Abstract
Past work has shown that brain structure and function differ between females and males. Males have larger cortical and sub-cortical volume and surface area (both total and subregional), while females have greater cortical thickness in most brain regions. Functional differences are also reported in the literature, yet to date little work has systematically considered whether patterns of brain activity indexed with functional magnetic resonance imaging (fMRI) differ between females and males. The current study sought to remediate this issue by employing task-based whole brain motor mapping analyses using an openly available dataset. We tested differences in patterns of functional brain activity associated with 12 voluntary movement patterns in females versus males. Results suggest that females exhibited smaller volumes of brain activation across all 12 movement tasks, and lower patterns of variability in 10 of the 12 movements. We also observed that females had greater cortical thickness, which is in alignment with previous analyses of structural differences. Overall, these findings provide a basis for considering biological sex in future fMRI research and provide a foundation of understanding differences in how neurological pathologies present in females vs males.
Subject terms: Neuroscience, Sensorimotor processing
Introduction
Females and males have anatomical and functional differences in their nervous systems1–5. These differences, attributed to differential sex chromosome expression and sex hormones as early as in utero6–8, manifest in lateralization, motor planning, motor skill, language, memory, and spatial ability9–14. However, our understanding of how biological sex affects whole brain motor mapping is limited. Recently, there has been a push to modernize our understanding of the classic sensorimotor “homunculus”15–19, including the identification of a previously undescribed motor association area deep within the midlateral aspect of the central sulcus20. However, most studies have not considered sex differences. When sex has been included as a factor, the focus was largely on the sexual and reproductive dimorphisms and their respective neuronal representations19,21,22. More recent work has investigated the role of menstrual hormones on the cyclical change in functional connectivity in the female brain, however methodological limitations make it hard to generalize these findings, therefore meaningful conclusions cannot yet be made23–25.
A comparison of female and male brain structure reveals differences in volume, cortical surface area, cortical thickness, and structural variability26. Previous research suggests that males have significantly greater grey matter volume in areas such as the amygdala, temporal pole, fusiform gyrus, putamen, and premotor cortex3,4. Further, a large meta-analysis of 16,683 healthy participants found that males exhibit greater subcortical volume as compared to females, with the largest differences found in the thalamus and pallidum, bilaterally5. Importantly, males not only exhibit greater mean differences in subcortical volume and cortical surface area as compared to females, but also significantly greater variability in these measures5,26. This variability in surface area is most pronounced in motor related regions such as the pallidum, right inferior parietal cortex, and paracentral region5. This suggests that, compared to females, males have less consistent brain structure, particularly in motor related regions.
While most studies suggest greater total and subregional volume in males, some research found regions with greater relative volume in females. When controlling for total brain volume, these studies suggest females show greater grey matter volume than males in prefrontal and superior parietal cortices, as well as the superior temporal sulcus, orbitofrontal cortex, and posterior insula3,4. Females also exhibit greater cortical thickness compared to males5,26. Further, Wierenga et al.5 noted regional differences in cortical thickness between females and males; females had greater cortex thickness in 38 of 68 regions, which were primarily in the frontal and parietal cortices. These structural differences suggest that brain function may also differ by sex.
Sex related differences may affect the efficiency of neuronal processing, with females being more localized and precise, and males more distributed and varied. In that, neural efficiency can be thought of as a process where lower neural resources are needed to achieve a given behavioural or cognitive output. Several sex differences in brain activation have been observed with functional magnetic resonance imaging (fMRI) across a range of tasks and resting-state brain activity12,27–29. However, to date, little work has considered whether sex differences affect whole brain activation associated with movements of various body parts. Researching sex specific patterns of brain activation with respect to area and variability across a range of motor tasks may shed light on key differences in brain function. For instance, in fMRI research, a common approach to investigate brain activation patterns is through region of interest (ROI) analyses. However, using ROIs that are derived from an atlas that is not sex-specific could potentially lead to data selection bias that does not fully capture differences in functional organization in females or males.
Using fMRI whole brain motor mapping from a previously published and openly available dataset [30, https://openneuro.org/datasets/ds004044/versions/2.0.3], the current study investigated differences in cortical activation patterns between females and males during a variety of motor tasks. Based on previous observations of structural differences, we hypothesized that across movement tasks, females would exhibit lower variability and smaller volumes of brain activation than males, reflecting more focal neural processing. This concerted effort to further quantify the anatomical localization and functional differences will aid our understanding of the inherent differences in motor maps between the sexes, and inform ROI selection strategies for future fMRI research by illustrating whether sex specific brain parcellations are needed.
Methods
Participants
A total of 68 neurologically intact right-handed young adults (age range 19–29 years) were recruited and screened for the Ma et al.30 study. Of the 68 participants, 61 were included in the present analyses, of which 33 were female (22.58 ± 2.19 years), and 28 were male (23.00 ± 2.34 years). Six participants were excluded from the study because they failed to master the movement patterns as determined by two experimenters prior to the MRI session, and one participant was excluded from the analyses in this work because we were unable to determine their sex based on the demographics tables in the data provided. Detailed demographic information is provided in original paper30. The original study from which these data were collected conformed to the Declaration of Helsinki and was approved by the Institutional Review Board at Beijing Normal University30, and written informed consent was provided by the participants.
Motor tasks
Twelve voluntary movement patterns were each performed for 16 seconds (s) twice during every fMRI scan. A total of six fMRI scans were acquired for each participant, resulting in 12 event blocks for each of the 12 movement types (i.e., 192 s of task evoked data for each movement). These movement conditions included bilateral toes, ankles, fingers, wrists, forearms, upper arms, and eyes, in addition to jaw, lip, and tongue movements, and separate unilateral movements for the left and right legs. Detailed movement instructions can be found in Table 1. All movements were performed at a self-selected pace between 0.5 and 5 Hz. Importantly, each participant underwent two behavioural training sessions before undergoing the MRI portion of the study. These sessions involved watching a video tutorial on how to perform each of movements, followed by lying supine and practicing each of the movements under experimenter supervision. During these training sessions, feedback was given to ensure mastery of movement patterns, magnitude, and speed, while avoiding head or unrelated body movements. To proceed to the MRI portion of the study, movement performance was evaluated and verified by two experimenters. For further details about the nature of the experiment and motor tasks please review the published dataset manuscript30.
Table 1.
Movement conditions and instructions.
| Body movements | Movement patterns and instructions |
|---|---|
| Eyes | Blink or saccade eyes |
| Jaw | Bite or twist jaws |
| Lips | Expand and contract the lips with the teeth being bitten and tongue still |
| Tongue | Circular tongue with the teeth being bitten and lips closed |
| Upper arms | Lift and lower the upper arms (maximum 20°) with the upper arms, forearms, wrist, and fingers straight |
| Forearms | Flex and release both forearms at the elbow (maximum 20°) with the wrists and fingers straight |
| Wrists | Pitch and roll both wrists with clenched fists |
| Fingers | Clench and loose both fists |
| Left leg | Lift and lower the left leg (maximum 10°) with the leg, ankle, and toes straight |
| Right leg | Lift and lower the right leg (maximum 10°) with the leg, ankle, and toes straight |
| Ankles | Dorsiflex and release both ankles |
| Toes | Flex and extend the toes from both feet |
| Rest | Fixate on the dot presented in the center of the screen |
MRI acquisition
A structural T1-weighted (T1w) image was acquired first [voxel size = 1 mm3 isotropic, repetition time (TR) = 2530 ms, echo time (TE) = 2.27 ms, field of view (FOV) = 256 × 256 × 208, duration = 6.00 min], followed by six fMRI runs [voxel size = 2 mm3, TR = 2000 ms, TE = 34 ms, FOV = 200 × 200 × 144, duration = 7.44 min each], and finally a field map image was acquired for distortion correction of the fMRI images [voxel size = 2 mm3, TR = 720 ms, TE = 4.92/7.38 ms, FOV = 200 × 200 × 144, duration = 2.27 min]. The entire session took approximately 60 min for each participant to complete.
Preprocessing structural MRI data
The T1w anatomical scans were preprocessed using FSL’s31 anatomical preprocessing script fsl_anat. Briefly, this script performs image reorientation to align with standard space template coordinates, crops the T1w image in the z-direction to remove the neck and other non-brain aspects of the image, implements bias-field correction using FSL’s FAST32, and then extracts the brain using a non-linear reverse transformation of a standard-space brain mask.
First level functional MRI processing
The previously preprocessed and manually denoised fMRI data (for more information regarding the original preprocessing and denoising please review Ma et al.30) were linearly (ridged body + affine) registered to the bias-field corrected and brain extracted T1w image and then non-linearly (rigid + affine + deformable syn) registered to the MNI152_2mm brain template using antsRegistrationSyN and antsApplyTransforms from Advanced Normalization Tools (ANTs)33,34. Each of the 12 motor tasks were modelled using the event timing files provided in the downloaded data with a double-gamma function and with a temporal derivative applied to the model in FSL’s FEAT35. White matter BOLD signal was not treated as a nuisance regressor in the present work as this remains a controversial technique36–52. Therefore, to prevent a bias towards grey matter BOLD signal and to truly investigate whole-brain fMRI signal we retained white matter BOLD in our analyses50.
Second level fixed effects functional MRI processing
Since each participant performed the same tasks over six separate runs, a fixed effects analysis was carried out in FEAT35 prior to performing group-level analyses. The fixed effects analysis was used to average the parameter estimates of the task-based activations across the six runs to create a single activation map and effect size for each motor task for every participant. These averaged parameter estimates were then carried forward for group level analyses in FEAT53.
Structural MRI analysis
T1w images for each participant were segmented using Fastsurfer54. Overall brain volume without ventricles was first analyzed to determine if there were differences between females and males in brain size. Next, cortical surface area, cortical thickness, and grey matter volume for each hemisphere were extracted for analysis and normalized as a ratio to overall brain volume on a participant-by-participant basis to account for differences in brain size. We then compared each of these normalized metrics for each hemisphere between females and males separately using two-tailed independent samples t-tests implemented using the permutation_test module from SciPy stats in Python 3.1055 with 20,000 permutations. A manual Bonferroni correction to adjust for multiple comparisons was performed and a result was deemed to be significant if the p-value was ≤ 0.007 (α = 0.05/7).
Group level functional MRI analysis
For group-level analyses, two one-sample t-tests were performed to compute spatial maps for females and males separately. Additionally, two one-tailed independent samples t-tests were carried out to determine if there were differences in the brain activation patterns for each of the 12 motor movements between female and males (t-test #1: females > males; t-test #2: males > females contrasts). Non-parametric permutation testing was performed on each contrast using randomise with 5,000 permutations, and threshold free cluster enhancement was used for statistical inference with a family wise error rate set to α ≤ 0.05 for each contrast.
Sex difference analyses
To address the question of sex differences for whole brain motor mapping, two approaches were taken. First, the two t-tests previously described (t-test #1: females > males; t-test #2: males > females) were assessed and reported when a significant contrast was detected (Fig. 1). Second, to further investigate sex differences, the mean activation map for each movement was computed separately for females and males and the spatial maps were correlated to assess their degree of similarity. To do this, for females and males the group-level p-value threshold statistical maps (α ≤ 0.05) for each movement condition were temporally merged together using fslmerge. Then correlation analyses between the spatial maps was carried out with fslcc. The correlation coefficients for same movement conditions between females and males were then reported and the inter-task correlations were discarded.
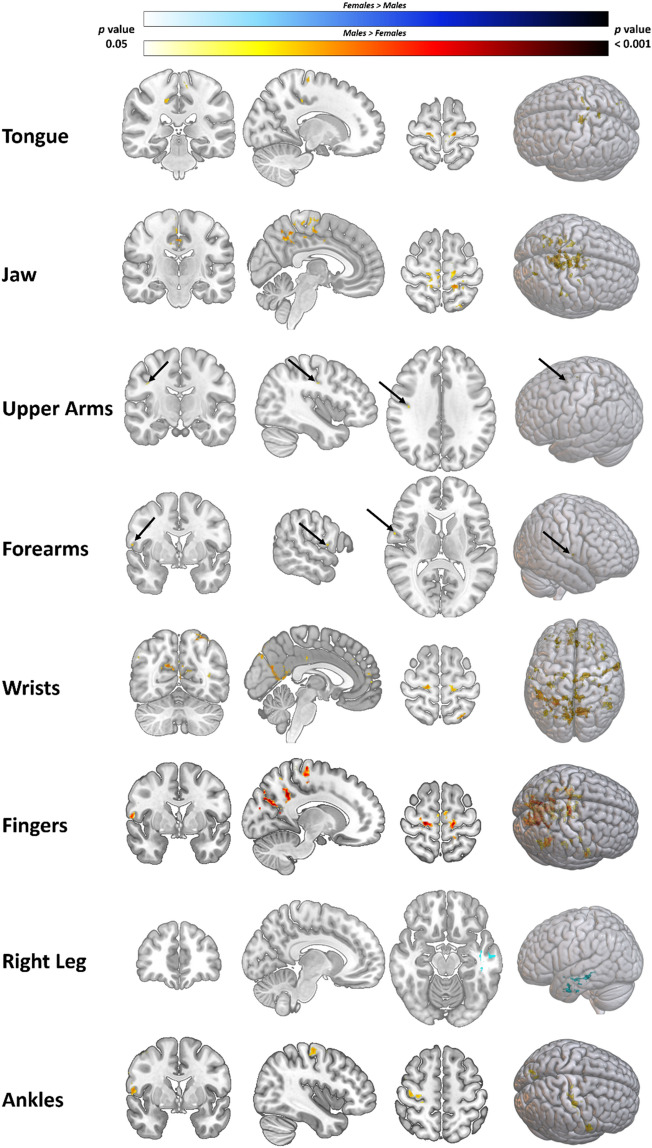
Figure 1.
Sex difference contrasts for 8 significant conditions. Significantly greater BOLD signal for males (Red/Yellow) and females (White/Blue) are plotted. Images are family-wise error corrected p-value maps with a p value threshold of 0.05. Figure was made in MRIcoGL57.
Sex differences in brain activation variability
Variability in brain activation was assessed in two ways. First, within group between-subject variability in brain activation was assessed for each motor task. To do this, we performed pairwise correlations for both females and males using the raw t-statistic image in standardized space for each participant and calculated the voxel-to-voxel correlation of these images for each pair of participants separately for each group using in-house python code. This process was repeated for each motor task. We then compared the pairwise correlations between females and males for each contrast using two-tailed independent samples t-tests implemented using the permutation_test module from SciPy stats in Python 3.1055 with 20,000 permutations. A manual Bonferroni correction to adjust for multiple comparisons was performed and a result was deemed to be significant if the p-value was ≤ 0.004 (α = 0.05/12).
Second, we analyzed brain activation variability within-subject between runs (6 runs). To do this, we once again performed pairwise correlations for each participant using the raw t-statistic image for each of the runs in subject space. We then calculated the mean correlation coefficient from these pairwise correlations (mean of 15 comparisons). This process was repeated for each of the 12 motor tasks and the rest condition. We then compared the pairwise correlations between females and males for each contrast using two-tailed independent samples t-tests implemented using the permutation_test module from SciPy stats in Python 3.1055 with 20,000 permutations. A manual Bonferroni correction to adjust for multiple comparisons was performed and a result was deemed to be significant if the p-value was ≤ 0.004 (α = 0.05/12).
Controlling for the effects of group-level template space on data interpretation
In addition to registering data to the MNI 152 template to carry out group-level analyses, we also replicated the analyses in three additional group-level templates. We used FSL tools FLIRT56 and fslmaths to create three additional group level mean template spaces: (1) a group level mean template space including all structural T1w scans, (2) a female only template space that included only the T1w scans from female participants, and finally (3) a male only template space that included only the T1w scans from male participants.
Results
Sex differences in brain structure
Permutation t-testing with 20,000 permutations revealed sex differences in total brain volume [Females: 1,099,719.242 ± 55,040.247 mm3; Males: 1,230,966.679 ± 110,273.860 mm3; t(59) = − 5.912, p = 1.00E−04, d = − 1.480]. There was also a significant difference in normalized cortical thickness in the left [Females: 2.18E-06 ± 1.17E−07; Males: 1.99E−06 ± 1.75E−07; t(59) = 4.950, p = 1.00E−04, d = 1.251] and right [Females: 2.19E−06 ± 1.17E−07; Males: 1.99E−06 ± 1.70E−07; t(59) = 5.268, p = 1.00E−04, d = 1.333] hemispheres, with females having thicker cortex. However, no differences were observed for normalized grey matter volume in the left [Females: 0.211 ± 0.008; Males: 0.214 ± 0.007; t(59) = 1.451, p = 0.155, d = − 0.376], or right [Females: 0.212 ± 0.008; Males: 0.215 ± 0.007; t(59) = − 1.740, p = 0.089, d = − 0.451] hemisphere, or normalized surface area in the left [Females: 0.075 ± 0.002; Males: 0.075 ± 0.002; t(59) = 0.653, p = 0.509, d = 0.165], or right [Females: 0.075 ± 0.002; Males: 0.075 ± 0.002; t(59) = 0.653, p = 0.509, d = 0.165] hemisphere. In line with previous findings5,26, these results suggest that males have larger overall brains, while females exhibited thicker cortex in both hemispheres.
Sex differences for group-level contrasts
Significant differences were found in the males > females contrast in seven out of the 12 movements including tongue, jaw, upper arms, forearms, wrists, fingers, and ankles (Fig. 1; supplementary data tables S1–S7). The only contrast that showed a significant females > males difference was right leg movements (data table S8).
Sex differences for group-level spatial maps
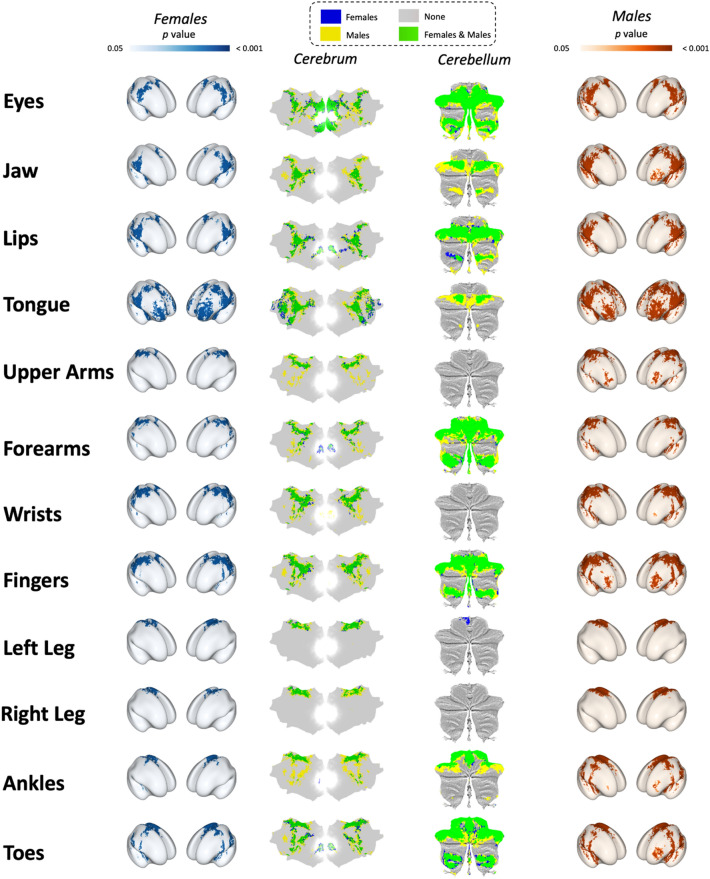
Across all 12 movement tasks, males (mean across 12 tasks: 36,632.58 ± 14,469.06 voxels) exhibited larger volumes of activation compared to females (mean across 12 tasks: 21,315.33 ± 11,738.90 voxels), which equates to males having a mean 92.68 ± 66.81% larger volumes of activation across the whole brain (Fig. 2; Table 2). The spatial maps between females and males were moderately correlated58 with a mean Pearsons correlation strength r = 0.61 ± 0.06, and a range across the 12 tasks from r = 0.48 for upper arms movements to r = 0.70 for left leg movements (Table 2).
Figure 2.
p-value spatial maps for all 12 movement tasks. Female spatial maps are in blue on the left, male spatial maps are in red/orange on the right. Cerebral and cerebellar flatmaps showing unique and overlap are in the centre two columns respectively. Cerebral inflated brains and flatmaps were made in pycortex59, and cerebellar flatmaps were made in SUIT60.
Table 2.
Group-level whole brain spatial maps and correlations between sexes.
| Condition | Total number of significant voxels* | Spatial map similarity | ||
|---|---|---|---|---|
| Females | Males | Difference (%)** | r-values*** | |
| Eyes | 38,843 | 53,649 | 38.12 | 0.66 |
| Jaw | 14,336 | 34,774 | 142.56 | 0.56 |
| Lips | 27,593 | 36,828 | 33.47 | 0.64 |
| Tongue | 37,443 | 52,374 | 39.88 | 0.64 |
| Upper Arms | 9434 | 30,045 | 218.48 | 0.48 |
| Forearms | 22,018 | 38,586 | 75.25 | 0.60 |
| Wrists | 26,317 | 47,832 | 81.75 | 0.64 |
| Fingers | 27,359 | 47,557 | 73.83 | 0.65 |
| Left Leg | 6267 | 8892 | 41.89 | 0.70 |
| Right Leg | 5325 | 10,933 | 105.31 | 0.64 |
| Ankles | 11,112 | 35,301 | 217.68 | 0.51 |
| Toes | 29,737 | 42,820 | 44.00 | 0.64 |
*Voxel size = 2 × 2 × 2 mm.
**Percent difference of voxels activated in each condition for males compared to females.
***Pearson’s r correlation coefficients from a cross-correlation analyses between females and males.
Sex differences in brain activation variability
Using permutation testing to assess sex differences in correlations derived from whole-brain voxel wise t-statistics for each contrast, we found that females had significantly higher between subject consistency in 10 of the 12 motor tasks (Table 3; p < 0.004, two-tailed, 20,000 permutations). However, an analysis of within subject between run variability resulted in no differences between sex for any of the motor tasks (Table 4; two-tailed, 20,000 permutations), suggesting that there were no differences between females and males in how consistent their patterns of brain activation were across the six runs for each of the motor tasks.
Table 3.
Permutation testing of within group, between subject whole-brain voxel wise brain activation correlations.
| Condition | Mean correlation ± Standard deviation | Independent samples t-tests | |
|---|---|---|---|
| Females | Males | ||
| Eyes | 0.259 ± 0.085 | 0.226 ± 0.064 | t(904) = 6.247, p = 1.00E−04, d = 0.430* |
| Jaw | 0.228 ± 0.056 | 0.187 ± 0.055 | t(904) = 11.021, p = 1.00E−04, d = 0.744* |
| Lips | 0.231 ± 0.066 | 0.201 ± 0.063 | t(904) = 6.883, p = 1.00E−04, d = 0.465* |
| Tongue | 0.278 ± 0.069 | 0.264 ± 0.056 | t(904) = 3.274, p = 0.001, d = 0.224* |
| Upper arms | 0.216 ± 0.079 | 0.200 ± 0.061 | t(904) = 3.483, p = 5.00E−04, d = 0.240* |
| Forearms | 0.239 ± 0.072 | 0.209 ± 0.047 | t(904) = 7.253, p = 1.00E−04, d = 0.505* |
| Wrists | 0.302 ± 0.063 | 0.266 ± 0.062 | t(904) = 8.530, p = 1.00E−04, d = 0.576* |
| Fingers | 0.303 ± 0.068 | 0.280 ± 0.066 | t(904) = 5.179, p = 1.00E−04, d = 0.350* |
| Left leg | 0.217 ± 0.085 | 0.183 ± 0.083 | t(904) = 5.886, p = 1.00E−04, d = 0.398* |
| Right leg | 0.199 ± 0.096 | 0.209 ± 0.083 | t(904) = − 1.598, p = 0.108, d = − 0.109 |
| Ankles | 0.216 ± 0.072 | 0.188 ± 0.066 | t(904) = 5.906, p = 1.00E−04, d = 0.401* |
| Toes | 0.244 ± 0.070 | 0.184 ± 0.055 | t(904) = 9.023, p = 1.00E−04, d = 0.620* |
Note: * indicates significant result. Significance set to Bonferroni corrected α = 0.004 [α = 0.004; (0.05/12)], two-tailed, 20,000 permutations.
Table 4.
Permutation testing of within subject, between runs whole-brain voxel wise brain activation correlations.
| Condition | Mean correlation ± Standard deviation | Independent samples t-tests | |
|---|---|---|---|
| Females | Males | ||
| Eyes | 0.190 ± 0.101 | 0.181 ± 0.100 | t(59) = 0.369, p = 0.700, d = 0.095 |
| Jaw | 0.174 ± 0.063 | 0.172 ± 0.092 | t(59) = 0.096, p = 0.929, d = 0.024 |
| Lips | 0.189 ± 0.086 | 0.180 ± 0.082 | t(59) = 0.452, p = 0.638, d = 0.116 |
| Tongue | 0.269 ± 0.085 | 0.226 ± 0.103 | t(59) = 1.774, p = 0.081, d = 0.452 |
| Upper arms | 0.241 ± 0.079 | 0.225 ± 0.089 | t(59) = 0.700, p = 0.489, d = 0.179 |
| Forearms | 0.195 ± 0.080 | 0.186 ± 0.073 | t(59) = 0.452, p = 0.654, d = 0.116 |
| Wrists | 0.223 ± 0.092 | 0.219 ± 0.087 | t(59) = 0.161, p = 0.870, d = 0.041 |
| Fingers | 0.206 ± 0.092 | 0.199 ± 0.071 | t(59) = 0.335, p = 0.731, d = 0.087 |
| Left leg | 0.195 ± 0.067 | 0.219 ± 0.099 | t(59) = − 1.129, p = 0.264, d = − 0.285 |
| Right leg | 0.181 ± 0.054 | 0.242 ± 0.120 | t(59) = − 2.588, p = 0.012, d = − 0.646 |
| Ankles | 0.148 ± 0.073 | 0.161 ± 0.071 | t(59) = − 0.705, p = 0.479, d = − 0.181 |
| Toes | 0.154 ± 0.073 | 0.141 ± 0.079 | t(59) = 0.685, p = 0.494, d = 0.175 |
| Rest | 0.059 ± 0.033 | 0.048 ± 0.022 | t(59) = 1.511, p = 0.138, d = 0.394 |
Note: Significance set to Bonferroni corrected α = 0.004 [α = 0.004; (0.05/12)], two-tailed, 20,000 permutations.
Effects of group-level template space on data interpretation
Overall, reanalyzing the data in the three additional study derived template spaces (i.e., study template, female only template, or male only template space) did not alter the findings or overall take-home message. All data tables from these additional analyses can be found in the supplemental materials.
Discussion
In this study, we sought to determine if there were sex differences in whole brain motor maps with a range of movements across the human body. Based on previous research, there are distinct structural differences in cortical organization between females and males, with females having greater cortical thickness5,26, and males having larger cortical surface area and cortical volume; this is particularly the case for the motor and somatosensory regions5,26 which are known to have somatotopic organization61. Given these previously observed differences, we hypothesized that females would exhibit more focal (i.e., smaller volumes of activation) and consistent (i.e., lower variability between females for each task) patterns of brain activation compared to males across the 12 different body movements. To answer these questions, we used a freely available fMRI dataset that included a total of 61 participants, of which 33 were females and 28 were males.
Males generally exhibit larger magnitudes of brain activation
In seven of the movements under investigation (jaw, tongue, upper arms, forearms, wrists, fingers, and ankles) we observed greater BOLD signal in males than females. In contrast, females only exhibited greater activation than males during right leg movements, and no differences were seen with the remaining four movements (eyes, lips, left leg, and toes). The observation that right leg movements resulted in a cluster of significantly greater BOLD signal for females than males should be interpreted with caution. In the original study by Ma et al.30, the authors noted higher head motion during right leg movements compared to the other movement tasks. Although sex differences were not analyzed specifically for the right leg movement aspects of the individual fMRI runs, mean motion parameters (relative and absolute motion) did not differ between sex. Regardless, the findings of greater BOLD signal in females during right leg movements may be a product of motion and should be interpreted with caution.
While clusters were distributed throughout the brain, several regions showed different activation patterns between females and males across multiple movements. In six of the movements, males showed greater activation in the precuneus, a brain region with complex structural and functional connectivity62,63. Many studies report sex differences in activation of the precuneus in various tasks, including mental rotation, body perception and memory recall tasks64–66. Further, sex differences in functional connectivity of the precuneus with many regions, including the thalamus, hippocampus, and middle occipital gyrus has been observed62. These results indicate a potential key-role for the precuneus in sex differences in brain functional organization.
Other regions that showed greater activation in males across multiple movements are the lateral occipital cortex (in 6 different movements), frontal pole (4 of the movements) and the precentral gyrus (4 of the movements). Interestingly, previous studies suggest larger grey-matter volumes in females compared to males in all of these regions4,67. Further, a recent study suggested increased activation in males compared to females in these regions during a body-perception task66. It is possible that differences in activation are related to other, non-motor, aspects of the task, such as increased awareness to own-body following task performance.
Females exhibit smaller volumes of brain activation across all movement conditions
Smaller activation maps were observed in females across all 12 motor tasks (on average males had 92.68 ± 66.81% larger activation maps than females, ranging from 33.47% larger for lip movements to 218.48% larger for upper arms movements; Table 2, Fig. 2). These data support our hypothesis that females would have more focal volumes of brain activation given the previous findings that males have larger overall cortical and subcortical volume and surface area5,26, and provide support for females having better cortical efficiency68 during simple movements across the body.
Lower whole-brain activation variability in females during all movement conditions
Based on our novel whole-brain voxel wise correlation analysis, females consistently showed higher correlations between-subjects compared to males (Table 2); these differences were statistically significant. These findings should be viewed as complementary to the volume of activation data reported in this work, given the smaller volumes of brain activation paired with the lower between-subject variability seen in females may be indicative of greater neural efficiency compared to males. Additionally, to determine if there were sex differences for how consistent patterns of brain activation were during the 12 motor tasks, we assessed intra-subject variability for each motor task across the six separate fMRI runs. Based on these intra-subject variability analyses, there were no sex differences and both females and males exhibited similar levels of variability. These analyses were important to ensure any sex differences observed throughout the study were not a product of intra-subject variability differences between sexes.
Our findings contribute to the growing body of evidence indicating that extensive sex differences in brain organization and function exist3,26,69,70. Importantly, Ritchie et al.26 noted that the observed sex differences in cortical measures in their work may be related to compensatory interactions between sex-specific hormones and the neural underpinnings that govern human voluntary movement71–73. It is plausible that these hormonal differences may impact both behaviour and the structure and function of the human brain, which could explain at least in part the differences observed in the present study.
There is accumulating evidence from recent years suggesting there are sex differences in brain structure, and specifically in motor areas, including the premotor cortex, putamen, and cerebellum [GM volume higher in males > females4,26,67]. Yet, the majority of these findings refer to structural differences in volume, surface area or cortical thickness, with limited data regarding functional imaging differences or their associations with motor behaviour. Greater spatial distributions and/or magnitudes of BOLD signal have been observed, without differences in task performance, for males during verbal and spatial tasks74, in addition to cognitive and motor tasks75. These findings paired with the present work suggest that females often show lower magnitudes, and smaller volumes of brain activation across cognitive-motor tasks.
Implications of sex differences in functional brain imaging research analyses
Importantly, there are many methods to analyze functional brain imaging data. These analysis and interpretation approaches often rely on previous findings, and as a result neglect to acknowledge sex differences. For example, a ROI based analysis that is derived from an atlas could prevent true ROI identification if it does not consider sex differences in functional organization of the brain, and is instead a product of group averaging. Some brain atlases that are commonly used in fMRI research for ROI selection include the Harvard–Oxford cortical and subcortical atlases (16 females, 21 males, between the ages of 18–50), the Brainnetome atlas (10 females, 10 males, all right handed, between the ages of 19–25), and the Schaefer atlas (665 males, 905 females, between the ages of 18–35). Each of these atlases were created by mixing female and male participants, which may lead to suboptimal parcellations given our findings that males tend to exhibit larger volumes of BOLD signal across a range of motor tasks. Our data suggests that future studies should consider selection of sex specific ROIs based on functional activation76–78, when possible.
Implications of sex differences in neurological impairments
As discussed, a knowledge gap exists regarding the dimorphisms in motor maps between females and males. While filling this gap is generally useful for research purposes, it cannot be understated how important these findings may be for the understanding of the etiology, prognosis, and recovery from injury or disease in females versus males. The incidence and presentation of many neurological conditions and movement disorders differ between sexes, suggesting underlying differences in brain function or structure79. For example, females experience increased severity, more impairment, and longer recovery from stroke80–83. Although many explanatory factors have been identified, incorporating motor mapping differences into this phenomenon should be discussed. As identified in this research, females use less of their cortex to generate voluntary movements, and do so with significantly lower brain activation variability. Although this may be beneficial in healthy, neurologically intact populations, this approach may be detrimental in the presence of a neurological injury. For example, with stroke, injury to the central nervous system interrupts the normal motor output pathways and leads to motor impairment. Further, our data showing that females have smaller volumes of cortical activation and lower variability than males suggests that comparisons using standard ROI atlases may lead to misinterpretation of data and fundamentally limit our understanding of brain function in these neurological conditions.
Limitations and future directions
As this study used a publicly available data, our analysis was restricted to the previously collected data. Therefore, we were unable to include gender in our analysis. Recent studies indicate that gender differences in both brain structure and brain function exist84, and future studies should collect the appropriate data to allow for both sex- and gender-based analyses. Additionally, force output can influence brain activation85–87, and in the present data there are no behavioural metrics to quantify the movement force, velocity, or quality. This is a noted limitation that future work should consider measuring to better link movement kinematics to cortical measures of activation. However, past work85 suggests that small differences in force (e.g., between 25 and 50%, or 50% and 75% MVC) are not associated with significant differences in brain activation. Only large differences in movement characteristics (e.g., 25% and 75% MVC) result in significant activation changes. Additionally, work by Wüthrich et al.88 recently observed good fMRI activation overlap and reliability between sessions with a finger tapping task, even between conditions where pacing was fast unpaced or paced. Regardless we believe such differences are unlikely to occur in this study, as participants were instructed to perform the movements at a comfortable self-selected pace, without any additional resistance. Further, even if individual differences exist, group differences in movement characteristics are not assumed, especially in light of the lack of sex differences in intra-subject variability. In light of our sample size, we presume that such differences, if they exist, will be balanced between the two groups.
Conclusions
In this study, we found significant sex differences in the magnitude of brain activation for eight out of the 12 movement conditions, with males having significantly greater brain activation in seven out of the 12 conditions and females showing greater brain activation in one condition. Interestingly, we observed smaller volumes of brain activation in all 12 movement conditions and significantly lower whole-brain activation variability in 10 of the 12 movement conditions for females. With a cross-correlation analysis to compare the spatial map similarity between sexes, we found that spatial maps were moderately correlated for each movement conditions between sexes. The difference in volume is an important consideration when creating ROIs based on functional localizers, as clear and visible differences can be observed between sexes, and has potentially significant implications for understanding the neurobiology of various neurological impairments in females and males (Fig. 2). We propose these data provide important insight into the need for sex specific ROIs for functional localization to accurately study brain-behaviour relationships using fMRI, and likely have important clinical implications for understanding impairment severity and rehabilitation strategies for each sex independently.
Supplementary Information
Acknowledgements
The authors would like to acknowledge the original authors of the open dataset Sai Ma, Taicheng Huang, Yukun Qu, Xiayu Chen, Yajie Zhang and Zonglei Zhen. Without their support of open science practices, this work would not have been made possible.
Author contributions
J.W.A. processed and analyzed the data, and assisted in writing the manuscript. S.R. processed and analyzed the data and assisted in writing the manuscript. E.K. created the figures and assisted in writing the manuscript. J.D. assisted with writing the manuscript and approving the final version. C.E. assisted in data analysis and writing the manuscript, and J.W.A., C.E. and L.B. were involved with the overall conception and direction of this study, and edited and approved the manuscript.
Funding
This work was funded by the Canadian Institutes of Health Research (PJT-148535) to L.A.B. and an NSERC Discovery Grant and NSERC Discovery Launch Supplement to C.E. (RGPIN-2021-03568 and DGECR-2021-00297). J.W.A., is funded by a Canadian Institutes of Health Research (CIHR) fellowship and a Michael Smith Foundation for Health Research postdoctoral award, S.R. is supported by awards from the University of British Columbia, E. K. is funded by an NSERC Canada Graduate Master’s award, J.D. is funded by a CIHR Canada Graduate Master’s award.
Data availability
Data are available at https://openneuro.org/datasets/ds004044/versions/2.0.3.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Chelsea Ekstrand and Lara A. Boyd.
Contributor Information
Chelsea Ekstrand, Email: Chelsea.ekstrand@uleth.ca.
Lara A. Boyd, Email: lara.boyd@ubc.ca
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-44871-4.
References
- 1.Williams CM, Peyre H, Toro R, Ramus F. Sex differences in the brain are not reduced to differences in body size. Neurosci. Biobehav. Rev. 2021;130:509–511. doi: 10.1016/j.neubiorev.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 2.DeCasien AR, Guma E, Liu S, Raznahan A. Sex differences in the human brain: A roadmap for more careful analysis and interpretation of a biological reality. Biol. Sex Differ. 2022;13:1. doi: 10.1186/s13293-022-00448-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S, Seidlitz J, Blumenthal JD, Clasen LS, Raznahan A. Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc. Natl. Acad. Sci. U. S. A. 2020;117:18788–18798. doi: 10.1073/pnas.1919091117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotze M, et al. Novel findings from 2,838 adult brains on sex differences in gray matter brain volume. Sci. Rep. 2019;9:1671. doi: 10.1038/s41598-018-38239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wierenga LM, et al. Greater male than female variability in regional brain structure across the lifespan. Hum. Brain Mapp. 2022;43:470–499. doi: 10.1002/hbm.25204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook KM, et al. Robust sex differences in functional brain connectivity are present in utero. Cereb. Cortex. 2023;33:2441–2454. doi: 10.1093/cercor/bhac218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallard TT, et al. X-chromosome influences on neuroanatomical variation in humans. Nat. Neurosci. 2021;24:1216–1224. doi: 10.1038/s41593-021-00890-w. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy MM, Pickett LA, VanRyzin JW, Kight KE. Surprising origins of sex differences in the brain. Horm. Behav. 2015;76:3–10. doi: 10.1016/j.yhbeh.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hegarty M. Ability and sex differences in spatial thinking: What does the mental rotation test really measure? Psychon. Bull. Rev. 2018;25:1212–1219. doi: 10.3758/s13423-017-1347-z. [DOI] [PubMed] [Google Scholar]
- 10.Yuan L, et al. Gender differences in large-scale and small-scale spatial ability: A systematic review based on behavioral and neuroimaging research. Front. Behav. Neurosci. 2019;13:896. doi: 10.3389/fnbeh.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogojin A, Gorbet DJ, Sergio LE. Sex differences in the neural underpinnings of unimanual and bimanual control in adults. Exp. Brain Res. 2023;241:793–806. doi: 10.1007/s00221-023-06561-5. [DOI] [PubMed] [Google Scholar]
- 12.Spets DS, Slotnick SD. Are there sex differences in brain activity during long-term memory? A systematic review and fMRI activation likelihood estimation meta-analysis. Cogn. Neurosci. 2021;12:163–173. doi: 10.1080/17588928.2020.1806810. [DOI] [PubMed] [Google Scholar]
- 13.Xu M, et al. Sex differences in functional brain networks for language. Cereb. Cortex. 2020;30:1528–1537. doi: 10.1093/cercor/bhz184. [DOI] [PubMed] [Google Scholar]
- 14.Gur RC, Gur RE. Complementarity of sex differences in brain and behavior: From laterality to multimodal neuroimaging. J. Neurosci. Res. 2017;95:189–199. doi: 10.1002/jnr.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon EM, et al. A somato-cognitive action network alternates with effector regions in motor cortex. Nature. 2023;2023:1–9. doi: 10.1038/s41586-023-05964-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roux FE, Niare M, Charni S, Giussani C, Durand JB. Functional architecture of the motor homunculus detected by electrostimulation. J. Physiol. 2020;598:5487–5504. doi: 10.1113/JP280156. [DOI] [PubMed] [Google Scholar]
- 17.Zeharia N, Hertz U, Flash T, Amedi A. Negative blood oxygenation level dependent homunculus and somatotopic information in primary motor cortex and supplementary motor area. Proc. Natl. Acad. Sci. U. S. A. 2012;109:18565–18570. doi: 10.1073/pnas.1119125109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kell CA, Von Kriegstein K, Rösler A, Kleinschmidt A, Laufs H. The sensory cortical representation of the human penis: Revisiting somatotopy in the male homunculus. J. Neurosci. 2005;25:5984–5987. doi: 10.1523/JNEUROSCI.0712-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima SQ. Genital cortex: Development of the genital homunculus. Curr. Biol. 2019;29:R1122–R1124. doi: 10.1016/j.cub.2019.09.051. [DOI] [PubMed] [Google Scholar]
- 20.Jensen MA, et al. A motor association area in the depths of the central sulcus. Nat. Neurosci. 2023;26:1165–1169. doi: 10.1038/s41593-023-01346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Noto PM, Newman L, Wall S, Einstein G. The hermunculus: What is known about the representation of the female body in the brain? Cereb. Cortex. 2013;23:1005–1013. doi: 10.1093/cercor/bhs005. [DOI] [PubMed] [Google Scholar]
- 22.Komisaruk BR, et al. Women’s clitoris, vagina, and cervix mapped on the sensory cortex: fMRI evidence. J. Sex. Med. 2011;8:2822–2830. doi: 10.1111/j.1743-6109.2011.02388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller JM, et al. Dynamic community detection reveals transient reorganization of functional brain networks across a female menstrual cycle. Netw. Neurosci. 2021;5:125–144. doi: 10.1162/netn_a_00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pritschet L, et al. Functional reorganization of brain networks across the human menstrual cycle. Neuroimage. 2020;220:117091. doi: 10.1016/j.neuroimage.2020.117091. [DOI] [PubMed] [Google Scholar]
- 25.Pritschet L, Taylor CM, Santander T, Jacobs EG. Applying dense-sampling methods to reveal dynamic endocrine modulation of the nervous system. Curr. Opin. Behav. Sci. 2021;40:72–78. doi: 10.1016/j.cobeha.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritchie SJ, et al. Sex differences in the adult human brain: Evidence from 5216 UK biobank participants. Cereb. Cortex. 2018;28:2959–2975. doi: 10.1093/cercor/bhy109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonelli C, et al. Sex differences in brain homotopic co-activations: A meta-analytic study. Brain Struct. Funct. 2022;227:2839–2855. doi: 10.1007/s00429-022-02572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuptsova SV, Ivanova MV, Petrushevsky AG, Fedina ON, Zhavoronkova LA. Sex-related differences in task switching: An fMRI study. Hum. Physiol. 2015;41:611–624. [PubMed] [Google Scholar]
- 29.Xu C, et al. Gender differences in cerebral regional homogeneity of adult healthy volunteers: A resting-state fMRI study. Biomed Res. Int. 2015;2015:8. doi: 10.1155/2015/183074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma S, et al. An fMRI dataset for whole-body somatotopic mapping in humans. Sci. Data. 2022;9:515. doi: 10.1038/s41597-022-01644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 33.Avants B, Tustison N, Johnson H. Advanced normalization tools (ANTS) Insight J. 2009 doi: 10.54294/uvnhin. [DOI] [Google Scholar]
- 34.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 36.Gore JC, et al. Functional MRI and resting state connectivity in white matter—a mini-review. Magn. Reson. Imaging. 2019;63:1–11. doi: 10.1016/j.mri.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gawryluk JR, Mazerolle EL, D’Arcy RCN. Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Front. Neurosci. 2014;8:239. doi: 10.3389/fnins.2014.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rostrup E, et al. Regional differences in the CBF and BOLD responses to hypercapnia: A combined PET and fMRI study. Neuroimage. 2000;11:87–97. doi: 10.1006/nimg.1999.0526. [DOI] [PubMed] [Google Scholar]
- 39.Helenius J, et al. Cerebral hemodynamics in a healthy population measured by dynamic susceptibility contrast MR imaging. Acta radiol. 2003;44:538–546. doi: 10.1080/j.1600-0455.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 40.Tettamanti M, et al. Interhemispheric transmission of visuomotor information in humans: fMRI evidence. J. Neurophysiol. 2002;88:1051–1058. doi: 10.1152/jn.2002.88.2.1051. [DOI] [PubMed] [Google Scholar]
- 41.Newman AJ, Supalla T, Hauser P, Newport EL, Bavelier D. Dissociating neural subsystems for grammar by contrasting word order and inflection. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7539–7544. doi: 10.1073/pnas.1003174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gawryluk JR, D’Arcy RCN, Mazerolle EL, Brewer KD, Beyea SD. Functional mapping in the corpus callosum: A 4 T fMRI study of white matter. Neuroimage. 2011;54:10–15. doi: 10.1016/j.neuroimage.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 43.Gawryluk JR, Mazerolle EL, Brewer KD, Beyea SD, D’Arcy RCN. Investigation of fMRI activation in the internal capsule. BMC Neurosci. 2011;12:1–7. doi: 10.1186/1471-2202-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weis S, et al. Functional neuroanatomy of sustained memory encoding performance in healthy aging and in alzheimer’s disease. Int. J. Neurosci. 2011;121:384–392. doi: 10.3109/00207454.2011.565892. [DOI] [PubMed] [Google Scholar]
- 45.Mazerolle EL, et al. Sensitivity to white matter fMRI activation increases with field strength. PLoS One. 2013;8:e58130. doi: 10.1371/journal.pone.0058130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fabri M, Pierpaoli C, Barbaresi P, Polonara G. Functional topography of the corpus callosum investigated by DTI and fMRI. World J. Radiol. 2014;6:895–906. doi: 10.4329/wjr.v6.i12.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peer M, Nitzan M, Bick AS, Levin N, Arzy S. Evidence for functional networks within the human brain’s white matter. J. Neurosci. 2017;37:6394–6407. doi: 10.1523/JNEUROSCI.3872-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frizzell TO, et al. White matter neuroplasticity: Motor learning activates the internal capsule and reduces hemodynamic response variability. Front. Hum. Neurosci. 2020;14:456. doi: 10.3389/fnhum.2020.509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Y, et al. Function–structure coupling: White matter functional magnetic resonance imaging hyper-activation associates with structural integrity reductions in schizophrenia. Hum. Brain Mapp. 2021;42:4022–4034. doi: 10.1002/hbm.25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grajauskas LA, Frizzell T, Song X, D’Arcy RCN. White matter fMRI activation cannot be treated as a nuisance regressor: Overcoming a historical blind spot. Front. Neurosci. 2019;13:1024. doi: 10.3389/fnins.2019.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding Z, et al. Spatio-temporal correlation tensors reveal functional structure in human brain. PLoS One. 2013;8:e82107. doi: 10.1371/journal.pone.0082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazerolle EL, et al. Confirming white matter fMRI activation in the corpus callosum: Co-localization with DTI tractography. Neuroimage. 2010;50:616–621. doi: 10.1016/j.neuroimage.2009.12.102. [DOI] [PubMed] [Google Scholar]
- 53.Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 54.Henschel L, et al. FastSurfer—a fast and accurate deep learning based neuroimaging pipeline. Neuroimage. 2020;219:117012. doi: 10.1016/j.neuroimage.2020.117012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Virtanen P, et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods. 2020;17:261–272. doi: 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 57.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav. Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- 58.Akoglu H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018;18:91–93. doi: 10.1016/j.tjem.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao JS, Huth AG, Lescroart MD, Gallant JL. Pycortex: An interactive surface visualizer for fMRI. Front. Neuroinform. 2015;9:8. doi: 10.3389/fninf.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diedrichsen J, Zotow E. Surface-based display of volume-averaged cerebellar imaging data. PLoS One. 2015;10:7. doi: 10.1371/journal.pone.0133402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Penfield W, Rasmussen T. The cerebral cortex of man: A clinical study of localization of function. Macmillan. 1950 doi: 10.1001/jama.1950.02920160086033. [DOI] [Google Scholar]
- 62.Zhang S, Li C, Shan R. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012;59:3548. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cunningham SI, Tomasi D, Volkow ND. Structural and functional connectivity of the precuneus and thalamus to the default mode network. Hum. Brain Mapp. 2017;38:938. doi: 10.1002/hbm.23429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young KD, Bodurka J, Drevets WC. Functional neuroimaging of sex differences in autobiographical memory recall in depression. Psychol. Med. 2017;47:2640. doi: 10.1017/S003329171700112X. [DOI] [PubMed] [Google Scholar]
- 65.Butler T, et al. Sex differences in mental rotation: Top-down versus bottom-up processing. Neuroimage. 2006;32:445. doi: 10.1016/j.neuroimage.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 66.Burke SM, et al. Sex differences in own and other body perception. Hum. Brain Mapp. 2019;40:474. doi: 10.1002/hbm.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruigrok ANV, et al. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luders E, et al. Gender effects on cortical thickness and the influence of scaling. Hum. Brain Mapp. 2006;27:314–324. doi: 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cahill L. An issue whose time has come. J. Neurosci. Res. 2017;95:12–13. doi: 10.1002/jnr.23972. [DOI] [PubMed] [Google Scholar]
- 70.Murray SO, et al. Sex differences in visual motion processing. Curr. Biol. 2018;28:2794–2799.e3. doi: 10.1016/j.cub.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- 72.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hyde JS. Gender similarities and differences. Annu. Rev. Psychol. 2014;65:373–398. doi: 10.1146/annurev-psych-010213-115057. [DOI] [PubMed] [Google Scholar]
- 74.Gur RC, et al. An fMRI study of sex differences in regional activation to a verbal and a spatial task. Brain Lang. 2000;74:157–170. doi: 10.1006/brln.2000.2325. [DOI] [PubMed] [Google Scholar]
- 75.Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH. Males and females differ in brain activation during cognitive tasks. Neuroimage. 2006;30:529–538. doi: 10.1016/j.neuroimage.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 76.Nieto-Castañón A, Fedorenko E. Subject-specific functional localizers increase sensitivity and functional resolution of multi-subject analyses. Neuroimage. 2012;63:1646–1669. doi: 10.1016/j.neuroimage.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nieto-Castanon A, Ghosh SS, Tourville JA, Guenther FH. Region of interest based analysis of functional imaging data. Neuroimage. 2003;19:1303–1316. doi: 10.1016/s1053-8119(03)00188-5. [DOI] [PubMed] [Google Scholar]
- 78.Poldrack RA. Region of interest analysis for fMRI. Soc. Cogn. Affect. Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meoni S, Macerollo A, Moro E. Sex differences in movement disorders. Nat. Rev. Neurol. 2020;16:84–96. doi: 10.1038/s41582-019-0294-x. [DOI] [PubMed] [Google Scholar]
- 80.Phan HT, et al. Sex differences in severity of stroke in the INSTRUCT study: A meta-analysis of individual participant data. J. Am. Heart Assoc. 2019;8:1. doi: 10.1161/JAHA.118.010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Persky RW, Turtzo LC, McCullough LD. Stroke in women: Disparities and outcomes. Curr. Cardiol. Rep. 2010;12:6. doi: 10.1007/s11886-009-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim JS, Lee KB, Roh H, Ahn MY, Hwang HW. Gender differences in the functional recovery after acute stroke. J. Clin. Neurol. 2010;6:183. doi: 10.3988/jcn.2010.6.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomita H, et al. Impact of sex difference on severity and functional outcome in patients with cardioembolic stroke. J. Stroke Cerebrovasc. Dis. 2015;24:2613–2618. doi: 10.1016/j.jstrokecerebrovasdis.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 84.Zhang X, et al. Gender differences are encoded differently in the structure and function of the human brain revealed by multimodal MRI. Front. Hum. Neurosci. 2020;14:244. doi: 10.3389/fnhum.2020.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andrushko JW, et al. High force unimanual handgrip contractions increase ipsilateral sensorimotor activation and functional connectivity. Neuroscience. 2021;452:111–125. doi: 10.1016/j.neuroscience.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 86.Noble JW, Eng JJ, Kokotilo KJ, Boyd LA. Aging effects on the control of grip force magnitude: An fMRI study. Exp. Gerontol. 2011;46:453–461. doi: 10.1016/j.exger.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Noble JW, Eng JJ, Boyd LA. Effect of visual feedback on brain activation during motor tasks: an FMRI study. Motor Control. 2013;17:298–312. doi: 10.1123/mcj.17.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wüthrich F, et al. Test–retest reliability of a finger-tapping fMRI task in a healthy population. Eur. J. Neurosci. 2023;57:78. doi: 10.1111/ejn.15865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at https://openneuro.org/datasets/ds004044/versions/2.0.3.