Abstract
Objective
Several studies have suggested a potential link between use of proton pump inhibitors (PPIs) and the risk of kidney stones, attributed to alterations in urine mineral levels. Our study aimed to investigate the association between PPI use and kidney stones in US adults.
Design
Cross-sectional study.
Setting
National Health and Nutrition Examination Survey (2007–2018).
Participants
27 075 individuals with complete information on PPI use and history of kidney stones were included in this study.
Outcomes and analyses
Non-linear analysis, logistic regression analysis and subgroup analysis were conducted to estimate the relationship between PPI use and the occurrence and recurrence of kidney stones, after adjusting for potential confounding factors.
Results
Multivariable logistic regression analysis revealed a significant association between PPI use and kidney stones (OR 1.31, 95% CI 1.07 to 1.60), with a 4% increase in the prevalence of kidney stones for each additional year of PPI use (p<0.001). Similarly, PPI use was significantly associated with recurrent kidney stones (OR 1.49, 95% CI 1.04 to 2.13), with a 7% increase in the recurrence of kidney stones for each additional year of PPI use (p<0.001). Furthermore, these associations remained significant even after conducting propensity score matching analysis on a subset of PPI users and non-users (all p≤0.001). Subgroup analyses showed that the effects of PPI use on kidney stones differed by age, sex, race and body mass index.
Conclusions
This study indicated that long-term use of PPI was associated with a higher risk of both the presence and recurrence of kidney stones.
Keywords: risk factors, urolithiasis, adverse events
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The National Health and Nutrition Examination Survey (NHANES) dataset comprises a representative sample of the national population to ensure that our findings can be extrapolated to the broader population.
Multiple potential confounders were adjusted for and a propensity score matching analysis was performed to ensure the reliability of the results.
It is difficult to draw causal conclusions from cross-sectional analyses.
NHANES did not record information regarding the time and type of kidney stones or the dosage and type of proton pump inhibitor use.
Introduction
Kidney stones are a common disease in the USA, with a prevalence of 12% in men and 10% in women, and have a substantial impact in terms of cost and morbidity.1 2 Some drugs may affect the risk of kidney stones by altering active compounds crystallising in urine or substances impairing urine composition.3–5
Proton pump inhibitors (PPIs) are commonly prescribed medications worldwide for the treatment of gastric acid-related diseases such as gastroesophageal reflux disease (GERD), Helicobacter pylori infection and gastric ulcers.6 However, the escalating prevalence of PPI overuse, especially for long-term therapy, has become a concerning issue.6 7 Long-term PPI intake is associated with a reduction in intestinal absorption of essential vitamins and minerals and increased susceptibility to infections, chronic kidney disease and dementia.7 Given that PPI can inhibit gastric acid secretion, thereby affecting the intestinal absorption of essential minerals and altering the levels of calcium, magnesium and citrate,8 9 several studies have investigated the impact of PPI use on the risk of kidney stones.10–12 For instance, Sui et al found that PPI use might elevate the risk of kidney stones by lowering the levels of urinary citrate and magnesium, which could compromise their inhibitory effect on kidney stone formation.11 However, it should be noted that all participants in their study were patients with GERD. Similarly, Simonov et al identified a correlation between PPI use and kidney stones primarily based on a sample of young individuals and males,10 thereby limiting the generalisability of their findings to not only the general population but also specific patient groups, such as recurrent stone formers.13
This study aimed to investigate the potential association between PPI use and kidney stones by analysing National Health and Nutrition Examination Survey (NHANES) data from 2007 to 2018. Our hypothesis was that PPI use increases the risk of both kidney stone formation and recurrence.
Methods
Study design and population
The NHANES is an ongoing cross-sectional survey that employs a sophisticated multistage sample methodology to investigate the health and nutritional status of the non-institutionalised population in the USA. Demographic characteristics, clinical history and self-reported dietary data were collected from participants using a structured household interview. Physical examinations, including anthropometric measurements and blood samples, were collected within a mobile examination centre. Additional information regarding data collection can be accessed on the NHANES website.14
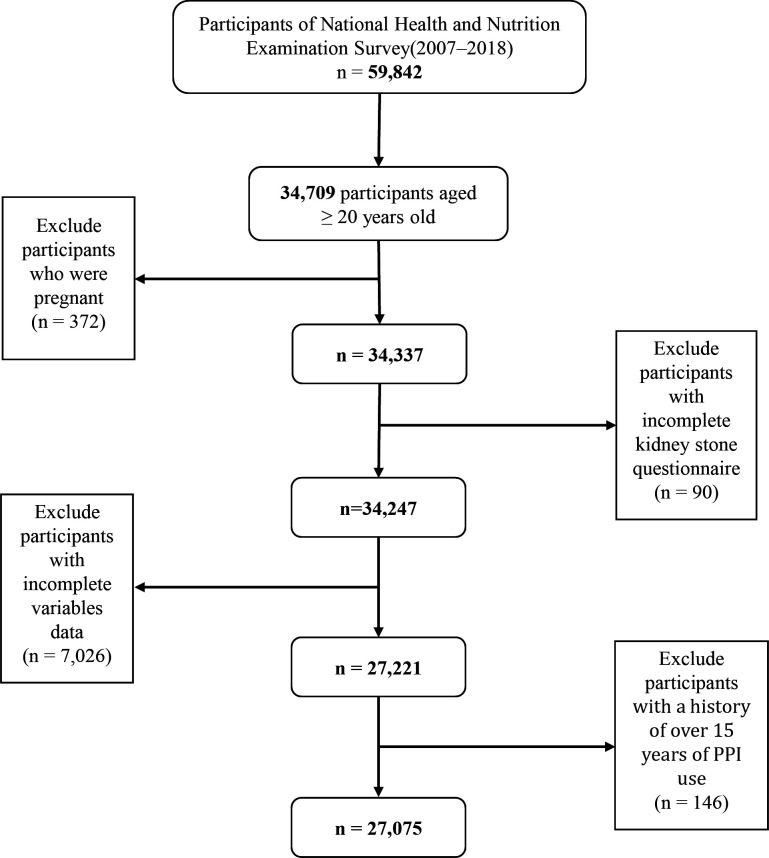
Six NHANES cycles were used in the study from 2007 to 2018. Initially, 34 709 participants aged 20 years and older were included. However, some participants were excluded: 372 participants who were pregnant, 90 participants with incomplete kidney stone questionnaire and 7026 participants with incomplete variables. In addition, given the limited number of participants who had taken PPI for more than 15 years, the SEs for model estimates increased substantially;15 thus, 146 participants were excluded. Finally, 27 075 participants were included in the analysis, consisting of 13 711 females and 13 364 males. Figure 1 illustrates the filtering process used in this study.
Figure 1.
Study flow chart. Of 59 842 participants in the 2007–2018 National Health and Nutrition Examination Survey, 27 075 remained after fulfilling inclusion and exclusion criteria. PPI, proton pump inhibitor.
Outcome assessment
The primary outcome was the response to the question, ‘Have you ever had kidney stones?’ (NHANES 2007–2018). Participants who responded ‘yes’ were defined as kidney stone formers. The secondary outcome was the response to the question, ‘How many times have you passed a kidney stone?’ (NHANES 2007–2014). Participants who reported passing at least two stones were classified as recurrent stone formers.
Medication use
The independent variables in this study were whether participants had taken PPI and the duration of their PPI use. Information on the types and duration of acid suppressant medication was obtained through prescription medication questionnaires. The types of PPI in this study included omeprazole, esomeprazole, lansoprazole, pantoprazole and rabeprazole. For participants using PPI, the duration of use was equal to the years since initiating therapy. Participants who did not use PPI had duration of use recorded as zero. Data on specific dosages or previously discontinued prescription medications were unavailable.
Covariates
The study collected three types of detailed information about covariates through standardised personal interviews. The first group included demographic factors including age, sex, race, education level, smoking status and alcohol consumption. The second group consisted of factors that impact the body’s metabolism level, including body mass index (BMI), mean arterial pressure, HbA1c, triglyceride levels, history of cardiovascular disease (CVD), thiazide use, loop diuretic use and histamine-2 receptor antagonist (H2RA) use. The third group focused on risk factors related to kidney stone formation, including sedentary time, total water intake, albumin-adjusted calcium levels, estimated glomerular filtration rate (eGFR) and history of gout. Education level was categorised as follows: grades 0–12, high school graduate/General Equivalency Diploma, and some college or above. Smokers were defined as smoking at least 100 cigarettes during their lifetime. BMI was calculated by dividing weight in kilograms (kg) by height in metres squared (m2). History of CVD (including congestive heart failure, coronary heart disease, myocardial infarction and stroke) was defined if participants self-reported a history of these conditions. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate eGFR.16 The CKD-EPI equation is as follows: eGFR=141×min (Scr/κ, 1)α×max (Scr/κ, 1)−1.209×0.993Age×1.018 (if female) 1.159 (if black), where Scr is serum creatinine, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1. Gout was defined as a self-reported diagnosis of gout, and/or the use of anti-gout medication.
Statistical analyses
All statistical analyses considered NHANES design characteristics with sampling weights. Descriptive statistics were used to evaluate the demographic and clinical characteristics of the study population. The variance inflation factor (VIF) was used to evaluate multicollinearity among covariates and between covariates and kidney stones. A VIF value over 10 indicates multicollinearity, but none was observed in this study (online supplemental table 1).17 To explore the relationship between PPI use and kidney stones, we performed four weighted logistic regression models and controlled for the aforementioned explanatory variables by modelling PPI as continuous variables based on the time of use. We used restricted cubic splines to explore the potential non-linear link between PPI use duration and kidney stones. Assessing model fit, we employed the Akaike Information Criterion (AIC). Our knot selection process prioritised the model with the lowest AIC value, leading us to choose a model with three knots located at the 5th, 50th and 95th centiles, as detailed in online supplemental table 2. Subgroup analyses were also performed to explore whether the relationship between the time of PPI use and kidney stones differed by age, sex, race and BMI, and potential effect modifiers were tested using the Wald test for multiplicative interactions. Additionally, a 1:1 propensity score matching (PSM) analysis was performed to balance population differences between PPI users and non-users while adjusting for all confounding variables. Previous studies have established links between kidney stones and dietary factors, such as vitamin C intake, caffeine consumption and the Dietary Inflammatory Index (DII).18–20 To address potential confounding effects, a sensitivity analysis was conducted using model 3 as the baseline, with additional adjustments made for three variables: vitamin C intake, caffeine intake and DII. We conducted a meta-analysis using the ‘meta’ package, which allowed us to combine data from relevant studies and estimate an overall effect size for the association between PPI use and kidney stones. All statistical tests were two sided, and p values of <0.05 (two-sided) were considered statistically significant. R V.4.2.2 software was used for modelling.
bmjopen-2023-075136supp001.pdf (52.7KB, pdf)
bmjopen-2023-075136supp002.pdf (35.2KB, pdf)
Patient and public involvement
None.
Results
Population characteristics
This analysis included 27 075 participants aged 20 years and older from the NHANES database (2007–2018), representing 203 076 872 adults. Table 1 presents their demographic and clinical characteristics based on PPI use. The mean age of all participants was 47.46±0.26 (SE) years, with roughly equal representation of females (51.13%) and males (48.87%). PPI users were more likely to be older; female; non-Hispanic white; obese; have lower education level, alcohol consumption, total water intake and eGFR; and higher sedentary time, mean arterial pressure, HbA1c, triglycerides and albumin-adjusted calcium. PPI users were taking more thiazide, loop diuretics and H2RA medications compared with non-users. Furthermore, CVD, gout and kidney stone diseases were more common in PPI users (all p<0.05).
Table 1.
Demographic and clinical characteristics according to PPI use, NHANES 2007–2018*
| Characteristics | Total adults (N=27 075) |
PPI non-user (N=24 643) |
PPI user (N=2432) |
P value |
| Age, years, mean (SE) | 47.46 (0.26) | 46.38 (0.25) | 59.05 (0.47) | <0.001 |
| Female, n (%) | 13 711 (51.13) | 12 335 (50.63) | 1376 (56.43) | <0.001 |
| Race (non-Hispanic white), n (%) | 11 470 (66.93) | 10 153 (65.94) | 1317 (77.61) | <0.001 |
| Education, n (%) | <0.001 | |||
| Grades 0–12 | 6368 (23.03) | 5671 (14.72) | 697 (17.85) | |
| High school graduate/GED | 6189 (14.99) | 5593 (22.69) | 596 (26.69) | |
| Some college or above | 14 518 (61.98) | 13 379 (62.58) | 1139 (55.46) | |
| Smoking†, n (%) | 5477 (19.65) | 5035 (19.81) | 442 (17.93) | 0.143 |
| Alcohol consumption, n (%) | 6469 (26.38) | 6010 (26.73) | 459 (22.63) | 0.017 |
| BMI, kg/m2‡, mean (SE) | 29.05 (0.09) | 28.88 (0.09) | 30.89 (0.22) | <0.001 |
| Weight status (≥25 kg/m2), n (%)‡ | 19 423 (70.54) | 17 439 (69.47) | 1984 (81.95) | <0.001 |
| Sedentary time, hours/day, mean (SE) | 368.10 (2.86) | 365.58 (2.96) | 395.13 (5.96) | <0.001 |
| Mean arterial pressure, mm Hg, mean (SE) | 87.98 (0.16) | 87.83 (0.17) | 89.59 (0.35) | <0.001 |
| Total water intake, g, mean (SE) | 1171.48 (15.90) | 1180.99 (16.29) | 1069.41 (30.63) | <0.001 |
| HbA1c, %, mean (SE) | 5.63 (0.01) | 5.61 (0.01) | 5.89 (0.03) | <0.001 |
| Triglycerides, mmol/L, mean (SE) | 1.75 (0.02) | 1.73 (0.02) | 1.97 (0.04) | <0.001 |
| Albumin-adjusted calcium, mmol/L, mean (SE) | 2.28 (0.00) | 2.28 (0.00) | 2.30 (0.00) | <0.001 |
| eGFR, mL/min, mean (SE) | 94.33 (0.33) | 95.55 (0.33) | 81.23 (0.61) | <0.001 |
| Gout, n (%) | 403 (1.25) | 309 (1.07) | 94 (3.23) | <0.001 |
| CVD, n (%) | 2595 (9.584) | 2050 (6.641) | 545 (17.438) | <0.001 |
| Congestive heart failure | 805 (2.20) | 589 (1.76) | 216 (6.88) | <0.001 |
| Coronary heart disease | 1080 (3.34) | 829 (2.87) | 251 (8.39) | <0.001 |
| Myocardial infarction | 1082 (3.01) | 828 (2.56) | 254 (7.82) | <0.001 |
| Stroke | 984 (2.78) | 788 (2.43) | 196 (6.47) | <0.001 |
| Thiazide user, n (%) | 2748 (8.66) | 2256 (7.81) | 492 (17.75) | <0.001 |
| Loop diuretic user, n (%) | 876 (2.46) | 626 (1.91) | 250 (8.35) | <0.001 |
| H2RA user, n (%) | 643 (2.33) | 550 (2.26) | 93 (3.12) | 0.030 |
| Kidney stones, n (%) | 2589 (9.80) | 2217 (9.23) | 372 (15.88) | <0.001 |
*Means and percentages were adjusted for survey weights of NHANES.
†Smoking was defined as smoking at least 100 cigarettes during their lifetime.
‡BMI was calculated by dividing weight in kilograms (kg) by height in metres squared (m2). Participants were classified as normal weight (BMI <25 kg/m2) and overweight/obese (BMI ≥25 kg/m2).
BMI, body mass index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; GED, General Equivalency Diploma; H2RA, histamine-2 receptor antagonist; NHANES, National Health and Nutrition Examination Survey; PPI, proton pump inhibitor.
Multivariable logistic regression analyses
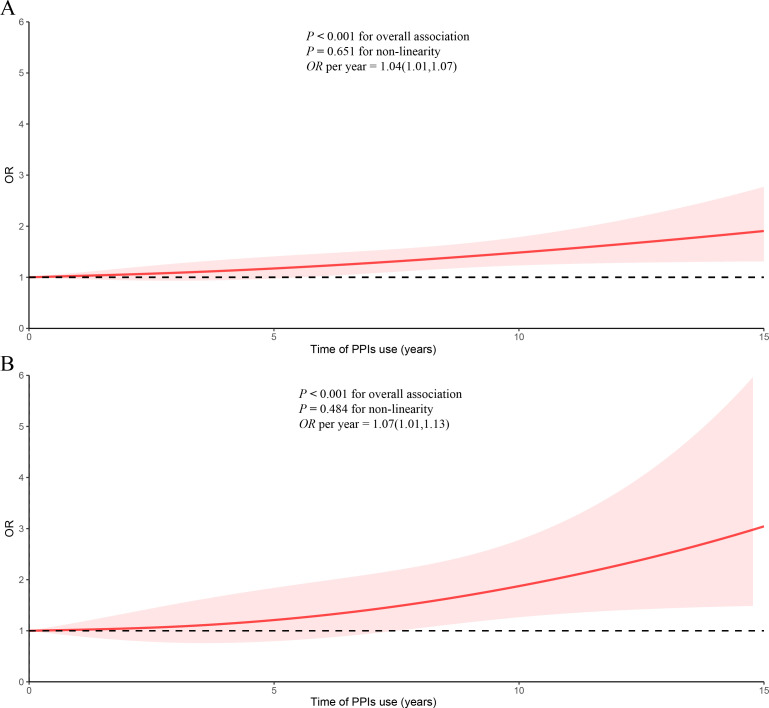
Weighted univariable and multivariable logistic regression models were used to investigate the independent association between PPI use and the risk of kidney stones, with PPI non-user as the reference group (table 2). In the crude model, PPI use showed a significantly positive association with the prevalence of kidney stones (OR=1.86, 95% CI=1.55 to 2.22). In the fully adjusted model (model 3), the association between PPI use and the prevalence of kidney stones remained significant (OR=1.31, 95% CI=1.07 to 1.60). When considering PPI use as a continuous variable, the restricted cubic spline analyses indicated a linear relationship between the duration of PPI use and the prevalence of kidney stones (p for non-linearity=0.651) (figure 2A). With each additional year of PPI use, the prevalence of kidney stones increased by 4% (table 2). Additionally, we explored the association between PPI use and recurrent kidney stones. In the crude model, PPI use showed a significantly positive association with the recurrence of kidney stones (OR=1.49, 95% CI=1.05 to 2.09). This positive association persisted in the fully adjusted model (model 3) (OR=1.49, 95% CI=1.04 to 2.13). The duration of PPI use exhibited a linear correlation with the recurrence of kidney stones (p for non-linearity=0.484) (figure 2B), with a 7% increase for each additional year of PPI use (table 2).
Table 2.
OR (95% CI) for kidney stones across PPI use*
| Crude model | Model 1 | Model 2 | Model 3 | |
| Kidney stones (N=2589) vs non-kidney stones (N=24 486) (NHANES 2007–2018) | ||||
| PPI use | ||||
| No | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Yes | 1.86 (1.55, 2.22) | 1.42 (1.18, 1.72) | 1.32 (1.09, 1.61) | 1.31 (1.07, 1.60) |
| Time of use (years) | 1.09 (1.07, 1.12) | 1.05 (1.02, 1.08) | 1.04 (1.01, 1.07) | 1.04 (1.01, 1.07) |
| Recurrent kidney stones (N=550) vs first kidney stone (N=1138) (NHANES 2007–2014) | ||||
| PPI use | ||||
| No | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Yes | 1.49 (1.05, 2.09) | 1.49 (1.05, 2.13) | 1.47 (1.03, 2.10) | 1.49 (1.04, 2.13) |
| Time of use (years) | 1.07 (1.01, 1.12) | 1.06 (1.01, 1.12) | 1.06 (1.01, 1.12) | 1.07 (1.01, 1.13) |
Model 1 was adjusted for age, sex, race, education level, smoking and alcohol consumption.
Model 2 was adjusted for age, sex, race, education level, smoking, alcohol consumption, BMI, mean arterial pressure, HbA1c, triglycerides, history of CVD, thiazide use, loop diuretic use and H2RA use.
Model 3 was additionally adjusted for sedentary time, total water intake, albumin-adjusted calcium, eGFR and history of gout.
*Values are numerical values or weighted OR (95% CI).
BMI, body mass index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; H2RA, histamine-2 receptor antagonist; PPI, proton pump inhibitor.
Figure 2.
Dose–response relationships between time of PPI use and kidney stones: (A) time of PPI use and kidney stones; (B) time of PPI use and recurrent kidney stones. Adjusted for age, sex, race, education level, smoking, alcohol consumption, BMI, mean arterial pressure, HbA1c, triglycerides, history of CVD, gout, thiazide use, loop diuretic use and H2RA use, sedentary time, total water intake, albumin-adjusted calcium and eGFR. The shaded part represents the 95% CI. BMI, body mass index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; H2RA, histamine-2 receptor antagonist; PPIs, proton pump inhibitors.
Subgroup analyses
Moreover, subgroup analyses were performed to assess whether the relationship between the duration of PPI use and kidney stones was influenced by age, sex, race and BMI (table 3). After adjusting for all covariates, it was found that the duration of PPI use was significantly associated with the prevalence of kidney stones in participants aged 50 years or older, females, non-Hispanic white and those with a BMI of 25 kg/m2 or higher. On the other hand, a significant positive association between time of PPI use and recurrent kidney stones was observed only in non-Hispanic white participants, and those with a BMI of 25 kg/m2 or higher (all p for interaction>0.05).
Table 3.
OR (95% CI) for kidney stones across time of PPI use stratified by selected factors*
| Kidney stones vs non-kidney stones | Recurrent kidney stones vs first kidney stone | |||||
| OR (95% CI) | P value | P for interaction | OR (95% CI) | P value | P for interaction | |
| Age | 0.439 | 0.419 | ||||
| <50 years | 1.05 (0.98, 1.11) | 0.150 | 1.11 (0.98, 1.27) | 0.104 | ||
| ≥50 years | 1.04 (1.01, 1.07) | 0.004 | 1.07 (1.00, 1.14) | 0.053 | ||
| Sex | 0.856 | 0.623 | ||||
| Female | 1.06 (1.02, 1.10) | 0.004 | 1.08 (0.99, 1.18) | 0.099 | ||
| Male | 1.02 (0.98, 1.07) | 0.258 | 1.06 (0.98, 1.14) | 0.156 | ||
| Race | 0.365 | 0.282 | ||||
| Non-Hispanic white | 1.04 (1.01, 1.07) | 0.005 | 1.11 (1.01, 1.22) | 0.037 | ||
| Other | 1.02 (0.98, 1.06) | 0.422 | 1.07 (1.00, 1.13) | 0.038 | ||
| BMI | 0.684 | 0.922 | ||||
| <25 kg/m2 | 1.06 (0.98, 1.14) | 0.134 | 1.04 (0.90, 1.22) | 0.569 | ||
| ≥25 kg/m2 | 1.04 (1.01, 1.06) | 0.013 | 1.07 (1.01, 1.15) | 0.029 | ||
Adjusted for age, sex, race, education level, smoking, alcohol consumption, BMI, mean arterial pressure, HbA1c, triglycerides, history of CVD, gout, thiazide use, loop diuretic use and H2RA use, sedentary time, total water intake, albumin-adjusted calcium and eGFR, if not already stratified.
*Values are numerical values or weighted OR (95% CI).
BMI, body mass index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; H2RA, histamine-2 receptor antagonist; PPI, proton pump inhibitor.
Sensitivity analyses and meta-analysis
A 1:1 matched cohort analysis was conducted through PSM to minimise potential bias, given the significant difference in PPI use and non-PPI use groups (table 1). This approach confirmed 4864 participants in the matched cohort. The descriptive statistics results showed that no significant differences were observed in most variables between the PPI non-user and PPI user groups (online supplemental table 3). In the fully adjusted model, the dose–response curve still displayed a positive association between the duration of PPI use and kidney stones (OR=1.05, 95% CI=1.02 to 1.08, p for non-linearity=0.956) (online supplemental figure 1A) and recurrent kidney stones (OR=1.10, 95% CI=1.02 to 1.18, p for non-linearity=0.488) (online supplemental figure 1B). Moreover, the results remained significant after making additional adjustments for vitamin C intake, caffeine consumption and DII (online supplemental table 4). Furthermore, we performed a meta-analysis based on our findings and previously published research, confirming a positive association between PPI use and the risk of kidney stones (OR=1.49, 95% CI=1.05 to 2.10) (online supplemental figure 2).10–12
bmjopen-2023-075136supp003.pdf (103.6KB, pdf)
bmjopen-2023-075136supp004.pdf (127.1KB, pdf)
bmjopen-2023-075136supp005.pdf (88.8KB, pdf)
bmjopen-2023-075136supp006.pdf (64.8KB, pdf)
Discussion
In this large cross-sectional study based on NHANES data from 2007 to 2018, we found that PPI use was associated with an increased risk of kidney stones. The duration of PPI use demonstrated a dose–response association with kidney stones. Furthermore, our study uncovered a novel association between long-term PPI use and recurrent kidney stones in patients with a history of kidney stones, demonstrating a significant linear correlation. Additionally, subgroup analysis found that age, sex, race and BMI varied in their influence on the relationship between PPI use and the prevalence of kidney stones.
Several studies have shown that PPI use could increase the risk of kidney stones, with a dose–response relationship.10–12 A retrospective study conducted on the Women’s Veterans Cohort, which included 465 891 individuals, revealed that PPI use was linked to a 1.25-fold higher risk of kidney stones (95% CI=1.19 to 1.33).10 It should be noted that this study included mainly young individuals (with a median age of 32 years) who were predominantly males (86%), thus having a certain degree of selection bias.13 Another study by Sui et al also found a positive association between PPI use and kidney stones in patients with GERD, with a 1.46-fold increased risk (95% CI=1.38 to 1.55), which could help in assessing the potential risk of kidney stones associated with PPI exposure.11 Nevertheless, both studies were limited to specific populations, limiting the generalisability of their findings to the general population. In contrast, a nationwide population cohort from Korea, without selection bias, also showed a positive association between PPI use and kidney stones, displaying a dose–response relationship.12 Similarly, the current study, based on data from the NHANES database representing over 203 million individuals, found that PPI use was significantly associated with not only a higher risk of kidney stones, but also recurrent kidney stones. The findings from the meta-analysis conducted in this study have confirmed the positive association between PPI use and the risk of kidney stones. Furthermore, the risk of developing kidney stones was found to be higher in individuals who used PPI for longer duration, highlighting the importance of monitoring this potential side effects of long-term PPI treatment, especially for patients with a history of kidney stones.
The mechanisms underlying the impact of PPI on kidney stone formation remain unclear. Studies have suggested that PPI can elevate gastric pH, leading to a decrease in magnesium absorption and urinary magnesium levels.9 Magnesium has been known to inhibit the formation of calcium oxalate crystals in urine.21 22 A meta-analysis of nine observational studies found a significant increased risk of hypomagnesaemia among patients using PPI.23 It should be noted that magnesium absorption occurs through both active and passive mechanisms, and alterations in pH do not affect passive absorption.23 Therefore, PPI use does not always result in hypomagnesaemia, but patients with impaired gastrointestinal absorptive capacity may have an increased risk of developing hypomagnesaemia. On the other hand, research has shown that citrate can inhibit the crystallisation of calcium salts in urine, and a deficiency of citrate can increase the risk of stone formation.24 25 A study of 301 patients with nephrolithiasis with 24-hour urine data found that PPI exposure significantly reduced urinary citrate excretion, but did not affect urinary magnesium, pH or other urinary minerals.8 Similarly, another study on patients with GERD reported a significant correlation between PPI use and lower levels of urinary citrate and magnesium.11 Therefore, given the association of PPI use with hypomagnesuria and hypocitraturia, it may monitor the levels of urinary magnesium and citrate when using PPI.
PPIs are commonly prescribed for acid-related disorders, and patients with these conditions may be at higher risk of kidney stone formation.26 In this study, we employed the PSM analysis to minimise potential differences between PPI users and non-users, yet still identified a significant association between PPI use and the occurrence and recurrence of kidney stones. Subgroup analyses further revealed that certain patient groups, including the elderly, females, non-Hispanic white and those with a BMI of 25 kg/m2 or higher, exhibited a stronger positive association between PPI use and the prevalence of kidney stones, highlighting the importance of considering potential side effects of PPI use in these populations. While it is undeniable that PPI therapy has improved the quality of life for many patients with acid-related disorders,27 a growing body of literature suggested a relationship between long-term PPI use and adverse events.28 Caution should be exercised when discontinuing PPI use for evidence-based indications,29 but global concerns over long-term PPI overuse should not be overlooked,6 7 especially in individuals with a history of kidney stones and high-risk factors, such as the elderly, females, non-Hispanic white and those with a BMI of 25 kg/m2 or higher, in order to reduce unnecessary use.
This study has several strengths. First, the NHANES dataset comprises a representative sample of the national population, and we use NHANES-provided weights to ensure that our findings can be extrapolated to the broader population. Second, this study not only elucidates the correlation between PPI use and the prevalence of kidney stones but also probes its association with the recurrence of renal calculi in individuals with a history of nephrolithiasis. Furthermore, multiple potential confounders were adjusted and PSM design was performed to ensure the reliability of the results. However, this study also has several limitations. First, it is difficult to draw causal conclusions from such cross-sectional analyses. Although we adjusted for three types of detailed covariate information, there may still be unmeasured potential factors that could affect the association between PPI and nephrolithiasis. Second, the questionnaire survey may have been prone to recall bias and reporting bias, which could affect the accuracy of the data collected. Third, NHANES lacks objective diagnostic imaging for the identification of kidney stones, potentially resulting in the omission of asymptomatic cases. Additionally, the dataset does not provide details on the timing and specific type of kidney stones. Finally, the lack of information about the dosage and type of PPI use may limit the interpretability of the results.
Conclusions
In conclusion, our study revealed a relationship between PPI use and the prevalence of kidney stones, as well as an increased risk of recurrent kidney stones in patients with a history of nephrolithiasis. To mitigate this potential adverse effect, caution should be exercised regarding unnecessary long-term use of PPI.
Supplementary Material
Acknowledgments
We appreciate the American Centers for Disease Control and Prevention for conducting the survey and making it available online freely, and all the participants for providing these data.
Footnotes
WL and JW contributed equally.
Contributors: WL—conceptualisation, methodology, data analysis and manuscript writing. JW—methodology, data collection, data analysis and manuscript writing. MiaoW—methodology, data collection and data analysis. MiaomiaoW—data analysis, manuscript writing and supervision. ML—conceptualisation, supervision, manuscript editing, funding acquisition and the guarantor for the overall content.
Funding: This work was supported by the National Key Research and Development Program of China (2022YFC3602900) and National High Level Hospital Clinical Research Funding (BJ-2022-115).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Publicly available datasets were analysed in this study. These data can be downloaded at https://www.cdc.gov/nchs/nhanes/ (NHANES 2005–2006 and 2007–2008).
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethical review and approval for the original research involving human participants were obtained from the Ethics Review Board of the National Center for Health Statistics (protocol #98-12). Written informed consent was obtained from all patients or participants who were part of the study.
References
- 1.Saigal CS, Joyce G, Timilsina AR, et al. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management Kidney Int 2005;68:1808–14. 10.1111/j.1523-1755.2005.00599.x [DOI] [PubMed] [Google Scholar]
- 2.Abufaraj M, Xu T, Cao C, et al. Prevalence and trends in kidney stone among adults in the USA: analyses of national health and nutrition examination survey 2007-2018 data. Eur Urol Focus 2021;7:1468–75. 10.1016/j.euf.2020.08.011 [DOI] [PubMed] [Google Scholar]
- 3.Dauw CA, Yi Y, Bierlein MJ, et al. Factors associated with preventive pharmacological therapy adherence among patients with kidney stones. Urology 2016;93:45–9. 10.1016/j.urology.2016.03.030 [DOI] [PubMed] [Google Scholar]
- 4.Daudon M, Frochot V, Bazin D, et al. Drug-induced kidney stones and crystalline nephropathy: pathophysiology. Drugs 2018;78:163–201. 10.1007/s40265-017-0853-7 [DOI] [PubMed] [Google Scholar]
- 5.Cohen AJ, Adamsky MA, Nottingham CU, et al. Impact of Statin intake on kidney stone formation. Urology 2019;124:57–61. 10.1016/j.urology.2018.01.029 [DOI] [PubMed] [Google Scholar]
- 6.Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Expert Rev Clin Pharmacol 2018;11:1123–34. 10.1080/17512433.2018.1531703 [DOI] [PubMed] [Google Scholar]
- 7.Eusebi LH, Rabitti S, Artesiani ML, et al. Proton pump inhibitors: risks of long-term use. J Gastroenterol Hepatol 2017;32:1295–302. 10.1111/jgh.13737 [DOI] [PubMed] [Google Scholar]
- 8.Patel PM, Kandabarow AM, Aiwerioghene E, et al. Proton-pump inhibitors associated with decreased urinary citrate excretion. Int Urol Nephrol 2021;53:679–83. 10.1007/s11255-020-02719-0 [DOI] [PubMed] [Google Scholar]
- 9.Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep 2010;12:448–57. 10.1007/s11894-010-0141-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonov M, Abel EA, Skanderson M, et al. Use of proton pump inhibitors increases risk of incident kidney stones. Clin Gastroenterol Hepatol 2021;19:72–9. 10.1016/j.cgh.2020.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sui W, Miller NL, Gould ER, et al. Proton pump inhibitors use and risk of incident nephrolithiasis. Urolithiasis 2022;50:401–9. 10.1007/s00240-022-01326-1 [DOI] [PubMed] [Google Scholar]
- 12.Kim SY, Yoo DM, Bang WJ, et al. Association between urolithiasis and history proton pump inhibitor medication: A nested case-control study. J Clin Med 2022;11:5693. 10.3390/jcm11195693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pella E, Chalkidou M, Sarafidis P. Proton pump inhibitors, histamine-2 receptor antagonists, and the risk of kidney stones: negligible or not? Clin Gastroenterol Hepatol 2021;19:624–5. 10.1016/j.cgh.2020.04.079 [DOI] [PubMed] [Google Scholar]
- 14.NHANES questionnaires, datasets, and related documentation. Available: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx [Accessed 14 Apr 2023].
- 15.Chang SL, Harshman LC, Presti JC. Impact of common medications on serum total prostate-specific antigen levels: analysis of the national health and nutrition examination survey. J Clin Oncol 2010;28:3951–7. 10.1200/JCO.2009.27.9406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charidimou A, Martinez-Ramirez S, Reijmer YD, et al. Total magnetic resonance imaging burden of small vessel disease in cerebral amyloid angiopathy: an imaging-pathologic study of concept validation. JAMA Neurol 2016;73:994–1001. 10.1001/jamaneurol.2016.0832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferraro PM, Curhan GC, Gambaro G, et al. Total, dietary, and supplemental vitamin C intake and risk of incident kidney stones. Am J Kidney Dis 2016;67:400–7. 10.1053/j.ajkd.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geng J, Qiu Y, Kang Z, et al. The association between caffeine intake and risk of kidney stones: a population-based study. Front Nutr 2022;9:935820. 10.3389/fnut.2022.935820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu N, Feng Y, Li J, et al. Relationship between the dietary inflammatory index and kidney stone prevalence. World J Urol 2022;40:1545–52. 10.1007/s00345-022-03998-1 [DOI] [PubMed] [Google Scholar]
- 21.Schwartz BF, Bruce J, Leslie S, et al. Rethinking the role of urinary magnesium in calcium urolithiasis. J Endourol 2001;15:233–5. 10.1089/089277901750161638 [DOI] [PubMed] [Google Scholar]
- 22.Johansson G, Backman U, Danielson BG, et al. Effects of magnesium hydroxide in renal stone disease. J Am Coll Nutr 1982;1:179–85. 10.1080/07315724.1982.10718985 [DOI] [PubMed] [Google Scholar]
- 23.Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail 2015;37:1237–41. 10.3109/0886022X.2015.1057800 [DOI] [PubMed] [Google Scholar]
- 24.Goldberg H, Grass L, Vogl R, et al. Urine citrate and renal stone disease. CMAJ 1989;141:217–21. [PMC free article] [PubMed] [Google Scholar]
- 25.Pak CY. Citrate and renal calculi: an update. Miner Electrolyte Metab 1994;20:371–7. [PubMed] [Google Scholar]
- 26.Bapir R, Bhatti KH, Eliwa A, et al. Risk of urinary stone formation associated to proton pump inhibitors: a systematic review and Metanalysis. Arch Ital Urol Androl 2022;94:507–14. 10.4081/aiua.2022.4.507 [DOI] [PubMed] [Google Scholar]
- 27.Moayyedi P, Armstrong D, Hunt RH, et al. The gain in quality-adjusted life months by switching to esomeprazole in those with continued reflux symptoms in primary care: encompass--a cluster-randomized trial. Am J Gastroenterol 2010;105:2341–6. 10.1038/ajg.2010.368 [DOI] [PubMed] [Google Scholar]
- 28.Elias E, Targownik LE. The clinician's guide to proton pump inhibitor related adverse events. Drugs 2019;79:715–31. 10.1007/s40265-019-01110-3 [DOI] [PubMed] [Google Scholar]
- 29.Boghossian TA, Rashid FJ, Thompson W, et al. Deprescribing versus continuation of chronic proton pump inhibitor use in adults. Cochrane Database Syst Rev 2017;3:CD011969. 10.1002/14651858.CD011969.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-075136supp001.pdf (52.7KB, pdf)
bmjopen-2023-075136supp002.pdf (35.2KB, pdf)
bmjopen-2023-075136supp003.pdf (103.6KB, pdf)
bmjopen-2023-075136supp004.pdf (127.1KB, pdf)
bmjopen-2023-075136supp005.pdf (88.8KB, pdf)
bmjopen-2023-075136supp006.pdf (64.8KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Publicly available datasets were analysed in this study. These data can be downloaded at https://www.cdc.gov/nchs/nhanes/ (NHANES 2005–2006 and 2007–2008).