Abstract
Insulin receptors are expressed throughout the adult brain, and insulin from the periphery reaches the central nervous system. In humans and rodents, actions of insulin in the brain decrease food intake. Furthermore, insulin receptor activation alters dopamine and glutamate transmission within mesolimbic regions that influence food-seeking and feeding including the nucleus accumbens (NAc). Here we determined how intra-NAc insulin affects conditioned approach (a measure of cue-triggered food-seeking), free food intake, and the motivation to obtain food in hungry rats using Pavlovian and instrumental approaches. Intra-NAc insulin did not affect conditioned approach but did reduce home cage chow intake immediately following conditioned approach testing. Consistent with reduced chow intake, intra-NAc insulin also reduced the motivation to work for flavored food pellets (assessed by a progressive ratio procedure). This effect was partially reversed by insulin receptor blockade and was not driven by insulin-induced sickness or malaise. Taken together, these data show that insulin within the NAc does not alter behavioral responses to a food cue, but instead reduces the motivation to work for and consume food in hungry animals. These data are discussed in light of insulin’s role in the regulation of feeding, and its dysregulation by obesity.
Keywords: Nucleus accumbens, Insulin, Conditioned approach, Break point, Motivation, Feeding
1. Introduction
The nucleus accumbens (NAc) is an integrative hub between cortical, limbic, and motor regions [45] that influences food-seeking and feeding behaviors [12, 18, 34]. While many transmitter systems contribute, glutamate provides the primary excitatory drive to the NAc, while the neuromodulator dopamine tunes NAc activity. Within the NAc, the core and shell sub-regions are involved in different aspects of feeding behavior. For example, reducing activity in the NAc shell, [7, 36, 44, 53, 59] increases food intake, whereas reducing activity in the NAc core attenuates cue- or sugar-primed food-seeking [3, 25, 40]. Furthermore, increasing dopamine activity within the NAc core selectively enhances the motivation to work for food [11, 38] while actions of dopamine in the shell increase food intake [31]. Taken together, these data demonstrate the importance of the NAc in driving cue-induced food-seeking, the willingness to work for food, and food consumption.
In addition to classical transmitters, several peripheral hormones influence feeding behavior via actions within the central nervous system, these include ghrelin, leptin, and insulin [37, 55]. Insulin is classically considered a satiety signal that reduces food intake via its actions in the hypothalamus [8, 29, 41, 60]. However, insulin from the periphery rapidly reaches the NAc [5], which strongly expresses insulin receptors [28]. Activation of NAc insulin receptors enhances dopamine and glutamate transmission. Specifically, activation of NAc core insulin receptors enhances excitatory neurotransmission via local feedback that increases presynaptic glutamate release [21]. In regard to dopamine, activation of insulin receptors on cholinergic inter-neurons enhances stimulated dopamine release within the NAc core and shell [48, 58]. However, insulin-induced increases in dopamine transporter function and surface expression have also been found [30, 48]. Thus, insulin is poised to strongly influence various aspects of food-seeking and feeding via its actions within the NAc. Indeed, there is evidence that NAc insulin receptors play an important role in flavor nutrient learning [58, 63]. However, no studies have directly examined the effects of intra-NAc insulin on cue-triggered food-seeking, food intake, or the motivation for food.
The goal of the current study was to determine the effects of direct intra-NAc insulin infusion on cue-induced food-seeking using conditioned approach, free chow intake, and the willingness to work for flavored food pellets using progressive ratio procedures. Within the same test session and across two cohorts, insulin infusion did not alter conditioned approach, but did reduce food intake in hungry male rats. Consistent with this, intra-NAc insulin also reduced the motivation to obtain food; this effect was partially blocked by co-infusion of an insulin receptor blocker. Additional data show that reductions in motivation to work for food were not due insulin-induced sickness or malaise. Together these data demonstrate novel roles for the regulation of feeding by NAc insulin and reveal dissociations between the ability of this peripheral hormone to influence food-seeking vs. feeding behavior.
2. Materials and methods
General methods are given first followed by detailed methods and Ns for each experiment (see also simplified timelines Fig. 1).
Fig. 1.
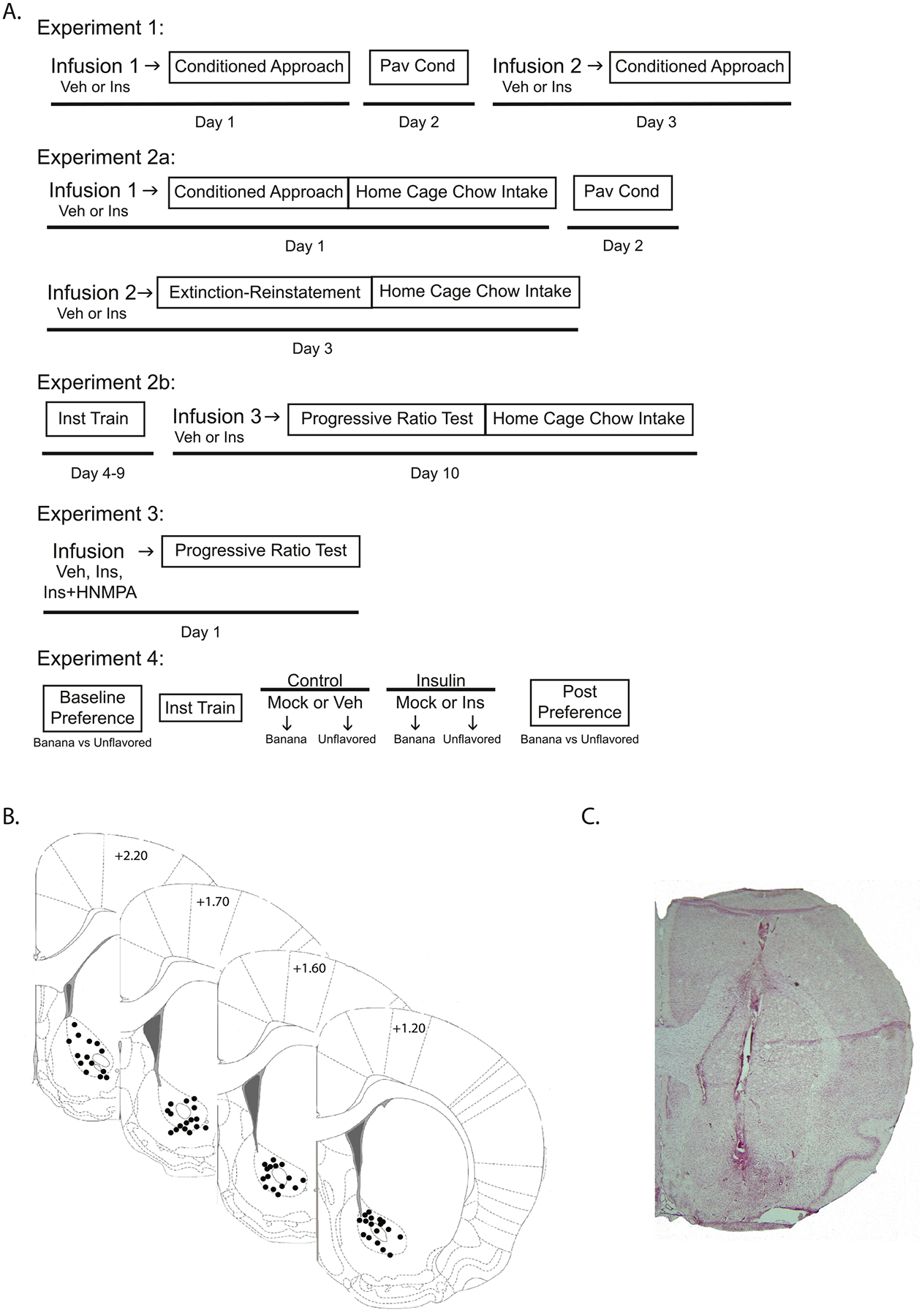
Experimental timelines and cannula placements. (A) Timelines of Pavlovian conditioning (Pav Cond); instrumental training (Inst Train) and key testing days for all experiments (vehicle [Veh]; control [Con] insulin [Ins]; Picropodophyllin [PPP]; HNMPA-(AM)3 [HNMPA]). (B) Schematic of cannula placements ranging from +1.2 mm to +2.20 mm from Bregma. Images adapted from Paxinos and Watson [49]. (C) Representative image of cannula placement from a neutral red stained slice.
2.1. Subjects
Male Sprague Dawley rats (Envigo; Indianapolis IN), aged 50–55 days on arrival, were individually housed in a vivarium maintained on a reverse (12/12) light dark cycle (lights off at 7am), at 22 °C (73°F), and 45% humidity with ad libitum access to water. All behavioral experiments were conducted within the first 3–4 h of the dark cycle. Ad libitum access to standard lab chow (5LOD; LabDiet; St. Louis, MO) was provided after the completion of daily training/testing (10–11 am) and food was removed at 5 pm each day. Thus, rats were tested and trained hungry. This modified feeding schedule limits potential confounds due to increases in insulin resulting from home cage food intake prior to testing. For all studies, rats were placed on this modified feeding schedule for 1 week prior to the onset of training. For experiments using a between subject design, experimental groups were counter-balanced based on weight and behavioral performance during acquisition.
2.2. Behavioral training and testing
All training and testing occurred in standard operant boxes (Med Associates; St. Albans City, VT) housed within sound attenuating chambers. Each box was equipped with a house light, a speaker, 2 levers, food hoppers, and a recessed food cup (i.e., magazine). A 50/50 mix of sucrose pellets (LabTab Sucrose Tablets 45 mg; TestDiet; St. Louis, MO) and unflavored pellets (Dustless Precision Pellets 45 mg; BioServ; Flemington, NJ) were used unless otherwise noted. Training and testing started between 9 and 10 am each day.
2.2.1. Pavlovian conditioning
Rats were conditioned to associate the presentation of a discrete auditory tone (conditioned stimulus; CS, 10 s) with the delivery of two food pellets (unconditioned stimulus; US). The US was delivered at CS offset. Each session consisted of 25 CS-US pairings that were separated by an average inter trial interval (ITI) of 60 s (range 30–90 s). Each training session lasted 30 min with one session given per day (10–12 sessions total). Magazine entries during the CS and the ITI were measured throughout each session.
2.2.2. Instrumental training and progressive ratio testing
Rats were first trained to lever press for food pellets using a fixed ratio 1 (FR) schedule of reinforcement where each response resulted in the delivery of one pellet (3 sessions, 1 session/day). The work requirement was then increased to an FR3 (three responses/1 pellet) for an additional 3 sessions. Each session ended when the rat received 50 pellets, or 40 min had elapsed. Completion time, magazine entries, and lever presses were recorded throughout. During progressive ratio (PR) testing, the number of active lever responses required to receive one pellet increased exponentially across the testing session (e0.1: 1, 2, 3, 4, 5, 6, […], [54]). Break point was defined as the last ratio completed within 30 min. Testing ended when rats failed to complete the next ratio within 30 min or after a maximum duration of 6 h.
2.3. Cannula implantation, infusion, and histology
To minimize the duration of cannula implantation, all cannulae were implanted following initial Pavlovian conditioning or instrumental training. Standard stereotaxic procedures were used to implant bilateral guide cannulae aimed at the NAc core [19]. Briefly, rats were anesthetized with isoflurane (induction: 5% maintenance: 2.5%) and given carprofen pre-operatively and once per day for 2 days post-operatively (5 mg/kg, s.c.). Guide cannulae (24 GA cut 9 mm below the pedestal; C316G/SPC, Plastics One; Roanoke, VA) were bilaterally implanted above the NAc (+ 1.4 A/P, ± 2.5 M/L from Bregma; - 6.0 D/V from the top of the skull, 10° angle). Rats were given ad libitum access to home cage chow the day prior to surgery and throughout recovery (7 days). The modified feeding schedule described above was then reintroduced 5 days prior to behavioral retraining or testing.
Intra-NAc infusions were delivered at a volume of 0.5 μL/side (infusion rate: 0.5 μL/2 min, injectors left in place for 1 min post-infusion to allow for diffusion), and testing began 10 min after the start of the infusion. Three concentrations of insulin (Cat. #91077C; Millipore Sigma; Burlington, MA) were examined: 100 nM (0.29 ng, 0.0084 mU), 500 nM (1.45 ng, 0.042 mU), and 60 μM (174.24 ng, 5.0 mU). These concentrations were based on previous studies showing that 100–500 nM insulin alters neural activity in the VTA [39] and NAc [21, 58], and that 60 μM insulin (5 mU) given intraventricularly or directly into the arcuate nucleus or NAc shell altered instrumental responding for sucrose pellets [22, 23]. Because insulin can act at insulin receptors and the receptor for insulin-like growth factor (IGFR) [56], the IGFR antagonist Picropodophyllin (PPP; 500 nM, Cat. #2956, Tocris Bioscience; Bristol, United Kingdom) was co-infused with insulin (Experiment 2). However, given that there could be unintended effects of this co-administration, we moved to using insulin alone in the presence or absence of the insulin receptor blocker HNMPA-(AM)3 (350 μM; Cat. #sc-221,730, Santa Cruz; Dallas, TX) in follow up studies to more directly examine the role of insulin receptors. Importantly, the goals of these studies were to evaluate the effect of insulin on behavior, and not necessarily to understand how physiological changes in insulin affect behavior.
At the end of the study, brains were collected and cannula placement within the NAc was verified using standard histological procedures [24]. Coronal sections (30 μm; −18 °C; Leica CM1850 cryostat; Wetzlar, Germany) were collected in series beginning ~ 2.76 mm from bregma, extending though the cannula tracts and ending ~0.48 mm from bregma [49].
2.4. Details of individual experiments
2.4.1. Experiment 1: Effects of intra-NAc insulin on conditioned approach
Rats (N = 10) were given 6 sessions of Pavlovian conditioning prior to guide cannula implantation, as described above. After surgical recovery (7 days), rats were given 4 additional conditioning sessions to ensure stable behavioral responding prior to testing. As such, 17 days elapsed between initial and post-surgical conditioning sessions. Conditioned approach in the absence of pellets (25 CS presentations, 60 s variable ITI) was measured using a within subject design following infusion of vehicle (ACSF: 122.5 mM NaCl, 25 mM NAHCO3, 1 mM NaH2PO4, 1 mM Ascorbic acid, 2.5 mM KCl, 2.5 mM CaCl, 1 mM MgCl, 7.4 pH, 295–305 mOsm) or insulin (100 nM; Cat. #91077C, Sigma-Aldrich; St. Louis, MO). Infusion order was counter balanced with one additional conditioning session between each test (Fig. 1 Experiment 1).
2.4.2. Experiment 2a: Effect of intra-NAc insulin on conditioned approach, extinction-reinstatement, and home cage chow intake
Rats (N = 16) were given 8 sessions of Pavlovian conditioning prior to guide cannula implantation and 4 sessions of post-operative conditioning. Identical to experiment 1, 17 days elapsed between initial and post-operative conditioning sessions. Rats were then tested for conditioned approach using a between subject design in which half received vehicle and the other half received 500 nM insulin co-infused with the IGFR antagonist PPP prior to testing. In addition, home cage chow intake was measured during the first hour immediately following testing (i.e., the first-time rats had access to chow that day; Fig. 1– Experiment 2a Infusion 1).
Because we did not observe any effect of insulin on conditioned approach in the absence of food, we next examined the effects of a higher concentration of insulin (60 μM) on reinstatement and extinction behavior. Importantly, this concentration is within the range of those previously shown to reduce food intake (see also above). Briefly, 24 h after the first test described above rats were given a single Pavlovian conditioning session to re-establish the CS-US relationship. The next day, rats were given an infusion of vehicle or insulin and responses to 5 presentations of the CS alone were assessed. This was immediately followed by 5 CS-US pairings identical to the initial conditioning, and an additional 5 presentations of the CS alone. Magazine entries during the CS and ITI were measured throughout. In addition, home cage chow intake was measured immediately following testing as described above (Fig. 1– Experiment 2a Infusion 2).
2.4.3. Experiment 2b: Effects of intra-NAc insulin on break point and home cage chow intake
Motivation for food was assessed in rats from Experiment 2a using a PR procedure. Briefly, rats were trained to lever press to receive food pellets as described above. They were then given one PR test session following intra-NAc infusion of vehicle or 60 μM insulin. Home cage chow intake was measured immediately after this test (Fig. 1– Experiment 2b). In total, each rat in Experiment 2 received three intra-NAc infusions and treatment groups were maintained throughout (vehicle treated rats only received vehicle [N = 8] and insulin-treated rats only received insulin [N = 8]). This allowed us to monitor for behavioral drift and directly assess effects of repeated infusion and testing within the vehicle group.
2.4.4. Experiment 3: Effect of NAc insulin on instrumental responding with and without insulin receptor blockade
Rats in Experiment 2b were subjected to repeated testing. Thus, to verify and expand upon these findings, a separate set of rats (N = 24) were trained to lever press for food pellets prior to guide cannula implantation. For this experiment, active and inactive levers were included in the testing chamber. Only responses on the active lever resulted in pellet delivery while responses on the inactive lever had no consequence. Rats in experimental groups underwent PR testing following infusion of control (N = 8), insulin (60 μM, N = 8), or insulin co-infused with the insulin receptor blocker HNMPA-(AM)3, (350 μM, N = 8; Cat. #sc-221,730, Santa Cruz; Dallas, TX). Half the rats in the control group received HNMPA-(AM)3 alone (N = 4; 350 μM) while the other half received vehicle (N=4) (Fig. 1– Experiment 3).
2.4.5. Experiment 4: Effects of repeated intra-NAc insulin on food devaluation
Reductions in food intake and break point observed in experiments above could be due to the induction of sickness or malaise by intra-NAc insulin infusion. To address this possibility, a separate cohort of rats (N = 17) was used to determine whether intra-NAc insulin results in devaluation. We first confirmed that there was a similar preference for two distinctly flavored food pellets (banana and unflavored pellets) in a 20-min free consumption test. Next, rats underwent instrumental training in which they could earn each type of pellet (details below). We then repeatedly paired one type of pellet with intra-NAc insulin or intra-NAc vehicle infusion and the other with a mock infusion. In order to keep conditions as similar to previous experiments as possible, rats were allowed to work for one pellet type after each infusion or mock infusion. We then re-evaluated preference for each pellet in a second 20-min free consumption test.
Instrumental training: Rats first learned to press one lever to obtain one type of pellet and a second lever to obtain the other (left/right counterbalanced). Rats were given two consecutive 30-minute training sessions each day with one active lever at a time (5 min between each session, 3 FR1 sessions, 3 FR3 sessions). A maximum of 50 pellets could be earned per session. After this training, rats underwent surgery for guide cannula implantation and instrumental re-training after recovery (2 sessions FR1, 3 sessions FR3). Rats were then assigned to control (N = 8) or insulin groups (N = 9).
Control or Insulin Treatment: Rats in the control group received intra-NAc infusion of vehicle (ACSF) before being allowed to lever press for one type of flavored pellet (e.g., unflavored), and mock infusion (handled identically to infusion days, but no infusion given) prior to lever pressing for the other flavored pellet (e.g., banana). Rats in the insulin group received an intra-NAc infusion of insulin (60μM) or mock infusion prior to each session. All rats received three sets of mock and infusion sessions (1 session/day) with each set of infusions separated by a 24-hour wash out period. For all groups, rats were given one 40 min FR3 session 10 min following mock or real infusions in which they could work for one type of food pellet. No limits were set on the number of pellets earned per session. Approximately half of the rats in each treatment group were assigned to lever press for unflavored pellets following mock infusion, while the other half worked for banana flavored pellets. Food pairings were reversed for the vehicle/insulin infusion days. Finally, the effects of repeated insulin vs. vehicle infusion on preference were assessed 48 h after the final infusion session (Fig. 1– Experiment 4).
2.5. Statistical analyses
All statistical analysis was conducted using Prism 9 software (Graph Pad; San Dieog, CA). Direct comparisons of the behavioral effects of insulin or vehicle were only conducted in groups tested under the same conditions. Two-tailed unpaired t-tests or one-way, two-way, and three-way ANOVAs followed by Šidák post hoc or Dunnettt’s multiple comparisons tests were used as appropriate.
3. Results
Fig. 1 shows cannula placements for all studies (panel B) and a representative image of neutral red stained slices (panel C). Data from any animals with cannula placements outside the NAc were excluded from statistical analyses (N = 8 of 71).
3.1. Experiment 1: Effects of intra-NAc insulin on conditioned approach
Fig. 2 shows the average rate of magazine entries (±SEM) during acquisition (panel A), and testing in extinction conditions following intra-NAc infusion (panel B). As expected, CS-US pairings resulted in significant increases in magazine entries during CS presentation (and prior to US delivery) compared to the ITI, and this behavior was not affected by guide cannula implantation (Fig. 2A; two-way RM ANOVA, main effect of session: F(11, 99) = 6.51, p < 0.0001; main effect of period (CS vs. ITI): F(1, 9) = 38.26, p < 0.001; session x period interaction: F(11, 90) = 7.80, p < 0.0001). Next, the effects of intra-NAc infusion of vehicle or 100 nM insulin on conditioned approach in the absence of food was assessed. While magazine entry rates during approach testing were significantly greater during the CS compared with the ITI (Fig. 2B; two-way ANOVA, main effect of period: F(1, 5) = 15.95, p < 0.05), behavior was unaffected by insulin infusion (Fig. 2B; two-way ANOVA, no main effect of treatment: F(1, 5) = 0.0004, p = 0.98; no treatment x period interaction: F(1, 5) = 0.49, p = 0.51). In addition, there were no order effects on behavior (i.e., insulin or vehicle infusion first).
Fig. 2.
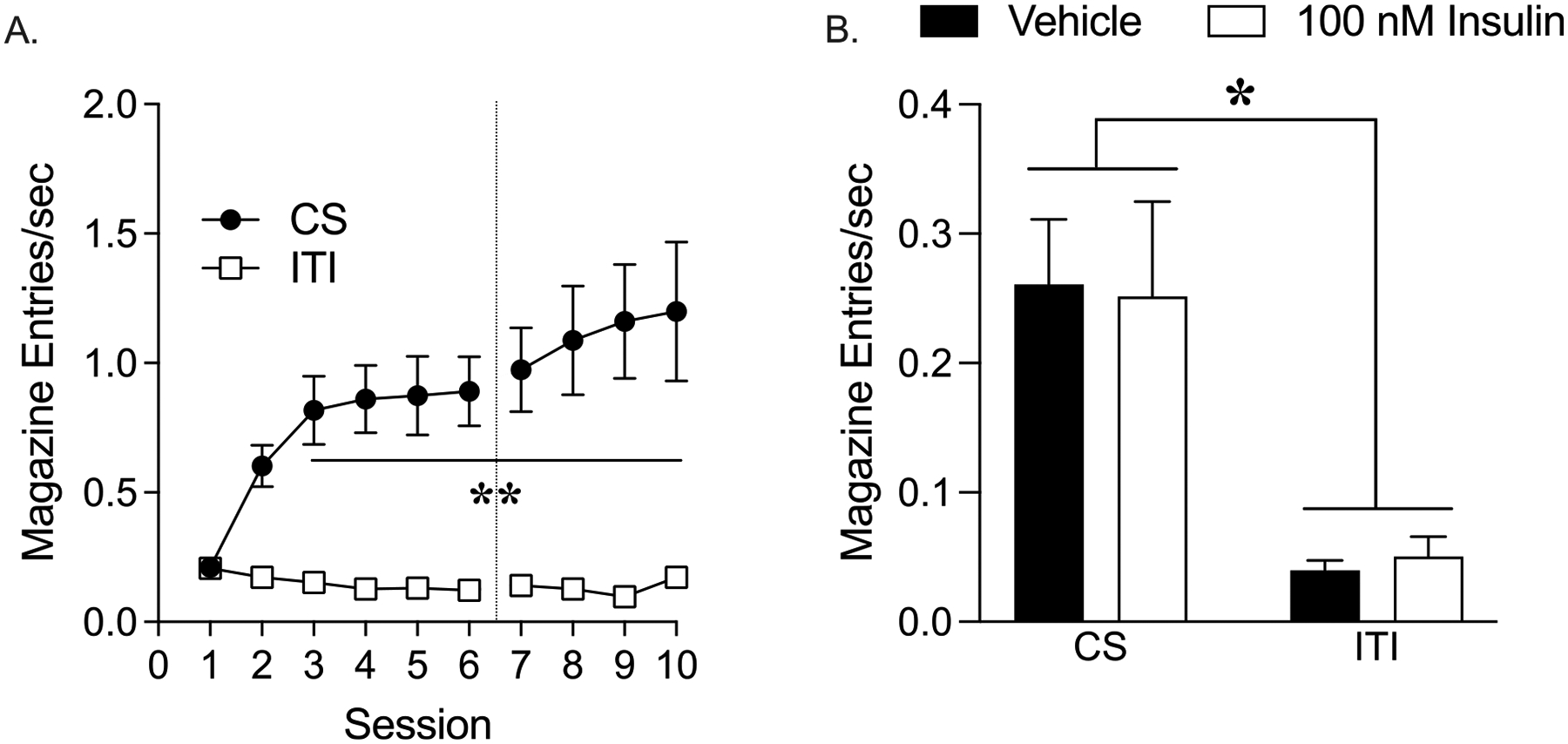
Intra-NAc infusion of 100 nM insulin does not alter conditioned approach. (A) Acquisition: Magazine entries per second during the CS and ITI periods. The dotted line shows when cannula implantation occurred. (B) Testing in extinction conditions: Magazine entries per second during the CS and ITI periods following infusion of vehicle or 100 nM insulin. All data shown as average ± SEM (*p < 0.05, **p < 0.01).
3.2. Experiment 2a: Effect of intra-NAc insulin on conditioned approach, extinction-reinstatement, and home cage chow intake
Rats in Experiment 2 quickly learned to associate the presentation of the CS with the delivery of the US as evidenced by a rapid rise in magazine entry rate during the CS vs. ITI period (Fig. 3A; two-way RM ANOVA, main effect of period: F(1, 15) = 69.70, p < 0.0001; main effect of session: F(11, 165) = 7.33, p < 0.0001; session x period interaction: F(11, 154) = 18.78, p < 0.0001). In this cohort, conditioned approach was reduced during the first conditioning session after surgical recovery (Fig. 3A: CS+ Session 8 vs. 9 Dunnett’s p = 0.03), but rapidly returned to pre-operative levels (CS+ Session 8 vs. 10–12 CS Dunnett’s P = 0.84–0.99). Next, the effect of intra-NAc infusion of vehicle or 500 nM insulin in the presence of PPP on conditioned approach was assessed. Similar to results in Experiment 1, direct infusion of 500 nM insulin into the NAc did not alter conditioned approach, with similar enhancements in the rate of magazine entries during the CS vs. ITI in vehicle and insulin groups (Fig. 3B; two-way ANOVA, main effect of period: F(1, 20) = 20.03, p < 0.001; no effect of treatment p = 0.54, no treatment x period interaction p = 0.99). However, when home cage food intake immediately following testing was measured, there was a slight visual trend towards reduced food intake in the insulin vs. vehicle group (Fig. 3C).
Fig. 3.
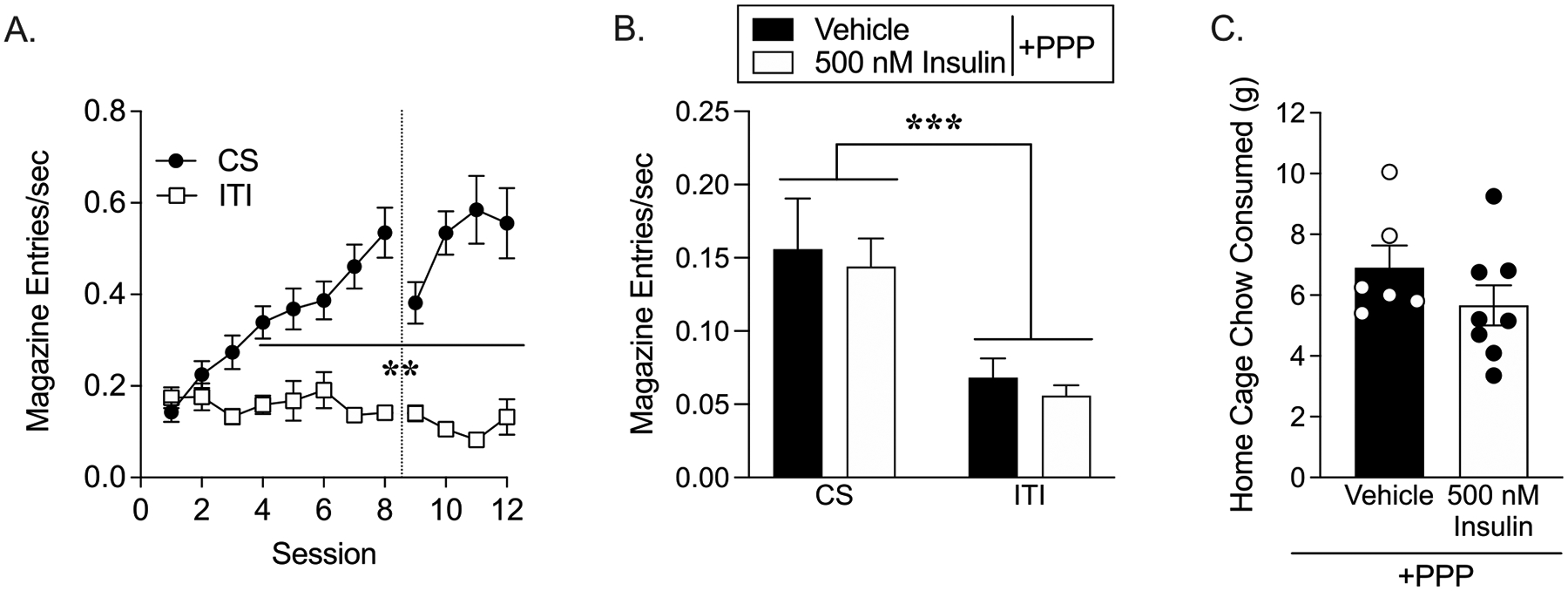
Infusion of 500 nM insulin into the NAc does not alter conditioned approach. (A) Acquisition: Magazine entry rate during the CS and ITI period before and after cannula implantation, denoted by the dotted line. (B) Testing in extinction conditions: Magazine entries per second during the CS and ITI following infusion of vehicle or 500 nM insulin infusion in the presence of PPP. (C) Chow consumed in the home cage during 1 hour immediately following conditioned approach testing. (CS vs ITI: **p < 0.01, ***p < 0.001).
Given the slight trend towards reduced food intake seen in Fig. 3C, these same rats were re-tested following infusion of a higher concentration of insulin (60 μM) that has previously been shown to alter instrumental responding for sucrose pellets [22, 23]. In addition, to further examine potential effects on Pavlovian motivation, rats underwent extinction-reinstatement testing immediately followed by assessment of home cage food intake. Prior to extinction-reinstatement testing, all rats were given a single Pavlovian conditioning session. Magazine entry rates during this “reminder” session were comparable to post-surgical behavior with greater entry rates during the CS vs. ITI (data not shown).
During extinction-reinstatement testing, the CS was presented alone (5 times), followed by 5 CS-US pairings, and an additional 5 presentations of the CS alone (Fig. 4A). Consistent with results above, insulin infusion did not alter behavior during any phase of extinction-reinstatement testing (Fig. 4A; two-way RM ANOVA, no main effect of treatment: F(1, 11) = 0.12, p = 0.74; no trial x treatment interaction F(14, 154) = 0.53, p = 0.91). However, reintroduction of CS-US pairings did enhance responding during the CS vs ITI, as expected (Fig. 4A; two-way REML ANOVA Trial 6–10: main effect of trial F(1.2,47.09) = 7.71, p < 0.002; main effect of period: F(1,24) = 18.75, p < 0.002; significant trial x period interaction F(4,96) = 3.47, p < 0.01), and was followed by rapid extinction when the CS was presented alone (Fig. 4A: two-way RELM ANOVA, Trial 11–15: main effect of trial: F(1.65,39.7) = 13.66, p < 0.0001, significant trial by period interaction: F(4,96) = 5.51, p < 0.0005).
Fig. 4.
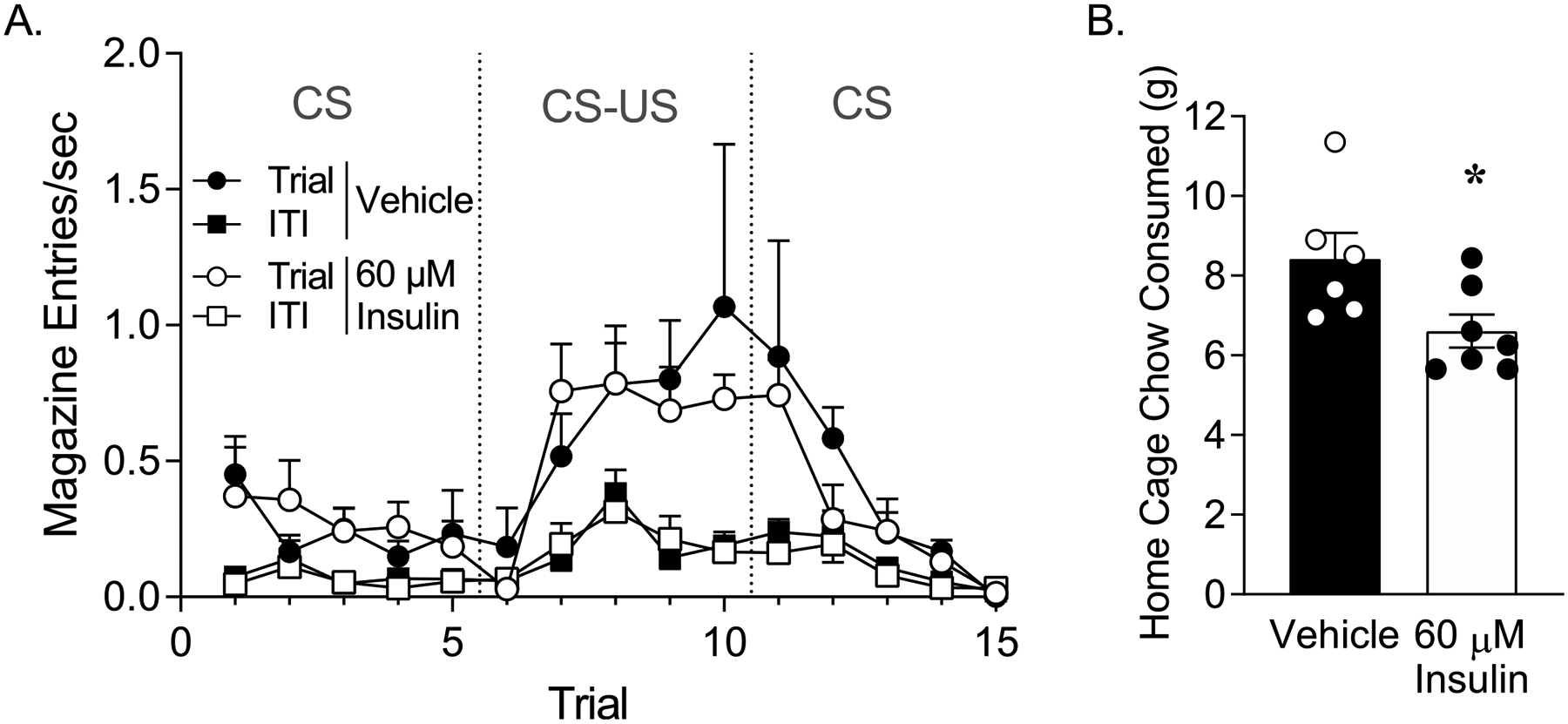
Infusion of 60 μM insulin did not alter extinction-reinstatement behavior but did reduce chow intake. (A) Extinction-reinstatement testing: Rate of magazine entries during presentation of the CS alone (Trials 1–5 and 11–15), and CS-US pairings (Trials 6–10) in vehicle (closed symbols) and insulin (open symbols) treated groups. Magazine entries during CS or CS-US presentations are shown by closed and open circles (Trial) and responses during the inter-trial-interval (ITI) are shown by closed and open squares. (B) Chow consumed in the home cage 1 hour immediately following the completion of extinction-reinstatement testing. (*p < 0.05).
While extinction-reinstatement was unaffected by 60 μM insulin infusion, home cage chow intake measured in these same rats immediately following the completion of extinction-reinstatement testing was significantly reduced in insulin vs. vehicle groups (Fig. 4B; unpaired two-tailed t-test, t(11) = 2.39, p < 0.05). Taken with results from Experiment 1, these data indicate that intra-NAc insulin infusion does not alter conditioned approach or extinction-reinstatement, but does reduce food intake.
3.3. Experiment 2b: Effects of intra-NAc insulin on break point and home cage chow intake
Given the results on home cage chow intake above, we next determined whether intra-NAc insulin would reduce motivation to work for food pellets. The same rats from Experiment 2a were trained to lever press to receive food pellets (Fig. 5A). All but one rat reached training criteria by the 3rd training session. After training, the effect of 60 μM insulin on the willingness to work for the food pellets was tested using a PR schedule. Infusion of insulin into the NAc significantly reduced break point (Fig. 5B; break point: unpaired two-tailed t-test, t(10) = 3.10, p < 0.01). Accordingly, the total number of lever presses were also reduced in insulin vs. vehicle groups (average ± SEM: vehicle 190 ± 21.84; 60 μM insulin 82.5 ± 21.26; unpaired two-tailed t-test, t(10) = 3.53, p < 0.01). Consistent with results in Experiment 2A, chow intake in the home cage measured immediately following PR testing was also reduced by intra-NAc insulin (Fig. 5C; unpaired two-tailed t-test, t(10) = 2.08, p = 0.06). It is important to note that home cage chow intake following the first test in these rats was higher than what was observed after their second test (Vehicle test 1: 8.417 ± 0.66, Vehicle test 2: 3.83 ± 0.31). This is likely due to the consumption of pellets during the PR testing vs. only 5 pellets during the extinction-reinstatement test. However, the magnitude of insulin-induced reductions in home cage chow intake was comparable between these two tests (22% reduction compared to vehicle in test 1, and 35% reduction compared to vehicle in test 2).
Fig. 5.
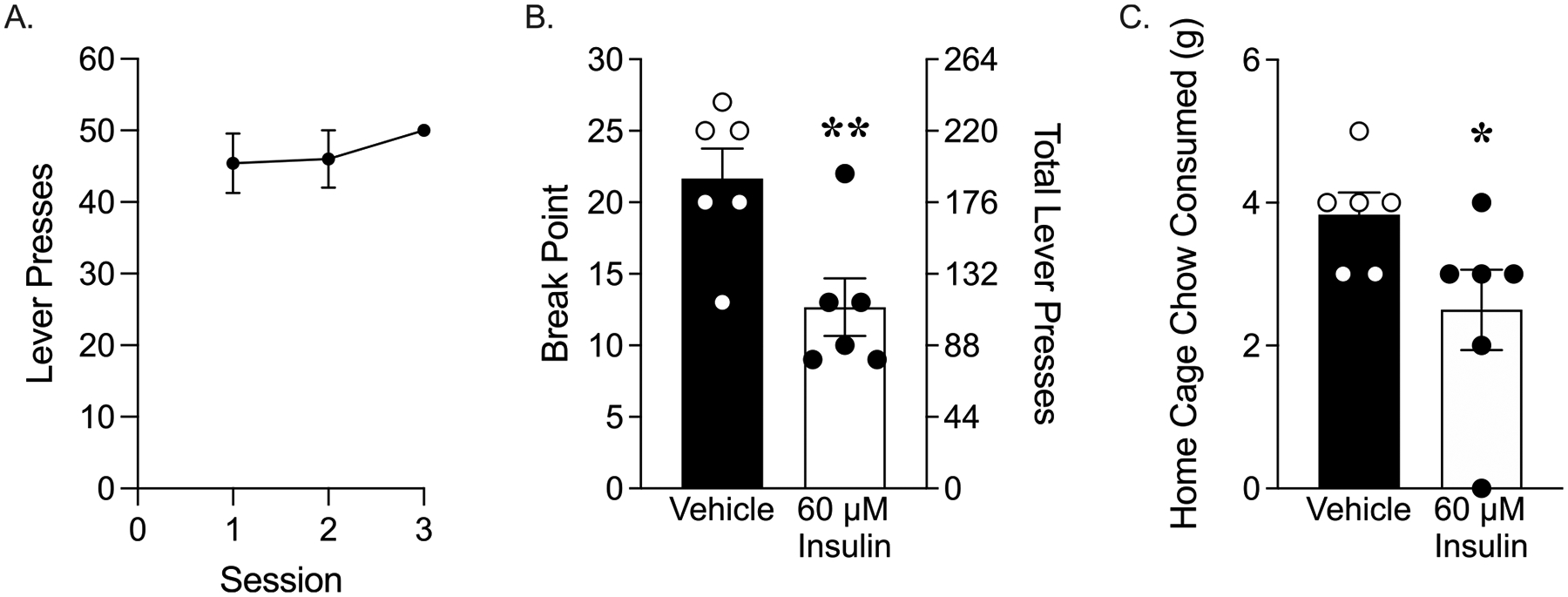
Intra-NAc infusion of 60 μM insulin reduced motivation for pellets and reduced home cage chow intake. (A) Acquisition: Number of lever presses during each of the three FR1 training sessions. (B) Progressive ratio testing: break point and total lever presses in vehicle and insulin-treated groups. (C) Chow consumed in the home cage for 1 hour immediately following the completion of PR testing. (*p = 0.06, **p < 0.01).
3.4. Experiment 3: Effect of NAc insulin with and without insulin receptor blockade on break point
Data above show that intra-NAc insulin reduces motivation to work for flavored food pellets, and reduces home cage chow intake. Next, we determined whether effects of insulin on break point require insulin receptor activation. Therefore, PR testing was repeated in a separate cohort of rats following insulin alone or co-infusion of insulin with the insulin receptor blocker HNMPA-(AM)3. Controls received HNMPA-(AM)3 alone or vehicle. Rats readily learned to discriminate between the active and inactive levers during both FR1 (Fig. 6A; sessions 1–3: two-way RM ANOVA, main effect of lever F(1, 24) = 1872, p < 0.0001; no main effect of session F(2, 48) = 0.65, p = 0.53, session x lever interaction F(2, 48) = 14.24, p < 0.0001) and FR3 training (Fig. 6A; sessions 3–6: two-way RM ANOVA, main effect of lever F(1, 24) = 27,936, p < 0.0001; main effect of session F(2, 48) = 9.42, p < 0.001; session x lever interaction F(2, 48) = 22.53, p < 0.0001). In addition, no differences in pre- vs. post-operative behavior were found (Fig. 6A, B).
Fig. 6.
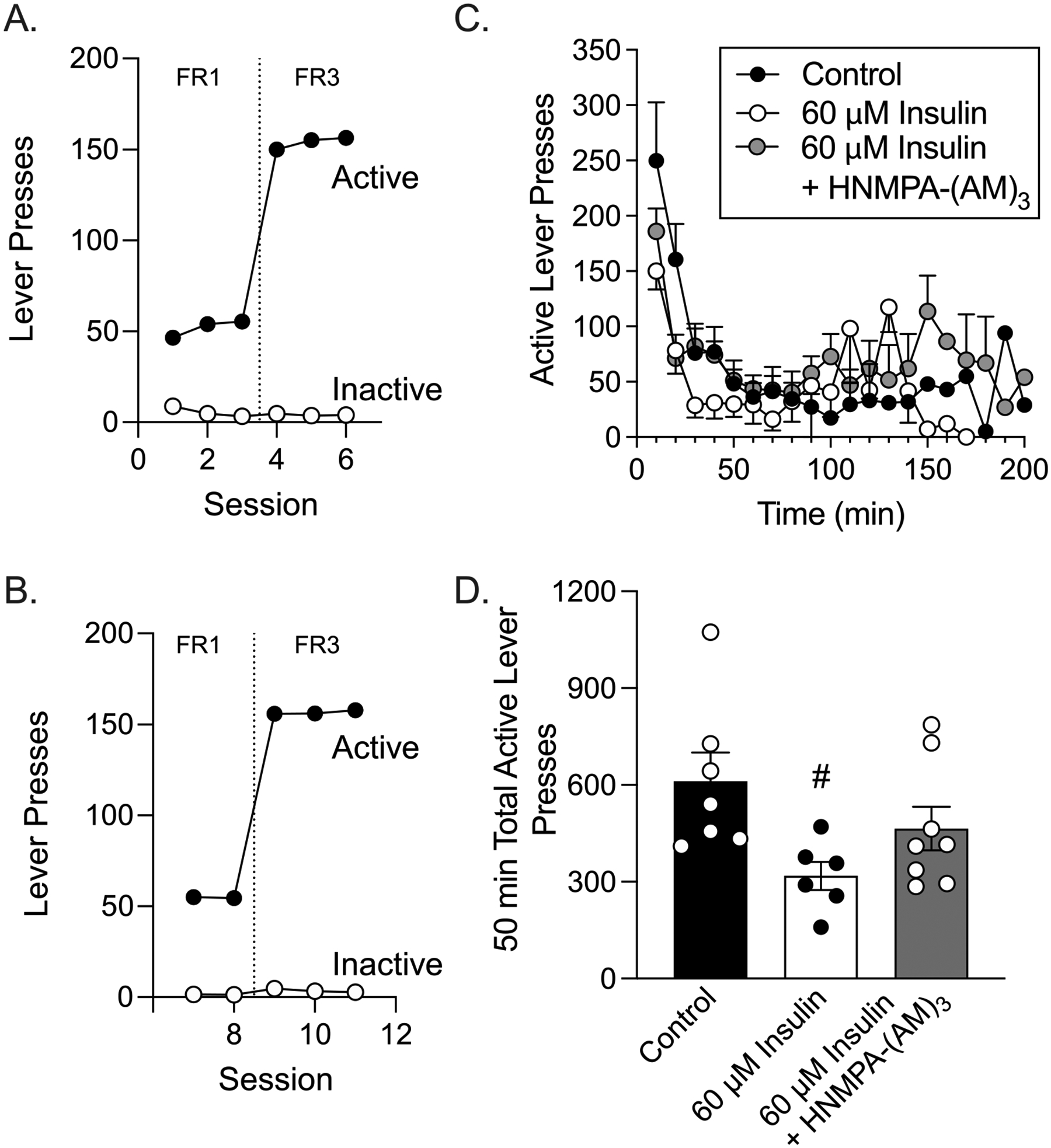
Co-infusion of 60 μM insulin with HNMPA-(AM)3 partially blocks instrumental responding during the first 50 min of PR testing. (A) Number of active and inactive lever presses across pre-surgical FR1 and FR3 training sessions. (B) Number of active and inactive lever presses across post-surgical FR1 and FR3 training sessions. (C) Number of active lever presses occurring in 10-minute bins across the PR testing. (D) Total number of active lever presses made within the first 50 min of PR testing (#p < 0.05 vs. control).
During testing, vehicle and HNMPA-(AM)3 only control rats exhibited similar break points (unpaired two-tailed t-test: t(6) = 0.80, p = 0.23), instrumental responding (two-way RM ANOVA; main effect of treatment: F(1, 6) = 0.62, p = 0.46), completion time (unpaired two-tailed t-test: t(6) = 1.10, p = 0.16), and number of US presentations (unpaired two-tailed t-test: t(6) = 0.59, p = 0.29). As such, data from these groups were collapsed into a single control group. Because it is unknown how long insulin and HNMPA-(AM)3 remain active in the brain following infusion, we first assessed active lever responding in 10 min bins across the PR test session (Fig. 6C). Behavior across the first ~50 min of testing showed a gradual decline in responding across all groups, and stable variance across time. However, behavior in both experimental groups became increasingly erratic beginning ~ 80 min after the start of the test, and responding began to increase alongside this increased variance. This pattern suggests that drug effects may be waning. Therefore, we limited our analysis to the first 50 min of the test session, before the onset of erratic behavior and just before the first rat reached its break point. Examination of responses during this 50 min period showed that infusion of insulin into the NAc blunted active lever pressing compared to controls and that co-infusion of HNMPA-(AM)3 partially blocked this effect (Fig. 6C; two-way REML ANOVA, main effect of treatment: F(2, 18) = 3.90, p < 0.05; treatment x time interaction: F(8, 72) = 1.37, p = 0.23). A similar pattern was present when total active lever presses were examined (Fig. 6D: one-way ANOVA, F(2, 18) = 3.90, p < 0.05, Sidak’s post-test control vs. 60 μM insulin p < 0.05, control vs. 60 μM insulin + HNMPA-(AM)3 p = 0.39). Importantly, inactive lever presses remained consistently low across the PR test (two-way RM ANOVA, no main effect of time: F(20, 230) = 1.14, p = 0.31; data not shown) and did not differ between groups (Avg ± SEM: Control 23.63 ± 3.34, Insulin 28.00 ± 11.53, Insulin & HNMPA-(AM)3 41.71 ± 11.08; one-way ANOVA, F(2, 20) = 0.99, p = 0.39; data not shown).
3.5. Experiment 4: Effects of repeated intra-NAc insulin on devaluation of food
Results from Experiment 2 and 3 demonstrate that intra-NAc insulin reduces willingness to work for food and free food intake. However, it’s possible that this could result from devaluation of the food by the induction of sickness or malaise following insulin infusion. To address this possibility, we assessed the effects of repeated insulin infusion on food devaluation in a separate cohort of rats. During the baseline free choice test, rats consumed similar amounts of banana vs. unflavored food pellets (Fig. 7A; open bars). Next, rats underwent instrumental training where they could earn banana pellets by responding on one lever, and unflavored pellets by responding on another lever (see methods for details). All rats readily acquired the task (data not shown).
Fig. 7.
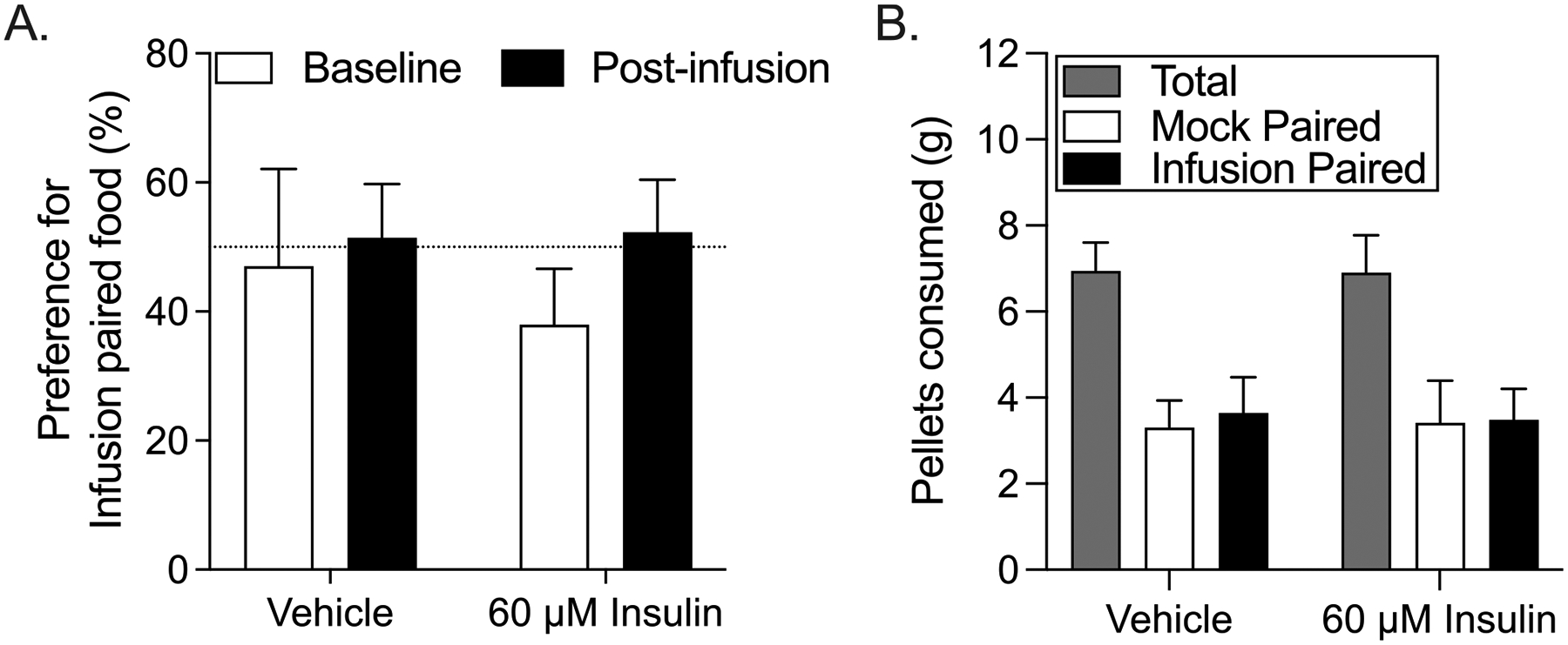
Repeated intra-NAc insulin infusion does not alter the preference for food paired with insulin. (A) Percent preference for infusion paired food in vehicle and insulin groups at baseline (pre-infusion) and post-infusion. (B) Grams of food consumed (total, mock-paired, and infusion-paired) during the post-infusion preference test for control and insulin groups.
Rats were counterbalanced into vehicle and insulin treatment groups based on their body weights and free consumption of banana vs. unflavored pellets. Despite our counterbalancing (including the type of food paired with mock infusion), active lever pressing was greater during the first mock session in the insulin vs. vehicle group (unpaired two-tailed t-test, t(12) = 2.12, p = 0.055; vehicle mock: 593.2 ± 111.2, insulin mock: 816.0 ± 38.31, data not shown). Although this difference was no longer present during the second or third mock infusion session, we hesitate to make comparisons of behavior during the treatment sessions. However, the purpose was to pair infusion of insulin, vehicle, or mock with one type of food pellet, not to examine effects on instrumental responding per se. Importantly, consumption of each pellet type was similar across all groups and conditions.
After three sessions of mock, vehicle, or insulin infusion pellet preference was reassessed. Within subjects comparisons to pre-infusion baseline showed no effect of infusion on preference for the mock vs. infusion paired food (Fig. 7A: two-way RM ANOVA, no main effect of group: F(1,12) = 0.11, p = 0.75; no main effect of time: F(1,12) = 3.30, p = 0.20; no treatment x time interaction: F(1,12) = 0.52, p = 0.48). Additionally, between subjects comparisons showed no differences in food consumption between control and insulin-treated groups during the post-infusion preference test (Fig. 7B). Thus, intra-NAc insulin did not result in devaluation.
4. Discussion
Although insulin reduces food intake, rather little is known about its broader effects on feeding behavior and the majority of studies have focused on its actions in hypothalamic regions. Here we show that direct infusion of insulin into NAc does not alter the expression, extinction, or reinstatement of conditioned approach. However, intra-NAc insulin did reduce free chow intake in the home cage immediately following conditioned approach testing. Consistent with this, the willingness to work for flavored food pellets was also reduced following intra-NAc insulin. Furthermore, insulin-induced reductions in motivation were partially driven by activity of the insulin receptor. Reductions in food intake and break point do not appear to be related to potential feelings of sickness or malaise, as we found no evidence for devaluation of food paired with insulin infusion. Taken together, these data are the first to demonstrate that intra-NAc insulin influences the motivation for food and food intake, without altering conditioned motivational responses to food cues.
The observed dissociation between the ability of insulin to regulate food intake but not cue-triggered food-seeking has important implications for the regulation of feeding behavior. Insulin is often thought of as a post-prandial satiety signal that conveys information about energy intake, ultimately resulting in reduced food intake [6, 20]. Indeed, effects on food intake and motivation observed here are consistent with this (discussed further below). However, stimuli in the environment that signal food availability can trigger food-seeking even in sated animals [15, 52]. Here we modeled cue-triggered food-seeking using a simplified Pavlovian procedure in which the offset of a discrete tone was immediately followed by pellet delivery. When tested in the absence of food, rats readily approached the food cup in anticipation of the pellets appearing (conditioned approach). Across several different concentrations, the magnitude of this behavior was unaffected by intra-NAc insulin, even though home cage chow intake immediately following this same test session was reduced. Thus, null effects on conditioned approach are not due to poor infusion or an ineffective concentration of insulin in the target tissue. In addition, extinction-reinstatement data demonstrate that approach behavior is contingent upon CS-US pairings, and that intra-NAc insulin does not disrupt extinction or reinstatement processes. Thus, while insulin influenced feeding, it did not alter cue-triggered food-seeking. This may contribute to the ability of food-cues to override the homeostatic regulation of feeding.
In three separate tests, we found that home cage chow intake was reduced by intra-NAc insulin. In addition, in two separate cohorts, insulin reduced the motivation to work for flavored food pellets (a 50/50 mix of sucrose pellets and unflavored pellets). This is a rather powerful demonstration of insulin’s anorectic effects within the NAc, given that this was the first opportunity to eat following the 15-hour fast. The NAc receives gustatory and visceral information from the brainstem, gustatory cortex, and amygdala andinteracts with the broader basal ganglia networks, the ventral tegmental area, and lateral hypothalamus to discretely modulate motor and visceral output and provide information about energy balance [34]. NAc control of food intake is generally thought to be restricted to foods that are calorically dense such as those high in fats and sugars [35, 50, 65]. These data and others have led to the overall suggestion that the NAc is critical in driving opportunistic or “hedonic” feeding [43], while the hypothalamus, which exerts global control of intake regardless of caloric content or palatability [4], is critical for the homeostatic regulation of food intake [4, 43, 57]. We found reductions in chow intake and in motivation for sweet pellets, suggesting that effects of NAc insulin may not be specific to food type. However, additional studies are needed to draw more firm conclusions.
We used a progressive ratio procedure to evaluate the effects of intra-NAc insulin on motivation to obtain food. In the first study, insulin reduced break point and subsequent home cage food intake (Fig. 5). Importantly, repeated insulin infusion did not result in the devaluation of insulin-paired food (Experiment 4, Fig. 7). Thus, effects of insulin on instrumental responding during PR testing are not likely due to malaise or aversion to the insulin-paired food. The absence of insulin-induced devaluation is also consistent with a role for NAc insulin receptor activation in flavor-nutrient learning [58, 63].
We also evaluated the ability of insulin-receptor blockade to prevent insulin-induced reductions in instrumental responding for food in a separate cohort of rats. Examination of the time course of active lever pressing showed that the variance in behavior was low and stable across the initial 50 min of testing, but increased in the latter portion of the session, particularly in rats that received a co-infusion of insulin and the insulin receptor blocker HNMPA-(AM)3. This pattern strongly suggests that the pharmacological action of HNMPA-(AM)3 may subside more quickly than that of insulin, and/or that the drug may be cleared faster than insulin. Given this, we focused our analysis on behavior during the first 50 min of the session. We found that active lever responding was reduced by intra-NAc insulin, and that this effect was partially blocked by co-infusion of HNMPA-(AM)3. It’s possible that this partial blockade was the result of competing effects of insulin. Insulin can also activate insulin like growth factor receptors (IGFRs) and IGFR-induced reductions of food intake have been demonstrated in chicks [27], female rats [61], and diabetic rats [42]. Thus, activation of IGFRs within the NAc may also contribute to effects observed here. Alternatively, this partial blockade could be due incomplete inhibition of insulin receptors by the concentration of HNMPA-(AM)3 used. However, this same concentration of HNMPA-(AM)3 completely prevented effects of insulin on NAc glutamate and dopamine in previous studies [21, 46]. Nonetheless, results here support the idea that NAc insulin receptor activation accounts for at least part of the observed reduction in motivation.
Only two previous studies have examined the effect of insulin on instrumental responding for sucrose (i.e. sucrose self-administration). One found that intraventricular infusion of the same concentration of insulin used here (60 μM) reduced lever pressing for sucrose pellets [23]. A follow-up study from this same group found that infusion of insulin into the NAc shell modestly increased sucrose self-administration compared to controls [22]. As mentioned in the introduction, NAc core and shell sub-regions have distinct roles in feeding, with the NAc shell predominantly influencing food intake, and the NAc core influencing food-seeking and instrumental responding for food [33, 34]. This stems in part from classic studies showing that reducing activity in the NAc shell (by enhancing inhibition or blocking excitation) increases food intake [36, 53]. In contrast, these same manipulations in NAc core did not affect free food intake [36]. However, inhibiting excitation in the NAc core via NMDA receptor blockade reduced food self-administration, while this same manipulation in the NAc shell had no effect [17]. Thus, manipulations of the NAc core generally influence food self-administration, but not free food intake, whereas manipulations of the NAc shell show the opposite pattern. In regard to cue-triggered food-seeking, manipulations of either the core or the shell can affect these behaviors [51], although there is evidence that these effects are more pronounced in the core [3, 13, 62]. Cannula here were aimed at the NAc core. Placements based on histology show that while the majority of infusion sites fell within the core, some fell on the core/shell border and a few within the shell. While it is difficult to estimate infusion spread, the rather extensive literature described briefly above suggests that behavioral effects of insulin on instrumental responding could be primarily mediated by the core, while effects on food intake could rely on the shell. This warrants further investigation.
Another important consideration is the concentration of insulin administered. In the current study, infusion of 60 μM insulin produced the strongest and most reliable effects on food intake and food self-administration. This is comparable to the concentration used in previous studies of insulin administered directly into the hypothalamus, NAc shell or intraventricularly [2, 10, 14, 22, 23, 47]. It is also within the range used in recent ex vivo studies examining the effects of insulin on dopamine and glutamate transmission (for review see [20]). While these concentrations are likely outside the physiological range, antagonist studies (here and prior research) confirm that effects are specific to insulin receptor activation.
Results here also have important implications for obesity and Type II diabetes that are characterized by insulin resistance, enhanced food intake and food craving [9, 16, 26]. While the current study does not directly address obesity or diabetes, diet-induced obesity reduces NAc insulin receptor expression and blunts the ability of insulin to influence NAc neurotransmission [21, 58].Thus, obesity may result in a reduction in insulin’s ability to influence food intake via actions in the NAc, although this requires direct testing.
In the current studies, all rats were maintained on a modified fixed feeding schedule to ensure that they had not eaten prior to behavioral training or testing, and to facilitate comparison to the existing literature. Acute food deprivation is known to alter several hormones (e.g., ghrelin, insulin, glucagon) [1, 32], and can induce a Hypothalamic-Pituitary-Adrenal (HPA) axis mediated stress response (see [64]). However, no similar studies have examined potential effects of prolonged modified feeding schedules, like the one used here, on HPA mediated hormonal responses. Thus, it’s possible that behavioral effects of insulin may differ in ad lib fed animals or in animals fasted for shorter periods of time prior to testing. This is an important consideration that should be systematically evaluated in the future.
In sum, studies here demonstrate for the first time that intra-NAc insulin regulates distinct aspects of feeding behavior: reducing the motivation to work for food and food intake without altering conditioned motivational responses to food cues. Importantly, effects on the motivation to work for food were partially driven by insulin receptor activation and were not due to the induction of sickness or malaise. Moreover, the specificity of insulin-induced behavioral effects adds to the existing literature showing a dissociation of processes that underlie cue-triggered urges to seek out food vs. motivation to consume food itself [15].
Acknowledgements
We thank Nathan Chan for their assistance with animal maintenance and data collection for the devaluation study, and Dr. Kent C. Berridge for his thoughtful comments about the manuscript. This work was supported by NIHNIDDK R01-DK106188 and R01-DK115526 to CRF; and T32-DK101357 to JEF.
Footnotes
Declaration of Competing Interest
The authors have nothing to disclose.
Data Availability
Data will be made available on request.
References
- [1].Aguilar-Parada E, Eisentraut AM, Unger RH, Effects of starvation on plasma pancreatic glucagon in normal man, Diabetes 18 (1969) 717–723. [DOI] [PubMed] [Google Scholar]
- [2].Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC, Acute third ventricular administration of insulin decreases food intake in two paradigms, Pharmacol. Biochem. Behav 72 (2002) 423–429. [DOI] [PubMed] [Google Scholar]
- [3].Ambroggi F, Ghazizadeh A, Nicola SM, Fields HL, Roles of nucleus accumbens core and shell in incentive-cue responding and behavioral inhibition, J. Neurosci 31 (2011) 6820–6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Anand BK, Brobeck JR, Localization of a “feeding center” in the hypothalamus of the rat, Proc. Soc. Exp. Biol. Med 77 (1951) 323–324. [DOI] [PubMed] [Google Scholar]
- [5].Banks WA, Kastin AJ, Differential permeability of the blood–brain barrier to two pancreatic peptides: insulin and amylin, Peptides 19 (1998) 883–889. [DOI] [PubMed] [Google Scholar]
- [6].Banks WA, Owen JB, Erickson MA, Insulin in the brain: there and back again, Pharmacol. Ther 136 (2012) 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Basso AM, Kelley AE, Feeding induced by GABAA receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference, Behav. Neurosci 113 (1999) 324–336. [DOI] [PubMed] [Google Scholar]
- [8].Belsham DD, Dalvi PS, Insulin signalling in hypothalamic neurones, J. Neuroendocrinol 33 (2021) e12919. [DOI] [PubMed] [Google Scholar]
- [9].Boswell RG, Kober H, Food cue reactivity and craving predict eating and weight gain: a meta-analytic review, Obesity Rev. 17 (2016) 159–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brief DJ, Davis JD, Reduction of food intake and body weight by chronic intraventricular insulin infusion, Brain Res. Bull 12 (1984) 571–575. [DOI] [PubMed] [Google Scholar]
- [11].Carlson HN, Murphy C, Pratt WE, Shifting motivational states: the effects of nucleus accumbens dopamine and opioid receptor activation on a modified effort-based choice task, Behav. Brain Res 399 (2021), 112999. [DOI] [PubMed] [Google Scholar]
- [12].Castro DC, Cole SL, Berridge KC, Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry, Front. Syst. Neurosci 9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chaudhri N, Sahuque LL, Schairer WW, Janak PH, Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking, Neuropsychopharmacology 35 (2010) 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE, Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet, Am. J. Physiol.-Regulatory, Integrative and Comparative Physiol 288 (2005) R981–R986. [DOI] [PubMed] [Google Scholar]
- [15].Derman RC, Ferrario CR, Junk-food enhances conditioned food cup approach to a previously established food cue, but does not alter cue potentiated feeding; implications for the effects of palatable diets on incentive motivation, Physiol. Behav 192 (2018) 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Drummen M, Dorenbos E, Vreugdenhil ACE, Raben A, Westerterp-Plantenga MS, Adam Tanja C, Insulin resistance, weight, and behavioral variables as determinants of brain reactivity to food cues: a prevention of diabetes through lifestyle intervention and population studies in europe and around the world – a preview study, Am. J. Clin. Nutr 109 (2018) 315–321. [DOI] [PubMed] [Google Scholar]
- [17].D’Souza MS, Markou A, Differential role of N-methyl-D-aspartate receptor-mediated glutamate transmission in the nucleus accumbens shell and core in nicotine seeking in rats, Eur. J. Neurosci 39 (2014) 1314–1322. [DOI] [PubMed] [Google Scholar]
- [18].Ferrario CR, Laboùebe G, Liu S, Nieh EH, Routh VH, Xu S, O’Connor EC, Homeostasis meets motivation in the battle to control food intake, J. Neurosci 36 (2016) 11469–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME, The role of glutamate receptor redistribution in locomotor sensitization to cocaine, Neuropsychopharmacology 35 (2010) 818–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ferrario CR, Reagan LP, Insulin-mediated synaptic plasticity in the CNS: anatomical, functional and temporal contexts, Neuropharmacology 136 (2018) 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fetterly TL, Oginsky MF, Nieto AM, Alonso-Caraballo Y, Santana-Rodriguez Z, Ferrario CR, Insulin bi-directionally alters NAc glutamatergic transmission; interactions between insulin receptor activation, endogenous opioids, and glutamate release, J. Neurosci (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ, Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats, Am. J. Physiol. Regulatory, Integrative and Comparative Physiol 295 (2008) R388–R394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW, Intraventricular insulin and leptin decrease sucrose self-administration in rats, Physiol. Behav 89 (2006) 611–616. [DOI] [PubMed] [Google Scholar]
- [24].Finnell JE, Lombard CM, Padi AR, Moffitt CM, Wilson LB, Wood CS, Wood SK, Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences, PLoS ONE 12 (2017), e0172868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Floresco SB, McLaughlin RJ, Haluk DM, Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior, Neuroscience 154 (2008) 877–884. [DOI] [PubMed] [Google Scholar]
- [26].Fordahl SC, Jones SR, High-fat-diet-induced deficits in dopamine terminal function are reversed by restoring insulin signaling, ACS Chem. Neurosci 8 (2017) 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fujita S, Honda K, Hiramoto D, Gyu M, Okuda M, Nakayama S, Yamaguchi M, Saneyasu T, Kamisoyama H, Central and peripheral administrations of insulin-like growth factor-1 suppress food intake in chicks, Physiol. Behav 179 (2017) 308–312. [DOI] [PubMed] [Google Scholar]
- [28].Havrankova J, Roth J, Brownstein M, Insulin receptors are widely distributed in the central nervous system of the rat, Nature 272 (1978) 827–829. [DOI] [PubMed] [Google Scholar]
- [29].Honda K, Kewan A, Osada H, Saneyasu T, Kamisoyama H, Central administration of insulin-like growth factor-2 suppresses food intake in chicks, Neurosci. Lett 751 (2021) 5. [DOI] [PubMed] [Google Scholar]
- [30].Jones KT, Woods C, Zhen J, Antonio T, Carr KD, Reith ME, Effects of diet and insulin on dopamine transporter activity and expression in rat caudate-putamen, nucleus accumbens, and midbrain, J. Neurochem 140 (2017) 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kalyanasundar B, Perez CI, Arroyo B, Moreno MG, Gutierrez R, The appetite suppressant D-norpseudoephedrine (Cathine) acts via D1/D2-like dopamine receptors in the nucleus accumbens shell, Front. Neurosci 14 (2020) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Karami KJ, Coppola J, Krishnamurthy K, Llanos DJ, Mukherjee A, Venkatachalam KV, Effect of food deprivation and hormones of glucose homeostasis on the acetyl CoA carboxylase activity in mouse brain: a potential role of acc in the regulation of energy balance, Nutr. Metab. (Lond) 3 (2006) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kelley AE, Neural integrative activities of nucleus accumbens subregions in relation to learning and motivation, Psychobiology 27 (1999) 198–213. [Google Scholar]
- [34].Kelley AE, Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning, Neurosci. Biobehav. Rev 27 (2004) 765–776. [DOI] [PubMed] [Google Scholar]
- [35].Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M, Opioid modulation of taste hedonics within the ventral striatum, Physiol. Behav 76 (2002) 365–377. [DOI] [PubMed] [Google Scholar]
- [36].Kelley AE, Swanson CJ, Feeding induced by blockade of AMPA and kainate receptors within the ventral striatum: a microinfusion mapping study, Behav. Brain Res 89 (1997) 107–113. [DOI] [PubMed] [Google Scholar]
- [37].Klok MD, Jakobsdottir S, Drent ML, The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review, Obesity Rev. 8 (2007) 21–34. [DOI] [PubMed] [Google Scholar]
- [38].Ko D, Wanat MJ, Phasic dopamine transmission reflects initiation vigor and exerted effort in an action- and region-specific manner, J. Neurosci 36 (2016) 2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Laboùebe G, Liu S, Dias C, Zou H, Wong JC, Karunakaran S, Clee SM, Phillips AG, Boutrel B, Borgland SL, Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids, Nat. Neurosci 16 (2013) 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lin P, Pratt WE, Inactivation of the nucleus accumbens core or medial shell attenuates reinstatement of sugar-seeking behavior following sugar priming or exposure to food-associated cues, PLoS ONE 9 (2014) e99301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Loh K, Zhang L, Brandon A, Wang Q, Begg D, Qi Y, Fu M, Kulkarni R, Teo J, Baldock P, Brüning JC, Cooney G, Neely G, Herzog H, Insulin controls food intake and energy balance via NPY neurons, Mol. Metab 6 (2017) 574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lu H, Martinez-Nieves B, Lapanowski K, Dunbar J, Intracerebroventricular insulin-like growth factor-1 decreases feeding in diabetic rats, Endocrine 14 (2001) 349–352. [DOI] [PubMed] [Google Scholar]
- [43].Lutter M, Nestler EJ, Homeostatic and hedonic signals interact in the regulation of food intake, J. Nutr 139 (2009) 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Maldonado-Irizarry CS, Swanson CJ, Kelley AE, Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus, The J. Neurosci 15 (1995) 6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mannella F, Gurney K, Baldassarre G, The nucleus accumbens as a nexus between values and goals in goal-directed behavior: a review and a new hypothesis, Front. Behav. Neurosci 7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mebel DM, Wong JC, Dong YJ, Borgland SL, Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake, Eur. J. Neurosci 36 (2012) 2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Meńendez J, Atrens DM, Insulin and the paraventricular hypothalamus: modulation of energy balance, Brain Res. 555 (1991) 193–201. [DOI] [PubMed] [Google Scholar]
- [48].Patel JC, Stouffer MA, Mancini M, Nicholson C, Carr KD, Rice ME, Interactions between insulin and diet on striatal dopamine uptake kinetics in rodent brain slices, Eur. J. Neurosci 49 (2019) 794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates, 6 Edition. London: Academic Press. [Google Scholar]
- [50].Peciña S, Berridge KC, Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes, Brain Res. 863 (2000) 71–86. [DOI] [PubMed] [Google Scholar]
- [51].Peciña S, Berridge KC, Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered ‘wanting’ for reward: entire core and medial shell mapped as substrates for PIT enhancement, Eur. J. Neurosci 37 (2013) 1529–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Petrovich GD, Gallagher M, Control of food consumption by learned cues: a forebrain-hypothalamic network, Physiol. Behav 91 (2007) 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Reynolds SM, Berridge KC, Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of gaba-elicited defensive behavior versus eating behavior, The J. Neurosci 21 (2001) 3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Richardson NR, Roberts DC, Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy, J. Neurosci. Methods 66 (1996) 1–11. [DOI] [PubMed] [Google Scholar]
- [55].Sallam NA, Borgland SL, Insulin and endocannabinoids in the mesolimbic system, J. Neuroendocrinol 33 (2021) 13. [DOI] [PubMed] [Google Scholar]
- [56].Schumacher R, Mosthaf L, Schlessinger J, Brandenburg D, Ullrich A, Insulin and insulin-like growth factor-1 binding specificity is determined by distinct regions of their cognate receptors, J. Biol. Chem 266 (1991) 19288–19295. [PubMed] [Google Scholar]
- [57].Stellar E, The physiology of motivation, Psychol. Rev 61 (1954) 5–22. [DOI] [PubMed] [Google Scholar]
- [58].Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, Machold RP, Jones KT, de Vaca SC, Reith ME, Carr KD, Rice ME, Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward, Nat. Commun 6 (2015) 8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Stratford TR, Kelley AE, GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior, The J. Neurosci 17 (1997) 4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Timper K, Brüning JC, Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity, Dis. Model Mech 10 (2017) 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Todd BJ, Fraley GS, Peck AC, Schwartz GJ, Etgen AM, Central Insulin-like growth factor 1 receptors play distinct roles in the control of reproduction, food intake, and body weight in female rats1, Biol. Reprod 77 (2007) 492–503. [DOI] [PubMed] [Google Scholar]
- [62].Valyear MD, Glovaci I, Zaari A, Lahlou S, Trujillo-Pisanty I, Andrew Chapman C, Chaudhri N, Dissociable mesolimbic dopamine circuits control responding triggered by alcohol-predictive discrete cues and contexts, Nat. Commun 11 (2020) 3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Woods CA, Guttman ZR, Huang D, Kolaric RA, Rabinowitsch AI, Jones KT, Cabeza de Vaca S, Sclafani A, Carr KD, Insulin receptor activation in the nucleus accumbens reflects nutritive value of a recently ingested meal, Physiol. Behav 159 (2016) 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Yau YHC, Potenza MN, Stress and eating behaviors, Minerva Endocrinol. 38 (2013) 255–267. [PMC free article] [PubMed] [Google Scholar]
- [65].Zhang M, Kelley AE, Enhanced intake of high-fat food following striatal muopioid stimulation: microinjection mapping and fos expression, Neuroscience 99 (2000) 267–277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


