Abstract
Applications of nanotechnology have increased the importance of research and nanocarriers, which have revolutionized the method of drug delivery to treat several diseases, including cancer, in the past few years. Cancer, one of the world’s fatal diseases, has drawn scientists’ attention for its multidrug resistance to various chemotherapeutic drugs. To minimize the side effects of chemotherapeutic agents on healthy cells and to develop technological advancement in drug delivery systems, scientists have developed an alternative approach to delivering chemotherapeutic drugs at the targeted site by integrating it inside the nanocarriers like synthetic polymers, nanotubes, micelles, dendrimers, magnetic nanoparticles, quantum dots (QDs), lipid nanoparticles, nano-biopolymeric substances, etc., which has shown promising results in both preclinical and clinical trials of cancer management. Besides that, nanocarriers, especially biopolymeric nanoparticles, have received much attention from researchers due to their cost-effectiveness, biodegradability, treatment efficacy, and ability to target drug delivery by crossing the blood–brain barrier. This review emphasizes the fabrication processes, the therapeutic and theragnostic applications, and the importance of different biopolymeric nanocarriers in targeting cancer both in vitro and in vivo, which conclude with the challenges and opportunities of future exploration using biopolymeric nanocarriers in onco-therapy with improved availability and reduced toxicity.
Keywords: Theranostic, Nanomedicines, Antitumor, Drug Delivery, Nanotherapeutics
1. Introduction
Cancer is one of the leading causes of mortality worldwide, accounting for nearly 10 million deaths until 2020. Furthermore, World Health Organization (WHO) has predicted that it will rise by up to three times by 2040.1−3 Due to lifestyle changes, around 70% of these fatalities occur in low- and middle-income nations. Standard treatment methods for cancer include chemotherapy, surgery, radiation, immunotherapy, and hormone therapy. However, these treatments adversely affect patients.4−6 For example, chemotherapy has several hazardous side effects, such as myelotoxicity, leukopenia, cardiotoxicity, blood vessel constriction, and lack of targeted delivery. To overcome these limitations, researchers are trying to find out the potent anticancer drugs with the fewest side effects to develop a better alternative with lower toxicity.7−11
Nanomedicine has been fashioned as a dimension of significance in pharmaceutical research, particularly in drug delivery systems.12−15 Nanoencapsulation of bioactive compounds is a salient technique for ensuring sustainable bioavailability due to an increase in surface-to-volume ratio through a significant reduction in particle size to a nano level. Biopolymers with dimensions ranging between 10 and 1000 nm can be used as nanoparticles (NPs).16−20 Recently, many researchers have sought the attention of biopolymers due to their biocompatibility, easy design and preparation, surface variations, and interesting biomimetic characteristics.21 A few recent studies through the application of nanomedicines from natural sources encompassing lipid nanocarriers for phenolic drug delivery,19,22−25 polysaccharide nanocarriers26 and composite nanocarriers for better specificity, prolonged circulation, better bioavailability, and enhanced permeation rate (EPR) have been discussed. These polymers have created a unique niche and inefficient ability in drug delivery. However, the possibility of synthetic polymers exhibiting cytotoxicity should not be overlooked. Gopi et al. performed a detailed study on the mechanism and role of effective drug delivery systems.27,28
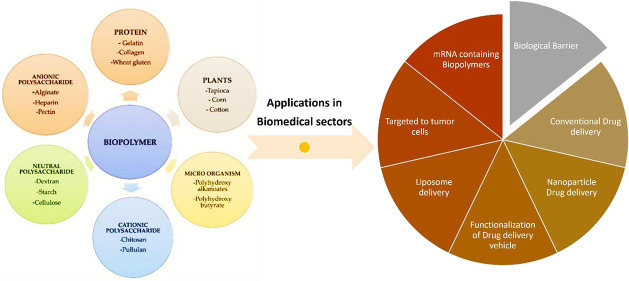
Moreover, the application of polymer conjugates for nanomedicines has been reported in the past decades. The application of nanosystems facilitates the delivery of drug agents in a systematic manner at the targeted site. Calzoni et al. mentioned various kinds of biopolymers used to formulate nanomedicines for controlled release formulations for anticancer therapy, as shown in Figure 1.29−33 Many researchers have also tried to develop a series of polylactic acid (PLA) conjugates through functionalization click chemistry for attaining better results in target binding and enhanced penetration of drugs to the target site.34−37 Recently, therapeutic and diagnostic methods based on a biopolymer have demonstrated great promise for improving cancer treatment. Significant advancements in cancer detection, prevention, and therapy focus on a new field of integrated study in biology, chemistry, engineering, and medicine known as cancer nanotechnology.38−41 Due of their increased efficacy and safety, biopolymers have drawn the attention of scientists in recent years. Recent developments in nanotechnology assisted anticancer medications include Myocet (Perrigo, Dublin, Ireland), DaunoXome (Gilead Sciences, Foster City, CA, USA), Doxil (Johnson & Johnson, New Brunswick, NJ, USA), and Abraxane which have been authorized by the US FDA as a result of these applications (Celgene, Summit, NJ, USA).42−49
Figure 1.
Schematic representation of biopolymers used to overcome the traditional barrier methods from conventional drug delivery methods. EPR enhances tumor-killing activity efficiently.
This review focuses on various techniques of nanodrug design, mechanisms, different biomaterials used, photodynamic therapy, and image analysis in an in vivo model. In addition, this review also explores the possibility of using novel biomaterials for antitumor/anticancer agents and PDT in in vivo models.
2. Characterization and Synthesis of Biopolymeric NPs Used in Drug Delivery Systems (DDSs)
Nanocarriers (NCs) loaded with anticancer drugs have several advantages over free drugs. They protect drugs’ premature degradation and nonspecific interactions, thereby ensuring target-specific killing of tumor cells.50−52 The biocompatible feature of NCs is of prime importance, and this feature makes the NCs more effective in enhancing efficacy and extending the shelf life of the drug in circulation.53 In this review, our focus is on the biopolymers that are currently being used as NC-DDSs. Biopolymers are naturally occurring substances possessing nontoxicity, biocompatibility, and biodegradability properties. To date, only polymers have been researched for NC formulation development.54−57 Polymers can either be synthetic or natural, wherein natural polymers such as chitosan, silk, alginate, albumin, starch, carbohydrate, proteins, and lipid materials may be incorporated for encapsulation without the requirement for chemical modification of the drug.48,58 Several formulations and techniques of the biopolymeric NPs resulted in efficient nanotransporting properties, which exhibited excellent antitumor effects in the past few decades. From Table 2, we can obtain insight into the techniques that depict the effect on particle size for biomedical applications, shown in Figure 2.47,59−61
Table 2. Nanoparticle Formulation Techniques to Understand the Processes That Provide the Desired Particle Size for Biomedical Applications.
| Techniques | Nanoparticles | Dimension | References |
|---|---|---|---|
| Electrospinning | Elastin-like polypeptides | 110–680 nm | (109) |
| Polymerization under nonlinear substructuring | Gelatin core with quantum dot on surface | 30–100 nm | (107) |
| Low energy throughput (mechanical stirring @800 rpm without heat) | PEGylated NP | 174–184 nm | (108) |
| Enzymatic synthesis | Polyglycerol adipate | 136 nm | (70) |
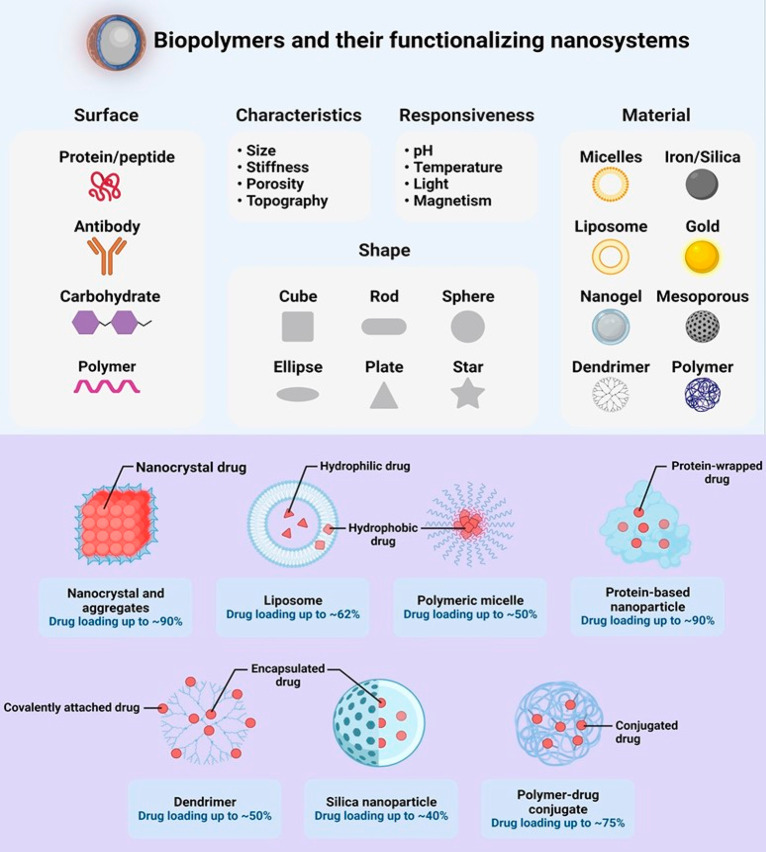
Figure 2.
Graphical representation of different kinds of biopolymers and their working nanosystems.
Lipid-Based Biopolymer
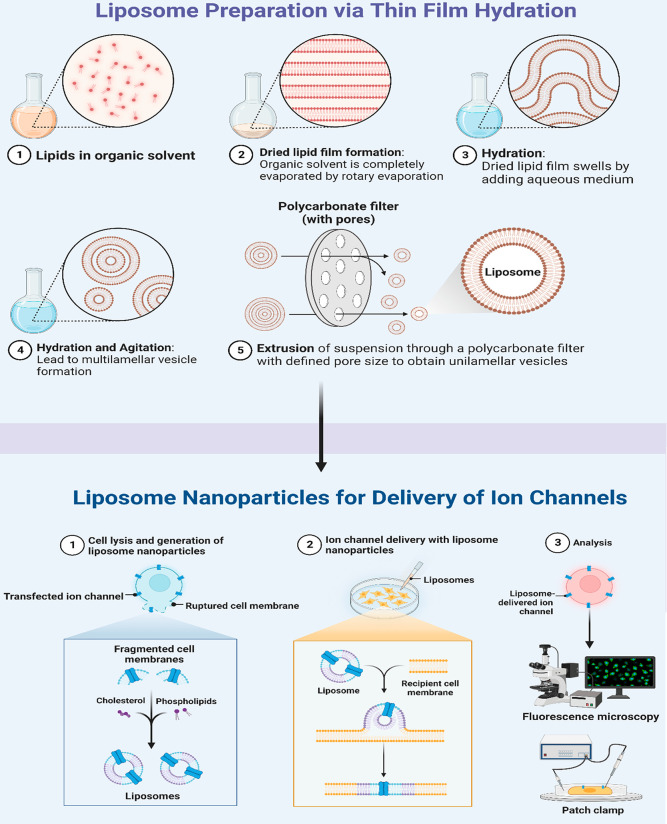
The use of organic polymers has already been practiced in the last few decades.62 Organic polymers, including solid lipid NPs, liposomes, and proteins, have been considered suitable nanocarriers in DDSs.57,63,64 Lipid polymers are commonly applied, possibly due to their efficient capacity to encapsulate both hydrophobic and hydrophilic drugs.65−68 Liposomes are self-assembled concentric lipid bilayers with an aqueous core. Recently, lipid nanoparticles (LPNs) have been used to deliver water-insoluble drugs.69 Weiss et al.70 showed that lipid material such as stearic acid-modified polyglycerol adipate (PGAS) can be used as a promising carrier for drug delivery and did not require surfactant.70 Only coating covalently or non-covalently with N-(2-hydroxypropyl) methacrylamide (HPMA) copolymers was performed.70 These nanoparticles have equivalent particle sizes but sometimes exhibit lower or minus zeta-potentials.70 Double labeling of the NPs was done with the fluorescent dyes DiR in a non-covalent manner and DYOMICS-676 which was bound covalently to a copolymer of HPMA. The bio distribution was investigated noninvasively through multiple-spectra-based optical imaging. Both coatings caused variations in the pharmacokinetics and bio distribution of healthy and cancer-bearing mice. We have already discussed the spray drying technique in the formulation of NPs; nowadays this technique is being modified by electro-hydrodynamic atomization,71 as shown in Figure 3.
Figure 3.
Liposome preparation and nanocarrier production using film hydration and ion channel methods.
Zhen et al. observed a successful gene delivery using crystalline lipids coupled with a photosensitive agent, and this combination has been proven to be an excellent nanotheranostic agent.72 Although lipid NPs have created a domain in nano-DDSs, their delivery and release of drugs at tumor sites are found to be erratic. Several techniques were thus applied to enhance drug delivery, the significant one being the ultrasound mechanism adopted by Nahire et al.73 They observed a 76% targeted drug release when the lipid nanocarrier is in the presence of cytosolic glutathione.73 However, if the nanocarrier is subjected to 3 MHz for 2 min, then the release of the drug was found to increase to 96%. Thus, from the above scheme presented by Nahire et al., it is evident that lipid NPs can be used as an effective DDS and in ultrasound imaging through modification of their environment.73 The current trends to enhance the efficacy of anticancer drugs are remarkable. Despite having favorable characteristics of lipid nanocarriers in therapeutic delivery, they also have several drawbacks such as the absence of quality control and self-assembly formulation inconsistency leading to inhibition of therapeutic efficacy at the target site.74 Thus, to overcome such issues, researchers reported new dimension of LNC synthesis using the electrospray ionization mass spectrometry (EIMS) fragmentation along with matrix assisted laser desorption/ionization (MALDI) and time-of-flight (ToF)-based mass spectrometry.75 A reliable synthesis of pure phospholipid peptide bioconjugates (peptide amphiphiles) was developed.76 This technique avoided unwarranted hydrolyzed byproduct formation that could dampen the therapeutic efficacy of the polymorphic nanotherapeutics.56,77,78
Recent biopolymer approaches in DDSs are being introduced wherein the nanocarriers cause a half burst release before accumulating at the tumor site, thereby causing toxicity due to the circulation of materials.79 Therefore, researchers have shifted focus to stimuli-responsive carriers. Michelle Stollzoff et al. reported a novel, lipid-coated nanoparticle which is pH sensitive and provides a 100–1000 nm expansion in size when placed in a mild acidic pH environment and in the presence of PEGylated lipids (PEG = polyethylene glycol).80 The modification of the surface PEG-L-eNPs allows the introduction of folic acid (FA) and folate receptor targeting.80 Therefore, these resultant polymer/lipid hybrid nanocarriers, FA-PEG-L-eNPs, provide an enhanced potency and uptake when loaded with paclitaxel in vitro and when compared to nontargeted PEG-L-eNPs.80 Another study on surface modification of the nanocarrier for better targeting was also reported by Biao Kang et al.81 These researchers utilized hydroxyethyl starch to prepare the PEGylated nanocarrier and modified the outer PEG layer with mannose to target dendritic cells, as shown in Figure 4.81 The human plasma interaction and a distinct pattern of protein adsorption and enhanced affinity ensure better targeting behavior with dendritic cells.81
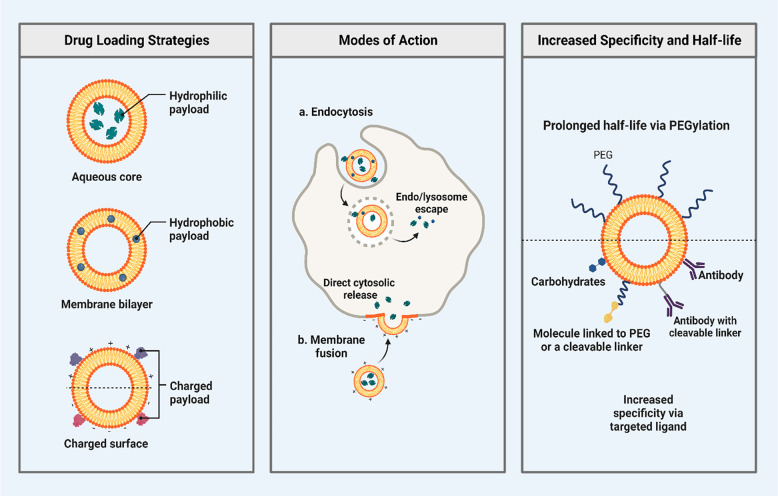
Figure 4.
Drug loading activity and mode of action in a cell, with a proper strategic mode of action and representing specificity to target cells.
Researchers are now developing self-organizable assemblies along with varying amphiphile phase structures with polymer NPs, as this is believed to be promising for designing and developing efficient nanocarriers.82 Angayarkanny et al. developed assemblies of micelles containing lauryl esters of tyrosine (LET) coated with polymer nanoparticles which behave as potential nanocarriers for the model solid lipid stearyl alcohol.83−86 It is essential to mention that amino acid surfactant dispersions in pure lauryl ester of tyrosine and lauryl esters of phenylalanine micelles separated spontaneously, thus suggesting a minuscule encapsulation of amino acid surfactant in the micelles.83−86 Through this, sufficient proof has been obtained to believe that polymer-coated LET micelles act as suitable matrixes for SA encapsulation, the strength of which is attributed to a H bonding interaction between the phenolic group present in LET and the hydroxyl (OH) group current in the SA.83−86 Many researchers are thus keen to develop NPs for anticancer therapy from natural silk.87 The silk NP was depicted by F. P. Seib et al.,87 who conducted an in vitro study of silk NPs loaded with doxorubicin to establish the nontoxic property of NPs on healthy cells with increased efficacy that overcomes the drug resistance mechanism, which is shown in Table 1.87
Table 1. Surface Modifications of Nanoparticles by Biopolymers for Enhanced Drug Delivery.
| Nanoparticle | Biopolymers | Drug | Biomedical Applications | References |
|---|---|---|---|---|
| PEG | Lipid | Paclitaxel | In vitro uptake enhanced | (80) |
| Hydroxylated ethyl starch | PEG layer and mannose | N/A | Targeting dendritic cells | (81) |
| MMT clay | Starch/d-l-lactic acid | DMSO (dimethyl sulfoxide) | In vitro studies revealed sustained release | (88) |
| Gold nanoparticles | Gelatin | Doxorubicin | In vitro studies revealed sustained release | (89) |
| Gold nanorods | Lipids | N/A | Enhanced bioimaging | (90) |
Polysaccharide-Based Biopolymer
Polysaccharides have been found to be significant components for stimuli responsiveness in DDSs based on them being biocompatible and less toxic.91 Alginates and chitosan (polysaccharides) are safe for use alone or with specific surface modification in DDSs.92−94 Wang et al.95 suggested some interesting manufacturing and surface modification techniques to formulate the biopolymers like polylactic acid and chitosan NPs for nanomedicine application.95 The processes for PLA involved the emulsion diffusion method where the fabrication of both single emulsion and double emulsion systems was undertaken. This technique was undertaken for the formulation and development of lipophilic (hydrophobic) substances, while the other method adopts (i) hydrophilic chemical entrapment: the salting out method, where aqueous and organic phases are emulsified in O/W and further distilled water was added which resulted in raw NPs, (ii) nanoprecipitation: where a syringe pump is used in the oil bath containing the magnetic stirrer and (iii) the emulsion evaporation method: where drug agent is dissolved in the polymer in organic solvent in which the emulsion is carried out in high shear phase and further followed by solvent evaporation in a vacuum resulting in NPs. The chitosan NPs (CNPs) were developed using the ionic gelation method whereby sodium tripolyphosphate is added to the chitosan solution and homogenized at high speed forming nanoparticles. For the reverse micelles method, chitosan with cross-linking agent is added to surfactant dissolved in organic solvent, stirred overnight, and later purified to obtain CNPs. These CNPs were also obtained through applying the compressed air to the chitosan solution, known as the spray drying technique. In addition to that, a hot air chamber and blow-drying chitosan into an alkali solution is known as the coacervation process at an adjusted pH of more than 6.5. Surface modification techniques of the nanopolymers by improving hydrophilicity, chitosan functionalization, target functionalization, pH-sensitive coating, and plasma treatment under pressure gauge in a gaseous environment presented a better result in in vivo treatment. These were thereby followed by observational studies of addition of trimethyl chloride, galactoside, polyethene glycol, thiolation, and target modifying agents to chitosan.
Alvarez-Lorenzo et al.96 showed that ionic polysaccharides exhibit sensitivity to pH and this ion sensitivity is transferable physically or chemically through cross-linking networks. Chitosan, the only naturally available cationic polysaccharide, tends to swell at acidic pH and shrinks at neutral/alkaline pH, thus assisting in faster drug release at acidic pH. The reversal of this property is observed with anionic polysaccharides. The osmotic effect of ions enhances the cross-linking density, thereby enhancing the release rate. Owing to ionic interactions, an affinity-controlled mechanism takes place, which results in the release of the drug. These features thus make polysaccharides a suitable choice for site-specific oral drug delivery, particularly for release into the colon. Therefore, resistance to the degradation of the drug by the enzymes of the upper gastrointestinal tract and susceptibility to enzymes present in the large intestine may thus synergistically cause efficient site-specific release. The report also mentioned that the variations in pH between healthy cells and tumor cells can also lead to the triggering effect of the carriers. The electric field may also be essential in drug release’s operating cycles. The other factors that can act as a stimulus, such as light, temperature, and redox reactions, can also affect release rate. Polysaccharide-based NPs have significant usage in theragnostics. Surface modifications through self-assembly and cross-linkers are seeking more attention nowadays. Researchers have been trying to industrialize polymeric nanocarriers. Kim et al.97 synthesized and developed an uncoated, chitosan-based coating and starch-based coating of magnetic NPs to use as a hyperthermic thermo seed. The chitosan-coated magnetic NPs were synthesized at 23 °C in the presence of an alternate current (AC) magnetic field compared to the starch-coated magnetite. The particle capture rate was 96% when an external magnetic field of 0.4 T was present. As the normal viability of 93.7% of L929 cells was compared to that of the KB carcinoma cells, the rate of capture of the KB cells relatively surged by 10.8% with the chitosan-coated magnetic NPs. The in vitro studies revealed that chitosan-coated magnetic NPs showed high compatibility, thus proving beneficial and significantly promising for use in magnetically targeted hyperthermia.
The latest work by Alkanawati et al.98 depicts a method of laboratory scale production of nanocarriers which can indeed be applied to an industrial scale for efficiency of production of such nanocarriers. They successfully produced nanocapsules of enhanced quality (low PDI and batch variability) along with a 300-fold surge in product output. However, the size of the produced nanocarriers could be easily manipulated through variations in the operating conditions. These findings were coupled with the knowledge that inline implementation of microfluidization could provide a beneficial way for the upscale production of nanocapsules for delivery of the drug at an industrial level.99 Suarasan et al.89 developed a doxorubicin-loaded nanocarrier made up of gelatin and Au NPs. The free drug showed acute toxicity and self-chemical modifications. Thus, loading the encapsulated drug particle between the positively charged gelatin layer and the negatively charged Au NPs avoids the requirement of any chemical change of the drug, which further ensures maximal antitumor activity. Furthermore, gold nanorods are being implanted in lipid nanodiscs, which can be enhanced by clustering and be used in sensing and imaging applications.90 Acetylated rapeseed protein isolate (ARPI) with chitosan coupled anticancer drug doxorubicin (DOX) are being designed as nanocarriers along with cathepsin B. Cathepsin B is a lysosomal acid protease that is ubiquitously expressed in all lysosomes of mammals and is enormously upregulated and active in various kinds of malignant cancers. The bioactive peptides that are ARPI-derived exhibit an anticancer effect synergistically with DOX through regulation of various pro- and anti-apoptotic proteins such as p53, Bax, Bcl-2, and pro-caspase-3 which are present in the mitochondria. In a CathB-overexpressing orthotropic breast tumor model, DOX-ARPI/CS NPs significantly inhibited tumor enlargement, enhanced tumor cell apoptosis, and increased hosts’ survival without producing any systemic toxicity.90
Polysaccharides, including chitosan and contrast agents, are also actively used in drug delivery and in image guided radiation therapy.100 Starch being biocompatible, inexpensive, readily available, and biodegradable has sought attention in DDSs as an alternative nanocarrier. Its hydrophilicity, however, can sometimes create problems in stability, and thus, acetylation may be required. To bypass this, Namazi et al.88 suggested using montmorillonite (MMt) clay, a biodegradable nanomaterial, along with d and l lactic acids as a better substitute. Reports showed high thermal stability of the MMt-induced nanocarrier and an efficient encapsulation and drug release pattern. A similar statement was also observed by a mini-review of cellulosic starch being reinforced by nanocrystals to enhance the biocompatibility of the polymers.101 Mukwaya et al.102 discussed saccharide-based nanocarriers for DDSs. According to their work, saccharides exhibited good encapsulating efficiency and low toxicity to increase the drug accumulation in the targeted site for a prolonged period.102 One- or two-pot syntheses have recently been developed for polysaccharide nanocarrier synthesis.102 This is inclusive of the self-assembly of nanovectors through reversible fragmentation chain transfer addition or anionic polymerization.102 However, these techniques possess certain limitations due to their nonemployment in the biomedical nanocarrier synthesis originating from natural macromolecules, which makes free radical copolymerization unique compared to other techniques due to its ability to be utilized in purely aqueous media devoid of surfactants.102
Nanomaterials are much more efficient than chemotherapeutics in delivery to the target site.103 However, further research is being conducted to improve the penetration of large sized NPs through introducing cross-linking methods.104−106 Ju et al.107 reported the formulation of a nanogel containing a polyelectrolyte core cross-linkage comprised of N-lysinal-N′-succinyl chitosan and poly(N-isopropylacrylamide) along with a cross-linked shell of bovine serum albumin which showed swelling in an acidic environment followed by shrinking under neutral conditions.107 This swelling was responsible for quickly releasing the encapsulated chemotherapeutic agent into the cancer cells for effective cytotoxicity. These nanogels also had a better effect on the neighboring cells in the vicinity of the tumor site. The design and development of a unique nanomedicine by Ren et al.108 showcased several advantages such as ease of preparation and reproducibility, increased payload of drug, superior and enhanced stability, prolonged circulation, and enhanced therapeutic effect.108 The high reproducibility rate of the 18 assemblies of molecules should, therefore, motivate the design and development of new nanomedicine, which was done through nanoparticles that are self-assembled devoid of aid of surface-active substances and based on the conjugation of docetaxel to d-α-tocopherol succinate.108 Reduction-sensitive prodrugs were developed through synthesis with introducing a disulfide bond into the linker and were compared with reduction-insensitive prodrugs as the control.108 The investigation of the morphology and stability of self-assembled nanoparticles was done. The researchers were keen on developing nanomedicine from biodegradable polymers due to their enhanced compatibility and bioavailability.108 They were also approved by the FDA and the European Medicine Agency (EMA), thus making them great candidates for clinical oral and parental administration. Wu et al.109 reported using formulations of NPs comprised of elastin-like polypeptides (ELPs) that are genetically engineered. They possess characteristics of biodegradability, biocompatibility and bio responsiveness.109 The study involved using NPs ranging from 300 to 400 nm in diameter and ELPs and drugs which are priorly co-dissolved in an organic solvent followed by acceleration through a gradient-based voltage.109 They are then dried through the process of evaporation during transit and collected from the surface of the target.109 The results indicate that the diameter and size of the particle, polydispersity, and morphology strongly correlate to the solvent concentration, voltage of spraying, and polymer’s molecular weight.109 Surprisingly, drug loading at 20 w/w% did not affect the morphology of the particle. An aspect that is noteworthy with this technique is the fact that the release of drug from these particles was related to pH-based solubility of the ELPs of the parent which suggests that electro spraying is a flexible and efficient technique for the generation of stimuli-responsive drug NPs.109
Free radical copolymerization’s eco-friendly, inexpensive, and straightforward characteristics allowed for developing a one-pot synthesis scheme for nanocarriers named “graft copolymerization-induced self-assembly” (GISA).110 Using a two-pot approach involves a modification in methodology and the usage of postmodification or postpolymerization self-assembly-based techniques.111 Techniques such as micellization, nanoprecipitation, dialysis, solvent evaporation, and emulsification can be used for the synthesis of nanocarriers from polysaccharides.112 However, the one-pot approach is preferentially utilized for the doxorubicin drug delivery at the anticancer site. The development of biodegradable drug carrier formulation by the electrospinning method was reported recently.113 Electrospinning is another technique in which the particles are synthesized by applying an electrohydrodynamic force that is uniform to break up liquids into fine jets. This is an exciting technique for quick and increased production of NPs that possess a controlled morphology that can assist with controlled release in the drug delivery of cancer. This resultant product of NPs produced through electrospinning must display (1) reliable entrapment of the drug, (2) uniform particulate size, (3) sufficient stability under physiological conditions, (4) adequate release or in response to a stimulus in a time-dependent manner, and (5) biological compatibility.114,115
3. Mechanism of Drug Delivery through Biopolymer
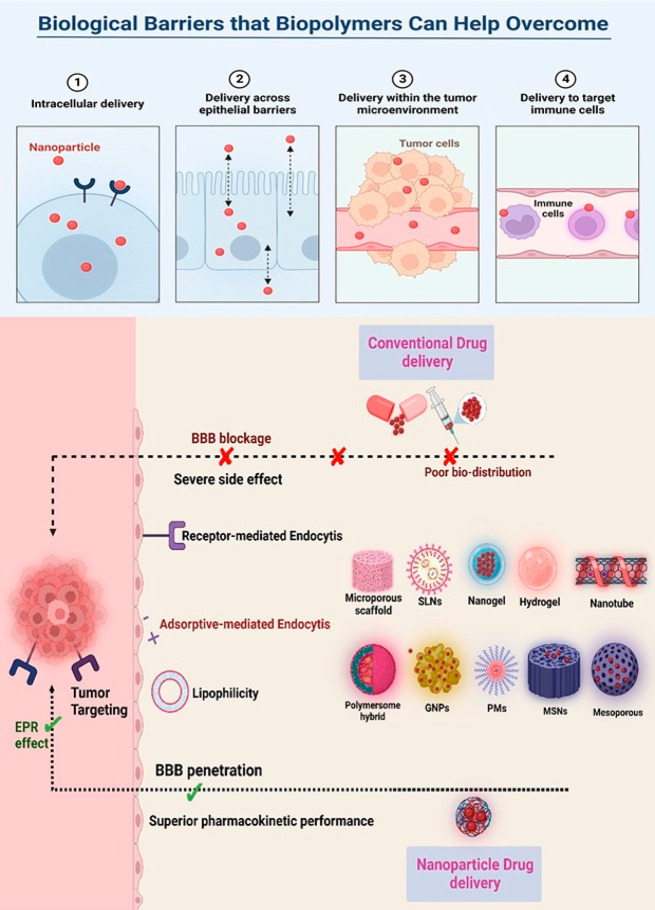
Many studies were reported which were based on the usage of polymeric materials for drug delivery.116−119 Biopolymers can be injected in the body via syringe, and once injected into the body, they solidify and form a depot of semisolid nature.120 Depending upon the solidification process, injectable implant systems are divided into five types: thermoplastic pastes, cross-linked systems in situ, gelling systems that are thermally induced, precipitates in situ, and hydrophobic lipophilic fatty acid-based injectable pastes.120 The method for studying this involves the process of extraction of solvent around the surrounding tissue resulting in precipitation which protects the protein and amide-based polymers from being damaged by heat and shear stress (shown in Figure 5).120
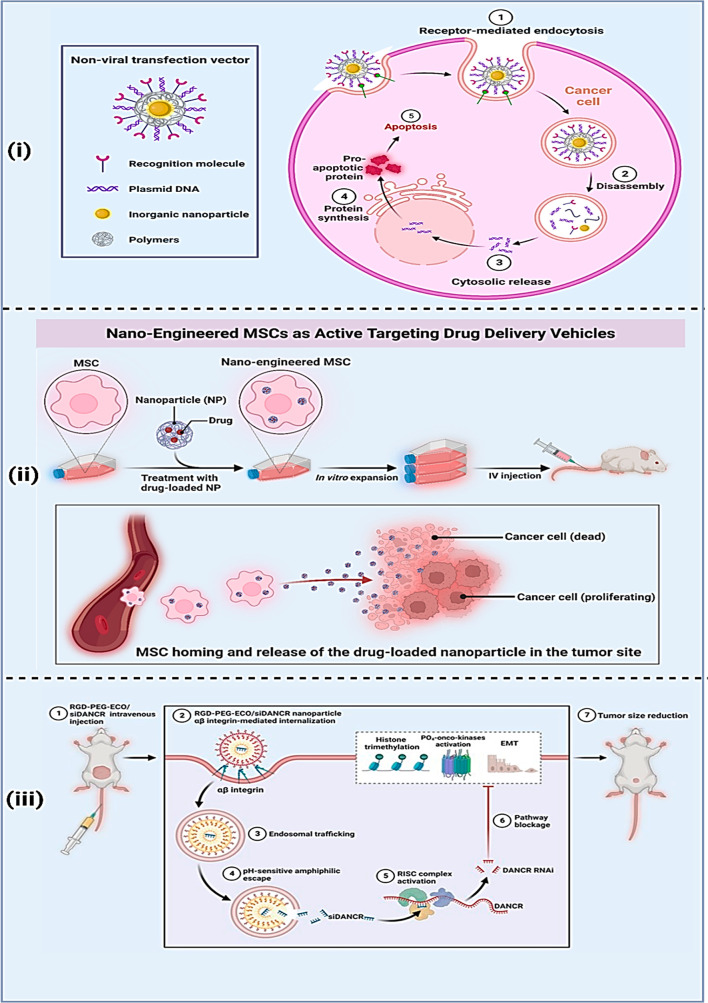
Figure 5.
Biopolymer assisted nanodrug delivery mechanisms: (i) nonviral transfection methods, (ii) nanoengineered MSC methods, and (iii) integrin mediated initialization to the targeted tumor cells.
Biocompatibility and biodegradability are desirable properties of the materials used in the development of nanocarriers.121 Various natural polymers have been investigated, like starch, cellulose, chitosan, albumin, and gliadin fulfilling these requirements.122 Starch has already been transformed into NPs and is used widely in delivery of drugs.123 For better efficacy, instead of direct targeting, doxorubicin can be delivered into the DU145 cell by entrapping into the hydroxyethyl starch, which is a synthetic polymer derived from starch.124 It enables the drug to circulate through the blood in a prolonged period.
M. Gulfam et al.125 extracted gliadin particles from gluten by the desolation method, which were already being used for drug delivery earlier. However, they resulted in low drug loading efficiency and less ability to separate the particles from the aqueous phase.125 Therefore, researchers processed the gliadin NPs through the electrospinning technique, which does not require the utilization of a surfactant. Gliadin NPs and gliadin gelatin nanocomposites loaded with cyclophosphamide were the target NPs in the drug delivery for this study.125 The study revealed that cyclophosphamide was slowly released from nanoparticles of gliadin for 48 h, and composite nanoparticles of gliadin–gelatin caused drug release rapidly. Moreover, 24-h cultured breast cancer cells with 7% gliadin nanoparticles loaded with cyclophosphamide became apoptotic.125 Also, the downregulation of the Bcl-2 protein was confirmed through analysis by Western blotting. As a result, this anticancer drug-loaded gliadin nanoparticle can be used as a powerful tool for cancer therapeutics.125
A DDS utilizing polymers extracted from the exoskeleton of crustaceans or any shell animals is also the most versatile substances chitosan.126 According to Uchegbu et al.,127 research of chitosan drug delivery began in 1990. Chitosan is deacetylated chitin (the exoskeleton of many animals). It has shown promising results as a film forming material required for drug delivery.127 The amphiphilic character of chitosan provides the ability to form NPs in aqueous solution instead of using any cross-linking agents or ionic gelation agents.127 The CNP less than 1 μm in dimension is stable for six months after drug loading.127 Chitosan NPs showed oral bioavailability of hydrophobic drugs up to 6-fold and when bound to anticancer agents effectively delivered the drug to the tumor site by exhibiting tumoricidal activity without being toxic to normal cells.127 When 200 nm dimension NPs were also administered to rabbit retinal cells, they were nontoxic, showing benefit as an ocular agent. It is also able to deliver peptides to the brain through the intravenous and oral route.127
Another new investigation was performed by Yi Tian et al.128 using silk fibroin. They developed silk fibroin NPs through a magnetizing method from the cocoon fiber of Bombyx mori.128 The past decade has witnessed the application of silk fibroin NPs as a DDS for release and entrapment of drugs. It has also shown its efficacy as a lysosomotropic anticancer nanodrug carrier when loaded with doxorubicin and has overcome multidrug resistance.128 However, the nonspecificity of tumor targeting could bring about an accumulation of therapeutic agents. Therefore, a single-step potassium phosphate salting-out technique included certain amounts of magnetic iron oxide NPs (MNPs) in phosphate solution that were hydrophilic in nature.128 As a result, the magnetic silk fibroin NPs loaded with doxorubicin showed suppressed tumor growth and a 100% survival rate even on day 30. Therefore, testing this in an in vivo model can serve better for clinical trials in the future.128 Mohapatra et al.129 studied a new formulation incorporating magnetic NPs in chitosan along with the antibiotic vancomycin to observe drug release through magnetic stimulation. The MNP embedded in chitosan showed more effective drug release in a controlled manner as compared to the nonstimulated one.129 These characteristic features would greatly benefit clinicians to have control with drug delivery, dosage timings, and local concentration which could be tailored to the clinical needs of the patient.129
Sun et al.130 critically reviewed the rational design and analyzed nanocarrier properties in DDSs. After intravenous administration, he concluded that nanomedicine functions through a five-step CAPIR mechanism. This includes (1) circulation through the compartments of blood, (2) accumulation and aggregation in the tumor tissue through its leaky, hastily built vasculature, (3) greater penetration into the tissues of tumors, (4) internalization, and (5) intracellular drug release .130 Further, researchers also observed that the nanomedicines involved per step differ in surface charge, dimensions, and stability dilemmas, thereby proposing accumulating these properties into one nanomedicine (3S transitions for short) to eliminate such dilemmas.130 With reference to the previous context and through understanding of CAPIR and 3S transitions of nanocarriers several modifications on the surface, stability, and size are being conducted.130 Moreover, many researchers have synthesized amphiphilic self-assembled glycyrrhizic acid-biotin-starch NPs (GaBS NPs) through a single-step esterification reaction and they observed the cellular uptake of free doxorubicin as well as GaBS NP loaded doxorubicin.130 The cellular internalization of drug loaded NPs was confirmed to be more rapid than that of the pure drug. A surface modification study was reported by Simon et al.;131 they synthesized a completely carbohydrate-based nanocarrier through surface-based modification using various sugar derivatives (hydroxyethyl starch, dextran, or glucose) through copper-free click chemistry.131 This study revealed a strong interaction between sugar moieties and plasma proteins.131 The cellular uptake showed no nonspecific interaction between the carbohydrate NPs and phagocytic cells.131 The advancement of peptide-based nanocarriers was also discussed by Wei et al.132 According to them, peptides have multiple biomedical applications, like self-assembling them into nanocarriers in drug delivery, or as a targeted ligand for tumor epithelial cells, or for enhancing the penetrating ability of drug loaded in them at the tumor site, or a triggering enzyme-responsive group to create a microenvironment for the tumor to resolve the problem of nanoparticle degradation.132 This study also revealed that sometimes there are limitations of these NPs as drug agents due to their inability to accumulate at the tumor site and penetration issues, which peptides or surface modified peptides can resolve,132 as shown in Figure 6. The pancreatic cancer cell target by some active biomolecules entrapped in pectin derived peptide by Sanna et al.133 formulated some quinoxalinediones and entrapped them into pectin derived peptide to observe their anticancer efficacy. Antiproliferative studies were performed where the targeted NP proved to be more potent than the nontargeted one.133
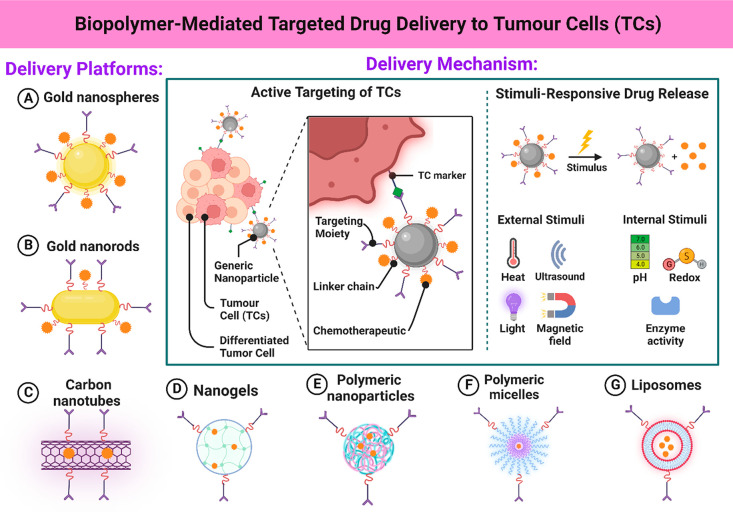
Figure 6.
Biopolymer-based targeted drug delivery inside the tumor cells, explanting the mechanism via different stimuli responsive platforms.
Nanopolymeric materials exhibited a better and more effective drug delivery than chemotherapeutic agents.134 However, there were certain limitations about the penetration of nanocarriers into solid tumors of big diameters.36 Traditional polymers can only penetrate up to 50 μm; however, nanopolymers associated with anticancer agents can penetrate into tumors of 100 μm diameter.135 Dextran nanopolymers embedded in aldehyde functionalized doxorubicin NPs (aldehyde dextran doxorubicin conjugates) showed such properties. 2D nanocarriers’ monolayer studies also provided huge discrepancies when administered into in vivo models.135 As the efficacy was insignificant, the developed 3D cell structure was used to better understand drug (doxorubicin) release with anticancer therapy for use in childhood stage. The drug use to eradicate the extra cranial tumor (neuroblastoma) in the expensive in vivo model, time consumption, and discrepancies.135 The role of the glycocalyx in the uptake of the ald-dex-dox NPs was also studied. The study involved understanding the interaction between the dextran-based nanocarrier and the highly glycosylated tumor cell surface and their penetration ability.135
Wang et al.136 extensively reviewed drug loading strategies for nanocarriers. They stated that major nanocarriers are developed to overcome problems of hydrophobic drugs, and researchers mainly focus on environment or on specific components without paying much attention to drug loading capacities.136 There are three types of loading capacities of nanocarriers wherein each system possesses its own release mechanism. In the case of the loading system on the surface, the loading of the drug is dependent upon absorption, while its release depends on desorption.137 The matrix loading system is determined by degradation of the carriers or diffusion since molecules of the drug are embedded in the nanocarriers.138 The cavity loading system is dependent upon diffusion controlled by the shells, which provides release of the drug. They conclude that further research and understanding can assist in rational designing of nanocarriers for DDSs.139 The prime function of nanocarriers is to encapsulate the drug within itself until it reaches the tumor site following controlled drug release. The method used to understand this process of drug release is through dialysis.140 This technique however cannot be applied to hydrophobic nanocarrier drug loaded substances. Thus, to overcome such a problem Bouchaala et al.141 developed a novel technique for the quantification of release of fluorescent moieties from LNCs using fluorescence correlation spectroscopy (FCS). For this study, LNCs that were nanoemulsion droplets were encapsulated by the hydrophobic Nile red derivative NR668.141 The study showed that sharp contrast classical FCS parameters are more effective in drug release compared to less bright light.141 Therefore, the researchers made use of the standard deviation of fluorescence fluctuations for analysis of release of the dye from the nanocarriers quantitatively. The drug release was found to be temperature dependent, and the rate declined at 37 °C after a 6-h duration of 50% delivery.141
Similarly, a focused effort on newer techniques like the production of nanocrystals in a confined environment can be achieved within microfluidics channels and was reported by Fontana et al.142 We have previously cited that modified peptide moieties can serve as good nanocarriers for DDSs. In the latest work by Medea Neek et al.,143 researchers found that the peptide alone fails to act as a nanocarrier at the targeted tumor site. They concluded that the peptides could only function effectively as tumoricidal to the target cells, if that embedded in a caged protein which has virus-like structures.143 Moreover, the interaction of these virus-like structures and cage protein with the immune system has concluded that they have a better efficacy as a DDS143 shown in Figure 7.
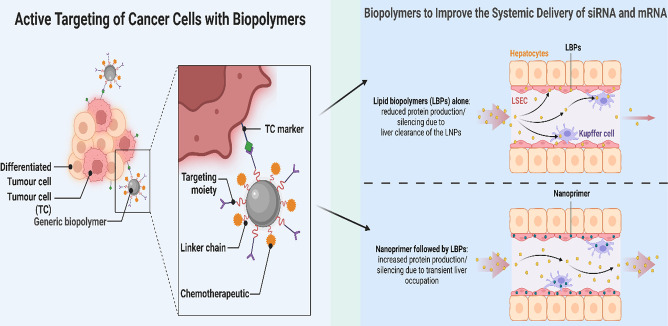
Figure 7.
Active targeting of tumor cells in the application of biopolymers with proper delivery of siRNA and mRNA.
4. Intracellular Trafficking of Biopolymer-Based Nanoparticles in Cancer Cells
The process of intracellular trafficking of biopolymer-based nanoparticles by cancer cells is a complex and dynamic phenomenon that holds significant importance in the targeted delivery of therapeutic drugs and imaging agents to specific locations within cancer cells. The complex process entails intricate interactions between nanoparticles and diverse biological components, which play a crucial role in determining the efficacy of cancer treatment. Biopolymer-based nanoparticles, which are generated from polymers such as poly(lactic-co-glycolic acid) (PLGA), chitosan, or polydopamine (PDA), offer distinct benefits such as biocompatibility, adjustable characteristics, and the capacity for functionalization. The aforementioned attributes make them highly desirable candidates for the purpose of cancer therapy since they enhance the effectiveness of medication delivery and imaging techniques.
The transportation of biopolymer-based nanoparticles within cancer cells occurs through a series of consequential phases:
-
(a)
Cellular uptake: The primary phase focuses on the uptake of nanoparticles by cancer cells. The process under consideration involves various processes, including endocytosis (such as clathrin-mediated endocytosis, caveolae-mediated endocytosis, and macropinocytosis) and phagocytosis. The precise mechanism of absorption is contingent upon various elements, including the size of the nanoparticles, their surface charge, and the process of functionalization. The nanoparticles have the potential to be modified in order to enhance the process of cellular uptake by including ligands that specifically bind to receptors that are excessively expressed on the surfaces of cancer cells.
-
(b)
Endosomal escape: Following internalization, nanoparticles frequently become sequestered within endosomes or lysosomes, leading to a restricted environment. In order for nanoparticles to achieve their desired therapeutic or imaging effects, it is imperative that they successfully evade these compartments and gain entry into the cytoplasm. Specific biopolymer-based nanoparticles are intentionally engineered to react to the acidic environment found in endosomes, thereby triggering the discharge of their payload into the cytoplasm. This particular phase holds significant importance in preventing the breakdown of cargo within lysosomes.
-
(c)
Intracellular trafficking: Once nanoparticles have evaded endosomes, they proceed to navigate within the cytoplasm, progressing along a sequence of intracellular trafficking routes. The aforementioned pathways encompass engagements with microtubules, molecular motors, and other constituents of the cytoskeleton. The use of cellular pathways can be leveraged in the design of nanoparticles composed of biopolymers, resulting in an increased ability to move within the cell.
-
(d)
Nuclear localization: In certain circumstances, nanoparticles are designed to specifically target the cell nucleus, serving purposes such as imaging or gene delivery therapy. This requires the surmounting of other obstacles, such as the nuclear envelope. The introduction of surface changes and the use of specific cargo molecules can enhance the nuclear entrance of nanoparticles, hence providing precise and targeted functionalities.
-
(e)
Cargo delivery and release: Once biopolymer-based nanoparticles have reached their designated subcellular locations, it is crucial for them to effectively transport their cargo, which may include therapeutic medicines, imaging agents, or genetic material. Controlled release techniques can be customized according to characteristics such as pH sensitivity, enzymatic breakdown, or external stimuli such as light or heat.
-
(f)
Interaction with subcellular organelles: The involvement of nanoparticles in cellular processes may require interactions with different subcellular organelles, such as mitochondria or the endoplasmic reticulum. These interactions exert a significant impact on cellular processes and, as a result, have implications for therapeutic outcomes.
-
(g)
Biodegradation and clearance: These are crucial aspects of the cellular fate of biopolymer-based nanoparticles, as they contribute to the nanoparticles’ safety and biocompatibility. As the nanoparticles navigate the complex environment within the cell, a natural process of biodegradation occurs over time, leading to the slow breakdown of the nanoparticles. The capacity of biopolymer-based nanoparticles to undergo degradation over time is a noteworthy characteristic that distinguishes them, thereby reducing the potential risks associated with extended accumulation inside the cellular milieu. The relevance of biodegradation is rooted in its substantial consequences for both the efficacy of therapeutic interventions and the long-term safety of such interventions. The progressive disintegration of the nanoparticles into smaller components facilitates a synergistic alignment between the therapeutic efficacy and cellular uptake. Upon the disintegration of nanoparticles, they undergo a transformation into metabolites and fragments, which possess a higher degree of assimilation and can be more efficiently digested by the cellular machinery. The deliberate coordination of this change guarantees that the nanoparticles do not persist indefinitely within the cellular boundaries, reducing the probability of any detrimental consequences arising from prolonged nanoparticle existence.
-
(h)
Cellular responses: The complex interaction between biopolymer-based nanoparticles and cancer cells elicits a wide range of significant cellular responses, including intricate changes in gene expression, modification of signaling pathways, and dynamic immunological reactions. The inclusion of nanoparticles in the cellular milieu initiates a series of interconnected processes that play a crucial role in defining these sophisticated therapeutic agents’ overall therapeutic effectiveness and safety. Gaining a thorough comprehension of these complex and diverse reactions is crucial, as it forms the fundamental basis for enhancing and optimizing the utilization of these nanoparticles in cancer treatment.
-
(i)
Immune reactions and immunomodulation: In addition to their direct effects on cancer cells, nanoparticles made from biopolymers also engage with the immune system, resulting in a diverse range of immunological reactions. The ability of nanoparticles to activate various immune cells, including dendritic cells, macrophages, and T cells, has the potential to elicit an immunological reaction against cancerous cells. The capacity of nanoparticles to regulate immune responses presents opportunities for synergistic combination therapy, wherein the immune system is utilized to enhance the effects mediated by nanoparticles. On the other hand, nanoparticles can suppress immunological responses to prevent excessive inflammation, thus underscoring their potential for immunomodulation.
5. Theragnostic Application of Biopolymer for Anticancer Therapy
As Anticancer Agents
Due to the large number of fatalities caused due to cancer, it is necessary to identify and develop therapeutic agents that are efficacious and have less side effects.144 Research conducted on chemotherapeutic agents shows many enhancements and modifications. However, they still possess detrimental effects such as harming of normal cells as well as poor delivery to the target site.145−148 Starch, among other polymers, has been incorporated into nanodrug formulations with anticancer agents due to many advantages such as its abundance in nature, non-cytotoxicity, biocompatibility, biodegradability, non-immunogenicity, stability in air, as well as compatibility with most drugs.149Table 3 provides some examples of biopolymer-based nanocarriers used as anticancer drug delivery agents.
Table 3. Biopolymer-Based Nanocarrier Targeting Tumor/Cancer Cell Lines.
| Nanocarrier | Dimension | Drug moieties | Studies | Cancer cell | References |
|---|---|---|---|---|---|
| Propyl starch | 229 nm | Docetaxel | In vitro | Colon cancer | (150) |
| Amine functionalized polyacrylamide | 39.4–46.3 nm | Photosensitized drug (Photofrin conjugated) | In vivo | Breast cancer | (154) |
| RGD peptide (arginine-glycine-aspartic acid) conjugated triblock polymer | 99.8 ± 17.9 nm | Camptothecin | In vitro/in vivo | Brain cancer/glioma | (174) |
| Amine modified layered double hydroxide | NIR optical contrast agent, Indocyanine green | In vitro/in vivo | Colon cancer | (187) | |
| Chitosan | 9.4–23.4 nm | Quantum dots | In vitro | Non-Hodgkin lymphoma cancer cells | (198) |
| PEG functionalized melanin nanoparticles | 7.5 nm | Image contrast agents | In vivo | Hepatocellular carcinoma | (205) |
Prajakta Dandekar et al.150 synthesized a hydrophobic starch derivative named propyl-starch to understand its application in NP formulations that encapsulated anticancer agent docetaxel using the solvent emulsification diffusion technique.150 The researchers observed an enhancement in efficacy and availability of the drug in the cancer cell line due to its “nano size”. Moreover, cytotoxicity was also not observed in in vivo studies and perinuclear localization of the NPs also confirmed its targeted association with the nucleus (peri/intra).150
Proteins like gliadin, soya, bovine serum albumin (BSA), milk protein, zein, elastin, and gelatin show efficient drug delivery characteristics at a nano level. Protein-based polymers have also been used by researchers as anticancer and antitumor delivery agents.151 EPR has been proved in many animal models and was also discussed by Taurin et al.160 Nanomedicines showed effectiveness and accumulation at the tumor site up to 27-fold more than normal drug even after 6 h of administration,152 as shown in Figure 8. In general, besides biocompatibility and biodegradability, protein NPs offer an advantage in synthesis as they can be prepared under mild conditions without harmful chemicals either through a coacervation/dissolvation technique, emulsion solvent extraction technique, or complex coacervation techniques.153
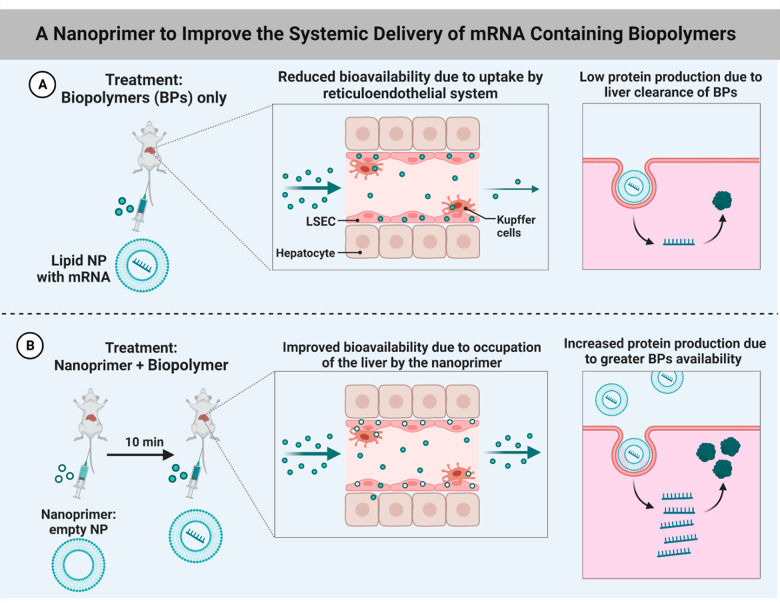
Figure 8.
mRNA modified biopolymer-based assisted drug delivery: (A) lipid NP with mRNA method and (B) nanoprimer + biopolymer approch.
Shouyan Wang et al.154 extensively researched biodegradable polyacrylamide NPs with an amine functionality as a cancer theragnostic and included studies based on active cancer cell targeting, imaging via fluorescence, and photodynamic therapy.154 The nanodrug development involved the addition of primary amino moieties and cross-linkers that were biodegradable during the polymerization process of NPs along with the introduction of photodynamic and fluorescent agents in the NP matrix that were conjugated with PEG and ligands of tumor on the surface.154 Studies performed in vitro on human breast cancer cells resulted in efficient drug delivery to the site. In conclusion, the benefits of polymer-based NPs as nanocarriers for tumor theragnostics that are biodegradable and multifunctional were demonstrated in the study.154 According to Lohcharoenkal et al.,155 proteins are much safer as nanocarriers of anticancer agents. An albumin-bound nanocarrier with a particle size distribution of 130 nm showed effectiveness as an anticancer drug.155 Their study also revealed the FDA approval of albumin-bound paclitaxel (Abraham, ABI-008) for metastatic breast cancer which exemplifies the clinical feasibility of this approach.155 When subjected to high pressurized homogenization, albumin’s dimensions are 100–200 nm which is effective in loading and delivering paclitaxel to the site.155 The study also revealed that cationic bovine serum albumin (CBSA) can be used as a new siRNA delivery system for the treatment of metastatic lung cancer. Milk protein casein NPs that encapsulated doxorubicin showed very interesting results in oral delivery, as it was highly effective in hepatocellular carcinoma treatment. The overview also helped to understand the various approaches undertaken for the development of protein-based anticancer therapeutics and their mechanisms.155
Polydopamine (PDA) has received considerable attention in the scientific community due to its remarkable characteristics, including biocompatibility, facile production, strong near-infrared absorption, high photothermal conversion efficiency, and efficient binding of metal ions. Extensive research has been conducted on multifunctional nanosystems containing programmable logic devices (PDA), primarily for biomedical applications, due to the notable characteristics exhibited by these systems. A thorough examination is essential to delineate the synthetic methodologies employed in producing diverse nanoparticles (NPs) incorporating polymeric drug carriers (PDA) for advanced cancer diagnosis and treatment. It also examines the diverse applications of these NPs in different imaging modalities and their potential therapeutic implications in cancer treatment.156 The genesis of the PDA (polydopamine) phenomenon can be traced back to the adhesive properties exhibited by mussel adhesive proteins when they interact with solid surfaces. The versatile substance known as polydopamine (PDA) was first produced by Messersmith et al. by the process of auto-oxidative polymerization of dopamine (DA) in mildly alkaline circumstances.157 Although the precise mechanism is not yet fully understood, the distinctive characteristics of PDA have led to its use in several domains such as healthcare, renewable energy, and environmental sciences.148 The utilization of PDA exhibits desirable characteristics, including a preparation process that is both mild and solvent-free, the attainment of homogeneous particle size, and a wide range of optical absorption. The utilization of this nanoplatform for tumor imaging has been observed to be endogenous, particularly in the context of photoacoustic and fluorescence imaging techniques.158 The significance of this issue lies in the limitations of conventional tumor detection procedures, such as biopsies, which lack both specificity and sensitivity. Additionally, imaging techniques are not only expensive but also subject to certain restrictions. PDA-based nanoplatforms have been found to provide a noninvasive and highly effective means of diagnosing tumors (10.1038/nature06917). In addition, the ability of PDA to interact with metal ions such as Cu2+, Fe2+/Fe3+, Mn2+, and Zn2+ through its catechol and amino groups allows for its use in several medical imaging techniques, including magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET).149,159 The capacity of PDA to effectively encapsulate medicinal or contrast materials enhances its efficacy as a delivery mechanism for imaging-guided therapy. The surface-modifying properties, biocompatibility, and biodegradability of PDA contribute to its increased suitability for many applications. It is worth mentioning that polydopamine (PDA) demonstrates the ability to convert light into heat and scavenge reactive oxygen species (ROS), rendering it highly useful in wound healing and cancer therapy. Therefore, the multifarious features of PDA have stimulated substantial research in the field of biomedical applications, including cancer diagnosis and therapy.41 The ease of synthesis, adaptability in surface modification, imaging capabilities, and potential for therapeutic applications of nanosystems containing polydopamine (PDA) render them highly promising instruments in the field of biomedicine.
Polymer conjugates, liposomes, micelles, and metal NPs have also been used as nanomedicines for anticancer therapeutics. Taurin et al.160 studied enhanced permeability rate (EPR) and effectiveness of such nanomedicines on tumor cells. They listed advantages and drawbacks of the EPR in their report. According to their view, tumors consist of defective endothelial tissue with dimensions between 300 and 4700 nm. They also lacked a smooth muscle layer or innervations and a wide lumen, and they possessed impaired and nonfunctional receptors for angiotensin II.160 They also observed that the nanomedicine accumulation in the target cell is dependent on factors such as biodistribution, circulation time, tumor uptake, bradykinin, nitric oxide (NO), prostaglandins, peroxynitrite, and matrix metalloproteinases.160 Variations between normal and tumor tissue were observed with respect to nanomedicine targeting owing to the enhanced permeability and retention (EPR) effect.161 This occurrence is expected since normal tissue contains endothelial cells that are tightly connected thereby preventing the diffusion of nanomedicine to the exterior of the blood vessel, while tumor tissue has large openings or fenestrations between the endothelial cells thus allowing nanomedicines to reach the matrix and the tumor cells through the EPR effect.162 VEGF secretion by tumor cells, stroma cells, and macrophages causes an enhancement in permeability and stimulates angiogenesis and endothelial cell migration toward the tumor.160 However, a considerable proportion of nanomedicine does not reach the targeted tumor area due to entrapment like, nonspecific interaction with matrix, matrix-composed collagen, or removal through macrophage endocytosis.38 These nanomedicines tend to accumulate and concentrate in the periphery of the tumor, while only a small proportion will diffuse to the tumor’s center. They also stated that NPs are easily identified by the body as foreign particles and, as a result, undergo a rapid uptake and elimination by specialized cells belonging to the reticulo-endothelial system (RES).163 To improve cellular uptake, uniform coating with bioligands could be performed as per the reviewer’s view. It is well-known that polymer conjugates behave as covalent ligands and therefore release of the drug is based on their bond structure and on factors such as pH, acidic pH of lysosome, temperature, or enzymatic cleavage.164
The lipid, liposomes, and micelles165 were also found to take part actively as anticancer agents to target breast cancer. Breast cancer patients prefer an oral drug administration route, according to Andey et al.166 A study was performed to observe the oral efficacy of lipid conjugated estrogenic derivative (ESC8) loaded with the anticancer agent cisplatin in a NU/NU xenografted mice model.166 It was observed that when ESC8 was administered alone it cured 74% of the breast cancer cells but when cisplatin was loaded with ESC8 the percentage cure increased to 87%.166 Indocyanine (IC)10 was also incorporated, which resulted in effective imaging analysis when in vivo studies were performed. Miao et al.167 reported the cellular mechanisms and treatment for the resistance of anticancer drugs using dual nanomedicines from liposomes, lipid nanocarriers, micelles, polymer conjugates, lipid coated drug loaded calcium phosphate, and combinations of siRNA chemotherapeutics in single nanocarriers.167 The dual functionality of drug liposomes in treatment of resistant cancers was extensively studied by Mu et al.168 These described the drug as possessing phospholipid biliary vesicles of dual functionality: (1) basic therapeutic efficacy of drug and (2) extended effect of the drug carrier.168 As a result, this dual functional liposome can therefore eliminate drug resistance observed in cancer through the circumvention of the efflux of the drug produced by the adenosine triphosphate binding cassette (ABC) transporters, elimination of cancer stem cells, destruction of mitochondria, initiation of apoptosis, regulation of autophagy, destruction of supply channels, utilization of the microenvironment, as well as genetic silencing of cancer cells.168 Mitochondrial targeting by dual-function drug liposomes and apoptosis induction has also been observed in drug resistant cancer. As mentioned earlier, lipid nanocarriers have good efficiency for delivering the drug at the tumor site.168 Recent studies dealt with understanding the molecular basis of epithelial ovarian cancer and its treatment. In this regard, Bondi et al.169 performed research on the development of curcumin-based NPs entrapped in lipid nanocarriers. The nanosystem helped in overcoming the drawback of poor bioavailability and enhanced the antitumoral activity of curcumin.169 In the study, three combinations of curcumin-loaded lipid nanocarriers were developed, including (1) compritol–captex, (2) compritol–miglyol, and (3) compritol NLCs. Dissolution of curcumin was done in the hot lipid phase followed by high-speed homogenization at 13000 rpm for 10 min further followed by lyophilization.169 Experiments performed in vitro using cisplatin sensitive (A2780S) and resistant (A2780CP) EOC cells showed that curcumin loaded into NLCs provided therapeutic efficacy that was comparable to that of free curcumin as assessed by cell viability.169 Moreover, in both of these cell lines, curcumin loaded NLCs showed an enhanced anticancer potential in clonogenicity assays as compared to free curcumin. So far, the works cited portrayed the usage of lipids and polymers as nanocarriers.169 According to Date et al.,170 lipids are cost-effective but have limitations such as instability, a burst release of the drug, and limited surface functionalization. In contrast, polymeric systems offer diverse chemical modifications and enhanced stability along with release of drug in a controlled manner.170 Conversely, the inability to scale up and limited drug loading capacities limit their use. Nanocarriers that are hybrid and contain a combination of lipids and polymers overcame these disadvantages and maintained the advantages of both systems170 shown in Figure 9. Date et al. also presented a description of the mechanism involved in drug delivery of lipid polymer hybrid nanomedicine formulations both in vivo and in vitro.170
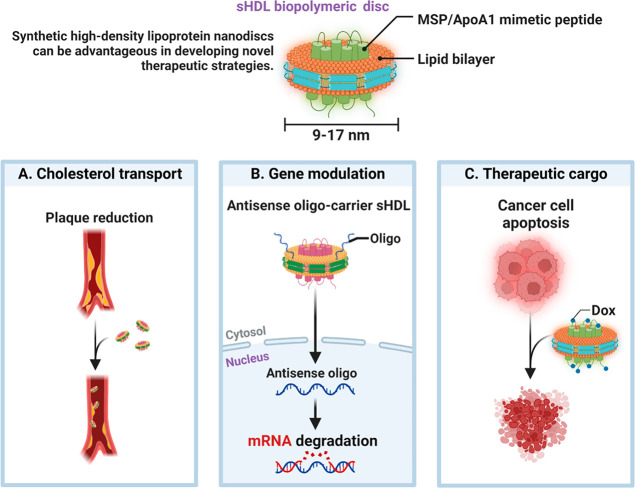
Figure 9.
Biopolymer-based nanocarriers (9–17 nm) are hybrid and contain a combination of lipids as well as genes like (A) cholesterol, (B) gene, and (C) other therapeutic cargo.
Nowadays, combination therapy has become an essential strategy for cancer treatment. It is due to the short half-lives of chemotherapeutic drugs. Dai et al.171 focused on dual nanomedicine combination therapy wherein a combination of therapeutic strategies between various nanomedicines or drug-loaded nanocarriers was focused on rather than the codelivery of different drugs via a single nanocarrier.171 The present treatment strategies using combination therapy are based on targeted nanomedicines. These strategies can be grouped into two major categories: (1) combination therapy based on tumor cell killing and (2) combination therapy based on tumor cell targeting.172 The schematic illustration describes the nanomedicines targeting the same or sometimes different subtypes of tumor cells. As the nanomedicines targeting tumor cells with multidrug resistance (MDR), tumor stem cells, and extracellular matrix (ECM), thus a high chance to kill all the cancer cells.
Nanomedicines showed extravagant properties in active or passive targeting of tumor cells in cancer therapy.173 However, most nanomedicines had several drawbacks such as the suboptimal targeting effect and leakage of the drug, which resulted in an unsatisfactory treatment outcome. Therefore, to overcome this situation, Wang et al.174 developed a hierarchical responsive nanomedicine (HRNM) for the programmed delivery of chemotherapeutics using cyclic Arg-Gly-Asp (RGD) peptide conjugated with a triblock copolymer such as poly(2-(hexamethyleneimino)ethyl methacrylate)-poly(oligo-(ethylene glycol)monomethyl ether methacrylate)-poly[reduction-responsive camptothecin (PC7A-POEG-PssCPT) through self-assembly.174 This study revealed the effective passive targeting of HRNM to the tumor site. The in vitro and in vivo studies showed antitumor activity with reduced normal cytotoxicity.174
Here, we present very recent and interesting research works besides the traditional methods followed and provide concepts of some newer approaches initiated by several groups of researchers. Yi Li et al.175 reported the ability of charge convertible polymers as nanocarriers for disease cure. Polymers that are negatively or neutrally charged become active at the tumor site, disabling cancer cells and increasing the viability of normal cells. Joint research work of scientists from India and USA presented by Padmakumar et al.176 showed some remarkable observations in late-stage ovarian cancer cells wherein anticancer agents were delivered at the tumor site by nanocarriers formulated by nanotextile implants woven through the electrospinning of biopolymeric materials. The recent developments on enzymes at the nanoscale also attracted great attention as a drug delivery system. Dheer et al.177 pointed out some remarkable features of the cathepsin-based nanodrugs and their mechanism in anticancer treatment.
The review already focused on the types of biopolymers and their nano range utility in drug design. Nanopolymers also serve as nanocarriers for cancer therapy. Dong et al.178 extensively surveyed new techniques of nanocarrier formulation for the effective tumoricidal activity. They reported various techniques of nanoparticle formulation which includes techniques like ionic stabilization and steric stabilization, use of polymeric ligands and small-molecule ligands, phase transfer (PT), the ligand exchange and ligand addition effect, and coupling strategies for bio functionalization.178
They even focused on magnetism-induced anticancer NPs and magnetic thermal NPs for drug delivery. According to the review, the release of heat by magnetic NPs, in the presence of a high frequency magnetic field, causes the apoptosis of the cancer cell without affecting normal cells. Furthermore, they described stimuli sensitive polymers and zwitterionic polymers which, following entry into cells of the tumor, the positive and negative charge balance will be broken bringing about changes in conformation, drug release in cells of tumors, and near infrared (NIR) sensitized smart ligand antibody interaction. Figure 7 depicts the 3 mechanisms, (i) a penetration enhancer targeting the moiety of interest, (ii) apoptosis due to the antitumor drug, and (iii) through utilization of a carrier comprised of lipids, metals, and ceramics in NPs. The study also revealed the usage of photoresponsive materials that are NIR-light-based and various other target-based receptors for cancer therapy. They also focused on modification of the surface of the NPs as well as the active and passive targeting mechanism.
In PDT
Chemotherapeutic drugs have shown difficulty releasing the drug at the tumor site and have been found to be harmful to normal healthy cells.179 Radiation therapy was also shown to be detrimental to healthy cells, and therefore, to bypass these limitations, a new theory named photodynamic theory (PDT) has emerged recently.180 This theory involves utilizing components such as a photosensitizer, drug-activating light at a specific wavelength, and oxygen.181 Photosensitizer light brings about energy generation thus causing development and formation of ROS which results in death of the cancerous cells.182
Porphyrin is one such promising sensitizer being used so far along with a chlorine-based nanoformulation to enhance the EPR of nanomedicines at the tumor site. Porphyrin has been loaded with polymeric NP, magnetized NP, silicon particles, and liposomes.183 Fluorescence NPs have served extensively in targeting breast, lung, and hepatic carcinoma according to Alyssa Master et al.184 The study also reveals that chlorinated NPs loaded with chitosan NP and human serum albumin worked efficiently. Other photosensitizers, namely, phycocyanin, indocyanine, hypericin, and methylene blue, have worked brilliantly when loaded with several NPs of polymeric conjugates leading to apoptosis of cancer cells.184 Nanoparticles have provided various methods to enhance the photosensitizer delivery of the targeted-tumor component through optimum encapsulation of the photosensitizer, drug inactivation protection that is induced by plasma, premature leakage of the drug, an enhancement in the uptake within the tumor tissue and cell along with specific drug release in the tumor environment and distribution.184
Indocyanine green (ICG), an amphiphilic tricarbocyanine dye, has been considered safe for use by the FDA. The emission maxima of ICG are around 800 nm; as a result, it is very suitable for bioimaging applications along with a high signal-to-background ratio.185 According to Sheng et al.,186 ICG combined with DoX loaded in nanoagents proved more stable and showed prolonged circulation in blood even in laser radiation.186 The study also revealed that in vivo biodistribution of ICG containing NPs with 50 nm particulate size can be used to target the liver.186 Moreover, PDT of ICG combined with poly(propargyl acrylate) NPs coupled with radiation of 780 nm decreased tumor cell growth significantly and statistically. The review also mentioned the use of modified ICG with BSA along with core magnetic nanoparticles for high optical imaging performance in DDSs.186
According to Pei Wei et al.,187 an amine modified into a layered double dihydroxide (LDH) along with indocyanine green as a contrast agent was declared safe for usage by the FDA for optical imaging in in vivo studies. LDH showed better biocompatibility and non-cytotoxicity. It also enhanced the solubility of drug. LDH–NH2–ICG coated chitosan confirmed the stability of nanocomposites in the in vivo studies.187 Furthermore, the noninvasive in vitro imaging studies of LDHs–NH2–ICG nanoparticles coupled with varying amounts of coatings of chitosan which were administered intravenously in nude mice that were anesthetized showed a visible fluorescence in the liver and lungs. The pairing of chitosan and LDHs–NH2–ICG showed an enhanced potential for the development of organ-specific DDSs and in vivo contrast agents for cancer diagnosis and chemotherapy.187
Nowadays PDT is very effective in cancer therapy, and palladium NPs are not only cost-effective but also provide photoacoustic imaging and photothermal therapy.188 Phan189 designed a porous flower-shaped palladium nanostructure using chitosan vitamin C, which, when administered in vitro, the photons absorbed by the nanostructure convert into heat energy, thus destroying cancer cells.189 In a similar investigation, sugar-based moieties due to their richness in hydroxyl protons can be used for phototherapy in cancers as per Han et al.190 These moieties make use of novel MRI technology and chemical exchange saturation transfer (CEST), therefore preventing the need of chemical labeling.190
Jin et al.191 reported that, though Au NPs attained great importance in cancer phototherapy, they have a limitation of surface toxicity. To reduce this, Au NPs need to be coated with organic polymers.191 There are advantages and disadvantages of organic polymer coating on gold nanorods; thus, further modification is essential to coating gold nanorods with polymer.191 Conjugation with target molecules could be done that can enhance the photothermal therapy (PTT). Combination therapy like chemotherapy, gene therapy, PTT, and the organic polymeric coating would enhance the DDS of gold nanorods.191
The use of PDT has now become an emerging trend in cancer therapy. Over the years, the revolutionary technologies of PDT have shifted sources moving from ultraviolet/visible (UV/vis) light to near-infrared (NIR) light up conversion fluorescence to NIR two-photon excitation to X-ray radiation and finally self-illumination (chemiluminescence, bioluminescence, and Cerenkov luminescence).192 This fact has brought about the promising development of PDT that offers better therapeutic efficacy. The use of these technologies, however, offers drawbacks. These include injury caused due to ionizing radiation, ablation of tissue which occurs due to long-term irradiation, and insufficient self-illumination efficiency.193 Therefore, the use of persistence luminescence has become important to eradicate these problems. The material of persistent luminescence (PersLum) can emit fluorescence even after the light source has ceased.194 Sun et al.195 developed a PersLum calcium alginate-based hydrogel for highly efficient PDT. This hydrogel possesses favorable characteristics such as biological compatibility, persistent luminescence which is bright, renewability of red light, acceptable syringe ability along with a strong fixing ability in cancers which can be introduced easily as a powerful localized light source via injection in vivo for superior persistent luminescence-sensitized photodynamic therapy.195
In Cancer Imaging
Despite the innovative research conducted in the field of cancer, it has taken millions of lives around the globe, especially in developing countries. Early stage cancer detection has now become one of the main focuses of research in the field of nanomedicines by scientists.196 The nanomaterial semiconductor known as quantum dots was found to be very potent in the detection of cancer when bound to biological molecule as it is very effective in the optical imaging of necrotic cells.197
Mansur et al.198 designed, synthesized, and characterized new multifunctional immunoconjugates comprised of a fluorescent inorganic core containing quantum dots (QDs) along with an organic shell composed of antibody-modified polysaccharide chitosan and applied them for the in vitro diagnosis of cancers such as non-Hodgkin lymphoma (NHL).198 The images of transmission electron microscopy (TEM) and UV–vis absorption results showed the development of ultrasmall nanocrystals whose average diameters ranged between 2.5 and 3.0 nm. Results of photoluminescence also showed that the immunoconjugates produced a “green” fluorescence under ultraviolet excitation. The laser light scattering immunoassay also confirmed binding of antigen to the quantum dots, thereby proving to be promising for the early stage detection of carcinoma.198
The glycol chitosan has been one of the most interesting target substances that have gained attention as a theragnostic agent.199 According to Rhee et al.,199 when glycol chitosan is combined with siRNA, stability is observed in vivo. Fullerene, an essential luminescence agent, has proved ineffective when combined with a biological molecule.199 However, it was found to enhance optical imaging characteristics in detecting tumor cells when doped with glycol chitosan NPs.199 Similarly, porphyrins also obtained better results when glycol chitosan NPs were loaded with it. Photosensitizes are crucial for PTT as they absorb NIR followed by the conversion of luminous energy to thermal energy thereby producing cytotoxic heat to tumor cells.199 Since chitosan and other polymer conjugates can be incorporated with ICG and are deemed safe for use by the FDA, they are already being used as nanocarriers for PTT.199
In a similar investigation, Song et al.200 designed a new nanocarrier. The nanocarrier is comprised of a coating of chitosan near-infrared (NIR) layered double hydroxide–indocyanine green nanocomposites.200 This nanocarrier which contained a double layer of chitosan exhibited a more enhanced phototherapeutic effect when compared with uncoated LDH–NH2–ICG as well as when compared with LDH–NH2–ICG with a single layer chitosan coating due to the superior photosensitizer protection against photo and thermal degradations.200 ICG approved by the FDA is the most common contrast agent known for PDT and photoacoustic image guidance chemotherapy.200 However, the only drawback that ICG offers is poor blood retention, which has been found to cause loss of drug through the excreta of xenografted mice models. To stabilize ICG, a lot of research was conducted and it was observed that liposomal PLGA offers stability to ICG and thus remains for a prolonged duration in blood circulation.200 Wang et al.201 showed that PLGA ICG nanocarriers can also be used as potential nanocarriers as theranostic agents.
Park et al.202 observed some controversies in Förster energy resonance transfer (FRET) technology in DDSs. According to them the FRET inhibits luminescence from the gold nanoclusters thereby causing inefficiency in tumor image guidance chemotherapy.202 They therefore designed heat- as well as fluorescence-emitting gold nanoclusters that were loaded with albumin nanoparticles (Au NCs/Cy5.5-BSA-NPs), through the optimization of the quantity of gold nanoparticles, gold nanoclusters, and albumin taking into consideration interparticle distances.202 These gold clusters/BSA hybrid nanoparticles of dimensions of ∼150 nm displayed an acceptable hyper thermal effect in the presence of NIR light of 808 nm and possessed a well-preserved fluorescence intensity due to the contribution of the Cy 5.5’s surface modification. Au NCs/BSA-NPs significantly caused a suppression of the tumors and due to a decrease in FRET allowed visualization of tumor sites.202
Researchers found it challenging to study the interactions of nanoparticles with biological systems. As a result, control of the delivery systems in vivo is unpredictable. Donahue et al.203 proposed the thought of internalization of pathways and summarized the reasons for which the physicochemical properties of nanoparticles correspond and can affect cellular interactions.203
As a Nanocarrier
Nanocarriers for image guidance have received tremendous attention as theragnostic agents. Polymeric materials and organic nanosystems have also been developed for PTT.204 Zhang et al.205 researched the ability of melanin NPs as efficient drug delivery systems for image guided chemotherapy.205 Thus, melanin is a biopolymer that possesses acceptable biocompatibility, biodegradability, and photoacoustic properties that are intrinsic in nature and the capability to bind to drugs which can thus be used in the development of an endogenous nano-DDS that is efficient for image-guided chemotherapy.205 NPs of melanin were formulated by loading them with the drug sorafenib to increase its hydrophilicity. Melanin–sorafenib shares π (pi) bond interactions.205 The SRFMNPs showed an equivalent anticancer effect as compared to the NPs that were polymeric, while the final loading dose that was used in the MNPs system was less (SRF 4 mg kg–1, one time every 2 days for MNPs vs SRF 3 mg kg–1, three times every 4 d for polymeric NPs), which shows that the MNP-based drug-delivery system was more advantageous and could at least provide the same antitumor effect which is seen with traditional polymeric-NP-based DDSs205 shown in Figure 10. Moreover, the traditional nanoplatform for imaging-guided therapy requires functionalization, and the introduction of contrast agents is complicated and can cause toxicity. MNP formulation involves a simple preparative procedure and is thus more appropriate to safe imaging guided therapy.39
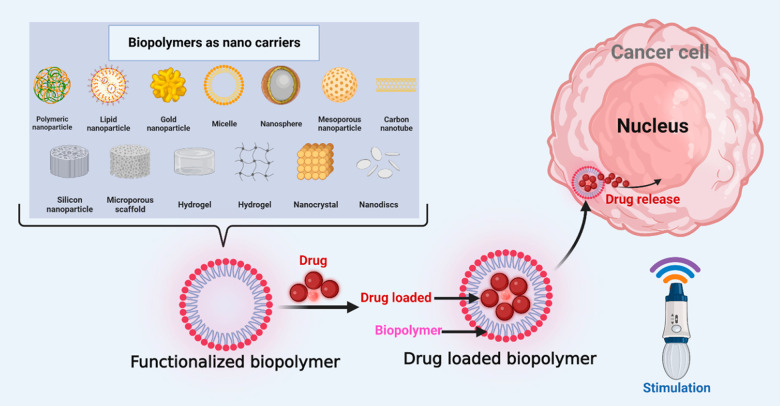
Figure 10.
Nanoparticles work as functionalized nanocarriers with a combination of biopolymers.
Bovine serum albumin has been established as a nanocarrier for efficient drug delivery. Recent studies portray its significance in fluorescence studies as it is inexpensive, is sensitive, and can be used for noninvasive optical imaging in biomedical applications.206 Bovine serum albumin (BSA) alone is insignificant and does not have photoluminescence properties. Thus, Pan et al.207 researched a nano-DDS comprised of BSA doped with gold nanoclusters, iron NPs, and gold nanorods as fluorescence agents. Hence, gold nanorods and clusters are nontoxic, inert, and superior as fluorescent probes rather than photobleachable agents and can also show potential for imaging in vivo due to their emission as well as two-photon excitation that lie within the near-infrared (NIR) “biological window” between 650 and 900 nm.207 Disease detection imaging can be performed by magnetic resonance imaging (MRI). The combination loaded with drug DOX showed efficient delivery in hepatocarcinoma cells significantly leading to apoptosis, whereas the photoluminescence of the nanocarriers benefited in visualization of the delivery via utilization of fluorescence microscopy.207
The understanding of the fate of nanocarriers in vivo is limited. Zhao et al.208 presented a detailed survey on the fate of nanocarriers in vivo. The study reflected the relationship between the properties of NPs and that of drug accumulation at the tumor site as well as enhanced retention.208 It described that nanocarrier circulation depends on the size, shape, and surface charge. Nanomaterials with 30 nm dimension can permeate deeply in tumors, and spherically shaped particles have better penetration rather than rod shaped particles.208 The surface charge is also significant and is related with drug accumulation.208 The increase in surface charge densities that resulted in a decline in tumor accumulation was observed with negatively charged NPs, while higher charge density positively charged particles showed an enhanced accumulation in tumors.208 The authors attributed this observation to the fact that NPs that possessed higher positive charge density could depart from the interstitial space more efficiently and could be internalized by tumor cells along with the associated endothelium.208 The hydrophobicity effect is also very important in the DDS nanocarrier mechanism wherein hydrophobicity enhances plasma protein’s absorbance, thereby providing faster blood clearance.209
Chitosan with fucoidan gold nanorods working as a nanocarrier for PTT is now a fascinating area of research for cancer therapy.210 Manivasagan et al.210 studied the in vitro and in vivo efficacies of the nanocarrier. They observed that fucoidan gold nanorods encamped in chitosan (dimensions of 51.87 ± 3.03 nm), in a NIR absorbance after intravenous injection to breast cancer Balb/c mice model (MDA-MB-231 mice model) followed by irradiation for 5 min post injection and after 6 h post injection at 54.4 °C. The study revealed that after 20 days of the laser treatment total ablation of tumor cells was achieved.210
6. Challenges and Outlook
Despite recent advancements in the drug delivery system and the utilization of a wide variety of nanomaterials with appropriate properties, significant problems still persist in cancer management and treatment. The enormous progress made in delivering drugs over the last several years has led many people to believe that nanomaterials will alter the whole healthcare system. However, only a limited number of nanoformulations have entered into clinical trials due to the difficulty in designing effective cancer nanotherapeutics. The physicochemical features of nanomaterials strongly influence biocompatibility and toxicity in biological systems. Therefore, it is important to take precautions during the fabrication and characterization of the nano-biomaterials used in delivering medicine to reduce the risk of unintended toxicity to normal cells. Because of their interactions with biomolecules, nanocarriers may tend to accumulate into protein aggregates, which disrupt the normal operation of formulating nanomedicine and render those nanodrugs useless for preventing the proliferation of cancer cells. In addition, the pharmacological efficacy of nanomaterials may be affected by how they are stored. Concerns about nanomaterial-related side effects, which may or may not have an immediate impact or be perceptible, provide another difficulty in drug delivery. Nanocarriers have the potential to cause unintended toxicity in the treatment of cancer by interacting with cells in a way that is detrimental to the treatment’s efficacy. Drug release profiles have not been shown to correlate well between in vitro and in vivo settings, which is a significant unresolved issue. Although targeted drug delivery has shown promise in cancer therapy and diagnosis, there is still much to discover about analyzing events during drug interaction at the intercellular level and making predictions. Important data on the potential of nanoformulations as therapeutic medicines are gleaned through animal studies. Enormous research is needed in several areas, including the cost-effectiveness of anticancer medicinal molecules and the immunogenic effects of these drugs on humans and the environment. Another significant hurdle is the scaling-up of the production of nanomedicine or biopolymeric products for commercial use.
Out of many, one important concern is the ability of crossing the blood–brain barrier (BBB) as many chemotherapeutic drugs are unable or inefficient to cross the barrier and thus fail to bind to their respective target receptor. To overcome this challenge, scientists develop some nanocarrier molecules capable of integrating or carrying both hydrophilic and hydrophobic drug molecules and delivering it target-specifically by crossing the BBB. These nanocarriers are stable compounds having minimal toxicity and an immunogenic effect on our body, which are protected from being degraded enzymatically before reaching their target location.
Although biopolymers offer unique advantages, large-scale production remains a challenge. Ensuring consistent quality and reproducibility of biopolymer-based nanoparticles on a commercial scale requires robust manufacturing processes. Innovations in scalable production methods, process optimization, and quality control will be crucial to bridging the gap between laboratory research and industrial applications.
Lastly, one of the major challenges is to obtain approval from the FDA for the in vivo application of bio-nanocarriers. Since the FDA has not issued any standards for products containing nanomaterials, another major problem is the difficulty of getting these medicines approved for use. Currently, utilized parameters are taken exactly from bulk material specifications. Due to the fact that regulatory decisions on nanoformulated therapeutics are reliant on individual assessments of benefits and risks, evaluations are time-consuming and add delays to commercialization. The emergence of multifunctional nanoplatforms will also likely make getting regulatory approval more challenging.
7. Future Directions and Perspectives of Biopolymer-Based Cancer Formulations
The realm of cancer therapy is witnessing a paradigm shift with the advent of biopolymer-based formulations. The combination of biology and materials science has given rise to a class of adaptable materials that show great potential in transforming cancer treatment tactics. As we contemplate the future, a multitude of captivating avenues and viewpoints arise, exerting an influence on the course of biopolymer-based cancer formulations.
-
(a)
Personalised Therapies: The field of cancer treatment is undergoing a transformation from a uniform approach to tailored therapies that accommodate the unique genetic characteristics and illness profiles of individual patients. Biopolymers provide a platform for the implementation of personalized medicine, enabling the customization of formulations to meet the specific requirements of individual patients. The customization of biopolymer-based formulations through the fine-tuning of characteristics such as nanoparticle size, surface qualities, and cargo payloads enables the optimization of drug delivery, the minimization of adverse effects, and the enhancement of therapeutic outcomes.
-
(b)
Combination Therapies: Combination therapies have become increasingly prominent in the field of medicine due to their potential to synergistically combine multiple treatment modalities. These therapies are particularly valuable in overcoming drug resistance and improving the overall effectiveness of treatment. Biopolymer-based systems have the potential to enable the simultaneous administration of diverse agents, including chemotherapeutic medicines, immunomodulators, and gene treatments. These platforms have the capability to coordinate complex therapy sequences, facilitating the controlled release of various medicines at precise stages of cancer growth. The inherent versatility of biopolymers allows for the development of formulations that effectively enhance synergistic effects while simultaneously reducing unfavorable interactions.
-
(c)
Targeting and Image Precision: The future of cancer therapy is contingent upon the attainment of precise targeting and imaging capabilities. Biopolymer-based nanoparticles possess the capability to be manipulated in such a way that they can bear ligands with a high degree of specificity toward cancer cell surface indicators. This enables them to facilitate targeted administration, hence mitigating the occurrence of off-target effects. Additionally, the functionalization of biopolymers with imaging agents offers the capability to monitor treatment responses in real time, hence facilitating the customization of therapy by physicians in a dynamic manner.
-
(d)
Overcoming Biological Barriers: The successful distribution of drugs encounters obstacles presented by biological barriers, including the blood–brain barrier and the stromal barrier found in solid tumors. Biopolymers have the ability to be engineered in a manner that allows them to effectively overcome these obstacles. Biopolymer-based formulations have the potential to open up novel possibilities for treating previously inaccessible areas by manipulating the size, surface charge, and functional groups of engineered nanoparticles to overcome existing obstacles.
-
(e)
Immunomodulation Strategies: The utilization of immunomodulation strategies to leverage the innate capabilities of the human immune system in the fight against cancer is an emerging and rapidly growing area of research. The utilization of biopolymer-based formulations presents an opportunity to effectively administer immunomodulatory drugs to immune cells or the tumor microenvironment. These formulations have the potential to be developed in order to regulate immune responses, modulate inflammation associated with tumors, and improve the effectiveness of immune checkpoint inhibitors, thus introducing a new age of immunotherapies.
-
(f)
Continuous Drug Release: Continuous release of drugs is a persistent obstacle in the field of cancer therapy, particularly in the context of chronic ailments. Biopolymers possess the capability to be manipulated in a manner that enables the provision of continuous and regulated release of drugs, hence facilitating the maintenance of therapeutic concentrations for prolonged durations. The aforementioned feature holds significant relevance in the context of slow-growing tumors, as it reduces the necessity for frequent administration of medication and enhances patient adherence to treatment protocols.
-
(g)
Biodegradability and Safety: The future of cancer formulations places significant emphasis on the biodegradability and safety aspects, with a particular focus on ensuring low occurrence of adverse effects. Biopolymers, frequently sourced from natural origins, possess inherent characteristics of biocompatibility and biodegradability. As scientific progress continues, it is anticipated that there will be a refinement of biopolymer compositions in order to minimize the likelihood of eliciting immune reactions and to enhance their effective elimination from the body.
-
(h)
Clinical Translation and Regulatory Approval: The process of translating cancer formulations based on biopolymers from laboratory experiments to practical application involves successfully navigating the intricate realm of regulatory approvals and conducting clinical trials. In the coming years, there is a growing expectation for enhanced cooperation among researchers, doctors, and regulatory bodies. This collaborative effort aims to optimize the approval process and facilitate the timely accessibility of groundbreaking therapies for patients.
-
(i)
Hybrid Approaches: In the future, it is possible that biopolymers might be combined with other modern technologies, such as nanomedicine, gene editing, and 3D printing, in order to develop new formulations. The integration of hybrid methodologies has the potential to facilitate the development of versatile platforms that effectively tackle multiple facets of cancer treatment, encompassing diagnostic procedures, imaging techniques, targeted drug administration, and tailored therapeutic interventions.
8. Conclusion
Nanomedicine for biomedical application has sought enormous attraction nowadays not only in the scientific forum but also among the public. The newest approach of design principles has been reported in an animal model but was not actuated or translated. The natures of EP and R and their dynamics need to be clarified in a better perspective in clinical settings. Several attempts to enhance EP and R were carried out in an artificial rodent tumor model, which does not depict human physiology or pathology in terms of transport or delivery. Therefore, scientists must place emphasis on this aspect. The studies on nanocarriers conducted as DDSs and performed in vitro and in vivo showed discrepancies in clinical trials. Therefore, nanoparticles should be used with due caution. Thus, instead of developing better or enhanced DDS target site drugs, researchers must give enormous importance in overcoming the adverse effects of existing nanomedicines, thereby widening the prospective for anticancer nanomedicines with better efficacy. In the past decades, researchers wanted to bridge the gap of the inconsistencies observed in in vitro and in vivo studies by chalking out alternate ways like 3D cancer models. Thus, from this extensive literature review our team aims to develop existing nanomedicines with enhanced drug delivery characteristics along with the design of a clinical trial model based on a rodent tumor model that is of close resemblance to human physiology.
Glossary
Abbreviations
- NPs
nanoparticles
- EPR
enhanced permeation rate
- PLA
polylactic acid
- DDS
drug delivery system
- NC
nanocarrier
- LPN
lipid nanoparticles
- LET
lauryl ester of tyrosine
- MMt
montmorillonite
- Au NPs
gold nanoparticles
- HG NPs
hollow gold nanoparticles
- ARPI
acetylated rapeseed protein isolate
- CNPs
chitosan nanoparticles
- HPMA
N-(2-hydroxypropyl) methacrylamide
- MNPs
ferric oxide nanoparticles
- GaBS NPs
glycyrrhizic acid-biotin-starch NPs
- FCA
fluorescence correlation spectroscopy
- CBSA
cationic bovine serum albumin
- ESC8
estrogenic derivative
- (IC)10
indocyanine
- ABC transporters
adenosine triphosphate binding cassette transporters
- MDR
multidrug resistance
- ECM
extracellular matrix
- HRNM
hierarchical responsive nanomedicine
- NIR
near infrared
- DOX
doxorubicin
- ICG
indocyanine green
- QDs
quantum dots
- NHL
non-Hodgkin lymphoma
- TEM
transmission electron microscopy
- SEM
scanning electron microscopy
- AFM
atomic fluorescence microscopy
- FRET
Förster energy resonance transfer
- PTT
photothermal therapy
- BSA
bovine serum albumin
- LDH
amine modified layered double dihydroxide
- Au NCs/Cy5.5-BSA-NPs
gold nanocluster loaded albumin nanoparticles
- NM
lymph node metastasis
- MiRNA
MicroRNAs
- PAMAM
fourth generation poly(amidoamine) with thiol at the terminal end
- PEG
polyethylene glycol
- PLD
PEGylated liposomal doxorubicin
- ELR
electro spun release
- ED
embryonic lethal/mesodermal defects
- PTX
paclitaxel
- scFv
single chain antibody fragments
- Fabs
antigen binding fragments
- Hsp
heat shock protein
- BBB
blood–brain barrier
- LCT
lower critical solution temperature
- UCT
upper critical solution temperature
- PMIL
photoactive multi-inhibitor nano liposomes
- VEGEF
vascular endothelial growth factor
- ROS
reactive oxygen species
- HA
hyaluronic acid
Author Contributions
& T.B. and S.P. contributed equally.
N.D. Thorat acknowledges funding under the Science Foundation Ireland and Irish Research Council (SFI-IRC) pathway program (21/PATH-S/9634).
The authors declare no competing financial interest.
References
- Sung H.; et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2021, 71 (3), 209–249. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Al Sufyani N. M.; Hussien N. A.; Hawsawi Y. M. Characterization and anticancer potential of silver nanoparticles biosynthesized from Olea chrysophylla and Lavandula dentata leaf extracts on HCT116 colon cancer cells. J. Nanomater. 2019, 2019, 7361695. 10.1155/2019/7361695. [DOI] [Google Scholar]
- Ratan Z. A.; et al. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers 2020, 12 (4), 855. 10.3390/cancers12040855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A.; et al. Global cancer statistics. CA: a cancer journal for clinicians 2011, 61 (2), 69–90. 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Patra C. R.; Mukherjee S.; Kotcherlakota R. Biosynthesized silver nanoparticles: a step forward for cancer theranostics?. Nanomedicine 2014, 9 (10), 1445–1448. 10.2217/nnm.14.89. [DOI] [PubMed] [Google Scholar]
- Ovais M.; et al. Green synthesis of silver nanoparticles via plant extracts: beginning a new era in cancer theranostics. Nanomedicine 2016, 11 (23), 3157–3177. 10.2217/nnm-2016-0279. [DOI] [PubMed] [Google Scholar]
- Zivyar N.; et al. Evaluation of the green synthesis, characterization and antibacterial activity of silver nanoparticles from corm extract of Crocus sativus var. Haussknechtii. Journal of Horticulture and Postharvest Research 2021, 4, 19–32. 10.22077/jhpr.2021.3812.1177. [DOI] [Google Scholar]
- Khan S.; et al. Ellagic acid attenuates bleomycin and cyclophosphamide-induced pulmonary toxicity in Wistar rats. Food and chemical toxicology 2013, 58, 210–219. 10.1016/j.fct.2013.03.046. [DOI] [PubMed] [Google Scholar]
- Wang M.; Thanou M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62 (2), 90–99. 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A. Ligand-based active targeting strategies for cancer theranostics. Naunyn-Schmiedeberg's Arch. Pharmacol. 2023, 10.1007/s00210-023-02612-4. [DOI] [PubMed] [Google Scholar]
- Nahak B. K.; et al. Advances in organ-on-a-chip materials and devices. ACS applied bio materials 2022, 5 (8), 3576–3607. 10.1021/acsabm.2c00041. [DOI] [PubMed] [Google Scholar]
- Halwani A. A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14 (1), 106. 10.3390/pharmaceutics14010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D.; et al. Docetaxel (DTX)-loaded polydopamine-modified TPGS-PLA nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Acta Biomater 2016, 30, 144–154. 10.1016/j.actbio.2015.11.031. [DOI] [PubMed] [Google Scholar]
- Qin X.; et al. Artificial Nucleotide-containing Aptamers Used in Tumor Therapy. Chemical Research in Chinese Universities 2020, 36 (2), 164–170. 10.1007/s40242-019-0033-2. [DOI] [Google Scholar]
- Yang Y.; et al. Smart materials for drug delivery and cancer therapy. VIEW 2021, 2 (2), 20200042. 10.1002/VIW.20200042. [DOI] [Google Scholar]
- Vasanth K.; et al. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf., B 2014, 117, 354–359. 10.1016/j.colsurfb.2014.02.052. [DOI] [PubMed] [Google Scholar]
- Govindaraju K.; et al. Green synthesis of silver nanoparticles for selective toxicity towards cancer cells. IET nanobiotechnology 2015, 9 (6), 325–330. 10.1049/iet-nbt.2015.0001. [DOI] [PubMed] [Google Scholar]
- Vaid P.; et al. Biogenic silver, gold and copper nanoparticles-A sustainable green chemistry approach for cancer therapy. Sustainable Chemistry and Pharmacy 2020, 16, 100247. 10.1016/j.scp.2020.100247. [DOI] [Google Scholar]
- Barabadi H.; et al. Anti-cancer green bionanomaterials: present status and future prospects. Green Chemistry Letters and Reviews 2017, 10 (4), 285–314. 10.1080/17518253.2017.1385856. [DOI] [Google Scholar]
- Manikandan R.; et al. Biosynthesis of silver nanoparticles using ethanolic petals extract of Rosa indica and characterization of its antibacterial, anticancer and anti-inflammatory activities. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2015, 138, 120–129. 10.1016/j.saa.2014.10.043. [DOI] [PubMed] [Google Scholar]
- Mishra R. K.; et al. Recent progress in selected bio-nanomaterials and their engineering applications: An overview. Journal of Science: Advanced Materials and Devices 2018, 3 (3), 263–288. 10.1016/j.jsamd.2018.05.003. [DOI] [Google Scholar]
- Faridi Esfanjani A.; Jafari S. M. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–43. 10.1016/j.colsurfb.2016.06.053. [DOI] [PubMed] [Google Scholar]
- Utreja P.; Jain S.; Tiwary A. K. Novel drug delivery systems for sustained and targeted delivery of anti-cancer drugs: current status and future prospects. Curr. Drug Delivery 2010, 7 (2), 152–161. 10.2174/156720110791011783. [DOI] [PubMed] [Google Scholar]
- Ovais M.; et al. Current state and prospects of the phytosynthesized colloidal gold nanoparticles and their applications in cancer theranostics. Applied microbiology and biotechnology 2017, 101 (9), 3551–3565. 10.1007/s00253-017-8250-4. [DOI] [PubMed] [Google Scholar]
- Liu J.; Cui L.; Losic D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta biomaterialia 2013, 9 (12), 9243–9257. 10.1016/j.actbio.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Khan W. Polysaccharide Based Nanoparticles. Isr. J. Chem. 2018, 58 (12), 1315. 10.1002/ijch.201800051. [DOI] [Google Scholar]
- Gopi S. Biopolymers and Their Composites for Drug Delivery: A Brief Review. Macromol. Symp. 2018, 380 (1), 1800114. 10.1002/masy.201800114. [DOI] [Google Scholar]
- Song H.; et al. Current status and prospects of camrelizumab, a humanized antibody against programmed cell death receptor 1. Recent Patents on Anti-Cancer Drug Discovery 2021, 16 (3), 312–332. 10.2174/22123970MTE0eMDY90. [DOI] [PubMed] [Google Scholar]
- Calzoni E. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct Biomater 2019, 10 (1), 4. 10.3390/jfb10010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S.; et al. High drug-loading nanomedicines: progress, current status, and prospects. International journal of nanomedicine 2017, 12, 4085. 10.2147/IJN.S132780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M. Z.; et al. Nanometric gold in cancer nanotechnology: current status and future prospect. J. Pharm. Pharmacol. 2013, 65 (5), 634–651. 10.1111/jphp.12017. [DOI] [PubMed] [Google Scholar]
- Kim C. S.; et al. Inorganic nanosystems for therapeutic delivery: Status and prospects. Advanced drug delivery reviews 2013, 65 (1), 93–99. 10.1016/j.addr.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Fattah W. I.; Ali G. W. On the anti-cancer activities of silver nanoparticles. J. Appl. Biotechnol Bioeng 2018, 5 (1), 43–46. 10.15406/jabb.2018.05.00116. [DOI] [Google Scholar]
- Luo S.-H. Direct Metal-Free Preparation of Functionalizable Polylactic Acid-Ethisterone Conjugates in a One-Pot Approach. Macromol. Chem. Phys. 2019, 220 (5), 1800475. 10.1002/macp.201800475. [DOI] [Google Scholar]
- Andleeb A.; et al. A systematic review of biosynthesized metallic nanoparticles as a promising anti-cancer-strategy. Cancers 2021, 13 (11), 2818. 10.3390/cancers13112818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua S.; Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano today 2014, 9 (2), 223–243. 10.1016/j.nantod.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preetam S.; et al. Emergence of microfluidics for next generation biomedical devices. Biosensors and Bioelectronics: X 2022, 10, 100106. 10.1016/j.biosx.2022.100106. [DOI] [Google Scholar]
- Li W.; et al. Tumor-Associated Fibroblast-Targeting Nanoparticles for Enhancing Solid Tumor Therapy: Progress and Challenges. Mol. Pharmaceutics 2021, 18 (8), 2889–2905. 10.1021/acs.molpharmaceut.1c00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; et al. Magnetic resonance imaging-guided and targeted theranostics of colorectal cancer. Cancer Biol. Med. 2020, 17 (2), 307–327. 10.20892/j.issn.2095-3941.2020.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.; et al. Magnetic nanoparticles coated with polyphenols for spatio-temporally controlled cancer photothermal/immunotherapy. J. Controlled Release 2020, 326, 131–139. 10.1016/j.jconrel.2020.06.015. [DOI] [PubMed] [Google Scholar]
- Zeng Q.; et al. Polydopamine nanoparticle-dotted food gum hydrogel with excellent antibacterial activity and rapid shape adaptability for accelerated bacteria-infected wound healing. Bioactive Materials 2021, 6 (9), 2647–2657. 10.1016/j.bioactmat.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto T.; Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nature Reviews Cancer 2017, 17 (2), 93–115. 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Esteban L. C.; Fajas L. Cell cycle regulators in cancer cell metabolism. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2020, 1866 (5), 165715. 10.1016/j.bbadis.2020.165715. [DOI] [PubMed] [Google Scholar]
- Kontomanolis E. N.; et al. Role of oncogenes and tumor-suppressor genes in carcinogenesis: a review. Anticancer research 2020, 40 (11), 6009–6015. 10.21873/anticanres.14622. [DOI] [PubMed] [Google Scholar]
- Lipsick J. A history of cancer research: tumor suppressor genes. Cold Spring Harbor Perspectives in Biology 2020, 12 (2), a035907. 10.1101/cshperspect.a035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptain S.; Tan L. K.; Chen B. Her-2/neu and breast cancer. Diagnostic molecular pathology 2001, 10 (3), 139–152. 10.1097/00019606-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Chen L.; Liu S.; Tao Y. Regulating tumor suppressor genes: post-translational modifications. Signal transduction and targeted therapy 2020, 5 (1), 90. 10.1038/s41392-020-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahak B. K. Advances in Organ-on-a-Chip Materials and Devices. ACS Applied Bio Materials 2022, 5, 3576. 10.1021/acsabm.2c00041. [DOI] [PubMed] [Google Scholar]
- Preetam S.; et al. Application of Nanobiosensor in Health Care Sector. Bio-Nano Interface 2022, 251–270. 10.1007/978-981-16-2516-9_14. [DOI] [Google Scholar]
- Slavin Y. N.; et al. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15 (1), 65. 10.1186/s12951-017-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. K. Comparative therapeutic effects of plant-extract synthesized and traditionally synthesized gold nanoparticles on alcohol-induced inflammatory activity in SH-SY5Y cells in vitro. Biomedicines 2017, 5 (4), 70. 10.3390/biomedicines5040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabadi H.; et al. Efficacy of green nanoparticles against cancerous and normal cell lines: a systematic review and meta-analysis. IET nanobiotechnology 2018, 12 (4), 377–391. 10.1049/iet-nbt.2017.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum D.; et al. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9 (1), 1410. 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R.; et al. Current development of biodegradable polymeric materials for biomedical applications. Drug Des Devel Ther 2018, 12, 3117–3145. 10.2147/DDDT.S165440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preetam S.; et al. Therapeutic potential of Lipid Nanosystems for the treatment of Parkinson’s disease: an updated review. Ageing Research Reviews 2023, 89, 101965. 10.1016/j.arr.2023.101965. [DOI] [PubMed] [Google Scholar]
- Pradhan A. K.; et al. Green synthesis of silver nanoparticles from various plant extracts and its applications: A mini review. World Journal of Biology Pharmacy and Health Sciences 2022, 11 (1), 050–061. 10.30574/wjbphs.2022.11.1.0100. [DOI] [Google Scholar]
- Bhattacharjee R.; et al. The emergence of metal oxide nanoparticles (NPs) as a phytomedicine: A two-facet role in plant growth, nano-toxicity and anti-phyto-microbial activity. Biomedicine & Pharmacotherapy 2022, 155, 113658. 10.1016/j.biopha.2022.113658. [DOI] [PubMed] [Google Scholar]
- Sharma K.; Porat Z.e.; Gedanken A. Designing Natural Polymer-Based Capsules and Spheres for Biomedical Applications—A Review. Polymers 2021, 13 (24), 4307. 10.3390/polym13244307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal A.; et al. Natural antioxidants as defense system against cancer. Asian Journal of Pharmaceutical and Clinical Research 2018, 11, 38–44. 10.22159/ajpcr.2018.v11i5.24119. [DOI] [Google Scholar]
- Abdalla A. M.; et al. Current challenges of cancer anti-angiogenic therapy and the promise of nanotherapeutics. Theranostics 2018, 8 (2), 533. 10.7150/thno.21674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbiah R.; Veerapandian M.; Yun K. S. Nanoparticles: functionalization and multifunctional applications in biomedical sciences. Curr. Med. Chem. 2010, 17 (36), 4559–4577. 10.2174/092986710794183024. [DOI] [PubMed] [Google Scholar]
- Ulbricht M. Design and synthesis of organic polymers for molecular separation membranes. Current Opinion in Chemical Engineering 2020, 28, 60–65. 10.1016/j.coche.2020.02.002. [DOI] [Google Scholar]
- Patra J. K.; et al. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnology 2018, 16 (1), 71. 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samal S. K.; Preetam S.. Synthetic Biology: Refining Human Health, in Microbial Engineering for Therapeutics; Springer: 2022; pp 57–70. [Google Scholar]
- Salehi S.; et al. Phytosynthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. International journal of nanomedicine 2016, 11, 1835. 10.2147/IJN.S99882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanra K.; et al. Evaluation of antibacterial activity and cytotoxicity of green synthesized silver nanoparticles using Scoparia dulcis. Nano Biomed. Eng. 2015, 7 (3), 128–133. 10.5101/nbe.v7i3.p128-133. [DOI] [Google Scholar]
- Venugopal K.; et al. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. Journal of Photochemistry and Photobiology B: Biology 2017, 167, 282–289. 10.1016/j.jphotobiol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Kanipandian N.; Thirumurugan R. A feasible approach to phyto-mediated synthesis of silver nanoparticles using industrial crop Gossypium hirsutum (cotton) extract as stabilizing agent and assessment of its in vitro biomedical potential. Industrial Crops and Products 2014, 55, 1–10. 10.1016/j.indcrop.2014.01.042. [DOI] [Google Scholar]
- Lee M. K. Liposomes for Enhanced Bioavailability of Water-Insoluble Drugs: In Vivo Evidence and Recent Approaches. Pharmaceutics 2020, 12 (3), 264. 10.3390/pharmaceutics12030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss V. M. Intended and Unintended Targeting of Polymeric Nanocarriers: The Case of Modified Poly(glycerol adipate) Nanoparticles. Macromol. Biosci 2018, 18 (1), 1700240. 10.1002/mabi.201700240. [DOI] [PubMed] [Google Scholar]
- Sverdlov Arzi R.; Sosnik A. Electrohydrodynamic atomization and spray-drying for the production of pure drug nanocrystals and co-crystals. Adv. Drug Deliv Rev. 2018, 131, 79–100. 10.1016/j.addr.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Zhen G.; et al. Glycerol monooleate-based nanocarriers for siRNA delivery in vitro. Mol. Pharmaceutics 2012, 9 (9), 2450–7. 10.1021/mp200662f. [DOI] [PubMed] [Google Scholar]
- Nahire R.; et al. Polymer-coated echogenic lipid nanoparticles with dual release triggers. Biomacromolecules 2013, 14 (3), 841–53. 10.1021/bm301894z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scioli Montoto S.; Muraca G.; Ruiz M. E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front Mol. Biosci 2020, 7, 587997. 10.3389/fmolb.2020.587997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorenberg M. R.; et al. Synthesis and Purification of Homogeneous Lipid-Based Peptide Nanocarriers by Overcoming Phospholipid Ester Hydrolysis. ACS Omega 2018, 3 (10), 14144–14150. 10.1021/acsomega.8b01772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehsorkhi A.; Castelletto V.; Hamley I. W. Self-assembling amphiphilic peptides. J. Pept Sci. 2014, 20 (7), 453–67. 10.1002/psc.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H.; Webber M. J.; Stupp S. I. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers 2010, 94 (1), 1–18. 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S.; et al. Exploring Microbial-Based Green Nanobiotechnology for Wastewater Remediation: A Sustainable Strategy. Nanomaterials 2022, 12 (23), 4187. 10.3390/nano12234187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaly N.; et al. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116 (4), 2602–63. 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzoff M.; et al. Synthesis and Characterization of Hybrid Polymer/Lipid Expansile Nanoparticles: Imparting Surface Functionality for Targeting and Stability. Biomacromolecules 2015, 16 (7), 1958–66. 10.1021/acs.biomac.5b00336. [DOI] [PubMed] [Google Scholar]
- Kang B.; et al. Carbohydrate-Based Nanocarriers Exhibiting Specific Cell Targeting with Minimum Influence from the Protein Corona. Angew. Chem., Int. Ed. Engl. 2015, 54 (25), 7436–40. 10.1002/anie.201502398. [DOI] [PubMed] [Google Scholar]
- Begines B. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials (Basel) 2020, 10 (7), 1403. 10.3390/nano10071403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angayarkanny S.; Baskar G.; Mandal A. B. Nanocarriers of solid lipid from micelles of amino acids surfactants coated with polymer nanoparticles. Langmuir 2013, 29 (23), 6805–14. 10.1021/la400605v. [DOI] [PubMed] [Google Scholar]
- Telrandhe R. Anti-Cancer Potential of Green Synthesized Silver Nanoparticles-A Review. Asian J. Pharm. Technol. 2019, 9, 260–266. 10.5958/2231-5713.2019.00043.6. [DOI] [Google Scholar]
- Jurj A.; et al. The new era of nanotechnology, an alternative to change cancer treatment. Drug design, development and therapy 2017, 11, 2871. 10.2147/DDDT.S142337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hira I.; et al. Pectin-guar gum-zinc oxide nanocomposite enhances human lymphocytes cytotoxicity towards lung and breast carcinomas. Materials Science and Engineering: C 2018, 90, 494–503. 10.1016/j.msec.2018.04.085. [DOI] [PubMed] [Google Scholar]
- Seib F. P.; et al. pH-dependent anticancer drug release from silk nanoparticles. Adv. Healthc Mater. 2013, 2 (12), 1606–11. 10.1002/adhm.201300034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namazi H.; Belali S. Starch-g-lactic acid/montmorillonite nanocomposite: Synthesis, characterization and controlled drug release study. Starch 2016, 68 (3–4), 177–187. 10.1002/star.201400226. [DOI] [Google Scholar]
- Suarasan S.; et al. Doxorubicin-Incorporated Nanotherapeutic Delivery System Based on Gelatin-Coated Gold Nanoparticles: Formulation, Drug Release, and Multimodal Imaging of Cellular Internalization. ACS Appl. Mater. Interfaces 2016, 8 (35), 22900–13. 10.1021/acsami.6b07583. [DOI] [PubMed] [Google Scholar]
- Sharma H.; Dormidontova E. E. Lipid Nanodisc-Templated Self-Assembly of Gold Nanoparticles into Strings and Rings. ACS Nano 2017, 11 (4), 3651–3661. 10.1021/acsnano.6b08043. [DOI] [PubMed] [Google Scholar]
- Pushpamalar J. Development of a Polysaccharide-Based Hydrogel Drug Delivery System (DDS): An Update. Gels 2021, 7 (4), 153. 10.3390/gels7040153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S.-S.; et al. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug delivery 2017, 24 (1), 1909–1926. 10.1080/10717544.2017.1410256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Tang L. The application of lncRNAs in cancer treatment and diagnosis. Recent patents on anti-cancer drug discovery 2018, 13 (3), 292–301. 10.2174/1574892813666180226121819. [DOI] [PubMed] [Google Scholar]
- da Silva Luz G. V.; et al. Nanorobotics in drug delivery systems for treatment of cancer: a review. J. Mat Sci. Eng. A 2016, 6, 167–180. 10.17265/2161-6213/2016.5-6.005. [DOI] [Google Scholar]
- Wang Y. Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer. Nanomaterials (Basel) 2016, 6 (2), 26. 10.3390/nano6020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Lorenzo C.; et al. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv Rev. 2013, 65 (9), 1148–71. 10.1016/j.addr.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Kim D. H.; et al. Targeting to carcinoma cells with chitosan- and starch-coated magnetic nanoparticles for magnetic hyperthermia. J. Biomed Mater. Res. A 2009, 88 (1), 1–11. 10.1002/jbm.a.31775. [DOI] [PubMed] [Google Scholar]
- Alkanawati M. S. Large-Scale Preparation of Polymer Nanocarriers by High-Pressure Microfluidization. Macromol. Mater. Eng. 2018, 303 (1), 1700505. 10.1002/mame.201700505. [DOI] [Google Scholar]
- Wang Z.; et al. In Situ Proapoptotic Peptide-Generating Rapeseed Protein-Based Nanocomplexes Synergize Chemotherapy for Cathepsin-B Overexpressing Breast Cancer. ACS Appl. Mater. Interfaces 2018, 10 (48), 41056–41069. 10.1021/acsami.8b14001. [DOI] [PubMed] [Google Scholar]
- Swierczewska M.; et al. Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv. Drug Deliv Rev. 2016, 99 (Pt A), 70–84. 10.1016/j.addr.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne A.; Castaño J. Polysaccharide nanomaterial reinforced starch nanocomposites: A review. Starch 2017, 69 (1–2), 1500307. 10.1002/star.201500307. [DOI] [Google Scholar]
- Mukwaya V.; Wang C.; Dou H. Saccharide-based nanocarriers for targeted therapeutic and diagnostic applications. Polym. Int. 2019, 68 (3), 306–319. 10.1002/pi.5702. [DOI] [Google Scholar]
- Cheng Z.; et al. Nanomaterials for cancer therapy: current progress and perspectives. J. Hematol Oncol 2021, 14 (1), 85. 10.1186/s13045-021-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyril N.; et al. Assessment of antioxidant, antibacterial and anti-proliferative (lung cancer cell line A549) activities of green synthesized silver nanoparticles from Derris trifoliata. Toxicology research 2019, 8 (2), 297–308. 10.1039/C8TX00323H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perveen S.; Al-Taweel A. M. Green chemistry and synthesis of anticancer molecule. Green Chem. 2018, 51–72. 10.5772/intechopen.70419. [DOI] [Google Scholar]
- Singh J.; Singh T.; Rawat M. Green synthesis of silver nanoparticles via various plant extracts for anti-cancer applications. Nanomedicine 2017, 2 (3), 555590. 10.19080/GJN.2017.02.555590. [DOI] [Google Scholar]
- Ju C.; et al. Sequential intra-intercellular nanoparticle delivery system for deep tumor penetration. Angew. Chem., Int. Ed. Engl. 2014, 53 (24), 6253–8. 10.1002/anie.201311227. [DOI] [PubMed] [Google Scholar]
- Ren G.; et al. A unique highly hydrophobic anticancer prodrug self-assembled nanomedicine for cancer therapy. Nanomedicine 2016, 12 (8), 2273–2282. 10.1016/j.nano.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Wu Y.; et al. Fabrication of elastin-like polypeptide nanoparticles for drug delivery by electrospraying. Biomacromolecules 2009, 10 (1), 19–24. 10.1021/bm801033f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbera V.; et al. Environmentally Friendly and Regioselective One-Pot Synthesis of Imines and Oxazolidines Serinol Derivatives and Their Use for Rubber Cross-Linking. ACS Sustainable Chem. Eng. 2020, 8 (25), 9356–9366. 10.1021/acssuschemeng.0c01603. [DOI] [Google Scholar]
- Ntoukam D. H. S.; Mutlu H.; Theato P. Post-polymerization modification of Poly(vinylcyclopropanes): A potential route to periodic copolymers. Eur. Polym. J. 2020, 122, 109319. 10.1016/j.eurpolymj.2019.109319. [DOI] [Google Scholar]
- Pulingam T.; et al. Exploring Various Techniques for the Chemical and Biological Synthesis of Polymeric Nanoparticles. Nanomaterials 2022, 12 (3), 576. 10.3390/nano12030576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senapati S.; et al. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther 2018, 3, 7. 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S.; et al. Electrohydrodynamics: A facile technique to fabricate drug delivery systems. Adv. Drug Deliv Rev. 2009, 61 (12), 1043–54. 10.1016/j.addr.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng L.; Xie J. Smart electrospun nanofibers for controlled drug release: recent advances and new perspectives. Curr. Pharm. Des 2015, 21 (15), 1944–59. 10.2174/1381612821666150302151959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.; et al. RAD54B potentiates tumor growth and predicts poor prognosis of patients with luminal A breast cancer. Biomedicine & Pharmacotherapy 2019, 118, 109341. 10.1016/j.biopha.2019.109341. [DOI] [PubMed] [Google Scholar]
- Yasuhara T.; et al. Rad54B serves as a scaffold in the DNA damage response that limits checkpoint strength. Nat. Commun. 2014, 5 (1), 5426. 10.1038/ncomms6426. [DOI] [PubMed] [Google Scholar]
- Sharma D.; Kanchi S.; Bisetty K. Biogenic synthesis of nanoparticles: a review. Arabian journal of chemistry 2019, 12 (8), 3576–3600. 10.1016/j.arabjc.2015.11.002. [DOI] [Google Scholar]
- Aziz N.; et al. Facile algae-derived route to biogenic silver nanoparticles: synthesis, antibacterial, and photocatalytic properties. Langmuir 2015, 31 (42), 11605–11612. 10.1021/acs.langmuir.5b03081. [DOI] [PubMed] [Google Scholar]
- Chitkara D.; et al. Biodegradable Injectable In Situ Depot-Forming Drug Delivery Systems. Macromol. Biosci. 2006, 6 (12), 977–990. 10.1002/mabi.200600129. [DOI] [PubMed] [Google Scholar]
- Witika B. A. Biocompatibility of Biomaterials for Nanoencapsulation: Current Approaches. Nanomaterials (Basel) 2020, 10 (9), 1649. 10.3390/nano10091649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A.; Shah P. A.; Shrivastav P. S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. 10.1016/j.ijpharm.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Alp E.; et al. Starch nanoparticles for delivery of the histone deacetylase inhibitor CG-1521 in breast cancer treatment. Int. J. Nanomedicine 2019, 14, 1335–1346. 10.2147/IJN.S191837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleos C. M.; et al. Carboxylated hydroxyethyl starch: a novel polysaccharide for the delivery of doxorubicin. Chem. Biol. Drug Des 2015, 85 (5), 653–8. 10.1111/cbdd.12447. [DOI] [PubMed] [Google Scholar]
- Gulfam M.; et al. Anticancer drug-loaded gliadin nanoparticles induce apoptosis in breast cancer cells. Langmuir 2012, 28 (21), 8216–23. 10.1021/la300691n. [DOI] [PubMed] [Google Scholar]
- Baharlouei P.; Rahman A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Marine Drugs 2022, 20 (7), 460. 10.3390/md20070460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchegbu I. F; et al. Chitosan Amphiphiles Provide New Drug Delivery Opportunities. Polym. Int. 2014, 63 (7), 1145–1153. 10.1002/pi.4721. [DOI] [Google Scholar]
- Tian Y.; et al. Doxorubicin-loaded magnetic silk fibroin nanoparticles for targeted therapy of multidrug-resistant cancer. Adv. Mater. 2014, 26 (43), 7393–8. 10.1002/adma.201403562. [DOI] [PubMed] [Google Scholar]
- Mohapatra A.; et al. Magnetic stimulus responsive vancomycin drug delivery system based on chitosan microbeads embedded with magnetic nanoparticles. J. Biomed Mater. Res. B Appl. Biomater 2018, 106 (6), 2169–2176. 10.1002/jbm.b.34015. [DOI] [PubMed] [Google Scholar]
- Sun Q. Rational Design of Cancer Nanomedicine: Nanoproperty Integration and Synchronization. Adv. Mater. 2017, 29 (14), 1606628. 10.1002/adma.201606628. [DOI] [PubMed] [Google Scholar]
- Simon J.; et al. Protein Corona Mediated Stealth Properties of Biocompatible Carbohydrate-based Nanocarriers. Isr. J. Chem. 2018, 58 (12), 1363–1372. 10.1002/ijch.201800166. [DOI] [Google Scholar]
- Wei G. Peptide-Based Nanocarriers for Cancer Therapy. Small Methods 2018, 2 (9), 1700358. 10.1002/smtd.201700358. [DOI] [Google Scholar]
- Sanna V.; et al. Targeted Nanoparticles for the Delivery of Novel Bioactive Molecules to Pancreatic Cancer Cells. J. Med. Chem. 2016, 59 (11), 5209–20. 10.1021/acs.jmedchem.5b01571. [DOI] [PubMed] [Google Scholar]
- Yan L.; et al. Nanoparticle-Based Drug Delivery System: A Patient-Friendly Chemotherapy for Oncology. Dose Response 2020, 10.1177/1559325820936161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagnella S. M.; et al. Dextran-based doxorubicin nanocarriers with improved tumor penetration. Biomacromolecules 2014, 15 (1), 262–75. 10.1021/bm401526d. [DOI] [PubMed] [Google Scholar]
- Wang N.; et al. Nanocarriers and Their Loading Strategies. Adv. Healthc Mater. 2019, 8 (6), 1801002. 10.1002/adhm.201801002. [DOI] [PubMed] [Google Scholar]
- Ahuja G.; Pathak K. Porous carriers for controlled/modulated drug delivery. Indian J. Pharm. Sci. 2009, 71 (6), 599–607. 10.4103/0250-474X.59540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrigue P. M. Degradation of Drug Delivery Nanocarriers and Payload Release: A Review of Physical Methods for Tracing Nanocarrier Biological Fate. Pharmaceutics 2021, 13 (6), 770. 10.3390/pharmaceutics13060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Yeo Y. Controlled Drug Release from Pharmaceutical Nanocarriers. Chemical engineering science 2015, 125, 75–84. 10.1016/j.ces.2014.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din F. U.; et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomedicine 2017, 12, 7291–7309. 10.2147/IJN.S146315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchaala R.; et al. Quantifying Release from Lipid Nanocarriers by Fluorescence Correlation Spectroscopy. ACS Omega 2018, 3 (10), 14333–14340. 10.1021/acsomega.8b01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana F.; et al. Production of pure drug nanocrystals and nano co-crystals by confinement methods. Adv. Drug Deliv Rev. 2018, 131, 3–21. 10.1016/j.addr.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Neek M.; Kim T. I.; Wang S. W. Protein-based nanoparticles in cancer vaccine development. Nanomedicine 2019, 15 (1), 164–174. 10.1016/j.nano.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski K.; Kciuk M.; Kontek R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21 (9), 3233. 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pećina-Šlaus N.; et al. Mismatch repair pathway, genome stability and cancer. Frontiers in molecular biosciences 2020, 7, 122. 10.3389/fmolb.2020.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiano A.; Ramadan K. DNA–protein crosslink proteases in genome stability. Communications Biology 2021, 4 (1), 11. 10.1038/s42003-020-01539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ui A.; Chiba N.; Yasui A. Relationship among DNA double-strand break (DSB), DSB repair, and transcription prevents genome instability and cancer. Cancer science 2020, 111 (5), 1443–1451. 10.1111/cas.14404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imre B.; Pukánszky B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49 (6), 1215–1233. 10.1016/j.eurpolymj.2013.01.019. [DOI] [Google Scholar]
- Chenthamara D.; et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019, 23, 20. 10.1186/s40824-019-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar P.; et al. A hydrophobic starch polymer for nanoparticle-mediated delivery of docetaxel. Macromol. Biosci 2012, 12 (2), 184–94. 10.1002/mabi.201100244. [DOI] [PubMed] [Google Scholar]
- Voci S. Antitumor Features of Vegetal Protein-Based Nanotherapeutics. Pharmaceutics 2020, 12 (1), 65. 10.3390/pharmaceutics12010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasool M.; et al. New challenges in the use of nanomedicine in cancer therapy. Bioengineered 2022, 13 (1), 759–773. 10.1080/21655979.2021.2012907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohcharoenkal W.; et al. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy. BioMed. Research International 2014, 2014, 180549. 10.1155/2014/180549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; et al. Multifunctional biodegradable polyacrylamide nanocarriers for cancer theranostics--a ″see and treat″ strategy. ACS Nano 2012, 6 (8), 6843–51. 10.1021/nn301633m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohcharoenkal W.; et al. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res. Int. 2014, 2014, 180549. 10.1155/2014/180549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; et al. Polydopamine-containing nano-systems for cancer multi-mode diagnoses and therapies: A review. Int. J. Biol. Macromol. 2023, 247, 125826. 10.1016/j.ijbiomac.2023.125826. [DOI] [PubMed] [Google Scholar]
- Lee H.; et al. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318 (5849), 426–430. 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L.; et al. Complementary autophagy inhibition and glucose metabolism with rattle-structured polydopamine@mesoporous silica nanoparticles for augmented low-temperature photothermal therapy and < i> in vivo</i> photoacoustic imaging. Theranostics 2020, 10 (16), 7273–7286. 10.7150/thno.44668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q.; et al. Transferring Biomarker into Molecular Probe: Melanin Nanoparticle as a Naturally Active Platform for Multimodality Imaging. J. Am. Chem. Soc. 2014, 136 (43), 15185–15194. 10.1021/ja505412p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurin S.; Nehoff H.; Greish K. Anticancer nanomedicine and tumor vascular permeability; Where is the missing link?. J. Controlled Release 2012, 164 (3), 265–75. 10.1016/j.jconrel.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Wu J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11 (8), 771. 10.3390/jpm11080771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.; et al. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm. Sin B 2021, 11 (8), 2265–2285. 10.1016/j.apsb.2021.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y.; et al. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer?. Bioconjug Chem. 2016, 27 (10), 2225–2238. 10.1021/acs.bioconjchem.6b00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A.; Davidson J. M. Nanovehicular intracellular delivery systems. J. Pharm. Sci. 2008, 97 (9), 3518–90. 10.1002/jps.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya T.; Wang C.; Ai Z. Cubic Lipids:A Bliss For Drug Delivery. International Journal of Engineering Inventions 2020, 9 (8), 1–8. [Google Scholar]
- Andey T.; et al. Lipid nanocarriers of a lipid-conjugated estrogenic derivative inhibit tumor growth and enhance cisplatin activity against triple-negative breast cancer: pharmacokinetic and efficacy evaluation. Mol. Pharmaceutics 2015, 12 (4), 1105–20. 10.1021/mp5008629. [DOI] [PubMed] [Google Scholar]
- Miao L.; et al. Nanoformulations for combination or cascade anticancer therapy. Adv. Drug Deliv Rev. 2017, 115, 3–22. 10.1016/j.addr.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu L. M.; et al. Dual-functional drug liposomes in treatment of resistant cancers. Adv. Drug Deliv Rev. 2017, 115, 46–56. 10.1016/j.addr.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Bondi M. L.; et al. Biocompatible Lipid Nanoparticles as Carriers To Improve Curcumin Efficacy in Ovarian Cancer Treatment. J. Agric. Food Chem. 2017, 65 (7), 1342–1352. 10.1021/acs.jafc.6b04409. [DOI] [PubMed] [Google Scholar]
- Date T.; et al. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. J. Controlled Release 2018, 271, 60–73. 10.1016/j.jconrel.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Dai W.; et al. Combination antitumor therapy with targeted dual-nanomedicines. Adv. Drug Deliv Rev. 2017, 115, 23–45. 10.1016/j.addr.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Kydd J. Targeting Strategies for the Combination Treatment of Cancer Using Drug Delivery Systems. Pharmaceutics 2017, 9 (4), 46. 10.3390/pharmaceutics9040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N.; et al. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv Rev. 2014, 66, 2–25. 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; et al. Hierarchical Tumor Microenvironment-Responsive Nanomedicine for Programmed Delivery of Chemotherapeutics. Adv. Mater. 2018, 30, 1803926. 10.1002/adma.201803926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; et al. Charge-convertible polymers for improved tumor targeting and enhanced therapy. Biomaterials 2019, 217, 119299. 10.1016/j.biomaterials.2019.119299. [DOI] [PubMed] [Google Scholar]
- Padmakumar S.; et al. Enhanced anti-tumor efficacy and safety with metronomic intraperitoneal chemotherapy for metastatic ovarian cancer using biodegradable nanotextile implants. J. Controlled Release 2019, 305, 29–40. 10.1016/j.jconrel.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheer D.; Nicolas J.; Shankar R. Cathepsin-sensitive nanoscale drug delivery systems for cancer therapy and other diseases. Adv. Drug Deliv Rev. 2019, 151–152, 130–151. 10.1016/j.addr.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Dong P.; et al. Innovative nano-carriers in anticancer drug delivery-a comprehensive review. Bioorg Chem. 2019, 85, 325–336. 10.1016/j.bioorg.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Mansoori B.; et al. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7 (3), 339–348. 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin G.; Gedik M. E.; Ayan S. Photodynamic Therapy-Current Limitations and Novel Approaches. Front Chem. 2021, 9, 691697. 10.3389/fchem.2021.691697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski S.; et al. Photodynamic therapy – mechanisms, photosensitizers and combinations. Biomedicine & Pharmacotherapy 2018, 106, 1098–1107. 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- Sai D. L.; et al. Tailoring photosensitive ROS for advanced photodynamic therapy. Exp Mol. Med. 2021, 53 (4), 495–504. 10.1038/s12276-021-00599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiee N.; et al. Recent advances in porphyrin-based nanocomposites for effective targeted imaging and therapy. Biomaterials 2020, 232, 119707. 10.1016/j.biomaterials.2019.119707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master A.; Livingston M.; Sen Gupta A. Photodynamic nanomedicine in the treatment of solid tumors: perspectives and challenges. J. Controlled Release 2013, 168 (1), 88–102. 10.1016/j.jconrel.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alander J. T.; et al. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed Imaging 2012, 2012, 940585. 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z.; et al. Indocyanine Green Nanoparticles for Theranostic Applications. Nano-Micro letters 2013, 5, 145–150. 10.1007/BF03353743. [DOI] [Google Scholar]
- Wei P.-R.; et al. Synthesis of chitosan-coated near-infrared layered double hydroxide nanoparticles for in vivo optical imaging. J. Mater. Chem. 2012, 22 (12), 5503–5513. 10.1039/c2jm16447g. [DOI] [Google Scholar]
- Liu Y.; et al. Palladium-based nanomaterials for cancer imaging and therapy. Theranostics 2020, 10 (22), 10057–10074. 10.7150/thno.45990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan T. T. V.; et al. Chitosan as a stabilizer and size-control agent for synthesis of porous flower-shaped palladium nanoparticles and their applications on photo-based therapies. Carbohydr. Polym. 2019, 205, 340–352. 10.1016/j.carbpol.2018.10.062. [DOI] [PubMed] [Google Scholar]
- Han Z.; Liu G. Sugar-based biopolymers as novel imaging agents for molecular magnetic resonance imaging. Wiley Interdiscip Rev. Nanomed Nanobiotechnol 2019, 11 (4), e1551. 10.1002/wnan.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin N.; et al. Detoxification and functionalization of gold nanorods with organic polymers and their applications in cancer photothermal therapy. Microsc Res. Tech 2019, 82 (6), 670–679. 10.1002/jemt.23213. [DOI] [PubMed] [Google Scholar]
- Wang C.; et al. Near-Infrared Light Duced In Vivo Photodynamic Therapy of Cancer Based on Upconversion Nanoparticales. Biomaterials 2011, 32, 6145–54. 10.1016/j.biomaterials.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Algorri J. F. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers (Basel) 2021, 13 (14), 3484. 10.3390/cancers13143484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H.; et al. Crucial Breakthrough of Functional Persistent Luminescence Materials for Biomedical and Information Technological Applications. Front Chem. 2019, 7, 387. 10.3389/fchem.2019.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. K.; et al. Turning solid into gel for high-efficient persistent luminescence-sensitized photodynamic therapy. Biomaterials 2019, 218, 119328. 10.1016/j.biomaterials.2019.119328. [DOI] [PubMed] [Google Scholar]
- Bhatia S. N. Cancer nanomedicine. Nature Reviews Cancer 2022, 22, 550. 10.1038/s41568-022-00496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z.; et al. Bio-Conjugated Quantum Dots for Cancer Research: Detection and Imaging. Front Oncol 2021, 11, 749970. 10.3389/fonc.2021.749970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur A. A.; et al. Fluorescent nanohybrids based on quantum dot-chitosan-antibody as potential cancer biomarkers. ACS Appl. Mater. Interfaces 2014, 6 (14), 11403–12. 10.1021/am5019989. [DOI] [PubMed] [Google Scholar]
- Rhee J. K.; et al. Glycol chitosan-based fluorescent theranostic nanoagents for cancer therapy. Mar Drugs 2014, 12 (12), 6038–57. 10.3390/md12126038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.; et al. Ultrasmall Chitosan-Genipin Nanocarriers Fabricated from Reverse Microemulsion Process for Tumor Photothermal Therapy in Mice. Biomacromolecules 2015, 16 (7), 2080–90. 10.1021/acs.biomac.5b00511. [DOI] [PubMed] [Google Scholar]
- Wang H.; et al. Indocyanine green-incorporating nanoparticles for cancer theranostics. Theranostics 2018, 8 (5), 1227–1242. 10.7150/thno.22872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.; et al. Gold nanocluster-loaded hybrid albumin nanoparticles with fluorescence-based optical visualization and photothermal conversion for tumor detection/ablation. J. Controlled Release 2019, 304, 7–18. 10.1016/j.jconrel.2019.04.036. [DOI] [PubMed] [Google Scholar]
- Donahue N. D.; Acar H.; Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv Rev. 2019, 143, 68–96. 10.1016/j.addr.2019.04.008. [DOI] [PubMed] [Google Scholar]
- Yang K.; et al. FeS nanoplates as a multifunctional nano-theranostic for magnetic resonance imaging guided photothermal therapy. Biomaterials 2015, 38, 1–9. 10.1016/j.biomaterials.2014.10.052. [DOI] [PubMed] [Google Scholar]
- Zhang R.; et al. Engineering Melanin Nanoparticles as an Efficient Drug-Delivery System for Imaging-Guided Chemotherapy. Adv. Mater. 2015, 27 (34), 5063–9. 10.1002/adma.201502201. [DOI] [PubMed] [Google Scholar]
- Radwan S. E.; et al. Chitosan-coated bovine serum albumin nanoparticles for topical tetrandrine delivery in glaucoma: in vitro and in vivo assessment. Drug Deliv 2022, 29 (1), 1150–1163. 10.1080/10717544.2022.2058648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan U. N.; et al. Protein-Based Multifunctional Nanocarriers for Imaging, Photothermal Therapy, and Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9 (23), 19495–19501. 10.1021/acsami.6b06099. [DOI] [PubMed] [Google Scholar]
- Zhao Z.; et al. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Delivery Rev. 2019, 143, 3–21. 10.1016/j.addr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Anselmo A. C.; et al. Elasticity of Nanoparticles Influences Their Blood Circulation, Phagocytosis, Endocytosis, and Targeting. ACS Nano 2015, 9 (3), 3169–3177. 10.1021/acsnano.5b00147. [DOI] [PubMed] [Google Scholar]
- Manivasagan P.; et al. Chitosan/fucoidan multilayer coating of gold nanorods as highly efficient near-infrared photothermal agents for cancer therapy. Carbohydr. Polym. 2019, 211, 360–369. 10.1016/j.carbpol.2019.01.010. [DOI] [PubMed] [Google Scholar]