Abstract
Objectives:
The Cow’s Milk-related Symptom Score (CoMISS) is an awareness tool for evaluating cow’s milk-related symptoms in otherwise healthy infants <1 year of age. This study assessed whether replacing the Bristol Stool Form Scale (BSFS) with the Brussels Infants and Toddlers Stool Scale (BITSS) in non-toilet-trained infants would modify the overall CoMiSS and change the clinical approach regarding potential cow’s milk allergy.
Methods:
Non-toilet-trained infants aged <13 months were assessed by CoMiSS using the 7 images from the BSFS (CoMiSS-BSFS) compared to the 4 images of stools from BITSS (CoMiSS-BITSS). The Wilcoxon signed-rank test and Pearson correlation coefficient were calculated. A post hoc analysis using identical tests was performed in subsets of CoMiSS-BSFS scores ≥10, ≥12, ≤5, and ≥6.
Results:
Eight hundred forty-four pairwise scores were collected. Applying the Wilcoxon test over the complete dataset, the difference between CoMiSS-BSFS and CoMiSS-BITSS was statistically significant (P < 0.001). However, there was no significant difference in the subsets with CoMiSS-BSFS ≥10, ≥12, and ≥6 (P = 0.84, P = 0.48, and P = 0.81, respectively). The significant difference remained restricted to the group with CoMiSS-BSFS ≤5, considered at low risk for CM-related symptoms (P < 0.001).
Conclusion:
Replacing BSFS with BITSS does not change the cutoff for awareness of possible CM-related symptoms and will not impact the use of CoMiSS in clinical practice. Changes in CoMiSS remained limited to the subgroup with a low risk for CM-related symptoms.
Keywords: Bristol Stool Scale, Brussels Infant and Toddlers Stool Scale, cow’s milk related symptom score, infant, stool consistency
What Is Known
The Cow’s Milk-related Symptom Score (CoMiSS) is a validated awareness tool for cow’s milk allergy.
The Brussels Infants and Toddlers Stool Scale (BITSS) is a validated tool to describe stool consistency in non-toilet trained children.
BITSS was developed after CoMiSS; as a consequence, stool consistency in CoMiSS used the Bristol Stool Scale
What Is New
The BITSS has been integrated in the CoMiSS without the necessity to redefine a new cutoff to arise awareness of possible cow’s milk related symptoms.
The Cow’s Milk-related Symptom Score (CoMiSS) (Table 1, Supplemental Digital Content 2, http://links.lww.com/MPG/D298) is a clinical tool developed to improve the awareness of health care professionals (HCPs) of the possible association between clinical manifestations and cow’s milk (CM) exposure and, potentially, cow’s milk allergy (CMA) in infants (1). The original CoMiSS quantified 5 clinical items: crying time, number and volume of regurgitation episodes, stool consistency, skin manifestations (atopic dermatitis/urticaria), and respiratory symptoms, with a total score ranging from 0 to 33 (2). Originally, an arbitrary cutoff of ≥12 was suggested as possibly indicating CMA, warranting an elimination diet followed by cow’s milk protein (CMP) reintroduction (2). A recent review evaluated the performance of CoMiSS in 25 clinical studies and concluded that lowering the cutoff to ≥10 was justified (3). Previous studies on CoMiSS also demonstrate that scores ≤5 are unlikely to be found in infants with CMA. Scores from 6 to 9 are present in different clinical conditions: in healthy infants, those with functional gastrointestinal (GI) symptoms as well as those with CMA (4).
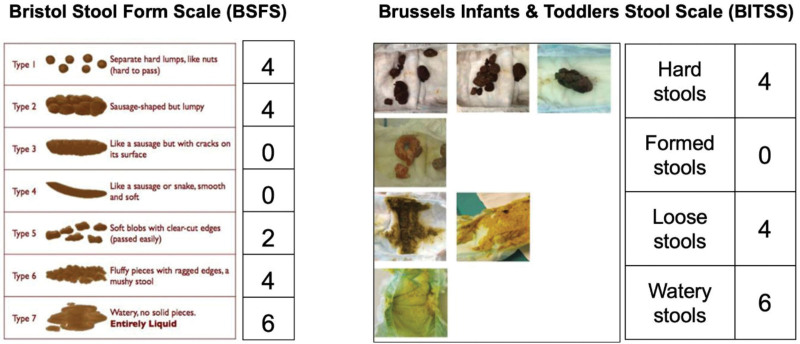
In the original CoMiSS (2), stool consistency was scored with the Bristol Stool Form Scale (BSFS), which consists of 7 pictures of different stool forms and facilitates recording of stool consistency (Fig. 1). This scale has long been used in adults and reflects the GI transit time (5). For children, the modified Bristol Stool Form Scale (m-BSFS), consisting of the BSFS types 1, 2, 4, 6, and 7 was developed (6) and validated (6–8).
FIGURE 1.
. CoMiSS rating for Bristol Stool Form Scale and Brussels Infants and Toddlers Stool Scale.
More recently, the Brussels Infant and Toddlers Stool Scale (BITSS) has been developed and validated specifically for non-toilet-trained children (9). In contrast to the BSFS, the BITSS (Fig. 1) consists of 7 photos of stools in diapers representing 4 groups of stool consistency: hard, formed, loose, and watery (9). An excellent agreement between photographic and real-time assessment ranging between 71.1% and 83.3% among observers using BITSS was reported (10). Furthermore, when assessing photographed stools, intra-rater reliability for HCPs ranged from 0.64 to 0.78 and from 0.68 to 0.94 in the caregiver’s group (11). If automatic intelligence using machine learning is applied, the accuracy of stool consistency recognition rises to 95% and offers the opportunity for BITSS validation via an application (12,13).
Since CoMiSS is intended to be used in infants, thus non-toilet-trained subjects, BITSS is more age-appropriate for CoMiSS than the BSFS. However, to keep the existing CoMiSS data valid (4), the use of BITSS instead of BSFS should not modify CoMiSS outcomes, especially when the cutoffs for CMA risk are considered. This study explores the possibility of replacing the BSFS with BITSS in CoMiSS without changing its results related to CMA awareness in non-toilet-trained children under 13 months of age.
MATERIAL AND METHODS
This was a prospective observational European multicenter study including subjects aged between birth and 13 months. Exclusion criteria were prematurity, dietary restrictions, chronic disease, or presence of any acute infection. Presumed healthy children attending a well-baby clinic for a scheduled visit were assessed in the Czech Republic and Spain and were not considered symptomatic by caregivers. Additionally, an Italian cohort was recruited, including a convenience sample of participants referred to a pediatric gastroenterology clinic for symptoms suggestive of CMA or functional GI disorders (colic, vomiting, diarrhea), with an intent to increase observations with higher scores. Data on the subsequent diagnosis of CMA were not collected as this was not the aim of the study.
Data were collected from December 2019 to February 2022. The Spanish and Italian infants were evaluated during a single assessment, while the Czech infants were assessed repetitively by the HCP (in an irregular manner 3 to 7 times per infant) during follow-up clinical check-ups at different ages (1.5, 3, 4, 6, 8, 10, and 12 months ± 4 weeks). All of these repeated observations were included, as they involved the assessment of stools using either BITTS or BSFS at each time point, which is what this study aimed to achieve. Moreover, we presumed that repeated assessment of the same subject at different ages with different feeding modes and psychomotor development would generate a difference. Each defecation was scored only once using each stool score. Age, sex, type of feeding, consistency of stools and CoMiSS-BSFS were recorded by the HCP during each clinical assessment according to information obtained from the caregiver.
COMISS-stool-values for BSFS types were assigned to individual stool categories according to BITSS, as shown in Table 1: 4 for hard, 0 for formed, 4 for loose, and 6 for watery stools. Type 3 of BSFS is categorized as hard by BITSS and thus scores differently. Stools 5 and 6 by BSFS are considered loose by BITSS and score 4 points. The value of 4 points was assigned for 2 different stool categories: hard and loose stools. Authors of the original CoMiSS considered these 2 stool types to have the same clinical impact regarding CMA-risk (2). The scoring was assigned to BITSS in respect to the BSFS scoring used in the original CoMiSS. A pairwise CoMiSS value with BSFS substituted by BITSS (CoMiSS-BITSS) was calculated for each observation.
TABLE 1.
BITSS scoring in relation to BSFS
| BITSS | BSFS type | CoMISS score |
|---|---|---|
| Hard | 1; 2; 3 | 4 |
| Formed | 4 | 0 |
| Loose | 5;6 | 4 |
| Watery | 7 | 6 |
CoMISS = Cow’s Milk-related Symptom Score; BITSS = Brussels Infants and Toddlers Stool Scale; BSFS = Bristol Stool Form Scale.
Statistical Analysis
A descriptive statistical analysis was performed. To compare the CoMiSS-BSFS with the CoMiSS-BITSS, the Wilcoxon signed-rank test and Pearson correlation coefficient (r) were calculated. In addition, a post hoc analysis using the same statistical tests was performed in subsets of CoMiSS-BSFS scores ≥10 and ≥12, respectively (new and original cutoff), and of CoMiSS-BSFS ≤5 and ≥6 since a score of ≤5 has been recently reported as the best threshold of an unlikely diagnosis CMA (4). Both tests were repeated for all CoMiSS-BSFS scores (≤1, ≤2, ≤3, ≤ 4, ≤5, ≥6, ≥7, ≥8, ≥9, ≥10, ≥11, and ≥12). We also performed an analysis to check how many CoMiSS-BITSS scores changed from “under the cutoff limit” to “above the cutoff limit” CoMiSS for both cutoffs (≥10 and ≥12) and the opposite, compared to the pairwise CoMiSS-BSFS. Moreover, we assessed how many scores with CoMiSS-BSFS ≤5 changed to ≥6 values pairwise CoMiSS-BITSS since ≤5 is the cutoff for the unlikelihood of CMA. We included all the Czech observations repeated in each subject without testing their correlation, as the CoMiSS-BSFS value is a sum of five different subscores and thus the same CoMiSS-BSFS value may be a result of different pattern of subscores’ values. Statistical analysis was conducted using statistical software (Microsoft-Excel 2016, SPSS Statistics, version 28, IBM and on-line calculators for Cohen d and Holm method, https://www.statskingdom.com).
A sample size calculation could not be performed in the absence of similar work. A number of 200 infants was considered adequate for testing both reliability and validity since the BSFS was reported on 191 ratings (7), and, more recently, 89 toddlers were recruited by Wegh et al for the same outcome (8). Studies reporting data on CoMiSS in presumed healthy infants included either 226 with 11 (4.9%) CoMiSS values ≥12 (14) or 563 observations with 9 (1.5%) CoMiSS values ≥12 and 28 (5.0%) CoMiSS values ≥10, respectively (15). Published validation studies reporting data on symptomatic infants enroll from 47 to 250 subjects (4). In addition, the Cohen d of effect size of our cohort was calculated, the d exceeding Cohen convention (16) for small effect was found in CoMiSS-BITSS values ≤5 (d = 0.43). Cohen d for CoMiSS-BSFS ≥6 was 0.04, thus below the threshold 0.2 of Cohen convention (Table 2, Supplemental Digital Content 3, http://links.lww.com/MPG/D299). Holm method was used to elucidate the effect of repeated assessments in subgroups of CoMiSS-BSFS ≤5 and subgroups of CoMiSS-BSFS ≥6. No statistically significant impact on obtained results was found, and corrections of significance level were not required.
The respective Hospital Ethical Committees approved the study. Written informed consent to the anonymized utilization of the infant data was obtained from all caregivers participating to the study.
RESULTS
We recruited 309 infants, 175 in Spain, 114 in the Czech Republic, and 20 in Italy. We included 294 infants since 15 subjects did not meet the study criteria (3 with previously diagnosed CMA recruited in Spain, and 12 recruited in the Czech Republic: 4 premature babies, 7 with previous dietary self-management, and 1 with GI infection). Finally, 844 CoMiSS-BSFS ratings and CoMiSS-BITSS pairwise scores were analyzed.
The descriptive statistics are summarized in Table 2.
TABLE 2.
Descriptive statistics of the recruited cohort
| No. 844 | Mean | Standard deviation | Median | Minimal value | Maximal value | Q1 | Q3 | P95 |
|---|---|---|---|---|---|---|---|---|
| Age, mo | 6.3 | 3.7 | 6.1 | 1.0 | 12.9 | 3.1 | 10.0 | 12.3 |
| CoMiSS—BSFS | 4.8 | 3.0 | 4.0 | 0.0 | 17.0 | 2.0 | 6.0 | 10.0 |
| CoMiSS—BITSS | 4.3 | 3.5 | 4.0 | 0.0 | 17.0 | 1.0 | 6.0 | 10.0 |
CoMISS = Cow’s Milk-related Symptom Score; BITSS = Brussels Infants and Toddlers Stool Scale; BSFS = Bristol Stool Form Scale; P95 = 95th centile; Q1 = 1st quartile; Q3 = 3rd quartile.
We checked the number of repeated observations among the Czech cohort: in the subset of CoMiSS-BSFS values ≥10 we found 17 scores out of 57 were from the Czech group. Fourteen infants of 17 underwent a single assessment, and 1 was assessed 3 times with different pairwise values of CoMiSS-BSFS/CoMiSS-BITSS: 11 of 11 (age: 3 months), 12 of 12 (age: 4 months), and 12 of 10 (age: 6 months). There was no repeated assessment in the group of CoMiSS-BSFS values ≥12. Four-hundred eighty-five (out of 540; 89%) observations with CoMiSS-BSFS values ≤5 were obtained in the Czech cohort (with maximum of 7 repeated assessments) and finally 150 of Czech observations were presenting with CoMiSS-BSFS values ≥6 (with maximum of 4 repeated assessments), representing 49% of the CoMiSS-BSFS ≥6.
Using the original CoMiSS awareness cutoff ≥12 for CM-related symptoms, changing from BSFS to BITSS switched only in 2 of 844 (0.24%) infants from <12 to ≥12. Furthermore, for the same cutoff, only 1 of 844 infants (0.12%) switched from above to below the cutoff. When the new cutoff ≥10 was considered to determine awareness for CM-related symptoms, 3 of 844 (0.36%) infants switched from <10 to ≥10. No infant switched from above to below the cutoff ≥10. In 7 cases out of 540 (1.30%) CoMiSS-BSFS ≤5, the CoMiSS-BITSS increased to scores ≥6.
The difference between CoMiSS-BSFS and CoMiSS-BITSS was significant (P < 0.001) for the complete dataset of 844 pairwise scores, with a correlation factor (r) of 0.96 and a 95% CI [0.95–0.96].
However, for the subsets of CoMiSS-BSFS ≥10, ≥12, and ≥6, no significant difference was detected, and a strong positive correlation was found (P = 0.48, r = 0.94, 95% CI [0.89–0.96]; P = 0.84, r = 0.85, 95% CI [0.68–0.94]; and P = 0.81, r = 0.98, 95% CI [0.97–0.98], respectively). Only in the group with CoMiSS-BSFS ≤5, a significant difference was observed (P < 0.001) with a Pearson correlation coefficient of 0.81. The results are summarized in Table 3. Both tests were repeated for all CoMiSS-BSFS scores (≤1, ≤2, ≤3, ≤4, ≤5, ≥6, ≥7, ≥8, ≥9, ≥10, ≥11, and ≥12) (Table 2, Supplemental Digital Content 3, http://links.lww.com/MPG/D299 and Figure 1, Supplemental Digital Content 1, http://links.lww.com/MPG/D297). The changes in CoMiSS values and their frequencies in subgroups are listed in Table 3, Supplemental Digital Content 4, http://links.lww.com/MPG/D300.
TABLE 3.
Comparison of CoMiSS-BSFS and COMISS-BITSS sets
| CoMiSS | No. CoMiSS-BSFS | Pearson correlation coefficient | 95% CI | CoMiSS-BSFS vs CoMiSS-BITSS* |
|---|---|---|---|---|
| ≤5 | 540 | 0.81 | 0.79; 0.84 | P < 0.001 |
| ≥6 | 304 | 0.98 | 0.97; 0.98 | P = 0.81 |
| ≥10 | 57 | 0.94 | 0.89; 0.96 | P = 0.48 |
| ≥12 | 23 | 0.85 | 0.68; 0.94 | P = 0.84 |
| All scores | 844 | 0.96 | 0.95; 0.96 | P < 0.001 |
CI = confidence interval; CoMISS = Cow’s Milk-related Symptom Score; BITSS = Brussels Infants and Toddlers Stool Scale; BSFS = Bristol Stool Form Scale; No = number; vs = versus.
Wilcoxon signed-rank test for the CoMiSS-BSFS value and pairwise CoMiSS-BITSS.
DISCUSSION
This study assessed whether replacing the BSFS with the BITSS in the CoMiSS in non-toilet-trained infants under 13 months of age would change the CoMiSS outcome. The hypothesis was that changing the stool scoring would not change the CoMiSS values and thus not change the number of infants for which the HCP should be aware of the possibility of CM-related symptoms for both cutoffs ≥10 and ≥12, respectively. We confirmed the hypothesis that using BITSS (with scoring 4 points for hard, 0 points for formed, 4 points for loose, and 6 for watery stools) instead of BSFS in CoMiSS does not modify CoMiSS for values ≥6, and thus does not change the cutoff for infants in whom CM-related symptoms cannot be excluded.
The detailed analysis demonstrated that changes of CoMiSS with BITSS are found almost exclusively in infants with a CoMiSS-BSFS ≤5, which has been recently reported as a negative predictive cutoff for CMA (4). Furthermore, a comparison of CoMiSS-BSFS ≥6 to pairwise CoMiSS-BITSS did not reveal any statistical difference and showed strong Pearson correlation coefficients. Since more than 50% of all scores were in infants within the group with CoMiSS ≤5, the overall statistical analysis was also significantly different between CoMiSS with BSFS or BITSS. Thus, switching from BSFS to BITSS changed CoMiSS only in the group of infants with a CoMiSS-BSFS within the normal physiologic range.
Considering the cutoff ≥10, no infant changed from above to below the cutoff by changing from BSFS to BITSS. Likewise, for the cutoff of ≥12, 1 infant (0.12%) switched from above to below the cutoff. Using CoMiSS-BITSS instead of CoMiSS-BSFS, only 2 of 844 (0.24%) infants changed from below to above the cutoff of ≥12. For a cutoff of ≥10, this was the case for 3 of 844 (0.36%). Moreover, only 7 of 540 (1.30%) of CoMiSS-BSFS ≤5 raised to the ≥6 CoMiSS-BITSS. Therefore, the switch from CoMiSS-BSFS to CoMiSS-BITSS does not change the previously proposed CoMiSS cutoff value for CMA awareness. As CoMiSS is an awareness tool and not a diagnostic one, the standardized diagnostic work-up remains crucial for CMA diagnosis.
The strength of this study is the high number of pairwise ratings, the analysis of the different CoMiSS cutoffs (high- and low-risk infants), and the inclusion of both healthy (824 out of 844, 98.6%) and symptomatic (20 out of 844, 1.4%) observations. The small number of high-scoring and symptomatic subjects is a limitation; however, the statistical analysis has shown low impact sample size in subsets of CoMiSS ≥6. Presumed healthy infants represent the major part of our cohort, so the descriptive statistics outcomes [median (interquartile range, IQR) 4 (2–6), 95th centile 10] are in line with previous data on presumed healthy infants: median (IQR) 4 (2–7), 95th centile of 11 (14) and 3 (1–5), 95th centile of 9 in multicentric European study (15), respectively. The repeated assessment of the Czech infants and absent analysis of CoMiSS-BSFS correlation of those repeated assessments might be a source of bias, especially in the subset of CoMiSS-BSFS values ≤5, where the Czech repeated assessments represented 89% (485/540 single observations), however CoMiSS-BSFS value is a sum of 5 different subscores (crying, vomiting, stool, respiratory, and skin subscore) thus even if the final value equals, the subscores variables takes on different values. Among observations with CoMiSS-BSFS values ≥10 were 17 assessments out of 57 from the Czech group. Fourteen infants of 17 (82%) underwent a single assessment, and one was assessed 3 times with different pairwise values of CoMiSS-BSFS/CoMiSS-BITSS: 11 of 11, 12 of 12, and 12 of 10, as there was each time different combination of the subscores values. There was no repeated assessment in the group of CoMiSS-BSFS values ≥12. The risk of repeated assessment bias is low when considering the at-risk cutoffs. Healthy infants present with CoMiSS-BSFS values ≤5 and median (IQR) of stools 2 (0–4) (4). CoMiSS-BSFS and CoMiSS-BITSS assign different values for the type 3 stool of BSFS (CoMiSS-BSFS value 0 in contrast to CoMiSS-BITSS value 4) but this shift was not a numerous one in our dataset. Furthermore, CoMiSS-BITSS does not include the stool value of 2, which is a frequent one among CoMiSS-BSFS in healthy infants and this is also a source of difference between the pairwise scorings, preferably among those with CoMiSS-BSFS values ≤5 as documented in Table 3, Supplemental Digital Content 4, http://links.lww.com/MPG/D300. The same table shows that obviously the only item scoring other than 0 in CoMiSS-BSFS ≤5 is the stool. Symptomatic infants, when scored using CoMiSS-BSFS, present with median (IQR) from 8 (5–10) (17) up to 13 (12–15) (18) and median (IQR) stool values 4 (4–6) (19) and 4 (2–4), respectively (17). The different distribution of stool types among healthy and symptomatic infants is evident. Shifting from CoMiSS-BSFS to CoMiSS-BITSS does not cause a change of assigned values to BSFS type 1, 2, 6 (4 points), and 7 (6 points) when assessed by CoMiSS-BITSS which are the dominant types in CoMiSS values above the cutoff (≥10 and ≥12). This finding allows us to presume that even though the number of high-scoring observations in our data set is limited, the change in stool validation will not influence the clinical approach and will not cause a decrease in CMA awareness. We did not test the infants on possible CMA, as this was not the goal of our study. However, we cannot deny that some of our subjects presenting with CoMiSS-BSFS ≥10 were allergic to CM. One study on presumed healthy infants confirmed by food challenge 10 subjects with CMA among 13 with CoMiSS-BSFS values ≥10 (20). The multicenter design might cause an interobserver variability of the CoMiSS, as well as show the potential of CoMiSS to be used in different settings and areas.
CONCLUSIONS
Replacing BSFS with BITSS in CoMiSS does modify the values of CoMiSS-BITSS compared to CoMiSS with BSFS but does not change the cutoff for awareness of possible CM-related symptoms. Particularly, it does not decrease the number of infants scoring ≥10, which are considered at risk of CMA-related symptoms. The changes of scores are limited to the subgroup of CoMiSS-BSFS ≤5, considered as subjects at very low risk of possible CM-related symptoms. Therefore, the use of CoMiSS with the suggested BITSS scoring (4 points for hard, 0 for formed, 4 points for loose, and 6 points for watery stools) will not have an impact on the use of CoMiSS in clinical practice. Since the number of symptomatic infants was low, further testing is needed to confirm the results in this subgroup of subjects.
Supplementary Material
Footnotes
Dr Bajerova has received honorarium lectures and participated as consultant for Danone-Nutricia and Nestlé Health Science. Dr Dupont has participated as scientific advisory board member for Abbott, Biostime, Danone, Nestlé Health Science, Nestlé France, and Sodilac. Dr Kuitunen has participated as advisory board member for Nestlé Health Science. Dr Meyer has participated as advisory board member for Nestlé Health Science and Abbott Nutrition, and received honorarium for lectures for Abbott Nutrition, Mead Johnson, Nutricia/Danone, and Nestlé. Dr Ribes-Koninckx has participated as advisory board member for Nestlé Health Science, and received honorarium for lectures for Mead Johnson, Nutricia/Danone, Nutriben, and Nestlé. Dr Salvatore has participated as a clinical investigator, and/or consultant, and/or speaker for Danone-Mellin, DVA, Noos, and Nestlé Health Science. Dr Shamir has participated as a clinical investigator, and/or advisory board member, and/or consultant, and/or speaker for Abbott, Else Nutrition, Nestlé Nutrition Institute, Nestlé Health Science, NGS, Nutricia, Soremartec, and Ukko. Dr Szajewska has participated as a clinical investigator, and/or advisory board member, and/or consultant, and/or speaker for: Ausnutria, Arla, Else Nutrition, Danone, Nestlé Health Science, Nestlé Nutrition Institute, Nutricia, and Mead Johnson. Dr Staiano is clinical investigator for Janssen Biologics B.V., Eli Lilly Cork Limited, and Novalac; consultant for Aboca, Angelini e Novalac, speaker for Novartis, Bromatech, Sanofi, and Vyvalife; was clinical investigator for Aboca and PAREXEL International Srl. Dr Vandenplas has participated as a clinical investigator, and/or advisory board member, and/or consultant, and/or speaker for Abbott Nutrition, Arla, Ausnutria, Biogaia, By Heart, CHR Hansen, Danone, ELSE Nutrition, Friesland Campina, Nestlé Health Science, Nestlé Nutrition Institute, Nutricia, Mead Johnson Nutrition, Phathom Pharmaceuticals, Pileje, United Pharmaceuticals (Novalac), and Yakult.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
REFERENCES
- 1.Saad K, Elgenidy A, Atef M, et al. Cow’s milk-related symptom score for cow’s milk allergy assessment: a meta-analysis for test accuracy. Pediatr Res. 2022;93:772–9. [DOI] [PubMed] [Google Scholar]
- 2.Vandenplas Y, Dupont C, Eigenmann P, et al. A workshop report on the development of the cow’s milk-related symptom score awareness tool for young children. Acta Paediatr. 2015;104:334–9. [DOI] [PubMed] [Google Scholar]
- 3.Vandenplas Y, Bajerova K, Dupont C, et al. The cow’s milk related symptom score: the 2022 update. Nutrients. 2022;14:2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajerova K, Salvatore S, Dupont C, et al. The Cow’s Milk-Related Symptom Score (CoMiSSTM): a useful awareness tool. Nutrients. 2022;14:2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4. [DOI] [PubMed] [Google Scholar]
- 6.Chumpitazi BP, Lane MM, Czyzewski DI, Weidler EM, Swank PR, Shulman RJ. Creation and initial evaluation of a stool form scale for children. J Pediatr. 2010;157:594–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane MM, Czyzewski DI, Chumpitazi BP, Shulman RJ. Reliability and validity of a modified Bristol Stool Form Scale for children. J Pediatr. 2011;159:437–41.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wegh CAM, Hermes GDA, Schoterman MHC, et al. The modified Bristol Stool Form Scale: a reliable and valid tool to score stool consistency in Dutch (non)toilet-trained toddlers. J Pediatr Gastroenterol Nutr. 2021;73:210–6. [DOI] [PubMed] [Google Scholar]
- 9.Huysentruyt K, Koppen I, Benninga M, et al. The Brussels Infant and Toddler Stool Scale: a study on interobserver reliability. J Pediatr Gastroenterol Nutr. 2019;68:207–13. [DOI] [PubMed] [Google Scholar]
- 10.Aman BA, Levy EI, Hofman B, Vandenplas Y, Huysentruyt K. Real time versus photographic assessment of stool consistency using the Brussels Infant and Toddler Stool Scale: are they telling us the same? Pediatr Gastroenterol Hepatol Nutr. 2021;24:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofman YMC, Vandenplas Y, Ludwig T, et al. Intra-rater variability of the Brussels Infants and Toddlers Stool Scale (BITSS) using photographed stools. J Pediatr Gastroenterol Nutr. 2022;75:584–8. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig T, Oukid I, Wong J, et al. Machine learning supports automated digital image scoring of stool consistency in diapers. J Pediatr Gastroenterol Nutr. 2021;72:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao F, Wang Y, Ludwig T, et al. Generation and application of a convolutional neural networks algorithm in evaluating stool consistency in diapers. Acta Paediatr. 2023;112:1333–40. [DOI] [PubMed] [Google Scholar]
- 14.Bigorajska K, Filipiak Z, Winiarska P, et al. Cow’s milk-related symptom score in presumed healthy polish infants aged 0–6 months. Pediatr Gastroenterol Hepatol Nutr. 2020;23:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandenplas Y, Salvatore S, Ribes-Koninckx C, Carvajal E, Szajewska H, Huysentruyt K. The Cow Milk Symptom Score (CoMiSSTM) in presumed healthy infants. PLoS One. 2018;13:e0200603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York: Routledge; 1988. [Google Scholar]
- 17.Vandenplas Y, Zhao Z-Y, Mukherjee R, et al. Assessment of the Cow’s Milk-related Symptom Score (CoMiSS) as a diagnostic tool for cow’s milk protein allergy: a prospective, multicentre study in China (MOSAIC study). BMJ Open. 2022;12:e056641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandenplas Y, Steenhout P, Järvi A, Garreau A-S, Mukherjee R. Pooled analysis of the Cow’s Milk-related-Symptom-Score (CoMiSSTM) as a predictor for cow’s milk related symptoms. Pediatr Gastroenterol Hepatol Nutr. 2017;20:22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sirin Kose S, Atakul G, Asilsoy S, Uzuner N, Anal O, Karaman O. The efficiency of the symptom-based score in infants diagnosed with cow’s milk protein and hen’s egg allergy. Allergol Immunopathol (Madr). 2019;47:265–71. [DOI] [PubMed] [Google Scholar]
- 20.Vandenplas Y, Carvajal E, Peeters S, et al. The Cow’s Milk-Related Symptom Score (CoMiSSTM): health care professional and parent and day-to-day variability. Nutrients. 2020;12:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.