Abstract
Polycystic ovary syndrome is characterised by excessive levels of androgens and ovulatory dysfunction, and is a common endocrine disorder in women of reproductive age. Polycystic ovary syndrome arises as a result of polygenic susceptibility in combination with environmental influences that might include epigenetic alterations and in utero programming. In addition to the well recognised clinical manifestations of hyperandrogenism and ovulatory dysfunction, women with polycystic ovary syndrome have an increased risk of adverse mental health outcomes, pregnancy complications, and cardiometabolic disease. Unlicensed treatments have limited efficacy, mostly because drug development has been hampered by an incomplete understanding of the underlying pathophysiological processes. Advances in genetics, metabolomics, and adipocyte biology have improved our understanding of key changes in neuroendocrine, enteroendocrine, and steroidogenic pathways, including increased gonadotrophin releasing hormone pulsatility, androgen excess, insulin resistance, and changes in the gut microbiome. Many patients with polycystic ovary syndrome have high levels of 11-oxygenated androgens, with high androgenic potency, that might mediate metabolic risk. These advances have prompted the development of new treatments, including those that target the neurokinin-kisspeptin axis upstream of gonadotrophin releasing hormone, with the potential to lessen adverse clinical sequelae and improve patient outcomes.
Keywords: Endocrinology, Obstetrics, Metabolic diseases, Reproductive medicine, Pharmacology, Medicine
Introduction
Polycystic ovary syndrome is a common metabolic and reproductive disorder characterised variably by high levels of androgens, insulin resistance, and ovulatory dysfunction, with not all patients affected by these three parameters. These changes manifest as hyperandrogenism (hirsutism, acne, or scalp hair loss, or a combination of these), oligomenorrhoea or amenorrhoea, and morphological features of polycystic ovaries on ultrasound. Long recognised as a reproductive disorder, polycystic ovary syndrome is now also established as a metabolic condition associated with long term health risks, including type 2 diabetes and cardiovascular disease.1 Adverse mental health outcomes and reduced quality of life have also been reported.1 Polycystic ovary syndrome is therefore associated with substantial healthcare costs and resource utilisation. International surveys report a high level of dissatisfaction with care,2 not least because current treatments are often only modestly effective in alleviating symptoms and minimising long term risks. International guidelines recognise the low quality of evidence and the critical need for better research.3
An improved understanding of the pathogenesis of the disease might result in the development of new treatments and better patient outcomes. Recent studies have advanced our understanding of these pathophysiological processes. Neuroendocrine dysregulation leads to abnormal high frequency pulsatile secretion of gonadotrophin releasing hormone, and hypothalamic kisspeptin, neurokinin B, and dynorphin A neurons (so-called KNDy neurons) are integral regulators of this process.4 Although increased production of ovarian and adrenal androgens contribute to hyperandrogenism, peripherally generated 11-oxygenated androgens are emerging as important predictors of metabolic risk.5 6 Together with advances in our understanding of adipocyte biology, insulin resistance, the gut microbiome, and insights from genome-wide association studies, these studies could improve our understanding of the pathogenesis of this disease. New treatments based on these observations are now in various stages of preclinical or clinical development.
This review outlines our current understanding of the key pathophysiological processes in polycystic ovary syndrome. We discuss the significance of new research into neuroendocrine dysfunction, disrupted steroidogenesis, and changes in adipocyte biology, and the potential implications for the diagnosis and management of polycystic ovary syndrome. Finally, we consider the benefits and limitations of current drug treatments, along with a review of the evidence for emerging drug treatments.
Epidemiology
Polycystic ovary syndrome is a common endocrine disorder in women of reproductive age,7 with a prevalence of 4-21% depending on the diagnostic criteria used.8 A systematic review of 13 studies found a slightly higher estimate of the prevalence in black and Middle-Eastern than in Chinese and white populations,9 although inconsistencies in diagnostic criteria and recruitment methods make comparisons between ethnic groups challenging.10 The global disease burden seems to be increasing at a high rate. In 2019, an age standardised point prevalence of 1677.8 per 100 000 and an annual incidence of 59.8 per 100 000 population were reported based on data from 204 countries, representing increases of 30.4% and 29.5%, respectively, since 1990.11 The rising incidence, and accompanying morbidity,11 emphasises the importance of recognising polycystic ovary syndrome as an international public health priority.
Sources and selection criteria
We searched PubMed, Medline, and Embase from 1 January 2010 to 28 February 2023 for articles in the English language, with the search terms: "polycystic ovary syndrome," "polycystic ovarian syndrome," "PCOS," "aetiology," "etiology," "cause," "pathogenesis," and "pathophysiology." We excluded articles on endocrine conditions that might lead to secondary polycystic ovary syndrome, including acromegaly, androgen secreting tumours, congenital adrenal hyperplasia, and Cushing's syndrome. To identify registered clinical trials, we searched ClinicalTrials.gov, the Cochrane Central Register of Controlled Trials (CENTRAL), and the International Standard Randomised Controlled Trial Number (ISRCTN) registry with the search terms "PCOS," "polycystic ovary syndrome," and "polycystic ovarian syndrome." We prioritised large scale, randomised controlled trials and systematic reviews. We also included relevant articles identified from reference lists of retrieved articles.
Pathogenesis
Neuroendocrine disruption
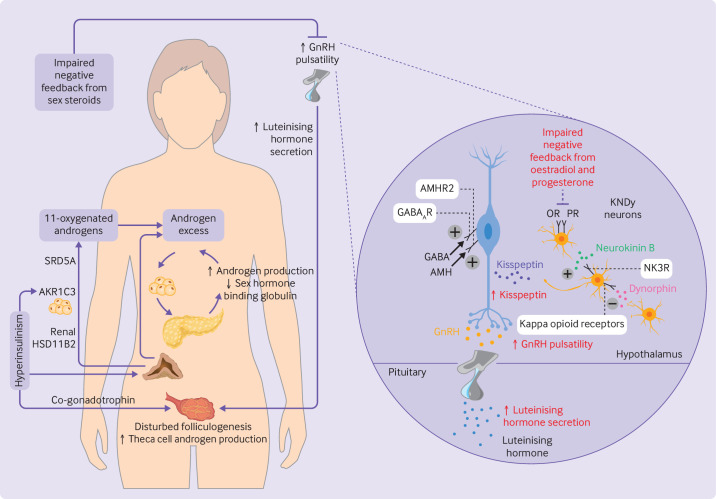
Polycystic ovary syndrome is characterised by increased pulse frequency of gonadotrophin releasing hormone and reduced negative feedback from sex steroids at the level of the hypothalamus.4 12 Gonadotrophin releasing hormone is released from neurons in the hypothalamic infundibular nucleus in a pulsatile manner, resulting in increased secretion of luteinising hormone and follicle stimulating hormone. The pulse frequency of gonadotrophin releasing hormone is controlled by multiple upstream endocrine and neural factors, with a higher frequency favouring secretion of luteinising hormone and a lower frequency favouring secretion of follicle stimulating hormone. In women with polycystic ovary syndrome, raised levels of luteinising hormone cause excess production of ovarian thecal androgens, whereas relative deficiency of follicle stimulating hormone causes follicular arrest, polycystic ovarian morphology, and oligo-ovulation.4 The reduction in sex steroid feedback on release of gonadotrophin releasing hormone is thought to occur upstream of the hormone itself because gonadotrophin releasing hormone neurons do not have receptors for oestrogens or progesterone13 (figure 1). KNDy neurons have an important role in this regard (figure 1).
Figure 1.
Pathophysiology and neuroendocrine disruption of the hypothalamo-pituitary-gonadal axis in polycystic ovary syndrome. (Left) Increased pulsatility of gonadotrophin releasing hormone (GnRH) causes increased secretion of luteinising hormone, consequent disrupted folliculogenesis, and increased production of ovarian androgens. Adrenal androgens are also increased, including 11-oxygenated androgens which are activated peripherally by renal 11β-hydroxysteroid dehydrogenase type 2 (HSD11B2) and aldo-keto reductase 1C3 (AKR1C3) in adipocytes. Steroid-5α-reductase (SRD5A) converts 11-ketotestosterone to 11-ketodihydrotestosterone. Excess levels of androgens stimulate deposition of abdominal adipose tissue which subsequently increases insulin resistance and hyperinsulinism. Hyperinsulinism stimulates AKR1C3 activity, increases androgen production from the ovaries (by its action as a co-gonadotrophin) and adrenal cortex, reduces production of hepatic sex hormone binding globulin, and inhibits progesterone mediated negative feedback onto GnRH neurons, worsening androgen excess in a vicious cycle. (Right) Kisspeptin, neurokinin B, and dynorphin A neurons (KNDy neurons) act in a paracrine and autocrine way to regulate release of kisspeptin onto GnRH neurons and consequent GnRH pulsatility. Neurokinin B binds to neurokinin 3 receptors (NK3R) to stimulate release of kisspeptin whereas dynorphin binds to kappa opioid receptors to inhibit kisspeptin release. γ-aminobutyric acid (GABA) and anti-müllerian hormone (AMH) bind to GABAA receptors (GABAAR) and AMH receptor type 2 (AMHR2), respectively, to stimulate GnRH pulsatility. Impaired negative feedback from oestradiol and progesterone is seen at the level of the hypothalamus. Neuroendocrine abnormalities in the control of these components are shown in red. OR=oestrogen receptor; PR=progesterone receptor
Kisspeptins are a family of peptides encoded by the KISS1 gene which act on the neuronal G protein coupled receptor KISS1R. KISS1 encodes prepro-kisspeptin, which is cleaved to produce the biologically active peptides KP54, KP14, KP13, and KP10.14 Two discrete neuronal populations exist: KNDy neurons in the infundibular nucleus function as the gonadotrophin releasing hormone pulse generator15 and mediate negative feedback from oestradiol,16 whereas a separate kisspeptin population located in the preoptic area mediates oestradiol positive feedback to produce the mid-cycle surge in luteinising hormone.16 17 Kisspeptin neurons express sex steroid receptors (progesterone and oestrogen receptors) required for negative feedback on gonadotrophin releasing hormone pulsatility.17 18 KISS1 is also expressed in adipose tissue where it is regulated independently of hypothalamic KISS1.19 Circulating levels of kisspeptin are higher in patients with polycystic ovary syndrome than in controls20 and although the origin of this excess is not entirely clear, a raised pulse frequency of kisspeptin in women with oligomenorrhoea and polycystic ovary syndrome suggests a hypothalamic source.21 Moreover, physiological coupling of kisspeptin and luteinising hormone pulsatility is lost in these women.21 The exact mechanisms for these effects are unclear, with inconsistent data from preclinical models on the existence and direction of dysregulated gonadotrophin releasing hormone pulsatility mediated by kisspeptin.22
Neurokinin B and dynorphin are expressed by KNDy neurons and act in an autocrine and paracrine way to control release of kisspeptin (figure 1). Neurokinin B preferentially binds to the neurokinin 3 receptor (encoded by TACR3) to stimulate gonadotrophin releasing hormone pulsatility.4 23 Unlike KISS1 null mice, mice deficient in components of neurokinin B signalling can still generate surges in luteinising hormone and conceive, suggesting that compensatory pathways exist which contribute to the generation of kisspeptin and gonadotrophin releasing hormone pulses.17 24 25 This milder effect of neurokinin B blockade might avoid excessive reduction in gonadotrophin releasing hormone pulsatility, making it an attractive target for treatment.4 Dynorphin, which activates kappa opioid receptors on KNDy neurons to inhibit secretion of gonadotrophin releasing hormone,22 26 has been shown to mediate progesterone negative feedback on gonadotrophin releasing hormone neurons in sheep27 and humans.22 28
Neuronal activity of gonadotrophin releasing hormone is also regulated by other substances, including γ-aminobutyric acid (GABA) and anti-müllerian hormone, both of which stimulate gonadotrophin releasing hormone neurons directly. GABA exerts an excitatory effect on gonadotrophin releasing hormone neurons through GABAA receptors, and GABA levels in cerebrospinal fluid can be raised in patients with polycystic ovary syndrome.29 Anti-müllerian hormone is secreted by ovarian granulosa cells, where raised levels in women with polycystic ovary syndrome disrupt folliculogenesis and ovulation.30 Anti-müllerian hormone might also have neuroendocrine effects: 50% of gonadotrophin releasing hormone neurons in mice and humans express anti-müllerian hormone receptor type 2,31 with studies implicating anti-müllerian hormone in neuronal migration of gonadotrophin releasing hormone,32 gonadotrophin releasing hormone pulsatility, and secretion of luteinising hormone.30
Classical pathway of androgen synthesis
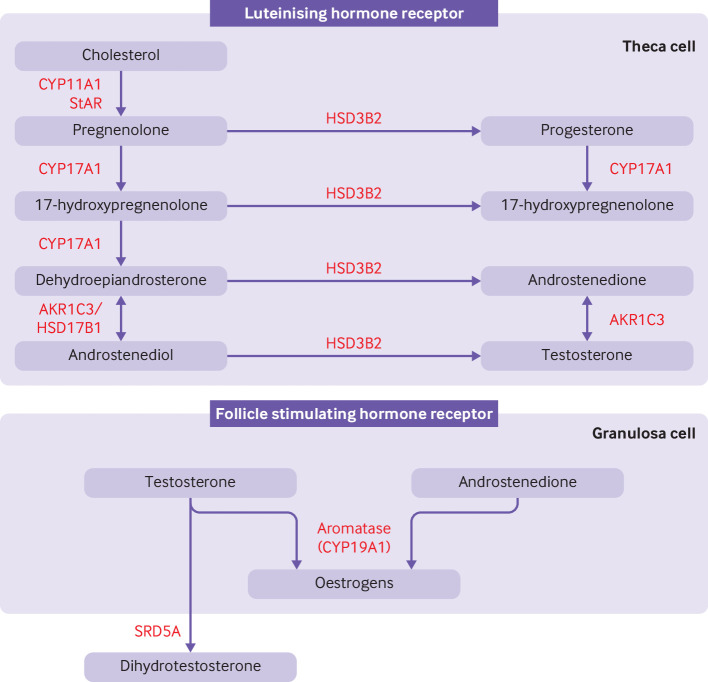
High levels of androgens is a primary defect in polycystic ovary syndrome. Cholesterol is converted to androgens by a cascade of enzymes common to all steroid producing organs, with tissue specific variations resulting in different steroid hormone profiles.33 In polycystic ovary syndrome, increased production of ovarian androgens by the classical pathway is driven by increased secretion of pituitary luteinising hormone, the action of insulin as a co-gonadotrophin, and increased thecal cell hypersensitivity to luteinising hormone.34–36 Figure 2 summarises the classical pathway of steroidogenesis. Through a sequence of reactions, cholesterol is converted to dehydroepiandrosterone, which is then converted to androstenedione by 3β-hydroxysteroid dehydrogenase type II and subsequently to testosterone by aldo-keto reductase type 1C3 (AKR1C3).35
Figure 2.
Classical pathway of androgen synthesis. Luteinising hormone stimulates the classical pathway of androgen synthesis in ovarian theca cells. Cholesterol is transported to the inner mitochrondrial membrane by steroidogenic acute regulatory protein (StAR). A cleavage system of the cytochrome P450 enzyme, CYP11A1, ferrodoxin, and ferrodoxin reductase converts cholesterol to pregnenolone. Expression of CYP11A1 is stimulated by activation of the luteinising hormone receptor. Pregnenolone is transported to smooth endoplasmic reticulum where it is converted to 17-hydroxypregnenolone and subsequently to dehydroepiandrosterone by the 17-hydroxylase and 17,20-lyase subunit of the CYP17A1 enzyme, respectively. Dehydroepiandrosterone is then converted to androstenedione or androstenediol and subsequently to testosterone by a combination of 3β-hydroxysteroid dehydrogenase type II (HSD3B2) and aldo-keto reductase type 1C3 (AKR1C3). 17β-hydroxysteroid dehydrogenase 1 (HSD17B1) also catalyses the conversion of dehydroepiandrosterone to androstenediol. HSD3B2 converts pregnenolone and 17-hydroxypregnenolone to progesterone and 17-hydroxyprogesterone, respectively, which are substrates for a back door alternative pathway of androgen synthesis. Androstenedione and testosterone diffuse into granulosa cells where they are converted to oestrogens by the action of aromatase (CYP19A1), under the control of follicle stimulating hormone receptor activation. Testosterone can be converted to dihydrotestosterone by steroid 5α-reductase (SRD5A) in peripheral tissues
Increased activity of ovarian 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), which converts inactive cortisone to active cortisol, might also have a role in the pathogenesis of polycystic ovary syndrome.37 Overexpression of ovarian 11β-HSD1 in rats caused polycystic ovarian morphology, oestrous cycle, and reproductive hormone abnormalities.37 Although 11β-HSD1 is widely expressed, dysregulation seems to be tissue specific, because hepatic 11β-HSD1 activity is impaired and expression of 11β-HSD1 in subcutaneous adipose tissue is increased in patients with polycystic ovary syndrome.38 Raised circulating levels and ovarian expression of vascular endothelial growth factor also contribute to the hypervascular, hyperplastic appearance of the ovarian stroma and theca interna in polycystic ovary syndrome, and might contribute to increased ovarian androgen synthesis.39
Androgen synthesis in adrenal glands and peripheral tissues
Polycystic ovary syndrome was previously thought to be primarily a disease of excess production of androgens in the ovaries, but the adrenal glands and peripheral tissues are now considered important sources of androgens in patients with polycystic ovary syndrome. Increased concentrations of dehydroepiandrosterone sulphate, an almost exclusive product of the adrenal cortex,40 are apparent in 20-30% of patients with polycystic ovary syndrome.41 This finding seems to be the result of increased secretory activity of the adrenal cortex because no change in pituitary responsiveness to corticotrophin releasing hormone or reduction in the minimal stimulatory dose of adrenocorticotropic hormone required for adrenal hormone production is seen.42 Changes in steroidogenesis, such as increased enzymatic activity of the 17-hydroxylase subunit of the cytochrome P450 enzyme, CYP17A1, might account for this hyper-responsiveness.43
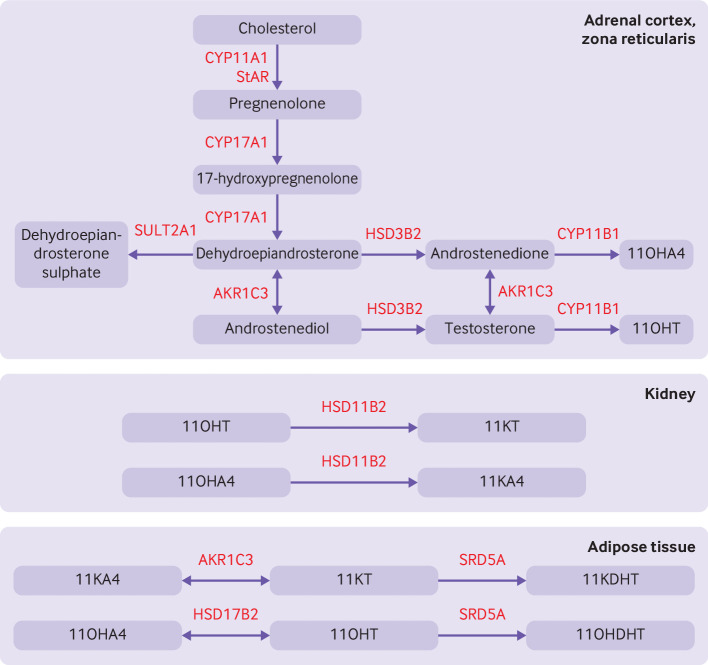
Other adrenal androgens are also secreted in excess, including 11β-hydroxyandrostenedione and 11β-hydroxytestosterone.5 44 The adrenal androgen 11β-hydroxyandrostenedione is abundant and was previously thought to have little physiological importance because of its weak androgenic activity. Recent studies, however, have shown that 11β-hydroxyandrostenedione can be metabolised to 11-ketotestosterone and 11-ketodihydrotestosterone, termed 11-oxygenated androgens, because of the presence of an oxygen atom on carbon 11.45 Both 11-ketotestosterone and 11-ketodihydrotestosterone bind to androgen receptors with similar affinity and potency to testosterone and dihydrotestosterone.46 47 Mass spectrometry analyses have shown that 11-oxygenated androgens are the dominant circulating androgens in women with polycystic ovary syndrome and correlate substantially with markers of metabolic risk.5 The synthesis of 11-oxygenated androgens is reliant on the peripheral activation of adrenal derived androgens (figure 3). 11β-hydroxysteroid dehydrogenase type 2 is an enzyme expressed by the kidney that converts 11β-hydroxyandrostenedione to 11-ketoandrostenedione, and 11β-hydroxytestosterone to 11-ketotestosterone.45 Adipose tissue also has enzymes responsible for potent androgen formation, however, and might represent the dominant source of circulating 11-oxygenated androgens.45 48
Figure 3.
Pathway for 11-oxygenated androgen synthesis, which begins in the adrenal cortex. Androstenedione and testosterone are produced by the classical pathway (figure 2). Dehydroepiandrosterone is diverted to downstream androgens or sulphonated to dehydroepiandrosterone sulphate by the sulphotransferase, SULT2A1. Androstenedione and testosterone are hydroxylated by 11β-hydroxylase (CYP11B) to produce abundant 11β-hydroxyandrostenedione (11OHA4) and smaller amounts of 11β-hydroxytestosterone (11OHT). Renal 11β-hydroxysteroid dehydrogenase type 2 (HSD11B2) converts 11OHT to 11-ketotestosterone (11KT) and 11OHA4 to 11-ketoandrostenedione (11KA4). In adipose tissue, 11KA4 is metabolised to 11KT and 11-ketodihydrotestosterone (11DHKT) by aldo-keto reductase type 1C3 (AKR1C3) and steroid-5α-reductase (SRD5A), respectively. 11OHA4 is metabolised to 11OHT and 11β-hydroxydihydrotestosterone (11OHDHT) by 17β-hydroxysteroid dehydrogenase 2 (HSD17B2) and SRD5A, respectively. 11KT and 11KDHT are potent agonists of the androgen receptor whereas 11OHT and 11OHDHT have milder potency. StAR=steroidogenic acute regulatory protein; HSD3B2=3β-hydroxysteroid dehydrogenase type II; CYP11A1, CYP17A1, CYP11B1=cytochrome P450 enzymes
Expression of the androgen activating enzyme, AKR1C3, in subcutaneous adipose tissue is increased in women with polycystic ovary syndrome compared with matched controls.6 49 Thus concentrations of androgens in adipose tissue are increased in women with polycystic ovary syndrome, accompanied by inhibition of lipolysis and increased de novo lipogenesis.6 These observations suggest that inhibition of AKR1C3 might be an attractive therapeutic target in patients with polycystic ovary syndrome.
Hyperinsulinism
Insulin resistance, and the consequent hyperinsulinism, have an important role in driving androgen synthesis in many endocrine tissues. Insulin acts as a co-gonadotrophin in the ovaries,36 impairs progesterone mediated inhibition of the gonadotrophin releasing hormone pulse generator,50 and facilitates synthesis of androgens in the adrenal glands by increasing adrenocorticotropic hormone stimulated steroidogenesis.51 AKR1C3 expression and activity in adipocytes is increased by insulin, contributing to increased synthesis of androgens in adipocytes in polycystic ovary syndrome.52 Insulin also inhibits sex hormone binding globulin, facilitating hyperandrogenism by increasing the percentage of free biologically active androgens.53 Excess production of androgens then stimulates hyperinsulinism, leading to a vicious cycle between androgen and insulin excess.7 54 Several studies have also implicated hyperandrogenism in the accumulation of abdominal and visceral adipose tissue in polycystic ovary syndrome55 56; this hyperandrogenism further drives insulin resistance and consequent production of androgens (figure 1).
In common with hyperandrogenism, insulin resistance is not a universal feature of polycystic ovary syndrome, although a systematic review of hyperinsulinaemic-euglycaemic clamp studies of 1224 women with polycystic ovary syndrome and 741 controls showed that insulin sensitivity was lower in women with polycystic ovary syndrome than in controls (mean effect size −27%, 99% confidence interval −21 to −33).57 Studies exploring steroid metabolomics in patients with polycystic ovary syndrome might give more information. One such cross sectional study (n=488) combining machine learning with mass spectrometry multisteroid profiling has identified three distinct groups of patients based on the predominant source of androgens.58 These subgroups have distinct steroid metabolomes and risk of metabolic complications: a gonadal derived classical androgen excess group, an adrenal derived androgen excess group (comprising 11-oxygenated androgens), and a group with comparably mild androgen excess.58 The adrenal derived androgen group had the highest rates of hirsutism, insulin resistance, and type 2 diabetes. These insights challenge our understanding of polycystic ovary syndrome as one entity and might prompt a reconsideration of the classification of the disease based on the metabolomic signature.
Changes in adipocyte structure and function
Changes in white adipose tissue morphology and function is seen in women with polycystic ovary syndrome, including enlarged adipocytes, reduced lipoprotein lipase activity,59 and increased secretion of proinflammatory cytokines.60 The function of brown adipose tissue might also be disrupted because women with polycystic ovary syndrome showed reduced postprandial thermogenesis compared with controls matched for body mass index.61 This defect could be driven by androgen excess, because prenatally androgenised sheep have reduced postprandial thermogenesis in adulthood,62 accompanied by reduced adipose expression of thermogenic uncoupling proteins and sympathetic activity. Adolescent prenatally androgenised sheep also showed reduced hepatic expression and circulating levels of fibroblast growth factor 21,63 a hormone that regulates adipocyte function, insulin sensitivity, and energy balance. Targeting expression of fibroblast growth factor 21 during an appropriate period in development might be a therapeutic option.
Gut microbiota and bile acid metabolism
Recent studies have implicated changes in the gut microbiome in the pathogenesis of polycystic ovary syndrome. Women with polycystic ovary syndrome have higher intestinal levels of Bacteroides vulgatus and lower levels of glycodeoxycholic acid and tauroursodeoxycholic acid.64 Oral gavage of wild-type mice with faecal microbiota from individuals with polycystic ovary syndrome or pure B vulgatus caused insulin resistance, changes in bile acid metabolism, reduced secretion of interleukin 22, and disrupted oestrous cycle and ovarian morphology.64 Administration of interleukin 22 or glycodeoxycholic acid to mice treated with B vulgatus improved insulin sensitivity, testosterone levels, and oestrous cycles. Hence modifying the gut microbiota or bile acid metabolism, increasing levels of interleukin 22, or a combination of these actions, might be therapeutically valuable in polycystic ovary syndrome.64
Insights from genome-wide association studies
Genome-wide association studies have identified numerous susceptibility loci for polycystic ovary syndrome, including 11 in Han Chinese populations,65 66 eight in European populations,67 68 and eight in a Korean population.69 Robust candidate susceptibility loci are near genes belonging to metabolic (insulin receptor (INSR), insulin gene-variable number of tandem repeats (INS-VNTR), and DENN domain containing protein 1A (DENND1A))70 and neuroendocrine (follicle stimulating hormone receptor, luteinising hormone receptor, and thyroid adenoma associated (THADA)) pathways.70 Meta-analyses of genome-wide association studies have shown that the genetic architecture of polycystic ovary syndrome is consistent across different diagnostic criteria and ethnic groups.71 72 These observations indicate a shared ancestry for polycystic ovary syndrome and reinforce the importance of neuroendocrine and metabolic pathways in the pathogenesis of the disease.
Developmental programming
Genetic loci identified by genome-wide association studies currently account for only 10% of the known heritability (about 70%) of polycystic ovary syndrome,73 74 suggesting other influences on the pathogenesis of the disease. Emerging evidence indicates that polycystic ovary syndrome might have its origins in utero, and thus could be subject to developmental programming and epigenetic modifications. Prenatal exposure to androgens in several preclinical models caused a permanent polycystic ovary syndrome-like phenotype postnatally.75–77 A programming effect might also persist transgenerationally, because pregnant mice treated with dihydrotestosterone produced female offspring with polycystic ovary syndrome-like phenotypes from the first to the third generations of offspring.78 Cautious interpretation is needed, however, because these models might not accurately reflect the human phenotype. Anti-müllerian hormone might also be involved in in utero programming: levels of anti-müllerian hormone increased significantly in pregnant women with polycystic ovary syndrome (P<0.001), and use of this hormone caused gonadotrophin releasing hormone neuronal hyperactivity and androgen excess in pregnant mice.79 Epigenetic mechanisms might also be involved in mediating susceptibility to polycystic ovary syndrome, with differential methylation patterns and microRNA expression detected in adipose tissue and ovarian tissue of patients with polycystic ovary syndrome compared with controls.80
Health risks
Polycystic ovary syndrome is well established as a reproductive disorder associated with hyperandrogenism, and is the leading cause of oligomenorrhoea and amenorrhoea.81 Patients with polycystic ovary syndrome are at increased risk of mental health disorders,82 83 endometrial cancer,84 and ovarian hyperstimulation syndrome after induction of ovulation.85 Consistent with our understanding of the pathogenesis, however, polycystic ovary syndrome is also recognised as a metabolic disorder, with long term health risks, including hypertension, type 2 diabetes, dyslipidaemia, insulin resistance, and obesity.1 These health risks could be associated with an increased risk of cardiovascular events86 and several adverse pregnancy outcomes.87 Although the reproductive aspects might diminish with age, metabolic features typically persist or can worsen.88
Therapeutic goals
Difficulty in losing weight, irregular menses, infertility, and excessive hair growth were the most important health problems reported by patients with polycystic ovary syndrome in an international survey.2 These problems should therefore represent the main targets for therapeutic intervention, although priority setting partnerships are still needed to help focus research priorities. Existing drug treatments have not been licensed specifically for polycystic ovary syndrome and are used off-label to target symptoms. Also, previous studies have not emphasised health related quality of life measures when evaluating response to treatment. An ideal treatment for polycystic ovary syndrome should look at the health risks, reduce key processes in the pathogenesis of the disease, and be responsive to the symptom profile and needs of the individual. Where relevant, treatments should reduce clinical and biochemical hyperandrogenism, restore ovulatory cycles and fertility, normalise the length of the menstrual cycle, improve insulin sensitivity, reduce weight and cardiometabolic risk, and improve condition specific quality of life.
Existing treatments
Non-pharmacological interventions
International guidelines highlight the importance of modifications to lifestyle in the management of the disease.3 Changes in lifestyle can improve fasting insulin levels and anthropometric outcomes, although benefits on hyperandrogenism are modest89 and adherence is often difficult to sustain in clinical practice. Data on reproductive benefits are limited,90 although a recent small randomised controlled trial of 68 women with polycystic ovary syndrome showed that a behavioural modification programme improved menstrual regularity compared with a minimal intervention group.91 Laser treatment might have a role in the treatment of facial hirsutism, although further trials are needed to confirm the benefits on quality of life and cost effectiveness.3
Contraceptive pill
In women not attempting to conceive, combined contraceptive pills are first line treatments for menstrual irregularity and hyperandrogenism.3 The oestrogen component increases sex hormone binding globulin, thus reducing free testosterone and improving hyperandrogenism. Because this stimulatory effect on hepatic production of proteins also causes hypercoagulability, ethinyloestradiol based contraceptive pills containing the lowest effective dose of oestrogen (eg, 20-30 μg of ethinyloestradiol) are recommended.3 Combined contraceptive pills containing newer, more physiological, oestrogenic compounds have recently been developed, and might have a lower risk of venous thromboembolism than ethinyloestradiol.92 The progestogen component reduces ovarian androgen production by inhibiting secretion of luteinising hormone and protects the endometrium from hyperplasia.93 Combined contraceptive pills containing androgenic progestogens, such as norethisterone, should be avoided because of the potential to aggravate hyperandrogenic symptoms. Furthermore, ethinyloestradiol based contraceptive pills containing cyproterone acetate, the most potent anti-androgenic progestogen, are not currently recommended as first line treatment because of the increased risk of venous thromboembolism.3 A recent systematic review of 19 randomised controlled trials, however, concluded that the ethinyloestradiol-cyproterone acetate combination improved serum testosterone (mean difference 0.38 nmol/L, 95% confidence interval 0.33 to 0.43) and hirsutism compared with conventional combined contraceptive pills.94 Thus combinations of cyproterone acetate and newer oestrogenic compounds might have the potential to improve hyperandrogenism in patients with polycystic ovary syndrome without the added risk of venous thromboembolism.
Anti-androgen agents
Currently available anti-androgen agents act by blocking androgen receptors (cyproterone acetate, spironolactone, and flutamide) or reducing production of androgens (finasteride and dutasteride). Guidance on specific preparations or doses in polycystic ovary syndrome is necessarily vague, because studies on these agents are few in number and small scale.3 Furthermore, although targeting excess production of androgens might be crucial to improved patient outcomes, the use of currently available anti-androgen drugs is limited by side effects. All anti-androgen drugs carry a risk of feminisation of a male fetus and therefore use must be restricted to patients with adequate contraception in place.3
Insulin sensitisers
Metformin modulates hepatic insulin sensitivity and glucose production by activating AMP activated protein kinase and AMP activated protein kinase independent pathways. More recently, metformin has also been shown to mediate its antiglycaemic effects by actions on the gastrointestinal tract and the gut microbiome.95 Metformin is used to manage weight and metabolic outcomes in adult women with polycystic ovary syndrome with a body mass index ≥25.3 Metformin might also improve ovulation and live birth rates but is less effective than clomifene citrate or letrozole.3 96 Nevertheless, because of its wide availability and low cost, metformin could still be valuable in improving reproductive outcomes in women with polycystic ovary syndrome, especially in healthcare economies where access to assisted reproduction is limited.
Thiazolidinediones improve insulin sensitivity by activating nuclear peroxisome proliferator activated receptor γ. A meta-analysis of eight randomised controled trials concluded that thiazolidinediones reduce insulin and fasting glucose levels in polycystic ovary syndrome, but do not seem to affect hirsutism scores or serum levels of androgens.97 Existing data on thiazolidinediones in polycystic ovary syndrome are limited. Thiazolidinediones are associated with intrauterine growth restriction in animal studies and weight gain in humans.98 Thiazolidinediones are thus not recommended for use in polycystic ovary syndrome outside of the licensed indication in type 2 diabetes. Preliminary studies suggest that the insulin sensitiser inositol might improve glycaemic control99 and new international guidelines recommend shared decision making on using inositol for its potential metabolic benefits in polycystic ovary syndrome. Specific doses, forms, or combinations of the substance, however, cannot be recommended because of a lack of high quality evidence.3
New therapeutic targets
Kisspeptin based treatment
Kisspeptin has a major role as a regulator of the hypothalamic-pituitary-gonadal axis, and therefore extensive efforts have been made in investigating the effects of kisspeptin based treatment in women with polycystic ovary syndrome and in other disorders of reproduction. KP54 and KP10 are the most studied native kisspeptins in humans and have been investigated for their potential role in optimising oocyte maturation in patients undergoing in vitro fertilisation.100 101 Although the two compounds bind to KISS1R with similar affinity, KP54 has a longer serum half-life than KP10 and a more profound effect on secretion of luteinising hormone.102 A bolus dose of KP54 induces oocyte maturation in patients with polycystic ovary syndrome without causing clinically significant ovarian hyperstimulation syndrome.100 101 Administration of a subcutaneous bolus injection of native kisspeptin is safe and well tolerated103 and also results in higher expression of gonadotrophin receptors (follicle stimulating hormone receptor and luteinising hormone receptor) and steroidogenic enzymes (including aromatase CYP19A1, steroidogenic acute regulatory protein, and 3β-hydroxysteroid dehydrogenase type II) in ovarian granulosa cells, potentially promoting an ovarian environment favouring progesterone synthesis and ovarian implantation.104
Use of KP54 as an ovulation induction agent, however, might be limited by tachyphylaxis.105 Because KP54 preferentially stimulates secretion of luteinising hormone over follicle stimulating hormone,106 concerns also exist that long term administration of kisspeptin might exacerbate pre-existing deficiency of follicle stimulating hormone in polycystic ovary syndrome.4 Nevertheless, KP54 effectively induced ovulation in neonatally androgenised rats but not in prenatal androgenisation or post-weaning androgenisation models.107 Simultaneous increases in luteinising hormone and follicle stimulating hormone were seen after KP54 use in the neonatal androgenisation model, suggesting that ovulation induced by kisspeptin is linked to its ability to stimulate increases in both luteinising hormone and follicle stimulating hormone.107 In a first-in-woman pilot study of 12 patients with polycystic ovary syndrome, twice daily use of KP54 over three weeks significantly increased levels of luteinising hormone (P=0.04) and oestradiol (P=0.03) but not follicle stimulating hormone or inhibin B.107 Two of the 12 women developed a dominant follicle with subsequent ovulation. Hence long term administration of KP54 might be suitable for follicular maturation in a subset of patients with polycystic ovary syndrome, but more studies are needed to identify the patient characteristics that predict response to treatment.
KISS1R agonists are currently in development with modifications that increase potency and are resistant to proteolytic degradation. KISS1R agonists might have a lower risk of tachyphylaxis because of their longer duration of action, allowing less frequent dosing. The KISS1R agonist, MVT-602, showed greater potency than KP54, increasing release of luteinising hormone in healthy women with a longer duration of gonadotrophin releasing hormone neuronal activation in vitro.108 When tested in patients with polycystic ovary syndrome, MVT-602 increased luteinising hormone with a similar amplitude but greater duration than KP54 in healthy women (area under the curve of luteinising hormone exposure 171.30 v 38.5 IU×h/L), similar to the natural mid-cycle surge in luteinising hormone.108 These findings warrant further investigation in polycystic ovary syndrome and in other female reproductive disorders.
Paradoxically, kisspeptin receptor antagonists have also been suggested as therapeutic agents in polycystic ovary syndrome based on their potential to normalise hypersecretion of luteinising hormone, restore folliculogenesis and ovulation, and improve ovarian hyperandrogenism.109 Existing KISS1R antagonists, such as P234 and P271, have inconsistent effects on kisspeptin induced stimulation of gonadotrophin releasing hormone-luteinising hormone across species.110 111 Compound 15 a is a small molecular KISS1R antagonist with antagonistic activity at the receptor and good permeability of the blood-brain barrier in rats.112 KISS1R antagonists have yet to be tested in humans, however, and concerns exist that these agents will overly suppress secretion of luteinising hormone and stop ovulation.4
Neurokinin 3 receptor antagonists
Inhibition of the neurokinin 3 receptor is believed to cause a tempered inhibition of gonadotrophin releasing hormone pulsatility without excessive reduction because of the presence of compensatory pathways.4 25 MLE4901 (also named AZD4901) had promising effects on levels of reproductive hormones in 65 women with polycystic ovary syndrome in a phase 2 randomised controlled trial.113 After seven days of treatment with MLE4901 80 mg/day, the area under the luteinising hormone curve was reduced by 52.0% (95% confidence interval 29.6% to 67.3%), total testosterone concentration was reduced by 28.7% (13.9% to 40.9%), and luteinising hormone pulses were reduced by 3.55 pulses/8 hours (2.0 to 5.1).113 MLE4901 was discontinued, however, because of increased levels of transaminases in some patients.114 115 Hepatotoxicity is believed to be specific to MLE4901 and has not been reported with other neurokinin 3 receptor antagonists.115
In a phase 2 randomised controlled trial in 64 women with polycystic ovary syndrome, treatment with the neurokinin 3 receptor antagonist, fezolinetant, for 12 weeks at 60 mg or 180 mg, reduced levels of testosterone by 17% (95% confidence interval −28.7% to −4.6%) and 33% (−45.91% to −20.4%), respectively, compared with 1% (−8.8% to 11.7%) with placebo.116 Levels of luteinising hormone but not follicle stimulating hormone were significantly reduced (P<0.001) in a dose dependent manner, reducing the ratio of luteinising hormone to follicle stimulating hormone.
Preclinical studies have also highlighted the potential metabolic benefits of neurokinin 3 receptor antagonists. In a dihydrotestosterone induced mouse model of polycystic ovary syndrome, treatment with neurokinin 3 receptor antagonists decreased body weight and adiposity.117 No changes in food intake or energy expenditure were seen, although an increased respiratory exchange ratio suggested that neurokinin 3 receptor antagonists cause a shift to a carbohydrate predominant utilisation of fuel.117 These promising observations suggest that neurokinin 3 receptor antagonists fulfil many of the properties of an ideal treatment for polycystic ovary syndrome, and further clinical trials are awaited with interest.
Dynorphin, γ-aminobutyric acid, and anti-müllerian hormone based treatment
When dynorphin binds to kappa opioid receptors, release of kisspeptin onto gonadotrophin releasing hormone neurons is inhibited, and therefore selective kappa receptor agonists with a central action might reduce gonadotrophin releasing hormone pulsatility.4 A new generation of peripherally selective kappa receptor agonists have been developed that might access brain regions, including the infundibular nucleus, by fenestrated capillaries in the median eminence.118 The kappa receptor agonist, difelikefalin, does not seem to cause the centrally mediated side effects of dysphoria and sedation of previous kappa receptor agonists, and has recently been approved in the US for the treatment of moderate-to-severe pruritus in adults undergoing haemodialysis.119 120 In a prenatally androgenised mouse model of polycystic ovary syndrome, difelikefalin reduced serum levels of luteinising hormone and testosterone, restored oestrous cyclicity and ovulation, and reduced overexpression of KISS1 mRNA in the hypothalamic preoptic area.121
Centrally acting GABAA antagonists might also benefit patients with polycystic ovary syndrome. Although weight gain could in part explain the increased incidence of polycystic ovary syndrome in women receiving sodium valproate, this drug also increases levels of GABAA in the central nervous system.122 In contrast, peripheral levels of GABA are reduced in patients with polycystic ovary syndrome,123 and enteral administration of GABAA reduced body mass index and levels of testosterone in a letrozole induced polycystic ovary syndrome model.124 Antagonism of the anti-müllerian hormone pathway might also be therapeutically useful; recent insights into the structural basis for binding of anti-müllerian hormone to anti-müllerian hormone receptor type 2 could facilitate the rational design of anti-müllerian hormone antagonists.125
Targeting key enzymes in steroidogenesis
AKR1C3 functions as the gatekeeper in classical and 11-oxygenated androgen synthesis by mediating enzymatic conversion of androstenedione to testosterone and 11-ketoandrostenedione to 11-ketotestosterone.6 Various AKR1C3 inhibitors have been developed, with mixed results for their antineoplastic effects and ability to inhibit prostaglandin F synthase activity in castration resistant prostate cancer, acute myeloid leukaemia, and oestrogen receptor positive breast cancers.126–128 Although steroidal based inhibitors of AKR1C3 are in development, the therapeutic potential in preclinical models of polycystic ovary syndrome has not been examined.129 Selective inhibition of 11β-HSD1 with BVT.2733 in a rodent model of polycystic ovary syndrome improved insulin resistance, reproductive hormone dysfunction, and polycystic ovarian morphology.37 These observations are encouraging but further preclinical work is needed before the potential therapeutic benefits of AKR1C3 and 11β-HSD1 inhibitors can be tested in patients.
Glucagon-like peptide 1 inhibitors
Glucagon-like peptide 1 (GLP-1) receptor agonists increase glucose dependent insulin secretion, suppress secretion of glucagon, and increase peripheral insulin sensitivity by weight loss (by stimulation of satiety) and suppression of inflammation in adipose tissue.130 Although these properties might be therapeutically attractive in patients with polycystic ovary syndrome, previous randomised controlled trials were largely small, single centre, and of limited duration.131 Nevertheless, in a network meta-analysis of 941 women with polycystic ovary syndrome and overweight or obesity, liraglutide was superior to metformin (mean difference −3.82, 95% confidence interval −4.44 to −3.20) and orlistat (−1.95, −3.74 to −0.16) in reducing body weight.132 Some studies showed that GLP-1 receptor agonists improved menstrual regularity or frequency, and that these improvements in menstrual frequency correlated with reduction in body weight.133 134 GLP-1 receptor agonists have also been shown to lower levels of androgens133 135 and improve markers of cardiovascular risk.136 137 A meta-analysis of eight randomised controlled trials concluded that GLP-1 agonists were more effective than metformin in improving homeostasis model assessment-insulin resistance (standard mean difference −0.40, 95% confidence interval −0.74 to −0.06), abdominal circumference (−0.45, −0.89 to −0.00), and body mass index (−1.02, −1.85 to −0.19), but not in improving menstrual frequency (0.15, –0.24 to 0.54) or serum levels of testosterone (0.64, –0.08 to 1.35).138 Newer longer acting GLP-1 analogues, such as semaglutide or dulaglutide,139 140 or dual GLP-1-glucose dependent insulinotropic polypeptide agonists, such as tirzepatide,141 could provide more therapeutic opportunities, with the potential benefits of greater effects on weight loss, longer duration of action, and improved adherence. Semaglutide, the only GLP-1 agonist currently available in an oral formulation, is being investigated in a clinical trial in adolescent girls with polycystic ovary syndrome and obesity (Treating PCOS With Semaglutide vs Active Lifestyle Intervention (TEAL), NCT03919929). More adequately powered trials with a focus on core outcomes of polycystic ovary syndrome142 are needed to establish whether these new drugs have a role in clinical management.
Sodium-glucose co-transporter inhibitors
Sodium-glucose co-transporter 2 inhibitors reduce reabsorption of glucose in the proximal convoluted tubules of the kidney, promoting excretion of urinary glucose, and also reduce weight and cardiovascular events in other populations.143 Current data in patients with polycystic ovary syndrome are limited to four small randomised controlled trials.144–148 Canagliflozin showed greater improvements in body mass index (P=0.006), basal metabolic rate (P=0.02), and fat mass (P=0.02) than metformin in women with polycystic ovary syndrome, but not in hormonal or metabolic parameters.144 In overweight and obese patients with polycystic ovary syndrome, combined canagliflozin-metformin treatment for three months produced greater reductions than metformin monotherapy in total testosterone (−0.33 v −0.18 ng/mL, P=0.02), area under the curve for glucose (−158.00 v 2.63 mmol/L×min, P=0.02), and the ratio of the area under the curve for insulin and glucose (−2.86 v 0.51, P=0.02),146 but no significant differences were found in menstrual frequency, body mass index, or homeostasis model assessment-insulin resistance between the treatment groups.146
Licogliflozin is a dual inhibitor of sodium-glucose co-transporter 1 (SGLT1) and 2 (SGLT2). Simultaneous SGLT1 and SGLT2 inhibition could provide more effective weight loss because SGLT1 inhibition alone stimulates intestinal secretion of GLP-1.131 In a phase 2 randomised controlled trial of 29 patients, licogliflozin reduced levels of androstenedione by 19%, dehydroepiandrosterone sulphate by 24%, and hyperinsulinaemia by 70% in women with polycystic ovary syndrome.148 The outcome of a recently completed randomised controlled trial of dapagliflozin on insulin resistance and serum levels of androgens in patients with polycystic ovary syndrome (Dapagliflozin Efficacy and Action in PCOS (DEAP), NCT04213677) is awaited with interest. Table 1 summarises the emerging treatments for polycystic ovary syndrome described in this review.
Table 1.
Emerging drug treatments for polycystic ovary syndrome
| Therapeutic class (references) | Drug | Phase of testing | Outcome measures | Main findings | Potential clinical benefits | Adverse effects (observed or potential) |
| Native kisspeptin100 101 104 105 107 | KP54, KP10 | Phase 1-2 | Phase 1 and 2 clinical studies: Oocyte maturation (in women at high risk of ovarian hyperstimulation syndrome, including polycystic ovary syndrome) |
KP54 triggered oocyte maturation without causing ovarian hyperstimulation syndrome. A second dose of KP54 improved oocyte yield | Oocyte maturation with reduced risk of ovarian hyperstimulation syndrome. Promotion of progesterone rich ovarian environment favouring ovarian implantation |
Tachyphylaxis after repeated and long term dosing. Follicle stimulating hormone deficiency in polycystic ovary syndrome |
| Pilot study in 12 women with polycystic ovary syndrome: Hormonal (gonadotrophin, oestradiol, inhibin B) and ovulatory responses to repeated KP54 administration |
Increases in luteinising hormone and oestradiol but not follicle stimulating hormone or inhibin B. Development of a dominant follicle and subsequent ovulation in 2 of 12 women | |||||
| In women with infertility undergoing in vitro fertilisation: Changes in gene expression in granulosa cells from women with infertility (most of whom had polycystic ovary syndrome) |
Compared with human chorionic gonadotropin or gonadotrophin releasing hormone agonists, KP54 increased expression of gonadotropin receptors (follicle stimulating hormone receptor and luteinising hormone receptor) and steroidogenic enzymes (including aromatase CYP19A1, StAR, and HSD3B2) in ovarian granulosa cells | |||||
| KP10 analogues108 | MVT-602 (TAK-448) |
Phase 1 | Pharmacokinetic and pharmacodynamic properties of MVT-602 | Compared with KP54, MVT-602 increased luteinising hormone with similar amplitude but later peak, resulting in greater AUC of luteinising hormone exposure | Oocyte maturation with less frequent dosing than native kisspeptins | No serious adverse effects identified so far |
| KISS1R antagonist110 111 | P234, P271, P354, P356, compound 15a | Preclinical | Basal and kisspeptin stimulated secretion of luteinising hormone | In female dogs, P234, P271, P354, and P356 did not affect basal or kisspeptin stimulated luteinising hormone secretion. P234 inhibited gonadotrophin releasing hormone neuron firing in mice and inhibited pulsatile gonadotrophin releasing hormone secretion in female monkeys. P234 inhibited kisspeptin stimulated luteinising hormone secretion in rats and mice |
Improvement in ovulatory dysfunction and fertility. Improvement in biochemical and clinical hyperandrogenism |
Oversuppression of luteinising hormone secretion leading to anovulation |
| Neurokinin 3 receptor antagonist116 117 150 | Fezolinetant | Phase 2a | Fezolinetant v placebo: Change in total testosterone. Change in gonadotrophins, oestradiol, progesterone, anti-müllerian hormone, ovarian function and volume |
Reduction in total testosterone. Reduction in luteinising hormone, reduction in luteinising hormone:follicle stimulating hormone ratio |
Improvement in biochemical and clinical hyperandrogenism. Improvement in ovulatory dysfunction and fertility. Improvement in body weight and composition. Improvement in insulin sensitivity |
Thrombophlebitis, headache, and gastrointestinal symptoms |
| Dihydrotestosterone mouse model: Oestrous cycle regularity. Ovarian follicle count. Adipocyte morphology and adipose tissue mass. Serum adiponectin, leptin, cholesterol, triglyceride, fasting blood glucose levels. Food intake, energy expenditure, locomotor activity (metabolic cage study). Glucose homeostasis |
Reduction in body weight. Reduction in inguinal, mesenteric, parametrial, and retroperitoneal fat pad weights. Reversal of adipocyte hypertrophy. Increased respiratory exchange ratio. Improvement in glucose tolerance |
|||||
| Opioid receptor agonists119 121 | Difelikefalin | Preclinical | Prenatally androgenised mouse model: Oestrous cycle regularity. Hypothalamic KISS1 expression. Luteinising hormone basal and peak levels, luteinising hormone pulse frequency. Testosterone levels |
Oestrous cycle reversed to normal state. KISS1 expression normalised in arcuate nucleus. Reduction in luteinising hormone pulse magnitude. Reduction of testosterone levels |
Improvement in ovulatory dysfunction and fertility. Improvement in biochemical and clinical hyperandrogenism |
Diarrhoea, dizziness, and vomiting |
| 11β-HSD1 inhibitors37 | BVT.2733 | Preclinical | DHEA rodent model: Insulin sensitivity. Ovulatory function |
Improvements in insulin resistance, ovulatory dysfunction, reproductive hormone dysfunction, and polycystic ovarian morphology | Improvement in insulin sensitivity. Improvement in ovulatory dysfunction and fertility |
Yet to be tested in humans |
| GLP-1 agonists*132–140 | Exenatide, liraglutide, dulaglutide, semaglutide | Phase 2-3 | Systematic review of GLP-1 agonists v metformin: Changes in body weight and anthropometric parameters. Insulin sensitivity |
GLP-1 agonists produced greater improvements in body weight, waist circumference, and insulin sensitivity | Improvement in weight and body composition. Improvement in cardiometabolic risk |
Nausea, vomiting, and diarrhoea; safety in pregnancy unclear |
| Liraglutide v placebo: Body weight. Ovarian morphology. Changes in menstrual pattern (bleeding ratio). Hyperandrogenism |
Reduction in body weight. Improvement in bleeding ratio. Reduction in free androgen index, sex hormone binding globulin and free testosterone. Reduction in ovarian volume |
|||||
| Markers of cardiovascular risk: Serum endothelial markers (ICAM-1, P-selectin, E-selectin). Changes in endothelial function. Lipid levels |
Exenatide improved serum markers of endothelial function without a change in endothelial function. Liraglutide improved thrombin generation test parameters. Liraglutide improved triglyceride levels and triglyceride:high density lipoprotein ratio |
|||||
| SGLT2 inhibitors†144 146 147 | Canagliflozin, dapagliflozin, empagliflozin | Clinical | Empagliflozin v placebo in humans: Changes in anthropometric parameters and body composition. Hormonal (total testosterone, androstenedione, sex hormone binding globulin, DHEAS) and metabolic (HOMA-IR, fasting lipids) parameters |
Reduction in body weight, and hip and waist circumference. Reduction in fat mass. Reduction in basal metabolic rate. No change in hormonal and metabolic parameters |
Improvement in weight and body composition. Improvement in cardiometabolic risk |
Urinary tract infection and ketoacidosis |
| Combination canagliflozin-metformin v metformin in humans: Anthropometric parameters, gonadotrophins, androgen levels, menstrual pattern, glucose and lipid homeostasis |
Compared with metformin alone, combination canagliflozin-metformin produced greater reductions in total testosterone, AUC for glucose, and AUC ratio for insulin to glucose | |||||
| Markers of cardiovascular risk in humans: Changes in serum endothelial markers (ICAM-1, E-selectin, VCAM-1, PECAM-1). Endothelial function |
Empagliflozin reduced ICAM-1, E-selectin, and VCAM-1 but not PECAM-1, without a change in endothelial function | |||||
| SGLT1-2 inhibitor148 | Licogliflozin | Phase 2 | Licogliflozin v placebo: Androgen levels. Insulin sensitivity (HOMA-IR). Hyperinsulinaemia (maximum (insulin), insulin AUC) |
Reduction in androstenedione and DHEAS. Reduction in HOMA-IR and fasting glucose. Reduction in maximum (insulin) and insulin AUC. No change in free testosterone, total testosterone, sex hormone binding globulin, free androgen index, or DHEA |
Improvement in insulin resistance. Reduction in androgen excess. Improvement in weight and cardiometabolic risk |
Urinary tract infection, ketoacidosis, diarrhoea, and nausea |
AUC=area under the curve; CYP19A1=cytochrome P450 family 19 subfamily A member 1; DHEA=dehydroepiandrosterone; DHEAS=dehydroepiandrosterone sulphate; GLP-1=glucagon-like peptide 1; HOMA-IR=homeostatic model assessment of insulin resistance; HSD3B2=3β-hydroxysteroid dehydrogenase; ICAM-1=intercellular adhesion molecule 1; KISS1=gene encoding kisspeptins in humans; KISS1R= KISS1 receptor; KP=kisspeptin; PECAM-1=platelet endothelial cell adhesion molecule; SGLT=sodium-glucose co-transporter; StAR=steroidogenic acute regulatory protein; VCAM-1=vascular cell adhesion molecule 1; 11β-HSD1=11β-hydroxysteroid dehydrogenase type 1.
*Clinical trial in progress: NCT03919929, Treating PCOS With Semaglutide vs Active Lifestyle Intervention (TEAL).
†Clinical trials in progress: NCT04213677, Dapagliflozin Efficacy and Action in PCOS (DEAP); NCT05200793, Efficacy of Empagliflozin or Linagliptin as an Alternative to Metformin for Treatment of Polycystic Ovary Syndrome.
Guidelines
Guidelines on polycystic ovary syndrome vary in their methodological quality, approach to diagnosis, approach to screening for health risks, and recommendations for the use of drug treatments.149 The 2023 update to the international polycystic ovary syndrome guidelines, which uses consensus methodology and clear grading systems for clinical recommendations, has now been released.3 These evidence based guidelines were developed after consultation with international multidisciplinary and consumer bodies to support clinicians and patients in the diagnosis and management of polycystic ovary syndrome and reduce variation in care.
Conclusions
Polycystic ovary syndrome is a common reproductive and metabolic disorder resulting from polygenic and environmental influences. Key pathological changes include neuroendocrine dysregulation, excess production of androgens, insulin resistance, and changes in adipose tissue biology, with variation in dysfunction of these pathways contributing to differences in phenotypic expression and severity of the disease. Advances in genetic understanding, together with new techniques to assess the steroid metabolome, have identified new biological targets, challenged the perception of polycystic ovary syndrome as one entity, and could facilitate an individualised approach to long term cardiometabolic surveillance based on the metabolomic signature. These advances could, for the first time, enable the development of specific drug treatments for the disorder based on an improved understanding of the underlying pathophysiology. Well designed, multicentre, patient centred clinical trials of neurokinin receptor antagonists, kisspeptin based treatments, and repurposed antidiabetic drugs are now needed to investigate new therapeutic options for polycystic ovary syndrome.
Questions for future research.
Should polycystic ovary syndrome be classified based on the steroid metabolomic signature, and specific treatments developed accordingly?
Does the steroid metabolome predict which patients are at increased risk of cardiometabolic disease?
Can reduction of 11-oxygenated androgens (eg, by inhibition of aldo-keto reductase type 1C3 (AKR1C3)) improve metabolic risk in patients with polycystic ovary syndrome?
Can later phase clinical trials of neurokinin 3 receptor antagonists show improvements in clinical hyperandrogenism and reproductive outcomes?
Patient involvement.
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Footnotes
Contributors: JD drafted the manuscript, table, and figures. AR did the outline, and critically reviewed the manuscript, table and figures. AR is the guarantor.
Funding: Funded in part by an anonymous benefactor grant (521809) to Cardiff University. The sponsor did not have a role in study design or performance, or analysis, or interpretation of the data.
Competing interests: We have read and understood the BMJ Policy on declaration of interests and declare the following interests: AR has undertaken educational activities funded by Pfizer and Diurnal, and received grant funding from the Waterloo Foundation. AR has participated as principal investigator in clinical trials funded by Neurocrine Biosciences, Sparrow Pharmaceuticals, Diurnal, and Ascendis.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Allen LA, Shrikrishnapalasuriyar N, Rees DA. Long-term health outcomes in young women with polycystic ovary syndrome: A narrative review. Clin Endocrinol (Oxf) 2022;97:187–98. 10.1111/cen.14609 [DOI] [PubMed] [Google Scholar]
- 2. Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2017;102:604–12. 10.1210/jc.2016-2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teede HJ, Tay CT, Laven JJE, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J Clin Endocrin Metab 2023;108:2447–69. 10.1210/clinem/dgad463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garg A, Patel B, Abbara A, et al. Treatments targeting neuroendocrine dysfunction in polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf) 2022;97:156–64. 10.1111/cen.14704 [DOI] [PubMed] [Google Scholar]
- 5. O’Reilly MW, Kempegowda P, Jenkinson C, et al. 11-oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J Clin Endocrinol Metab 2017;102:840–8. 10.1210/jc.2016-3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Reilly MW, Kempegowda P, Walsh M, et al. Akr1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2017;102:3327–39. 10.1210/jc.2017-00947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018;14:270–84. 10.1038/nrendo.2018.24 [DOI] [PubMed] [Google Scholar]
- 8. Lizneva D, Suturina L, Walker W, et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 2016;106:6–15. 10.1016/j.fertnstert.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 9. Ding T, Hardiman PJ, Petersen I, et al. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget 2017;8:96351–8. 10.18632/oncotarget.19180 Available: https://www.oncotarget.com/lookup/doi/10.18632/oncotarget.v8i56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolf WM, Wattick RA, Kinkade ON, et al. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health 2018;15:2589. 10.3390/ijerph15112589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Safiri S, Noori M, Nejadghaderi SA, et al. Prevalence, incidence and years lived with disability due to polycystic ovary syndrome in 204 countries and territories, 1990–2019. Hum Reprod 2022;37:1919–31. 10.1093/humrep/deac091 [DOI] [PubMed] [Google Scholar]
- 12. Pastor CL, Griffin-Korf ML, Aloi JA, et al. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 1998;83:582–90. 10.1210/jcem.83.2.4604 [DOI] [PubMed] [Google Scholar]
- 13. Walters KA, Edwards MC, Tesic D, et al. The role of central androgen receptor actions in regulating the hypothalamic-pituitary-ovarian axis. Neuroendocrinology 2018;106:389–400. 10.1159/000487762 [DOI] [PubMed] [Google Scholar]
- 14. Hu K-L, Chen Z, Li X, et al. Advances in clinical applications of kisspeptin-GNRH pathway in female reproduction. Reprod Biol Endocrinol 2022;20:81. 10.1186/s12958-022-00953-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clarkson J, Han SY, Piet R, et al. Definition of the hypothalamic Gnrh pulse generator in mice. Proc Natl Acad Sci U S A 2017;114:E10216–23. 10.1073/pnas.1713897114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stevenson H, Bartram S, Charalambides MM, et al. Kisspeptin-neuron control of LH pulsatility and ovulation. Front Endocrinol (Lausanne) 2022;13:951938. 10.3389/fendo.2022.951938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clarkson J, d’Anglemont de Tassigny X, Moreno AS, et al. Kisspeptin-Gpr54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 2008;28:8691–7. 10.1523/JNEUROSCI.1775-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith JT, Dungan HM, Stoll EA, et al. Differential regulation of Kiss-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 2005;146:2976–84. 10.1210/en.2005-0323 [DOI] [PubMed] [Google Scholar]
- 19. Brown RE, Imran SA, Ur E, et al. Kiss-1 mRNA in adipose tissue is regulated by sex hormones and food intake. Mol Cell Endocrinol 2008;281:64–72. 10.1016/j.mce.2007.10.011 [DOI] [PubMed] [Google Scholar]
- 20. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 21. Katulski K, Podfigurna A, Czyzyk A, et al. Kisspeptin and LH pulsatile temporal coupling in PCOS patients. Endocrine 2018;61:149–57. 10.1007/s12020-018-1609-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruddenklau A, Campbell RE. Neuroendocrine impairments of polycystic ovary syndrome. Endocrinology 2019;160:2230–42. 10.1210/en.2019-00428 [DOI] [PubMed] [Google Scholar]
- 23. Keen KL, Petersen AJ, Figueroa AG, et al. Physiological characterization and transcriptomic properties of Gnrh neurons derived from human stem cells. Endocrinology 2021;162:1–26. 10.1210/endocr/bqab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang JJ, Caligioni CS, Chan YM, et al. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology 2012;153:1498–508. 10.1210/en.2011-1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Talbi R, Ferrari K, Choi JH, et al. Characterization of the action of tachykinin signaling on pulsatile LH secretion in male mice. Endocrinology 2021;162:1–9. 10.1210/endocr/bqab074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and Κ-opioid receptors in adult male mice. Endocrinology 2013;154:2761–71. 10.1210/en.2013-1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 2004;145:2959–67. 10.1210/en.2003-1305 [DOI] [PubMed] [Google Scholar]
- 28. Casper RF, Alapin-Rubillovitz S. Progestins increase endogenous opioid peptide activity in postmenopausal women. J Clin Endocrinol Metab 1985;60:34–6. 10.1210/jcem-60-1-34 [DOI] [PubMed] [Google Scholar]
- 29. Kawwass JF, Sanders KM, Loucks TL, et al. Increased cerebrospinal fluid levels of GABA, testosterone and estradiol in women with polycystic ovary syndrome. Hum Reprod 2017;32:1450–6. 10.1093/humrep/dex086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dewailly D, Barbotin AL, Dumont A, et al. Role of anti-Müllerian hormone in the pathogenesis of polycystic ovary syndrome. Front Endocrinol (Lausanne) 2020;11:641. 10.3389/fendo.2020.00641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cimino I, Casoni F, Liu X, et al. Novel role for anti-Müllerian hormone in the regulation of Gnrh neuron excitability and hormone secretion. Nat Commun 2016;7:10055. 10.1038/ncomms10055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malone SA, Papadakis GE, Messina A, et al. Defective AMH signaling disrupts Gnrh neuron development and function and contributes to hypogonadotropic hypogonadism. Elife 2019;8:e47198. 10.7554/eLife.47198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011;32:81–151. 10.1210/er.2010-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev 2016;37:467–520. 10.1210/er.2015-1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Naamneh Elzenaty R, du Toit T, Flück CE. Basics of androgen synthesis and action. Best Pract Res Clin Endocrinol Metab 2022;36:101665. 10.1016/j.beem.2022.101665 [DOI] [PubMed] [Google Scholar]
- 36. Nestler JE, Jakubowicz DJ, de Vargas AF, et al. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 1998;83:2001–5. 10.1210/jcem.83.6.4886 [DOI] [PubMed] [Google Scholar]
- 37. Li X, Hu S, Zhu Q, et al. Addressing the role of 11Β-hydroxysteroid dehydrogenase type 1 in the development of polycystic ovary syndrome and the putative therapeutic effects of its selective inhibition in a preclinical model. Metabolism 2021;119:154749. 10.1016/j.metabol.2021.154749 [DOI] [PubMed] [Google Scholar]
- 38. Gambineri A, Fanelli F, Tomassoni F, et al. Tissue-specific dysregulation of 11Β-hydroxysteroid dehydrogenase type 1 in overweight/obese women with polycystic ovary syndrome compared with weight-matched controls. Eur J Endocrinol 2014;171:47–57. 10.1530/EJE-13-1030 [DOI] [PubMed] [Google Scholar]
- 39. Peitsidis P, Agrawal R. Role of vascular endothelial growth factor in women with PCO and PCOS: A systematic review. Reprod Biomed Online 2010;20:444–52. 10.1016/j.rbmo.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 40. Endoh A, Kristiansen SB, Casson PR, et al. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 beta-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab 1996;81:3558–65. 10.1210/jcem.81.10.8855801 [DOI] [PubMed] [Google Scholar]
- 41. Yildiz BO, Azziz R. The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord 2007;8:331–42. 10.1007/s11154-007-9054-0 [DOI] [PubMed] [Google Scholar]
- 42. Azziz R, Black V, Hines GA, et al. Adrenal androgen excess in the polycystic ovary syndrome: sensitivity and responsivity of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab 1998;83:2317–23. 10.1210/jcem.83.7.4948 [DOI] [PubMed] [Google Scholar]
- 43. Moran C, Reyna R, Boots LS, et al. Adrenocortical hyperresponsiveness to corticotropin in polycystic ovary syndrome patients with adrenal androgen excess. Fertil Steril 2004;81:126–31. 10.1016/j.fertnstert.2003.07.008 [DOI] [PubMed] [Google Scholar]
- 44. Steinberger E, Smith KD, Rodriguez-Rigau LJ. Testosterone, dehydroepiandrosterone, and dehydroepiandrosterone sulfate in hyperandrogenic women. J Clin Endocrinol Metab 1984;59:471–7. 10.1210/jcem-59-3-471 [DOI] [PubMed] [Google Scholar]
- 45. Turcu AF, Rege J, Auchus RJ, et al. 11-oxygenated androgens in health and disease. Nat Rev Endocrinol 2020;16:284–96. 10.1038/s41574-020-0336-x Available: https://pubmed.ncbi.nlm.nih.gov/32203405/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Storbeck K-H, Bloem LM, Africander D, et al. 11Β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer? Mol Cell Endocrinol 2013;377:135–46. 10.1016/j.mce.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 47. Pretorius E, Africander DJ, Vlok M, et al. 11-Ketotestosterone and 11-ketodihydrotestosterone in castration resistant prostate cancer: potent androgens which can no longer be ignored. PLoS One 2016;11:e0159867. 10.1371/journal.pone.0159867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Paulukinas RD, Mesaros CA, Penning TM. Conversion of classical and 11-oxygenated androgens by insulin-induced Akr1C3 in a model of human PCOS adipocytes. Endocrinology 2022;163:bqac068. 10.1210/endocr/bqac068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang L, Li S, Zhao A, et al. The expression of sex steroid synthesis and inactivation enzymes in subcutaneous adipose tissue of PCOS patients. J Steroid Biochem Mol Biol 2012;132:120–6. 10.1016/j.jsbmb.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 50. Blank SK, McCartney CR, Chhabra S, et al. Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls--implications for regulation of pubertal maturation. J Clin Endocrinol Metab 2009;94:2360–6. 10.1210/jc.2008-2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moghetti P, Castello R, Negri C, et al. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol Metab 1996;81:881–6. 10.1210/jcem.81.3.8772544 [DOI] [PubMed] [Google Scholar]
- 52. O’Reilly M, Gathercole L, Capper F, et al. Effect of insulin on Akr1C3 expression in female adipose tissue: in-vivo and in-vitro study of adipose androgen generation in polycystic ovary syndrome. Lancet 2015;385 Suppl 1:S0140-6736(15)60331-2. 10.1016/S0140-6736(15)60331-2 [DOI] [PubMed] [Google Scholar]
- 53. Nestler JE, Powers LP, Matt DW, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 1991;72:83–9. 10.1210/jcem-72-1-83 [DOI] [PubMed] [Google Scholar]
- 54. Escobar-Morreale HF, San Millán JL. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metab 2007;18:266–72. 10.1016/j.tem.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 55. Borruel S, Fernández-Durán E, Alpañés M, et al. Global adiposity and thickness of intraperitoneal and mesenteric adipose tissue depots are increased in women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab 2013;98:1254–63. 10.1210/jc.2012-3698 [DOI] [PubMed] [Google Scholar]
- 56. Dumesic DA, Akopians AL, Madrigal VK, et al. Hyperandrogenism accompanies increased intra-abdominal fat storage in normal weight polycystic ovary syndrome women. J Clin Endocrinol Metab 2016;101:4178–88. 10.1210/jc.2016-2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cassar S, Misso ML, Hopkins WG, et al. Insulin resistance in polycystic ovary syndrome: a systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum Reprod 2016;31:2619–31. 10.1093/humrep/dew243 [DOI] [PubMed] [Google Scholar]
- 58. Melson E, Rocha TP, Veen RJ, et al. Machine learning-based steroid metabolome analysis reveals three distinct subtypes of polycystic ovary syndrome and implicates 11-oxygenated androgens as major drivers of metabolic risk. EJEA 2023:2. 10.1530/endoabs.90.OC11.2 [DOI] [Google Scholar]
- 59. Mannerås-Holm L, Leonhardt H, Kullberg J, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab 2011;96:E304–11. 10.1210/jc.2010-1290 [DOI] [PubMed] [Google Scholar]
- 60. Lemaitre M, Christin-Maitre S, Kerlan V. Polycystic ovary syndrome and adipose tissue. Ann Endocrinol 2023;84:308–15. 10.1016/j.ando.2022.11.004 [DOI] [PubMed] [Google Scholar]
- 61. Robinson S, Chan S-P, Spacey S, et al. Postprandial thermogenesis is reduced in polycystic ovary syndrome and is associated with increased insulin resistance. Clin Endocrinol 1992;36:537–43. 10.1111/j.1365-2265.1992.tb02262.x [DOI] [PubMed] [Google Scholar]
- 62. Siemienowicz K, Rae MT, Howells F, et al. Insights into manipulating postprandial energy expenditure to manage weight gain in polycystic ovary syndrome. iScience 2020;23:101164. 10.1016/j.isci.2020.101164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Siemienowicz KJ, Furmanska K, Filis P, et al. Pubertal Fgf21 deficit is central in the metabolic pathophysiology of an ovine model of polycystic ovary syndrome. Mol Cell Endocrinol 2021;525:111196. 10.1016/j.mce.2021.111196 [DOI] [PubMed] [Google Scholar]
- 64. Qi X, Yun C, Sun L, et al. Gut microbiota–bile acid–Interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med 2019;25:1459. 10.1038/s41591-019-0562-8 [DOI] [PubMed] [Google Scholar]
- 65. Shi Y, Zhao H, Shi Y, et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet 2012;44:1020–5. 10.1038/ng.2384 [DOI] [PubMed] [Google Scholar]
- 66. Chen Z-J, Zhao H, He L, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2P16.3, 2P21 and 9Q33.3. Nat Genet 2011;43:55–9. 10.1038/ng.732 [DOI] [PubMed] [Google Scholar]
- 67. Hayes MG, Urbanek M, Ehrmann DA, et al. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun 2015;6:1–13. 10.1038/ncomms8502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Day FR, Hinds DA, Tung JY, et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun 2015;6:1–7. 10.1038/ncomms9464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee H, Oh J-Y, Sung Y-A, et al. Genome-wide association study identified new susceptibility loci for polycystic ovary syndrome. Hum Reprod 2015;30:723–31. 10.1093/humrep/deu352 [DOI] [PubMed] [Google Scholar]
- 70. Hiam D, Moreno-Asso A, Teede HJ, et al. The genetics of polycystic ovary syndrome: an overview of candidate gene systematic reviews and genome-wide association studies. J Clin Med 2019;8:1606. 10.3390/jcm8101606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Day F, Karaderi T, Jones MR, et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLOS Genet 2018;14:e1007813. 10.1371/journal.pgen.1007813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Louwers YV, Stolk L, Uitterlinden AG, et al. Cross-ethnic meta-analysis of genetic variants for polycystic ovary syndrome. J Clin Endocrinol Metab 2013;98:E2006–12. 10.1210/jc.2013-2495 [DOI] [PubMed] [Google Scholar]
- 73. Azziz R. PCOS in 2015: new insights into the genetics of polycystic ovary syndrome. Nat Rev Endocrinol 2016;12:183. 10.1038/nrendo.2016.9 [DOI] [PubMed] [Google Scholar]
- 74. Vink JM, Sadrzadeh S, Lambalk CB, et al. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab 2006;91:2100–4. 10.1210/jc.2005-1494 [DOI] [PubMed] [Google Scholar]
- 75. Demissie M, Lazic M, Foecking EM, et al. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am J Physiol Endocrinol Metab 2008;295:E262–8. 10.1152/ajpendo.90208.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Abbott DH, Bruns CR, Barnett DK, et al. Experimentally induced gestational androgen excess disrupts glucoregulation in Rhesus monkey dams and their female offspring. Am J Physiol Endocrinol Metab 2010;299:E741–51. 10.1152/ajpendo.00058.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Manikkam M, Steckler TL, Welch KB, et al. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology 2006;147:1997–2007. 10.1210/en.2005-1338 [DOI] [PubMed] [Google Scholar]
- 78. Risal S, Pei Y, Lu H, et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med 2019;25:1894–904. 10.1038/s41591-019-0666-1 [DOI] [PubMed] [Google Scholar]
- 79. Tata B, Mimouni NEH, Barbotin A-L, et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med 2018;24:834–46. 10.1038/s41591-018-0035-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stener-Victorin E, Deng Q. Epigenetic inheritance of polycystic ovary syndrome — challenges and opportunities for treatment. Nat Rev Endocrinol 2021;17:521–33. 10.1038/s41574-021-00517-x [DOI] [PubMed] [Google Scholar]
- 81. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the LifeSpan. BMC Med 2010;8:41. 10.1186/1741-7015-8-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Brutocao C, Zaiem F, Alsawas M, et al. Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine 2018;62:318–25. 10.1007/s12020-018-1692-3 [DOI] [PubMed] [Google Scholar]
- 83. Berni TR, Morgan CL, Berni ER, et al. Polycystic ovary syndrome is associated with adverse mental health and neurodevelopmental outcomes. J Clin Endocrinol Metab 2018;103:2116–25. 10.1210/jc.2017-02667 [DOI] [PubMed] [Google Scholar]
- 84. Dumesic DA, Lobo RA. Cancer risk and PCOS. Steroids 2013;78:782–5. 10.1016/j.steroids.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 85. Wada I, Matson PL, Troup SA, et al. Assisted conception using buserelin and human menopausal gonadotrophins in women with polycystic ovary syndrome. Br J Obstet Gynaecol 1993;100:365–9. 10.1111/j.1471-0528.1993.tb12981.x [DOI] [PubMed] [Google Scholar]
- 86. Berni TR, Morgan CL, Rees DA. Women with polycystic ovary syndrome have an increased risk of major cardiovascular events: a population study. J Clin Endocrinol Metab 2021;106:e3369–80. 10.1210/clinem/dgab392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rees DA, Jenkins-Jones S, Morgan CL. Contemporary reproductive outcomes for patients with polycystic ovary syndrome: a retrospective observational study. J Clin Endocrinol Metab 2016;101:1664–72. 10.1210/jc.2015-2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. de Medeiros SF, Yamamoto MMW, Souto de Medeiros MA, et al. Changes in clinical and biochemical characteristics of polycystic ovary syndrome with advancing age. Endocr Connect 2020;9:74–89. 10.1530/EC-19-0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pundir J, Charles D, Sabatini L, et al. Overview of systematic reviews of non-pharmacological interventions in women with polycystic ovary syndrome. Hum Reprod Update 2019;25:243–56. 10.1093/humupd/dmy045 [DOI] [PubMed] [Google Scholar]
- 90. Lim SS, Hutchison SK, Van Ryswyk E, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2019;3:CD007506. 10.1002/14651858.CD007506.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Oberg E, Gidlöf S, Jakson I, et al. Improved menstrual function in obese women with polycystic ovary syndrome after behavioural modification intervention-a randomized controlled trial. Clin Endocrinol (Oxf) 2019;90:468–78. 10.1111/cen.13919 [DOI] [PubMed] [Google Scholar]
- 92. Heikinheimo O, Toffol E, Partonen T, et al. Systemic hormonal contraception and risk of venous thromboembolism. Acta Obstet Gynecol Scand 2022;101:846–55. 10.1111/aogs.14384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med 2005;352:1223–36. 10.1056/NEJMra041536 [DOI] [PubMed] [Google Scholar]
- 94. Forslund M, Melin J, Alesi S, et al. Different kinds of oral contraceptive pills in polycystic ovary syndrome: a systematic review and meta-analysis. Eur J Endocrinol 2023;189:S1–16. 10.1093/ejendo/lvad082 [DOI] [PubMed] [Google Scholar]
- 95. Foretz M, Guigas B, Viollet B. Metformin: update on mechanisms of action and repurposing potential. Nat Rev Endocrinol 2023;19:460–76. 10.1038/s41574-023-00833-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Morley LC, Tang T, Yasmin E, et al. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev 2017;2018. 10.1002/14651858.CD003053.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Du Q, Yang S, Wang YJ, et al. Effects of thiazolidinediones on polycystic ovary syndrome: A meta-analysis of randomized placebo-controlled trials. Adv Ther 2012;29:763–74. 10.1007/s12325-012-0044-6 [DOI] [PubMed] [Google Scholar]
- 98. Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004;351:1106–18. 10.1056/NEJMra041001 [DOI] [PubMed] [Google Scholar]
- 99. Pundir J, Psaroudakis D, Savnur P, et al. Inositol treatment of anovulation in women with polycystic ovary syndrome: a meta-analysis of randomised trials. BJOG 2018;125:299–308. 10.1111/1471-0528.14754 Available: https://obgyn.onlinelibrary.wiley.com/toc/14710528/125/3 [DOI] [PubMed] [Google Scholar]
- 100. Abbara A, Jayasena CN, Christopoulos G, et al. Efficacy of kisspeptin-54 to trigger oocyte maturation in women at high risk of ovarian hyperstimulation syndrome (OHSS) during in vitro fertilization (IVF) therapy. J Clin Endocrin Metab 2015;100:3322–31. 10.1210/jc.2015-2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Abbara A, Clarke S, Islam R, et al. A second dose of kisspeptin-54 improves oocyte maturation in women at high risk of ovarian hyperstimulation syndrome: a phase 2 randomized controlled trial. Hum Reprod 2017;32:1915–24. 10.1093/humrep/dex253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. d’Anglemont de Tassigny X, Jayasena CN, Murphy KG, et al. Mechanistic insights into the more potent effect of KP-54 compared to KP-10 in vivo. PLoS One 2017;12:e0176821. 10.1371/journal.pone.0176821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mills EG, Dhillo WS. Invited review: translating kisspeptin and neurokinin B biology into new therapies for reproductive health. J Neuroendocrinol 2022;34:e13201. 10.1111/jne.13201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Owens LA, Abbara A, Lerner A, et al. The direct and indirect effects of kisspeptin-54 on granulosa lutein cell function. Hum Reprod 2018;33:292–302. 10.1093/humrep/dex357 [DOI] [PubMed] [Google Scholar]
- 105. Jayasena CN, Nijher GMK, Chaudhri OB, et al. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab 2009;94:4315–23. 10.1210/jc.2009-0406 [DOI] [PubMed] [Google Scholar]
- 106. George JT, Seminara SB. Kisspeptin and the hypothalamic control of reproduction: lessons from the human. Endocrinology 2012;153:5130–6. 10.1210/en.2012-1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Romero-Ruiz A, Skorupskaite K, Gaytan F, et al. Kisspeptin treatment induces gonadotropic responses and rescues ovulation in a subset of preclinical models and women with polycystic ovary syndrome. Hum Reprod 2019;34:2495–512. 10.1093/humrep/dez205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Abbara A, Eng PC, Phylactou M, et al. Kisspeptin receptor agonist has therapeutic potential for female reproductive disorders. J Clin Invest 2020;130:6739–53. 10.1172/JCI139681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Skorupskaite K, George JT, Anderson RA. The kisspeptin-Gnrh pathway in human reproductive health and disease. Hum Reprod Update 2014;20:485–500. 10.1093/humupd/dmu009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Albers-Wolthers CHJ, de Gier J, Walen M, et al. In vitro and in vivo effects of kisspeptin antagonists P234, P271, P354, and P356 on Gpr54 activation. PLoS ONE 2017;12:e0179156. 10.1371/journal.pone.0179156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Roseweir AK, Kauffman AS, Smith JT, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 2009;29:3920–9. 10.1523/JNEUROSCI.5740-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kobayashi T, Sasaki S, Tomita N, et al. 2-Acylamino-4,6-diphenylpyridine derivatives as novel Gpr54 antagonists with good brain exposure and in vivo efficacy for plasma LH level in male rats. Bioorganic & Medicinal Chemistry 2010;18:5157–71. 10.1016/j.bmc.2010.05.061 [DOI] [PubMed] [Google Scholar]
- 113. George JT, Kakkar R, Marshall J, et al. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: A randomized, placebo-controlled trial. J Clin Endocrinol Metab 2016;101:4313–21. 10.1210/jc.2016-1202 [DOI] [PubMed] [Google Scholar]
- 114. Prague JK, Roberts RE, Comninos AN, et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2017;389:1809–20.:S0140-6736(17)30823-1. 10.1016/S0140-6736(17)30823-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Modi M, Dhillo WS. Neurokinin 3 receptor antagonism: A novel treatment for menopausal hot flushes. Neuroendocrinology 2019;109:242–8. 10.1159/000495889 [DOI] [PubMed] [Google Scholar]
- 116. Fraser GL, Obermayer-Pietsch B, Laven J, et al. Randomized controlled trial of neurokinin 3 receptor antagonist fezolinetant for treatment of polycystic ovary syndrome. J Clin Endocrinol Metab 2021;106:e3519–32. 10.1210/clinem/dgab320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sucquart IE, Nagarkar R, Edwards MC, et al. Neurokinin 3 receptor antagonism ameliorates key metabolic features in a hyperandrogenic PCOS mouse model. Endocrinology 2021;162:bqab020. 10.1210/endocr/bqab020 [DOI] [PubMed] [Google Scholar]
- 118. Miyata S. New aspects in fenestrated capillary and tissue dynamics in the sensory circumventricular organs of adult brains. Front Neurosci 2015;9:390. 10.3389/fnins.2015.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fishbane S, Jamal A, Munera C, et al. A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med 2020;382:222–32. 10.1056/NEJMoa1912770 [DOI] [PubMed] [Google Scholar]
- 120. Deeks ED. Difelikefalin: first approval. Drugs 2021;81:1937–44. 10.1007/s40265-021-01619-6 [DOI] [PubMed] [Google Scholar]
- 121. McCarthy EA, Dischino D, Maguire C, et al. Inhibiting Kiss1 neurons with kappa opioid receptor agonists to treat polycystic ovary syndrome and vasomotor symptoms. J Clin Endocrinol Metab 2022;107:e328–47. 10.1210/clinem/dgab602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hu X, Wang J, Dong W, et al. A meta-analysis of polycystic ovary syndrome in women taking valproate for epilepsy. Epilepsy Res 2011;97:73–82. 10.1016/j.eplepsyres.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 123. Radwan RA, Abuelezz NZ, Abdelraouf SM, et al. Decreased serum level of gamma-amino butyric acid in Egyptian infertile females with polycystic ovary syndrome is correlated with dyslipidemia, total testosterone and 25(OH) vitamin D levels. J Med Biochem 2019;38:512–8. 10.2478/jomb-2018-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ullah A, Jahan S, Razak S, et al. Protective effects of GABA against metabolic and reproductive disturbances in letrozole induced polycystic ovarian syndrome in rats. J Ovarian Res 2017;10:62. 10.1186/s13048-017-0359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hart KN, Stocker WA, Nagykery NG, et al. Structure of AMH bound to Amhr2 provides insight into a unique signaling pair in the TGF-Β family. Proc Natl Acad Sci USA 2021;118. 10.1073/pnas.2104809118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yin YD, Fu M, Brooke DG, et al. The activity of Sn33638, an inhibitor of Akr1C3, on testosterone and 17Β-estradiol production and function in castration-resistant prostate cancer and ER-positive breast cancer. Front Oncol 2014;4:159. 10.3389/fonc.2014.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Loriot Y, Fizazi K, Jones RJ, et al. Safety, tolerability and anti-tumour activity of the androgen biosynthesis inhibitor Asp9521 in patients with metastatic castration-resistant prostate cancer: multi-centre phase I/II study. Invest New Drugs 2014;32:995–1004. 10.1007/s10637-014-0101-x [DOI] [PubMed] [Google Scholar]
- 128. Khanim FL, Hayden RE, Birtwistle J, et al. Combined bezafibrate and medroxyprogesterone acetate: potential novel therapy for acute myeloid leukaemia. PLoS ONE 2009;4:e8147. 10.1371/journal.pone.0008147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Penning TM. Aldo-keto reductase (AKR) 1C3 inhibitors: a patent review. Expert Opin Ther Patents 2017;27:1329–40. 10.1080/13543776.2017.1379503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Abdalla MA, Deshmukh H, Atkin S, et al. The potential role of incretin-based therapies for polycystic ovary syndrome: a narrative review of the current evidence. Ther Adv Endocrinol 2021;12:204201882198923. 10.1177/2042018821989238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Helvaci N, Yildiz BO. Current and emerging drug treatment strategies for polycystic ovary syndrome. Expert Opin Pharmacother 2023;24:105–20. 10.1080/14656566.2022.2108702 [DOI] [PubMed] [Google Scholar]
- 132. Wang F-F, Wu Y, Zhu Y-H, et al. Pharmacologic therapy to induce weight loss in women who have obesity/overweight with polycystic ovary syndrome: a systematic review and network meta-analysis. Obes Rev 2018;19:1424–45. 10.1111/obr.12720 [DOI] [PubMed] [Google Scholar]
- 133. Nylander M, Frøssing S, Clausen HV, et al. Effects of liraglutide on ovarian dysfunction in polycystic ovary syndrome: a randomized clinical trial. Reprod Biomed Online 2017;35:121–7. 10.1016/j.rbmo.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 134. Elkind-Hirsch K, Marrioneaux O, Bhushan M, et al. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:2670–8. 10.1210/jc.2008-0115 [DOI] [PubMed] [Google Scholar]
- 135. Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition, and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril 2022;118:371–81. 10.1016/j.fertnstert.2022.04.027 [DOI] [PubMed] [Google Scholar]
- 136. Dawson AJ, Sathyapalan T, Vince R, et al. The effect of exenatide on cardiovascular risk markers in women with polycystic ovary syndrome. Front Endocrinol (Lausanne) 2019;10:189. 10.3389/fendo.2019.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Nylander M, Frøssing S, Kistorp C, et al. Liraglutide in polycystic ovary syndrome: a randomized trial, investigating effects on thrombogenic potential. Endocr Connect 2017;6:89–99. 10.1530/EC-16-0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Han Y, Li Y, He B. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reprod Biomed Online 2019;39:332–42.:S1472-6483(19)30394-3. 10.1016/j.rbmo.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 139. Jensterle M, Ferjan S, Vovk A, et al. Semaglutide reduces fat accumulation in the tongue: a randomized single-blind, pilot study. Diabetes Res Clin Pract 2021;178:108935. 10.1016/j.diabres.2021.108935 [DOI] [PubMed] [Google Scholar]
- 140. Zhang Y, Qu Z, Lu T, et al. Effects of a dulaglutide plus calorie-restricted diet versus a calorie-restricted diet on visceral fat and metabolic profiles in women with polycystic ovary syndrome: a randomized controlled trial. Nutrients 2023;15:556. 10.3390/nu15030556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021;385:503–15. 10.1056/NEJMoa2107519 [DOI] [PubMed] [Google Scholar]
- 142. Al Wattar BH, Teede H, Garad R, et al. Harmonising research outcomes for polycystic ovary syndrome: an international multi-stakeholder core outcome set. Hum Reprod 2020;35:404–12. 10.1093/humrep/dez272 [DOI] [PubMed] [Google Scholar]
- 143. Pereira MJ, Eriksson JW. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs 2019;79:219–30. 10.1007/s40265-019-1057-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Javed Z, Papageorgiou M, Deshmukh H, et al. Effects of empagliflozin on metabolic parameters in polycystic ovary syndrome: a randomized controlled study. Clin Endocrinol (Oxf) 2019;90:805–13. 10.1111/cen.13968 [DOI] [PubMed] [Google Scholar]
- 145. Elkind-Hirsch KE, Chappell N, Seidemann E, et al. Dapagliflozin, or phentermine/topiramate differentially affect metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab 2021;106:3019–33. 10.1210/clinem/dgab408 [DOI] [PubMed] [Google Scholar]
- 146. Zhang J, Xing C, Cheng X, et al. Canagliflozin combined with metformin versus metformin monotherapy for endocrine and metabolic profiles in overweight and obese women with Polycystic ovary syndrome: A single-center, open-labeled prospective randomized controlled trial. Front Endocrinol (Lausanne) 2022;13:1003238. 10.3389/fendo.2022.1003238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Javed Z, Papageorgiou M, Madden LA, et al. The effects of empagliflozin vs metformin on endothelial microparticles in overweight/obese women with polycystic ovary syndrome. Endocr Connect 2020;9:563–9. 10.1530/EC-20-0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Tan S, Ignatenko S, Wagner F, et al. Licogliflozin versus placebo in women with polycystic ovary syndrome: A randomized, double-blind, phase 2 trial. Diabetes Obesity Metab 2021;23:2595–9. 10.1111/dom.14495 Available: https://dom-pubs.onlinelibrary.wiley.com/toc/14631326/23/11 [DOI] [PubMed] [Google Scholar]
- 149. Al Wattar BH, Fisher M, Bevington L, et al. Clinical practice guidelines on the diagnosis and management of polycystic ovary syndrome: a systematic review and quality assessment study. J Clin Endocrinol Metab 2021;106:2436. 10.1210/CLINEM/DGAB232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Neal-Perry G, Cano A, Lederman S, et al. Safety of fezolinetant for vasomotor symptoms associated with menopause: a randomized controlled trial. Obstet Gynecol 2023;141:737–47. 10.1097/AOG.0000000000005114 [DOI] [PMC free article] [PubMed] [Google Scholar]