Abstract
Introduction
The global burden of age-related cognitive decline is increasing, with the number of people aged 60 and over expected to double by 2050. This study compares the acute effects of age-appropriate cognitively demanding aerobic exercises involving walking, on cognitive functions and exerkine responses such as brain-derived neurotrophic factor (BDNF) and cathepsin B (CTSB) in older, healthy adults.
Methods/design
Healthy older golfers (n=25, 16 male and 9 female, 69±4 years) were enrolled in a 5-day randomised cross-over study and completed three different exercise trials (18-hole golf round, 6 km Nordic walking, 6 km walking) in a real-life environment, in random order and at a self-selected pace. Differences in cognition (the Trail-Making Test (TMT) AB) and exerkines (BDNF and CTSB) were analysed within groups using the Wilcoxon signed-rank test and between groups using the Kruskal-Wallis test.
Results
All exercise types resulted in a significant decrease in the TMT A-test (p<0.05; golf: −4.43±1.5 s, Nordic walking: −4.63±1.6 s, walking: −6.75±2.26 s), where Nordic walking and walking demonstrated a decrease in the TMT B-test (p<0.05; Nordic walking: −9.62±7.2 s, walking: −7.55±3.2 s). In addition, all exercise types produced significant decreases in the TMT AB test scores (p<0.05), and Nordic walking (p=0.035) showed decreases in the TMTB-TMTA-test. There were no immediate postexercise changes in the levels of BDNF or CTSB.
Conclusion
Acute bouts of golf, Nordic walking and walking improved cognitive functions irrespective of exerkines in healthy older adults. In addition, Nordic walking and walking in general enhanced executive functions. No significant effects were seen on the levels of BDNF and CTSB.
Trial registration number
ISRCTN10007294.
Keywords: Golf, Walking, Aging, Exercise
WHAT IS ALREADY KNOWN ON THIS TOPIC
Previous research has highlighted the potential cognitive enhancements resulting from acute bouts of aerobic exercise, with the extent of improvement influenced by factors like exercise intensity, duration and modality.
WHAT THIS STUDY ADDS
The study assesses three popular types of age-appropriate aerobic exercise, namely golf, Nordic walking and walking, and examines their relationships with cognitive function and cognitive-related exerkines in healthy older adults.
This study indicates that a single session of 18 holes of golf, 6 km of Nordic walking or 6 km of regular walking may improve cognitive function in older adults, irrespective of the specific exercise modality.
Nordic walking and regular walking were shown to enhance more demanding executive functions such as set-shifting and cognitive flexibility.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The study results support that age-appropriate exercises, especially aerobic exercises such as golf, Nordic walking and regular walking can maintain and improve cognitive function in the general population of older adults.
Aerobic exercise, including Nordic walking, regular walking and golf, offers potential promise as a treatment strategy for those who already exhibit cognitive decline.
Introduction
The globally ageing population and expected increase in age-related cognitive decline1 and dementia2 all pose health, social care and economic challenges, with the cost of care projected to reach US$1.6 trillion by 2050.3 Exercise has become a crucial modifiable factor in preventing cognitive decline4 5 as it can help maintain cognitive performance during normal ageing and protects against neurogenerative diseases,5 in addition to improving executive functioning such as processing speed and visuospatial functions in older adults.6
Recent meta-analyses have indicated that an acute period (<60 min) moderate-intensity (40%–60% VO2max) aerobic exercise (AE) appears to have greater impact on the executive function of older adults than on younger adults regarding time-dependent tests,7 and it can also improve task-shifting performance in healthy adults,8 as measured by the Trail Making Test (TMT). The TMT is a commonly used evaluation of cognitive function for older adults that is sensitive to dementia-related cognitive decline.9 Although AE is a promising non-pharmaceutical method for preventing cognitive decline, the underlying mechanisms are not fully understood.10 Recent studies suggest that exercise-induced improvement in cognitive function6 may be related to exercise intensity and duration.10 11 Exerkines such as brain-derived neurotrophic factor (BDNF) and cathepsin B (CTSB) are gaining increasing attention due to their potential role in signalling possible contributions to cognitive/neurological health.12 13 In this regard, a recent meta-analysis reported higher-intensity (> 80%) exercise leading to increases of 60% in BDNF in older adults.11 The acute effects of AE on CTSB, however, are less well understood, although higher intensity (>80% VO2max) AE (30–40 min) has been shown to increased CTSB by 20% in younger individuals, while no response was observed after moderate intensity (40%–60% VO2max) AE in older adults.10
Before we are able to determine the possible beneficial effects of exercise as a preventive factor and treatment strategy for cognitive decline in older adults,4 14 15 more knowledge of the exercise-specific effectiveness of age-appropriate, popular and safe16–18 cognitively-demanding exercises such as golf and Nordic walking is required. Golf, Nordic walking and walking are all moderate-intensity outdoor AEs known for their safety and accessibility for older individuals.17–19 However, golf and Nordic walking stand out as more cognitively demanding activities compared with traditional walking, since golf involves strategic thinking and continuous cognitive involvement during play.20 Nordic walking requires synchronised rhythms with use of poles, accompanied by heightened engagement of the upper body extremities and trunk compared with regular walking.21 This study aims to investigate whether an acute bout of these different types of AEs affects cognitive function and exerkines such as BDNF and CTSB.
Methods
Study design
This study was a 5-day randomised cross-over trial involving 25 healthy older adults (16 males, 9 females; mean age 69±4 years) who were already active golfers. Each subject completed (in a random order) three exercise trials at a self-selected pace. The study involved a cross-over design over two separate weeks with 4–5 participants randomly assigned to each of the three exercise groups. The trials included an 18-hole round of golf on a flat course at Tarina Golf course, Finland (course profile: flat; length from red tees: 4477 m), while pulling a trolley in groups of 2–3 players; Nordic walking on a flat predetermined walking route of 6000 m and walking the same walking route of 6000 m without the use of Nordic walking poles. Each participant completed the three different exercise trials over a 5-day period, with a 1-day washout period between each trial to mitigate potential carryover effects. For example, one group began with an 18-hole golf round on day 1 (d1), followed by Nordic walking on day 3 (d3) and finally walking on day 5 (d5). Between each exercise trial on the second (d2) and fourth (d4) days, a 1-day washout phase was incorporated to minimise any carryover effects from the previous trial. Throughout the 5-day period, participants were instructed to maintain their regular physical activities and nutritional habits, which were recorded in a diary.
Sample size
The required calculation of the sample size was performed for the outcome variable BDNF. Based on a one-way analysis of variance, at least 21 participants were needed per group to achieve a statistically significant difference (p<0.05) between groups, assuming a BDNF concentration of 19 ng/mL after walking, 22 ng/mL after Nordic walking and 24 ng/mL after golf, with an SD of 5.
Participants
The study recruited 60 volunteers from Tarina Golf’s official membership register, out of whom 34 underwent a screening test at the University of Eastern Finland (Kuopio, Finland).
The inclusion criteria were male or female, over 65 years of age, body mass index <35 kg/m2, golf handicap (HCP) ≤36 and participation in at least 18 holes of golf per week. Participants were permitted to have long-term use of hyperlipidaemia and hypertension medication except those that may affect heart rate (HR), such as beta-blockers. Exclusion criteria were those diagnosed with dementia, Alzheimer’s disease, Parkinson’s disease or cardiovascular diseases. After evaluation by a doctor, 26 participants were deemed eligible, and 1 dropped out after randomisation, leaving a total of 25 eligible participants. Participants provided written consent to participate in the study.
All participants engaged in each exercise modality, except for one who did not complete the golf round due to health issues. The testing protocol also included collecting data on weight, height, body composition, waist and pelvis circumference, blood pressure, cardiorespiratory fitness and physical function, as detailed in a prior publication.18
Experimental procedure
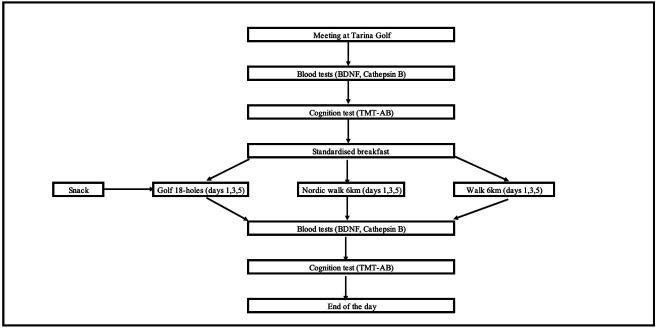
The daily experimental protocol is presented in figure 1. On experimental days, participants arrived at Tarina Golf Course (Siilinjärvi, Finland) at 7:00 hours following an overnight fast of 10 hours. Blood samples were taken first for BDNF and CTSB analysis. Next, participants completed the cognitive function TMT A and B test. They were then fitted with a Fitbit Versa 3 (California, USA) on the wrist22 23 to measure exercise-specific outcomes (distance, duration, pace, energy expenditure and number of steps). It has been previously reported and noted that Fitbits tend to overestimate energy expenditure during activity with an estimated mean error of 4% (SD 2%).24 Additionally, they were fitted with a Beat2Phone ECG sensor with a chest strap (VitalSignum, Helsinki, Finland)25 26 to measure HR. This device provided reliable HR data that is useful for monitoring cardiovascular health and guiding clinical interventions.26 After pre-exercise measurements, participants were provided with a standardised breakfast (530 kcal) before the exercise as well as a snack (150 kcal) during the golf round. Nordic walking and walking groups started between 8:00 and 9:00 hours, and the golf group started between 9:00 and 10:00 hours. All pre-exercise measurements were taken in the morning hours between 7:00 and 8:30 hours, and postexercise measurements were taken between 09:30 and 14:00 hours. Postexercise, measurements were repeated immediately (<15 min).
Figure 1.
Flow chart of experimental day protocol. BDNF, brain-derived neurotrophic factor; TMT, Trail-Making Test.
Blood samples and cognitive-related exerkines
Blood samples were taken before and after exercise from the brachial vein of participants who had fasted overnight (>10 hours) prior to testing and avoided caffeine, alcohol and physical activity the day before sampling. Samples were collected in EDTA tubes for BDNF and serum tubes for CTSB and stored at −80°C for further analysis. To minimise the impact of natural diurnal variations in BDNF levels,27 28 measurements were performed in the morning hours between 8:00 and 12:00 hours, except for postexercise blood samples taken from golfers, which were taken between 13:00 and 14:00 hours. The plasma levels of BDNF were measured by ELISA (Thermo Fisher Scientific, California, USA), and the serum levels of CTSB were also measured by ELISA (Elabscience, Texas, USA) according to the manufacturer’s instructions. Intra-assay variations were less than 10% in both analyses.
Cognitive function assessment
The TMT AB test is a widely used cognitive task for assessing visuospatial lower and higher cognitive function in older adults.29 It consists of two parts, TMT A and TMT B,9 where the TMT A test measures lower cognitive functions such as attention and processing speed,29 that are required for, for example, visual searching.9 TMT B test measures task-switching ability and assesses more executive function and higher cognitive functionality,29 requiring working memory and cognitive flexibility.9 In TMT A, participants use a pencil to connect randomly arranged numbers (1–25) in ascending order, while in TMT B, they were required to connect numbered (1–13) and lettered (A–M) circles, beginning with the number one and alternating between letters and numbers in ascending order as quickly as possible.9 29 30 Scores for both tests were obtained based on the time taken to complete each task, and the time to complete TMT A and TMT B was recorded using a handheld stopwatch.
Statistical analysis
The study involved pre-exercise and postexercise measurements using a cross-over design, resulting in 75 outcome values that were analysed using IBM SPSS Statistics V.27.0 (IBM). Means and SDs and SEs were calculated for continuous variables, while frequencies and percentages were calculated for categorical variables. TMT B-TMT A values and TMT B/TMT A ratio scores were calculated to measure cognitive efficiency.31 32 The normal distribution of the data was checked using the Kolmogorov-Smirnov test and visually observed from a histogram, which indicated that most variables did not distribute normally and sizes of groups were rather small. As a result, non-parametric tests were used to analyse data. The Wilcoxon signed-rank test was used to compare cognitive function and cognitive-related exerkines outcomes within groups for pre-exercise and postexercise sessions. The Kruskal-Wallis test was used to assess differences between all three groups for the variables at baseline and changes during the trials period. The Pearson correlation assessed the relationship between outcomes. The Mann-Whitney U test was also used to compare the outcomes between genders. Significance level was set at p<0.05 with 95% CIs.
Results
Characteristics of study participants
This study had 25 healthy older golfers as participants (n=25, 64% (n=16) males and 36% (n=9) females). The characteristics of the subjects are shown in table 1. The participants were considered normal weight to overweight (25±2.5 kg/m2) and had elevated blood pressure (144/89±18/12 mm Hg) as well as increased total cholesterol (5.5±1.2 mmol/L). The HCP value was 22±6, and participants had previous golf experience of 17±9 years on average, and they played 2±1 golf rounds (18 holes) per week during the summer season (April–October) in Finland, which would equate to meeting moderate to vigorous physical activity guidelines.
Table 1.
Participant characteristics at the baseline
| Men | Women | All | P value*† | Cohen’s d‡ | |
| n=16 | n=9 | n=25 | |||
| Demmographic outcomes | |||||
| Age (years) | 68±3.9 | 67±2.6 | 69±4.4 | 0.628 | 0.066 |
| Marital satus | 0.088 | ||||
| Married | 15 (94%) | 5 (56%) | 20 (80%) | ||
| Divorced | 0 (0%) | 2 (22%) | 2 (8%) | ||
| Widowed | 0 (0%) | 1 (11%) | 1 (4%) | ||
| Single | 1 (6%) | 1 (11%) | 2 (8%) | ||
| Employment status | 0.019 | ||||
| Retired | 14 (88%) | 8 (89%) | 22 (88%) | ||
| Working <40 hours/week | 2 (12%) | 1 (11%) | 3 (12%) | ||
| Physical health outcomes | |||||
| Weight (kg) | 79±8.9 | 70±5.3 | 76±8.9 | 0.024 | 1.153 |
| BMI (kg/m2) | 26±2.7 | 26±2.3 | 26±2.5 | 0.692 | 0.014 |
| The waist–hip ratio | 1±0.1 | 0.86±0.06 | 0.96±0.09 | <0.001 | 2.620 |
| Systolic blood pressure (mm Hg) | 151±17 | 135±16 | 150±18 | 0.044 | 0.924 |
| Diastolic blood pressure (mm Hg) | 92±12 | 83±12 | 89±13 | 0.126 | 0.723 |
| Total cholesterol (mmol/L) | 5.0±1.3 | 5.6±0.6 | 5.5±1.2 | 0.088 | 0.478 |
| Triglycerides (mmol/L | 1.0±0.4 | 1.1±0.3 | 1.0±0.4 | 0.444 | 0.988 |
| LDL-cholesterol (mmol/L) | 2.8±1.0 | 3.0±0.4 | 2.9±0.9 | 0.164 | 1.030 |
| HDL-cholesterol (mmol/L) | 1.6±0.5 | 2.0±0.6 | 1.7±0.5 | 0.034 | 1.751 |
| Haemoglobin A1c (mmol/L) | 37.9±3.6 | 36.6±3.5 | 37.4±3.6 | 0.333 | 0.386 |
| Blood glucose (mmol/L) | 5.9±0.4 | 5.7±0.7 | 5.8±0.5 | 0.163 | 0.499 |
| Physical fitness outcomes | |||||
| 6 min walk test (m) | 671±65 | 625±61 | 654±66 | 0.066 | 0.714 |
| VO2max (mL/kg/min) | 35±3 | 31±4 | 33±4 | 0.079 | 1.032 |
| SPPB score | 11.6±0.5 | 11.7±0.5 | 11.6±0.5 | 0.838 | 0.081 |
| Golf outcomes | |||||
| Handicap value (WHS) | 19.4±5.7 | 27.6±3.2 | 22.2±6.4 | 0.004 | 1.458 |
| Golf exerperience (years) | 17.6±8.7 | 14.13±9.8 | 16.6±9.0 | 0.712 | 0.184 |
| Golf round-activity (week) | 2.4±0.8 | 2.3±1.1 | 2.3±0.9 | 1.000 | 0.066 |
| Practice swing (during play) | 0.412 | ||||
| Yes | 13 (81%) | 6 (67%) | 19 (76%) | ||
| No | 3 (19%) | 3 (33%) | 6 (24%) |
n (total)=25, n (men)=16 (64%), n (women)=9 (36%).
Quantitative variables were expressed as means ±SD and expressed in mean (95% CI).
Categorical variables were expressed as numbers and percentage values.
Bold values indicates a statistically significant difference with a p-value less than 0.05.
*To determine gender difference, data were analysed by Mann-Whitney U test.
†To determine gender difference, data were analysed by χ2 tests.
‡To determine gender difference effect sizes, data were analysed by independent t-test.
BMI, body mass index; HDL-cholesterol, high-density lipoprotein cholesterol; LDL-cholesterool, low-density lipoprotein cholesterol; SPPB, short physical performance test; WHS, world handicap system.
Exercise/physical activity outcomes
The mean distance (p<0.001), time spent exercising/completing the exercise bout (p<0.001) and steps (p<0.001) were significantly greater for golf compared with both Nordic walking and walking.18 However, the average HR of golf (p=0.050) was significantly lower. The average exercise intensity based on estimated HR max (220 bpm—age) during each trial was 61% in the golf group, 77% in the Nordic walking group, and 76% in the walking group.18
Acute responses to cognitive function
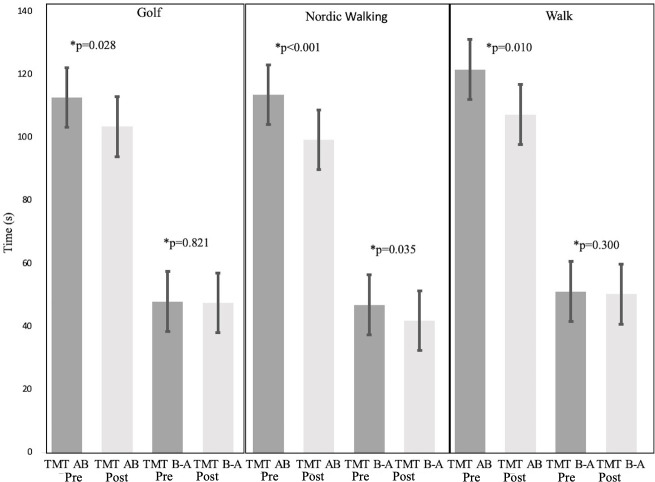
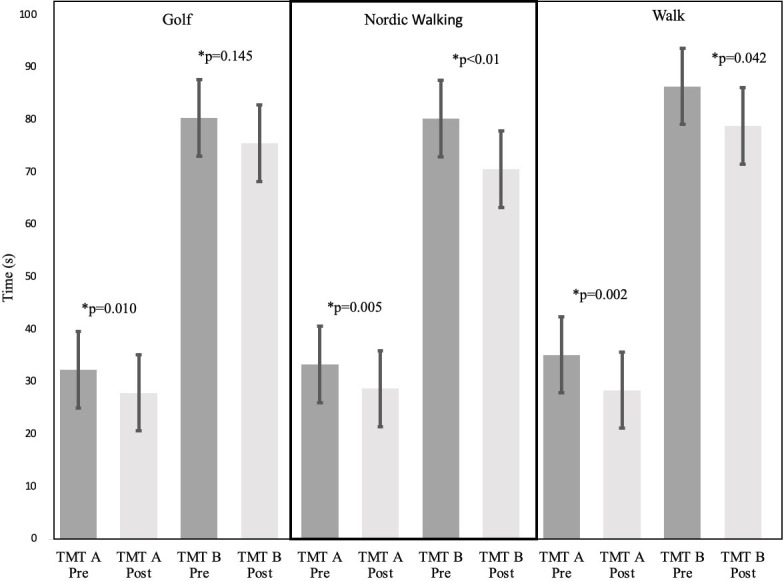
TMT test completion time values are shown in figures 2 and 3. Between-group comparison showed no significant differences in TMT test results (p>0.05). In the within-group comparisons, significant decreases were observed in all groups in both TMT (p<0.05) tests from pre-exercise to postexercise, corresponding to improved outcomes. Statistically significant decreases in (p<0.05) TMT A time values were observed in all groups (golf: −4.43±1.5 s, Nordic walking: −4.63±1.6 s, walking: −6.75±2.26 s), while decreased TMT B time values were found in the Nordic walking (−9.62±7.2 s, p<0.001) and walking (−7.55±3.2 s, p=0.042) groups. TMT AB total time values statistically decreased (p<0.05) in all groups (golf: −9.23±4.7, Nordic walking: −14.26±7.4, walking: −14.28±8.20). TMT B - TMT A time values statistically decreased (−4.97±7.26 s, p=0.035) only in the Nordic walking group. In TMT B/TMT A, the ratio values remained between 2.0–3.0 in all groups. In addition, no significant gender differences were observed.
Figure 2.
Measn±SEM Trial-Making Test (TMT) A and B scores pre-exercises and post-exercises in each gourp. To determine group differences in pre-exercise and post-exercise, data were analysed via the Wilcoxon signed-rank test. * = p-value
Figure 3.
Measn±SEM Trial-Making Test (TMT) corrected B scores pre-exercises and post-exercises in each gourp. To determine group differences in pre-exercise and post-exercise, data were analysed via the Wilcoxon signed-rank test. * = p-value.
Acute responses to BDNF
The average BDNF changes pre-exercise and postexercise are presented in table 2. In the between-group comparison, there were no significant differences in BDNF test results (p=0.745). In the within-group comparisons, BDNF slightly decreased non-significantly in all groups (golf: −6.11±7.35 ng/mL Nordic walking: −2.56±7.56 ng/mL, walking: −2.31±7.29 ng/mL), but we did not observe a significant change in BDNF levels after the exercise session between groups (p=0.745) or within the groups (golf p=0.391, Nordic walking p=0.968, walking=0.523). Data from the washout period between d1 (pre-exercise) and d3 (pre-exercise) is presented in table 3. Approximately 40 hours after exercise, Nordic walking demonstrated significantly increased BDNF values (p=0.046) in the participants. No correlations were observed between changes across the TMT test, BDNF and CTSB. In addition, no significant gender differences were observed in the acute response to exercise.
Table 2.
Acute responses to BDNF and CTSB tests
| Golf | Nordic walk | Walk | |||||||||||
| Pre-exercise | Postexercise | Change | P value* | Pre-exercise | Postexercise | Change | P value* | Pre-exercise | Postexercise | Change | P value* | P value† | |
| BDNF (ng/mL) | 40.61±6.53 | 34.49±6.28 | 6.11±7.35 | 0.391 | 45.11±8.34 | 43.83±9.23 | 2.56±7.56 | 0.968 | 44.11±9.66 | 37.67±5.13 | 2.31±7.29 | 0.523 | 0.745 |
| CTSB (ng/mL) | 66.26±6.20 | 69.26±8.22 | 3.0±4.00 | 0.615 | 68.69±8.56 | 71.22±8.56 | 2.55±3.07 | 0.693 | 73.55±8.45 | 77.14±9.99 | 3.59±3.82 | 0.927 | 0.927 |
BDNF golf (n=20 pre and n=20 post), NW (n=20 pre, n=19 post), W (n=18 pre, n=19 post).
CTSB golf (n=22 pre and n=22 post), NW (n=23 pre, n=13 post), W (n=23 pre, n=23 post).
Quantitative variables were expressed as means ±SEM and expressed in mean (95% CI).
*To determine group difference in pre-exercise and post-exercise, data were analysed by Wilcoxon signed rank test.
†To compare the difference in pre-exercise and change value between all groups, data were analysed by Kruskal-Wallis test.
BDNF, brain-derived neurotrophic factor; CTSB, cathepsin B.
Table 3.
Wash-out period to days d1 and d3 to BDNF and CTSB tests
| Golf | Nordic walk | Walk | |||||||||||
| d1 | d3 | Change | P value* | d1 | d3 | Change | P value* | d1 | d3 | Change | P value* | P value† | |
| BDNF (ng/mL) | 26.88±7.36 | 39.25±6.30 | 12.37±11.58 | 0.310 | 37.86±15.48 | 54.00±24.85 | 18.12±7.91 | 0.046 | 52.17±23.61 | 51.77±14.82 | 0.39±22.28 | 0.779 | 0.769 |
| CTSB (ng/mL) | 58.75±5.80 | 62.27±10.75 | 3.52±11.6 | 0.674 | 102.40±15.48 | 98.73±12.37 | 3.68±6.76 | 0.500 | 84.23±16.84 | 66.77±13.05 | 17.46±10.42 | 0.173 | 0.322 |
Golf n (BDNF)=7, n (CTSB)=8; NW n (BDNF)=7, n (CTSB)=5; W n (BDNF)=8, n (CTSB)=9.
Quantitative variables were expressed as means ±SEM and expressed in mean (95% CI).
Bold values indicates a statistically significant difference with a p-value less than 0.05.
*To determine group difference in days d1 (pre-exercise) and d3 (pre-exercise), data were analysed by Wilcoxon signed rank test.
†To compare the difference in pre-exercise and change value between all groups, data were analysed by Kruskal-Wallis test.
BDNF, brain-derived neurotrophic factor; CTSB, cathepsin B; d1, day 1; d3, day 3.
Acute responses to cathepsin-B
The average CTSB changes pre-exercise to postexercise are presented in table 2. In the between-group comparison, there were no significant differences in CTSB test results (p=0.927). In the within-group comparisons, CTSB values increased non-significantly in all groups (golf: 3.0±4.0 ng/mL Nordic walking: 2.55±3.07 ng/mL, walking: 3.59±3.82 ng/mL), but no significant changes in CTSB levels were observed after the exercise session between groups (p=0.927) or within the groups (golf p=0.615, Nordic walking p=0.693, walking=0.927). Approximately 40 hours after exercise, golf demonstrated a non-significant increase in CTSB values (3.52±11.6 ng/mL), while after Nordic walking, values slightly decreased (−3.68±6.76 ng/mL). Similarly, a non-significant reduction was observed in CTSB (−17.46±10.42 ng/mL) after walking.
Discussion
To our knowledge, this is the first study to assess the immediate cognitive effects of golf, Nordic walking and walking in older adults without underlying cognitive impairment. Our findings indicate that an acute bout of AE popular in this age group is able to improve cognitive function in older adults. Moreover, Nordic walking and walking also improve more demanding executive functions, which may be related to the higher relative exercise intensity. While no significant increase in cognition-related exerkines was observed immediately after exercise, Nordic walking produced significantly longer-lasting increases in BDNF.
Acute responses to cognitive function
We observed significant improvements in the TMT A test, which measures lower cognitive functions such as psychomotor speed, visuospatial searching and target-director motor tracking,33 with all exercise modalities. In addition, improvements in the TMT B test, which measures high-order processes, executive functions and set-switching ability,33 and these findings were statistically significant after Nordic walking and walking. From previous study, it is known that acute AE can improve task-shifting performance measured by the TMT A and B tests, while low to moderate intensity exercise may provide better results in task-shifting performance.8 Meta-analysis11 proves that moderate and vigorous exercise induces fatigue and dehydration. Longer duration of a cognitively demanding activity may induce more fatigue, cognitive fatigue and other factors (eg, dehydration) that can influence cognition, and this may explain the lack of improvement in the TMT B test results after golf trials as golf is a longer duration activity.
Studies have supported that a subject’s performance on TMT B is influenced by the low-level processes that are also involved in TMT A, such as visual scanning, motor speed, and basic visuomotor tracking. To account for this, we calculated corrected TMT B scores ((TMT B−TMT A) or (TMT B/TMT A)) to provide a more efficient measure of cognitive flexibility and processing speed that isolates the specific demands of set-switching in TMT B.31 32 We observed significant improvements in the total time of both the TMT A and B tests, which describes generally more low-level processes improvements. It is important to highlight that the TMT A test has an impact on both its own time values and those of TMT B, owing to the test protocol’s.31–33 Additionally, the enhancement in overall time following Nordic walking for the TMT B-TMT A test could potentially be attributed to the fact that Nordic walking places higher cognitive demands compared with regular walking. Among all exercise types, the TMT B/TMT A ratio score was between 2 and 3, suggesting essentially equal performance for these tests.32
The TMT-A and TMT-B time of our study participants were approximately 60% faster compared with normative data of TMT tests on older adults, which reported TMT A (53±26 s) and TMT B (137±54 s).9 It appears that our senior golfers may have better cognitive function than matched members of the general population, which could be due to their habitual physical activity and regular participation in cognitively demanding AE (golf),20 although confounding factors could include socio-economic status, education level and other factors, which may mean the baseline of a golf-playing cohort is different to the general population. Based on prior investigations7–9 as well as those of this study, low to moderate intensity physical activity seems to be positively associated with improved acute cognitive function for older healthy adults; this is particularly important as these types of exercise are more popular and perhaps more practical than high-intensity training/ exercise, which may not be feasible for older adults considering their health status, and activities participated in by their friends and peers.
Acute responses of BDNF to AE
BDNF is synthesised primarily in the brain and also in skeletal muscle.6 It has been suggested as a mediator of acute exercise-cognitive performance relationships.34 It is known that a single session of at least 30 min of moderate intensity (> 60% VO2max) AE can increase BDNF concentrations11 in healthy younger adults.34 However, it remains unclear whether acute cognitively demanding exercise has a similar impact on BDNF in healthy older adults.11 35 In our study, we were not able to find an immediate post-exercise effect of acute exercise on BDNF levels. Only one study directly measured the effect of playing an 18-hole golf round on BDNF levels in healthy younger adults (n=9, 31±4 years) and reported a modest but significant increase in BDNF (20%) immediately after the golf round, with levels returning to baseline after 1 hour of recovery.36 However, the duration of their golf round was longer (5 hours) in this past study than in our study (3.5 hours), and participants differed in age and prior exposure, where in our current study, all participants were regular weekly senior golfers.11 Interestingly, BDNF levels have been shown to decrease by about 6% in older women who participated in NW training for the first time after 1 hour of training, but BDNF increased if the person regularly practised Nordic walking.37 In our study, most of the golfers did not regularly practice Nordic walking. However, Nordic walking led to a significant increase in BDNF expression, while golf also produced higher BDNF levels after approximately 35–40 hours after the first exercise; this makes biological sense given that the maximum concentration of BDNF was found 24 hours after blood collection, and this concentration can remain stable up to 42 hours postexercise.38
Some studies have determined that acute AE can lead to a reduction in BDNF concentration.39 40 It has been suggested that this reduction may be due to the utilisation of BDNF in the repair of exercise-induced muscle damage or a reduction of BDNF release by the brain during recovery.40 41 The release of BDNF in response to exercise appears to be intensity-dependent, with higher-intensity exercise resulting in a greater release of BDNF.35 40 However, in our study, the intensity of AE was largely moderate (60%–76% HRmax), which might have affected the immediate lack of BDNF response. In addition, previous studies have indicated that BDNF levels may also vary within individuals over days and that physical fitness, sex, age and body weight can all influence BDNF concentrations.11 28 35
Acute responses of cathepsin-B to AE
In addition to BDNF, we also investigated the effect of acute AE on CTSB. However, CTSB is released from skeletal muscle cells, and we know that CTSB circulation increases in an AE intensity-dependent manner.10 The acute effects of AE on CTSB circulation in humans requires further study, especially since there are conflicting results regarding exercise and CTSB.6 In our study we observed non-significant increases immediately postexercise in this population. A recent past study observed no change in serum CTSB in response to acute low and moderate (40% and 65% VO2max) intensity AE, but there was a 20%±7% (p=0.02) and 30%±18% (p=0.04) increase in CTSB in response to higher intensity (80% of VO2max and VO2max) AEs.10 In another recent study, where acute moderate-intensity (50%–60% of HRR) postexercise effects of open-skill and closed-skill on CTSB were studied in healthy younger (age 18–25 years) athletes (n=45), an increase in CTSB was reported immediately (3 min) postexercise.6
From a clinical perspective, this study’s findings focused on acute changes in cognitive function and cognitive-related exerkines. In keeping with the wider literature on the role of health-enhancing physical activity on cognitive function, our study showed that all types of studied AE produce favourable effects on cognitive function in older adults, regardless of intensity or duration. However, no significant changes were observed in the cognitive-related exerkines. Further research is needed on the exercise dose-response relationship in exerkines, including BDNF and CTSB, based on exercise-specific parameters such as intensity, duration and type in populations including healthy older adults.
Study limitations
We conducted this randomised cross-over study in a real-world environment. This is advantageous for its generalisability to real-life contexts. However, due to the field-test conditions, differences in exercise modulation, such as distance and terrain, may influence the findings. Not all factors could be controlled as precisely as in a laboratory setting. The use of one postexercise measurement for BDNF and CTSB and blood samples taken after 12:00 hours may also impact the interpretation of the study’s findings due to potential biomarker changes over time and BDNF’s circadian rhythm expression. Additionally, it is important to take into consideration the small sample size when evaluating the results.
Considering the trial protocol, we recruited only golfers for the study as it was impossible to assign non-golfers to play a round of golf. Nordic walking was a new type of exercise for most participants, which may have led to poor technique and thereby exercise parameters of the activity. Those who regularly play golf may have better overall health and physical fitness, limiting the generalisability of the results to all older adults or those with chronic conditions.
Conclusion
All three forms of AE studied (golf, Nordic walking and walking) improved cognitive functions regardless of the specific type of exercise engaged in. Interestingly, Nordic walking and regular walking were shown to enhance more demanding executive functions. However, it may be that the intensity of these exercises was not high enough to effect BDNF and CTSB, and other factors that merit further study may have contributed to these findings.
Golf, Nordic walking and walking can be recommended to healthy older adults as AE for their protective effects on cognitive function. The best available evidence suggests they can be recommended as a preventive factor for older adults and potentially as a treatment strategy for those presenting decline.
Footnotes
Twitter: @kettinenjulia, @HiltunenLab, @docandrewmurray, @w_r_taylor, @venojarvi
Contributors: JK, MV and HT contributed to the design of the study. JK coordinated the trial and data collection and is the study’s guarantor. MV assisted with recruitment and data collection as well as biochemical analysis. JK wrote the statistical analysis plan and the paper. MV, HT, MH, AM, NH and WRT reviewed and edited the paper. All authors approved the final version of the manuscript.
Funding: This research was supported by the Strategic Neuroscience Funding of the University of Eastern Finland and by grants from the Aarne Koskelo Foundation and the Paulo Foundation. Vital Signum donated the Beats2Phone devices.
Competing interests: The authors declare that they have no competing interests, except AM, who is a medical advisor to The R&A, a global governing body for golf.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and the study protocol was approved by the Ethics Committee, Hospital District of Northern Savo Committee (reference number 1073/2021). Every potential participant was informed about the aims and procedures of the study both verbally and in writing. Participants gave informed consent to participate in the study before taking part.
References
- 1.Ageing and health. Available: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health [Accessed 06 Mar 2023].
- 2.Nichols E, Steinmetz JD, Vollset SE, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health 2022;7:e105–25. 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velandia PP, Miller-Petrie MK, Chen C, et al. Global and regional spending on dementia care from 2000–2019 and expected future health spending scenarios from 2020–2050: an economic modelling exercise. EClinicalMedicine 2022;45:101337. 10.1016/j.eclinm.2022.101337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alty J, Farrow M, Lawler K. Exercise and dementia prevention. Pract Neurol 2020;20:234–40. 10.1136/practneurol-2019-002335 [DOI] [PubMed] [Google Scholar]
- 5.Kirk-Sanchez NJ, McGough EL. Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging 2014;9:51–62. 10.2147/CIA.S39506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gökçe E, Güneş E, Arı F, et al. Comparison of the effects of open- and closed-skill exercise on cognition and peripheral proteins: a cross-sectional study. PLoS One 2021;16:e0251907. 10.1371/journal.pone.0251907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludyga S, Gerber M, Brand S, et al. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: a meta-analysis. Psychophysiology 2016;53:1611–26. 10.1111/psyp.12736 [DOI] [PubMed] [Google Scholar]
- 8.Oberste M, Sharma S, Bloch W, et al. Acute exercise-induced set shifting benefits in healthy adults and its moderators: a systematic review and meta-analysis. Front Psychol 2021;12:528352. 10.3389/fpsyg.2021.528352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki H, Sakuma N, Kobayashi M, et al. Normative data of the trail making test among urban community-dwelling older adults in Japan. Front Aging Neurosci 2022;14:832158. 10.3389/fnagi.2022.832158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazo CE, Miranda ER, Shadiow J, et al. High intensity acute aerobic exercise elicits alterations in circulating and skeletal muscle tissue expression of neuroprotective exerkines. Brain Plast 2022;8:5–18. 10.3233/BPL-220137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinoff A, Herrmann N, Swardfager W, et al. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. Eur J Neurosci 2017;46:1635–46. 10.1111/ejn.13603 [DOI] [PubMed] [Google Scholar]
- 12.Babaei P, Azari HB. Exercise training improves memory performance in older adults: a narrative review of evidence and possible mechanisms. Front Hum Neurosci 2021;15:771553. 10.3389/fnhum.2021.771553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T, Maltez MT, Lee HW, et al. Effect of exercise training on the FNDC5/BDNF pathway in spontaneously hypertensive rats. Physiol Rep 2019;7:e14323. 10.14814/phy2.14323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . Global status report on the public health response to dementia. Geneva: World; 2021. 251. Available: https://digitalcommons.fiu.edu/cgi/viewcontent.cgi?article=1962&context=srhreports [Accessed 25 Feb 2023]. [Google Scholar]
- 15.Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 2018;90:126–35. 10.1212/WNL.0000000000004826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray A, Junge A, Robinson PG, et al. Cross-sectional study of characteristics and prevalence of musculoskeletal complaints in 1170 male golfers. BMJ Open Sport Exerc Med 2023;9:e001504. 10.1136/bmjsem-2022-001504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bullo V, Gobbo S, Vendramin B, et al. Nordic walking can be incorporated in the exercise prescription to increase aerobic capacity, strength, and quality of life for elderly: a systematic review and meta-analysis. Rejuvenation Res 2018;21:141–61. 10.1089/rej.2017.1921 [DOI] [PubMed] [Google Scholar]
- 18.Kettinen J, Tikkanen H, Venojärvi M. Comparative effectiveness of playing golf to nordic walking and walking on acute physiological effects on cardiometabolic markers in healthy older adults: a randomised cross-over study. BMJ Open Sport Exerc Med 2023;9:e001474. 10.1136/bmjsem-2022-001474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray AD, Daines L, Archibald D, et al. The relationships between golf and health: a scoping review. Br J Sports Med 2017;51:12–9. 10.1136/bjsports-2016-096625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bliss RR, Church FC. Golf as a physical activity to potentially reduce the risk of falls in older adults with Parkinson’s disease. Sports (Basel) 2021;9:72. 10.3390/sports9060072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellegrini B, Boccia G, Zoppirolli C, et al. Muscular and metabolic responses to different Nordic walking techniques, when style matters. PLoS One 2018;13:e0195438. 10.1371/journal.pone.0195438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitbit versa 3 user manual. Available: https://help.fitbit.com/manuals/manual_versa_3_en_US.pdf [Accessed 11 Jan 2022].
- 23.Henriksen A, Haugen Mikalsen M, Woldaregay AZ, et al. Using fitness trackers and smartwatches to measure physical activity in research: analysis of consumer wrist-worn wearables. J Med Internet Res 2018;20:e110. 10.2196/jmir.9157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feehan LM, Geldman J, Sayre EC, et al. Accuracy of Fitbit devices: systematic review and narrative syntheses of quantitative data. JMIR Mhealth Uhealth 2018;6:e10527. 10.2196/10527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.User manual, Beat2Phone ECG. 2020. Available: www.beat2phone.com [Accessed 11 Jan 2022].
- 26.Lumikari TJ, Pirinen J, Putaala J, et al. Prolonged ECG with a novel recorder utilizing electrode belt and mobile device in patients with recent embolic stroke of undetermined source: a pilot study. Ann Noninvasive Electrocardiol 2020;25:e12802. 10.1111/anec.12802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giese M, Beck J, Brand S, et al. Fast BDNF serum level increase and diurnal BDNF oscillations are associated with therapeutic response after partial sleep deprivation. J Psychiatr Res 2014;59:1–7. 10.1016/j.jpsychires.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 28.Choi S-W, Bhang S, Ahn J-H. Diurnal variation and gender differences of plasma brain-derived Neurotrophic factor in healthy human subjects. Psychiatry Research 2011;186:427–30. 10.1016/j.psychres.2010.07.028 [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Cubillo I, Periáñez JA, Adrover-Roig D, et al. Construct validity of the trail making test: role of task-switching, working memory, inhibition/interference control, and Visuomotor abilities. J Int Neuropsychol Soc 2009;15:438–50. 10.1017/S1355617709090626 [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Yamashiro D, Ogawa S, et al. Intake of seven essential amino acids improves cognitive function and psychological and social function in middle-aged and older adults: a double-blind, randomized, placebo-controlled trial. Front Nutr 2020;7:586166. 10.3389/fnut.2020.586166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin TA, Hoffman NM, Donders J. Clinical utility of the trail making test ratio score. Appl Neuropsychol 2010. 10.1207/S15324826AN1003_05 [DOI] [PubMed] [Google Scholar]
- 32.Martin TA, Hoffman NM, Donders J. Clinical utility of the trail making test ratio score. Appl Neuropsychol 2003;10:163–9. 10.1207/S15324826AN1003_05 [DOI] [PubMed] [Google Scholar]
- 33.Varjacic A, Mantini D, Demeyere N, et al. Neural signatures of trail making test performance: evidence from lesion-mapping and neuroimaging studies. Neuropsychologia 2018;115:78–87. 10.1016/j.neuropsychologia.2018.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piepmeier AT, Etnier JL. Brain-derived neurotrophic factor (BDNF) as a potential mechanism of the effects of acute exercise on cognitive performance. J Sport Health Sci 2015;4:14–23. 10.1016/j.jshs.2014.11.001 [DOI] [Google Scholar]
- 35.Walsh EI, Smith L, Northey J, et al. Towards an understanding of the physical activity-BDNF-cognition Triumvirate: a review of associations and dosage. Ageing Res Rev 2020;60:101044. 10.1016/j.arr.2020.101044 [DOI] [PubMed] [Google Scholar]
- 36.Yasuoka Y, Nakamura T, Umemoto Y, et al. An 18-hole round of golf acutely elevates serum Interleukin-6 and brain-derived neurotrophic factor concentration-a pilot study. JPFSM 2022;11:1–7. 10.7600/jpfsm.11.1 [DOI] [Google Scholar]
- 37.Gmiąt A, Jaworska J, Micielska K, et al. Improvement of cognitive functions in response to a regular Nordic walking training in elderly women - A change dependent on the training experience. Exp Gerontol 2018;104:105–12. 10.1016/j.exger.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 38.Knaepen K, Goekint M, Heyman EM, et al. Neuroplasticity-exercise-induced response of peripheral brain-derived neurotrophic factor A systematic review of experimental studies in human subjects. Sports Med 2010;40:765–801. 10.2165/11534530-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 39.Laske C, Banschbach S, Stransky E, et al. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. Int J Neuropsychopharm 2010;13:595–602. 10.1017/S1461145709991234 [DOI] [PubMed] [Google Scholar]
- 40.Nofuji Y, Suwa M, Sasaki H, et al. Different circulating brain-derived Neurotrophic factor responses to acute exercise between physically active and sedentary subjects. J Sports Sci Med 2012;11:83–8. [PMC free article] [PubMed] [Google Scholar]
- 41.Rasmussen P, Brassard P, Adser H, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise [Experimental Physiology-Research Paper;94:1062–9]. Experimental Physiology 2009;94:1062–9. 10.1113/expphysiol.2009.048512 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available.