this study highlights the diverse effect of premature death from specific cancer types worldwide and in individual countries according to sex and national HDI, with transitioning countries having the highest burden of premature cancer mortality and YLLs from the disease. These indicators are key measures of national health systems throughout the whole cancer care continuum and can be used to examine cancer burden disparities and issues raised within the Lancet Commission on women, power, and cancer.34 Our findings highlight the need for priority-setting within national cancer planning and implementation. The high proportion of preventable premature cancer deaths calls for greater policy emphasis on primary and secondary prevention, specifically also of the cancer types responsible for the most premature deaths, such as lung, cervical, colorectal, and breast cancer. Public health policies could facilitate a reduction in exposures to major cancer risk factors, such as tobacco consumption (any form), alcohol drinking, environmental and occupational hazards, and infectious agents. The variability we found indicates that priorities of cancer control should be made specific to context, and efforts could be tailored to available resources: nations could draw from these findings for strategic cost-effective resource mobilisation according to the degrees of benefit from primary and secondary prevention, in balance with therapeutic capacities building. International and national efforts that prioritise greater investments in risk factor reduction and vaccination as an effective means for primary prevention of specific cancers are needed to ensure optimal societal and economic gains from reducing premature avoidable mortality from cancer.
Data sharing
All data are publicly available at https://gco.iarc.fr.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This project is funded by the Erasmus Mobility programme (paid to CF) and the International Agency for Research on Cancer. Where authors are identified as personnel of the International Agency for Research on Cancer and WHO, the authors alone are responsible for the views expressed in this Article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer and WHO. The opinions expressed by the authors are their own and this material should not be interpreted as representing the official viewpoint of the US Department of Health and Human Services, the National Institutes of Health, or the National Cancer Institute.
Contributors
CF and IS designed the study. CF and HR verified all the underlying data. CF and HR did all analyses supported by IS and JV. CF and HR drafted the first version of the paper. OG, EN, and FB provided technical and specific expertise on methodology, public health, clinical input, and data quality input, and regional assessment of included data and results. All authors commented critically on the manuscript. All authors have full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Supplementary Materials
References
- 1.Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 2.Ortega-Ortega M, Hanly P, Pearce A, Soerjomataram I, Sharp L. Paid and unpaid productivity losses due to premature mortality from cancer in Europe in 2018. Int J Cancer. 2022;150:580–593. doi: 10.1002/ijc.33826. [DOI] [PubMed] [Google Scholar]
- 3.Pearce A, Sharp L, Hanly P, et al. Productivity losses due to premature mortality from cancer in Brazil, Russia, India, China, and South Africa (BRICS): a population-based comparison. Cancer Epidemiol. 2018;53:27–34. doi: 10.1016/j.canep.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Martinez R, Lloyd-Sherlock P, Soliz P, et al. Trends in premature avertable mortality from non-communicable diseases for 195 countries and territories, 1990–2017: a population-based study. Lancet Glob Health. 2020;8:e511–e523. doi: 10.1016/S2214-109X(20)30035-8. [DOI] [PubMed] [Google Scholar]
- 5.Nolte E, McKee M. The Nuffield Trust; London: 2004. Does health care save lives? Avoidable mortality revisited. [Google Scholar]
- 6.Organisation for Economic Co-operation and Development . OECD Publishing; Paris: 2021. Health at a glance 2021: OECD indicators: avoidable mortality (preventable and treatable) [Google Scholar]
- 7.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 8.International Agency for Research on Cancer Cancer Today: data & methods. 2020. https://gco.iarc.fr/today/data-sources-methods
- 9.WHO The Global Health Observatory: premature mortality from noncommunicable disease. https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3411
- 10.Organisation for Economic Co-operation and Development. Eurostat . OECD Publishing; Paris: 2022. Avoidable mortality: OECD/Eurostat lists of preventable and treatable causes of death (January 2022 version) [Google Scholar]
- 11.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 12.O'Sullivan DE, Brenner DR, Villeneuve PJ, et al. The current burden of non-melanoma skin cancer attributable to ultraviolet radiation and related risk behaviours in Canada. Cancer Causes Control. 2021;32:279–290. doi: 10.1007/s10552-020-01382-1. [DOI] [PubMed] [Google Scholar]
- 13.Soerjomataram I, Shield K, Marant-Micallef C, et al. Cancers related to lifestyle and environmental factors in France in 2015. Eur J Cancer. 2018;105:103–113. doi: 10.1016/j.ejca.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Arnold M, de Vries E, Whiteman DC, et al. Global burden of cutaneous melanoma attributable to ultraviolet radiation in 2012. Int J Cancer. 2018;143:1305–1314. doi: 10.1002/ijc.31527. [DOI] [PubMed] [Google Scholar]
- 15.Institute for Health Metrics and Evaluation GBD Compare. 2019. https://vizhub.healthdata.org/gbd-compare/
- 16.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 17.European Commission . European Commission; Brussels: 2022. Europe's Beating Cancer Plan: Communication from the commission to the European Parliament and the Council. [Google Scholar]
- 18.International Agency for Research on Cancer. Nordcan: Association of the Nordic Cancer Registries 5-year age-standardised relative survival (%), females: lung. 2022. https://nordcan.iarc.fr/en/dataviz/survival?cancers=160&set_scale=0&sexes=2
- 19.Murray CJL. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72:429–445. [PMC free article] [PubMed] [Google Scholar]
- 20.Doll R, Payne P, Waterhouse JAH. volume I. IARC Publications; Lyon: 1966. Cancer incidence in five continents. [Google Scholar]
- 21.WHO . World Health Organization; Geneva: 2017. WHO report on the global tobacco epidemic, 2017: monitoring tobacco use and prevention policies. [Google Scholar]
- 22.Fidler-Benaoudia MM, Torre LA, Bray F, Ferlay J, Jemal A. Lung cancer incidence in young women vs young men: a systematic analysis in 40 countries. Int J Cancer. 2020;147:811–819. doi: 10.1002/ijc.32809. [DOI] [PubMed] [Google Scholar]
- 23.GBD 2019 Cancer Risk Factors Collaborators The global burden of cancer attributable to risk factors, 2010–19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400:563–591. doi: 10.1016/S0140-6736(22)01438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rumgay H, Shield K, Charvat H, et al. Global burden of cancer in 2020 attributable to alcohol consumption: a population-based study. Lancet Oncol. 2021;22:1071–1080. doi: 10.1016/S1470-2045(21)00279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simms KT, Steinberg J, Caruana M, et al. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–99: a modelling study. Lancet Oncol. 2019;20:394–407. doi: 10.1016/S1470-2045(18)30836-2. [DOI] [PubMed] [Google Scholar]
- 26.WHO . World Health Organization; Geneva: 2016. Assessing national capacity for the prevention and control of noncommunicable diseases: report of the 2015 global survey. [Google Scholar]
- 27.International Atomic Energy Agency Directory of Radiotherapy Centres Number of radiotherapy machines per million people. https://dirac.iaea.org/Query/Map2?mapId=0
- 28.Broeders M, Moss S, Nyström L, et al. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14–25. doi: 10.1258/jms.2012.012078. [DOI] [PubMed] [Google Scholar]
- 29.Lauby-Secretan B, Vilahur N, Bianchini F, Guha N, Straif K. The IARC perspective on colorectal cancer screening. N Engl J Med. 2018;378:1734–1740. doi: 10.1056/NEJMsr1714643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan T, Sullivan R, Ginsburg OM, et al. In: Cancer: disease control priorities. 3rd edn. Gelband H, Jha P, Sankaranarayanan R, et al., editors. The International Bank for Reconstruction and Development, The World Bank; Washington, DC: 2015. Screening for cancer: considerations for low- and middle-income countries; pp. 211–222. [PubMed] [Google Scholar]
- 31.Dal Maso L, Panato C, Tavilla A, et al. Cancer cure for 32 cancer types: results from the EUROCARE-5 study. Int J Epidemiol. 2020;49:1517–1525. doi: 10.1093/ije/dyaa128. [DOI] [PubMed] [Google Scholar]
- 32.Antoni S, Soerjomataram I, Bjørn Møller, Bray F, Ferlay J. An assessment of GLOBOCAN methods for deriving national estimates of cancer incidence. Bull World Health Organ. 2016;94:174–184. doi: 10.2471/BLT.15.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sedeta E, Sung H, Laversanne M, Bray F, Jemal A. Recent mortality patterns and time trends for the major cancers in 47 countries worldwide. Cancer Epidemiol Biomarkers Prev. 2023;32:894–905. doi: 10.1158/1055-9965.EPI-22-1133. [DOI] [PubMed] [Google Scholar]
- 34.Ginsburg O, Vanderpuye V, Beddoe AM, et al. Women, power, and cancer: a Lancet Commission. Lancet. 2023 doi: 10.1016/S0140-6736(23)01701-4. published online Sept 26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are publicly available at https://gco.iarc.fr.