Abstract
Paramacrobiotus fairbanksi was described from Alaska (USA) based on integrative taxonomy and later reported from various geographical localities making it a true cosmopolitan species. The ‘Everything is Everywhere’ (EiE) hypothesis assumes that the geographic distribution of microscopic organisms is not limited by dispersal but by local environmental conditions, making them potentially cosmopolitan. In the present work we report four new populations of P. fairbanksi from the Northern Hemisphere which suggests that the ‘EiE’ hypothesis is true, at least for some tardigrade species. We also compared all known populations of P. fairbanksi at the genetic and morphological levels. The p-distances between COI haplotypes of all sequenced P. fairbanksi populations from Albania, Antarctica, Canada, Italy, Madeira, Mongolia, Spain, USA and Poland ranged from 0.002 to 0.005%. In total, twelve haplotypes (H1-H12) of COI gene fragments were identified. We also report statistically significant morphometrical differences of species even though they were cultured and bred in the same laboratory conditions. Furthermore, we also discuss differences in the potential distribution of two Paramacrobiotus species.
Subject terms: Biodiversity, Biogeography, Molecular ecology
Introduction
The Phylum Tardigrada currently consists of ca. 1500 species1 that inhabit terrestrial and aquatic environments throughout the world2. Currently there are 33 families, 159 genera, 1464 species and 21 additional subspecies within this phylum1. Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf, 20103 was described from Alaska (USA) and reported from the Antarctic, Italy, Poland and Spain4 (reported as Macrobiotus richtersi Murray, 19115)6–9. It is a large-size (up to 800 μm) parthenogenetic Paramacrobiotus found mostly in mosses and can be shortly characterized by white or transparent cuticle without pores, three bands of teeth in the oral cavity, three macroplacoids and a microplacoid in pharynx (richtersi group), smooth lunules under all claws, granulation on all legs, and eggs with reticulated conical processes without caps or spines. Paramacrobiotus fairbanksi is a triploid species8 inhabiting various locations throughout the globe. The species is an omnivore, i.e., it feeds on algae, cyanobacteria, fungi, nematodes and rotifer10. However, dietary preferences have been observed to differ between juveniles and adults (juveniles prefer green alga and adults favour rotifers and nematodes10).
The ‘Everything is Everywhere’ hypothesis, which was proposed at the beginning of the twentieth century11,12 suggests that microorganisms and small invertebrate are not dispersal-limited on large geographical scales and have potentially cosmopolitan distribution. Microscopic organisms are often considered cosmopolitan species, as, the presence of specific adaptations allows them to being disperse easily. These adaptations include (a) the possibility of easy passive dispersion (by wind, rivers, sea currents, other animals, etc.), (b) the presence of very resistant spore stages (which include spores, cysts, eggs or cryptobiotic individuals) that help to survive extreme conditions, and (c) the presence of asexual or parthenogenetic reproduction, allowing for rapid increase in the number of individuals12–16. Cosmopolitism was strongly suggested for many tardigrade species in the past, however, the suggestion was later undermined (e.g., refs.17–19). At present, we have strong and compelling evidence of a wide distribution of some tardigrade taxa, which means that we return to the concept of cosmopolitism of at least some species of tardigrades (e.g., refs.8,9,20–22) which can support the hypothesis ‘Everything is Everywhere’ (EiE) for tardigrades. According to Gąsiorek et al.21, “a species may be termed as cosmopolitan if it was recorded in more than one zoogeographic realm”. There are 19 tardigrade species known from more than one zoogeographic realm, i.e., Cornechiniscus madagascariensis23 Maucci, 1993, Echiniscus africanus24 Murray, 1907, E. baius25 Marcus, 192826, E. blumi27 Richters, 1903, E. cavagnaroi28 Schuster & Grigarick, 1966, E. lichenorum29 Maucci, 1983, E. lineatus30 Pilato, Fontoura, Lisi & Beasley, 200831, E. marginatus32 Binda & Pilato, 1994, E. merokensis Richters, 190426, E. perarmatus Murray, 190724, E. pusae25 Marcus, 1928, E. scabrospinosus33 Fontoura, 1982, E. testudo34 (Doyère, 1840), Milnesium inceptum35 Morek, Suzuki, Schill, Georgiev, Yankova, Marley & Michalczyk, 2019, Nebularmis cirinoi36 (Binda & Pilato, 1993)28, P. gadabouti22 Kayastha, Stec, Mioduchowska and Kaczmarek. 2023 and P. fairbanksi, Pseudechiniscus (Meridioniscus) wallacei26 Vončina, Gąsiorek, Morek & Michalczyk, 2022, and Viridiscus viridissimus37 (Péterfi, 1956)38 (including V. miraviridis which was concluded as junior synonym for V. viridissimus by Gąsiorek et al.38). Furthermore, two parthenogenetic species from the genus Paramacrobiotus, i.e., P. fairbanksi and P. gadabouti, are contenders as they show a wide distribution that supports the hypothesis EiE.
In the present paper we compare morphological and genetic variability of different populations of P. fairbanksi from all known localities of this species in Albania, the Antarctica, Canada, Italy, Mongolia, Poland, Portugal (Madeira) and the USA. We also discuss genetic and morphological differences between them and consider the general distribution of P. fairbanksi.
Materials and methods
Sample processing
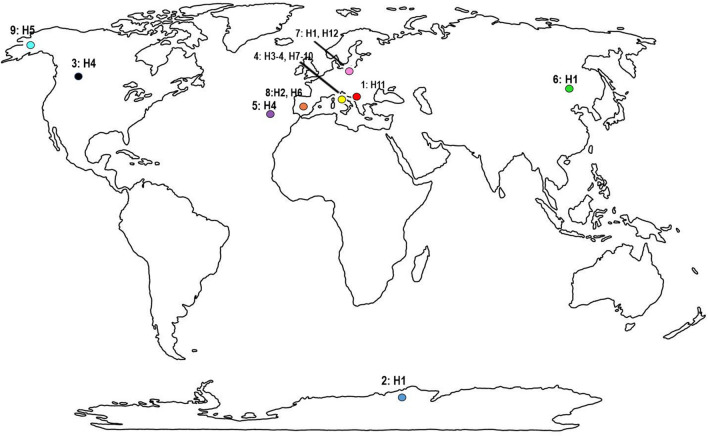
Four moss samples from trees and rocks were collected in 2018 (Mongolia) and 2019 (Albania, Canada and Madeira) (for details, see Table 1, Fig. 1). The samples were packed in paper envelopes, dried at room temperature and delivered to the laboratory at the Faculty of Biology, Adam Mickiewicz University in Poznań, Poland. Tardigrades were extracted from the samples and studied following the protocol of Stec et al.39. The moss samples (Alb, CN8, M85 and MN01) were dried post extractions and were deposited at the Department of Animal Taxonomy and Ecology, Institute of Environmental Biology, Adam Mickiewicz University, Poznań, Uniwersytetu Poznańskiego 6, 61–614 Poznań, Poland. Additionally, we used morphometric and genetic data of P. fairbanksi populations from the Antarctic, Italy, Spain, the USA and Poland9.
Table 1.
Studied populations of Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf, 20103 (see also Fig. 1).
| Sample no | Coordinates | Locality and sample description | Remarks |
|---|---|---|---|
| 1 | 41°19′ 36″ N, 19°49′ 08″ E; 112 m asl | Albania, Tirana County, Tirana, near Bunk’Art 2; moss on tree | Present study |
| 2 | ca. 67°39′ S, 46°09′ E; 0 m asl | Antarctic, near Vechernia Mt Base; moss (Ceratodon purpureus40 (Hedw.) Brid., 1826) | Kaczmarek et al.9 |
| 3 | 51°24′ 21″ N, 116°14′ 27″ W; 1900 m asl |
Canada, Alberta, Banff National Park, near east end of the Louise Lake; moss on stone |
Present study |
| 4 | ca. 44°26′ N, 10°51′ E; 510 m asl | Riccò, Modena Province, Italy; beech leaf litter | Kaczmarek et al.9 |
| 5 | 32°44′ 37.3″ N, 16°54′ 14.4″ W; 710 m asl | Portugal, Madeira, Ribera de Brava; moss on rock | Present study |
| 6 | 47°49′ 57.0″ N, 107°31′ 26.8″ E; 1 432 m asl | Mongolia, Töv Province; moss on rocky hill | Roszkowska et al.41 |
| 7 | 50°03′ 44″ N, 19°57′ 26″ E; 205 m asl | Poland, Lesser Poland Province, Kraków, Jagiellonian University Botanical Garden, Kopernika 27 street; moss on tree | Kaczmarek et al.9 |
| 8 | 40°52′ 42″ N, 03°50′ 45″ W; 1382 m asl | Spain, Madrid; litter, oaks | Guil and Giribet6 |
| 9 | ca. 64°50′ N, 147°43′ E; 135 m asl | USA, Alaska, Fairbanks; moss | Kaczmarek et al.9 |
Figure 1.
A World map with indicated sample number from Table 1 along with haplotypes of Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf 20103 found in different localities (see also Fig. 8). The world map is from https://www.wpmap.org/blank-world-map-with-antarctica/blank-world-map-jpg/ and the figure was prepared in Corel Photo-Paint 2021.
Culture procedure
Specimens of the populations from Albania, Canada, Madeira and Mongolia were cultured in the Department of Animal Taxonomy and Ecology (Faculty of Biology, Adam Mickiewicz University in Poznań) according to the protocol described by Roszkowska et al.41. In summary, tardigrades were cultured in small Petri dishes in spring water mixed with distilled water (1:3) with the rotifers and nematodes added as food ad libitum. All cultures were kept in the environmental chamber at a temperature of 18 °C and in darkness.
Microscopy, morphometrics and morphological nomenclature
Specimens were extracted from cultures and prepared for light microscopy analysis. They were mounted on microscope slides in a small drop of Hoyer’s medium and secured with a cover slip42,43. Slides were then placed in an incubator and dried for two days at ca. 60 °C. Dried slides were sealed with transparent nail polish and examined under an Olympus BX41.
All measurements are given in micrometers [μm]. Structures were measured only if their orientation was suitable. Body length was measured from the anterior extremity to the end of the body, excluding the hind legs. Buccal tubes, claws and eggs were measured according to Kaczmarek and Michalczyk44. Macroplacoid length sequence is given according to Kaczmarek et al.45. The pt ratio is the ratio of the length of a given structure to the length of the buccal tube, expressed as a percentage46. The pt values always provided in italics. Morphometric data were handled using the “Parachela” ver. 1.8 template available from the Tardigrada Register47. Tardigrade taxonomy follows Bertolani et al.48.
Genotyping
Before genomic DNA extraction, each specimen of P. fairbanksi was identified in vivo using light microscopy (LM). To obtain voucher specimens, DNA extractions were made from individuals using a Chelex® 100 resin (Bio-Rad) extraction method provided by Casquet et al.49 with modifications described in Stec et al.39. We sequenced three molecular markers, which differ in effective mutation rates: two nuclear fragments (18S rRNA and 28S rRNA) and one mitochondrial fragment (COI). All DNA fragments were amplified according to the protocols described in Kaczmarek et al.9, with primers listed in Table 2. Alkaline phosphatase FastAP (1 U/μl, Thermo Scientific) and exonuclease I (20 U/μl, Thermo Scientific) were used to clean the PCR products. Sequencing in both directions was carried out using the BigDyeTM terminator cycle sequencing method and ABI Prism 3130xl Genetic Analyzer (Life Technologies).
Table 2.
Primers with their original references used for sequencing of three molecular markers of Paramacrobiotus fairbanksi.
| DNA molecular marker | Primer name and direction | Primer sequence (5′–3′) | Source |
|---|---|---|---|
| COI | LCO1490 (forward) | GGTCAACAAATCATAAAGATATTGG | Folmer et al.50 |
| HCO2198 (reverse) | TAAACTTCAGGGTGACCAAAAAATCA | ||
| 18S rRNA | SSU01_F (forward) | AACCTGGTTGATCCTGCCAGT | Sands et al.51 |
| SSU82_R (reverse) | TGATCCTTCTGCAGGTTCACCTAC | ||
| 28S rRNA | 28SF0001 (forward) | ACCCvCynAATTTAAGCATAT | Mironov et al.52 |
| 28SR0990 (reverse) | CCTTGGTCCGTGTTTCAAGAC |
Molecular data analysis
The amplified nuclear and mitochondrial barcode sequences were edited using the BioEdit software53. Comparison of obtained molecular markers with those deposited in GenBank and homology search were performed using Blast application (Basic Local Alignment Search Tool54). The COI haplotypes were generated using the DnaSP v5.10.01 program55 and were translated into amino acid sequences using the EMBOSS-TRANSEQ application56 to check for internal stop codons and indels. Then all sequences obtained in our study, and the sequences downloaded from the GenBank database as originating from P. fairbanksi, were aligned with ClustalW using default settings. Alignment sequences were trimmed to 689, 572 and 574 bp for 28S rRNA, 18S rRNA and COI barcodes, respectively. The calculation for the uncorrected pairwise distances (p-distances) was performed for COI sequences using the MEGA X57.
All obtained sequences have been deposited in GenBank (for the accession numbers please see Table 3). The slides prepared from exoskeleton/voucher after DNA extraction of P. fairbanksi were deposited at the Department of Animal Taxonomy and Ecology, Institute of Environmental Biology, Adam Mickiewicz University, Poznań, Uniwersytetu Poznańskiego 6, 61–614 Poznań, Poland.
Table 3.
GenBank accession numbers of sequences obtained in the present study along with the slide numbers of voucher specimens.
| Populations of Paramacrobiotus fairbanksi | GenBank accession number; bp long DNA molecules | Voucher numbers | ||
|---|---|---|---|---|
| COI mtDNA | 18S rRNA | 28S rRNA | ||
| Albania | ON911917-18; 623–678 | ON872386; 1480 | ON872380-81; 805 | Alb2/S, Alb3/S, Alb4/S |
| Canada | ON911919; 625 | ON872387; 1480 | ON872382; 793 | CN8.2/S |
| Madeira | ON911920-21; 678–679 | ON872388; 1547 | ON872383; 744 | M85.11/S, M85.12/S |
| Mongolia | ON911922-23; 687–689 | ON872389; 917 | ON872384-85; 694–711 | MNO101/S, MNO103/S |
Reconstruction of genetic relationships among COI haplotypes and genealogical connections was carried out using the Network 4.6.1.3 software (www.fluxuxengineering.com). The median-joining algorithm (MJ)58 and substitution rates with the weight of 3 for transitions and 1 for transversions (transition: transversion ratio (ti:tv)) were applied. The star contraction pre-processing was generated to delete all superfluous median vectors and links. Additionally, the maximum parsimony post-processing was calculated. In turn, signatures of population expansion, equilibrium or decline in P. fairbanksi were inferred from the neutrality tests calculation (Tajima D59 and Fu FS60, respectively) computed in the DnaSP v5.10.01 program and Arlequin v.3.5. software61. Analyses were performed with 1000 replicates.
Statistical analysis
We used the Analysis of Variance (ANOVA) test with post hoc comparison of pairs of measurements, applying Benjamini–Hochberg correction to statistically analyze the differences in morphometrics between different populations of P. fairbanksi. Measurements of the body and buccal tube length (BL and BTL, respectively) were used as the dependent and the populations as grouping variables. Normal distribution in residuals was checked using the Shapiro test. Other morphometric traits, i.e., stylet support insertion points (SSIP), external width of buccal tube (BTEW) and placoids (M1—macroplacoid 1, M2—macroplacoid 2, M3—macroplacoid 3, Mi—microplacoid, MR—macroplacoid row, PR—placoid row) were also analysed. All the analyses were performed in R 4.1.362. The level of statistical significance was considered at p < 0.05. Principal Component Analysis (PCA) was performed using the R script from Stec et al.63. The analysis was performed for data from eggs and animals. For animals, both absolute values (raw measurements in μm) (BLm, BTLm, SSIPm, BTEWm, M1m, M2m, M3m, Mim, MRm and PRm) and relative pt values (BLpt, SSIPpt, BTEWpt, M1pt, M2pt, M3pt, Mipt, MRpt and PRpt) were used. For eggs, absolute values (raw measurements in μm) were used. All analyses were carried out using the R software program48. The “imputePCA” function of the R package “missMDA ver. 1.17” was used to impute missing data in the animal data set using the PCA imputation technique64. Cross-validation (function “estim_ncpPCA”) was used to determine the number of components utilized to impute the missing data. The PCA function of the software “FactoMineR ver. 2.3”65 was used to perform PCAs on the scaled data. The software “ggplot2 ver. 3.3.2”, “plyr ver. 1.8.6”, and “gridExtra ver. 2.3” were used to depict PCAs66,67. The presence of a structure in the PCA data was tested using a randomization approach on the eigenvalues and statistics according to Björklund68 and an in-house R script developed by MV in Stec et al.63. PERMANOVA was done on the PCs with the R packages “vegan ver. 2.5.6” and “pairwiseAdonis ver. 0.3”69, with the species hypothesis generated by phylogenetic techniques as the independent variable. Using the Benjamini–Hochberg correction, the level for multiple post hoc comparisons was adjusted independently for adults and eggs70. In total, 106 tardigrade specimens (16 Albanian, 16 Antarctic, 17 Canadian, 15 Madeiran, 14 Mongolian, 15 Polish, 4 Italian and 9 Alaskan) were measured and later used in the analyses for animals. Furthermore, differences in egg morphology between populations were studied and tested using ANOVA. Egg bare diameter (EBD), full diameter (EFD) and processes height (PH) were characters for the populations used as the dependent variable to determine compared groups and Benjamini-Hochberg corrections. In total, 100 tardigrade eggs (15 Albanian, 16 Antarctic, 15 Canadian, 15 Madeiran, 6 Mongolian, 15 Polish and 18 Alaskan) were measured and used in the analyses. All the analyses were performed in R 4.1.0. The level of statistical significance was considered at p < 0.05.
Potential distribution of cosmopolitan P. fairbanksi and P. gadabouti
A map of the known distribution of P. fairbanksi populations was assembled in Corel Photo-Paint 2021.
An ecological niche modelling (ENM) approach was used to predict the current potential distribution of P. fairbanksi and P. gadabouti. The ENM was performed with the use of the MaxEnt software, ver. 3.2.0. ( https://biodiversityinformatics.amnh.org/open_source/maxent/). MaxEnt performs the model with the fewest possible occurrence data and takes presence-only (PO) data. The model generates models of habitat appropriateness by handling continuous and categorical variables using regularization parameters71,72. The raster package in R was used to extract climatic raster values, and for ENM evaluation, version 0.3.1 of ENMevaluate in R was used. The bioclimatic variables available in MERRAclim Dataset 19 were used as environmental variables for MaxEnt modelling. We used MERRAclim Dataset because it provides a global set of satellite-based bioclimatic variables that includes Antarctica, which is one of the localities for P. fairbanksi. The 19 global bioclimatic datasets from the 2000s at 5 arcminutes resolution (mean value)73 consist of temperature layers (BIO1-BIO11) and humidity layers (BIO12-BIO19). Using ENMTools74 correlations among environmental layers were tested for all 19 variables and correlation coefficients >|0.7| were removed. Based on correlation results, six bioclimatic variables with correlation coefficients <|0.7| were selected. The temperature layers are in degrees Celsius multiplied by 10 and the humidity layers are in kg of water/kg of air multiplied by 100,00073. The receiver operating characteristic (ROC) plot’s area under curve (AUC) was used to assess the model’s accuracy71. AUC describes the relationship between the proportion of correctly anticipated presences and the proportion of absences of mistakenly-projected species in the model75. The AUC gauges the effectiveness of the model with a value between 0 and 1. Furthermore, AUC values > 0.9 indicate excellent accuracy, 0.7 to 0.9 indicate good accuracy, and values below 0.7 indicate low accuracy71,76,77. The jackknife test was used to estimate the model’s variable relevance. Later, the MaxEnt results were masked to the landmasses using shapefile from https://www.naturalearthdata.com/downloads/10m-physical-vectors/10m-land/. The localities for P. fairbanksi are from Table 1 and P. gadabouti from Kayastha et al.22. The coordinate list is provided in SM.01 and the R script for ENM in SM.02. The html MaxEnt output provided as SM.03a and SM.03b.
Ethics declarations
All procedures were conducted in accordance with the guidelines. Also, none of the moss samples were collected from the region which requires permission.
Results
Morphometric comparison of different P. fairbanksi populations
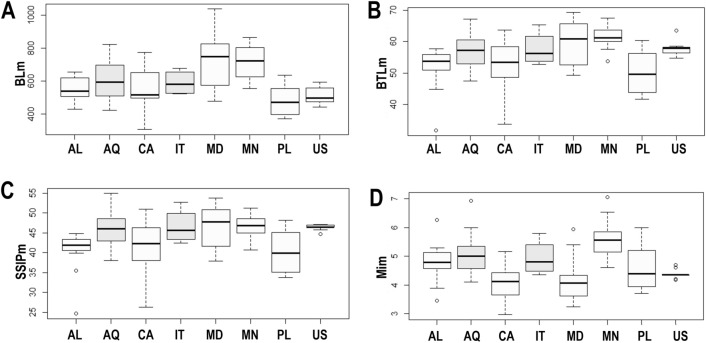
No significant differences were shown by the ANOVA test performed on BL between the studied populations (df = 7; F = 7.832; p = 0.902; N = 106; Tables 4, 5, 6, 7; Fig. 2A). However, significant differences were found on BTL between different populations (df = 7; F = 5.633; p = 0.010; N = 106; Tables 4, 5, 6, 7), where the buccal tube of the specimens from Mongolia was significantly longer than in specimens from the Albanian and Canadian populations (p = 0.001 and p = 0.001 respectively; Fig. 2B). The buccal tube of the specimens from Madeira was significantly longer than in specimens from the Polish population (p = 0.003; Fig. 2B). Analysis for SSIP length showed significant differences as well (df = 7; F = 4.812; p = 0.016; N = 106; Fig. 2C), with the specimens in the Polish population having significantly lower SSIP than in specimens from the Antarctic (p = 0.012), Madeiran (p = 0.012), and Mongolian (p = 0.012) populations. Analysis of Mi length showed significant differences as well (df = 7; F = 8.48; p = 0.020; N = 106; Tables 4, 5, 6, 7; Fig. 2D).
Table 4.
Measurements [in µm] and pt values of selected morphological structures of individuals of Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf 2010 Albanian population mounted in Hoyer’s medium (N—number of specimens/structures measured; RANGE refers to the smallest and the largest structure among all measured specimens; SD—standard deviation, pt—ratio of the length of a given structure to the length of the buccal tube expressed as a percentage).
| CHARACTER | N | RANGE | MEAN | SD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| µm | pt | µm | pt | µm | pt | ||||||
| Body length | 16 | 426 | – | 654 | – | 549 | 73 | ||||
| Buccopharyngeal tube | |||||||||||
| Buccal tube length | 16 | 31.7 | – | 57.8 | – | 52.1 | – | 6.4 | – | ||
| Stylet support insertion point | 15 | 24.6 | – | 44.8 | 76.1 | – | 80.8 | 40.7 | 78.7 | 5.0 | 1.4 |
| Buccal tube external width | 15 | 7.5 | – | 14.2 | 19.2 | – | 24.6 | 11.1 | 21.4 | 1.8 | 1.8 |
| Buccal tube internal width | 15 | 5.5 | – | 10.9 | 16.1 | – | 19.8 | 9.0 | 17.2 | 1.3 | 1.0 |
| Ventral lamina length | 14 | 18.2 | – | 34.1 | 57.4 | – | 61.5 | 30.3 | 58.7 | 4.1 | 1.3 |
| Placoid lengths | |||||||||||
| Macroplacoid 1 | 15 | 6.1 | – | 9.6 | 13.5 | – | 16.8 | 8.2 | 15.3 | 1.0 | 1.1 |
| Macroplacoid 2 | 16 | 3.8 | – | 8.3 | 11.0 | – | 14.5 | 6.6 | 12.7 | 1.1 | 0.9 |
| Macroplacoid 3 | 16 | 5.8 | – | 11.1 | 16.6 | – | 19.4 | 9.4 | 18.0 | 1.3 | 0.9 |
| Microplacoid | 16 | 3.5 | – | 6.3 | 8.3 | – | 10.9 | 4.8 | 9.2 | 0.6 | 0.9 |
| Macroplacoid row | 15 | 16.8 | – | 32.0 | 50.6 | – | 56.1 | 27.2 | 52.5 | 3.6 | 1.5 |
| Placoid row | 15 | 21.2 | – | 40.4 | 66.0 | – | 71.0 | 35.3 | 68.2 | 4.8 | 1.4 |
| Claw 1 heights | |||||||||||
| External primary branch | 16 | 10.1 | – | 16.0 | 23.4 | – | 31.9 | 13.9 | 26.9 | 1.6 | 2.2 |
| External secondary branch | 16 | 7.2 | – | 13.2 | 18.7 | – | 24.3 | 10.7 | 20.6 | 1.4 | 1.5 |
| Internal primary branch | 16 | 9.5 | – | 17.3 | 24.1 | – | 30.0 | 13.4 | 25.8 | 1.7 | 2.0 |
| Internal secondary branch | 16 | 6.6 | – | 13.7 | 18.5 | – | 23.6 | 10.6 | 20.4 | 1.6 | 1.4 |
| Claw 2 heights | |||||||||||
| External primary branch | 16 | 10.3 | – | 18.3 | 24.6 | – | 32.6 | 14.8 | 28.5 | 1.8 | 2.1 |
| External secondary branch | 16 | 8.2 | – | 13.3 | 19.8 | – | 25.8 | 11.5 | 22.2 | 1.4 | 1.4 |
| Internal primary branch | 16 | 9.8 | – | 16.6 | 23.9 | – | 31.1 | 13.5 | 26.1 | 1.6 | 1.8 |
| Internal secondary branch | 15 | 8.2 | – | 13.8 | 16.7 | – | 25.9 | 10.7 | 20.7 | 1.6 | 2.3 |
| Claw 3 heights | |||||||||||
| External primary branch | 16 | 11.5 | – | 19.0 | 27.9 | – | 36.5 | 15.4 | 29.7 | 1.6 | 2.2 |
| External secondary branch | 16 | 8.9 | – | 14.3 | 20.1 | – | 28.1 | 11.8 | 22.7 | 1.4 | 2.3 |
| Internal primary branch | 16 | 9.7 | – | 15.9 | 23.7 | – | 30.7 | 13.5 | 26.0 | 1.5 | 1.7 |
| Internal secondary branch | 15 | 7.7 | – | 13.1 | 18.2 | – | 24.2 | 10.9 | 21.0 | 1.3 | 1.6 |
| Claw 4 heights | |||||||||||
| Anterior primary branch | 16 | 11.5 | – | 20.0 | 26.3 | – | 36.3 | 15.8 | 30.4 | 1.9 | 2.5 |
| Anterior secondary branch | 16 | 8.3 | – | 14.4 | 20.0 | – | 26.1 | 11.9 | 22.9 | 1.7 | 1.8 |
| Posterior primary branch | 16 | 11.9 | – | 20.5 | 28.4 | – | 37.6 | 16.0 | 30.9 | 1.9 | 2.5 |
| Posterior secondary branch | 16 | 7.4 | – | 14.2 | 20.7 | – | 25.7 | 12.2 | 23.4 | 1.6 | 1.3 |
pt values are in italics.
Table 5.
Measurements [in µm] and pt values of selected morphological structures of individuals of Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf 2010 Canadian population mounted in Hoyer’s medium (N—number of specimens/structures measured; RANGE refers to the smallest and the largest structure among all measured specimens; SD—standard deviation, pt—ratio of the length of a given structure to the length of the buccal tube expressed as a percentage).
| CHARACTER | N | RANGE | MEAN | SD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| µm | pt | µm | pt | µm | pt | ||||||
| Body length | 17 | 303 | – | 772 | – | 564 | 133 | ||||
| Buccopharyngeal tube | |||||||||||
| Buccal tube length | 17 | 33.8 | – | 63.7 | – | 52.7 | – | 8.6 | – | ||
| Stylet support insertion point | 17 | 26.3 | – | 51.0 | 75.3 | – | 80.0 | 41.2 | 78.3 | 6.8 | 1.2 |
| Buccal tube external width | 17 | 6.2 | – | 13.7 | 15.4 | – | 23.8 | 11.3 | 21.3 | 2.3 | 1.9 |
| Buccal tube internal width | 17 | 4.5 | – | 11.4 | 11.0 | – | 18.1 | 8.2 | 15.4 | 1.9 | 1.8 |
| Ventral lamina length | 15 | 24.5 | – | 38.3 | 56.4 | – | 64.7 | 32.6 | 60.4 | 4.9 | 2.6 |
| Placoid lengths | |||||||||||
| Macroplacoid 1 | 17 | 4.7 | – | 11.7 | 12.5 | – | 19.9 | 8.9 | 16.7 | 2.1 | 1.8 |
| Macroplacoid 2 | 17 | 3.9 | – | 10.4 | 11.2 | – | 16.8 | 7.8 | 14.6 | 1.9 | 1.6 |
| Macroplacoid 3 | 17 | 5.0 | – | 12.1 | 14.9 | – | 20.9 | 9.7 | 18.2 | 2.0 | 1.6 |
| Microplacoid | 17 | 3.0 | – | 5.2 | 6.2 | – | 9.7 | 4.1 | 7.8 | 0.6 | 0.9 |
| Macroplacoid row | 16 | 21.5 | – | 36.0 | 51.8 | – | 59.5 | 30.2 | 55.9 | 4.8 | 2.3 |
| Placoid row | 17 | 23.6 | – | 46.8 | 68.5 | – | 75.8 | 37.8 | 71.6 | 6.9 | 2.4 |
| Claw 1 heights | |||||||||||
| External primary branch | 16 | 8.2 | – | 17.9 | 21.9 | – | 30.7 | 14.4 | 27.4 | 2.9 | 2.4 |
| External secondary branch | 16 | 5.5 | – | 14.8 | 16.2 | – | 25.2 | 10.9 | 20.6 | 2.3 | 1.9 |
| Internal primary branch | 16 | 8.4 | – | 16.1 | 20.7 | – | 29.0 | 13.2 | 25.2 | 2.5 | 2.2 |
| Internal secondary branch | 16 | 6.9 | – | 13.9 | 17.1 | – | 24.1 | 10.9 | 20.8 | 2.0 | 2.1 |
| Claw 2 heights | |||||||||||
| External primary branch | 17 | 9.2 | – | 18.1 | 22.7 | – | 30.3 | 14.6 | 27.6 | 3.0 | 2.7 |
| External secondary branch | 16 | 7.2 | – | 15.5 | 17.7 | – | 26.4 | 11.9 | 22.4 | 2.6 | 2.2 |
| Internal primary branch | 17 | 8.0 | – | 16.2 | 20.9 | – | 27.7 | 13.3 | 25.3 | 2.3 | 1.9 |
| Internal secondary branch | 17 | 6.9 | – | 13.8 | 19.0 | – | 23.6 | 11.1 | 21.1 | 2.1 | 1.3 |
| Claw 3 heights | |||||||||||
| External primary branch | 17 | 9.0 | – | 18.6 | 20.4 | – | 31.9 | 14.9 | 28.3 | 3.1 | 3.2 |
| External secondary branch | 17 | 7.1 | – | 14.4 | 17.0 | – | 25.9 | 11.7 | 22.0 | 2.5 | 2.4 |
| Internal primary branch | 17 | 8.5 | – | 18.3 | 23.0 | – | 31.5 | 14.2 | 26.9 | 2.7 | 2.0 |
| Internal secondary branch | 17 | 7.3 | – | 14.6 | 17.9 | – | 24.9 | 11.8 | 22.4 | 2.1 | 1.7 |
| Claw 4 heights | |||||||||||
| Anterior primary branch | 17 | 10.2 | – | 19.2 | 25.1 | – | 34.6 | 15.9 | 30.3 | 2.5 | 2.5 |
| Anterior secondary branch | 17 | 6.6 | – | 14.6 | 16.3 | – | 25.6 | 11.9 | 22.5 | 2.3 | 2.1 |
| Posterior primary branch | 17 | 10.9 | – | 21.2 | 27.1 | – | 36.2 | 16.3 | 31.1 | 2.8 | 2.6 |
| Posterior secondary branch | 17 | 7.8 | – | 15.0 | 20.4 | – | 27.8 | 12.4 | 23.5 | 2.0 | 1.9 |
pt values are in italics.
Table 6.
Measurements [in µm] and pt values of selected morphological structures of individuals of Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf 2010 Madeira population mounted in Hoyer’s medium (N—number of specimens/structures measured; RANGE refers to the smallest and the largest structure among all measured specimens; SD—standard deviation, pt—ratio of the length of a given structure to the length of the buccal tube expressed as a percentage).
| Character | N | RANGE | MEAN | SD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| µm | pt | µm | pt | µm | pt | ||||||
| Body length | 15 | 476 | – | 1036 | – | 714 | 174 | ||||
| Buccopharyngeal tube | |||||||||||
| Buccal tube length | 15 | 49.4 | – | 69.3 | – | 59.3 | – | 7.2 | – | ||
| Stylet support insertion point | 15 | 37.9 | – | 53.8 | 76.0 | – | 81.4 | 46.4 | 78.2 | 5.6 | 1.5 |
| Buccal tube external width | 15 | 10.4 | – | 16.5 | 21.0 | – | 25.9 | 13.8 | 23.2 | 2.2 | 1.6 |
| Buccal tube internal width | 15 | 7.0 | – | 12.2 | 14.1 | – | 19.1 | 9.8 | 16.4 | 1.9 | 1.5 |
| Ventral lamina length | 13 | 29.5 | – | 39.6 | 55.4 | – | 60.8 | 34.7 | 58.7 | 3.7 | 1.6 |
| Placoid lengths | |||||||||||
| Macroplacoid 1 | 15 | 8.3 | – | 14.1 | 16.7 | – | 21.8 | 11.5 | 19.3 | 2.2 | 1.6 |
| Macroplacoid 2 | 15 | 7.6 | – | 13.2 | 14.9 | – | 19.1 | 10.1 | 16.9 | 1.8 | 1.3 |
| Macroplacoid 3 | 15 | 8.6 | – | 16.5 | 16.7 | – | 24.0 | 13.1 | 21.8 | 2.6 | 2.1 |
| Microplacoid | 15 | 3.2 | – | 5.9 | 6.0 | – | 8.6 | 4.1 | 7.0 | 0.8 | 0.8 |
| Macroplacoid row | 15 | 31.0 | – | 47.5 | 60.1 | – | 71.7 | 38.8 | 65.2 | 6.1 | 2.9 |
| Placoid row | 15 | 40.4 | – | 60.1 | 78.8 | – | 86.8 | 49.6 | 83.4 | 7.4 | 2.8 |
| Claw 1 heights | |||||||||||
| External primary branch | 15 | 13.8 | – | 21.8 | 27.9 | – | 31.9 | 17.8 | 30.0 | 2.5 | 1.5 |
| External secondary branch | 15 | 9.5 | – | 15.7 | 18.5 | – | 25.3 | 12.9 | 21.8 | 1.7 | 1.8 |
| Internal primary branch | 15 | 13.4 | – | 19.6 | 24.6 | – | 31.2 | 16.3 | 27.5 | 2.2 | 1.6 |
| Internal secondary branch | 15 | 10.0 | – | 16.0 | 18.0 | – | 24.7 | 13.1 | 22.1 | 2.1 | 1.8 |
| Claw 2 heights | |||||||||||
| External primary branch | 15 | 14.2 | – | 22.3 | 28.4 | – | 32.7 | 18.3 | 30.8 | 2.5 | 1.3 |
| External secondary branch | 15 | 11.8 | – | 17.4 | 22.4 | – | 26.9 | 14.6 | 24.6 | 1.9 | 1.5 |
| Internal primary branch | 15 | 13.9 | – | 20.4 | 26.5 | – | 31.8 | 16.9 | 28.5 | 2.3 | 1.5 |
| Internal secondary branch | 15 | 10.2 | – | 17.6 | 20.6 | – | 25.8 | 13.6 | 22.9 | 2.2 | 1.7 |
| Claw 3 heights | |||||||||||
| External primary branch | 15 | 14.9 | – | 21.4 | 24.4 | – | 32.9 | 17.8 | 30.1 | 2.1 | 2.2 |
| External secondary branch | 15 | 11.6 | – | 16.5 | 20.1 | – | 27.7 | 14.3 | 24.3 | 1.7 | 2.0 |
| Internal primary branch | 15 | 14.0 | – | 21.2 | 25.5 | – | 33.1 | 17.3 | 29.2 | 2.5 | 1.9 |
| Internal secondary branch | 15 | 11.0 | – | 21.0 | 21.0 | – | 30.7 | 14.2 | 23.8 | 2.7 | 2.3 |
| Claw 4 heights | |||||||||||
| Anterior primary branch | 15 | 16.7 | – | 23.2 | 31.7 | – | 37.6 | 20.1 | 34.0 | 2.4 | 1.7 |
| Anterior secondary branch | 15 | 12.2 | – | 18.6 | 22.9 | – | 29.5 | 15.5 | 26.2 | 1.8 | 1.9 |
| Posterior primary branch | 15 | 14.6 | – | 22.2 | 28.8 | – | 35.5 | 19.0 | 32.1 | 2.7 | 1.6 |
| Posterior secondary branch | 15 | 10.3 | – | 18.1 | 20.4 | – | 26.2 | 14.6 | 24.5 | 2.4 | 1.5 |
pt values are in italics.
Table 7.
Measurements [in µm] and pt values of selected morphological structures of individuals of Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf 2010 Mongolian population mounted in Hoyer’s medium (N—number of specimens/structures measured; RANGE refers to the smallest and the largest structure among all measured specimens; SD—standard deviation, pt—ratio of the length of a given structure to the length of the buccal tube expressed as a percentage).
| CHARACTER | N | RANGE | MEAN | SD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| µm | pt | µm | pt | µm | pt | ||||||
| Body length | 14 | 553 | – | 864 | – | 717 | 104 | ||||
| Buccopharyngeal tube | |||||||||||
| Buccal tube length | 14 | 53.7 | – | 67.6 | – | 61.5 | – | 3.6 | – | ||
| Stylet support insertion point | 13 | 40.7 | – | 51.2 | 73.4 | – | 78.8 | 46.9 | 76.9 | 2.8 | 1.4 |
| Buccal tube external width | 14 | 12.3 | – | 18.1 | 20.1 | – | 26.8 | 13.7 | 22.2 | 1.6 | 1.9 |
| Buccal tube internal width | 14 | 9.0 | – | 14.8 | 14.3 | – | 21.9 | 10.5 | 17.0 | 1.4 | 1.7 |
| Ventral lamina length | 11 | 36.0 | – | 43.1 | 60.0 | – | 65.8 | 39.0 | 62.9 | 2.2 | 1.8 |
| Placoid lengths | |||||||||||
| Macroplacoid 1 | 14 | 10.4 | – | 13.1 | 16.2 | – | 21.8 | 11.4 | 18.6 | 0.9 | 1.5 |
| Macroplacoid 2 | 14 | 8.7 | – | 11.2 | 14.1 | – | 18.8 | 10.0 | 16.3 | 0.8 | 1.4 |
| Macroplacoid 3 | 14 | 11.0 | – | 15.0 | 17.5 | – | 25.0 | 12.7 | 20.8 | 1.1 | 1.8 |
| Microplacoid | 14 | 4.6 | – | 7.1 | 6.8 | – | 10.9 | 5.6 | 9.1 | 0.7 | 1.0 |
| Macroplacoid row | 14 | 33.2 | – | 41.4 | 56.6 | – | 64.9 | 37.0 | 60.2 | 2.0 | 2.5 |
| Placoid row | 14 | 43.9 | – | 54.8 | 74.1 | – | 84.6 | 49.4 | 80.5 | 2.6 | 3.1 |
| Claw 1 heights | |||||||||||
| External primary branch | 14 | 15.1 | – | 18.1 | 23.7 | – | 30.1 | 16.8 | 27.3 | 1.0 | 1.9 |
| External secondary branch | 14 | 11.7 | – | 15.2 | 18.7 | – | 24.3 | 13.6 | 22.2 | 1.1 | 1.9 |
| Internal primary branch | 14 | 14.8 | – | 18.2 | 23.6 | – | 29.9 | 16.5 | 27.0 | 1.1 | 2.2 |
| Internal secondary branch | 14 | 11.6 | – | 15.1 | 18.7 | – | 24.4 | 13.1 | 21.3 | 1.0 | 1.9 |
| Claw 2 heights | |||||||||||
| External primary branch | 14 | 16.2 | – | 21.0 | 26.2 | – | 35.1 | 18.0 | 29.4 | 1.3 | 2.5 |
| External secondary branch | 14 | 12.6 | – | 16.9 | 19.7 | – | 28.2 | 14.6 | 23.8 | 1.3 | 2.2 |
| Internal primary branch | 14 | 13.0 | – | 19.6 | 20.9 | – | 32.0 | 15.9 | 25.9 | 2.1 | 3.0 |
| Internal secondary branch | 14 | 9.6 | – | 15.0 | 15.5 | – | 24.6 | 13.0 | 21.2 | 1.7 | 2.3 |
| Claw 3 heights | |||||||||||
| External primary branch | 14 | 13.6 | – | 19.6 | 21.8 | – | 31.5 | 17.2 | 28.0 | 1.6 | 2.8 |
| External secondary branch | 14 | 10.7 | – | 16.4 | 18.5 | – | 25.2 | 13.4 | 21.7 | 1.7 | 2.3 |
| Internal primary branch | 14 | 13.6 | – | 18.0 | 21.8 | – | 30.8 | 16.5 | 26.8 | 1.4 | 2.2 |
| Internal secondary branch | 14 | 11.1 | – | 16.4 | 17.9 | – | 25.1 | 13.4 | 21.8 | 1.6 | 2.3 |
| Claw 4 heights | |||||||||||
| Anterior primary branch | 14 | 15.6 | – | 21.9 | 26.4 | – | 35.1 | 18.6 | 30.3 | 1.7 | 2.6 |
| Anterior secondary branch | 14 | 10.7 | – | 17.1 | 16.7 | – | 26.4 | 14.3 | 23.3 | 1.7 | 2.8 |
| Posterior primary branch | 14 | 14.5 | – | 22.8 | 23.6 | – | 34.5 | 18.5 | 30.1 | 2.0 | 2.9 |
| Posterior secondary branch | 14 | 11.0 | – | 17.5 | 18.5 | – | 28.5 | 14.2 | 23.1 | 2.2 | 3.3 |
pt values are in italics.
Figure 2.
(A) Differences in the body length (BLm); (B) differences in the buccal tube length (BTLm); (C) differences in the stylet support insertion point (SSIPm); (D) differences in the Microplacoid length (Mim). The studied populations of Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf 2010 are AL Albania; AQ Antarctic; CA Canada; IT Italy; MD Madeira; MN Mongolia; PL Poland; US USA. Minimum, maximum, median, first quartile and third quartile for each population are presented. All measurements are in micrometres [μm]. White boxplots represent cultured population and light grey boxplots represent wild population.
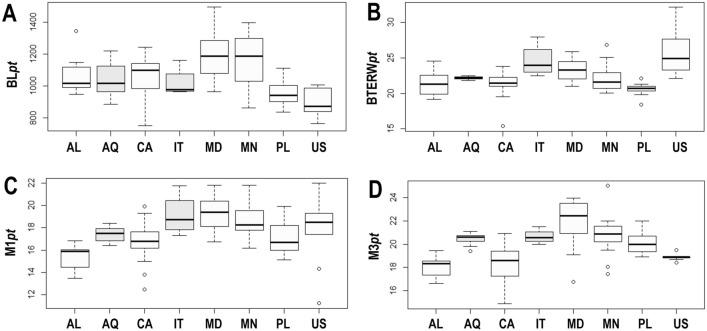
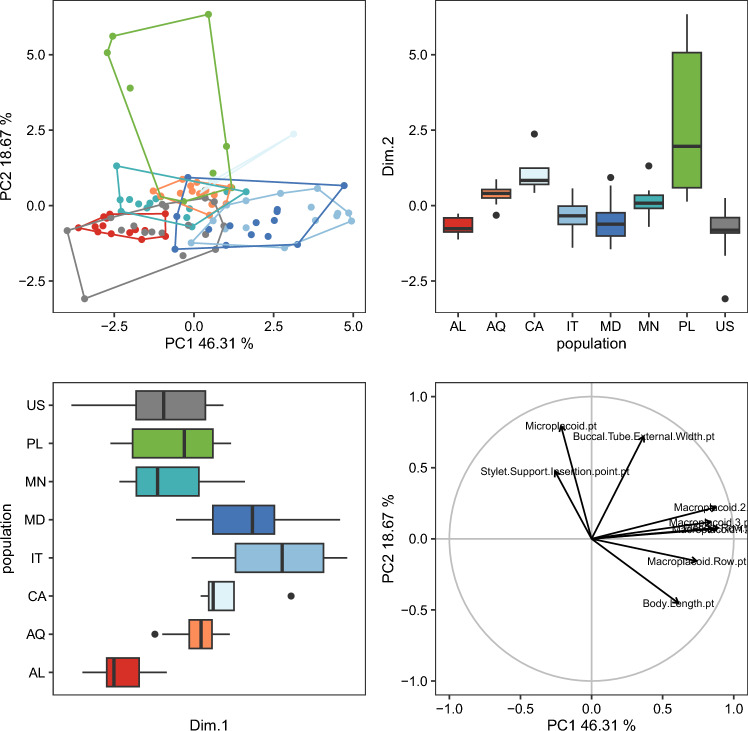
The ANOVA test showed, however, no statistical significance for pt of BL between populations (df = 7; F = 8.056; p = 0.678; N = 106; Tables 4, 5, 6, 7; Fig. 3A). The ANOVA test for pt values of the SSIP showed no statistically significant differences between studied populations (df = 7; F = 20.81; p = 0.112; N = 106; Tables 4, 5, 6, 7; Fig. 3A), whereas the ANOVA test for pt values of the BTEW showed statistically significant differences between studied populations (df = 7; F = 9.87; p = 0.001; N = 106; Tables 4, 5, 6, 7; Fig. 3B). The pt values specimens from the Italian population were higher than the Albanian population (p = 0.005), the Antarctic population (p = 0.035), the Canadian population (p = 0.004), the Mongolian population (p = 0.004) and the Polish population (p = 0.0005), while pt values of specimens from the Madeiran population were higher than the Albanian population (p = 0.014), the Canadian population (p = 0.009) and the Polish population (p = 0.0005), pt values of specimens from the Polish population were lower than the Antarctic population (p = 0.030) and pt values of specimens from the Alaskan population were higher than the Madeiran population (p = 0.002) (Fig. 3B). The ANOVA test for pt values of the M1 (df = 7; F = 8.38; p = 0.0.007; N = 106; Tables 4, 5, 6, 7; Fig. 3C) and M3 (df = 7; F = 14.53; p = 0.001; N = 106; Tables 4, 5, 6, 7; Fig. 3D) showed statistically significant differences between studied populations.
Figure 3.
(A) Differences in the pt of body length (BLpt); (B) differences in the pt of external width of buccal tube (BTEWpt); (C) differences in the pt of Macroplacoid 1 (M1pt); (D) differences in the pt of Macroplacoid 3 (M3pt). The studied populations of Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf 2010 are AL Albania, AQ Antarctic, CA Canada, IT Italy, MD Madeira, MN Mongolia, PL Poland, US USA. Minimum, maximum, median, first quartile and third quartile for each population are presented. White boxplots represent cultured population and light grey boxplots represent wild population.
The ANOVA performed on EFD measurements of eggs (df = 6; F = 22.92; p = 0.002; N = 100) showed significant differences between all the populations (Tables 8, 9, 10, 11). Eggs in the Polish population were significantly smaller than those from Antarctica (p = 0.007) and Canada (p = 0.0005), as well as, eggs from the Alaskan population were clearly smaller than those from Albania (p = 0.028), Antarctica (p = 0.014) and Canada (p = 0.008) (Fig. 4A). Analysis of EBD values, however, showed no statistically significant differences between eggs in different populations (df = 6; F = 9.192; p = 0.249; n = 100; Tables 8, 9, 10, 11; Fig. 4B). There were also no statistically significant differences between the studied populations (Tables 8, 9, 10, 11) in the size of egg processes (PH) (df = 6; F = 24.42; p = 0.260; n = 100; Fig. 4C).
Table 8.
Measurements [in µm] of selected morphological structures of eggs of Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf 2010 Albanian population mounted in Hoyer’s medium (N—number of specimens/structures measured, RANGE refers to the smallest and the largest structure among all measured eggs; SD—standard deviation).
| CHARACTER | N | RANGE | MEAN | SD | ||
|---|---|---|---|---|---|---|
| Egg bare diameter | 15 | 63.8 | – | 95.4 | 77.9 | 9.3 |
| Egg full diameter | 15 | 86.2 | – | 116.9 | 98.5 | 8.3 |
| Process height | 45 | 7.6 | – | 17.3 | 12.0 | 2.0 |
| Process base width | 45 | 9.7 | – | 20.3 | 15.5 | 2.2 |
| Process base/height ratio | 45 | 100% | – | 177% | 130% | 17% |
| Inter-process distance | 42 | 1.3 | – | 7.8 | 3.6 | 1.6 |
| Number of processes on the egg circumference | 15 | 13 | – | 16 | 14.1 | 1.1 |
Table 9.
Measurements [in µm] of selected morphological structures of eggs of Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf 2010 Canadian population mounted in Hoyer’s medium (N—number of specimens/structures measured, RANGE refers to the smallest and the largest structure among all measured eggs; SD—standard deviation).
| CHARACTER | N | RANGE | MEAN | SD | ||
|---|---|---|---|---|---|---|
| Egg bare diameter | 15 | 62.9 | – | 89.7 | 75.0 | 9.5 |
| Egg full diameter | 15 | 88.7 | – | 117.5 | 101.5 | 9.3 |
| Process height | 42 | 11.3 | – | 17.0 | 13.9 | 1.5 |
| Process base width | 42 | 13.6 | – | 18.9 | 15.9 | 1.2 |
| Process base/height ratio | 42 | 100% | – | 136% | 115% | 10% |
| Inter-process distance | 41 | 1.0 | – | 5.5 | 2.7 | 1.0 |
| Number of processes on the egg circumference | 14 | 10 | – | 12 | 10.8 | 0.8 |
Table 10.
Measurements [in µm] of selected morphological structures of eggs of Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf 2010 Madeira population mounted in Hoyer’s medium (N—number of specimens/structures measured, RANGE refers to the smallest and the largest structure among all measured eggs; SD—standard deviation).
| CHARACTER | N | RANGE | MEAN | SD | ||
|---|---|---|---|---|---|---|
| Egg bare diameter | 15 | 74.9 | – | 97.6 | 82.9 | 6.3 |
| Egg full diameter | 15 | 107.7 | – | 141.7 | 117.5 | 8.3 |
| Process height | 43 | 13.7 | – | 23.0 | 17.7 | 2.2 |
| Process base width | 43 | 15.1 | – | 23.8 | 18.7 | 2.0 |
| Process base/height ratio | 43 | 93% | – | 125% | 106% | 9% |
| Inter-process distance | 44 | 3.0 | – | 6.2 | 4.6 | 0.7 |
| Number of processes on the egg circumference | 15 | 11 | – | 14 | 12.4 | 0.9 |
Table 11.
Measurements [in µm] of selected morphological structures of eggs of Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf 2010 Mongolian population mounted in Hoyer’s medium (N—number of specimens/structures measured, RANGE refers to the smallest and the largest structure among all measured eggs; SD—standard deviation).
| CHARACTER | N | RANGE | MEAN | SD | ||
|---|---|---|---|---|---|---|
| Egg bare diameter | 6 | 64.0 | – | 83.5 | 73.0 | 8.0 |
| Egg full diameter | 6 | 87.4 | – | 112.4 | 96.8 | 9.5 |
| Process height | 16 | 11.0 | – | 16.9 | 14.2 | 1.9 |
| Process base width | 15 | 15.0 | – | 21.7 | 17.7 | 2.3 |
| Process base/height ratio | 15 | 114% | – | 137% | 124% | 7% |
| Inter-process distance | 12 | 2.2 | – | 3.8 | 3.1 | 0.5 |
| Number of processes on the egg circumference | 5 | 11 | – | 15 | 12.3 | 1.9 |
Figure 4.
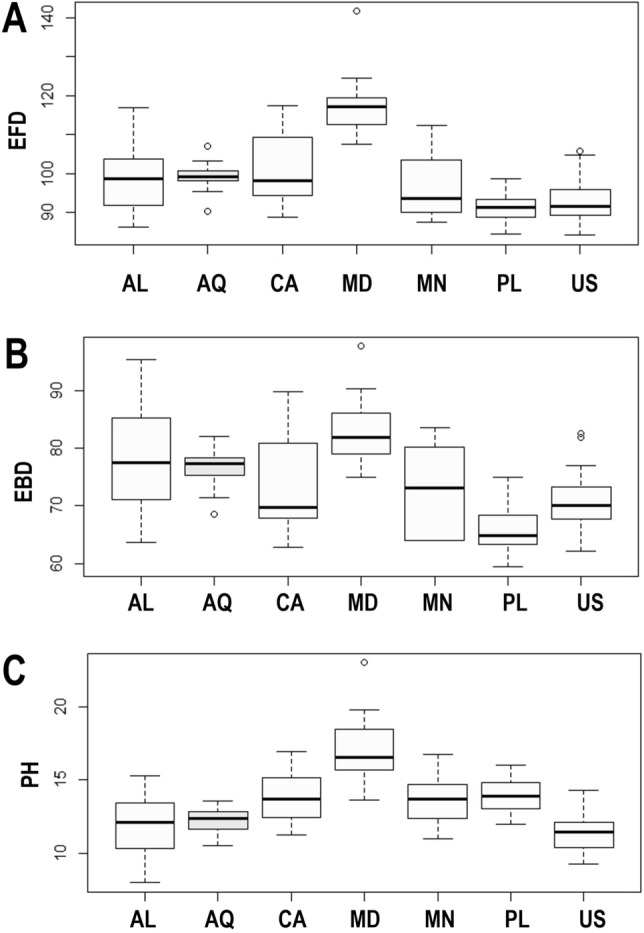
(A) Differences in the egg full diameter (EFD); (B) differences in the egg bare diameter (EBD); (C) differences in the egg processes height (PH). The studied populations of Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf 2010 are AL Albania; AQ Antarctic; CA Canada; MD Madeira; MN Mongolia; PL Poland; US USA. Minimum, maximum, median, first quartile and third quartile for each population are presented. All measurements are in micrometres [μm]. White boxplots represent cultured population and light grey boxplots represent wild population.
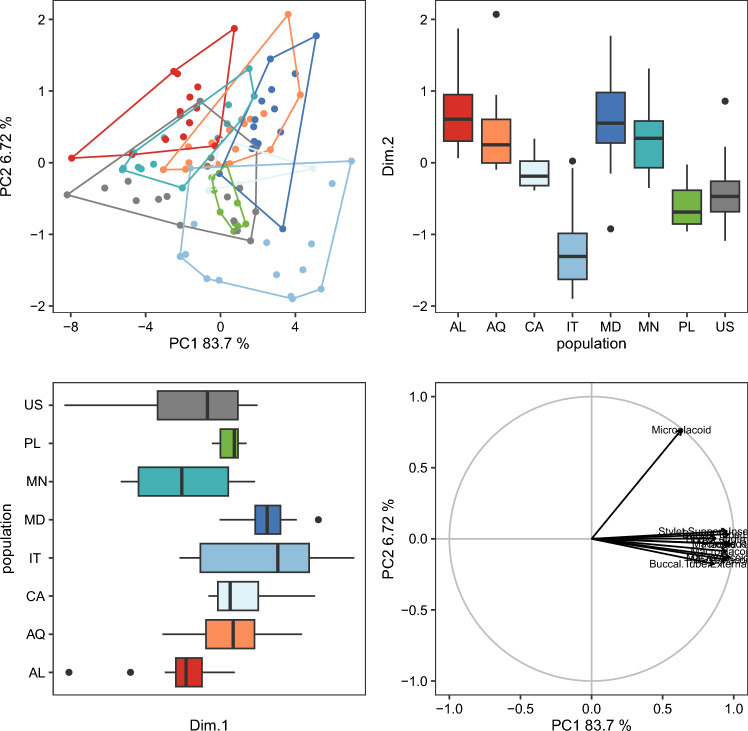
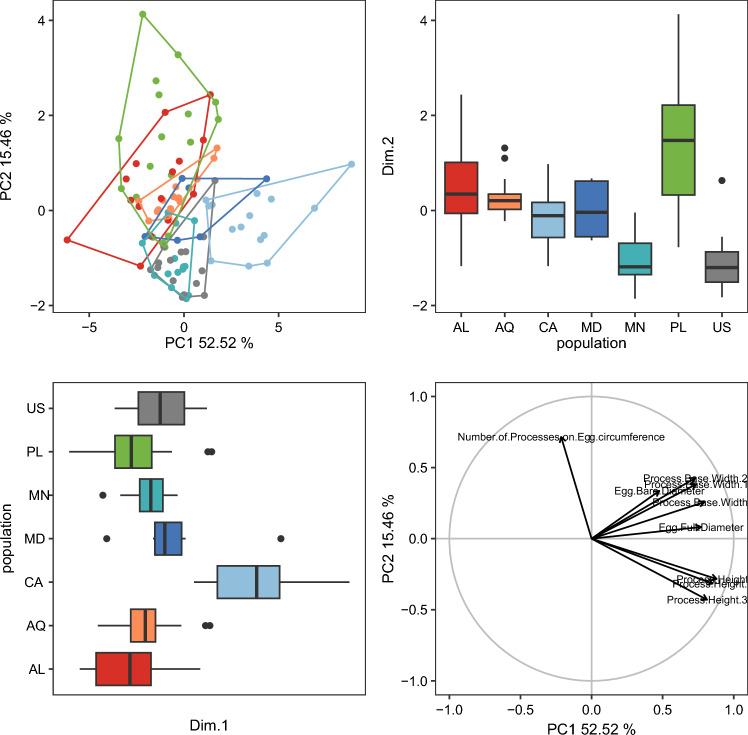
The randomization test in PCA demonstrated that only the first two PCs explained greater variation than the null model (no data structure) for both animal and egg datasets (SM.11). As a result, only the initial two PCs were maintained and used for additional investigation and interpretation. Furthermore, the ψ and ϕ statistics of the PCA were significantly distinct from what they anticipated under the null assumption (animals: ψ = 60.72 p < 0.001, ϕ = 0.82 p < 0.001; animals pt: ψ = 13.14 p < 0.001, ϕ = 0.43 p < 0.001; eggs: ψ = 17.62 p < 0.001, ϕ = 0.50 p < 0.001). The first two components of the PCA of animals’ absolute measured value (Fig. 5) explained 90% of the overall variation (83.7% for PC1 and 6.7% for PC2) and for animals’ pt indices (Fig. 6) explained 65% of the overall variation (46.3% for PC1 and 18.7% for PC2). PCA of egg measurements (Fig. 7) described 68% of the total variance with the first two components (52.5% for PC1 and 15.5% for PC2). PERMANOVA revealed that species identity has a substantial overall effect on PCs (p < 0.001, Tables 12, 13, 14). Raw morphometric data for all the populations in the present study are given in the Supplementary Materials (SM.04–SM.07). R script for single characters as well as measurement files for both adults and eggs, are provided in the Supplementary Materials (SM.08, SM.09a and SM.09b). All the test results from R are provided in Supplementary Materials (SM.10). Results of PCA randomization tests in the Supplementary Materials (SM.11).
Figure 5.
Results of PCA for animal measurements, 1st and 2nd Principal Components. Score scatterplots presented in top-left quadrants; boxplots of single component scores presented in top-right and bottom-left quadrants and loading plot presented in bottom-right.
Figure 6.
Results of PCA for animal pt indices, 1st and 2nd Principal Components. Score scatterplots presented in top-left quadrants; boxplots of single component scores presented in top-right and bottom-left quadrants and loading plot presented in bottom-right.
Figure 7.
Results of PCA for egg measurements, 1st and 2nd Principal Components. Score scatterplots presented in top-left quadrants; boxplots of single component scores presented in top-right and bottom-left quadrants and loading plot presented in bottom-right.
Table 12.
Results of PERMANOVA and post hoc pairwise PERMANOVA comparisons for the first two principal components (PC1 and PC2) of animal measured values; significant post hoc p-values adjusted with the Benjamini–Hochberg correction.
| Post hoc comparisons | df | SS | F | R2 | P |
|---|---|---|---|---|---|
| Poland vs Italy | 1 | 33.92 | 4.98 | 0.23 | 0.068 |
| Poland vs USA | 1 | 36.99 | 8.09 | 0.27 | 0.019 |
| Poland vs Antarctica | 1 | 49.69 | 8.77 | 0.23 | 0.017 |
| Poland vs Albania | 1 | 0.98 | 0.17 | 0.01 | 0.704 |
| Poland vs Canada | 1 | 5.39 | 0.65 | 0.02 | 0.485 |
| Poland vs Madeira | 1 | 144.31 | 16.56 | 0.37 | 0.003 |
| Poland vs Mongolia | 1 | 143.25 | 31.67 | 0.54 | 0.001 |
| Italy vs USA | 1 | 2.71 | 1.29 | 0.11 | 0.344 |
| Italy vs Antarctica | 1 | 2.50 | 0.52 | 0.03 | 0.502 |
| Italy vs Albania | 1 | 37.72 | 7.93 | 0.31 | 0.019 |
| Italy vs Canada | 1 | 26.61 | 2.96 | 0.13 | 0.145 |
| Italy vs Madeira | 1 | 6.09 | 0.62 | 0.04 | 0.496 |
| Italy vs Mongolia | 1 | 5.94 | 2.13 | 0.12 | 0.207 |
| USA vs Antarctica | 1 | 6.00 | 1.92 | 0.08 | 0.214 |
| USA vs Albania | 1 | 44.90 | 14.65 | 0.39 | 0.002 |
| USA vs Canada | 1 | 23.65 | 3.64 | 0.13 | 0.098 |
| USA vs Madeira | 1 | 18.70 | 2.72 | 0.11 | 0.150 |
| USA vs Mongolia | 1 | 30.82 | 21.91 | 0.51 | 0.001 |
| Antarctica vs Albania | 1 | 55.76 | 12.46 | 0.29 | 0.003 |
| Antarctica vs Canada | 1 | 42.92 | 6.06 | 0.16 | 0.033 |
| Antarctica vs Madeira | 1 | 39.28 | 5.29 | 0.15 | 0.041 |
| Antarctica vs Mongolia | 1 | 27.43 | 8.23 | 0.23 | 0.017 |
| Albania vs Canada | 1 | 11.33 | 1.61 | 0.05 | 0.256 |
| Albania vs Madeira | 1 | 164.10 | 22.24 | 0.43 | 0.001 |
| Albania vs Mongolia | 1 | 153.60 | 46.73 | 0.63 | 0.001 |
| Canada vs Madeira | 1 | 115.28 | 11.55 | 0.28 | 0.005 |
| Canada vs Mongolia | 1 | 133.00 | 21.73 | 0.43 | 0.001 |
| Madeira vs Mongolia | 1 | 23.85 | 3.72 | 0.12 | 0.085 |
Table 13.
Results of PERMANOVA and post hoc pairwise PERMANOVA comparisons for the first two principal components (PC1 and PC2) of animal pt values; significant post hoc p-values adjusted with the Benjamini–Hochberg correction.
| Post hoc comparisons | df | SS | F | R2 | P |
|---|---|---|---|---|---|
| Poland vs Italy | 1 | 14.60 | 7.12 | 0.30 | 0.0147 |
| Poland vs USA | 1 | 39.29 | 9.12 | 0.29 | 0.0016 |
| Poland vs Antarctica | 1 | 6.80 | 5.60 | 0.16 | 0.0218 |
| Poland vs Albania | 1 | 23.07 | 17.70 | 0.38 | 0.0002 |
| Poland vs Canada | 1 | 7.41 | 3.11 | 0.09 | 0.0620 |
| Poland vs Madeira | 1 | 96.12 | 37.76 | 0.57 | 0.0002 |
| Poland vs Mongolia | 1 | 45.99 | 19.38 | 0.42 | 0.0002 |
| Italy vs USA | 1 | 15.96 | 2.33 | 0.18 | 0.1088 |
| Italy vs Antarctica | 1 | 5.00 | 5.75 | 0.24 | 0.0214 |
| Italy vs Albania | 1 | 48.07 | 47.57 | 0.73 | 0.0003 |
| Italy vs Canada | 1 | 26.96 | 9.90 | 0.34 | 0.0025 |
| Italy vs Madeira | 1 | 15.25 | 5.02 | 0.23 | 0.0264 |
| Italy vs Mongolia | 1 | 9.39 | 3.38 | 0.17 | 0.0591 |
| USA vs Antarctica | 1 | 36.20 | 11.01 | 0.32 | 0.0013 |
| USA vs Albania | 1 | 85.73 | 25.23 | 0.52 | 0.0002 |
| USA vs Canada | 1 | 76.01 | 16.33 | 0.40 | 0.0002 |
| USA vs Madeira | 1 | 118.93 | 23.44 | 0.52 | 0.0002 |
| USA vs Mongolia | 1 | 88.02 | 17.70 | 0.46 | 0.0002 |
| Antarctica vs Albania | 1 | 55.98 | 90.28 | 0.75 | 0.0002 |
| Antarctica vs Canada | 1 | 22.28 | 13.23 | 0.30 | 0.0002 |
| Antarctica vs Madeira | 1 | 58.07 | 32.33 | 0.53 | 0.0002 |
| Antarctica vs Mongolia | 1 | 23.56 | 14.70 | 0.34 | 0.0003 |
| Albania vs Canada | 1 | 13.26 | 7.51 | 0.20 | 0.0080 |
| Albania vs Madeira | 1 | 199.01 | 105.68 | 0.78 | 0.0002 |
| Albania vs Mongolia | 1 | 116.03 | 68.54 | 0.71 | 0.0002 |
| Canada vs Madeira | 1 | 116.25 | 39.54 | 0.57 | 0.0002 |
| Canada vs Mongolia | 1 | 55.41 | 19.84 | 0.41 | 0.0002 |
| Madeira vs Mongolia | 1 | 9.33 | 3.12 | 0.10 | 0.0783 |
Table 14.
Results of PERMANOVA and post hoc pairwise PERMANOVA comparisons for the first two principal components (PC1 and PC2) of animal pt values; significant post hoc p-values adjusted with the Benjamini–Hochberg correction.
| Post hoc comparisons | df | SS | F | R2 | P |
|---|---|---|---|---|---|
| Poland vs USA | 1 | 47.08 | 17.87 | 0.37 | 0.0002 |
| Poland vs Antarctica | 1 | 13.96 | 12.27 | 0.30 | 0.0002 |
| Poland vs Albania | 1 | 25.55 | 9.43 | 0.25 | 0.0002 |
| Poland vs Canada | 1 | 1.19 | 1.04 | 0.04 | 0.3524 |
| Poland vs Madeira | 1 | 134.43 | 47.34 | 0.63 | 0.0002 |
| Poland vs Mongolia | 1 | 9.14 | 4.73 | 0.20 | 0.0254 |
| USA vs Antarctica | 1 | 10.77 | 3.79 | 0.11 | 0.0372 |
| USA vs Albania | 1 | 6.23 | 1.44 | 0.04 | 0.2496 |
| USA vs Canada | 1 | 54.20 | 18.64 | 0.38 | 0.0002 |
| USA vs Madeira | 1 | 198.41 | 44.72 | 0.59 | 0.0002 |
| USA vs Mongolia | 1 | 17.89 | 4.16 | 0.16 | 0.0294 |
| Antarctica vs Albania | 1 | 7.47 | 2.54 | 0.08 | 0.1235 |
| Antarctica vs Canada | 1 | 16.51 | 11.55 | 0.28 | 0.0004 |
| Antarctica vs Madeira | 1 | 135.88 | 44.34 | 0.60 | 0.0002 |
| Antarctica vs Mongolia | 1 | 4.74 | 2.06 | 0.09 | 0.1731 |
| Albania vs Canada | 1 | 33.87 | 11.25 | 0.29 | 0.0004 |
| Albania vs Madeira | 1 | 200.18 | 42.54 | 0.60 | 0.0002 |
| Albania vs Mongolia | 1 | 17.57 | 3.75 | 0.16 | 0.0697 |
| Canada vs Madeira | 1 | 111.85 | 35.60 | 0.56 | 0.0002 |
| Canada vs Mongolia | 1 | 7.01 | 2.95 | 0.13 | 0.0893 |
| Madeira vs Mongolia | 1 | 42.85 | 8.80 | 0.32 | 0.0105 |
Genetic comparisons and phylogeographical analyses of different populations of the P. fairbanksi
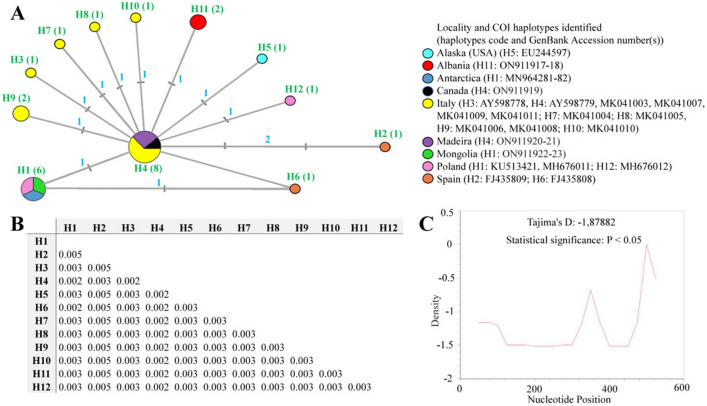
The COI sequences of P. fairbanksi from Albania, Canada, Madeira and Mongolia were 623–689 bp-long, and represented three haplotypes: haplotype H11 was observed in the population from Albania, haplotype H1 was identified in P. fairbanksi from Mongolia, and haplotype H4 was found in populations from Canada and Madeira (for details see Table 3 and Fig. 8A,B). No stop codons, insertions or deletions were observed. The translation was successfully carried out with the –2nd reading frame and the invertebrate mitochondrial codon table. The p-distances between COI haplotypes of all sequenced P. fairbanksi populations deposited in GenBank, i.e., from Antarctica, Italy, Spain, the USA and Poland ranged from 0.002 to 0.005% (an average distance of 0.003%) (Fig. 8B). In total, twelve haplotypes (H1–H12) of COI gene fragments were identified after comparing all available COI sequences of P. fairbanksi. Overall, the median joining COI haplotype network showed a star-like radiation. Interestingly, the most frequent haplotype H4 was present in populations from Italy, Madeira and Canada. This central haplotype H4 was surrounded by ten haplotypes (H1, H3, H5–H12) that differed from it by one mutational step. One haplotype (haplotype H2 from Spain) differed from central haplotype H4 by two mutational steps. In several geographical regions, i.e., the USA, Albania, Italy, Poland and Spain there were regional endemic haplotypes. Surprisingly, the second haplotype that occurred in different localities was haplotype H1 and this haplotype was common for three populations, from Mongolia, Poland and Antarctica.
Figure 8.
(A) Median-joining network based on the COI sequences: haplotypes marked as H1–H12 (the number of sequences is given in parentheses), the size of the circles is proportional to the number of sequences, the mutational steps values are indicated along the lines; (B) p-distance value based on the COI barcode sequences; (C) Tajima’s D neutrality test.
In turn, the 18S rRNA sequences of P. fairbanksi from Albania, Canada, Madeira and Mongolia were 917–1547 bp-long (Table 3) and no nucleotide substitution was found (except for a single undetermined “N” base). Compared with the data available in GenBank sequences of P. fairbanksi (sequences were aligned and trimmed to 572 bp), they showed only one nucleotide substitution. A comparison was performed with the sequences from the following geographical localities: Antarctica (GenBank: MN9603029), Poland (GenBank: MH664941-4278), USA (GenBank: EU03807879) and Italy (GenBank: MK041027-298). The 28S rRNA molecular marker (694–805 bp-long) was very conservative. No nucleotide substitution was found for all obtained sequences even after comparing (and trimmed to 689 bp) with GenBank sequences from Antarctica (GenBank: MN960306–MN9603079) and Poland (GenBank: MH66495078). Nevertheless, one unidentified base was found in the sequence originating from the Polish population.
Demographic expansion was preliminarily tested based on the value of neutrality tests that confirmed a neutral model of observed polymorphism. Negative significant values for Tajima’s D were found, indicating a high number of low-frequency polymorphisms in the COI sequences dataset and potential population size expansion (Fig. 8C). In turn, values of Fu’s Fs test statistic for COI data were negative, but non-significant: − 7.794, 0.10 > P > 0.05 (graphical results not shown).
Predictions of the distribution of the two parthenogenetic Paramacrobiotus species
Ecological niche modelling of potential distribution based on available location data was performed for two parthenogenetic species with verified records from various realms, i.e., P. fairbanksi and P. gadabouti. The study is limited to bioclimatic variables. The stimulated model predicted good accuracy for the overall model with an AUC for P. fairbanksi of 0.778 and the overall model with an AUC for P. gadabouti of 0.843. The suitability for P. fairbanksi seems moderate (peach areas on the map in Fig. 9A) to good (yellow areas on the map in Fig. 9A) with the most suitable habitats in the northern hemisphere (green areas on the map in Fig. 9A). Paramacrobiotus gadabouti shows maximal suitability around areas with a Mediterranean climate, although it also has a wider distribution than just the Mediterranean biomes (tropics, subtropics, and temperate regions) (Fig. 9B).
Figure 9.
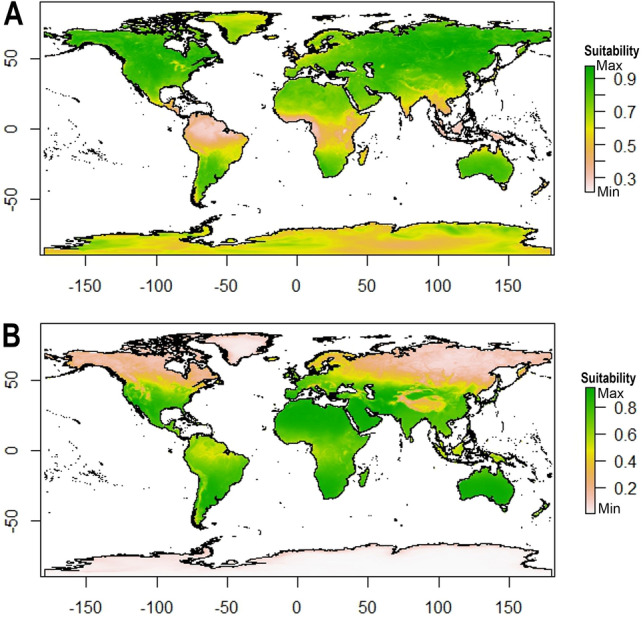
Ecological biogeography of two parthenogenetic Paramacrobiotus species with wide distributions—geographic ranges predicted by ecological niche modelling for: (A) Paramacrobiotus fairbanksi Schill, Förster, Dandekar & Wolf 20109, (B) Paramacrobiotus gadabouti Kayastha, Stec, Mioduchowska and Kaczmarek 202321. The maps were generated using MaxEnt software ver. 3.2.0: https://biodiversityinformatics.amnh.org/open_source/maxent/ and assembled in Corel Photo-Paint 2021.
Discussion
Morphometric comparison of different populations of the P. fairbanksi
Based on morphometric analyses, there is clear variation in measurements of morphological features between populations of P. fairbanksi from different regions of the world. However, the identification of this species is still possible based on morphological characters, in fact the morphometric data are shown to not affect the species identification because of the overlap in measurements of all measured structures. Therefore, it is valid to suggest the correct classification of all the specimens collected from different regions based on their morphology only. Even though the egg processes of Polish and Albanian populations are similar, the EBD of the Polish population are the smallest and those from the Albania are largest. The EFD as well as egg processes of the Madeiran population are largest while those of the Polish population are the smallest. Additionally, body length values of the Polish and USA populations of P. fairbanksi are smaller compared to the other populations studied.
Kaczmarek et al.9 suggested that the differences in measurements between different populations of this species are caused by conditions, i.e., specimens from cultures and specimens from wild populations. However, in the present study all measurements were based on specimens from cultured populations, i.e., Albanian, Canadian, Madeiran and Mongolian. Thus, we can suggest that the phenomenon described by Kaczmarek et al.9 (that dwarfing is caused by suboptimal conditions, high culture densities and inbreeding and that it might be due to the result of ongoing speciation) is unlikely to be true. Similarly, the suggestions that harsh conditions in Antarctica may favour laying larger eggs while in cultures the eggs are smaller because of the lack of such selective pressure9 seems untrue as the egg size of specimens from Antarctica overlaps with egg sizes of specimens from Albania, Canada and Mongolia, which were sampled from cultured populations in the present study.
Genetic comparison of different populations of the P. fairbanksi
Cytochrome oxidase subunit I gene (COI) sequences is one of the most reliable barcodes to investigate genetic variation with phenotypic plasticity since COI is a genetic marker with a high genetic variation compared to multiple other DNA barcodes80. Various studies combining COI variation and phenotypic plasticity were conducted throughout different invertebrates’ phyla, including tardigrades9,21,81–84, proving the marker’s accuracy in this group of organisms. The result showed high genetic homogeneity between organisms with wide geographical distribution together with clearly visible morphological differences known as phenotypic plasticity82. This phenomenon could be possible explanation for the subtle morphological variation observed in this study between P. fairbanksi populations, accompanied by very low mitochondrial barcode variation.
Furthermore, several studies uncovered data incongruence between mitochondrial and nuclear markers, e.g., for earthworms85 or corals86, suggesting that occasionally COI may fail as a barcode marker due to hybridization events. Many studies have already shown that Wolbachia (presence shown by Mioduchowska et al87 in P. fairbanksi) can increase the speciation rate and can affect COI haplotypes88. However, the nuclear markers tested for P. fairbanksi have been consistent for the studied populations.
The exact causes and mechanisms of the phenotypic plasticity in the morphology of adults and eggs of P. fairbanksi remains unknown, although, it has unsurprisingly been shown, that some physical traits differ in chosen cultured tardigrades depending on the temperature and food abundance7. If the morphological variation in P. fairbanksi is an effect of phenotypic plasticity, it is unclear which factors could cause various morphotype expressions. The specimens from Mongolia, Albania, Canada and Madeira that were measured in our study come from populations cultured in similar laboratory conditions but were started with different counts of founders of various ages, kept in variable densities and with different numbers of generations that had passed culture, so no answer can be proposed at this moment. No molecular markers that correspond with morphological features in tardigrades have been suggested so far. Future studies with higher-resolution markers designed for intrapopulation variation should be performed to determine if any pattern of genetic diversity concordant with morphological variation can be observed.
Phenotypic plasticity, in morphology and other aspects of phenotype, such as life history traits, is seen as an advantage for thriving in heterogeneous environments (e.g., ref89), which tardigrades’ habitats clearly are. Furthermore, phenotypic plasticity has been widely observed in other invertebrates like corals (e.g., Pseudopterogorgia bipinnata90) Verrill 1864, scallops (e.g., Pecten maximus91) Linnaeus, 1758, marine invertebrates, gastropods (e.g., Littorina littorea77) Linnaeus, 1758, rotifers (Keratella tropica92 (Apstein, 1907)) and many more. No concordant genetic variation was observed, but a large and discrete differentiation of morphotypes was present and was always associated with external environmental factors such as temperature, predation risk and food availability93–97.
Parthenogenesis and wide distribution
The phenomenon where parthenogenetic (asexual) lineages occupy a wider geographical range, but sexual populations are restricted to a limited area, is termed ‘geographical parthenogenesis’98. Guidetti et al.8 concluded that the difference in the dispersal potential of tardigrades is associated with the two types of reproduction, i.e., parthenogenetic species show a very wide distribution, inhabiting more continents, while the amphimictic species show a very limited or punctiform distribution. A similar pattern was shown for arthropods where parthenogenesis has been linked with higher dispersal abilities99 (for example, the freshwater ostracod Eucypris virens100 (Jurine, 1820) and the scorpion species Liocheles australasiae101 (Fabricus 1775) are parthenogenetic for multiple generations in captivity102,103 and are widely distributed104,105. Similar cases are found in many animals and plants (ref106–109)). However, Baker et al.99 also suggested that parthenogenesis indicates morphological variation as a result of epigenetic mechanisms. Furthermore, Mioduchowska et al.87 provided molecular evidence of the presence of the bacterial endosymbiont Wolbachia based on next generation sequencing in tardigrades. Wolbachia have an effect on the evolution as well as the ecology of their hosts and have been found to cause effects including cytoplasmic incompatibility, feminization, male killing, and induced parthenogenesis110 It has been noted that at the intraspecific level, even individuals from the same population can undergo morphological changes in their characters to diversify within niches available to the species111. Similarly, Kihm et al.104 proposed epigenetic factors as a main cause for variability in tardigrade Dactylobiotus ovimutans112 Kihm, Kim, McInnes, Zawierucha, Rho, Kang & Park, 2020 egg morphology, although the population was cultured under controlled laboratory conditions. Despite being rare, it is known that intraspecific variation is caused by external environmental conditions, epigenetics and seasonality112.
“Two faces” of cosmopolitism in the Paramacrobiotus
Ecological niche modelling is an important and useful tool that has been used to address issues in many fields of basic and applied ecology113. It effectively predicts habitat suitability for rare and poorly studied taxa114,115. P. fairbanksi presence is linked to the presence of suitable microhabitats, like moss patches, and their life strategy can make them less likely to be affected by general climatic conditions. However, bioclimatic variables used in the study may be a good predictor of the possibility of the occurrence of suitable microhabitats. We investigated the possible distribution of P. fairbanksi and compared it with other widely distributed species of the genus Paramacrobiotus, i.e., P. gadabouti. Paramacrobiotus fairbanksi already reported from various continents exhibit a cosmopolitan distribution covering different types of environments, whereas P. gadabouti, although also potentially cosmopolitan, has a clear affinity to areas with a Mediterranean climate. Its distribution is poorly known due to lack of sampling in many habitats. Such differences clearly show us that even when we consider some of the species to be cosmopolitan, specific patterns of distribution can be completely different. However, we must also stress that the number of known localities for both species is relatively low and, in the future, when the number of records of these species will be higher, a distribution pattern may look different.
Conclusions
Paramacrobiotus fairbanksi described originally from Alaska, USA, is now known from almost all zoogeographic realms. The identification of this species is still possible based on morphometric characters alone because the morphometric data are shown to not affect the species identification because of the overlap in measurements of all measured structures. Moreover, the analysis shows low genetic variability among P. fairbanksi populations from various geographical locations, which may in general suggest that interspecies genetic variability in tardigrades is very low too or could be’ the effects of Wolbachia infection. The species fits the ‘Everything is Everywhere’ hypothesis and is an example of a parthenogenetic species with wide distribution. Despite very low genetic variation, some indiscrete morphological variations were observed. Since all the studied populations were cultured and bred in the same laboratory conditions, such variation may have been caused by epigenetic effects, and were not the result of different temperatures, food sources and seasonality.
Supplementary Information
Acknowledgements
Studies have been partially conducted in the framework of activities of BARg (Biodiversity and Astrobiology Research group). The PK is scholarship holder of Passport to the future—Interdisciplinary doctoral studies at the Faculty of Biology, Adam Mickiewicz University, Poznań POWR.03.02.00-00-I006/17. Dr. Matteo Vecchi for helping me figure out masking landmasses to MaxEnt results. This research was funded in part by National Science Centre, Poland, grant no. 2021/43/D/NZ8/00344 ("Let’s dry up and survive together": is anhydrobiosis in water bears (Tardigrades) modulated by a specific microbiome community and does it depend on bacteria that survive desiccation together with them?) granted to MM. The authors also wish to thank Cambridge Proofreading LLC (http://proofreading.org) for their linguistic assistance. For the purpose of Open Access, the author has applied a CC-BY public copyright licence to any Author Accepted Manuscript (AAM) version arising from this submission.
Author contributions
Conceptualization, P.K. and Ł.K.; data curation, P.K.; formal analysis, P.K.; investigation, P.K., M.M., and Ł.K.; methodology, P.K., M.M. and Ł.K.; supervision, Ł.K.; validation, P.K., W.S., M.M. and Ł.K.; visualization, P.K.; writing—original draft, P.K., M.M. and Ł.K.; writing—review and editing, P.K., W.S., M.M. and Ł.K. All authors have read and agreed to the published version of the manuscript.
Data availability
The datasets generated and/or analysed during the current study are available in the GenBank repository (all accession numbers listed in Table 2: ON911917-18, ON872386, ON872380-81, ON911919, ON872387, ON872382, ON911920-21, ON872388, ON872383, ON911922-23, ON872389 and ON872384-85). The data of all sequences are available for public access.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-42653-6.
References
- 1.Degma, P. & Guidetti R. Actual checklist of Tardigrada species (42th Edition: 09–01–2023). Accessed: 7th February 2023 (2009–2023). 10.25431/11380_1178608.
- 2.Nelson, D. R., Guidetti, R., Rebecchi, L., Kaczmarek, Ł. & McInnes, S. Phylum Tardigrada. In Thorp and Covich’s Freshwater Invertebrates 505–522 (Elsevier, 2020). 10.1016/B978-0-12-804225-0.00015-0.
- 3.Schill RO, Förster F, Dandekar T, Wolf M. Using compensatory base change analysis of internal transcribed spacer 2 secondary structures to identify three new species in Paramacrobiotus (Tardigrada) Org. Divers. Evol. 2010;10:287–296. doi: 10.1007/s13127-010-0025-z. [DOI] [Google Scholar]
- 4.Guidetti R, Gandolfi A, Rossi V, Bertolani R. Phylogenetic analysis of Macrobiotidae (Eutardigrada, Parachela): A combined morphological and molecular approach. Zool. Scr. 2005;34:235–244. doi: 10.1111/j.1463-6409.2005.00193.x. [DOI] [Google Scholar]
- 5.Murray J. Arctiscoida. Proc. R. Irish. Acad. 1911;31:1–16. [Google Scholar]
- 6.Guil N, Giribet G. A comprehensive molecular phylogeny of tardigrades-adding genes and taxa to a poorly resolved phylum-level phylogeny. Cladistics. 2012;28:21–49. doi: 10.1111/j.1096-0031.2011.00364.x. [DOI] [PubMed] [Google Scholar]
- 7.Kosztyła P, Stec D, Morek W, Gąsiorek P, Zawierucha Z, Michno K, Małek D, Hlebowicz K, Ufir K, Laska A, Dudziak M, Frohme M, Prokop ZM, Kaczmarek Ł, Michalczyk Ł. Experimental taxonomy confirms the environmental stability of morphometric traits in a taxonomically challenging group of microinvertebrates. ZJLS. 2016;178:765–775. doi: 10.1111/zoj.12409. [DOI] [Google Scholar]
- 8.Guidetti R, Cesari M, Bertolani R, Altiero T, Rebecchi L. High diversity in species, reproductive modes and distribution within the Paramacrobiotus richtersi complex (Eutardigrada, Macrobiotidae) Zool. Lett. 2019 doi: 10.1186/s40851-018-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaczmarek Ł, Mioduchowska M, Kačarević U, Kubska K, Parnikoza I, Gołdyn B, Roszkowska M. New Records of Antarctic Tardigrada with comments on interpopulation variability of the Paramacrobiotus fairbanksi Schill, Förster, Dandekar and Wolf, 2010. Diversity. 2020;12:108. doi: 10.3390/d12030108. [DOI] [Google Scholar]
- 10.Bryndová M, Stec D, Schill RO, Michalczyk Ł, Devetter M. Dietary preferences and diet effects on life-history traits of tardigrades. ZJLS. 2020;188(3):865–877. doi: 10.1093/zoolinnean/zlz146. [DOI] [Google Scholar]
- 11.Beijerinck, M. W. De infusies en de ontdekking der bakterien. Jaarb.V.dek.Akad.V.Wetensch.Amst. 1–28 (1913).
- 12.Baas Becking, L. G. M. Geobiologie of Inleiding Tot de Milieukunde. (W.P. Van Stockum & Zoon N.V, 1934).
- 13.Cardillo M, Bromham L. Body size and risk of extinction in Australian mammals. Biol. Conserv. 2001;15:1435–1440. doi: 10.1046/j.1523-1739.2001.00286.x. [DOI] [Google Scholar]
- 14.Finlay BJ. Global dispersal of free-living microbial eukaryote species. Science. 2002;296:1061–1063. doi: 10.1126/science.1070710. [DOI] [PubMed] [Google Scholar]
- 15.Foissner W. Biogeography and dispersal of micro-organisms: A review emphasizing protists. Acta Protozool. 2006;45:111–136. [Google Scholar]
- 16.Schön, I., Martens, K. & Dijk, P. Lost Sex: The Evolutionary Biology of Parthenogenesis. (Springer Netherlands, 2009). 10.1007/978-90-481-2770-2.
- 17.Pilato G, Binda MG. Biogeography and limno-terrestrial tardigrades: Are they truly incompatible binomials? Zool. Anz. 2001;240:511–516. doi: 10.1078/0044-5231-00061. [DOI] [Google Scholar]
- 18.Guil N, Sánchez-Moreno S, Machordom A. Local biodiversity patterns in micrometazoans: Are tardigrades everywhere? Syst. Biodivers. 2009;7:259–268. doi: 10.1017/S1477200009003016. [DOI] [Google Scholar]
- 19.Faurby S, Jørgensen A, Kristensen RM, Funch P. Distribution and speciation in marine intertidal tardigrades: Testing the roles of climatic and geographical isolation: Distribution and speciation in tidal tardigrades. J. Biogeogr. 2012;39:1596–1607. doi: 10.1111/j.1365-2699.2012.02720.x. [DOI] [Google Scholar]
- 20.Jørgensen A, Møbjerg N, Kristensen RM. A molecular study of the tardigrade Echiniscus testudo (Echiniscidae) reveals low DNA sequence diversity over a large geographical area. J. Limnol. 2007;66:77–83. doi: 10.4081/jlim-nol.2007.s1.77. [DOI] [Google Scholar]
- 21.Gąsiorek P, Vončina K, Michalczyk Ł. Echiniscus testudo (Doyère, 1840) in New Zealand: Anthropogenic dispersal or evidence for the ‘Everything is Everywhere’ hypothesis? NZJZ. 2019;46:174–181. doi: 10.1080/03014223.2018.1503607. [DOI] [Google Scholar]
- 22.Kayastha P, Stec D, Sługocki Ł, Gawlak M, Mioduchowska M, Kaczmarek Ł. Integrative taxonomy reveals new, widely distributed tardigrade species of the genus Paramacrobiotus (Eutardigrada: Macrobiotidae) Sci. Rep. 2023;13:2196. doi: 10.1038/s41598-023-28714-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maucci W. Prime notizie su Tardigradi ‘terrestri’ del Madagascar con descrizione di tre specie nuove. Boll. Museo Civico Storia Nat. Verona. 1993;17:381–392. [Google Scholar]
- 24.Murray J. Some South African Tardigrada. JRMS. 1907;27(5):515–524. doi: 10.1111/j.1365-2818.1907.tb01665.x. [DOI] [Google Scholar]
- 25.Marcus, E. Spinnentiere oder Arachnoidea. IV. Bärtierchen (Tardigrada). Tierwelt Deutschlands und der angrenzenden Meeresteile Jena12, 1–230 (1928).
- 26.Gąsiorek P, Vončina K, Bochnak M, Surmacz B, Morek W, Michalczyk Ł. Echiniscidae (Heterotardigrada) of South Africa. Zootaxa. 2022;5156(1):1–238. doi: 10.11646/zootaxa.5156.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Richters F. Nordische Tardigraden. Zool. Anz. 1903;27:168–172. [Google Scholar]
- 28.Schuster RO, Grigarick AA. Tardigrada from the Galápagos and Cocos Islands. Proc. Calif. Acad. Sci. 1966;34:315–328. [Google Scholar]
- 29.Maucci, W. Echiniscus bisculptus sp. nov., del Marocco, ed E. lichenorum sp. nov., del Portogallo. Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale in Milano124 (3–4), 257–261 (1983).
- 30.Pilato G, Fontoura P, Lisi O, Beasley C. New description of Echiniscus scabrospinosus Fontoura, 1982, and description of a new species of Echiniscus (Heterotardigrada) from China. Zootaxa. 2008;1856(1):41–54. doi: 10.11646/zootaxa.1856.1.4. [DOI] [Google Scholar]
- 31.Gąsiorek P, Jackson KJ, Meyer HA, Zając K, Nelson DR, Kristensen RM, Michalczyk Ł. Echiniscus virginicus complex: The first case of pseudocryptic allopatry and pantropical distribution in tardigrades. Biol. JLS. 2019;128(4):789–805. doi: 10.1093/biolinnean/blz147. [DOI] [Google Scholar]
- 32.Binda MG, Pilato G. Notizie sui Tardigradi delle Isole Hawaii con descrizione di due specie nuove. Animalia. 1994;21(1/3):57–62. [Google Scholar]
- 33.Fontoura, A. P. Deux nouvelles espèces de tardigrades muscicoles du Portugal. Publicações do Instituto de Zoologia “Dr. Augusto Nobre” Faculdade de Ciências do Porto165, 5–19 (1982).
- 34.Doyère M. Memoire sur les tardigrades. Ann. Sci. Nat Zool. Ser. 1840;2:269–362. [Google Scholar]
- 35.Morek W, Suzuki AC, Schill RO, Georgiev D, Yankova M, Marley NJ, Michalczyk Ł. Redescription ofMilnesiumalpigenumEhrenberg, 1853 (Tardigrada: Apochela) and a description ofMilnesium inceptum sp. nov., a tardigrade laboratory model. Zootaxa. 2019;4586(1):035–064. doi: 10.11646/zootaxa.4586.1.2. [DOI] [PubMed] [Google Scholar]
- 36.Binda MG, Pilato G. Ridescrizione diEchiniscusreticulatus Murray, 1905 e descrizione di Echiniscus cirinoi, nuova specie di tardigrado della Tanzania. Animalia. 1993;20:55–58. [Google Scholar]
- 37.Péterfi F. Contributioni la cunoasterea Tardigradelor din RPR Studii si Cercetari de Biologia. Accademia RPR Filiola Cluj. 1956;7:149–155. [Google Scholar]
- 38.Gąsiorek P, Vončina K, Nelson DR, et al. The importance of being integrative: A remarkable case of synonymy in the genus Viridiscus (Heterotardigrada: Echiniscidae) Zool. Lett. 2021;7:13. doi: 10.1186/s40851-021-00181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stec D, Smolak R, Kaczmarek Ł, Michalczyk Ł. An integrative description ofMacrobiotuspaulinae sp. nov. (Tardigrada: Eutardigrada: Macrobiotidae: hufelandi group) from Kenya. Zootaxa. 2015;4052:501–526. doi: 10.11646/zootaxa.4052.5.1. [DOI] [PubMed] [Google Scholar]
- 40.WFO: Ceratodon purpureus (Hedw.) Brid. http://www.worldfloraonline.org/taxon/wfo-0001180270. Accessed on: 08 Sep 2023 (2023).
- 41.Roszkowska M, Wojciechowska D, Kmita H, Cerbin S, Dziuba MK, Fiałkowska E, Sobkowiak R, Szydło W, Kaczmarek Ł. Tips and tricks how to culture water bears: Simple protocols for culturing eutardigrades (Tardigrada) under laboratory conditions. EZJ. 2021;88:449–465. doi: 10.1080/24750263.2021.1881631. [DOI] [Google Scholar]
- 42.Ramazzotti, G. & Maucci, W. Il Phylum Tardigrada. III edizione riveduta e aggiornata. Mem Ist Ital Idrobiol 1–1012 (1983).
- 43.Beasley, C. W. The phylum Tardigrada. Third Edition by G. Ramazzotti and W. Maucci, English Translation P. Abilene, USA 1–1014 (1995).
- 44.Kaczmarek Ł, Michalczyk Ł. The Macrobiotus hufelandi group (Tardigrada) revisited. Zootaxa. 2017;4363:101–123. doi: 10.11646/zootaxa.4363.1.4. [DOI] [PubMed] [Google Scholar]
- 45.Kaczmarek Ł, Cytan J, Zawierucha K, Diduszko D, Michalczyk Ł. Tardigrades from Peru (South America), with descriptions of three new species of Parachela. Zootaxa. 2014;3790:357–379. doi: 10.11646/zootaxa.3790.2.5. [DOI] [PubMed] [Google Scholar]
- 46.Pilato G. Analisi di nuovi caratteri nello studio degli Eutardigradi. Animalia. 1981;8:51–57. [Google Scholar]
- 47.Michalczyk Ł, Kaczmarek Ł. The Tardigrada Register: A comprehensive online data repository for tardigrade taxonomy. J. Limnol. 2013;72(e22):175–181. doi: 10.4081/jlimnol.2013.s1.e22. [DOI] [Google Scholar]
- 48.Bertolani R, Guidetti R, Marchioro T, Altiero T, Rebechci L, Cesari M. Phylogeny of Eutardigrada: New molecular data and their morphological support lead to the identification of new evolutionary lineages. Mol. Phylogenet. Evol. 2014;76:110–126. doi: 10.1016/j.ympev.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Casquet J, Thebaud C, Gillespie RG. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol-stored spiders. Mol. Ecol. Resour. 2012;12:136–141. doi: 10.1111/j.1755-0998.2011.03073.x. [DOI] [PubMed] [Google Scholar]
- 50.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. Phylogenetic uncertainty. Mol. Mar. Biol. Biotechnol. 1994;3(5):294–299. [PubMed] [Google Scholar]
- 51.Sands CJ, McInnes SJ, Marley NJ, Goodall-Copestake WP, Convey P, Linse K. Phylum Tardigrada: An “individual” approach. Cladistics. 2008;24:861–871. doi: 10.1111/j.1096-0031.2008.00219.x. [DOI] [PubMed] [Google Scholar]
- 52.Mironov SV, Dabert J, Dabert M. A new feather mite species of the genus Proctophyllodes Robin, 1877 (Astigmata: Proctophyllodidae) from the long-tailed tit Aegithalos caudatus (Passeriformes: Aegithalidae)—morphological description with DNA barcode data. Zootaxa. 2012;3253:54–61. doi: 10.11646/zootaxa.3253.1.2. [DOI] [Google Scholar]
- 53.Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999;41:95–98. [Google Scholar]
- 54.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 55.Librado P, Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 56.Rice P, Longden I, Bleasby A. EMBOSS: The European molecular biology open software suite. TiG. 2000;16:276–277. doi: 10.1016/S0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 57.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Lankton. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bandelt HJ, Forster P, Sykes BC, Richards MB. Mitochondrial portraits of human populations using median networks. Genetics. 1995;141:743–753. doi: 10.1093/genetics/141.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu Y-X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics. 1997;147:915–925. doi: 10.1093/genetics/147.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Excoffier L, Lischer HEL. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 62.Team RC. A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2012). https://www.R-project.org
- 63.Stec D, Vecchi M, Dudziak M, Bartels PJ, Calhim S, Michalczyk Ł. Integrative taxonomy resolves species identities within the Macrobiotus pallarii complex (Eutardigrada: Macrobiotidae) Zool. Lett. 2021;7:9. doi: 10.1186/s40851-021-00176-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Josse J, Husson F. missMDA: A package for handling missing values in multivariate data analysis. J. Stat. Softw. 2016;70(1):1–31. doi: 10.18637/jss.v070.i01. [DOI] [Google Scholar]
- 65.Lê S, Josse J, Husson F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008;25(1):1–8. doi: 10.18637/jss.v025.i01. [DOI] [Google Scholar]
- 66.Wickham H. The split-apply-combine strategy for data analysis. J. Stat. Softw. 2011;40(1):1–29. doi: 10.18637/jss.v040.i01. [DOI] [Google Scholar]
- 67.Wickham, H. et al. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. R Package Version. 2(1) (2016).
- 68.Björklund M. Be careful with your principal components. Evolution. 2019;73(10):2151–2158. doi: 10.1111/evo.13835. [DOI] [PubMed] [Google Scholar]
- 69.Martinez Arbizu, P. pairwiseAdonis: Pairwise Multilevel Comparison Using Adonis. R Package Version 0.0 (2017).
- 70.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 71.Phillips SJ, Anderson RP, Dudík M, Schapire RE, Blair ME. Opening the black box: An open-source release of Maxent. Ecography. 2017;40:887–893. doi: 10.1111/ecog.03049. [DOI] [Google Scholar]
- 72.Phillips, S. J., Dudík, M. & Schapire, R. E. Maxent Software for Lankton Species Niches and Distributions (Version 3.4.1) (2020). http://biodiversityinformatics.amnh.org/open_source/maxent/.
- 73.Vega G, Pertierra L, Olalla-Tárraga M. MERRAclim, a high-resolution global dataset of remotely sensed bioclimatic variables for ecological modelling. Sci. Data. 2017;4:170078. doi: 10.1038/sdata.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Warren DL, Glor RE, Turelli M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography. 2010;33(3):607–611. doi: 10.1111/j.1600-0587.2009.06142.x. [DOI] [Google Scholar]
- 75.Chhogyel N, Kumar L, Bajgai Y, Jayasinghe LS. Prediction of Bhutan’s ecological distribution of rice (Oryza sativa L.) under the impact of climate change through maximum entropy modelling. J. Agric. Sci. 2020;158:25–37. doi: 10.1017/S0021859620000350. [DOI] [Google Scholar]
- 76.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy lankton of species geographic distributions. Ecol. Model. 2006;190:231–259. doi: 10.1016/j.ecolmodel.2005.03.026. [DOI] [Google Scholar]
- 77.Merow C, Smith MJ, Silander JA., Jr A practical guide to MaxEnt for lankton species’ distributions: What it does, and why inputs and settings matter. Ecography. 2013;36:1058–1069. doi: 10.1111/j.1600-0587.2013.07872.x. [DOI] [Google Scholar]
- 78.Stec D, Krzywański Ł, Zawierucha K, Michalczyk Ł. Untangling systematics of the Paramacrobiotus areolatus species complex by an integrative redescription of the nominal species for the group, with multilocus phylogeny and species delineation in the genus Paramacrobiotus. ZJLS. 2020;188:694–716. doi: 10.1093/zoolinnean/zlz163. [DOI] [Google Scholar]
- 79.Guidetti R, Schill RO, Bertolani R, Dandekar T, Wolf M. New molecular data for tardigrade phylogeny, with the erection of Paramacrobiotus gen. nov. J. Zool. Syst. Evol. 2009;47(4):315–321. doi: 10.1111/j.1439-0469.2009.00526.x. [DOI] [Google Scholar]
- 80.Sari SY, Ambarwati R, Rahayu DA. Molecular characteristics of Donax faba (Bivalvia: Donacidae) from Nepa Beach, Madura, based on cytochrome oxidase subunit I gene sequences. AACL Bioflux. 2021;14:2416–2426. [Google Scholar]
- 81.Caputo L, Fuentes R, Woelfl S, Castañeda LE, Cárdenas L. Phenotypic plasticity of clonal populations of the freshwater jellyfish Craspedacusta sowerbii (Lankester, 1880) in Southern Hemisphere lakes (Chile) and the potential role of the zooplankton diet. Austral. Ecol. 2021;46:1192–1197. doi: 10.1111/aec.13087. [DOI] [Google Scholar]
- 82.González MT, Leiva NV, Sepúlveda F, Asencio G, Baeza JA. Genetic homogeneity coupled with morphometric variability suggests high phenotypic plasticity in the sea louse Caligus rogercresseyi (Boxshall and Bravo, 2000), infecting farmed salmon (Salmo salar) along a wide latitudinal range in southern Chile. J. Fish Dis. 2021;44:633–638. doi: 10.1111/jfd.13341. [DOI] [PubMed] [Google Scholar]
- 83.Morek W, Surmacz B, López-López A, Michalczyk Ł. “Everything is not everywhere”: Time-calibrated phylogeography of the genus Milnesium (Tardigrada) Mol. Ecol. 2021;30:3590–3609. doi: 10.1111/mec.15951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu R, Liu X, Guo L, Zhou C, Ouyang S, Wu X. DNA barcoding, multilocus phylogeny, and morphometry reveal phenotypic plasticity in the Chinese freshwater mussel Lamprotula caveata (Bivalvia: Unionidae) Ecol. Evol. 2022;12:e9035. doi: 10.1002/ece3.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shekhovtsov SV, Golovanova EV, Peltek SE. Genetic diversity of the earthworm Octolasion tyrtaeum (Lumbricidae, Annelida) Pedobiologia. 2014;57:245–250. doi: 10.1016/j.pedobi.2014.09.002. [DOI] [Google Scholar]
- 86.Forsman ZH, Barshis DJ, Hunter CL, Toonen RJ. Shape-shifting corals: Molecular markers show morphology is evolutionarily plastic in Porites. BMC Evol. Biol. 2009;9:45. doi: 10.1186/1471-2148-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mioduchowska M, Nitkiewicz B, Roszkowska M, Kačarević U, Madanecki P, Pinceel T, Namiotko T, Gołdyn B, Kaczmarek Ł. Taxonomic classification of the bacterial endosymbiont Wolbachia based on next-generation sequencing: Is there molecular evidence for its presence in tardigrades? Genome. 2021;64:951–958. doi: 10.1139/gen-2020-0036. [DOI] [PubMed] [Google Scholar]
- 88.Klopfstein S, Kropf C, Baur H. Wolbachia endosymbionts distort DNA barcoding in the parasitoid wasp genus Diplazon (Hymenoptera: Ichneumonidae) ZJLS. 2016;177:541–557. doi: 10.1111/zoj.12380. [DOI] [Google Scholar]
- 89.Altiero T, Giovannini I, Guidetti R, Rebecchi L. Life history traits and reproductive mode of the tardigrade Acutuncus antarcticus under laboratory conditions: Strategies to colonize the Antarctic environment. Hydrobiologia. 2015;761:277–291. doi: 10.1007/s10750-015-2315-0. [DOI] [Google Scholar]
- 90.Verrill AE. List of the polyps and corals sent by the Museum of Comparative Zoology to other institutions in exchange, with annotations. Bull. Mus. Comp. 1864;1:29–60. [Google Scholar]
- 91.Linnaeus, C. Caroli Linnaei Systema naturae per regna tria naturae :secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. (Impensis Direct. Laurentii Salvii, 1758). 10.5962/bhl.title.542.
- 92.Apstein C. Das lankton im Colombo-See auf Ceylon. Zool. Jahrb. 1907;25:201–244. doi: 10.5962/bhl.part.11957. [DOI] [Google Scholar]
- 93.Charifson D. Phenotypic plasticity in gastropod shell remodelling. Invertebr. Biol. 2019;138:e12267. doi: 10.1111/ivb.12267. [DOI] [Google Scholar]
- 94.Broitman BR, Lagos NA, Opitz T, Figueroa D, Maldonado K, Ricote N, Lardies MA. Phenotypic plasticity is not a cline: Thermal physiology of an intertidal barnacle over 20° of latitude. J. Anim. Ecol. 2021;90:1961–1972. doi: 10.1111/1365-2656.13514. [DOI] [PubMed] [Google Scholar]
- 95.Ramos-Rodríguez E, Moreno E, Conde-Porcuna JM. Intraspecific variation in sensitivity to food availability and temperature-induced phenotypic plasticity in the rotifer Keratella cochlearis. J. Exp. Biol. 2020;223:jeb209676. doi: 10.1242/jeb.209676. [DOI] [PubMed] [Google Scholar]
- 96.Gilbert JJ. Temperature, kairomones, and phenotypic plasticity in the rotifer Keratella tropica (Apstein, 1907) Hydrobiologia. 2011;678:179–190. doi: 10.1007/s10750-011-0847-5. [DOI] [Google Scholar]
- 97.Hart MW, Strathmann RR. Functional consequences of phenotypic plasticity in Echinoid larvae. Biol. Bull. 1994;186:291–299. doi: 10.2307/1542275. [DOI] [PubMed] [Google Scholar]
- 98.Vandel AL. Parthénogénèse géographique. Bull. Biol. Fr. Belg. 1928;62:164–281. [Google Scholar]
- 99.Baker CM, Ballesteros JA, Aharon S, Gainett G, Steinpress IA, Wizen G, Sharma PP, Gavish-Regev E. Recent speciation and phenotypic plasticity within a parthenogenetic lineage of levantine whip spiders (Chelicerata: Amblypygi: Charinidae) Mol. Phylogenet. Evol. 2022;175:107560. doi: 10.1016/j.ympev.2022.107560. [DOI] [PubMed] [Google Scholar]
- 100.Jurine, L. Histoire des monocles qui se trouvent aux environs de Genève/par Louis Jurine 1–260. 10.5962/bhl.title.10137 (1820).
- 101.Fabricius, J.C. Systema entomologiae, sistens insectorum classes, ordines, genera, species, adjectis synonymis, locis, descriptionibus, observationibus. Flensburgi et Lipsiae (1775)
- 102.Makioka T. Reproductive biology of the viviparous scorpion, Liocheles australasiae (Fabricius) (Arachnida, Scorpiones, Ischnuridae) IV. Pregnancy in females isolated from infancy, with notes on juvenile stage duration. Invertebr. Reprod. Dev. 1993;24:207–211. doi: 10.1080/07924259.1993.9672353. [DOI] [Google Scholar]
- 103.Yamazaki K, Makioka T. Parthenogenesis through five generations in the scorpion Liocheles australasiae (Fabricius 1775) (Scorpiones, Ischnuridae) J. Arachnol. 2005;33:852–856. doi: 10.1636/S02-5.1. [DOI] [Google Scholar]
- 104.Lourenço WR. Reproduction in scorpions, with special reference to parthenogenesis. Eur. Arachnol. 2000;2002:71–85. [Google Scholar]
- 105.Monod L, Prendini L. Evidence for Eurogondwana: The roles of dispersal, extinction and vicariance in the evolution and biogeography of Indo-Pacific Hormuridae (Scorpiones: Scorpionoidea) Cladistics. 2015;31:71–111. doi: 10.1111/cla.12067. [DOI] [PubMed] [Google Scholar]
- 106.Bell G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality. California Press; 1982. [Google Scholar]
- 107.Van Dijk PJ. Ecological and evolutionary opportunities of apomixis: Insights from Taraxacum and Chondrilla. Philos. Trans. R. Soc. Lond. B. 2003;358:1113–1121. doi: 10.1098/rstb.2003.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haag CR, Ebert D. A new hypothesis to explain geographic parthenogenesis. Ann. Zool. Fenn. 2004;41:539–544. [Google Scholar]
- 109.Kearney M. Hybridization, glaciation and geographical parthenogenesis. Trends Ecol. Evol. 2005;20:495–502. doi: 10.1016/j.tree.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 110.Mioduchowska M, Konecka E, Gołdyn B, Pinceel T, Brendonck L, Lukić D, Kaczmarek Ł, Namiotko T, Zając K, Zając T, Jastrzębski JP, Bartoszek K. Playing peekaboo with a master manipulator: metagenetic detection and phylogenetic analysis of Wolbachia supergroups in freshwater invertebrates. Int. J. Mol. Sci. 2022;24:9400. doi: 10.3390/ijms24119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Robinson BW, Wilson DS. Character release and displacement in fishes: A neglected literature. Am. Nat. 1994;144:596–627. doi: 10.1086/285696. [DOI] [Google Scholar]
- 112.Kihm JH, Kim S, McInnes SJ, Zawierucha K, Rho HS, Kang P, Park TYS. Integrative description of a new Dactylobiotus (Eutardigrada: Parachela) from Antarctica that reveals an intraspecific variation in tardigrade egg morphology. Sci. Rep. 2020;10:9122. doi: 10.1038/s41598-020-65573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murphy HT, Lovett-Doust J. Accounting for regional niche variation in habitat suitability models. Oikos. 2007;116:99–110. doi: 10.1111/j.2006.0030-1299.15050.x. [DOI] [Google Scholar]
- 114.Cianfrani C, Le Lay G, Hirzel AH, Loy A. Do habitat suitability models reliably predict the recovery areas of threatened species? J. Appl. Ecol. 2010;47:421–430. doi: 10.1111/j.1365-2664.2010.01781.x. [DOI] [Google Scholar]
- 115.McCune JL. Species distribution models predict rare species occurrences despite significant effects of landscape context. J. Appl. Ecol. 2016;53:1871–1879. doi: 10.1111/1365-2664.12702. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the GenBank repository (all accession numbers listed in Table 2: ON911917-18, ON872386, ON872380-81, ON911919, ON872387, ON872382, ON911920-21, ON872388, ON872383, ON911922-23, ON872389 and ON872384-85). The data of all sequences are available for public access.