Abstract
Novel agents addressing non-amyloid, non-tau targets in Alzheimer’s Disease (AD) comprise 70% of the AD drug development pipeline of agents currently in clinical trials. Most of the target processes identified in the Common Alzheimer’s Disease Research Ontology (CADRO) are represented by novel agents in trials. Inflammation and synaptic plasticity/neuroprotection are the CADRO categories with the largest number of novel candidate therapies. Within these categories, there are few overlapping targets among the test agents. Additional categories being evaluated include apolipoprotein E 4 (APOE4) effects, lipids and lipoprotein receptors, neurogenesis, oxidative stress, bioenergetics and metabolism, vascular factors, cell death, growth factors and hormones, circadian rhythm, and epigenetic regulators. We highlight current drugs being tested within these categories and their mechanisms. Trials will be informative regarding which targets can be modulated to produce a slowing of clinical decline. Possible therapeutic combinations of agents may be suggested by trial outcomes. Biomarkers are evolving in concert with new targets and novel agents, and biomarker outcomes offer a means of supporting disease modification by the putative treatment. Identification of novel targets and development of corresponding therapeutics offer an important means of advancing new treatments for AD.
Key Points
| Seventy percent of AD clinical trials are assessing non-Aβ, non-tau targets with novel agents. |
| Inflammation and synaptic plasticity/neuroprotection are the CADRO categories with the largest number of novel candidate therapies. |
| Evaluation of non-canonical targets are novel therapeutic strategies for the disease-modifying treatment of AD. |
Introduction
Alzheimer's disease (AD) is rapidly increasing in frequency globally as the world population ages. It is currently estimated that the global population of AD dementia comprises 57.4 million individuals, and this will grow to an estimated 152.8 million by the year 2050 [1]. The increase in AD is accompanied by severe social, economic, family, and individual consequences. Therapeutic interventions that will prevent or delay the onset, slow the progression, or improve the symptoms of AD are urgently needed.
There are many potential targets for the treatment of AD recognized in the Common Alzheimer's Disease Research Ontology (CADRO) including amyloid beta (Aβ) peptide, tau protein, apolipoprotein E 4 (APOE4) effects, lipids and lipoprotein receptors, neurotransmitter receptors, neurogenesis, inflammation, oxidative stress, cell death, proteostasis, bioenergetics and metabolism, vascular factors, growth factors and hormones, synaptic plasticity and neuroprotection, gut-brain axis, circadian rhythm, epigenetic regulators, and multitarget interventions [2]. Amyloid beta and tau proteins are the canonical pharmacologic targets for disease modifying therapies (DMTs) of AD. No tau-related agents have been approved by regulatory authorities for the treatment of AD. Three agents—aducanumab (Aduhelm®), lecanemab (Leqembi®), and donanemab—have been approved by the US Food and Drug Administration (FDA) for treatment initiation in early AD comprising patients with mild cognitive impairment (MCI) and mild dementia due to AD with Aβ-based diagnostic confirmation using amyloid positron emission tomography (PET) or cerebrospinal fluid (CSF) biomarkers [3–5].
Therapeutic agents directed at Aβ and tau comprise approximately 30% of the AD drug development pipeline [6]. Drugs directed at novel targets constitute 70% of AD drug development. In this review we address the non-canonical targets representing novel therapeutic strategies for the disease-modifying treatment of AD. We emphasize those agents that are best represented in the therapeutic pipeline as revealed by a larger number of clinical trials devoted to understanding the therapeutic impact of diverse agents on the target pathological process. Within each of the CADRO categories, there are multiple targets that may represent a means of modulating that aspect of the disease pathophysiology (e.g., inflammation, synaptic plasticity, etc.). We will present these mechanisms within the CADRO categories. We also note the stage of development of the drugs addressing each target and the key outcomes of the trials of the agent. We do not discuss drugs that address treatment of neuropsychiatric symptoms or those that seek to produce cognitive enhancement and are not intended to be DMTs. We do not discuss agents directed at proteostasis and proteopathies since the mechanisms of these drugs overlap with those directed at amyloid and tau. The goal of this review is to enhance understanding of the novel targets of agents in the AD drug development pipeline including drawing attention to agents that might be used in combination with approved treatments or with other emerging drugs to optimize the therapeutic benefit for AD patients.
Inflammation
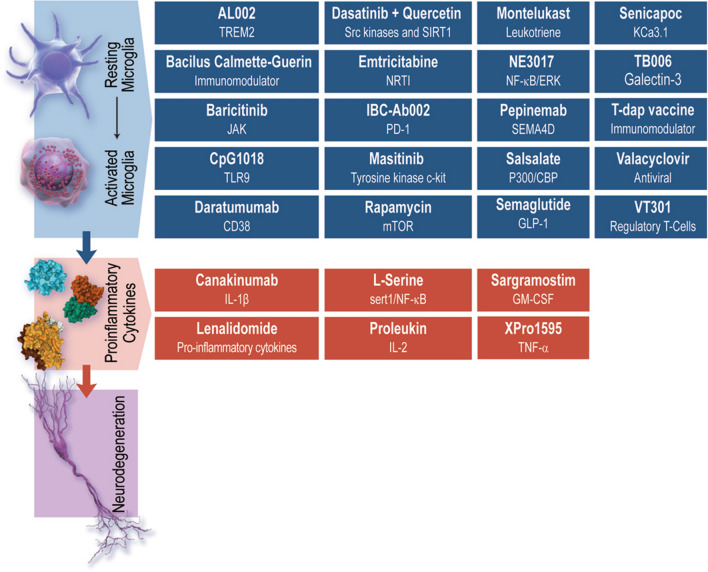
Recently, there has been a marked growth in our understanding of the role of inflammation in the brain in AD and other neurodegenerative disorders [7]. The inflammatory process is complex involving the transition of microglia from resting to activated states and the secretion of both pro-inflammatory and anti-inflammatory cytokines [8]. The role of other macrophage/immune cells in AD has also been shown to be relevant to AD onset and progression. This complexity produces a rich array of central and peripheral targets for potential intervention. Candidate treatments aimed at reducing neuroinflammation comprise the largest segment of the AD drug development pipeline following neurotransmitter modulators and Aβ-directed agents [6]. While the majority of these drugs are characterized as anti-inflammatory, a few activate the immune response to target enhanced clearance of aggregates, including sargramostim, proleukin, CpG1018, and IBC-Ab002. Table 1 shows the inflammation drug, its phase of development, the principal mechanism of action, and the primary outcomes of currently active clinical trials. Figure 1 represents the inflammation-related drugs and mechanisms.
Table 1.
Inflammation-related drugs in the current Alzheimer’s disease pipeline
| Drug | Phase | Mechanism of action | Trial number | Number of participants | Duration of treatment | Primary outcome measures |
|---|---|---|---|---|---|---|
| Masitinib | 3 | Tyrosine kinase c-kit inhibitor | NCT05564169 | 600 | 24 weeks | ADAS-Cog-11, ADCS-ADL |
| NE3107 | 3 | Inhibits NFκB/ERK pathway | NCT04669028 | 316 | 30 weeks | ADAS Cog-12, ADCS-CGIC |
| Semaglutide | 3 | GLP-1 agonist | NCT04777396 | 1840 | 173 weeks | CDR-sb |
| AL002 | 2 | TREM2 antibody | NCT04592874 | 265 | 96 weeks | MMSE, RBANS, ADAS-Cog13, ADCS-ADL-MCI, ADCOMS, Safety and tolerability |
| Bacillus Calmette-Guerin | 2 | Immunomodulator | NCT05004688 | 15 | 364 days | RBANS, cytokines in blood and CSF, biomarkers of ATN pathology in blood and CSF (Aβ-42/40, pTau, tau, NFL) |
| Baricitinib | 1/2 | JAK inhibitor | NCT05189106 | 20 | 24 weeks | CSF concentrations of baricitinib, CCL2 |
| Canakinumab | 2 | Anti-IL-1β antibody | NCT04795466 | 90 | 24 weeks | Change in NTB total score |
| Daratumumab | 2 | Anti-CD38 antibody | NCT04070378 | 15 | 24 weeks | ADAS-cog-11 |
| Dasatinib + quercetin | 2 | Scr kinases inhibitor and upregulates SIRT1, senolytics | NCT04685590 | 48 | 48 weeks | Adverse/serious adverse events |
| NCT04785300 | 20 | 11 weeks | Safety and tolerability | |||
| NCT05422885 | 12 | 12 weeks | Neurovascular coupling, executive function, gait speed | |||
| Lenalidomide | 2 | Inhibits pro-inflammatory cytokines | NCT04032626 | 30 | 18 months | ADAS-Cog, ADCS-ADL, CDR-SB, MMSE scores |
| L-serine | 2 | Reduces inflammation | NCT03062449 | 40 | 12 months | Cognitive assessment, health check (blood tests), adverse events |
| Montelukast | 2 | Leukotriene antagonist | NCT03402503 | 70 | 26 weeks | Global Neuropsychological Test Battery |
| Pepinemab | 1/2 | Anti-SEMA4D antibody | NCT04381468 | 40 | 40 weeks | Adverse events |
| Proleukin | 2 | Recombinant human IL-2 | NCT05468073 | 45 | 18 months | CDR |
| Rapamycin | 2 | mTOR inhibitor | NCT04200911 | 10 | 8 weeks | Blood brain barrier penetration of rapamycin |
| NCT04629495 | 40 | 12 months | Adverse events, metabolic panel | |||
| Sargramostim | 2 | Synthetic GM-CSF | NCT04902703 | 42 | 24 weeks | Adverse events |
| Senicapoc | 2 | KCa3.1 inhibitor | NCT04804241 | 55 | 52 weeks | ADAS-Cog 13 scores, CSF markers (IL-1β, IL-6, TNF-α, MCP-1, and IL-10), serum markers (IL-6, TNF-α, MCP-1, and IL-10, high sensitivity C-reactive protein) |
| TB006 | 2 | Anti-galectin-3 antibody | NCT05074498 | 140 | 104 days |
Part 1: Adverse events, C-SSRS, physical function, laboratory parameters, vital signs, PK, concentration of TB006, anti-TB006 antibodies, severity of dementia Part 2: CDR-SB |
| Tdap vaccine | 1/2 | Immunomodulator | NCT05183516 | 50 | 6 months | Change in Aβ42/40 ratio and tau in plasma |
| Valacyclovir | 2 | HSV antiviral | NCT03282916 | 120 | 18 months | ADAS-COG11 (modified version) |
| XPro1595 | 2 | sTNF inhibitor | NCT05318976 | 201 | 23 weeks | EMACC |
| CpG1018 | 1 | Actives TLR9 | NCT05606341 | 39 | 18 weeks | Adverse events, rheumatoid factor, antinuclear antibody, and antineutrophil antibody in their blood, ARIA-H and/or ARIA-E PET/MR, MRI |
| Emtricitabine | 1 | NRTI for HIV | NCT04500847 | 35 | 8 months | Adverse events |
| IBC-Ab002 | 1 | PD-1 inhibitor | NCT05551741 | 40 | 48 weeks | Adverse/serious adverse events, incidence of clinically significant changes in hematology, biochemistry, urinalysis, vital signs, physical examination, EEG, abnormalities on brain MRI, suicidality |
| Salsalate | 1 | p300/CBP inhibitor | NCT03277573 | 40 | 12 months | Adverse events |
| VT301 | 1 | Regulatory T-cells | NCT05016427 | 12 | 3 months | Adverse events, C-SSRS, ECG, physical examination, laboratory tests, vital signs |
ADAS-Cog Alzheimer’s Disease Assessment Scale-Cognitive, ADCOMS Alzheimer’s Disease Composite Score, ADCS-CGIC Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change, ADCS-ADL Alzheimer’s Disease Cooperative Study-Activities of Daily Living, ARIA-H Amyloid-Related Imaging Abnormalities of Hemosiderin, ARIA-E Amyloid-Related Imaging Abnormalities of Edema, ATN amyloid, tau, neurodegeneration, AUCtau area under the receiver operating characteristic curve for tau, CBC CREB-binding protein, CCL2 monocyte chemoattractant protein-1, CDR Commission on Dietetic Registration, CDR-sb Clinical Dementia Rating Scale-Sum of Boxes, Cmax maximum concentration, CSF cerebrospinal fluid, C-SSRS Columbia Suicide Severity Rating Scale, Ctrough pre-dose trough concentration, ECG electrocardiogram, EEG electroencephalography, EMACC Early AD/MCI Alzheimer’s Cognitive Composite, GM-CSF granulocyte-macrophage colony-stimulating factor, HSV herpes simplex virus, MCP-1 monocyte chemoattractant protein 1, MMSE Mini Mental State Examination, mTOR mechanistic target of rapamycin, NRTI , PD-l programmed death ligand, RBANS Repeatable Battery for the Assessment of Neuropsychological Status, sTNF soluble tumor necrosis factor, t1/2 half-life of the drug, tmax time to peak drug concentration, TNFα tumor necrosis factor alpha
Fig. 1.
Inflammation-related drugs and the targets of agents currently in development (©J Cummings; Illustrator, Mike de la Flor, PhD)
Phase 3
Masitinib (AB1010)
Masitinib is an oral, small-molecule drug that selectively inhibits tyrosine kinase c-kit, modulating survival, differentiation, and degranulation of mast cells, leading to the regulation of inflammatory mediators [9]. In a preclinical model of AD, masitinib recovered spatial learning performance and synaptic markers [10]
NE3107 (HE3286)
NE3107 is a small molecule drug that has demonstrated anti-inflammatory properties in several preclinical models of disease (rheumatoid arthritis, chronic obstructive pulmonary disease, diabetes mellitus, and Parkinson’s Disease). NE3107 inhibits the nuclear factor kappa B/extracellular signal-regulated kinase (NF-κB/ERK) pathway, reducing the release of inflammatory cytokines including tumor necrosis factor alpha (TNFα), interferon-gamma (IFNγ), interleukin (IL)-1α, and transforming growth factor beta [11]. Restricting the NF-κB/ERK pathway decreases glial activation, oxidative stress, and production of Aβ and pTau [12]. NE3107 inhibits activation of mitogen activated protein kinases (MAPK), which is involved in the mediation of insulin resistance, reducing hyperglycemia and hyperinsulinemia [12].
Semaglutide
Semaglutide is a glucagon-like peptide-1 agonist developed for the treatment of type 2 diabetes and obesity. Epidemiologic studies demonstrate that diabetics treated with semaglutide are less likely to develop dementia than those treated with other anti-diabetic agents [13]. The principal therapeutic effect of semaglutide in AD is hypothesized to be its anti-inflammatory affect exerted through decreased peripheral inflammation. Semaglutide does not cross the blood brain barrier, or does so in only small amounts and in specific brain regions.
Phase 2
AL002
Triggering receptor expressed on myeloid cells 2 (TREM2) is a highly expressed transmembrane receptor on microglia cells, involved in activation of glia cells, inflammation, and phagocytosis. Genetic variation in TREM2 increases the risk for developing late-onset AD [14]. Preclinical studies demonstrate that dysregulation of TREM2 reduces overall number of microglia, decreases microglial coalescence around Aβ plaques, ameliorates the activation of microglia into a phagocytic state, and increases apoptotic cell death [15]; overexpression of TREM2 attenuates AD pathology [16]. AL002 is a monoclonal IgG1 antibody that is an agonist for TREM2. Introduced in preclinical models, the murine isoform of the drug, AL002c, reduced filamentous Aβ plaques, decreased dystrophic neurites, and promoted microglia activation and phagocytosis of Aβ [16].
Bacillus Calmette-Guerin
Bacillus Calmette-Guerin (BCG) is a vaccine originally used against Mycobacterium tuberculosis and is a common treatment for bladder cancer. Research has demonstrated BCG’s ability to modulate the immune response through stimulation of T helper 1 cells to secrete several cytokines (IL-1, IL-2, IL-5,IL- 6, IL-8, IL-10, IL-12, IL-18, IFNγ, TNFα, and granulocyte-macrophage colony-stimulating factor [GM-CSF]) in the bladder [17]. Alterations to peripheral inflammation support the hypothesis that BCG could be used as a therapeutic treatment for AD. In a transgenic mouse model of AD, BCG immunization rescued cognitive decline, increased circulating IFNγ, recruited macrophages to cerebral Aβ plaques, and upregulated cerebral anti-inflammatory cytokines [17].
Baricitinib
Originally a small molecule compound used in rheumatoid arthritis and severe COVID, baricitinib is a Janus kinase (JAK) inhibitor. JAK inhibitors are enzymes that are bound to the intracellular domains of cytokine receptors, including interleukins, interferons, and colony-stimulating factors [18]. They are involved in signaling from the cell surface receptors to the nucleus, altering DNA transcription and recruiting signal transducer and activator of transcriptions (STATs) [19]. The JAK-STAT pathway operates downstream of more than 50 cytokines and growth factors, and is described as the central communication node for the immune system [20]. Baricitinib inhibits JAK and subsequent STAT pathways, suppressing plasmablasts, T helper 1 and T helper 17 cell differentiation, as well as T cell proliferation, overall affecting innate and adaptive immune responses [21].
Canakinumab
Interleukin-1β is a proinflammatory cytokine, considered to be a master regulator of inflammation that modulates a range of inflammatory processes associated with innate immunity. In postmortem AD patient brains, IL-1β accumulation was observed around Aβ plaques and is associated with inflammation-related neuronal damage and neurodegeneration [22]. Canakinumab is a humanized anti-IL-1β monoclonal antibody inhibitor of IL-1β.
Daratumumab
Cluster of differentiation 38 (CD38) is found on neurons, astrocytes, and microglia and plays a role in inflammatory responses and neurodegeneration [23]. In a preclinical model of AD, knockout of the CD38 receptor significantly decreased soluble Aβ and Aβ plaque load, reduced β-secretase and γ-secretase activity, and enhanced spatial learning [24, 25]. Daratumumab is a humanized monoclonal anti-CD38 IgG1 antibody.
Dasatinib + Quercetin
Aging is the greatest risk factor for AD, and senolytics—drugs involved in the clearance of senescent cells—are of interest as therapeutics for AD. Dasatinib is approved as an anti-cancer drug, functioning as a multi-kinase inhibitor of steroid receptor coactivator (src) family tyrosine kinases (SFKs). Family tyrosine kinases influence cellular metabolism, survival, and proliferation, and is involved in macrophage processes including phagocytosis, production of cytokines, and cellular migration [26]. Quercetin is a naturally derived flavonoid found in many fruits and vegetables that have antioxidant and anti-inflammatory activity. Quercetin targets sirtuin 1 to initiate anti-aging effects through inhibition of oxidative stress, inflammatory responses, mitochondrial damage, and autophagy [27]. Together, dasatinib and quercetin (D+Q) have been shown to have senolytic properties. In preclinical models of AD, D+Q alleviates inflammation associated with Aβ plaques and ameliorates cognitive decline [28]. Additionally, D+Q reduced neurofibrillary tangle burden and neurodegeneration in a mouse model of the disease [29]. Interim data from the SToMP clinical trial have been reported for five participants who have completed the treatment. The data show the drug to be well tolerated and have positive results in target engagement and treated-related outcomes [30].
Lenalidomide
Lenalidomide, is an FDA approved anti-cancer drug that has immunomodulatory, antineoplastic, and anti-angiogenic properties [31]. Lenalidomide inhibits proinflammatory cytokines that contribute to AD, including TNFα, IL-1, IL-6, and IL-12; it is also capable of enhancing the production of the anti-inflammatory cytokine, IL-10 [31].
l-serine
l-serine is a naturally occurring dietary amino acid decreased in patients with AD; it is generally recognized as safe (GRAS) by the FDA and is approved as a food additive and dietary supplement. l-serine is found primarily in glial cells, released to neurons for the synthesis of its enantiomer, d-serine. Deficits in glycolysis in astrocytes lead to reductions in the production of l-serine and ultimately disrupts neuronal function, including synaptic plasticity and memory [32]. In a preclinical model of AD, l-serine rescued long-term potentiation (LTP) and spatial memory impairment [32].
Montelukast
Leukotrienes are lipid mediators of inflammation, involved in several pro-inflammatory responses, including leukocyte migration, proliferation, reactive oxygen species, and the production of cytokines [33]. Leukotriene pathways may modulate Aβ formation and tau hyperphosphorylation in AD. Montelukast, a leukotriene receptor antagonist, with systemic anti-inflammatory properties, prevents binding of leukotrienes to cysteinyl leukotriene receptor 1. In preclinical models of AD, montelukast rescued neurons against Aβ neurotoxicity, reduced neuroinflammation and apoptosis, and improved cognitive function [33, 34].
Pepinemab (VX15)
Semaphorin 4D (SEMA4D or CD100) is a protein that binds to plexin receptors found on astrocytes and microglia, regulating several glial processes [35]. SEMA4D is upregulated in stressed or damaged neurons, leading to activation of glial cells, interruption of astrocytic metabolic and synaptic functions, and decreased migration and differentiation of glial progenitor cells [35]. SEMA4D is increased in brains of patients with AD. Pepinemab is a humanized IgG4 monoclonal antibody that targets SEMA4D.
Proleukin
Interleukin-2 (IL-2) is a pro-inflammatory cytokine that is decreased in the hippocampus of patients with AD. In transgenic AD mice, IL-2 treatment led to activation and recruitment of astrocytes around Aβ plaques, reductions in Aβ42/40 and plaque load, improvement in synaptic plasticity, and recovery of memory deficits [36]. Proleukin is a recombinant human IL-2 used to activate the immune system (in cancer immunotherapy and autoimmune disorders) and is being investigated as a therapeutic for AD. Furthermore, IL-2 may selectively restore functional regulatory T cells (Tregs) and Tregs-enhancement is a potential therapeutic strategy for AD [37]. Although there are currently no active clinical trials evaluating this mechanism, COYA 301, a low-dose IL-2 drug, has been tested in a preliminary clinical trial and results are anticipated (NCT05821153).
Rapamycin
Rapamycin is an inhibitor of mechanistic target of rapamycin (mTOR), a protein involved in cell growth, metabolism, and cellular processes including proliferation, differentiation, migration, and dendrite formation [38]. Glycogen synthase kinase 3β (GSK3-β) is involved in the mTOR pathway and promotes AD pathogenesis of Aβ and tau. Targeting GSK3-β through the mTOR pathway using rapamycin is proposed as a therapy for AD. Studies with preclinical models demonstrated rapamycin to decrease Aβ plaque load, reduce β- and γ-secretase activity, increase Aβ clearance through autophagy, and decrease pTau through upregulation of insulin-degrading enzyme [39].
Sargramostim
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a cytokine secreted by cells including microglia and astrocytes. GM-CSF is involved in inflammatory processes such as maturation and proliferation of cells, cell survival, phagocytosis, cell adhesion, chemotaxis, and production of other inflammatory mediators (i.e., cytokines). Treatment of GM-CSF in preclinical models of AD resulted in a 50% reduction of Aβ load, increased activated microglia and microglia surrounding plaques, and improved cognition [40, 41]. Sargramostim is a recombinant human GM-CSF.
Senicapoc
Potassium calcium-activated channel subfamily N member 4 (KCa3.1), is a protein expressed on T-lymphocytes, microglia, and macrophages, regulating cellular activation, migration, and proliferation. KCa3.1 is increased in the brains of AD patients, upregulated by Aβ oligomers [42]. Senicapoc is a KCa3.1 inhibitor that attenuates AD pathologies, such as LTP deficits, Aβ, and neuroinflammation in a preclinical model [42].
TB006
TB006 is a humanized monoclonal antibody that targets galectin-3, a TREM2 ligand. Galectin-3 is upregulated in patients with AD and involved in microglia activation [43]. Decreasing galecin-3 attenuated immune responses through toll-like receptor and TREM2 signaling, decreased Aβ load, and improved cognitive behavior in preclinical models.
Tdap Vaccine
Tdap is a combined multivariate vaccine that protects against tetanus, diphtheria, and pertussis (whooping cough). Bordetella pertussis, the bacterium known to cause whooping cough, may be associated with AD; three epidemiological studies suggest B. pertussis can account for hallmarks including Aβ plaques, tau tangles, inflammation, and neurodegeneration in AD [44]. Data from two cohorts of patients demonstrated that receiving the tdap vaccine reduced the risk for developing dementia by 42% [45].
Valacyclovir
Several lines of pre-clinical and epidemiological evidence suggest that herpes simplex virus (HSV) is involved in AD [46]. Valacyclovir is an antiviral drug that inhibits HSV replication, making it effective against HSV infections [46]. Given that viruses can evoke inflammation, and studies showing that valacyclovir can impact cognitive function in individuals with schizophrenia and multiple sclerosis, valacyclovir is currently in clinical trials for AD.
XPro1595
Soluble tumor necrosis factor (sTNF) is a cytokine involved in inflammation, blood brain barrier permeability, and metabolism. Studies have shown increases in sTNF in CSF and brains of patients with AD [47]. Preclinical investigations utilizing XPro1595, an sTNF-selective inhibitor, demonstrated beneficial effects in AD mouse models, reducing immune cells, rescuing impaired LTP, and decreasing Aβ in the hippocampus [47].
Phase 1
CpG1018
Cytosine-phosphate-guanosine oligodeoxynucleotides (CpGs) are involved in the innate immune response through interaction with Toll-like receptor 9. Cytosine-phosphate-guanosine oligodeoxynucleotides reduce Aβ plaques, tau pathology, and cerebral amyloid angiopathy (CAA), while enhancing cognition in mouse models of AD [48]. A long-term study using CpGs in elderly squirrel monkeys demonstrated mitigation of CAA and tau pathologies, with absence of microhemorrhages and improvements in behavior and cognition [48].
Emtricitabine
Human immunodeficiency virus (HIV) can pass the blood brain barrier and lead to neuronal dysfunction and cognitive decline [49]. Some HIV patients develop Aβ plaques or neurofibrillary tangles, indicating a relationship between HIV and AD, possibly through the modulation of Aβ and tau pathways by the virus; neuroinflammation is evident in both HIV and AD [49]. Emtricitabine is a nucleoside reverse transcriptase inhibitor used for the treatment of HIV by preventing replication of the virus, a potential therapeutic for AD.
IBC-Ab002
IBC-Ab002 is a humanized IgG1 antibody that inhibits programmed death ligand (PD-1), evoking the immune response. PD-1 inhibitors facilitate the conversion of proinflammatory monocytes to patrolling monocytes, reducing Aβ protein in the brain and enhancing cognition [50, 51]. Nonclinical observations of the utility of these agents have not been confirmed in human clinical trials. There is a first in human, Phase 1 clinical trial in progress.
Salsalate
Salsalate is a nonsteroidal anti-inflammatory drug (NSAID) that inhibits p300/cyclic adenosine monophosphate response element binding protein-binding protein (CBP) acetyltransferase. Inhibition of p300/CBP prevents tau acetylation, increases tau turnover, reduces tau levels, and protects against neurodegeneration and cognitive/behavioral impairments in transgenic mice [52, 53]. The current status of the salsalate clinical trial for AD is unknown.
VT301
Regulatory T-cells modulate immune responses and several studies in preclinical models demonstrate a relationship between T-cells and AD pathology. Depletion of T-cells in an amyloid mouse model promotes cognitive decline and decreases Aβ plaque-associated microglia [54]. VT301 is a regulatory T-cell drug being tested as a therapeutic treatment for AD.
Synaptic Plasticity/Neuroprotection
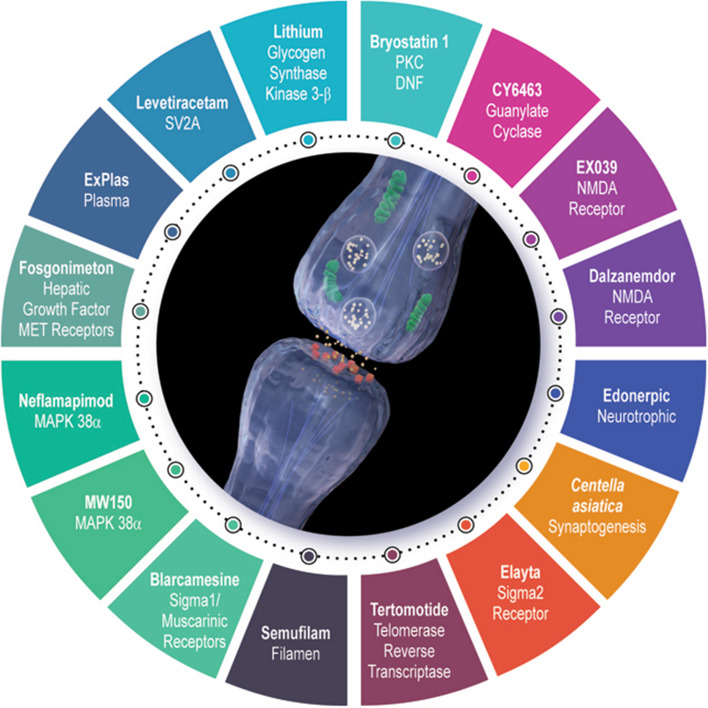
Drugs seeking to enhance synaptic plasticity or produce neuroprotection are among the most common candidate therapies in the AD drug development pipeline. The strategies described within this CADRO category are diverse and represent a multitude of approaches and underlying theoretical constructs about synaptic function. A unique aspect of the clinical trials of synaptic agents is that enhanced synaptic function can represent disease modification or might improve cognition or behavior, producing symptomatic benefit. Observation of these trial outcomes will be critical to understanding this dynamic between disease modification and symptomatic response. Table 2 lists the agents currently in clinical trials targeting synaptic plasticity neuroprotection, their phase of development, their known/hypothesized mechanism of action, and the primary outcomes of current clinical trials. Figure 2 represents the synaptic plasticity-related drugs and their targets.
Table 2.
Synaptic-plasticity drugs in the current Alzheimer’s disease pipeline
| Drug | Phase | Mechanism of action | Trial number | Number of participants | Duration of treatment | Primary outcome measures |
|---|---|---|---|---|---|---|
| AGB101 | 2/3 | SV2A inhibitor | NCT03486938 | 164 | 78 weeks | CDR-sb |
| Blarcamesine (Anavex 2-73) | 2/3 | Sigma 1/muscarinic agonist | NCT03790709 | 500 | 48 weeks | ADAS-Cog, ADCS-ADL |
| Fosgonimeton (ATH-1017) | 2/3 | Hepatocyte growth factor/MET activator | NCT04488419 | 475 | 26 weeks | Global Statistical Test (combined ADAS-cog and the ADCS ADL scores) |
| NCT04491006 | 77 | Event-related potential (ERP) P300 latency | ||||
| Simufilam (PTI-125) | 3 | Binds to filamin to prevent interaction of Aβ and the A7 nicotinic acetylcholine receptor | NCT04994483 | 750 | 52 weeks | ADAS-Cog, ADCS-ADL |
| NCT04388254 | 200 | 24 months | Safety and Tolerability, Changes in CSF markers (P-tau, Total Tau, Aβ-42, NfL, neurogranin, YKL-40, soluble TREM2 and HMGB1) change from baseline in ADAS-Cog-11 | |||
| Tertomotide (GV1001) | 3 | Telomerase reverse transcriptase mimic | NCT05303701 | 936 | 6 months | SIB, CDR-sb |
| NCT05189210 | 180 | 52 weeks | ADAS-Cog | |||
| AL 001 | 1/2 | Glycogen synthase kinase 3-β inhibitor | NCT05363293 | 72 | 14 days | Safety and tolerability |
| Bryostatin 1 | 2 | Protein kinase C activator | NCT04538066 | 100 | 30 days | SIB, Safety |
| CY6463 | 2 | Positive allosteric modulator of guanylate cyclase | NCT04798989 | 30 | 14 days | Safety and tolerability |
| Dalzanemdor (Sage 718) | 2 | N-Methyl-d-aspartate receptor allosteric modulator | NCT05619692 | 150 | 84 days | Wechsler Adult Intelligence Scale (edition IV) coding test |
| Edonerpic (T-817MA) | 2 | Specific target underdefined may be collapsin response mediator protein 2 | NCT04191486 | 200 | 78 weeks | Change in CSF p-tau 181 |
| Elayta CT1812 | 2 | Sigma 2 receptor antagonist | NCT05531656 | 540 | 18 months | CDR-sb |
| EX039 | 2 | Enhance N-methyl-d-aspartate receptor activity | NCT05413655 | 120 | 28 weeks | ADAS-Cog and the CDR-sb |
| ExPlas | 2 | Human plasma with multiple constituents | NCT05068830 | 60 | 1 year | Adverse events |
| Levetiracetam | 2 | SV2A modulator | NCT03875638 | 85 | 5 months | NTB, TMS resting motor threshold, TMS-evoked EEG hypersynchrony, EEG beta band power, EEG beta band connectivity, resting state MRI, evoked potential amplitude, change in beta power after theta-burst stimulation |
| NCT04004702 | 65 | 1 year | NPI | |||
| NCT03489044 | 30 | 4 weeks | Hippocampal function (standardized computer-based test) | |||
| MW150 | 2 | Mitogen activated protein kinase P38α inhibitor | NCT05194163 | 24 | 84 days | Safety Measures |
| Neflamapimod (VX-745) | 2 | Mitogen activated protein kinase P38α inhibitor | NCT03435861 | 40 | 12 weeks | Brain inflammation by translocator protein tracer (18F-DPA-714) |
| Centella asiatica | 1 | Multimodal herb-derives traditional Chinese medicine | NCT05591027 | 48 | 6 weeks | Brain NAA/Cr ratio assessed by MR spectroscopy |
ADAS-Cog Alzheimer’s Disease Assessment Scale-Cognitive, ADCS-ADL Alzheimer’s Disease Cooperative Study-Activities of Daily Living, CDR-sb Clinical Dementia Rating Scale-Sum of Boxes, Cr Creatinine, HMGB1 high mobility group box 1 protein, NAA N-acetyl aspartate, NPI Neuropsychiatric Inventory Score, SIB severe impairment battery, TMS transcranial magnetic stimulation, YKL-40 chitinase-3 like protein 1
Fig. 2.
Synaptic plasticity-related drugs and the targets of agents currently in development (©J Cummings; illustrator, Mike de la Flor, PhD)
Phase 3 and Phase 2/3
AGB101
AGB101 is a proprietary extended-release formulation of low-dose levetiracetam, an atypical anticonvulsant used for the treatment of epilepsy. The use of levetiracetam in this condition is based on the observation that medial temporal pathology in animal models of AD is associated with neuronal hyperactivity that can be reduced with therapy and leads to improvement in cognitive function. The putative mechanism of action of levetiracetam in this setting is to act as a synaptic vesicle glycoprotein 2A inhibitor. Improvement on neuropsychological tests and normalization of functional magnetic resonance imaging (fMRI) signals with levetiracetam was observed in a pilot study of patients with amnestic MCI [55]. These observations provided the foundation for the construction of the current Phase 2/3 HOPE for MCI study of AGB101 [56].
Blarcamesine (Anavex 2-73)
Blarcamesine (Anavex 2-73) is a ligand for sigma 1/muscarinic receptors. In rodent models the agent was shown to enhance memory-related behavioral performance and to exhibit neuroprotective effects including reducing tau hyperphosphorylation and protect mitochondria against injury induced by Aβ [57].
Fosgonimeton (ATH-1017)
Fosgonimeton (ATH-1017) enhances synaptic plasticity and neuronal survival by activating the hepatocyte growth factor (HGF)/mesenchymal-epithelial transition factor (MET) receptor system. MET receptors are decreased in AD; increased HGF activity ameliorated Aβ-induced cognitive deficits in a rodent model of AD [58]. A Phase 1/2 trial of fosgonimeton in healthy volunteers and patients with AD showed the agent to be well tolerated following subcutaneous administration. Improvement in electroencephalography (EEG) patterns and evoked potentials were observed in the small number of the AD patients assessed with these techniques [59, 60].
Simufilam (PTI-125)
Simufilam is a novel drug candidate that reduces filamen A’s binding to α7 nicotinic acetylcholine receptors (α7nAChRs); this interaction is posited to be critical to Aβ-related toxicity [61]. Simufilam binds to filamin to restore its shape and function and to stabilize its interaction of Aβ and the A7 nicotinic acetylcholine receptor that triggers tau phosphorylation and synaptic dysfunction. Simufilam reduced tau hyperphosphorylation, tau aggregation and deposition, Aβ aggregation, and neuroinflammation, and enhanced synaptic function in an animal model of AD. Memory-based behaviors were improved in the treated animals [62]. An open label trial of simufilam showed improvements in CSF biomarkers supportive of disease modification, including reductions in total tau, neurogranin, neurofilament light, and pTau 181 [63].
Tertomotide (GV1001)
Tertomotide (GV1001) makes up a part of the human enzyme telomerase reverse transcriptase, an enzyme with antioxidant, antiapoptotic, and pro-cellular survival functions. In cell systems, tertomotide exhibited similar features to full-length telomerase reverse transcriptase and blocked Aβ toxicity and exhibited anti-inflammatory and antioxidant properties [64]. A Phase 2 study of tertomotide administered subcutaneously demonstrated a better outcome on cognition using the Severe Impairment Battery (SIB) in the high-dose compared to the low-dose group. No other significant drug-placebo differences were observed [65].
Phase 2
AL 001
AL 001 consists of an ionic cocrystal combination of lithium, proline, and salicylate. Lithium is posited to have both disease-modifying properties and the potential to reduce neuropsychiatric symptoms. Lithium is an inhibitor of GSK3-β that is upregulated in AD and contributes to impairment of LTP, Aβ-induced neurotoxicity, and tau hyperphosphorylation [66]. Treatment of transgenic mice engineered to overproduce Aβ and tau showed that chronic doses of lithium reduced amyloid plaque formation, decreased hyperphosphorylation of tau, and improved learning and memory [67, 68].
Bryostatin 1
Bryostatin 1 is a natural marine-derived product that activates protein kinase C (PKC) leading to enhanced synthesis and secretion of brain derived neurotrophic factor (BDNF). Brain-derived neurotrophic factor is linked to improved synaptic growth, learning, and memory [69]. A Phase 2 proof-of-concept study demonstrated a higher dropout rate in the bryostatin 1 high dose (40 μg) compared to the bryostatin 1 low dose (20 μg), with evidence of cognitive benefit on the SIB in patients on the low dose, especially in those not on concomitant memantine [70]. A Phase 2 clinical trial on bryostatin was reported to have completed in November of 2022, although no results have been released; the status of another trial is currently unknown.
CY6463
CY6463 is a positive allosteric modulator of guanylate cyclase leading to increased endogenous nitrous oxide with decreased blood pressure, improved neuronal function, and neuroprotection. CY6463 raises cyclic guanosine monophosphate levels in CSF providing biomarker insight into the function and development of this agent [71].
Dalzanemdor (Sage 718)
Dalzanemdor is an N-methyl-D-aspartate (NMDA) receptor allosteric modulator derived from steroid 24(S)-hydroxycholesterol. It has been reported that 24(S)-hydroxycholesterol has diminished in the brains of individuals with AD, and restoration of levels is posited to restore cognition through effects on the NMDA receptor [72].
Edonerpic (T-817MA)
Edonerpic is a neurotrophic agent reported to promote neurite outgrowth, maintain synaptic plasticity, protect neurons against Aβ-induced neurotoxicity, and enhance LTP [73]. Preclinical models treated with endonerpic exhibit amelioration of memory deficits. The specific target of endonerpic may be collapsin response mediator protein 2 (CRMP2), a phosphoprotein that promotes neurite outgrowth and preserves synaptic plasticity.
Elayta (CT1812)
Elayta is a sigma-2 receptor antagonist that binds to the progesterone receptor membrane part 1 subunit of the receptor complex, reducing Aβ-induced synaptic toxicity through negative allosteric modulation of the receptor. In AD transgenic mice, Elayta improved cognition, and in human AD brain tissue, it was shown to displace Aβ oligomers [74].
EX039
EX039 is a formulation of sodium benzoate hypothesized to enhance NMDA receptor activity. Sodium benzoate was assessed in a randomized double-blind placebo-controlled trial of amnestic MCI and mild AD dementia treated over a 24-week period. Significant differences in favor of sodium benzoate were observed on the ADAS-cog, clinical global impression of change with caregiver input (CGIC+), and cognition composites included in the trial [75].
ExPlas
ExPlas is human plasma derived from young fit male donors. ExPlas is collected by plasmapheresis once a month for four months to create the treatment product. It is hypothesized that ExPlas will have rejuvenating properties measurable on clinical and biomarker assessments [76].
Levetiracetam
The biology of levetiracetam as a modulator of the synaptic vesicle SV2A protein was described above. Levetiracetam is being studied by other developers in several Phase 2 clinical trials.
MW150
MW150 has both anti-inflammatory and synaptic vesicle function properties. It is an inhibitor of the MAPK P38α. This enzyme stimulates release of inflammatory cytokines including TNFα and IL-1β. P38α MAPK also regulates the RAS-related protein Rab-5, a regulator of endosomes and synaptic vesicles. In transgenic mouse models of AD, MW150 suppressed release of inflammatory cytokines and improved synaptic function. Mice treated with MW150 exhibited electrophysiological evidence of improved long-term potentiation and behavioral evidence of enhanced memory [77].
Neflamapimod (VX-745)
Neflamapimod (VX475) is an inhibitor of MAPKα P38. As mentioned above, P38 MAPKα stimulates release of proinflammatory cytokines in microglia in response to cellular stresses including Aβ. In neurons, P38 MAPKα has been implicated in tau abnormalities and synaptic plasticity. A key dimension of P38 MAPKα is its regulation of the Ras-related protein, Rab-5, which in turn regulates endosomes and synaptic vesicle function. In transgenic mouse models of AD, neflamapimod reduced amyloid plaque burden, decreased inflammation, increased the post-synaptic marker PSD95, and improved performance in the Morris Water Maze task [78]. In an open-label study of 16 individuals with early AD treated with neflamapimod for 12 weeks, there were statistically significant improvements above baseline on tests of immediate and delayed recall. Changes in memory performance correlated with plasma drug concentrations. A modest effect on amyloid reduction was seen in some patients [79].
Phase 1
Centella asiatica
Centella asiatica is a herbal medication used in Ayurvedic and traditional Chinese medicine as a cognitive enhancer and brain therapeutic. Animal models and in vitro studies of C. asiatica have shown neuroprotection against Aβ toxicity, increased mitochondrial activity, improved antioxidant status, increased dendritic arborization, and synaptogenesis. Centella asiatica may enhance the potential of the nuclear factor-erythroid factor 2-related factor 2 (Nrf2)-antioxidant response pathway [80].
Apolipoprotein E 4 (APOE4) Effects, Lipids, and Lipoprotein Receptors
Apolipoprotein E 4 gene carrier status is the most influential risk factor for AD after an individual’s age, and is the single most important genetic risk for AD [81]. The APOE4 protein interacts strongly with Aβ, decreasing the age of amyloid brain accumulation and increasing the total Aβ burden in gene carriers [81]. Apolipoprotein E 4 is also known to exacerbate tau neurofibrillary tangle-related neurodegeneration, microglial responses and neuroinflammation, astroglial activity, and blood brain barrier disruption [82]. Despite the marked importance of APOE4 as a risk factor and key feature in the pathogenesis of AD, there have been few therapeutics that specifically address this target.
Phase 2 and Phase 1/2
Hydroxypropyl-beta-cyclodextrin (Trappsol® Cyclo™)
Hydroxypropyl-beta-cyclodextrin (Trappsol® Cyclo™) is a cyclic oligosaccharide that sequesters cholesterol, reducing intracellular levels. In mouse models of AD, hydroxypropyl-beta-cyclodextrin reduced amyloid plaque formation, inhibited Aβ aggregation and toxicity, decreased dystrophic neurites, and improved learning and memory. Genes involved in cholesterol transport were up regulated [83].
Hydroxypropyl-beta-cyclodextrin is being studied in a Phase 2 clinical trial of 90 patients with clinically diagnosed MCI, supported by a Precivity AD plasma biomarker showing high likelihood of a positive amyloid scan (NCT05607615). The 24-week clinical trial has safety and tolerability as its primary outcome.
LX 1001
LX1001 is one of the few APOE4-directed therapies in the AD pipeline and among the few gene therapies being developed for AD. LX1001 uses an adeno-associated viral vector expressing the complementary DNA coding for human APOE2 to increase human APOE2 and antagonize the effect of APOE4. LX1001 is injected into the spinal canal where it diffuses to reach the spinal fluid and brain.
LX 1001 is in an open-label Phase 1/2 trial of 15 APOE4 homozygous individuals with MCI or mild-to-moderate AD dementia (NCT03634007). All patients have amyloid PET and CSF biomarker studies to confirm the presence of AD pathology. They are observed for one year and are followed in a Phase 1 open-label extension (NCT05400330). The Phase 1/2 trial has the goal of proving safety of the intervention and of deciding the maximum tolerated dose.
Obicetrapib (TA-8995)
Obicetrapib is a cholesteryl ester transfer protein (CETP) inhibitor targeting reduction of low-density lipoprotein when given with a statin. Obicetrapib effects are being assessed in AD, atherosclerosis, dyslipidemia, heterozygous familial hypercholesterolemia, and diabetes. Epidemiologic studies suggest that statins may decrease the risk for AD and cholesterol may interact to exacerbate neurodegeneration in the disease [84]. These observations provide the basis for exploring the possible therapeutic benefit of obicetrapib in AD patients.
Currently, obicetrapib is in a Phase 2 open-label exploratory proof-of-concept clinical trial (NCT05161715). Thirteen participants with a clinical diagnosis of AD supported by biomarker evidence of amyloid and tau biomarker abnormalities with or without evidence of neurodegeneration will receive treatment. Patients must also have at least one copy of the APOE4 gene. Participants will be observed for 24 weeks, and primary outcome measures include apolipoproteins in plasma and CSF and high-density lipoprotein particle concentrations in plasma and CSF.
Neurogenesis
Few agents in the AD drug-development pipeline have neurogenesis as their novel target. Allopregnanolone and sovateltide are two agents currently in the pipeline that try to stimulate neurogenesis.
Phase 2
Allopregnanolone
Allopregnanolone is an allosteric modulator of inhibitory gamma aminobutyric acid A (GABA-A) receptors derived as a nonsteroidal metabolite of progesterone. In transgenic mouse models of AD, allopregnanolone increases neurogenesis, reduces amyloid deposition, and improves learning and memory [85].
Allopregnanolone is in a Phase 1 study designed to assess the pharmacokinetics of intramuscular administration of the agent (NCT03748303). Twelve participants with clinically diagnosed MCI or mild AD dementia will be engaged in the 14-week study. Outcome measures include safety, tolerability, and pharmacokinetic measures of the agent. A Phase 2 trial will engage 200 participants who are APOE4 gene carriers and manifest MCI or mild AD dementia (NCT04838301). Participants will be randomized to drug or placebo for 12 months. The primary outcome measure is hippocampal volume.
Magnetic resonance imaging studies of patients included in a Phase 1B/2A clinical trial showed slowing of the rate of decline in hippocampal volume in the treated group and evidence of increased hippocampal volume in APOE4 gene carriers. Diffusion tensor imaging measures of white matter integrity and resting state default mode network measures of functional connectivity suggested improvement in the treatment group [86].
Sovateltide (PMZ-1620; IRL-1620)
Sovateltide is an endothelin-B receptor agonist that promotes the differentiation of neuronal progenitors to produce mature neuronal cells and exhibits antioxidant, anti-apoptotic, and mitochondrial functional enhancement properties [87].
Sovateltide is in a Phase 2 randomized, double-blind placebo-controlled trial (NCT04052737). Eighty participants with clinically diagnosed AD will receive 3 doses of intravenous therapy on one day per month for six months following randomization. The primary outcome measures are safety and tolerability.
Oxidative Stress
Oxidative stress and production of reactive oxygen species reactive oxygen species (ROS) are both age- and disease-dependent and contributes to mitochondrial dysfunction, altered metal homeostasis, reduced antioxidant defense, and impaired synaptic function. Reactive oxygen species can adversely affect many cellular structures and processes including nuclear DNA, mitochondrial dynamics and function, lipids, proteins, calcium homeostasis, cellular architecture, receptor trafficking and endocytosis, and energy homeostasis. [88]. Diets rich in antioxidants, B vitamins, polyphenols, and polyunsaturated fatty acids have been associated with a reduced risk for AD, suggesting that optimization of antioxidant status and reducing ROS-related damage may prevent, reduce the risk, or treat AD [89]. Similarly, physical exercise may reduce the risk of AD and slow the progression of MCI or AD dementia in part through its effect on brain antioxidant status [90].
Clinical trials of antioxidant have been added to this foundation of biological and epidemiological observations. Antioxidants are in current Phase 3 clinical trials of hydralazine hydrochloride and omega-3 fatty acids; Phase 2 trials are assessing the efficacy of adaravone, and Flos gossypii flavonoids.
Phase 3
Hydralazine
Hydralazine activates the Nrf2 antioxidant pathway, enhances mitochondrial function, increases mitochondrial respiration and adenosine triphosphate production; and activates autophagy that may facilitate clearance of intracellular protein aggregates [91].
Hydralazine is being assessed in a Phase 3 placebo-controlled trial of 424 participants with clinically diagnosed mild to moderate AD (NCT04842552). The primary outcome is the progression of AD as measured on the ADAS-cog at 3, 6, 9, and 12 months.
Omega three (DHA/EPA)
Omega three (DHA/EPA) is being studied in a Phase 3 trial of 400 participants who have a low red blood cell DHA/eicosapentaenoic acid (EPA) index, subjective memory complaints, and a family history of AD (NCT03691519). The trial will be conducted with an 18-month treatment period. The primary outcome is a composite cognitive score.
Phase 2
Edaravone (MCI-186, Radicava®, Radicut®, MT-1186, FAB122)
Edaravone is a pyralozone free radical scavenger antioxidant that is approved by the FDA for treatment of amyotrophic lateral sclerosis (ALS). In transgenic AD mouse models, edaravone inhibited Aβ aggregation and attenuated Aβ-induced oxidation. Edaravone reduced downstream pathologies including tau hyperphosphorylation, glial activation, neuroinflammation, neuronal loss, and synaptic dysfunction. Behavioral deficits of the animals were rescued with therapy [92]. Edaravone is being assessed in a Phase 2 clinical trial including 60 participants with a clinical diagnosis of early AD, confirmed by CSF AD biomarkers or amyloid PET (NCT05323812). The participants will be assessed for four months, and the primary outcome measures will be safety and tolerability and plasma and CSF oxidative stress biomarkers.
Flos Gossypii Flavonoids
Flavonoids have antioxidant and anti-inflammatory properties and the beneficial effects of Flos gossypii flavonoids, a traditional Uygur ethnic medicine, is being assessed in a Phase 2 clinical trial (NCT05269173). Two-hundred and forty participants with clinically diagnosed mild to moderate AD will be assessed over a 26-week period. The primary outcome is the ADAS-cog.
Bioenergetics and Metabolism
Bioenergetics and metabolism embrace a large array of processes and possible interventions. There is some overlap with other CADRO categories such as oxidative injury to mitochondria and the putative benefits of antioxidants in mitochondrial preservation. Likewise, some bioenergetic and metabolic agents have substantial anti-inflammatory activity and overlap with the functions of inflammation-reducing treatments. Several of the bioenergetic drugs currently in clinical trials are drugs that are used for their metabolic effects in diabetes. This pertains to metformin, semaglutide, dapagliflozin, and insulin. These agents were developed to address insulin insensitivity in type 2 diabetes, and they may reduce insulin resistance characteristic of the brain in AD.
Phase 3
Metformin (Glucophage™)
Metformin is an insulin sensitizer that lowers blood glucose and reduces inflammation, decreases oxidative stress and increases neurogenesis in preclinical assessment. Metformin is a biguanide drug that decreases blood glucose by reducing glucose production in the liver, decreasing intestinal absorption, and increasing insulin sensitivity of target tissues. Metformin reduces all-cause mortality, cardiovascular disease, and cancer, as well as treating type 2 diabetes [93]. Metformin is in a Phase 2/3 clinical trial involving 370 individuals with clinically identified amnestic MCI (NCT04098666). Participants will be randomized to drug or placebo for 24 months. The primary outcome measure is the Free and Cued Selective Reminding Test.
Semaglutide
Semaglutide is approved for treatment of diabetes and obesity. It has peripheral anti-inflammatory effects that are posited to reduce central inflammation. Semaglutide is being assessed in two large Phase 3 trials described above in the section on anti-inflammatory drugs.
Tricaprilin (AC-1204)
Tricaprilin is a caprylic triglyceride metabolized to ketones including hydroxybutyric acid that can be converted to acetyl-CoA to produce energy by mitochondria. The conceptual framework for the development of this agent suggests that providing an alternate neuronal metabolic pathway through ketones will improve brain metabolism in the setting of the insulin resistance and glucose hypometabolism observed in AD [94]. Tricaprilin is in a Phase 3 randomized double-blind controlled trial (NCT04187547). Participants will have mild to moderate AD diagnosed clinically and supported by an AD-compatible pattern on FDG PET. All participants must be APOE4 gene non-carriers. The ADAS-cog will be the primary outcome. Tricaprilin is also in a Phase 1 clinical trial assessing the safety and tolerability of a new formulation in healthy volunteers.
Phase 2
Dapagliflozin
Dapagliflozin inhibits the sodium-glucose cotransporter-2 (SGLT2) and lowers blood glucose by facilitating its reabsorption into the kidney and excretion via the urine. Dapagliflozin is an acetylcholinesterase inhibitor as well as an SGLT2 inhibitor and may have symptomatic benefits in the treatment of AD [95]. Dapagliflozin is in a Phase 2 placebo-controlled trial involving 48 patients with clinically diagnosed mild to moderate AD and with a high basal metabolic index (BMI ≥ 23) (NCT03801642). The primary outcome measure is brain N-acetylaspartate as shown on magnetic resonance spectroscopy.
Traditional Chinese Medicine
Traditional Chinese Medicine is being assessed in a randomized controlled trial of 180 participants with MCI, mild to moderate AD, or severe AD (NCT05538507). Diagnoses are based on clinical criteria. Six treatment groups will be constructed to allow a comparison of a “smart soup” of three traditional Chinese medical herbs compared to memantine, donepezil and placebo. Patients will be on active therapy for a period of 1 year. Primary outcome measures include assessments of cognition, behavior, ADLs, general health status, Aβ and tau biomarkers in CSF and plasma, MRI, EEG, and oxidative and transmitter biomarkers in the blood. Traditional Chinese Medicine is reported to improve AD and anticipated to influence brain metabolism.
Insulin
Insulin has multiple actions in the brain and multiple potential therapeutic effects in AD. Insulin resistance is characteristic of neurons in AD, and insulin has a beneficial effect on brain metabolism and bioenergetics. In addition, insulin has multiple other roles observed in tissue studies and animal models of AD, including effects on synaptic plasticity, dendritic spine formation, transmitter turnover, proteostasis including clearance of Aβ and tau, vascular function, lipid metabolism, and inflammation [96]. Outcomes of trials so far have been largely negative; new trials are exploring novel delivery mechanisms and trial designs [97, 98].
Insulin is a large molecule biologic and must be delivered subcutaneously, intravenously, or through nasal inhalation. Developing effective delivery mechanisms for clinical trials with AD participants has been challenging. A trial of 40 cognitively normal amyloid-positive individuals will explore the feasibility of using an intranasal insulin delivery device (NCT05006599). The primary outcome of this study is the percentage of prescribed doses successfully taken by the participants.
Another insulin trial will explore the safety and efficacy of the combination of insulin plus an SGLT 2 inhibitor, empagliflozin (NCT05081219). Sixty participants who are cognitively normal or have MCI or mild AD dementia, and shown to be amyloid positive on amyloid PET or with CSF AD biomarkers, will be studied for 8 weeks. The primary outcomes are safety and the incidence of treatment-emergent events and treatment-emergent serious events.
T3D-959
T3D-959 is a dual delta gamma peroxisome proliferator activated receptor (PPAR) agonist. Peroxisome proliferator activated receptor agents improve insulin sensitivity. In rodent models of experimentally induced diabetes, T3D-959 normalizes the AD type biomarkers that evolve in these animals [99]. Peroxisome proliferator activated receptors play a significant role in energy homeostasis, oxidative stress, fatty acid metabolism by mitochondria, and inflammation. Peroxisome proliferator activated receptor gene expression is reduced in the AD brain, and this reduction may contribute to some of the metabolic abnormalities observed [100]. T3D-959 is in a Phase 2 clinical trial assessing 3 doses compared to placebo (NCT04251182). Participating individuals will have a diagnosis of mild to moderate AD based on clinical criteria. Primary outcomes include measures of cognition, function, safety, and tolerability.
Phase 1
Nicotinamide Riboside
Nicotinamide riboside is a proprietary form of nicotinamide or vitamin B3. Cellular and animal model studies suggest that raising intracellular nicotinamide adenine dinucleotide (NAD) levels improves mitochondrial function, decreases oxidative stress, and restores or maintains metabolic processing of aggregated proteins [101]. A study of nicotinamide riboside will assess 50 participants in a 12-week trial (NCT04430517). Participating individuals will have MCI or mild AD dementia supported by AD biomarkers and the presence of at least one copy of the APOE4 gene. Primary outcomes include magnetic resonance (MR) spectroscopy measures of brain NAD+ and changes in the brain redox state.
Vascular Factors
Vascular health plays a prominent role in AD. Vascular risk factors include stroke, hypertension, hypercholesterolemia, diabetes, and obesity, and amyloid is deposited in the blood vessels of AD patients [102]. There is also evidence of blood-brain barrier disruption in AD. Magnetic resonance imaging studies indicate that ischemic brain injury is a common accompanying feature of AD; microhemorrhages are frequently seen on MRI of patients with AD; and CAA is often accompanied by AD [103, 104]. The occurrence of amyloid-related imaging abnormalities (ARIA) in the context of treatment with anti-amyloid monoclonal antibodies has drawn attention to the importance of vascular pathology in the pathogenesis and treatment of AD [105]. Despite the common co-occurrence of AD and vascular risk factors, there are relatively few clinical trials addressing vascular risk among novel targets in the AD drug development pipeline.
Phase 2
Telmisartan or Perindopril
An active comparator trial investigates the effects of telmisartan (angiotensin receptor blocker [ARB]) or perindopril (angiotensin converting enzyme inhibitor [ACEI]) on brain atrophy as manifested by ventricular enlargement after 12 months of treatment (NCT02085265).
Yangxue qingnao
Yangxue qingnao is a traditional Chinese medicine posited to have effects on cerebral blood flow and of potential benefit for the treatment of AD. A trial will randomize 216 individuals with mild to moderate AD to a high dose, low dose, or placebo (NCT04780399). Alzheimer’s disease diagnosis includes clinical assessment, MRI scan demonstrating medial temporal atrophy, and positive amyloid imaging or CSF AD biomarkers. The primary outcome measures of the trial are the ADAS-cog and CDR-sb.
Cell Death
Phase 2
Deferiprone
Deferiprone is an iron chelator being studied to determine the benefit of reducing brain iron on the clinical course of AD. High iron content in the brain generates ROS leading to downstream cell death; elevated brain iron in AD has been associated with an accelerated clinical decline. Iron chelation is hypothesized to have neuroprotective effects in reducing damaging ROS, inhibiting the setting of Aβ and tau pathology, and decreasing levels of brain iron [106, 107]. A double-blind placebo-controlled trial will assess the efficacy of deferiprone in 171 individuals with MCI or mild AD dementia (NCT03234686). Participants will have a clinical diagnosis of AD supported by positive amyloid imaging. The primary outcome measure is the Neuropsychological Test Battery (NTB) assessed at after 12 months of therapy.
Growth Factors and Hormones
Phase 2
CORT108297
Cortisol excess has been identified as a risk factor for AD and a promoter of AD pathobiology [108]. Elevated cortisol has been associated with compromised memory, executive function, language, processing speed, and social cognition. Increased cortisol levels are associated with an increased risk for cognitive decline and cortisol levels are higher in patients with MCI than in cognitively normal older individuals. Elevated CSF cortisol has been associated with more rapid cognitive decline in MCI. Elevated cortisol may be an important mediator linking depression, sleep disturbances, and cardiovascular risk factors to AD. Biologically, high cortisol has been connected to hippocampal atrophy, oxidative stress, and Aβ toxicity [108].
CORT108297 is a glucocorticoid receptor modulator that has been shown to reduce corticosterone hypersecretion associated with physiological stress. The CORT-X trial will examine if mitigation of stress-mediated pathogenesis of AD is a possible target for intervention in individuals at risk for this disease (NCT04601038). This is a 2-week, randomized, placebo-controlled crossover study of the effects of CORT108297 on cognitive test performance in 26 individuals with MCI due to AD, and in 26 cognitively normal individuals with an increased risk for AD due to family history, genetics, and/or subjective memory complaints. All subjects will take part in a brief stressor (public speaking and mental arithmetic) and provide saliva samples to assess the stress hormone response. The primary aims will compare the effects of CORT108297 to placebo on tests of memory, executive function, and digit span.
Leuprolide (Leuprorelin; Lupron)
Leuprolide is a synthetic analogue of gonadotrophin releasing hormone (GnRH) and down-regulates luteinizing hormone (LH) and follicle stimulating hormone (FSH). Elevated LH has been linked to neurodegeneration and cognitive decline, while leuprolide has decreased amyloid and improved cognitive in some animal models of AD [109, 110].
Leuprolide is being studied in a Phase 2 double-blind randomized trial of women with clinically diagnosed MCI or mild-moderate AD dementia (NCT03649724). The primary outcome is performance on the ADAS-cog after 48 weeks of treatment.
GnRH
Gonadotrophin releasing hormone is in a clinical trial of participants with Down syndrome with evidence of concomitant AD (NCT04390646). Gonadotrophin releasing hormone improves cognition in a mouse model of Down syndrome and has shown beneficial effects on cognition and connectivity in a preliminary trial of adults with Down syndrome likely due to Aβ pathology [111].
Circadian Rhythm
Phase 2/3
Piromelatine (Neu-P11)
Piromelatine is a melatonin receptor agonist (MT1, MT2, MT3) and a serotonin receptor agonist (5-HT1A, 15-HT1D). Preliminary studies in rodent models of AD suggest that piromelatine decreased Aβ-related cell loss and improved memory [112]. There is currently a clinical trial underway with ADAS-cog as the primary outcome measure (NCT05267535).
Epigenetic Regulators
Phase 2
Lamivudine (3TC)
Lamivudine is a reverse transcriptase inhibitor approved for use for the treatment of HIV. In aged mice, the agent reduced inflammation and decreased the number of senescent cells [113]. The primary outcomes are the change in reverse transcriptase activity in blood and CSF and the penetration of lamivudine across the blood brain barrier as reflected in the plasma/CSF ratio of 3TC 5′-triphosphate (NCT04552795).
Discussion
There is a robust array of novel targets and agents being studied in the AD drug development pipeline. Many of the CADRO-identified processes of AD are being explored as possible points of disease intervention. The areas of inflammation and synaptic plasticity are notably well represented by multiple agents in the pipeline. Of the agents in each of these categories, there are very few with the same specific mechanism of action. This suggests that trials will be informative regarding which targets may be more vulnerable to therapeutic modulation and might provide insight into useful combinations of therapies within a therapeutic category.
Some processes critical to AD have few or no candidate therapies in the pipeline. APOE4 is one of the most important risk factors for AD and is present in 65%–70% of patients. There is only one agent, the gene therapy LEX 1001, in the pipeline targeting APOE4. In this trial, a viral vector carrying the APOE2 gene is being given to antagonize the effects of APOE4. The amyloid, tau, neurodegeneration (ATN) framework has provided a working model of the biology and related biomarkers of AD [114]. “A” and “T” are the canonical drug targets for AD and have associated candidate therapies in the pipeline. There is only one agent for “N” in the current pipeline, deferiprone, a drug targeting reduction of brain iron. Iron is hypothesized to exaggerate the effects on Aβ leading to cell death. There are many interactions between vascular disease and AD and few agents exploring therapeutic opportunities regarding this interaction. One trial compares cognitive effects of telmisartan (and angiotensin receptor blocker) to perindopril (an ACE inhibitor); the other is assessing the effects of the traditional Chinese medicine, Yangxue qingnao. Some categories such as epigenetic agents and circadian rhythm have few therapeutic approaches in the pipeline; understanding of the biology of these processes may not have matured sufficiently to suggest target mechanisms for DMTs.
The complexity of the biology of AD, and overlap in risk factors associated with the disease, suggest the need for combination therapies to offer the best opportunity to alter the disease course. There are currently no combination therapies in the pipeline. There are a few agents with multiple actions possibly beneficial for AD and representing “a combination therapy in a pill.” The success of anti-amyloid monoclonal antibodies validates amyloid plaques as a therapeutic target for AD therapeutics. Similarly, the success of tau antisense oligonucleotides suggest that therapies decreasing protein production may be effective [115]. The increasing understanding of brain inflammation has found several pathways that are promising as targets for monotherapies and could possibly guide development of combinations therapies. The advantage of developing different therapeutic approaches independently and then combining them is that the contribution of each agent can be understood and the target-dose relationships of both therapies can be assessed.
Biomarkers are revolutionizing AD drug development [116–118]. Multiple modalities of brain imaging, an array of CSF analytes, and an emerging repertoire of blood-based biomarkers are available to drug developers for a variety of contexts of use (CoU). Identified CoUs include biomarkers of risk (e.g., APOE4), diagnosis (e.g., amyloid PET, CSF AD biomarkers), monitoring (e.g., blood-based biomarkers of p-tau and NfL), pharmacodynamic response (e.g., amyloid PET, tau PET), prognosis (p-tau, Up53), treatment response prediction (none known for predicting benefit; APOE4 genotype predicts greater risk for amyloid-related imaging abnormalities [ARIA] with anti-amyloid monoclonal antibodies), and safety (MRI for ARIA). Some trials have integrated biomarkers into the design and others have not.
Most of the trials assessing novel agents use traditional clinical outcomes such as the ADAS-cog and the ADCS ADL (Tables 1 and 2). These tools have the advantage of long-term use in clinical trials and great depth of understanding how they perform in trials of different AD populations (e.g., MCI, mild and moderate AD dementia) and the expected rate of placebo decline can be calculated and used for sample size estimations for trials of agents with a predefined expected effect size. However, the ADAS-cog was published in 1984 and constructed before the current understanding of neuropsychological processes had evolved [119]. The ADCS-ADL reflects the daily life functions of 1997 when the tool was inaugurated [120]. More advanced neuropsychological approaches such as the NTB or more current functional measures such as the Amsterdam ADL scale may offer advantages and are increasingly used in trials [121, 122]. As more data evolve, there will more confidence in the performance of these new instruments in trials. Computerized neuropsychological measures and remote digital monitors are increasingly available and may offer insights into drug effects not available from other measures [123–125].
Summary and Conclusions
The canonical target of AD drug development – amyloid and tau – are together represented in approximately 30% of current trials [6]. Seventy percent of trials are assessing non-Aβ non-tau targets with novel agents. The array of novel drugs being assessed in the pipeline span most of the CADRO categories identified as processes in AD biology that comprise therapeutic targets. Most common in the pipeline are novel agents addressing inflammation and synaptic plasticity. Some novel designs, outcomes, and biomarkers are being integrated into the trials assessing these agents. More target engagement biomarkers, better clinical measures, and new approaches to assessment, such as computerized tests and digital biomarkers, may improve the ability to characterize drug-placebo differences and advance novel therapies for AD.
Acknowledgments
JC is supported by National Institute of General Medical Sciences (NIGMS) grant P20GM109025; National Institute of Neurological Disorders and Stroke (NINDS) grant U01NS093334; National Institute on Aging (NIA) grant R01AG053798; NIA grant P20AG068053; NIA grant P30AG072959; NIA grant R35AG71476; Alzheimer’s Disease Drug Discovery Foundation (ADDF); Ted and Maria Quirk Endowment; and the Joy Chambers-Grundy Endowment.
Declarations
Funding
None.
Conflicts of Interest
JC has provided consultation to Acadia, Actinogen, Acumen, AlphaCognition, Aprinoia, AriBio, Artery, Biogen, BioVie, Cassava, Cerecin, Diadem, EIP Pharma, Eisai, GemVax, Genentech, GAP Innovations, Janssen, Jocasta, Karuna, Lilly, Lundbeck, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Optoceutics, Ono, Otsuka, PRODEO, Prothena, ReMYND, Roche, Sage Therapeutics, Signant Health, Simcere, Suven, SynapseBio, TrueBinding, Vaxxinity, and Wren pharmaceutical, assessment, and investment companies. AL and JK have no disclosures.
Ethics Approval
Not applicable, no human subjects included.
Consent to Participate
Not applicable, no human subjects included.
Availability of Data
Available on public website clinicaltrials.gov.
Code Availability
Not applicable, no special programing was employed.
Author Contributions
All authors have taken part in the outline, revision of multiple drafts, and review and approval of the final version of the manuscript.
Consent for publication
Not applicable.
References
- 1.“Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022; 7(2): e105–e125. 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed]
- 2.Liggins C, et al. International Alzheimer’s Disease Research Portfolio (IADRP) aims to capture global Alzheimer’s disease research funding. Alzheimers Dement. 2014;10(3):405–408. doi: 10.1016/j.jalz.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Budd Haeberlein S, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9(2):197–210. doi: 10.14283/jpad.2022.30. [DOI] [PubMed] [Google Scholar]
- 4.van Dyck CH, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- 5.Mintun MA, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691–1704. doi: 10.1056/NEJMoa2100708. [DOI] [PubMed] [Google Scholar]
- 6.Cummings J, Zhou Y, Lee G, Zhong K, Fonseca J, Cheng F. Alzheimer’s disease drug development pipeline: 2023. Alz & Dem. 2023. [DOI] [PMC free article] [PubMed]
- 7.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y) 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandrekar S, Landreth GE. Microglia and Inflammation in Alzheimer’s Disease. CNS Neurol Disord Drug Targets. 2010;9(2):156–167. doi: 10.2174/187152710791012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagiani F, Lanni C, Racchi M, Govoni S. Targeting dementias through cancer kinases inhibition. Alzheimers Dement (N Y) 2020;6(1):e12044. doi: 10.1002/trc2.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T, et al. Effects of chronic masitinib treatment in APPswe/PSEN1dE9 transgenic mice modeling Alzheimer’s disease. Journal of Alzheimer’s Disease. 2020;76(4):1339–1345. doi: 10.3233/JAD-200466. [DOI] [PubMed] [Google Scholar]
- 11.Balzano T, Esteban-García N, Blesa J. Neuroinflammation, immune response and α-synuclein pathology: how animal models are helping us to connect dots. Expert Opin Drug Discov. 2022 doi: 10.1080/17460441.2023.2160440. [DOI] [PubMed] [Google Scholar]
- 12.Reading CL, Ahlem CN, Murphy MF. NM101 Phase III study of NE3107 in Alzheimer’s disease: rationale, design and therapeutic modulation of neuroinflammation and insulin resistance. Neurodegener Dis Manag. 2021;11(4):289–298. doi: 10.2217/nmt-2021-0022. [DOI] [PubMed] [Google Scholar]
- 13.Nørgaard CH, et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: Data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimers Dement (N Y) 2022;8(1):e12268. doi: 10.1002/trc2.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulland TK, Colonna M. TREM2—a key player in microglial biology and Alzheimer disease. Nat Rev Neurol. 2018;14(11):11. doi: 10.1038/s41582-018-0072-1. [DOI] [PubMed] [Google Scholar]
- 15.Price BR, et al. Therapeutic Trem2 activation ameliorates amyloid-beta deposition and improves cognition in the 5XFAD model of amyloid deposition. J Neuroinflammation. 2020;17(1):238. doi: 10.1186/s12974-020-01915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, et al. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J Exp Med. 2020;217(9):e20200785. doi: 10.1084/jem.20200785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gofrit ON, Bercovier H, Klein BY, Cohen IR, Ben-Hur T, Greenblatt CL. Can immunization with Bacillus Calmette-Guérin (BCG) protect against Alzheimer’s disease? Med Hypotheses. 2019;123:95–97. doi: 10.1016/j.mehy.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 18.O’Shea JJ, Gadina M. Selective Janus kinase inhibitors come of age. Nat Rev Rheumatol. 2019;15(2):2. doi: 10.1038/s41584-018-0155-9. [DOI] [PubMed] [Google Scholar]
- 19.Bechman K, Yates M, Galloway JB. The new entries in the therapeutic armamentarium: the small molecule JAK inhibitors. Pharmacol Res. 2019;147:104392. doi: 10.1016/j.phrs.2019.104392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak–STAT signaling in the immune system. Nat Immunol. 2017;18(4):4. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubo S, et al. Janus kinase inhibitor baricitinib modulates human innate and adaptive immune system. Front Immunol. 2018 doi: 10.3389/fimmu.2018.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Italiani P, et al. Circulating levels of IL-1 family cytokines and receptors in Alzheimer’s disease: new markers of disease progression? J Neuroinflammation. 2018;15(1):342. doi: 10.1186/s12974-018-1376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerreiro S, Privat A-L, Bressac L, Toulorge D. CD38 in neurodegeneration and neuroinflammation. Cells. 2020;9(2):471. doi: 10.3390/cells9020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blacher E, et al. Alzheimer’s disease pathology is attenuated in a CD38-deficient mouse model. Ann Neurol. 2015;78(1):88–103. doi: 10.1002/ana.24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onyango IG, Jauregui GV, Čarná M, Bennett JP, Stokin GB. Neuroinflammation in Alzheimer’s disease. Biomedicines. 2021;9(5):5. doi: 10.3390/biomedicines9050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byeon SE, Yi Y-S, Oh J, Yoo BC, Hong S, Cho JY. The role of Src kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2012 doi: 10.1155/2012/512926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui Z, et al. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front Immunol. 2022;13:943321. doi: 10.3389/fimmu.2022.943321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang P, et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci. 2019;22(5):719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musi N, et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 2018;17(6):e12840. doi: 10.1111/acel.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzales MM, et al. Senolytic therapy to modulate the progression of Alzheimer’s Disease (SToMP-AD)—outcomes from the first clinical trial of senolytic therapy for Alzheimer’s disease. Res Sq. 2023 doi: 10.21203/rs.3.rs-2809973/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syed YY. Lenalidomide: a review in newly diagnosed multiple myeloma as maintenance therapy after ASCT. Drugs. 2017;77(13):1473–1480. doi: 10.1007/s40265-017-0795-0. [DOI] [PubMed] [Google Scholar]
- 32.Le Douce J, et al. Impairment of glycolysis-derived l-serine production in astrocytes contributes to cognitive deficits in Alzheimer’s disease. Cell Metab. 2020;31(3):503–517.e8. doi: 10.1016/j.cmet.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Michael J, et al. The leukotriene receptor antagonist montelukast attenuates neuroinflammation and affects cognition in transgenic 5xFAD mice. Int J Mol Sci. 2021;22(5):2782. doi: 10.3390/ijms22052782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai J, et al. Montelukast rescues primary neurons against Aβ1-42-induced toxicity through inhibiting CysLT1R-mediated NF-κB signaling. Neurochem Int. 2014;75:26–31. doi: 10.1016/j.neuint.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Feigin A, et al. Pepinemab antibody blockade of SEMA4D in early Huntington’s disease: a randomized, placebo-controlled, phase 2 trial. Nat Med. 2022;28(10):10. doi: 10.1038/s41591-022-01919-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alves S, et al. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer’s disease mice. Brain. 2017;140(3):826–842. doi: 10.1093/brain/aww330. [DOI] [PubMed] [Google Scholar]
- 37.Faridar A, et al. Ex vivo expanded human regulatory T cells modify neuroinflammation in a preclinical model of Alzheimer’s disease. Acta Neuropathol Commun. 2022;10(1):144. doi: 10.1186/s40478-022-01447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LiCausi F, Hartman NW. Role of mTOR Complexes in Neurogenesis. Int J Mol Sci. 2018;19(5):1544. doi: 10.3390/ijms19051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J, Long Z, Li Y, Luo M, Luo S, He G. Alteration of the Wnt/GSK3β/β-catenin signalling pathway by rapamycin ameliorates pathology in an Alzheimer’s disease model. Int J Mol Med. 2019;44(1):313–323. doi: 10.3892/ijmm.2019.4198. [DOI] [PubMed] [Google Scholar]
- 40.Kiyota T, et al. Granulocyte-macrophage colony-stimulating factor neuroprotective activities in Alzheimer’s disease mice. J Neuroimmunol. 2018;319:80–92. doi: 10.1016/j.jneuroim.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Potter H, et al. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer’s disease. Alzheimers Dement (N Y) 2021;7(1):e12158. doi: 10.1002/trc2.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin L-W, et al. Repurposing the KCa3.1 inhibitor senicapoc for Alzheimer’s disease. Ann Clin Transl Neurol. 2019;6(4):723–738. doi: 10.1002/acn3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boza-Serrano A, et al. Galectin-3, a novel endogenous TREM2 ligand, detrimentally regulates inflammatory response in Alzheimer’s disease. Acta Neuropathol. 2019;138(2):251–273. doi: 10.1007/s00401-019-02013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubin K, Glazer S. The pertussis hypothesis: Bordetella pertussis colonization in the pathogenesis of Alzheimer’s disease. Immunobiology. 2017;222(2):228–240. doi: 10.1016/j.imbio.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Scherrer JF, Salas J, Wiemken TL, Jacobs C, Morley JE, Hoft DF. Lower risk for dementia following adult tetanus, diphtheria, and pertussis (Tdap) vaccination. J Gerontol A Biol Sci Med Sci. 2021;76(8):1436–1443. doi: 10.1093/gerona/glab115. [DOI] [PubMed] [Google Scholar]
- 46.Weidung B, et al. VALZ-Pilot: High-dose valacyclovir treatment in patients with early-stage Alzheimer’s disease. Alzheimers Dement (N Y) 2022;8(1):e12264. doi: 10.1002/trc2.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacPherson KP, et al. Peripheral administration of the soluble TNF inhibitor XPro1595 modifies brain immune cell profiles, decreases beta-amyloid plaque load, and rescues impaired long-term potentiation in 5xFAD mice. Neurobiol Dis. 2017;102:81–95. doi: 10.1016/j.nbd.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel AG, et al. Innate immunity stimulation via CpG oligodeoxynucleotides ameliorates Alzheimer’s disease pathology in aged squirrel monkeys. Brain. 2021;144(7):2146–2165. doi: 10.1093/brain/awab129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canet G, et al. HIV Neuroinfection and Alzheimer’s disease: similarities and potential links? Front Cell Neurosci. 2018;12:307. doi: 10.3389/fncel.2018.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baruch K, et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat Med. 2016;22(2):2. doi: 10.1038/nm.4022. [DOI] [PubMed] [Google Scholar]
- 51.Ghareghani M, Rivest S. The synergistic potential of combining PD-1/PD-L1 immune checkpoint inhibitors with NOD2 Agonists in Alzheimer’s disease treatment. Int J Mol Sci. 2023;24(13):10905. doi: 10.3390/ijms241310905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Min S-W, et al. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med. 2015;21(10):1154–1162. doi: 10.1038/nm.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shin M-K, et al. Reducing acetylated tau is neuroprotective in brain injury. Cell. 2021;184(10):2715–2732.e23. doi: 10.1016/j.cell.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stym-Popper G, et al. Regulatory T cells decrease C3-positive reactive astrocytes in Alzheimer-like pathology. J Neuroinflammation. 2023;20:64. doi: 10.1186/s12974-023-02702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 2015;7:688–698. doi: 10.1016/j.nicl.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenzweig-Lipson S, Barton R, Gallagher M, Edgar CJ, Maruff PT, Mohs R. HOPE4MCI trial: First trial targeting reduction of hippocampal overactivity to treat mild cognitive impairment due to Alzheimer’s disease with AGB101. Alzheimer’s Dementia. 2020;16(S9):e045331. doi: 10.1002/alz.045331. [DOI] [Google Scholar]
- 57.Lahmy V, Long R, Morin D, Villard V, Maurice T. Mitochondrial protection by the mixed muscarinic/σ1 ligand ANAVEX2-73, a tetrahydrofuran derivative, in Aβ25-35 peptide-injected mice, a nontransgenic Alzheimer’s disease model. Front Cell Neurosci. 2014;8:463. doi: 10.3389/fncel.2014.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamasaki H, et al. Down-regulation of MET in hippocampal neurons of Alzheimer’s disease brains. Neuropathology. 2014;34(3):284–290. doi: 10.1111/neup.12095. [DOI] [PubMed] [Google Scholar]
- 59.Hua X, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the positive modulator of HGF/MET, fosgonimeton, in healthy volunteers and subjects with Alzheimer’s disease: randomized, placebo-controlled, double-blind, phase I clinical trial. J Alzheimers Dis. 2022;86(3):1399–1413. doi: 10.3233/JAD-215511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnston JL, et al. Fosgonimeton, a Novel positive modulator of the HGF/MET system, promotes neurotrophic and procognitive effects in models of dementia. Neurotherapeutics. 2022 doi: 10.1007/s13311-022-01325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burns LH, Pei Z, Wang H-Y. Targeting α7 nicotinic acetylcholine receptors and their protein interactions in Alzheimer’s disease drug development. Drug Dev Res. 2023 doi: 10.1002/ddr.22085. [DOI] [PubMed] [Google Scholar]
- 62.Wang H-Y, Lee K-C, Pei Z, Khan A, Bakshi K, Burns LH. PTI-125 binds and reverses an altered conformation of filamin A to reduce Alzheimer’s disease pathogenesis. Neurobiol Aging. 2017;55:99–114. doi: 10.1016/j.neurobiolaging.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 63.Wang H-Y, et al. PTI-125 reduces biomarkers of Alzheimer’s disease in patients. J Prev Alzheimers Dis. 2020;7(4):256–264. doi: 10.14283/jpad.2020.6. [DOI] [PubMed] [Google Scholar]
- 64.Park H-H, et al. Neural stem cells injured by oxidative stress can be rejuvenated by GV1001, a novel peptide, through scavenging free radicals and enhancing survival signals. Neurotoxicology. 2016;55:131–141. doi: 10.1016/j.neuro.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 65.Koh S-H, et al. Efficacy and safety of GV1001 in patients with moderate-to-severe Alzheimer’s disease already receiving donepezil: a phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. Alzheimer’s Res Ther. 2021;13(1):66. doi: 10.1186/s13195-021-00803-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hampel H, et al. Lithium as a treatment for Alzheimer’s disease: the systems pharmacology perspective. J Alzheimers Dis. 2019;69(3):615–629. doi: 10.3233/JAD-190197. [DOI] [PubMed] [Google Scholar]
- 67.Zhang X, et al. Long-term treatment with lithium alleviates memory deficits and reduces amyloid-β production in an aged Alzheimer’s disease transgenic mouse model. J Alzheimers Dis. 2011;24(4):739–749. doi: 10.3233/JAD-2011-101875. [DOI] [PubMed] [Google Scholar]
- 68.Lei P, et al. Lithium suppression of tau induces brain iron accumulation and neurodegeneration. Mol Psychiatry. 2017;22(3):3. doi: 10.1038/mp.2016.96. [DOI] [PubMed] [Google Scholar]
- 69.Sun M-K, Nelson TJ, Alkon DL. Towards universal therapeutics for memory disorders. Trends Pharmacol Sci. 2015;36(6):384–394. doi: 10.1016/j.tips.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 70.Farlow MR, et al. A randomized, double-blind, placebo-controlled, phase II study assessing safety, tolerability, and efficacy of bryostatin in the treatment of moderately severe to severe Alzheimer’s disease. J Alzheimers Dis. 2019;67(2):555–570. doi: 10.3233/JAD-180759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Correia SS, et al. The CNS-penetrant soluble guanylate cyclase stimulator CY6463 reveals its therapeutic potential in neurodegenerative diseases. Front Pharmacol. 2021;12:656561. doi: 10.3389/fphar.2021.656561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gamba P, et al. The controversial role of 24-s-hydroxycholesterol in Alzheimer’s disease. Antioxidants (Basel) 2021;10(5):740. doi: 10.3390/antiox10050740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takamura Y, Ono K, Matsumoto J, Yamada M, Nishijo H. Effects of the neurotrophic agent T-817MA on oligomeric amyloid-β-induced deficits in long-term potentiation in the hippocampal CA1 subfield. Neurobiol Aging. 2014;35(3):532–536. doi: 10.1016/j.neurobiolaging.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 74.Izzo NJ, et al. Preclinical and clinical biomarker studies of CT1812: A novel approach to Alzheimer’s disease modification. Alzheimers Dement. 2021;17(8):1365–1382. doi: 10.1002/alz.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin C-H, et al. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer disease: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2014;75(9):678–685. doi: 10.1016/j.biopsych.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 76.Tari AR, et al. Safety and efficacy of plasma transfusion from exercise-trained donors in patients with early Alzheimer’s disease: protocol for the ExPlas study. BMJ Open. 2022;12(9):e056964. doi: 10.1136/bmjopen-2021-056964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rutigliano G, Stazi M, Arancio O, Watterson DM, Origlia N. An isoform-selective p38α mitogen-activated protein kinase inhibitor rescues early entorhinal cortex dysfunctions in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2018;70:86–91. doi: 10.1016/j.neurobiolaging.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alam JJ. Selective brain-targeted antagonism of p38 MAPKα reduces hippocampal IL-1β levels and improves morris water maze performance in aged rats. J Alzheimers Dis. 2015;48(1):219–227. doi: 10.3233/JAD-150277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scheltens P, et al. An exploratory clinical study of p38α kinase inhibition in Alzheimer’s disease. Ann Clin Transl Neurol. 2018;5(4):464–473. doi: 10.1002/acn3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gray NE, et al. Centella asiatica-Phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem Rev. 2018;17(1):161–194. doi: 10.1007/s11101-017-9528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu C-C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serrano-Pozo A, Das S, Hyman BT. APOE and Alzheimer’s disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021;20(1):68–80. doi: 10.1016/S1474-4422(20)30412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yao J, Ho D, Calingasan NY, Pipalia NH, Lin MT, Beal MF. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J Exp Med. 2012;209(13):2501–2513. doi: 10.1084/jem.20121239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Samant NP, Gupta GL. Novel therapeutic strategies for Alzheimer’s disease targeting brain cholesterol homeostasis. Eur J Neurosci. 2021;53(2):673–686. doi: 10.1111/ejn.14949. [DOI] [PubMed] [Google Scholar]
- 85.Chen S, Wang JM, Irwin RW, Yao J, Liu L, Brinton RD. Allopregnanolone promotes regeneration and reduces β-amyloid burden in a preclinical model of Alzheimer’s disease. PLoS ONE. 2011;6(8):e24293. doi: 10.1371/journal.pone.0024293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raikes AC, et al. Exploratory imaging outcomes of a phase 1b/2a clinical trial of allopregnanolone as a regenerative therapeutic for Alzheimer’s disease: structural effects and functional connectivity outcomes. Alzheimers Dement (N Y) 2022;8(1):e12258. doi: 10.1002/trc2.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ranjan AK, Gulati A. Sovateltide mediated endothelin B receptors agonism and curbing neurological disorders. Int J Mol Sci. 2022;23(6):6. doi: 10.3390/ijms23063146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tönnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis. 2017;57(4):1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu N, Yu J-T, Tan L, Wang Y-L, Sun L, Tan L. Nutrition and the risk of Alzheimer’s disease. Biomed Res Int. 2013 doi: 10.1155/2013/524820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McGurran H, Glenn JM, Madero EN, Bott NT. Prevention and treatment of Alzheimer’s disease: biological mechanisms of exercise. J Alzheimers Dis. 2019;69(2):311–338. doi: 10.3233/JAD-180958. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, et al. Pharmacological modulation of Nrf2/HO-1 signaling pathway as a therapeutic target of Parkinson’s disease. Front Pharmacol. 2021;12:757161. doi: 10.3389/fphar.2021.757161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiao S-S, et al. Edaravone alleviates Alzheimer’s disease-type pathologies and cognitive deficits. Proc Natl Acad Sci U S A. 2015;112(16):5225–5230. doi: 10.1073/pnas.1422998112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31–44. doi: 10.1016/j.arr.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 94.Henderson ST. Ketone bodies as a therapeutic for Alzheimer’s disease. Neurotherapeutics. 2008;5(3):470–480. doi: 10.1016/j.nurt.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shaikh S, Rizvi SMD, Shakil S, Riyaz S, Biswas D, Jahan R. Forxiga (dapagliflozin): plausible role in the treatment of diabetes-associated neurological disorders. Biotechnol Appl Biochem. 2016;63(1):145–150. doi: 10.1002/bab.1319. [DOI] [PubMed] [Google Scholar]
- 96.Kellar D, Craft S. Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19(9):758–766. doi: 10.1016/S1474-4422(20)30231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Craft S, et al. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical Trial. JAMA Neurol. 2020;77(9):1099–1109. doi: 10.1001/jamaneurol.2020.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rosenbloom M, et al. A phase II, single-center, randomized, double-blind, placebo-controlled study of the safety and therapeutic efficacy of intranasal glulisine in amnestic mild cognitive impairment and probable mild Alzheimer’s disease. Drugs Aging. 2021 doi: 10.1007/s40266-021-00845-7. [DOI] [PubMed] [Google Scholar]
- 99.de la Monte SM, Tong M, Schiano I, Didsbury J. Improved brain insulin/IGF signaling and reduced neuroinflammation with T3D–959 in an experimental model of sporadic Alzheimer’s disease. J Alzheimers Dis. 2017;55(2):849–864. doi: 10.3233/JAD-160656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wójtowicz S, Strosznajder AK, Jeżyna M, Strosznajder JB. The novel role of PPAR alpha in the brain: promising target in therapy of Alzheimer’s disease and other neurodegenerative disorders. Neurochem Res. 2020;45(5):972–988. doi: 10.1007/s11064-020-02993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lautrup S, Sinclair DA, Mattson MP, Fang EF. NAD+ in brain aging and neurodegenerative disorders. Cell Metab. 2019;30(4):630–655. doi: 10.1016/j.cmet.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Silva MVF, de Loures CMG, Alves LCV, de Souza LC, Borges KBG, Carvalho das MG. Alzheimer’s disease: risk factors and potentially protective measures. J Biomed Sci. 2019;26:33. doi: 10.1186/s12929-019-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.He J-T, Zhao X, Xu L, Mao C-Y. vascular risk factors and Alzheimer’s disease: blood-brain barrier disruption, metabolic syndromes, and molecular links. J Alzheimers Dis. 2020;73(1):39–58. doi: 10.3233/JAD-190764. [DOI] [PubMed] [Google Scholar]
- 104.Kapasi A, Schneider JA. Vascular contributions to cognitive impairment, clinical Alzheimer’s disease, and dementia in older persons. Biochim Biophys Acta. 2016;1862(5):878–886. doi: 10.1016/j.bbadis.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sperling RA, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimer’s Dementia. 2011;7(4):367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ayton S, et al. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol Psychiatry. 2020;25(11):2932–2941. doi: 10.1038/s41380-019-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nuñez MT, Chana-Cuevas P. New perspectives in iron chelation therapy for the treatment of neurodegenerative diseases. Pharmaceuticals (Basel) 2018;11(4):109. doi: 10.3390/ph11040109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ouanes S, Popp J. High cortisol and the risk of dementia and Alzheimer’s disease: a review of the literature. Front Aging Neurosci. 2019;11:43. doi: 10.3389/fnagi.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Atwood CS, Bowen RL. The endocrine dyscrasia that accompanies menopause and andropause induces aberrant cell cycle signaling that triggers re-entry of post-mitotic neurons into the cell cycle, neurodysfunction, neurodegeneration and cognitive disease. Horm Behav. 2015;76:63–80. doi: 10.1016/j.yhbeh.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bowen RL, Perry G, Xiong C, Smith MA, Atwood CS. A clinical study of lupron depot in the treatment of women with alzheimer’s disease: preservation of cognitive function in patients taking an acetylcholinesterase inhibitor and treated with high dose Lupron over 48 weeks. Journal of Alzheimer’s Disease. 2015;44(2):549–560. doi: 10.3233/JAD-141626. [DOI] [PubMed] [Google Scholar]
- 111.Manfredi-Lozano M, et al. GnRH replacement rescues cognition in Down syndrome. Science. 2022;377(6610):eabq4515. doi: 10.1126/science.abq4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He P, et al. A novel melatonin agonist Neu-P11 facilitates memory performance and improves cognitive impairment in a rat model of Alzheimer’ disease. Horm Behav. 2013;64(1):1–7. doi: 10.1016/j.yhbeh.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 113.De Cecco M, et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature. 2019;566(7742):7742. doi: 10.1038/s41586-018-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jack CR, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dementia. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mummery CJ, et al. Tau-targeting antisense oligonucleotide MAPTRx in mild Alzheimer’s disease: a phase 1b, randomized, placebo-controlled trial. Nat Med. 2023;29(6):1437–1447. doi: 10.1038/s41591-023-02326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Colom-Cadena M, et al. The clinical promise of biomarkers of synapse damage or loss in Alzheimer’s disease. Alzheimer’s Res Ther. 2020;12(1):21. doi: 10.1186/s13195-020-00588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cummings J. The role of biomarkers in Alzheimer’s disease drug development. Adv Exp Med Biol. 2019;1118:29–61. doi: 10.1007/978-3-030-05542-4_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ossenkoppele R, van der Kant R, Hansson O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 2022;21(8):726–734. doi: 10.1016/S1474-4422(22)00168-5. [DOI] [PubMed] [Google Scholar]
- 119.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 120.Galasko D, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S33–39. doi: 10.1097/00002093-199700112-00005. [DOI] [PubMed] [Google Scholar]
- 121.Harrison JE, Rentz DM, Brashear HR, Arrighi HM, Ropacki MT, Liu E. Psychometric evaluation of the neuropsychological test battery in individuals with normal cognition, mild cognitive impairment, or mild to moderate alzheimer’s disease: results from a longitudinal study. J Prev Alzheimers Dis. 2018;5(4):236–244. doi: 10.14283/jpad.2018.31. [DOI] [PubMed] [Google Scholar]
- 122.Jutten RJ, et al. Why a clinical trial is as good as its outcome measure: A framework for the selection and use of cognitive outcome measures for clinical trials of Alzheimer’s disease. Alzheimers Dement. 2023;19(2):708–720. doi: 10.1002/alz.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gold M, et al. Digital technologies as biomarkers, clinical outcomes assessment, and recruitment tools in Alzheimer’s disease clinical trials. Alzheimers Dement (N Y) 2018;4:234–242. doi: 10.1016/j.trci.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Robin J, Xu M, Kaufman LD, Simpson W. Using digital speech assessments to detect early signs of cognitive impairment. Front Digit Health. 2021 doi: 10.3389/fdgth.2021.749758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu C-Y, Beattie Z, Mattek N, Sharma N, Kaye J, Dodge HH. Reproducibility and replicability of high-frequency, in-home digital biomarkers in reducing sample sizes for clinical trials. Alzheimers Dement (N Y) 2021;7(1):e12220. doi: 10.1002/trc2.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]