Abstract
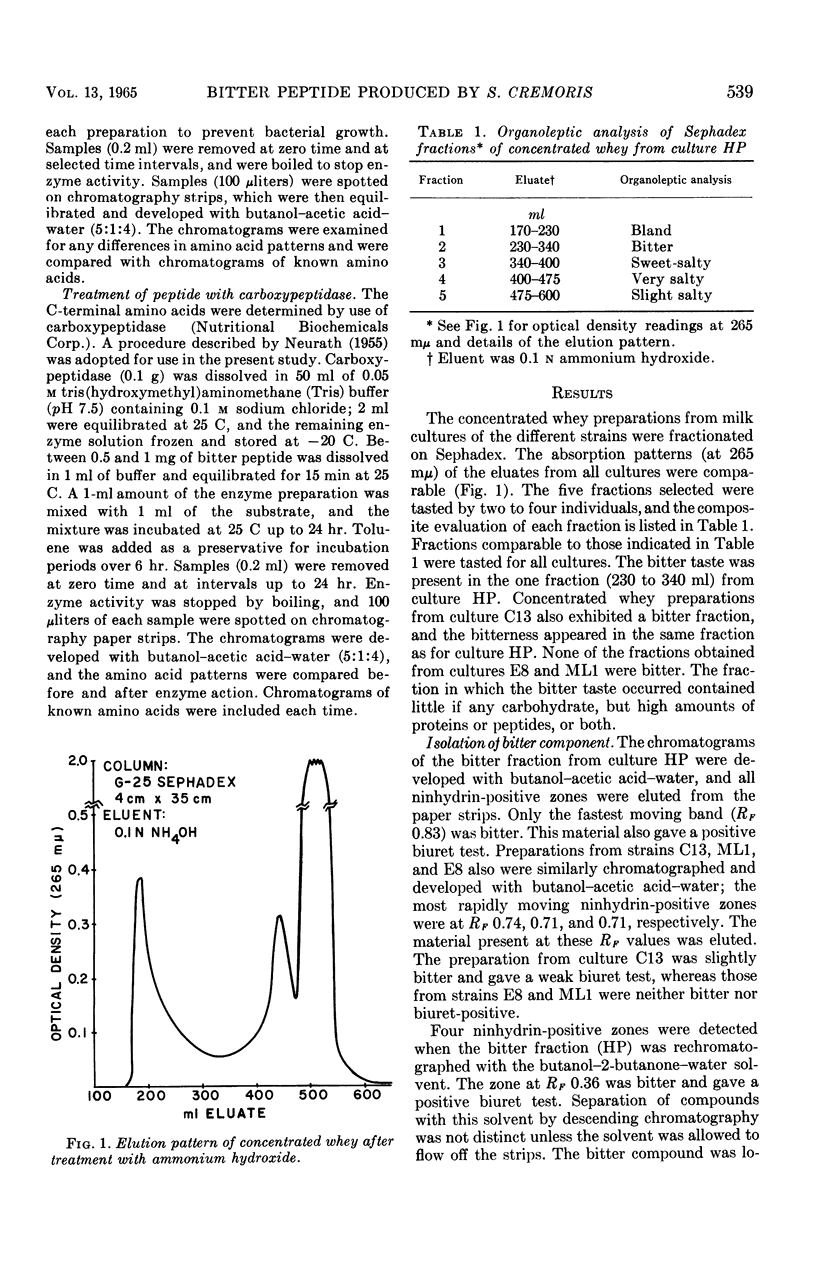
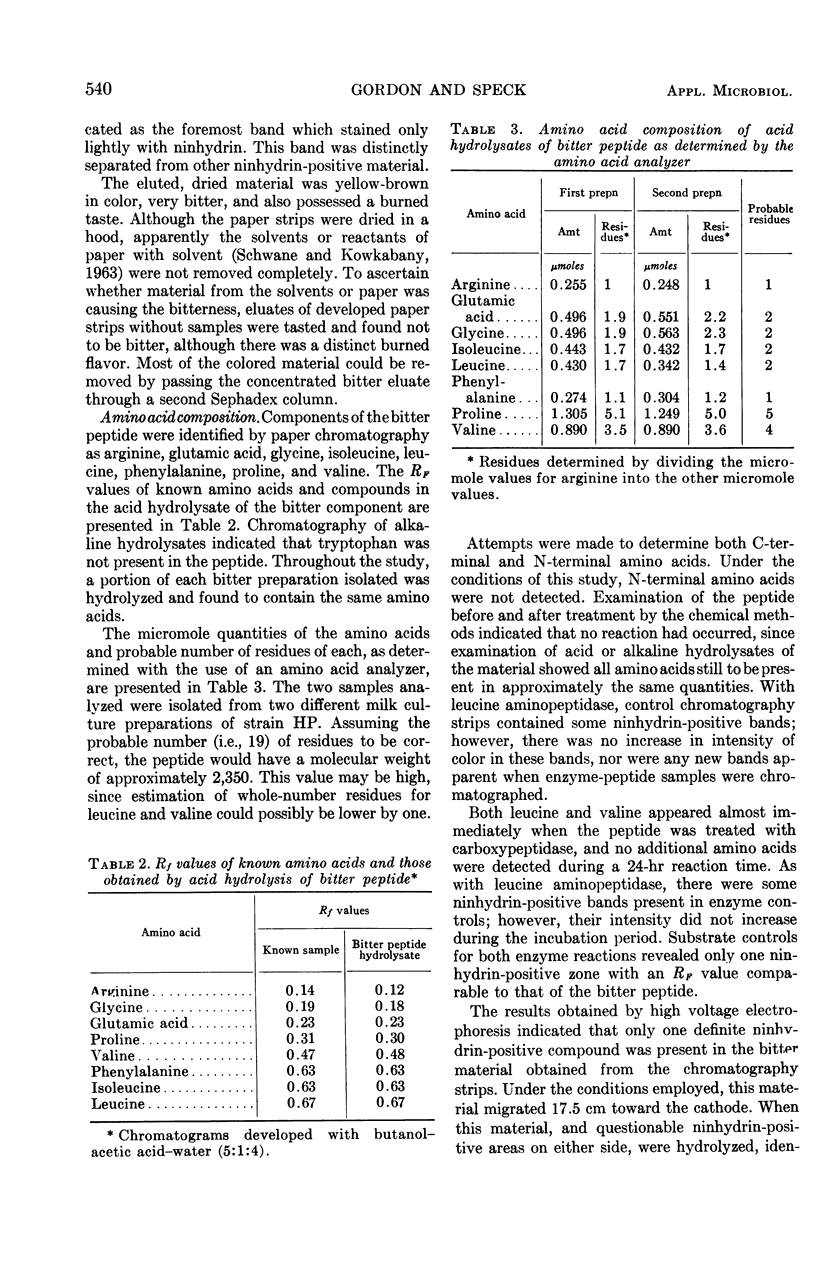
Certain cultures of Streptococcus cremoris produced a bitter taste that occurred in the whey portion of milk cultures. Whey from a culture which produced bitterness was fractionated on Sephadex. The fraction in which the bitter taste was concentrated was chromatographed successively on paper with butanol-acetic acid-water (5:1:4), and then butanol-2-butanone-water (2:2:1). In each instance, the bitter component was in the most rapidly moving band that gave a positive ninhydrin test. The bitterness was observed to be caused by a peptide containing the following numbers of each amino acid: arginine, 1; glutamic acid, 2; glycine, 2; isoleucine, 2; leucine, 2; phenylalanine, 1; proline, 5; and valine, 4. N-terminal amino acids could be detected by coupling with 2,4-dinitrofluorobenzene or phenylisothiocyanate, or by hydrolysis with leucine aminopeptidase. When treated with carboxypeptidase, only leucine and valine appeared at the C-terminal end, and these were detected simultaneously.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GLADNER J. A., NEURATH H. Carboxyl terminal groups of proteolytic enzymes. I. The activation of chymotrypsinogen to alpha-chymotrypsin. J Biol Chem. 1953 Nov;205(1):345–360. [PubMed] [Google Scholar]
- GORDON D. F., Jr, SPECK M. L. BITTERNESS IN MILK CULTURES OF STREPTOCOCCUS CREMORIS. J Dairy Sci. 1965 Apr;48:499–500. doi: 10.3168/jds.s0022-0302(65)88261-3. [DOI] [PubMed] [Google Scholar]
- HILL R. L., SMITH E. L. Leucine aminopeptidase. VII. Action on long chain polypeptides and proteins. J Biol Chem. 1957 Oct;228(2):577–600. [PubMed] [Google Scholar]
- HIRS C. H. The structure of ribonuclease. Ann N Y Acad Sci. 1960 Aug 31;88:611–641. doi: 10.1111/j.1749-6632.1960.tb20057.x. [DOI] [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- MARGOLIASH E. Amino acid sequence of chymotryptic peptides from horse heart cytochrome c. J Biol Chem. 1962 Jul;237:2161–2174. [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- SANGER F., THOMPSON E. O. P. The amino-acid sequence in the glycyl chain of insulin. I. The identification of lower peptides from partial hydrolysates. Biochem J. 1953 Feb;53(3):353–366. doi: 10.1042/bj0530353. [DOI] [PMC free article] [PubMed] [Google Scholar]


