Abstract
Background:
We aim to assess the impact of montelukast on paediatric patients with asthma/allergic rhinitis, measured using patient-reported outcome measures, compared with other treatments or placebo.
Methods:
Protocol registration CRD42020216098 (www.crd.york.ac.uk/PROSPERO). MEDLINE and Embase databases were used to conduct the search. Two authors independently selected studies and extracted data, and a third reviewer resolved discrepancies. Meta-analyses were constructed to estimate the standardised mean difference (SMD) using a random-effects model.
Results:
Out of 3937 articles identified, 49 studies met the inclusion criteria, mostly randomised clinical trials (sample sizes: 21–689 patients). The SMD of change pooled estimators for the global, mental and physical domains of health-related quality of life were not statistically significant. For daytime and night-time symptoms scores, the SMD (95% CI) was in favour of inhaled corticosteroids (−0.12, −0.20– −0.05 and −0.23, −0.41– −0.06, respectively). The pooled estimator for global asthma symptoms was better for montelukast when compared with placebo (0.90, 0.44–1.36).
Conclusions:
The synthesis of the available evidence suggests that, in children and adolescents, montelukast was effective in controlling asthma symptoms when compared with placebo, but inhaled corticosteroids were superior in controlling symptoms, especially at night-time. These findings of our systematic review concur with current guidelines for asthma treatment.
Tweetable abstract
This is the first systematic review focused on the impact of montelukast on children and adolescents with asthma, from the patients’ perspective, measured with patient-reported outcome measures (PROMs) https://bit.ly/3YwqfBb
Introduction
Asthma and allergic rhinitis affect >300 million people globally [1, 2], comprising 10–30% of all adults and up to 40% of children. Montelukast, a leukotriene receptor antagonist (LTRA), was approved by the United States Food and Drug Administration (FDA) in 1998 for the treatment of asthma and in 2002 for allergic rhinitis [3]. Currently, asthma treatment guidelines [4, 5] include LTRAs in the category of other controller options (in monotherapy or in combination with inhaled corticosteroids (ICS)), highlighting their limited indications and the lack of evidence for efficacy or safety. The guideline for allergic rhinitis [6] limits montelukast only as a treatment option for patients who are not effectively treated with alternative therapies.
In 2009, the FDA requested a review of the clinical trials with montelukast performed by Merck KGaA, whose results indicated that suicidality and behaviour-related adverse experiences were infrequent and similar to those seen in control subjects [7, 8]. Nonetheless, several cases were notified between 2014 and 2018 as suspected adverse neuropsychiatric events [9]. In 2019, a European Medicines Agency review [10] identified some cases in which there had been a delay in recognising neuropsychiatric events as possible adverse drug reactions, and the United Kingdom Medicines and Healthcare Products Regulatory Agency activated a warning including nightmares, depression, insomnia, aggression, anxiety and abnormal or changed behaviour. In 2020, the FDA required a boxed warning advising healthcare providers to avoid prescribing montelukast for patients with mild symptoms [11]. The majority of systematic reviews evaluating montelukast adverse events were performed before the release of the FDA's warning [12–14]. To the best of our knowledge, after the release there was only one systematic review aimed at identifying montelukast adverse drug reactions reported in the medical literature [15], which found a wide range of suspected reactions, predominantly gastrointestinal and neuropsychiatric, and a study reviewing reports of suspected adverse drug reactions to montelukast in the World Health Organization's database (VigiBase) [16], which found 1118 reports of nightmares; two-thirds of which concerned children aged 5–10 years.
The impact of montelukast adverse reactions could be captured through patient-reported outcomes measures (PROMs), which cover a variety of constructs from the patient's perspective, including symptoms and health-related quality of life (HRQoL) among others [17]. Asthma and rhinitis guidelines recommend the use of PROMs to monitor symptom control and to measure the impact of the disease on daily activities and HRQoL [4, 5, 18, 19].
We found five systematic reviews evaluating the efficacy of montelukast or LTRA specifically on paediatric asthma patients [13, 20–23], confirming the superiority of ICS in symptom control [20, 21, 24], symptom scores [13], symptom-free days [24], night-time awakening [24] and HRQoL [24]. Other systematic reviews compared LTRA with placebo [12], or as an addition to usual care [23]. PROMs were secondary end-points in most of these systematic reviews and no meta-analyses were constructed with them. We identified three systematic reviews including adolescents and adults with allergic rhinitis, which compared montelukast with placebo, antihistamines and intranasal corticosteroid [25–27], all considering PROMs as primary and secondary outcomes (symptoms and HRQoL, respectively), but none included a meta-analysis with paediatric patients.
There are few systematic reviews in children and adolescents evaluating montelukast with PROMs, especially for HRQoL in asthma patients. Our primary aim was to evaluate the impact of montelukast on paediatric patients with asthma and/or allergic rhinitis, measured with PROMs, when compared with other treatments or with placebo, through a systematic review and meta-analysis. In addition, we considered a synthesis of the evidence about adverse drug events and withdrawals in the selected studies.
Methods
Protocol and registration
We conducted a systematic review of the literature on the impact of montelukast in children and adolescents with asthma and/or allergic rhinitis, measured with PROMs (https://www.crd.york.ac.uk/PROSPERO identifier CRD42020216098), following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses methodological standards [28].
Eligibility criteria
We consider as inclusion criteria: studies including children and adolescents (aged <18 years) with a diagnosis of asthma or allergic rhinitis, comparing montelukast with other treatments or placebo, with or without randomisation, and with at least one end-point measured with a validated PROM. No limitation of language, region or year of publication was considered.
Studies were excluded if samples were composed of patients with multimorbidity, case–control, a longitudinal design without a comparison group, comparison groups with nonpharmacological intervention, data not stratified for patients aged <18 years, with a small sample size (n≤30) or not published as original articles.
Information sources and search
The search was conducted in Ovid MEDLINE and Ovid Embase databases on 1 June 2022, and updated last on 31 January 2023. We searched for both Medical Subject Headings (MeSH) and text word terms including “asthma”, “rhinitis”, “leukotriene antagonists” and “patient-reported outcome measures”. The detailed search strategy can be found in the supplementary material (supplementary figure S1). In addition, a manual search was performed of the reference list of selected articles and other published systematic reviews.
Selection process
Two members (K. Mayoral, V. Zamora) independently reviewed titles and abstracts using the Covidence software (www.covidence.org). A pilot test was conducted to standardise criteria among reviewers. Two pairs of members (K. Mayoral, C. Lizano-Barrantes; V. Zamora, C. Miret) reviewed the articles’ full text, to select the articles for data extraction. Disagreements in all phases were resolved through discussion, with the participation of a third party (M. Ferrer).
Data collection process and data items
Data extraction was carried out by one researcher (K. Mayoral, C. Lizano-Barrantes or V. Zamora) with independent verification performed by a statistician (A. Pont). We designed a pre-defined data collection form in Microsoft Excel with the information to be extracted: author and year of publication, study design, follow-up period, sample size and age, diagnosis and severity, intervention and control groups, PROMs administered, type of end-point and central tendency and dispersion statistics, adverse events and withdrawals. Authors were contacted via email to request any PROM information not reported in the manuscript.
Study risk-of-bias assessment
To assess the risk of bias we used RoB 2 version 2 [29], or the Risk of Bias in Non-randomized Studies of Interventions (ROBINS)-I [30] tool, according to the study design. Both tools have a common set of domains of bias: deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported result. For the nonrandomised studies, the bias due to confounding, selection of participants and classification of interventions was added and, for the randomised controlled trials (RCTs), the bias arising from the randomisation process. The risk of bias arising from each domain is classified for the RoB 2 as “low”, “high” or “some concerns”; and for ROBINS-I, “serious”, “moderate”, “low”, or “no information”. We used the Robvis tool [31] for presenting the risk-of-bias results in graphics.
Summary measures
The primary outcome was defined as the difference between treatment groups on the mean change of the PROM score. As studies differed in the instrument to gather PROMs and algorithms to construct scores also differed among instruments, PROMs results were standardised by applying the escalc function in R-4.2.0 [32]. Either the difference between treatment groups on the mean change of the PROM score or the mean change of each group was used to estimate the standardised mean difference (SMD). Otherwise, we calculated the mean change through a basic subtraction of the means at baseline and at follow-up evaluations for each group and the pooled standard deviation. Standardising PROM results in units of standard deviations allows them to be directly comparable, and to interpret the magnitude (effect size) of the SMD [33] as small for 0.2 sd, moderate for 0.5 sd and large for 0.8 sd, regardless of the instrument used to gather PROMs.
Synthesis of results
Aggregated data were described as part of the general narrative synthesis. Meta-analyses for quantitative syntheses were constructed to test the impact of montelukast on PROMs, by performing subgroup analyses according to type of asthma (considering severity or chronicity). A random-effects model (DerSimonian–Laird method) was employed due to expecting variation in study populations, diseases and data collection. Heterogeneity among studies was evaluated using the I2 statistic and categorised as 25–50% low, 50–75% moderate and >75% high [34]. To explore a possible publication bias, a funnel plot was planned when the number of studies pooled is ≥10. The statistical analysis software programme used was RevMan version 5.4 [35].
Additional analyses
Sensitivity analyses by risk of bias were carried out: studies rated as high risk of bias using the tool RoB 2 or the ROBINS-I were excluded as they were considered a potential source of heterogeneity.
Results
Study selection
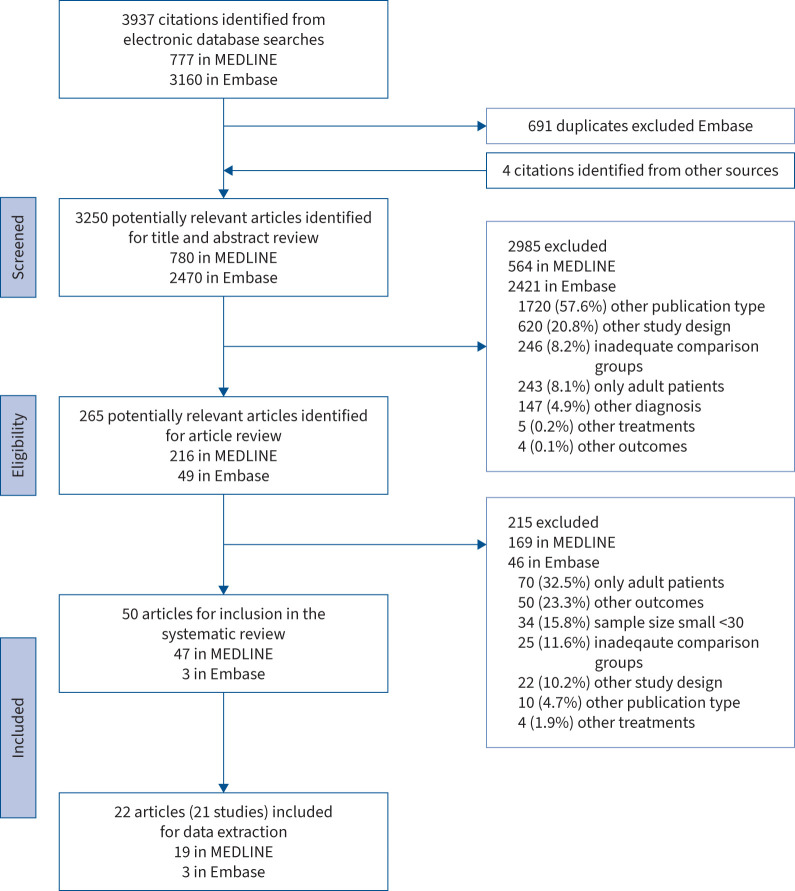
Figure 1 shows that 3937 articles were identified, 3250 titles and abstracts were reviewed and a complete reading of 265 articles was carried out. Of them, 215 were excluded mostly due to “only adult patients” (32.5%), “other outcomes” (23.3%) and “small sample size (n<30)” (15.8%). Finally, of the 50 articles that fulfilled the inclusion criteria, 28 did not report sufficient data to be included in the meta-analysis (supplementary table A) and 22 did [36–57]. Data from five articles [43, 44, 50, 53, 57] were obtained by contacting the sponsor (a pharmaceutical company) or authors.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of the systematic literature review.
Study characteristics
The 22 articles (21 studies) included in some of the meta-analyses (table 1) [36–57] were published between 1998 and 2015, and all assessed asthma, except for one study evaluating allergic rhinitis [54] and another one evaluating patients with both conditions [53]. All studies were performed only in paediatric samples except for four including adults, but with data stratified by age [43–45, 53]. Sample size ranged from 21 to 689, and the follow-up period was from 2 to 52 weeks. There were seven studies using ICS in monotherapy as the comparator group [36–42], five studies using diverse treatments [43–47] and nine studies (10 articles) comparing it only with placebo [48–57]. All administered PROMS were disease-specific, except for a RCT which used the generic questionnaire Child Health Questionnaire Parent Form-50 (CHQ-PF50) [40]. The constructs collected were symptoms (differentiating between daytime and night-time, or considered globally) and HRQoL.
TABLE 1.
Characteristics of the included studies
|
First author [reference], publication year;
study funding |
Study design; follow-up period |
Sample size;
age range (mean) |
Diagnosis |
Intervention
or control group |
PROM administered | PROM as primary, secondary or tertiary outcome | End-point measure | Adverse events reported |
| LTRA versus ICS | ||||||||
| Bukstein [36], 2003; Merck & Co, Clinical Development, US Human Health |
Observational; 12 months |
Total n=104 6–15 years (9.6 years) |
Mild persistent asthma | I: montelukast (10 mg) C: fluticasone (176 μg) |
ATAQ | Secondary | Mean±sd | No |
| Stelmach [37], 2005#; Medical University of Lodz, Poland |
RCT; 6 months |
Total n=51 6–18 years (11.8 years) |
Newly diagnosed atopic asthma | I: montelukast (5 or 10 mg) C: budesonide (400 or 800 μg) |
Asthma symptom score (0–9) (daytime/night-time/β2-agonist use) | Secondary | Mean±sem | No |
| García-García [38], 2005; Merck & Co |
RCT; 12 months |
Total n=541 6–14 years (median 9 years) |
Mild persistent asthma | I: montelukast (5 mg) C: fluticasone (200 μg) |
PATAQ overall | Tertiary | Mean change (95% CI) | Yes |
| Ostrom [39], 2005#; GlaxoSmithKline Inc |
RCT; 12 weeks |
Total n=342 6–12 years (9.35 years) |
Chronic asthma | I: montelukast (5 mg) C: fluticasone (100 μg) |
Daytime symptom score (0–5) Night-time symptom score (0–3) Satisfaction with treatment+ |
Not specified | Mean±sem Mean change±sem |
Yes |
| Szefler [40], 2007; AstraZeneca LP |
RCT; 52 weeks |
Total n=312 2–8 years (4.65 years) |
Mild persistent asthma | I: montelukast (5 mg) C: budesonide (500 μg) |
CHQ-PF50: general/ activities CHSA: physical/activities/emotional Asthma symptom score (am/pm) |
Secondary | Mean±sd Mean±sem Adjusted mean change±sem |
Yes |
| Olszowiec-Chlebna [41], 2010; Medical University of Lodz, Poland |
RCT; 6 months |
Total n=60 5–18 years (8.53 years) |
Newly diagnosed asthma | I: montelukast (5 or 10 mg) C: budesonide (400 and 800 μg) |
ATAQ | Not specified | Mean±sd | No |
| Parisi [42], 2022; University of Catania, Italy |
RCT; 12 weeks |
Total n=42 2–6 years (3.5 years) |
Recurrent wheezing | I: montelukast (4 or 5 mg) C: beclomethasone (200 μg) |
TRACK | Primary | Mean±sd | Yes |
| LTRA versus other treatment combinations | ||||||||
| Malmstrom [43], 1999¶; Merck Research Laboratories |
RCT; 12 weeks |
Total n=895 >15 years Paediatric n=45 15–18 years |
Chronic asthma |
I: montelukast (10 mg) C: beclomethasone (400 μg) C: placebo |
Daytime asthma symptoms Global evaluation question+ AQLQ+ |
Primary | Mean±sd Mean change±sd |
Yes |
| Williams [44], 2001¶; Merck Research Laboratories |
RCT; 16 weeks |
Total n=1055 15–85 years Paediatric n=245 6–14 years |
Chronic asthma | I: montelukast (5 or 10 mg) C: beclomethasone (300 μg) C: placebo |
PAQLQ: activity/symptom/emotional | Not specified | Mean Adjusted mean change (95% CI) |
No |
| Peters [45], 2007; GlaxoSmithKline, American Lung Association |
RCT; 16 weeks |
Total n=500 >15 years Paediatric n=245 6–14 years |
Mild persistent controlled asthma | I: montelukast (5 or 10 mg) C: fluticasone (200 μg) C: fluticasone (100 μg) + salmeterol (50 μg) |
Mini AQLQ ASUI+ ACQ+ |
Secondary | Adjusted mean±sd (95% CI) | No |
| Máspero [46], 2008; GlaxoSmithKline |
RCT; 12 weeks |
Total n=548 6–14 years (9.3 years) |
Persistent uncontrolled asthma | I: montelukast (5 mg) C: salmeterol/fluticasone (100/200 μg) |
PAQLQ Asthma symptom score (0–5)+ |
Secondary | Adjusted mean change±sem |
No |
| Bérubé [47], 2014; Merck Frosst Canada Ltd |
Observational; 12 weeks |
Total n=328 2–14 years (6.92 years) |
Uncontrolled asthma | I: montelukast (4 or 5 mg) C: montelukast (4 or 5 mg) ICS (different doses) |
ACQ | Primary and secondary | Mean±sd (95% CI) Mean change±sd |
No |
| LTRA versus placebo | ||||||||
| Knorr [48], 1998; Merck & Co Inc Becker [49], 2004; Merck & Co Inc |
RCT; 8 weeks |
Total n=336 6–14 years (median 11 years) |
Mild asthma | I: montelukast (5 mg) C: placebo |
PAQLQ: activity/symptom/emotional Daytime symptom score (0–5) Night-time symptom score (0–5) |
Primary | Mean±sd Mean change (95% CI) |
No |
| Knorr [50], 2001¶; Merck Research Laboratories |
RCT; 12 weeks |
Total n=689 2–5 years (3.6 years) |
Mild to severe persistent asthma | I: montelukast (4 mg) C: placebo |
Daytime-symptom score (0–5) Overnight asthma symptoms |
Secondary | Mean change (95% CI) | Yes |
| Stelmach [51], 2002; Medical University of Lodz, Poland |
RCT; 6 weeks |
Total n=32 6–18 years (13.5 years) |
Mild to moderate asthma | I: montelukast (5 or 10 mg) C: placebo |
Asthma symptom score (0–9) (daytime/night-time/β2-agonist use) | Secondary | Mean±sd (95% CI) |
No |
| Stelmach [52], 2002#; Medical University of Lodz, Poland |
RCT; 8 weeks |
Total n=49 9–15 years (11.9 years) |
Moderate atopic asthma | I: montelukast (5 or 10 mg) C: placebo |
Asthma symptom score (0–9) (daytime/night-time/β2-agonist use) PAQLQ+ |
Not specified | Mean±sd (95% CI) |
No |
| Philip [53], 2004¶; Merck Research Laboratories |
RCT; 2 weeks |
Total n=831 15–85 years Paediatric n=102 15–18 years |
Asthma and seasonal allergic rhinitis | I: montelukast (10 mg) C: placebo |
Daily rhinitis symptoms (0–3) Daytime symptom score (0–3) Night-time symptom score (0–3) RQLQ+ |
Primary | Mean change±sd | No |
| Chen [54], 2006; Chung Shan Medical University Hospital, Taiwan |
RCT; 12 weeks |
Total n=40 2–6 years (4.4 years) |
Rhinitis | I: montelukast (5 mg) C: placebo |
PRQLQ Symptom score |
Not specified | Mean±sd Mean change±sd |
No |
| Spahn [55], 2006;# Merck & Co Inc |
RCT; 8 weeks |
Total n=21 9–18 years (13.1 years) |
Mild to moderate asthma | I: montelukast (5 or 10 mg) C: placebo |
Daytime symptom score (0–3) Night-time symptom score (0–3) |
Not specified | Mean±sem | Yes |
| Stelmach [56], 2015; Medical University of Lodz, Poland. National Science Centre |
RCT; 30 weeks |
Total n=76 6–14 years (10.7 years) |
Allergic asthma | I: montelukast (5 mg) C: placebo |
ACT | Not specified | Mean±sd | No |
| Sahiner [57], 2021; Merck Sharp and Dohme Company |
RCT; 4 weeks |
Total n=46 6–18 years (11 years) |
Asthma | I: montelukast (10 mg) C: placebo |
ACT | Secondary | Mean±sd | No |
PROM: patient-reported outcome measure; LTRA: leukotriene receptor antagonist; ICS: inhaled corticosteroid; I: intervention group; C: control group; ATAQ: Asthma Therapy Assessment Questionnaire for children; RCT: randomised controlled trial; PATAQ: Pediatric Asthma Therapy Assessment Questionnaire; CHQ-PF50: Child Health Questionnaire Parent Form-50; CHSA: Children's Health Survey for Asthma; TRACK: Test for Respiratory and Asthma Control in Kids; AQLQ: Asthma Quality of Life Questionnaire; PAQLQ: Pediatric Asthma Quality of Life Questionnaire; ASUI: Asthma Symptom Utility Index; ACQ: Asthma Control Questionnaire; RQLQ: Rhinoconjunctivitis Quality of Life Questionnaire; PRQLQ: Pediatric Rhinoconjunctivitis Quality of Life Questionnaire; ACT: Asthma Control Test. #: citations identified from other reviews; ¶: data obtained after contacting sponsor; +: PROM information not available.
Authors’ judgements about each risk-of-bias item for each included study
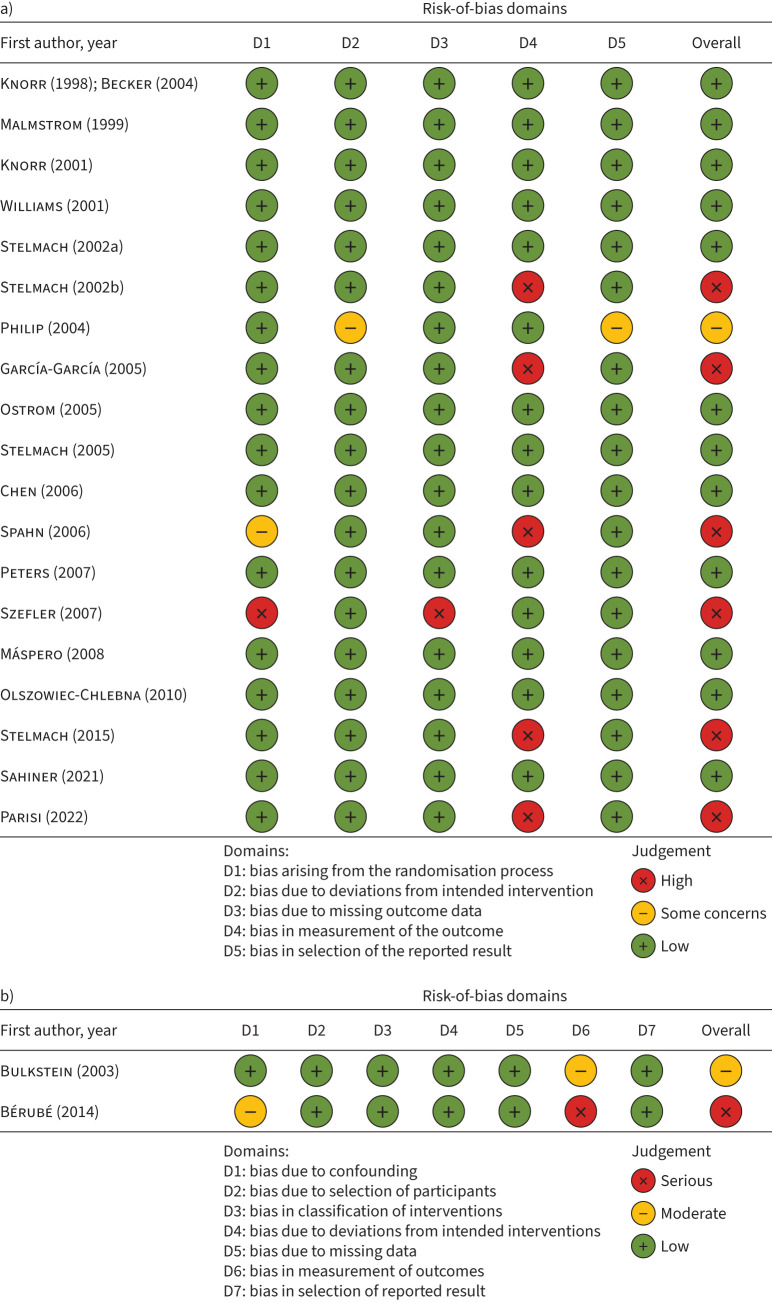
Figure 2 shows that, out of 20 RCTs, 12 had a low risk of bias [37, 39, 41, 43–46, 48, 50, 52, 54, 57], six had high risk [38, 40, 42, 52, 55, 56] and one study presented some concerns [53] (figure 2a). The most frequent domain causing downgrading was “bias in measurement of the outcome”, because the assessment of the outcome was probably being influenced by knowledge of the intervention received by patients [38, 40, 42, 52, 55, 56], and no information was reported in the articles. Figure 2b shows two nonrandomised studies [36, 47] which presented moderate and serious risk of bias.
FIGURE 2.
a) Risk-of-bias summary for randomised controlled trials using the revised tool for risk of bias (RoB 2) in randomised trials; b) risk of bias of nonrandomised studies assessed using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool.
Results of syntheses
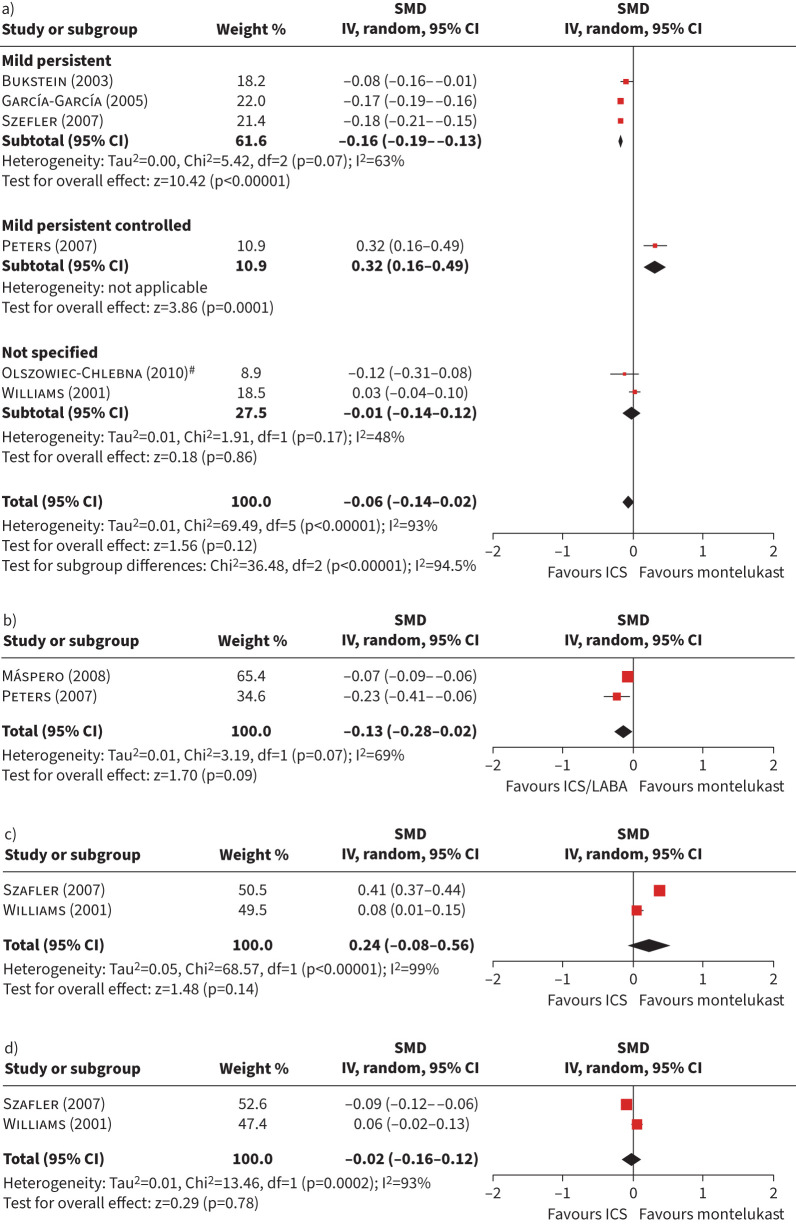
Figure 3 shows forest plots of studies comparing HRQoL of patients with asthma treated with ICS in monotherapy or combined with long-acting β-agonists (LABAs) versus montelukast. Figure 3a shows the forest plot of the SMD of change for the global HRQoL, which included six studies with a sample size of 656 participants for the intervention group, and 535 for the control group (ICS in monotherapy); the pooled estimator was −0.06 (95% CI −0.14–0.02). The subgroup analysis was statistically significant (p<0.001), showing that, in patients with mild persistent asthma and unspecified type of asthma, the change in HRQoL was in favour of ICS, but there was one study in favour of montelukast including patients with mild persistent controlled asthma.
FIGURE 3.
Forest plots of studies comparing patients with asthma treated with inhaled corticosteroids (ICS) in monotherapy or as a combined treatment with long-acting β-agonists (LABAs) versus montelukast in terms of health-related quality of life (HRQoL). a) Global scores of HRQoL for monotherapy; b) global scores of HRQoL for combined therapy; c) mental and d) physical dimension scores of HRQoL for monotherapy. SMD: standardised mean difference; IV: inverse variance. #: study including more than one dose of ICS; 400 μg budesonide was used.
Similarly, the HRQoL forest plot of the two studies comparing montelukast with ICS/LABA (figure 3b) was in favour of the latter group without statistically significant differences: overall estimator −0.13 (95% CI −0.28–0.02) with a moderate heterogeneity (I2=69%). Both studies were carried out among patients with persistent asthma [45, 46]. Figure 3c and d shows forest plots of mental and physical domains constructed with only two studies providing data at domain level (n=298 intervention, n=158 control), which used ICS in monotherapy as the comparator group. Pooled estimators were not statistically significant and heterogeneity was high in both cases.
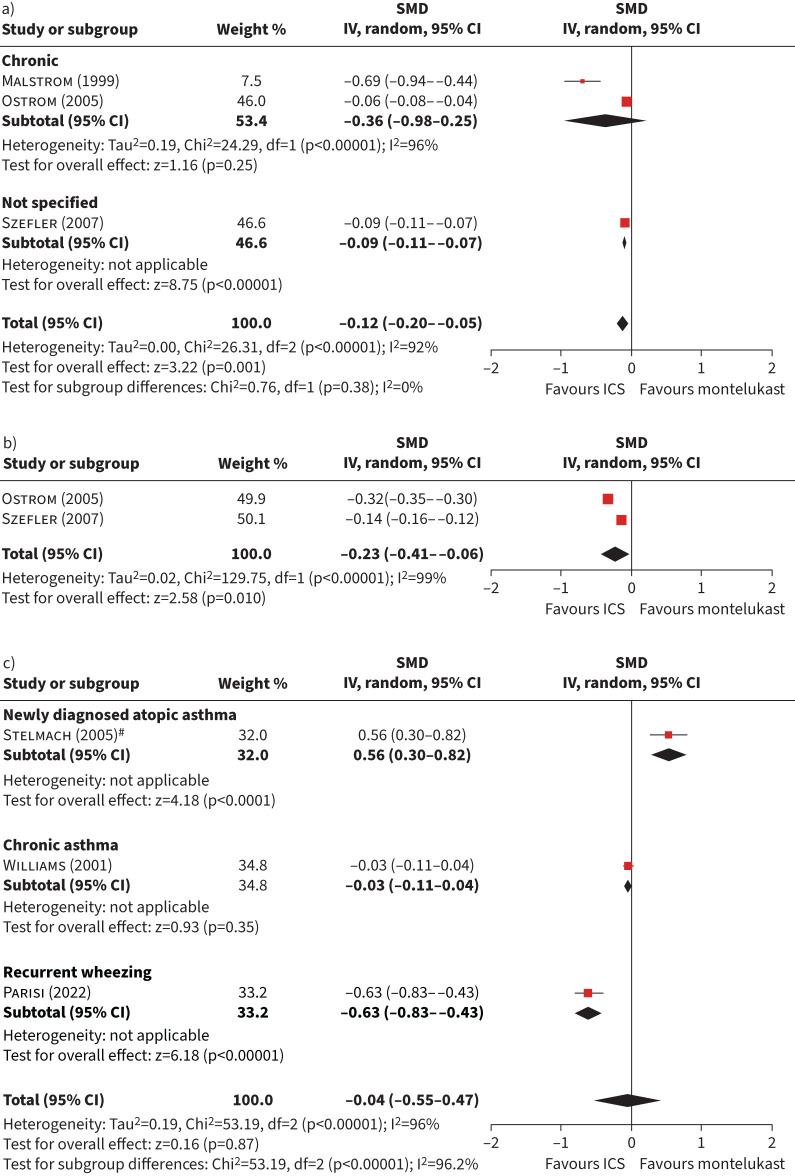
Figure 4a and b shows forest plots of studies comparing patients with asthma treated with ICS versus montelukast in terms of daytime (n=386 intervention, n=376 control) and night-time (n=363 intervention, n=363 control) symptoms, which showed a statistically significant difference in favour of ICS. When assessing the symptoms globally (figure 4c), there were no differences between montelukast and ICS (−0.04, 95% CI −0.55–0.47). The subgroup analysis by type of asthma was statistically significant (p<0.001), and heterogeneity was high (I2>90%) for the three meta-analyses. The change was statistically significant in favour of montelukast in a study among newly diagnosed atopic asthma patients, while favouring ICS in a study among patients with recurrent wheezing.
FIGURE 4.
Forest plot of studies comparing patients with asthma treated with inhaled corticosteroids (ICS) versus montelukast in terms of symptoms. a) Daytime symptom score; b) night-time symptom score; c) global symptom score. SMD: standardised mean difference; IV: inverse variance. #: study including more than one dose of ICS; 400 μg budesonide was used.
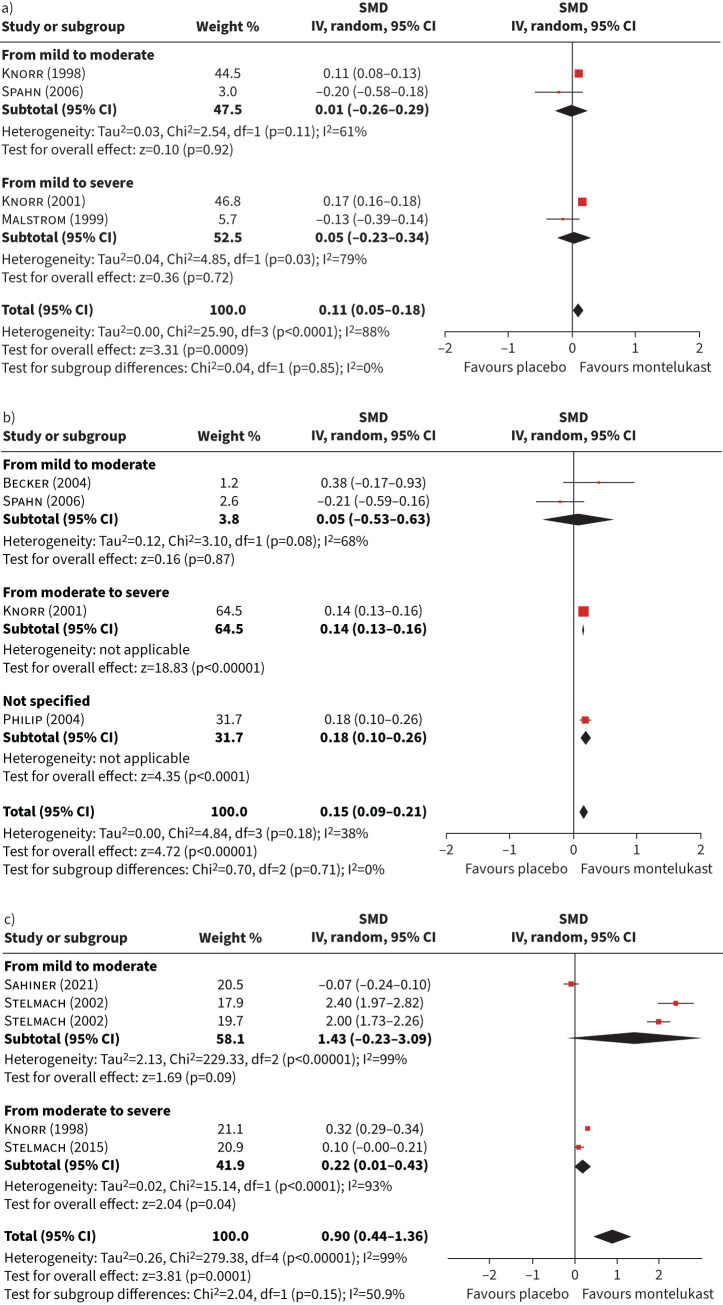
Meta-analyses of studies comparing montelukast with placebo are shown in figure 5. Pooled estimators of daytime, night-time symptoms and global symptoms scores (figure 5a, b and c) showed a statistically significant difference in favour of montelukast: 0.11 (95% CI 0.05–0.18), 0.15 (95% CI 0.09–0.21) and 0.90 (95% CI 0.44–1.36). The heterogeneity was high, except for the night-time symptoms score (I2 = 38%). The subgroup analysis by asthma severity was not statistically significant in any of these three meta-analyses.
FIGURE 5.
Forest plot of studies comparing patients with asthma treated with placebo versus montelukast in terms of symptoms. a) Daytime symptom score; b) night-time symptom score; c) global symptom score. SMD: standardised mean difference; IV: inverse variance.
Sensitivity analyses excluding studies with a high risk of bias are shown in supplementary figure S2. Pooled estimators obtained in these sensitivity analyses were in the same direction and similar magnitude to the meta-analyses constructed with all the studies.
Six studies [38–40, 47, 48, 50] provided information on adverse drug events and study withdrawals, and three other studies [37, 46, 55] provided information only on study withdrawals (supplementary table B). Most studies reporting adverse drug events concluded that those were not significantly different for montelukast when compared to other treatments [38–40, 48, 50], while some studies reported withdrawals more frequently in the montelukast group [38–40, 46, 48]. Asthma exacerbations, headaches and upper respiratory tract infections were among the most commonly reported events [38–40, 48, 50]. After having requested them from the authors, one study [50] provided adverse effects for 60 participants, the most frequent being headache (n=35), irritability (n=7), nervousness (n=4), behaviour disturbance (n=4) and drug overdose (n=4, presenting thirst, mydriasis and somnolence). There was one study [47] that reported both adverse events and withdrawals in general, but montelukast was included in both the intervention and control groups: nightmares and sleep terror (n=6), abdominal pain (n=5), insomnia (n=2) and headache (n=2). Another study [38] reported one death in the montelukast group, although it was not considered to be related to the drug.
Discussion
In this systematic review, we identified 50 articles (49 studies) evaluating the impact of montelukast on PROMs when compared with other treatments or placebo in children and adolescents with asthma/allergic rhinitis. The 21 studies finally included in some of the meta-analyses allowed for the construction of pooled estimators of montelukast impact on HRQoL (global, mental and physical domains) and symptoms (daytime, night-time and globally) with high heterogeneity. Results were in favour of ICS without statistically significant differences, in the global, mental and physical HRQoL mean changes. For daytime and night-time symptom scores, the change was significant and in favour of ICS. The incidence of adverse events and withdrawals was only provided by six and nine of these studies, respectively, showing infrequent adverse events, which were similar to those seen in the control group, and only one study reported neuropsychiatric events, but withdrawals were slightly more frequent in the montelukast group.
In our systematic review, of the 49 studies that fulfilled inclusion criteria, 11 (22%) included PROMs as a primary outcome, 21 (43%) as a secondary outcome, one (2%) as a tertiary outcome and 16 (33%) did not specify. Furthermore, of the 21 studies included in the meta-analysis, six did not include complete data for all the PROMs administered [39, 43, 45, 46, 52, 53]. The 28 studies whose data could not be extracted for meta-analysis were predominantly RCTs in asthma patients comparing montelukast with ICS [58–67] or placebo [64, 68–75] (supplementary table A). Reasons for noninclusion in the meta-analyses were results not being segregated for children and adults [59, 60, 64, 66, 67, 69, 72–74, 76–82], or PROM data not fully reported [58, 61–63, 65, 68, 70, 71, 75, 83–85]. These latter 12 studies with missing information on PROMs, which remained unavailable after contacting authors, suggest the poor relevance given to these end-points. This is in line with reviews [86, 87] highlighting the small proportion of asthma clinical trials including PROMs: only 20 out of 300 published RCTs [86], and only 27% of 96 736 registered trials [87]. There is growing evidence that PROM results are frequently omitted and not reported, and data are presented with suboptimal reporting standards [88, 89]. Issues such as lack of PROMs training, poor understanding of the purpose of PROMs assessment and questionnaire selection [90] should be contemplated to avoid research waste.
Global HRQoL pooled results of our meta-analyses were in favour of ICS in monotherapy or combined with LABAs, but they were not statistically significant, with a negligible magnitude of difference. The study that constituted the subgroup of mild persistent controlled asthma was the only one favouring montelukast, which is consistent with by the Global Initiative for Asthma (GINA)'s recommendation of using montelukast in patients with fewer frequency of symptoms (more than once a month, but less than daily) [4]. The studies included in our systematic review of HRQoL mostly administered asthma-specific questionnaires [36, 38, 40, 41, 43–46, 48, 52–54], similarly to findings of a review of PROMs in asthma [86], where the low use of generic questionnaires was highlighted. In fact, the National Asthma Education and Prevention Program [91] in the United States supported the use of generic HRQoL instruments to capture patients’ global health perceptions.
Results for both meta-analyses on mental and physical domains of HRQoL were also in favour of ICS, without statistically significant differences; however, the first one achieves a small magnitude of the difference between treatment groups. Mental health is an important component of health in children with asthma [92, 93]. The USA National Survey of Children's Health [94] reported higher odds ratios for developmental or behavioural problems in children with asthma. Furthermore, considering the lack of screening tools for anxious and depressive symptomatology validated among asthma populations remarked by GINA [4], mental dimensions covered by PROMs could be a valuable proxy, since they could capture mental adverse problems related to drugs or disease. Several studies included in our meta-analyses measured the mental health component of the HRQoL, such as those administering the Children's Health Survey for Asthma (CHSA) [40] (emotional health domain); the CHQ-PF50 (role/social emotional functioning, role/social behavioural functioning, mental health and behaviour domains); the Pediatric Asthma Quality of Life Questionnaire (PAQLQ) [44, 46, 48, 52], the Asthma Quality of Life Questionnaire (AQLQ) [43] or Mini-AQLQ [45] (emotional function domain); the Asthma Therapy Assessment Questionnaire for children [36, 41] and Pediatric Asthma Therapy Assessment Questionnaire [38] (attitude and behaviour domain); and the Pediatric Rhinoconjunctivitis Quality of Life Questionnaire [53, 54] (feeling irritable item). Unfortunately, the majority of the articles identified only included total scores [36, 38, 41, 44, 45, 54], and none provided information at dimension and item level, which may have limited the possibility of finding the real impact of montelukast on mental and physical components of health. Only three studies provided information on the dimension scores: one study that administered the CHSA [40], and two that administered the PAQLQ [44, 48]. The latter used placebo as the comparison group, and the first one used ICS.
The meta-analysis of global symptoms shows opposite results according to the type of asthma used as inclusion criteria in the studies (newly diagnosed atopic asthma, chronic asthma and recurrent wheezing). Results of daytime and night-time symptom meta-analyses were statistically significant, favouring ICS over montelukast, with only night-time achieving a small magnitude. This is in line with asthma guidelines recommendation of ICS therapy as the step-one treatment for paediatric patients with mild-to-moderate persistent asthma [4, 18].
Furthermore, our results are consistent with those obtained in previous systematic reviews of schoolchildren with mild to moderate asthma [13], preschool children [21] and paediatric patients [20], concluding that those treated with ICS had better symptom control than those with montelukast. Previous systematic reviews evaluating the impact of montelukast through PROMs were predominantly focused on symptom control [13, 20, 21], while there have been very few reviews gathering information on global HRQoL [24], and none were found summarising HRQoL mental and physical domains.
The synthesis of trials using placebo as a comparison group confirmed the efficacy of montelukast in terms of statistically significant improvement in symptoms during daytime, night-time and globally. However, the magnitude of the benefits was only large for symptoms when measured globally, but very small (<0.2 sd) for symptoms measured separately for daytime and night-time. The large magnitude for the global symptoms pooled estimator (0.9 sd) is explained by two [51, 52], of the five studies included. These studies [51, 52] measured asthma symptoms with an overall score covering daytime, night-time and also the use of β2-agonists, differing from the other studies which did not include the use of rescue inhalers [48, 56, 57]. Results of studies using placebo as comparison group, without enough information to be included in the meta-analysis, did not show significant differences for HRQoL [70] and symptom score [75] in asthma patients using montelukast, except for one study which obtained a statistically significant higher HRQoL improvement in this group [71]. Another study in children with allergic rhinitis found that montelukast's improvement of HQRL was significantly higher than placebo [68].
In this systematic review, we have not found any PROMs aiming to measure treatment adverse events, and only six studies reporting them [38–40, 47, 48, 50]. Only one of these studies reported neuropsychiatry-related events [50] such as irritability, nervousness, behaviour disturbance, insomnia, dream abnormality, anxiety, bipolar disorder and personality change, but they were more infrequent than in the control group (4.0% versus 5.1%). This is consistent with results of the FDA-requested review of behaviour-related adverse experiences in montelukast clinical trials [14], but it is important to consider that some neuropsychiatric events could have been under-reported, since patients/parents may have not associated the treatment with the adverse experience. Conversely, study withdrawals, extracted as a proxy to adverse events, were more frequent in the montelukast group in five out of eight studies [38–40, 46, 48]. This result is consistent with another systematic review [24] which found that LTRA associated with a 24% increased risk of overall withdrawals in paediatric patients, but also with no statistically significant group difference due to adverse effects. Although treatment may occasionally be discontinued for mild or moderate adverse events, withdrawals are especially important because they reflect the ultimate decision of the participant and/or physician to discontinue treatment.
The main limitation related to the systematic review process was that we may have failed to identify all articles assessing the impact of montelukast on PROMs in children/adolescents. However, we believe that this has been minimised due to the bibliographic database interface used (Ovid), the sensitive search strategy, and the additional hand search of references. Regarding limitation related to the studies included and pooled results obtained, first, heterogeneity was high (I2>75%) in all meta-analyses comparing montelukast with ICS in monotherapy, and moderate (I2= 69%) when compared with ICS combined with LABAs. Since we hypothesised asthma severity as a potential source of heterogeneity, we performed subgroup analyses according to this variable, but it was statistically significant only in one of the three meta-analyses where it could be tested: the one comparing HRQoL between montelukast and ICS. It is important to remark the lack of uniform criteria across studies for asthma severity and classification types, which limited our subgroup analyses, but many other possible reasons could explain such heterogeneity. Second, the internal validity of the pooled estimator provided by a meta-analysis depends on the quality of primary studies. Results of the sensitivity analyses excluding the 29.4% of studies qualified as high risk of bias were consistent with those obtained by the meta-analyses constructed with all the studies. Third, the follow-up period in most of trials could be considered short to detect montelukast's adverse events, which could appear after stopping treatment [16]. In fact, 11 studies were conducted over ≤12 weeks [39, 43, 46–48, 50–55]. Fourth, the ability to generalise our findings in preschool children (<5 years) is limited because only two studies examined this population; likewise in paediatric patients with allergic rhinitis, for which there were five studies and most of them without data to be included in the meta-analyses. Fifth, no funnel plot for publication bias assessment could be constructed because of the low number of studies pooled (n<10) in all forest plots. Finally, concerns about the sources of these studies are remarkable, as more than half of the studies included were sponsored by pharmaceutical companies.
Points for clinical practice
The results of this systematic review suggest that the benefit of ICS on night-time symptoms was statistically higher than that of montelukast, although the magnitude of the difference was small.
HRQoL did not present any statistically significant differences between montelukast and ICS, and their magnitude was negligible except for the mental domain, which was small.
Montelukast was effective in controlling asthma symptoms when compared with placebo.
These findings are in accordance with current guidelines for asthma treatment, which includes LTRAs in the category of other controller options, but remark upon their lack of evidence for safety.
Conclusion
Our results suggest that the benefit of ICS on night-time symptoms was statistically higher than that of montelukast, although the magnitude of the difference was small. HRQoL did not present any statistically significant differences between montelukast and ICS, and their magnitude was small only for the mental domain (negligible for the rest). Compared with placebo, montelukast was effective in controlling asthma symptoms. These findings of our systematic review are in accordance with current guidelines for asthma treatment, which recommend the use of ICS therapy as first step treatment for paediatric patients with mild-to-moderate persistent asthma; and considering LTRAs in the category of other controller options, but taking into account the lack of evidence for safety. It is essential to achieve a broader understanding of the safety of montelukast by long-term post-marketing studies, and improving the collection of adverse events in clinical trials, specifically measuring them through validated PROMs.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0124-2023.SUPPLEMENT (1.1MB, pdf)
Acknowledgements
The authors would like to acknowledge Raúl Castro (Universitat Autònoma de Barcelona, Barcelona, Spain) for helping with part of the search process, and Aurea Martin (CIBER en Epidemiología y Salud Pública, CIBERESP, Madrid, Spain and Health Services Research Group, Hospital del Mar Research Institute, Barcelona) for her help in the English editing and proofreading process and in finalising this manuscript.
Provenance: Submitted article, peer reviewed.
ARCA Group: Montse Ferrer, Karina Mayoral, Catalina Lizano, Olatz Garin, Yolanda Pardo, Àngels Pont (IMIM, Institut Hospital del Mar d'Investigacions Mèdiques); Maria Araceli Caballero (Hospital del Mar); Manuel Praena (Centro de Salud la Candelaria); Laura Valdesoiro (Hospital Universitario Parc Taulí); Ines de Mir (Hospital Vall d'Hebron); Gimena Hernandez, Camila Maroni (CAP La Sagrera); Alberto Bercedo (Centro de Salud Los Castros); Jose Antonio Castillo (Hospital Infantil Universitario Miguel Servet); María Teresa Guerra (Centro de Salud Jerez Sur), Olga Cortés (Centro de Salud Canillejas); Eva Tato (Hospital Universitario Araba); Pilar Ortiz, Marta Ortega, Alberto Servan (Centro de Salud Dos de Mayo); María Ángela Carrasco (Consultorio Sevilla la Nueva); Alexandra L. Dima (Institut de Recerca Sant Joan de Déu); Eric van Ganse (University Claude Bernard Lyon); Marijn de Bruin (Radboud University Medical Center).
Authors’ contributions: M. Ferrer conceived the research question, contributed to the conception and design of the article, oversaw all aspects, contributed to the statistical analyses, carried out the interpretation of data, and contributed to the writing of the article; K. Mayoral, C. Lizano-Barrantes and V. Zamora participated in the conception of the research question, conducted the literature search and data extraction, as well as the quality assessment, conceptualised and oversaw analyses, and wrote the article; C. Miret and C. Barrufet conducted the literature search and data extraction, as well as the quality assessment; A. Pont contributed to the analysis and provided statistical support and help in the interpretation of data; M.A. Caballero-Rabasco, M. Praena-Crespo, A. Bercedo, L. Valdesoiro-Navarrete and M.T. Guerra participated in the conception of the research question, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, and helped in the interpretation of data and drafting of the paper; O. Garin, Y. Pardo and M.J. Martínez Zapata revised important intellectual content and the draft versions of the manuscript. All the co-authors critically revised the manuscript and approved the final draft before submission, and can attest to the validity and legitimacy of the data in the manuscript and agree to be named as author of the manuscript.
Conflict of interest: All authors have nothing to disclose.
Support statement: Financial support for this study was provided through grants by the Instituto de Salud Carlos III FEDER: Fondo Europeo de Desarrollo Regional (PI15/00449) and DIUE of Generalitat de Catalunya (AGAUR 2021 SGR 00624, 2017 SGR 452). The following researchers have worked on this manuscript while funded by grants: K. Mayoral and V. Zamora (Instituto de Salud Carlos III FEDER: Fondo Europeo de Desarrollo Regional FI16/00071, FI19/00229), K. Mayoral also obtained financial support from CIBER of Epidemiology and Public Health CIBERESP for a short PhD stay abroad, and C. Lizano-Barrantes (University of Costa Rica OAICE-85-2019). The funding agreements ensure the authors’ independence in designing the study, interpreting the data, and writing and publishing the report. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–1259. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nur Husna SM, Tan HT, Md Shukri N, et al. Allergic rhinitis: a clinical and pathophysiological overview. Front Med 2022; 9: 874114. 10.3389/fmed.2022.874114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harmanci K. Montelukast: its role in the treatment of childhood asthma. Ther Clin Risk Manag 2007; 3: 885–892. [PMC free article] [PubMed] [Google Scholar]
- 4.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2022. Available from: http://ginasthma.org/
- 5.U.S. Department of Health and Human Services . 2020 Updates to the Asthma Management Guidelines. 2020. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management- guidelines [DOI] [PubMed]
- 6.Dykewicz MS, Wallace DV, Amrol DJ, et al. Rhinitis 2020: a practice parameter update. J Allergy Clin Immunol 2020; 146: 721–767. doi: 10.1016/j.jaci.2020.07.007 [DOI] [PubMed] [Google Scholar]
- 7.Philip G, Hustad C, Noonan G, et al. Reports of suicidality in clinical trials of montelukast. J Allergy Clin Immunol 2009; 124: 691–696. doi: 10.1016/j.jaci.2009.08.010 [DOI] [PubMed] [Google Scholar]
- 8.Philip G, Hustad CM, Malice MP, et al. Analysis of behavior-related adverse experiences in clinical trials of montelukast. J Allergy Clin Immunol 2009; 124: 699–706. doi: 10.1016/j.jaci.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 9.GOV.UK. Montelukast (Singulair): reminder of the risk of neuropsychiatric reactions. Date last accessed 17 February 2021. Date last updated: 19 September 2019. www.gov.uk/drug-safety-update/montelukast-singulair-reminder-of-the-risk-of-neuropsychiatric-reactions
- 10.European Medicines Agency (EMA) . Annex I Scientific Conclusions and Grounds for the Variation to the Terms of the Marketing Authorisation(s). 2019. Date last accessed: 19 February 2021. www.ema.europa.eu/en/documents/psusa/montelukast-cmdh-scientific-conclusions-grounds-variation-amendments-product-information-timetable/00002087/201807_en.pdf
- 11.U.S. Food and Drug Administration . FDA Requires Boxed Warning About Serious Mental Health Side Effects for Asthma and Aallergy Drug Montelukast (Singulair); Advises Restricting use for Allergic Rhinitis. Date last accessed: 21 February 2021. Date last updated: 13 March 2020. www.fda.gov/drugs/drug-safety-and-availability/fda-requires-boxed-warning-about-serious-mental-health-side-effects-asthma-and-allergy-drug
- 12.Miligkos M, Bannuru RR, Alkofide H, et al. Leukotriene-receptor antagonists versus placebo in the treatment of asthma in adults and adolescents: a systematic review and meta-analysis. Ann Intern Med 2015; 163: 756–767. doi: 10.7326/M15-1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro-Rodriguez JA, Rodrigo GJ. The role of inhaled corticosteroids and montelukast in children with mild–moderate asthma: results of a systematic review with meta-analysis. Arch Dis Child 2010; 95: 365–370. doi: 10.1136/adc.2009.169177 [DOI] [PubMed] [Google Scholar]
- 14.Song Q, Mu K, Yao Y, et al. Meta-analysis of the relationship between montelukast use and neuropsychiatric events in patients with allergic rhinitis and/or asthma. Authorea 2020; preprint [ 10.22541/au.159050469.93143403]. doi 10.22541/au.159050469.93143403 [DOI] [PMC free article] [PubMed]
- 15.Dixon EG, Rugg-Gunn CEM, Sellick V, et al. Adverse drug reactions of leukotriene receptor antagonists in children with asthma: a systematic review. BMJ Paediatr Open 2021; 5: e001206. doi: 10.1136/bmjpo-2021-001206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson S, Kaminsky E, Taavola H, et al. Montelukast and nightmares: further characterisation using data from VigiBase. Drug Saf 2022; 45: 675–684. Doi: 10.1007/s40264-022-01183-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvert M, Kyte D, Mercieca-Bebber R, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA 2018; 319: 483–494. doi: 10.1001/jama.2017.21903 [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence (NICE)/British Thoracic Society (BTS)/Scottish Intercollegiate Guidelines Network (SIGN) . British Guideline on the Management of Asthma. 2021. www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/
- 19.Scadding GK, Kariyawasam HH, Scadding G, et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (revised edition 2017; first edition 2007). Clin Exp Allergy 2017; 47: 856–889. doi: 10.1111/cea.12953 [DOI] [PubMed] [Google Scholar]
- 20.Massingham K, Fox S, Smaldone A. Asthma therapy in pediatric patients: a systematic review of treatment with montelukast versus inhaled corticosteroids. J Pediatr Health Care 2014; 28: 51–62. Doi: 10.1016/j.pedhc.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 21.Castro-Rodriguez JA, Rodriguez-Martinez CE, Ducharme FM. Daily inhaled corticosteroids or montelukast for preschoolers with asthma or recurrent wheezing: a systematic review. Pediatr Pulmonol 2018; 53: 1670–1677. doi: 10.1002/ppul.24176 [DOI] [PubMed] [Google Scholar]
- 22.Murphy KR, Hong JG, Wandalsen G, et al. Nebulized inhaled corticosteroids in asthma treatment in children 5 years or younger: a systematic review and global expert analysis. J Allergy Clin Immunol Pract 2020; 8: 1815–1827. Doi: 10.1016/j.jaip.2020.01.042 [DOI] [PubMed] [Google Scholar]
- 23.Watts K, Chavasse RJ. Leukotriene receptor antagonists in addition to usual care for acute asthma in adults and children. Cochrane Database Syst Rev 2012; 5: CD006100. doi: 10.1002/14651858.CD006100.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev 2012; 5: CD002314. doi: 10.1002/14651858.CD002314.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grainger J, Drake-Lee A. Montelukast in allergic rhinitis: a systematic review and meta-analysis. Clin Otolaryngol 2006; 31: 360–367. doi: 10.1111/j.1749-4486.2006.01276.x [DOI] [PubMed] [Google Scholar]
- 26.Wilson AM, O'Byrne PM, Parameswaran K. Leukotriene receptor antagonists for allergic rhinitis: a systematic review and meta-analysis. Am J Med 2004; 116: 338–344. doi: 10.1016/j.amjmed.2003.10.030 [DOI] [PubMed] [Google Scholar]
- 27.Krishnamoorthy M, Mohd Noor N, Mat Lazim N, et al. Efficacy of montelukast in allergic rhinitis treatment: a systematic review and meta-analysis. Drugs 2020; 80: 1831–1851. Doi: 10.1007/s40265-020-01406-9 [DOI] [PubMed] [Google Scholar]
- 28.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 30.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2021; 12: 55–61. doi: 10.1002/jrsm.1411 [DOI] [PubMed] [Google Scholar]
- 32.R Core Team . R: a Language and Environment for Statistical Computing. Vienna, R Foundation for Statistical Computing. Date last accessed: 16 May 2022. www.r-project.org/index.html [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 34.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Cochrane Collaboration . Review Manager (RevMan), version 5.4. 2020. Date last accessed: 27 April 2023. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-non-cochrane-reviews
- 36.Bukstein DA, Luskin AT, Bernstein A. “Real-world” effectiveness of daily controller medicine in children with mild persistent asthma. Ann Allergy Asthma Immunol 2003; 90: 543–549. Doi: 10.1016/S1081-1206(10)61848-0 [DOI] [PubMed] [Google Scholar]
- 37.Stelmach I, Bobrowska-Korzeniowska M, Majak P, et al. The effect of montelukast and different doses of budesonide on IgE serum levels and clinical parameters in children with newly diagnosed asthma. Pulm Pharmacol Ther 2005; 18: 374–380. doi: 10.1016/j.pupt.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Garcia ML, Wahn U, Gilles L, et al. Montelukast, compared with fluticasone, for control of asthma among 6- to 14-year-old patients with mild asthma: the MOSAIC study. Pediatrics 2005; 116: 360–369. doi: 10.1542/peds.2004-1172 [DOI] [PubMed] [Google Scholar]
- 39.Ostrom NK, Decotiis BA, Lincourt WR, et al. Comparative efficacy and safety of low-dose fluticasone propionate and montelukast in children with persistent asthma. J Pediatr 2005; 147: 213–220. doi: 10.1016/j.jpeds.2005.03.052 [DOI] [PubMed] [Google Scholar]
- 40.Szefler SJ, Baker JW, Uryniak T, et al. Comparative study of budesonide inhalation suspension and montelukast in young children with mild persistent asthma. J Allergy Clin Immunol 2007; 120: 1043–1050. doi: 10.1016/j.jaci.2007.08.063 [DOI] [PubMed] [Google Scholar]
- 41.Olszowiec-Chlebna M, Majak P, Brzozowska A, et al. Effect of inhaled steroid and montelukast on clinical symptoms in children with newly diagnosed asthma: a pilot study. Pediatr Allergy Immunol 2010; 21: e687–e690. doi: 10.1111/j.1399-3038.2009.00983.x [DOI] [PubMed] [Google Scholar]
- 42.Parisi GF, Manti S, Papale M, et al. Addition of a nutraceutical to montelukast or inhaled steroid in the treatment of wheezing during COVID-19 pandemic: a multicenter, open-label, randomized controlled trial. Acta Biomed 2022; 93: e2022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malmstrom K, Rodriguez-Gomez G, Guerra J, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Ann Intern Med 1999; 130: 487–495. doi: 10.7326/0003-4819-130-6-199903160-00005 [DOI] [PubMed] [Google Scholar]
- 44.Williams B, Noonan G, Reiss TF, et al. Long-term asthma control with oral montelukast and inhaled beclomethasone for adults and children 6 years and older. Clin Exp Allergy 2001; 31: 845–854. doi: 10.1046/j.1365-2222.2001.01085.x [DOI] [PubMed] [Google Scholar]
- 45.Peters S, Anthonisen N, Castro M, et al. Randomized comparison of strategies for reducing treatment in mild persistent asthma. N Engl J Med 2007; 356: 2027–2039. doi: 10.1056/NEJMoa070013 [DOI] [PubMed] [Google Scholar]
- 46.Máspero J, Guerra F, Cuevas F, et al. Efficacy and tolerability of salmeterol/fluticasone propionate versus montelukast in childhood asthma: a prospective, randomized, double-blind, double-dummy, parallel-group study. Clin Ther 2008; 30: 1492–1504. doi: 10.1016/j.clinthera.2008.07.018 [DOI] [PubMed] [Google Scholar]
- 47.Bérubé D, Djandji M, Sampalis JS, et al. Effectiveness of montelukast administered as monotherapy or in combination with inhaled corticosteroid in pediatric patients with uncontrolled asthma: a prospective cohort study. Allergy Asthma Clin Immunol 2014; 10: 21. doi: 10.1186/1710-1492-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knorr B, Matz J, Bernstein JA, et al. Montelukast for chronic asthma in 6- to 14-year-old children: a randomized, double-blind trial. JAMA 1998; 279: 1181–1186. doi: 10.1001/jama.279.15.1181 [DOI] [PubMed] [Google Scholar]
- 49.Becker A, Swern A, Tozzi CA, et al. Montelukast in asthmatic patients 6 years–14 years old with an FEV1 >75%. Curr Med Res Opin 2004; 20: 1651–1659. doi: 10.1185/030079904X4644 [DOI] [PubMed] [Google Scholar]
- 50.Knorr B, Franchi LM, Bisgaard H, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics 2001; 108: E48. doi: 10.1542/peds.108.3.e48 [DOI] [PubMed] [Google Scholar]
- 51.Stelmach I, Jerzynska J, Kuna P. A randomized, double-blind trial of the effect of treatment with montelukast on bronchial hyperresponsiveness and serum eosinophilic cationic protein (ECP), soluble interleukin 2 receptor (sIL-2R), IL-4, and soluble intercellular adhesion molecule 1 (sICAM-1) in children with asthma. J Allergy Clin Immunol 2002; 109: 257–263. doi: 10.1067/mai.2002.121456 [DOI] [PubMed] [Google Scholar]
- 52.Stelmach I, Jerzynska J, Kuna P. A randomized, double-blind trial of the effect of glucocorticoid, antileukotriene and β-agonist treatment on IL-10 serum levels in children with asthma. Clin Exp Allergy 2002; 32: 264–269. doi: 10.1046/j.1365-2222.2002.01286.x [DOI] [PubMed] [Google Scholar]
- 53.Philip G, Nayak AS, Berger WE, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin 2004; 20: 1549–1558. doi: 10.1185/030079904X3348 [DOI] [PubMed] [Google Scholar]
- 54.Chen ST, Lu KH, Sun HL, et al. Randomized placebo-controlled trial comparing montelukast and cetirizine for treating perennial allergic rhinitis in children aged 2–6 yr. Pediatr Allergy Immunol 2006; 17: 49–54. doi: 10.1111/j.1399-3038.2005.00351.x [DOI] [PubMed] [Google Scholar]
- 55.Spahn JD, Covar RA, Jain N, et al. Effect of montelukast on peripheral airflow obstruction in children with asthma. Ann Allergy Asthma Immunol 2006; 96: 541–549. Doi: 10.1016/S1081-1206(10)63548-X [DOI] [PubMed] [Google Scholar]
- 56.Stelmach I, Ozarek-Hanc A, Zaczeniuk M, et al. Do children with stable asthma benefit from addition of montelukast to inhaled corticosteroids: randomized, placebo controlled trial. Pulm Pharmacol Ther 2015; 31: 42–48. Doi: 10.1016/j.pupt.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 57.Sahiner UM, Arik Yilmaz E, Fontanella S, et al. The montelukast therapy in asthmatic children with and without food allergy: does it make any difference. Int Arch Allergy Immunol 2021; 182: 1212–1221. doi: 10.1159/000517865 [DOI] [PubMed] [Google Scholar]
- 58.Maspero JF, Dueñas-Meza E, Volovitz B, et al. Oral montelukast versus inhaled beclomethasone in 6- to 11-year-old children with asthma: results of an open-label extension study evaluating long-term safety, satisfaction, and adherence with therapy. Curr Med Res Opin 2001; 17: 96–104. Doi: 10.1016/j.jaci.2012.05.050 [DOI] [PubMed] [Google Scholar]
- 59.Meltzer EO, Lockey RF, Friedman BF, et al. Efficacy and safety of low-dose fluticasone propionate compared with montelukast for maintenance treatment of persistent asthma. Mayo Clin Proc 2002; 77: 437–445. doi: 10.1016/S0025-6196(11)62212-X [DOI] [PubMed] [Google Scholar]
- 60.Zeiger RS, Bird SR, Kaplan MS, et al. Short-term and long-term asthma control in patients with mild persistent asthma receiving montelukast or fluticasone: a randomized controlled trial. Am J Med 2005; 118: 649–657. doi: 10.1016/j.amjmed.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 61.Zeiger RS, Szefler SJ, Phillips BR, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol 2006; 117: 45–52. doi: 10.1016/j.jaci.2005.10.012 [DOI] [PubMed] [Google Scholar]
- 62.Kumar V, Ramesh P, Lodha R, et al. Montelukast vs. inhaled low-dose budesonide as monotherapy in the treatment of mild persistent asthma: a randomized double blind controlled trial. J Trop Pediatr 2007; 53: 325–330. doi: 10.1093/tropej/fmm038 [DOI] [PubMed] [Google Scholar]
- 63.Knuffman JE, Sorkness CA, Lemanske RF Jr, et al. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. J Allergy Clin Immunol 2009; 123: 411–416. doi: 10.1016/j.jaci.2008.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laviolette M, Malmstrom K, Lu S, et al. Montelukast added to inhaled beclomethasone in treatment of asthma. Am J Respir Crit Care Med 1999; 160: 1862–1868. doi: 10.1164/ajrccm.160.6.9803042 [DOI] [PubMed] [Google Scholar]
- 65.Karaman Ö, Sünneli L, Uzuner N, et al. Evaluation of montelukast in 8 to 14 year old children with mild persistent asthma and compared with inhaled corticosteroids. Allergol Immunopathol 2004; 32: 21–27. doi: 10.1016/S0301-0546(04)79219-8 [DOI] [PubMed] [Google Scholar]
- 66.Lu S, Liu N, Dass SB, et al. A randomized study comparing the effect of loratadine added to montelukast with montelukast, loratadine, and beclomethasone monotherapies in patients with chronic asthma. J Asthma 2009; 46: 465–469. doi: 10.1080/02770900902846323 [DOI] [PubMed] [Google Scholar]
- 67.Price D, Musgrave S, Wilson E, et al. A pragmatic single-blind randomised controlled trial and economic evaluation of the use of leukotriene receptor antagonists in primary care at steps 2 and 3 of the national asthma guidelines (ELEVATE study). Health Technol Assess 2011; 15: 1–132. doi: 10.3310/hta15suppl1-01 [DOI] [PubMed] [Google Scholar]
- 68.Hsieh JC, Lue KH, Lai DS, et al. A comparison of cetirizine and montelukast for treating childhood perennial allergic rhinitis. Pediatr Asthma Allergy Immunol 2004; 17: 59–69. doi: 10.1089/088318704322994958 [DOI] [Google Scholar]
- 69.American Lung Association Asthma Clinical Research Centers . Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am J Respir Crit Care Med 2007; 175: 235–242. doi: 10.1164/rccm.200603-416OC [DOI] [PubMed] [Google Scholar]
- 70.Simons FER, Villa JR, Lee BW, et al. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr 2001; 138: 694–698. doi: 10.1067/mpd.2001.112899 [DOI] [PubMed] [Google Scholar]
- 71.Strauch E, Moske O, Thoma S, et al. A randomized controlled trial on the effect of montelukast on sputum eosinophil cationic protein in children with corticosteroid-dependent asthma. Pediatr Res 2003; 54: 198–203. doi: 10.1203/01.PDR.0000072328.28105.06 [DOI] [PubMed] [Google Scholar]
- 72.Patel P, Philip G, Yang W, et al. Randomized, double-blind, placebo-controlled study of montelukast for treating perennial allergic rhinitis. Ann Allergy Asthma Immunol 2005; 95: 551–557. Doi: 10.1016/S1081-1206(10)61018-6 [DOI] [PubMed] [Google Scholar]
- 73.Wise RA, Bartlett SJ, Brown ED, et al. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J Allergy Clin Immunol 2009; 124: 436–444. doi: 10.1016/j.jaci.2009.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goh BS, Ismail MIM, Husain S. Quality of life assessment in patients with moderate to severe allergic rhinitis treated with montelukast and/or intranasal steroids: a randomised, double-blind, placebo-controlled study. J Laryngol Otol 2014; 128: 242–248. doi: 10.1017/S002221511400036X [DOI] [PubMed] [Google Scholar]
- 75.Kim JH, Lee S, Shin YH, et al. Airway mechanics after withdrawal of a leukotriene receptor antagonist in children with mild persistent asthma: double-blind, randomized, cross-over study. Pediatr Pulmonol 2020; 55: 3279–3286. doi: 10.1002/ppul.25085 [DOI] [PubMed] [Google Scholar]
- 76.Nelson HS, Busse WW, Kerwin E, et al. Fluticasone propionate/salmeterol combination provides more effective asthma control than low-dose inhaled corticosteroid plus montelukast. J Allergy Clin Immunol 2000; 106: 1088–1095. doi: 10.1067/mai.2000.110920 [DOI] [PubMed] [Google Scholar]
- 77.Pearlman DS, White M V, Lieberman AK, et al. Fluticasone propionate/salmeterol combination compared with montelukast for the treatment of persistent asthma. Ann Allergy Asthma Immunol 2002; 88: 227–235. Doi: 10.1016/S1081-1206(10)62001-7 [DOI] [PubMed] [Google Scholar]
- 78.Price DB, Hernandez D, Magyar P, et al. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax 2003; 58: 211–216. doi: 10.1136/thorax.58.3.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bjermer L, Bisgaard H, Bousquet J, et al. Montelukast and fluticasone compared with salmeterol and fluticasone in protecting against asthma exacerbation in adults: one year, double blind, randomised, comparative trial. BMJ 2003; 327: 891–895. doi: 10.1136/bmj.327.7420.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ilowite J, Webb R, Friedman B, et al. Addition of montelukast or salmeterol to fluticasone for protection against asthma attacks: a randomized, double-blind, multicenter study. Ann Allergy Asthma Immunol 2004; 92: 641–648. Doi: 10.1016/S1081-1206(10)61430-5 [DOI] [PubMed] [Google Scholar]
- 81.Koenig SM, Ostrom N, Pearlman D, et al. Deterioration in asthma control when subjects receiving fluticasone propionate/salmeterol 100/50 mcg Diskus are “stepped-down”. J Asthma 2008; 45: 681–687. doi: 10.1080/02770900802168695 [DOI] [PubMed] [Google Scholar]
- 82.Katial RK, Oppenheimer JJ, Ostrom NK, et al. Adding montelukast to fluticasone propionate/salmeterol for control of asthma and seasonal allergic rhinitis. Allergy Asthma Proc 2010; 31: 68–75. doi: 10.2500/aap.2010.31.3306 [DOI] [PubMed] [Google Scholar]
- 83.Narayanan S, Edelman JM, Berger ML, et al. Asthma control and patient satisfaction among early pediatric users of montelukast. J Asthma 2002; 39: 757–765. doi: 10.1081/JAS-120015800 [DOI] [PubMed] [Google Scholar]
- 84.O'Connor RD, Gilmore AS, Manjunath R, et al. Comparing outcomes in patients with persistent asthma: a registry of two therapeutic alternatives. Curr Med Res Opin 2006; 22: 453–461. doi: 10.1185/030079906X89793 [DOI] [PubMed] [Google Scholar]
- 85.Moeller A, Lehmann A, Knauer N, et al. Effects of montelukast on subjective and objective outcome measures in preschool asthmatic children. Pediatr Pulmonol 2008; 43: 179–186. doi: 10.1002/ppul.20753 [DOI] [PubMed] [Google Scholar]
- 86.Braido F, Baiardini I, Canonica GW. Patient-reported outcomes in asthma clinical trials. Curr Opin Pulm Med 2018; 24: 70–77. doi: 10.1097/MCP.0000000000000440 [DOI] [PubMed] [Google Scholar]
- 87.Vodicka E, Kim K, Devine EB, et al. Inclusion of patient-reported outcome measures in registered clinical trials: evidence from ClinicalTrials.gov (2007-2013). Contemp Clin Trials 2015; 43: 1–9. Doi: 10.1016/j.cct.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 88.Mercieca-Bebber R, King MT, Calvert MJ, et al. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas 2018; 9: 353–367. doi: 10.2147/PROM.S156279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rivera SC, Kyte DG, Aiyegbusi OL, et al. The impact of patient-reported outcome (PRO) data from clinical trials: a systematic review and critical analysis. Health Qual Life Outcomes 2019; 17: 156. doi: 10.1186/s12955-018-1072-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kyte D, Ives J, Draper H, et al. Inconsistencies in quality of life data collection in clinical trials: a potential source of bias? Interviews with research nurses and trialists. PLoS One 2013; 8: e76625. doi: 10.1371/journal.pone.0076625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.National Heart, Lung, and Blood Institute . 2020 Focused Updates to the Asthma Management Guidelines: A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. 2020. www.nhlbi.nih.gov/resources/2020-focused-updates-asthma-management-guidelines
- 92.Ye G, Baldwin DS, Hou R. Anxiety in asthma: a systematic review and meta-analysis. Psychol Med 2021; 51: 11–20. doi: 10.1017/S0033291720005097 [DOI] [PubMed] [Google Scholar]
- 93.Opolski M, Wilson I. Asthma and depression: a pragmatic review of the literature and recommendations for future research. Clin Pract Epidemiol Ment Health 2005; 1: 18. doi: 10.1186/1745-0179-1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blackman JA, Gurka MJ. Developmental and behavioral comorbidities of asthma in children. J Dev Behav Pediatr 2007; 28: 92–99. doi: 10.1097/01.DBP.0000267557.80834.e5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0124-2023.SUPPLEMENT (1.1MB, pdf)