Abstract
Plant pathogens cause great economic losses in agriculture. To reduce damage, chemical pesticides have been frequently used, but these compounds in addition to causing risks to the environment and health, its continuous use has given rise to resistant phytopathogens, threatening the efficiency of control methods. One alternative for such a problem is the use of natural products with high antifungal activity and low toxicity. Here, we present the production, isolation, and identification of cyclopaldic acid, a bioactive compound produced by Penicillium sp. CRM 1540, a fungal strain isolated from Antarctic marine sediment. The crude extract was fractionated by reversed-phase chromatography and yielded 40 fractions, from which fraction F17 was selected. We used 1D and 2D Nuclear Magnetic Resonance analysis in DMSO-d6 and CDCl3, together with mass spectrometry, to identify the compound as cyclopaldic acid C11H10O6 (238 Da). The pure compound was evaluated for antimicrobial activity against phytopathogenic fungi of global agricultural importance, namely: Macrophomina phaseolina, Rhizoctonia solani, and Sclerotinia sclerotiorum. The antifungal assay revealed the potential of cyclopaldic acid, produced by Penicillium sp. CRM 1540, as a leading molecule against M. phaseolina and R. solani, with more than 90% of growth inhibition after 96h of contact with the fungal cells using 100 µg mL−1, and more than 70% using 50 µg mL−1.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03792-9.
Keywords: Cyclopaldic acid, Penicillium, Macrophomina phaseolina, Rhizoctonia solani, Sclerotinia sclerotiorum
Introduction
Phytopathogenic fungi cause huge losses in agricultural production by decreasing crop yield and quality (Thambugala et al. 2020). Macrophomina phaseolina (Tassi) Gold [Rhizoctonia bataticola (Taub) Butler] is a soil-borne fungus with worldwide distribution, wide host range, and high persistence in soil (Marquez et al. 2021). At least 500 plant species are affected by this plant pathogen, which is responsible for diseases such as root rot, charcoal rot, stem rot, seedling blight, and damping-off (Ghosh et al. 2018; Lodha and Mawar 2020). Another fungus that causes significant damage to crops is Rhizoctonia solani Kühn [Thanatephorus cucumeris (A.B. Frank) Donk]. This microorganism causes brown patches, damping-off, root rot, black scurf, and stem canker on its hosts, which include rice, wheat, soybeans, potato, and corn (Erlacher et al. 2014; Hussain and Khan 2020; Nguyen et al. 2021).
Significant losses caused by R. solani have been reported through the years (Goulart 2018). For soybean yield, 48% losses were estimated in the USA (Tachibana et al. 1971; Dorrance et al. 2003) and 52% in Canada (Chang et al. 2018), for common bean the losses ranged from 10 to 60%, depending on whether more soil pathogens are involved or not (Zambolin et al. 1997) and for rice, losses of 58% have been reported (Chahal et al. 2003). Some isolates, such as AG2-1 can cause severe diseases pre and post-emergence with establishment losses up to 80–100% and final yield of 30% in some crops (Sturrock et al. 2015).
For crops affected by M. phaseolina, yield losses from 28 to 50% have been reported in Brazil for soybeans under high air temperature and dry weather (Ferreira et al. 1979; Almeida et al. 2001; Torres et al. 2007), in the USA reports shown losses up to 20% in 1970 (Sinclair and Gray 1972; Wyllie 1974; Wrather and Koenning 2006). The incidence of Sclerotinia sclerotiorum caused losses higher than 200 million dollars/year in the United States and for canola production in Canada, 600 million losses (Bolton et al. 2006; Dupont Pioner Report 2012). In the global market, yield losses by S. sclerotiorum range from 20 to 35%, but infection is widely spread, with reports of cases of 50% up to 80% incidences (Alkooranee et al. 2017).
To circumvent production losses and prevent diseases, producers have made use of many synthetic chemicals, whose prolonged use has resulted in environmental and ecological impacts, besides the emergence of resistant pathogenic strains (Keswani et al. 2019; Cheng et al. 2021). Given these concerns, strict regulation has been proposed for the use of fungicides to ensure toxicological food safety, in addition to the development of new antifungals for use in a more environmental-friendly way (Brauer et al. 2019; Keswani et al. 2019).
Natural products are secondary metabolites produced by living organisms that aid in survival and adaptation (O’Brien and Wright 2011). These compounds have varied biological activities and can be used as antibiotics, pigments, hormones, antitumors, antimicrobials, and antivirals (Newman and Cragg 2016; Singh et al. 2019). Microorganisms are known producers of bioactive molecules, and a relevant source for bioprospection of compounds for clinical, industrial, and agricultural applications (Dayan et al. 2009; Furbino et al. 2014). Furthermore, naturally occurring antifungal products are considered effective alternatives to pesticides (Silber et al. 2013; Henríquez et al. 2014; Encheva-Malinova et al. 2015; Lee 2016; Ngo et al. 2019).
Among the most promising sources of active natural products are metabolites from marine-derived fungi, and fungi from extreme habitats. A significant number of compounds with a wide variety of biological activity produced by these fungi have been reported (Melo et al. 2014; Gonçalves et al. 2015; Svahn et al. 2015; Deshmukh et al. 2018).
In recent years, filamentous fungi isolated from terrestrial and marine samples collected in the Antarctica region for antimicrobial activity against phytopathogens have been explored in bioprospection studies in our group (Purić et al. 2018; Vieira et al. 2018; Ferrarezi et al. 2019). The fungus Penicillium sp. strain CRM 1540 isolated from Antarctica marine sediment has already shown, in previous studies, antibacterial activity against phytopathogens, being isolated the penicillic acid as main compound with antibacterial activity (Vieira et al. 2022). These promising results led us now to determine the potential of the fungus Penicillium sp. strain CRM 1540 in the production and identify of compounds with activity as antifungal, in particular compounds with action against the phytopathogenic fungi global agricultural importance: M. phaseolina, R. solani, and S. sclerotiorum.
Materials and methods
Fungal strain
The fungus Penicillium sp. strain CRM 1540 was isolated from marine sediment collected at Admiralty Bay (King George Island, Antarctica) as reported by Wentzel et al. (2019). Molecular taxonomy analyses based on beta-tubulin sequencing and phylogeny (GenBank accession number MZ198745) suggested that this fungus is a new species from the genus Penicillium close related to P. commune and P. caseifulvum (Vieira et al. 2022). This strain has been maintained by cryopreservation (− 80 °C) at the UNESP Central of Microbial Resources (CRM-UNESP).
For the present study, Penicillium sp. CRM 1540 was reactivated in 2% malt extract (20 g L−1 malt extract, 20 g L−1 agar, KASVI) agar plates (60 × 15 mm) and incubated at 15 °C for 7 days. Following reactivation, three plugs of mycelium + agar (5 mm × 5 mm) were transferred from the culture borders to 250 mL Erlenmeyer flasks (× 15) containing 150 mL of 2% malt extract liquid medium and incubated at 15 °C and 150 rpm for 20 days to produce secondary metabolites.
Extraction and fractionation of crude extract
After growth, fungal biomass was transferred to a vacuum filtration system, and the culture media were subjected to liquid–liquid extraction with analytical grade ethyl acetate (EtOAc 3:1). This process was repeated five times. The solvent was removed by reduced pressure evaporation to yield the crude extract of Penicillium sp. CRM 1540.
The EtOAc extract was dissolved in methanol to 60 mg mL−1, and then aliquots (900 µL) were subjected to reversed-phase HPLC (Phenomenex Luna-C8 21.2 mm × 25 cm × 7 µm column) with standardized automated collection (~ 0.6 min interval), as described in Vieira et al. (2022). Scheme 1 shows the extraction and fractionation processes. The fractions obtained during the fractionation process (Fig. S1) were dried using a centrifugal evaporator and combined by similarity based on UPLC-DAD-QTOF analysis. After fractionation of the crude extract, the fractions obtained were analyzed for their purity in order to proceed with the identification of their components and antimicrobial activity assays.
Scheme 1.
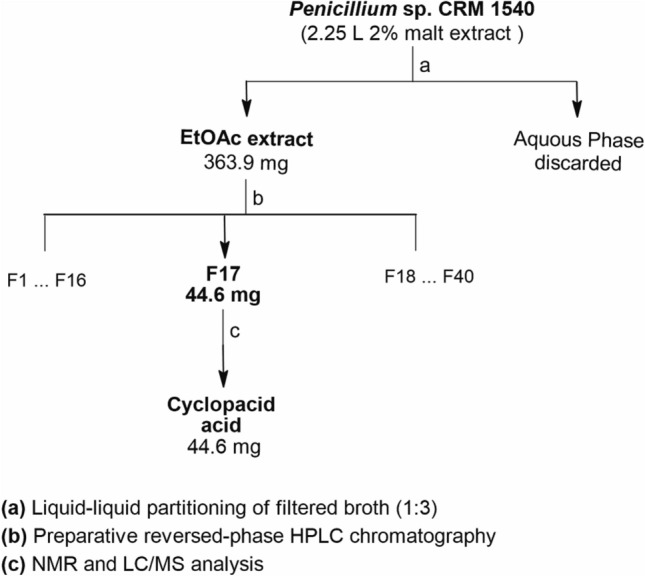
Isolation scheme of cyclopaldic acid. a Liquid–liquid extraction of the microbial broth using ethyl acetate; b crude extract fractionation by reversed-phase HPLC; c fractions from the same peak (F17) were grouped as one
Cyclopaldic acid identification
Fraction F17 was selected for further elucidation due to its relative purity in relation to the other fractions. For the LC-HRMS analysis, 1 µL of F17 was diluted in MeOH/H2O (1:9 v/v) to 1 µg mL−1 and injected in a C8 column (Betasil-C8 10 × 0.2 mm) with gradient elution of water and acetonitrile with 0.1% formic acid. Equipment and further experimental details are available in Supplementary Information. Sample F17 was subjected to several NMR experiments, involving 1H NMR, 13C NMR, 13C APT NMR, HSQC, HMBC, and COSY. The solvents used for analysis were dimethyl sulfoxide (DMSO-d6) and chloroform (CDCl3). NMR data were recorded on a Bruker Avance DRX400. NMR data were analysed by Bruker TopSpin 4.0.9 software (academic license); the compound structure was drawn with ChemSketch Freeware v 14.00 (ACD/ChemSketch 2019).
Plant pathogenic fungi
Antifungal activity in vitro was measured against three known fungal phytopathogens. The targeted fungi were cultivated in potato dextrose agar plates (27 g potato dextrose broth, 20 g L−1 agar, KASVI) for 4 days for R. solani MMBF30/11 (São Paulo, Brasil), 7 days for M. phaseolina MMBF12/18 (Paraná, Brasil), and 15 days for S. sclerotiorum MMBF04/19 (Paraná, Brasil) at 28 °C. The fungi belong and are preserved at the Biological Institute of São Paulo (Instituto Biológico de São Paulo—IB).
Antifungal activity assay
The assay was carried out in 96-well plates with a twofold dilution of the compound, allowing the evaluation of cyclopaldic acid from 100 to 0.78 µg mL−1. From the agar plate, a standardized inoculum of 104 cells for each culture was prepared in saline solution (0.85% NaCl) for each plant pathogen, followed by a dilution at 1:10 in PDB. To each well were added 50 µL of cyclopaldic acid and 50 µL of fungal phytopathogen. PDB medium with the respective inoculum was used as Negative Control (NC). The plates were incubated at 28 °C for 96 h, and OD600 was measured at 24 h intervals in a hybrid multi-mode microplate reader (Biotek Synergy H1MFD). The assay was performed in triplicate. Aliquots of 10 µL from each well were transferred to PDA plates after the test and then incubated at 28 °C to identify whether the compound had fungistatic or fungicide action. Growth inhibition (%) was calculated for each concentration according to the formula below:
wherein OD600 = optical density measured at 600 nm, NC: negative control.
Statistical analysis
The data were analysed by descriptive analysis and one-way ANOVA followed by Tukey’s multiple comparison test, using GraphPad Prism 8 software for Windows (GraphPad Software, San Diego, California USA, www.graphpad.com). The same software was used to plot inhibition graphics.
Results
Cyclopaldic acid isolation and identification
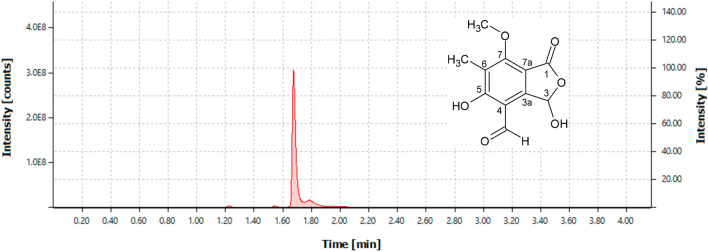
The EtOAc crude extract (363.90 mg) yielded 40 fractions after UPLC analysis and grouping by similarity. From these, fraction F17 (44.6 mg) was isolated (Fig. 1) as a white compound with the molecular formula C11H10O6 (238 Da), based on high-resolution ESIMS m/z 239.2365 [M + H]+ (calculated for C11H11O6, 239.2365) (Fig. S2). Analysis of the 1D and 2D NMR (400 MHz, DMSO-d6, CDCl3) data for F17 (Fig. S3–S9, and Table S1) and comparison with available literature data Achenbach et al. (1982) confirmed the structure of cyclopaldic acid.
Fig. 1.
Chromatogram of fraction F17 obtained by analysis of UPLC-DAD-QTOF at 290 nm and structure of cyclopaldic acid, compound present in fraction F17
Antifungal activity assay
No antifungal activity was detected against S. sclerotiorum, even with the phytopathogen initially having slow growth rate (24 h OD600 = 0.01; 48 h = 0.02) which started to increase after 72 h (72 h OD600 = 0.1; 96 h = 0.28. The growths of R. solani and M. phaseolina were affected by cyclopaldic acid at 100, 50, and 25 µg mL−1.
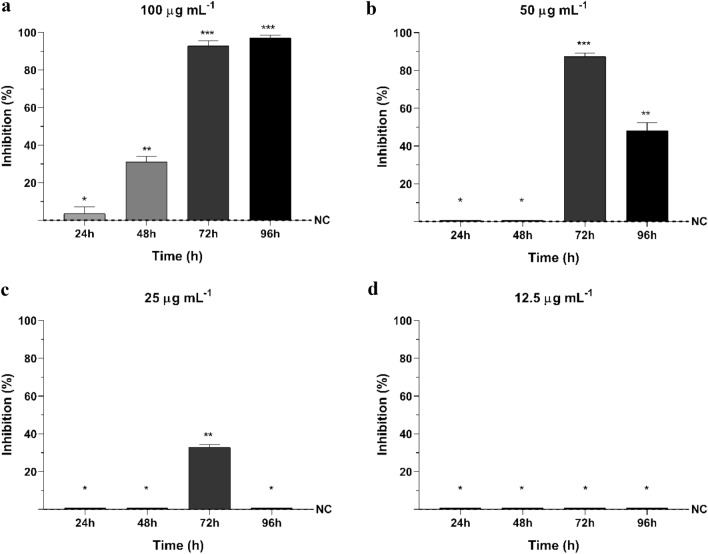
For R. solani, the 24 h interval measures of fungal growth showed that, at 100 µg mL−1, it took 48 h of contact for the compound to have its inhibitory effect (31.02%), reaching more than 90% inhibition after 72 h and 96 h (Fig. 2a). At 50 µg mL−1, inhibition started after 72 h and severely decreased in the subsequent interval (Fig. 2b). And at 25 µg mL−1, cyclopaldic acid affected growth only after 72 h, with a 32.85% inhibition (Fig. 2c). For 12.5 µg mL−1 and the subsequent concentrations evaluated, no activity was observed (Fig. 2d).
Fig. 2.
Growth Inhibition (%) of Rhizoctonia solani by different concentrations of cyclopaldic acid in vitro at 24, 48, 72 and 96 h. Data shown as means and standard deviation. NC: negative control of Rhizoctonia solani. a 100 µg mL−1 of cyclopaldic acid, b 50 µg mL−1 of cyclopaldic acid, c 25 µg mL−1 of cyclopaldic acid, d 12.5 µg mL−1 of cyclopaldic acid. Differing signals (*) denote significant differences among group means according to analysis of variance (ANOVA) and Tukey test for comparison between treatments with a confidence interval of 95% (α = 0.05)
Statistical analysis revealed no significant difference between 72 and 96 h of incubation for the compound at 100 µg mL−1, which promoted inhibitions of 92.83% and 97.18%, respectively (Fig. 2a). There was also a significant difference between 72 and 96 h of contact at 50 µg mL−1 (Fig. 2b).
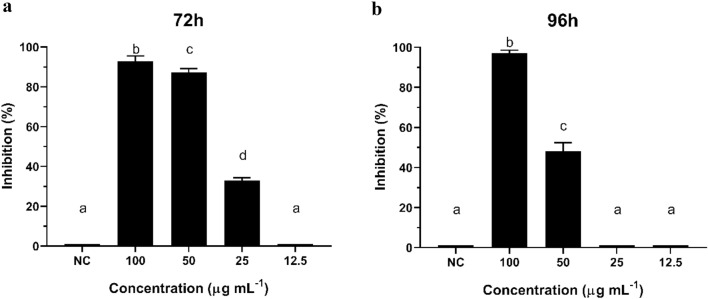
Additionally, at a 72 h interval, the inhibitory effect of cyclopaldic acid showed a slight difference between 100 and 50 µg mL−1 (p = 0.0122), which grew after 96 h (Fig. 3a, b). These results contribute to our conclusion that the inhibition activity of cyclopaldic acid on R. solani reaches its optimal effectiveness at 100 µg mL−1after 72 h of contact with the fungal cells in in vitro conditions. We may also infer that similar results can be achieved by applying a lower concentration of the compound, e.g., 50 µg mL−1.
Fig. 3.
Inhibitory effect of cyclopaldic acid at concentrations of 100, 50, 25 and 12.5 µg mL−1. a After 72 h and b after 96 h of contact with Rhizoctonia solani. NC: negative control of Rhizoctonia solani. Different letters denote significant differences between group means according to the analysis of variance (ANOVA) and Tukey test for comparison between treatments with a confidence interval of 95% (α = 0.05)
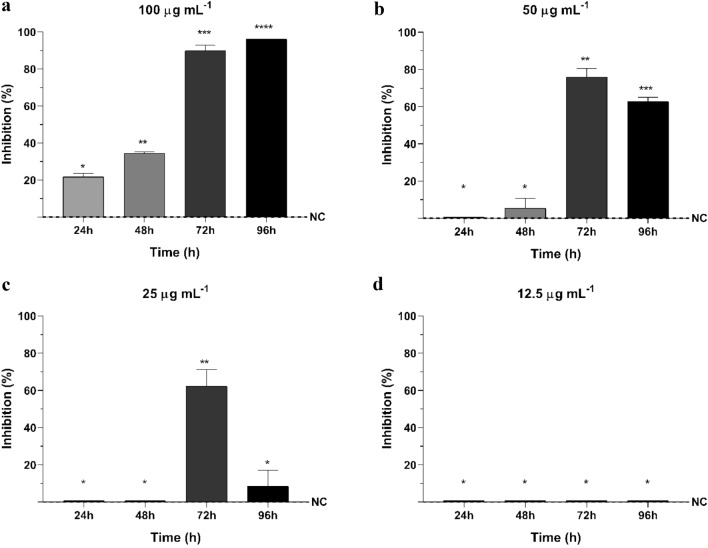
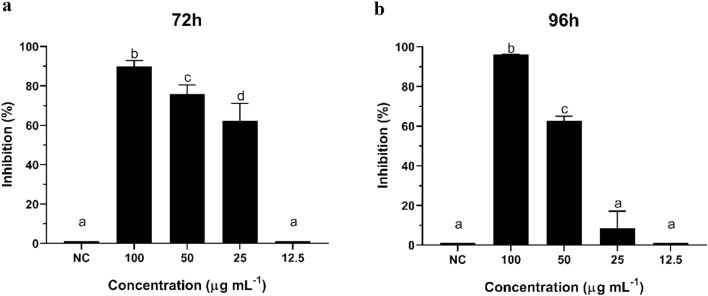
Akin to what was observed with R. solani, the highest inhibition of M. phaseolina growth (96.13%) was detected at 100 µg mL−1 after 96 h of incubation, and no activity was found at 12.5 µg mL−1 and lower concentrations (Fig. 4a, d). Cyclopaldic acid showed activity within the first 24 h of contact with M. phaseolina. Its growth was inhibited by 21.68% at 100 µg mL−1, which increased to 34.42% after 48 h. Afterwards, inhibition increased to 89.94% and 96.13% after 72 h and 96 h of incubation, respectively (Fig. 4a).
Fig. 4.
Growth Inhibition (%) of Macrophomina phaseolina by different concentrations of cyclopaldic acid in vitro at 24, 48, 72 and 96 h. Data shown as means and standard deviation. NC: negative control of Macrophomina phaseolina. a 100 µg mL−1 of cyclopaldic acid, b 50 µg mL−1 of cyclopaldic acid, c 25 µg mL−1 of cyclopaldic acid, d 12.5 µg mL−1 of cyclopaldic acid. Differing signals (*) denote significant differences between group means according to the analysis of variance (ANOVA) and Tukey test for comparison between treatments with a confidence interval of 95% (α = 0.05)
The antifungal activity of the compound at 50 and 25 µg mL−1against M. phaseolina differs from the negative control only after 72 h of incubation (Fig. 4b, c). Within this time interval, more than 60% inhibition was observed at the three concentrations, in which cyclopaldic acid was active against M. phaseolina. The activity decreased in the next 24 h to 75.95% at 50 µg mL−1 and to less than 10% at 25 µg mL−1, which was statically similar to the control.
Against M. phaseolina, the inhibitory activity of cyclopaldic acid differs significantly among concentrations after 72 h of contact with the phytopathogen, and this difference increased after 96 h (Fig. 5a, b). However, at 100 µg mL−1, inhibition means showed a small difference between the exposure times of 72 h and 96 h (p = 0.0123). Given those results, we conclude that the most effective treatment in vitro for M. phaseolina is applying 100 µg mL−1 for 96 h, which can be shortened to 72 h.
Fig. 5.
Inhibitory effect of cyclopaldic acid at concentrations of 100, 50, 25 and 12.5 µg mL−1. a After 72 h and b after 96 h of contact with Macrophomina phaseolina. NC: negative control of Macrophomina phaseolina. Different letters denote significant differences between group means according to the analysis of variance (ANOVA) and Tukey test for comparison between treatments with a confidence interval of 95% (α = 0.05)
Rhizoctonia solani and M. phaseolina grew in the agar plates after being treated with the active compound (Fig. 6).
Fig. 6.
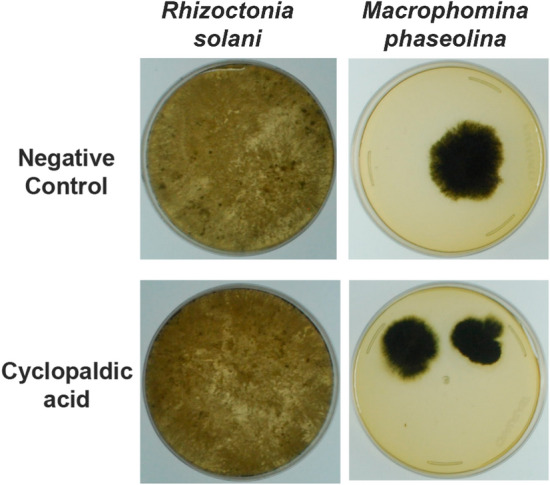
Evaluation of the fungistatic activity of cyclopaldic acid against Rhizoctonia solani and Macrophomina phaseolina. Four-day R. solani and 7-day M. phaseolina colonies developed on PDA medium after 96 h of treatment with cyclopaldic acid at 100 µg mL−1
Discussion
Traditionally regarded as a necrotrophic fungus, S. sclerotiorum acquires nutrients by progressively killing the host cells through cell-wall degrading enzymes and toxins secretion (Kabbage et al. 2015; Mbengue et al. 2016; Mccaghey et al. 2019). These molecules, known as effectors, include fungal secondary metabolites that range from small peptides to complex chemical structures and are important virulence components found in plant pathogens (O’Sullivan et al. 2021; Shao et al. 2021).
Among these molecules are oxalic and fumaric acids, which contribute to host defence suppression and pathogenesis by acidifying the environment and creating conditions for the expression of lytic and hydrolytic enzymes related to disease development (Frisvad 1989a, b; Boysen et al. 1996; Nicoletti and Trincone 2016; O’Sullivan et al. 2021). Furthermore, the growth of S. sclerotiorum is slowed down under neutral and alkaline pH but favoured under acidic conditions (Frisvad et al. 2004; Shah et al. 2020). The pathogen is also known to secret other organic acids such as glycolic, citric, glyoxylic, malic, and succinic acid when in neutral or alkaline environments although these substances have not been the object of many studies on S. sclerotiorum (Graniti et al. 1992; Nicoletti and Trincone 2016; Roscetto et al. 2020; Masi et al. 2021).
The link between S. sclerotiorum growth and increased virulence at lower pH, combined with slow development in neutral or alkaline environments, led us to hypothesize that cyclopaldic acid does not affect fungal growth as it may contribute to pathogen growth, acidifying in vitro culture media.
The growth inhibition patterns observed for R. solani and M. phaseolina suggest a slow fungistatic activity for cyclopaldic acid on the microbial cells studied. This type of activity was confirmed by the growth of treated fungi in PDA plates, showing that cyclopaldic acid delays fungal growth but does not kill the phytopathogens, regardless of the concentration at which the inhibitory activity was detected (Fig. 6).
Cyclopaldic acid is a known compound produced by species of Penicillium, Aspergillus, and Pestalotiopsis. Among the documented producers from the genus Penicillium are P. commune, P. carneum, P. mononematosum, P. viridicatum, and P. polonicum (Frisvad 1989a, b; Boysen et al. 1996; Frisvad et al. 2004). While novel antimicrobial compounds may be found in habitats geographically isolated such as Antarctica, known molecules can also be produced by fungal strains that inhabited this kind of extreme environment and, as a consequence, novel uses for previously known natural compounds might be found (Nicoletti and Trincone 2016; Shah et al. 2020).
The literature is scarce regarding biological activity, with limited reports on antifungal activity, larvicidal activity, and phytotoxicity for this molecule. More recent studies on the antimicrobial activity of cyclopaldic acid have shown a diverse pattern of inhibition. According to Roscetto et al. (2020), some strains of Staphylococcus aureus were inhibited by 90% using cyclopaldic acid at 100 µg mL−1, while other Gram-positive and Gram-negative bacteria of clinical interest showed no minimum inhibition of 50%.
For antifungal activities, Masi et al. (2021) showed that cyclopaldic acid had no effect against Aspergillus niger, Alternaria alternata, or Fusarium oxysporum isolated from lithic substrata. However, other authors have reported that, at 100 µg mL−1, cyclopaldic acid can inhibit from 40 to 65% of the growth of Geotrichum candidum, Botrytis cinerea, Fusarium solani, and F. oxysporum f. sp. lycopersici, while at 50 µg mL−1, it can reduce spore germination for these plant pathogenic fungi (Graniti et al. 1992). At 100 mM or lower concentrations, cyclopaldic acid inhibited the germination of Puccinia spp. and Uromyces spp. strains, which are causal agents of rust in plants (Barilli et al. 2016, 2017). Aside from that, cyclopaldic acid at 100 µg mL−1 also has shown activity against Penicillium roqueforti, a food contaminant, with 29.34% inhibition after 72 h and 59.48% after 96 h (Valerio et al. 2018).
Recent studies showed the potential of fungal metabolites from isolates such as Trichoderma longibrachiatum against M. phaseolina, with 22% antifungal activity of the crude extract (Sridharan et al 2021). Trichoderma viride has also been reported to inhibit in 63% the pathogen, T. koningii, T. hamatum, T. longipile have slowed down by 46–47% the growth of M. phaseolina and T. harzianum in 28% (Khan et al 2021). According to Motlagh et al (2022), in dual-cultivation assays Trichoderma virens inhibited growth of R. solani in 46%, T. harzianum and Aspergillus awamori in 37 and 39%, respectively, and A, fumigatus caused inhibition of 42% of the phytopathogen. The filtrate of Paecilomyces at 60% concentration inhibited in 56.25% the radial growth of R. solani in a study conducted by Hawar et al (2023). The reports on antifungal activity against M. phaseolina and R. solani have shown the potential of fungi as a source of inhibitory metabolites, but not many papers have elucidated which compounds are responsible for the slowing of mycelial growth of these phytopathogens.
As previously mentioned, R. solani and M. phaseolina are phytopathogens that can cause significant yield losses in several important crops, as they have a wide host range and high persistence in the soil (Sturrock et al. 2015; Feng et al. 2017; Marquez et al. 2021). Current control strategies have aimed to lower the incidence in plantations, but management methods remain a challenge despite research efforts (Almeida et al. 2014; AGROLINK 2021; Marquez et al. 2021). Many health and environmental issues are associated with the use of pesticides and chemical fungicides, residue accumulation and resistance development by pathogens. Dermatological, respiratory, endocrine and neurological negative effects have been associated with exposure to these types of chemicals or its residues, and the use of certain products have been discarded, but alternatives for sustainable practices are needed for many crop diseases (Nicolopoulou-Stamati et al 2016; Pathak et al 2022). The use of natural compounds and biocontrol strategies can help with avoiding fungal diseases outbreaks and can also benefit the plants in other ways, such as growth promotion. Besides that, there is a lesser risk of disrupting the chemical and biological balance of the environment depending on the molecule and mode of application (Rodrigo et al. 2022).
In this aspect, cyclopaldic acid can be a leading molecule for formulations against fungal plant pathogens as an alternative to other ineffective chemicals. The integration of bioactive molecules into modern agriculture practices is a sustainable and environmentally friendly alternative that also depends on efficacy, practicality and stability of the formulations, which can only be assessed by extensive research (Berestetskiy 2023).
To effectively apply cyclopaldic acid in a formulation, more details regarding the mechanism of action of the compound are needed to first increase its efficacy and a suitable formulation which can ensure stability, dispersion and release of the molecule is also required. Furthermore, it is important to work together with the agricultural industry to understand the performance of bioactive compounds in the field and redefine strategies of application (Cobb and Reade 2007; Mesnage 2021). After regulatory approval, a valid approach is to slowly introduce bioactive compounds as a component of integrated pest control and so reduce reliance on traditionally used chemicals (Berestetskiy 2023). Additionally, the search for secondary metabolites from extremophiles has proven that these microorganisms can be considered essential sources of compounds for agricultural use.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Prof Robert J Capon and Zeinab Khalil from the of the University of Queensland, Brisbane, QLD, Australia for valued help on the purification process of the crude extract at the Institute for Molecular Bioscience.
Author contributions
Conceptualization: DCS; methodology: DCS, GV; formal analysis and investigation: GV, DCS, LDS, DAdeA; writing—original draft preparation: GV; writing—review and editing: DCS, GV, LDS, DAdeA; funding acquisition: DCS; resources: DCS; supervision: DCS.
Funding
This study was financed in part by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [Grant #141988/2018-5], Brazil, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—PrInt [Grant #88887.467270/2019-00] and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [Grant numbers #2018/11747-3 and #2016/07957-7].
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to publication
Not applicable.
References
- ACD/ChemSketch version 14. 00 (2019) Advanced Chemistry Development, Inc.
- Achenbach H, Mühlenfeld A, Weber B, et al. Stoffwechselprodukte von Mikroorganismen, XXVII[1] Cyclopaldsäure und 3-O-methyl-cyclopolsäure, zwei antibiotisch wirksame Substanzen aus Aspergillus duricaulis/Investigations on Metabolites of Microorganisms, XXVII [1] Cyelopaldic acid and 3-O-methyl Cyc. Zeitschrift Für Naturforschung B. 1982;37:1091–1097. doi: 10.1515/znb-1982-0823. [DOI] [Google Scholar]
- AGROLINK (2021) Podridão-radicular: Tombamento, fungo de solo (Rhizoctonia solani). https://www.agrolink.com.br/problemas/podridao-radicular_1886.html. Accessed 20 Jan 2023
- Alkooranee JT, Aledan TR, Ali AK, et al. Detecting the hormonal pathways in oilseed rape behind induced systemic resistance by Trichoderma harzianum TH12 to Sclarotinia sclerotiorum. PLoS One. 2017;12:e0168850. doi: 10.1371/journal.pone.0168850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida AMR, Torres E, Farias JRB, et al. Macrophomina phaseolina em soja: sistema de semeadura, sobrevivência em restos cultura e diversidade genética. Londrina: Embrapa Soja; 2001. [Google Scholar]
- Almeida ÁMR, Seixas CDS, Farias JRB et al (2014) Macrophomina phaseolina em soja. Embrapa Soja, Londrina
- Barilli E, Cimmino A, Masi M, et al. Inhibition of spore germination and appressorium formation of rust species by plant and fungal metabolites. Nat Prod Commun. 2016;11:1934578X1601100. doi: 10.1177/1934578x1601100940. [DOI] [PubMed] [Google Scholar]
- Barilli E, Cimmino A, Masi M, et al. Inhibition of early development stages of rust fungi by the two fungal metabolites cyclopaldic acid and epi-epoformin. Pest Manag Sci. 2017;73:1161–1168. doi: 10.1002/ps.4438. [DOI] [PubMed] [Google Scholar]
- Berestetskiy A. Modern approaches for the development of new herbicides based on natural compounds. Plants. 2023;12(2):234. doi: 10.3390/plants12020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton MD, Thomma BP, Nelson BD (2006) Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathol 7:1–16. 10.1111/j.1364-3703.2005.00316.x [DOI] [PubMed]
- Boysen M, Skouboe P, Frisvad J, Rossen L. Reclassification of the Penicillium Roqueforti group into three species on the basis of molecular genetic and biochemical profiles. Microbiology (n y) 1996;142:541–549. doi: 10.1099/13500872-142-3-541. [DOI] [PubMed] [Google Scholar]
- Brauer VS, Rezende CP, Pessoni AM, et al. Antifungal agents in agriculture: friends and foes of public health. Biomolecules. 2019;9:521. doi: 10.3390/biom9100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal KS, Sokhi SS, Rattan GS. Investigations on sheath blight of rice in Punjab. Indian Phytopathol. 2003;56:22–26. [Google Scholar]
- Chang KF, Hwang SF, Ahmed HU, et al. Disease reaction to Rhizoctonia solani and yield losses in soybean. Can J Plant Sci. 2018;98(1):115–124. doi: 10.1139/CJPS2017-0053. [DOI] [Google Scholar]
- Cheng J, Peng Y, Yan J, et al. Research progress in phytopathogenic fungi and their role as biocontrol agents. Front Microbiol. 2021;12:1209. doi: 10.3389/fmicb.2021.670135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb AH, Reade JPH. Herbicides and plant physiology. 2. Oxford: Wiley-Blackwell; 2007. [Google Scholar]
- Dayan FE, Cantrell CL, Duke SO. Natural products in crop protection. Bioorg Med Chem. 2009;17:4022–4034. doi: 10.1016/j.bmc.2009.01.046. [DOI] [PubMed] [Google Scholar]
- Deshmukh SK, Prakash V, Ranjan N. Marine fungi: a source of potential anticancer compounds. Front Microbiol. 2018 doi: 10.3389/fmicb.2017.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrance AE, Kleinhenz MD, McClure SA, et al. Temperature, moisture, and seed treatment effects on Rhizoctonia solani root rot of soybean. Plant Dis. 2003;87(5):533–538. doi: 10.1094/PDIS.2003.87.5.533. [DOI] [PubMed] [Google Scholar]
- Dupont Pioneer Report . Economic impact of sclerotinia stem rot. In: Hacault K, Faust R, Butzen S, Jeschke M, editors. Agronomy sciences research summary 2012. Canadian. Johnston: Dupont Pioneer; 2012. pp. 34–37. [Google Scholar]
- Encheva-Malinova M, Vancheva T, Badzhinerov N, et al. Antimicrobial activity of Antarctic Streptomycetes against pepper bacterial spot causing agents. Annuaire De L’université De Sofia “st Kliment Ohridski” Faculte De Biologie. 2015;100:216–222. [Google Scholar]
- Erlacher A, Cardinale M, Grosch R, et al. The impact of the pathogen Rhizoctonia solani and its beneficial counterpart Bacillus amyloliquefaciens on the indigenous lettuce microbiome. Front Microbiol. 2014;5:175. doi: 10.3389/fmicb.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Shu C, Wang C, et al. Survival of Rhizoctonia solani AG-1 IA, the causal agent of rice sheath blight, under different environmental conditions. J Phytopathol. 2017;165:44–52. doi: 10.1111/jph.12535. [DOI] [Google Scholar]
- Ferrarezi JH, dos Santos AJ, Sette LD, et al. Anti-Xanthomonas activity of Antarctic fungi crude extracts. Afr J Biotechnol. 2019;18:713–718. doi: 10.5897/ajb2019.16886. [DOI] [Google Scholar]
- Ferreira LP, Lehman OS, Almeida AMR. Doenças da soja no Brasil. Londrina: Circular técnica; 1979. [Google Scholar]
- Frisvad JC. The connection between the Penicillia and Aspergilli and mycotoxins with special emphasis on misidentified isolates. Arch Environ Contam Toxicol. 1989;18:452–467. doi: 10.1007/bf01062373. [DOI] [PubMed] [Google Scholar]
- Frisvad JC. The use of high-performance liquid chromatography and diode array detection in fungal chemotaxonomy based on profiles of secondary metabolites. Bot J Linn Soc. 1989;99:81–95. doi: 10.1111/j.1095-8339.1989.tb00393.x. [DOI] [Google Scholar]
- Frisvad JC, Smedsgaard J, Larsen TO, Samson RA. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud Mycol. 2004;49:201–241. [Google Scholar]
- Furbino LE, Godinho VM, Santiago IF, et al. Diversity patterns, ecology and biological activities of fungal communities associated with the endemic macroalgae across the Antarctic Peninsula. Microb Ecol. 2014;67:775–787. doi: 10.1007/s00248-014-0374-9. [DOI] [PubMed] [Google Scholar]
- Ghosh T, Biswas MK, Guin C, Roy P. A review on characterization, therapeutic approaches and pathogenesis of Macrophomina phaseolina. Plant Cell Biotechnol Mol Biol. 2018;19:72–84. [Google Scholar]
- Gonçalves VN, Carvalho CR, Johann S, et al. Antibacterial, antifungal and antiprotozoal activities of fungal communities present in different substrates from Antarctica. Polar Biol. 2015;38:1143–1152. doi: 10.1007/s00300-015-1672-5. [DOI] [Google Scholar]
- Goulart ACP. Setting a rating scale for assess Rhizoctonia solani lesions on cotton, soybean and common bean seedlings. Biosci J. 2018;34(6):1632–1639. doi: 10.14393/BJ-v34n6a2018-42657. [DOI] [Google Scholar]
- Graniti A, Sparapano L, Evidente A. Cyclopaldic acid, a major phytotoxic metabolite of Seiridium cupressi, the pathogen of a canker disease of cypress. Plant Pathol. 1992;41:563–568. doi: 10.1111/j.1365-3059.1992.tb02454.x. [DOI] [Google Scholar]
- Hawar SN, Taha ZK, Hamied AS, et al. Antifungal activity of bioactive compounds produced by the endophytic fungus Paecilomyces sp. (JN227071.1) against Rhizoctonia solani. Int J Biomater. 2023 doi: 10.1155/2023/24115555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henríquez M, Vergara K, Norambuena J, et al. Diversity of cultivable fungi associated with Antarctic marine sponges and screening for their antimicrobial, antitumoral and antioxidant potential. World J Microbiol Biotechnol. 2014;30:65–76. doi: 10.1007/s11274-013-1418-x. [DOI] [PubMed] [Google Scholar]
- Hussain T, Khan AA. Bacillus subtilis HussainT-AMU and its Antifungal activity against Potato Black scurf caused by Rhizoctonia solani on seed tubers. Biocatal Agric Biotechnol. 2020;23:101443. doi: 10.1016/j.bcab.2019.101443. [DOI] [Google Scholar]
- Kabbage M, Yarden O, Dickman MB. Pathogenic attributes of Sclerotinia sclerotiorum: switching from a biotrophic to necrotrophic lifestyle. Plant Sci. 2015;233:53–60. doi: 10.1016/j.plantsci.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Keswani C, Singh HB, Hermosa R, et al. Antimicrobial secondary metabolites from agriculturally important fungi as next biocontrol agents. Appl Microbiol Biotechnol. 2019;103:9287–9303. doi: 10.1007/s00253-019-10209-2. [DOI] [PubMed] [Google Scholar]
- Khan IH, Javaid A, Ahmed D. Trichoderma viride controls Macrophomina phaseolina through its DNA disintegration and production of antifungal compounds. Int J Agric Biol. 2021;25:888–894. doi: 10.17957/IJAB/15.1743. [DOI] [Google Scholar]
- Lee Y. Lysimachia foenum-graecum Herba extract, a novel biopesticide, inhibits ABC transporter genes and mycelial growth of Magnaporthe oryzae. Plant Pathol J. 2016;32:8–15. doi: 10.5423/ppj.oa.08.2015.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodha S, Mawar R. Population dynamics of Macrophomina phaseolina in relation to disease management: a review. J Phytopathol. 2020;168:1–17. doi: 10.1111/jph.12854. [DOI] [Google Scholar]
- Marquez N, Giachero ML, Declerck S, Ducasse DA. Macrophomina phaseolina: general characteristics of pathogenicity and methods of control. Front Plant Sci. 2021;12:666. doi: 10.3389/fpls.2021.634397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi M, Petraretti M, de Natale A, et al. Fungal metabolites with antagonistic activity against fungi of lithic substrata. Biomolecules. 2021;11:295. doi: 10.3390/biom11020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbengue M, Navaud O, Peyraud R, et al. Emerging trends in molecular interactions between plants and the broad host range fungal pathogens Botrytis cinerea and Sclerotinia sclerotiorum. Front Plant Sci. 2016 doi: 10.3389/fpls.2016.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccaghey M, Willbur J, Smith DL, Kabbage M. The complexity of the Sclerotinia sclerotiorum pathosystem in soybean: virulence factors, resistance mechanisms, and their exploitation to control Sclerotinia stem rot. Trop Plant Pathol. 2019;44:12–22. doi: 10.1007/s40858-018-0259-4. [DOI] [Google Scholar]
- Melo IS, Santos SN, Rosa LH, et al. Isolation and biological activities of an endophytic Mortierella alpina strain from the Antarctic moss Schistidium antarctici. Extremophiles. 2014;18:15–23. doi: 10.1007/s00792-013-0588-7. [DOI] [PubMed] [Google Scholar]
- Mesnage R. Coformulants in commercial herbicides. In: Mesnage R, Zahler JG, editors. Herbicides: chemistry, efficacy, toxicology, and environmental impacts. Amsterdam: Elsevier Inc.; 2021. pp. 87–111. [Google Scholar]
- Motlagh MRS, Jahangiri B, Kulus D, et al. Endophytic fungi as potential biocontrol agents against Rhizoctonia solani J.G. Kühn, the causal agent of rice sheath blight disease. Biology. 2022;11:1282. doi: 10.3390/biology11091282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Ngo MT, Han JW, Yoon S, et al. Discovery of new triterpenoid saponins isolated from Maesa japonica with antifungal activity against rice blast fungus Magnaporthe oryzae. J Agric Food Chem. 2019;67:7706–7715. doi: 10.1021/acs.jafc.9b02236. [DOI] [PubMed] [Google Scholar]
- Nguyen CC, Nguyen TQC, Kanaori K, et al. Antifungal activities of Ageratum conyzoides L. extract against rice pathogens Pyricularia oryzae cavara and Rhizoctonia solani Kühn. Agriculture. 2021;11:1169. doi: 10.3390/agriculture11111169. [DOI] [Google Scholar]
- Nicoletti R, Trincone A. Bioactive compounds produced by strains of Penicillium and Talaromyces of marine origin. Mar Drugs. 2016;14:37. doi: 10.3390/md14020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolopoulou-Stamati P, Maipas S, Kotampasi C, et al. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health. 2016;4:148. doi: 10.3389/fpubh.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J, Wright GD. An ecological perspective of microbial secondary metabolism. Curr Opin Biotechnol. 2011;22:552–558. doi: 10.1016/j.copbio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- O’Sullivan CA, Belt K, Thatcher LF. Tackling control of a cosmopolitan phytopathogen: Sclerotinia. Front Plant Sci. 2021 doi: 10.3389/fpls.2021.707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak VM, Verma VK, Rawat BS, et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: a comprehensive review. Front Microbiol. 2022;13:962619. doi: 10.3389/fmicb.2022.962619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purić J, Vieira G, Cavalca LB, et al. Activity of Antarctic fungi extracts against phytopathogenic bacteria. Lett Appl Microbiol. 2018;66:530–536. doi: 10.1111/lam.12875. [DOI] [PubMed] [Google Scholar]
- Rodrigo S, García-Latorre C, Santamaria O. Metabolites produced by fungi against fungal phytopathogens: review, implementation and perspectives. Plants. 2022;11:81. doi: 10.3390/plants11010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscetto E, Masi M, Esposito M, et al. Anti-biofilm activity of the fungal phytotoxin sphaeropsidin a against clinical isolates of antibiotic-resistant bacteria. Toxins (basel) 2020;12:444. doi: 10.3390/toxins12070444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Sun C, Sun Z, et al. Antibacterial polyketides from Antarctica sponge-derived fungus Penicillium sp. HDN151272. Mar Drugs. 2020 doi: 10.3390/md18020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D, Smith DL, Kabbage M, Roth MG. Effectors of plant necrotrophic fungi. Front Plant Sci. 2021 doi: 10.3389/fpls.2021.687713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber J, Ohlendorf B, Labes A, et al. Calcarides A-E, antibacterial macrocyclic and linear polyesters from a Calcarisporium Strain. Mar Drugs. 2013;11:3309–3323. doi: 10.3390/md11093309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JB, Gray LE. Three fungi that can reduce soybean yields. Ill Res. 1972;14:5. [Google Scholar]
- Singh BP, Rateb ME, Rodriguez-Couto S, et al. Editorial: microbial secondary metabolites: recent developments and technological challenges. Front Microbiol. 2019 doi: 10.3389/fmicb.2019.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan AP, Sugitha T, Karthikeyan G, et al. Metabolites of Trichoderma longibrachiatum EF5 inhibits soil borne pathogen, Macrophomina phaseolina by triggering amino sugar metabolism. Microb Pathog. 2021;150:104714. doi: 10.1016/j.micpath.2020.104714. [DOI] [PubMed] [Google Scholar]
- Sturrock CJ, Woodhall J, Brown M, et al. Effects of damping-off caused by Rhizoctonia solani anastomosis group 2–1 on roots of wheat and oil seed rape quantified using X-ray computed tomography and real-time PCR. Front Plant Sci. 2015;6:461. doi: 10.3389/fpls.2015.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svahn KS, Chryssanthou E, Olsen B, et al. Penicillium nalgiovense Laxa isolated from Antarctica is a new source of the antifungal metabolite amphotericin B. Fungal Biol Biotechnol. 2015 doi: 10.1186/s40694-014-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana H, Jowett D, Fehr WR. Determination of losses in soybeans caused by Rhizoctonia solano. Phytopathology. 1971;61:1444–1446. doi: 10.1094/Phyto-61-1444. [DOI] [Google Scholar]
- Thambugala KM, Daranagama DA, Phillips AJL, et al. Fungi vs. fungi in biocontrol: an overview of fungal antagonists applied against fungal plant pathogens. Front Cell Infect Microbiol. 2020;10:604923. doi: 10.3389/fcimb.2020.604923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JP, Bueno JT, Afonso MC, et al. Danos provocados Macrophomina phaseolina na cultura da soja. Rev Fitopatol Bras. 2007;32(1):231. [Google Scholar]
- Valerio F, Masi M, Cimmino A, et al. Antimould microbial and plant metabolites with potential use in intelligent food packaging. Nat Prod Res. 2018;32:1605–1610. doi: 10.1080/14786419.2017.1385018. [DOI] [PubMed] [Google Scholar]
- Vieira G, Purić J, Morão LG, et al. Terrestrial and marine Antarctic fungi extracts active against Xanthomonas citri subsp. citri. Lett Appl Microbiol. 2018;67:64–71. doi: 10.1111/lam.12890. [DOI] [PubMed] [Google Scholar]
- Vieira G, Khalil ZG, Capon RJ, et al. Isolation and agricultural potential of penicillic acid against citrus canker. J Appl Microbiol. 2022 doi: 10.1111/jam.15413. [DOI] [PubMed] [Google Scholar]
- Wentzel LCP, Inforsato FJ, Montoya QV, et al. Fungi from Admiralty Bay (King George Island, Antarctica) Soils and Marine Sediments. Microb Ecol. 2019;77:12–24. doi: 10.1007/s00248-018-1217-x. [DOI] [PubMed] [Google Scholar]
- Wrather JA, Koenning SR. Estimates of diseases effects on soybean yields in the United States 2003–2005. J Nematol. 2006;38:173–180. [PMC free article] [PubMed] [Google Scholar]
- Wyllie TD. Worst soybean disease. Crop Soils Mag. 1974;27:10–11. [Google Scholar]
- Zambolin L, Costa H, Vale FXR. Feijão Comum (Phaseolus vulgaris L.): Controle de doenças—podridão, tombamento e murcha causados por fungos de solo. In: do Vale FXR, Zambolin L, editors. Controle de doenças de plantas: grandes culturas. Viçosa: UFV Imprensa Universitária; 1997. pp. 375–402. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.