Abstract
Context
It remains uncertain whether aging before late adulthood and menopause are associated with fat-free mass and fat mass–adjusted resting energy expenditure (REEadj).
Objectives
We investigated whether REEadj differs between middle-aged and younger women and between middle-aged women with different menopausal statuses. We repeated the age group comparison between middle-aged mothers and their daughters to partially control for genotype. We also explored whether serum estradiol and FSH concentrations explain REEadj in midlife.
Methods
We divided 120 women, including 16 mother-daughter pairs, into age groups; group I (n = 26) consisted of participants aged 17 to 21, group II (n = 35) of those aged 22 to 38, and group III (n = 59) of those aged 41 to 58 years. The women in group III were further categorized as pre- or perimenopausal (n = 19), postmenopausal (n = 30), or postmenopausal hormone therapy users (n = 10). REE was assessed using indirect calorimetry, body composition using dual-energy X-ray absorptiometry, and hormones using immunoassays.
Results
The REEadj of group I was 126 kcal/day [95% confidence interval (CI): 93-160] higher than that of group III, and the REEadj of group II was 88 kcal/day (95% CI: 49-127) higher. Furthermore, daughters had a 100 kcal/day (95% CI: 63-138 kcal/day) higher REEadj than their middle-aged mothers (all P < .001). In group III, REEadj was not lower in postmenopausal women and did not vary by sex hormone concentrations.
Conclusions
We demonstrated that REEadj declines with age in women before late adulthood, also when controlling partially for genetic background, and that menopause may not contribute to this decline.
Keywords: resting energy expenditure, menopause, estrogen, hormone replacement therapy
Energy expenditure is often assumed to begin declining in early to middle adulthood, but Pontzer et al (1) challenged this assumption by showing that fat-free mass (FFM) and fat mass (FM)-adjusted total energy expenditure (TEEadj) were stable between the ages of 20 and 63. However, they found that similarly adjusted resting energy expenditure (REEadj) stabilizes at adult levels at age 18 and declines from age 46 onward, although the limited numbers of middle-aged participants with a measured REE prevented the authors from making definitive inferences about the onset of REEadj decline, leading to a conclusion that age does not affect energy expenditure in adults before the age of 60 (1). Nevertheless, previous studies are consistent with an earlier turning point for REEadj (2–4), and we therefore sought to assess whether REEadj declines before late adulthood. Our dataset also included mother-daughter dyads, some of which had TEE measured with doubly labeled water, enabling us to partly control the analyses for genetic background and to explore whether increasing age showed similar associations with TEEadj as it does with REEadj.
Like aging, menopause is widely believed to reduce REEadj, and the topic has broad interest because many women gain FM during the menopausal transition (5, 6) and associate the change in body composition with slowing metabolism. During the menopausal transition, ovarian follicular activity ceases, causing a striking shift in women's sex hormone profile. The decline in systemic estradiol (E2) concentration in particular is thought to decrease REEadj, potentially via both central (7) and peripheral (8) mechanisms, while the increase in FSH secretion may also play a role (9, 10). Menopausal hormone therapy (MHT) can restore E2 and decrease FSH levels to some extent, which should reverse the potential menopause-associated decline in REEadj. However, whether menopause truly decreases REEadj is still uncertain because longitudinal studies following women over the menopausal transition (11, 12), cross-sectional studies comparing women with different menopausal states (13–16), and MHT interventions (15, 17–19) have been inconclusive. Therefore, in addition to investigating whether REEadj differs between young and middle-aged women, we also assessed whether REEadj differs between middle-aged women with different menopause statuses. We restricted the menopause analysis to middle-aged participants to limit the confounding effects of age. We also explored whether serum E2 and FSH concentrations explain REEadj in midlife.
Materials and Methods
Participants
The participants were 120 women who had taken part in 1 of 4 studies performed at the Faculty of Sport and Health Sciences of the University of Jyväskylä (Fig. 1). They were required to be healthy and not taking medications that could affect metabolism, although hormonal contraception and MHT use were allowed.
Figure 1.
Description of the study participants, outcomes, and statistical approaches.
The Calex study (data collection 2008-2011) investigated whether lifestyle factors influence muscle and adipose tissues (20). The current study used data from 17 middle-aged and 21 younger women with measured REE. This dataset included 16 mother-daughter pairs; 10 pairs also had their TEEs measured. The data has been partly used in an earlier validation study (21). The Estrogen and microRNAs as Modulators of Women's Metabolism study (EsmiRs; 2019-2020) examined resting and exercise metabolism in middle-aged women (22), and we included all 42 participants with measured REEs from that study. The Physique study (23) investigated the effects of competition weight loss in normal-weight participants; here, we used the baseline data from 23 young women collected before their weight loss. Finally, the Athletic Performance and Nutrition study (NO RED-S, 2021, Ihalainen et al unpublished) studied the health of winter sports athletes; from that study, we used baseline data from 17 young women, measured after the transition season when their training load was the lowest, ranging from 6 to 8 hours/week without high-intensity exercise.
Studies were conducted according to the Declaration of Helsinki and approved by the Ethics Committee of the Central Finland Health Care District (Calex; memo 22/8/2008 and 5/2009, EsmiRs; 9U/2018, Physique; 19U/2018) or the Ethics Committee of the University of Jyväskylä (NO RED-S 514/13.00.04.00/2021). Participants gave informed consent.
Age Categorization
We used age as a continuous variable and categorized the participants into 3 age groups, based on previous findings. REEadj plateaued at age 18.0 (95% CI: 16.8-19.2) and started to decline at age 46.5 (95% CI: 40.6-52.4), while TEEadj plateaued at 20.5 (95% CI: 19.8-21.2) and started to decline at 63.0 years (95% CI: 60.1-65.9) (1). We therefore assigned participants aged 17 to 21 years to group I, 22 to 39 years to group II, and 40 to 60 years to group III.
Hormonal and Menopausal Status
Participants in groups I and II were either naturally menstruating women at different menstrual cycle phases or hormonal contraception users. Whether REE varies slightly according to the menstrual cycle phase is still questionable (24). In group I, 12 participants reported using combination oral contraceptives, 1 used a hormonal ring, and detailed information on contraceptive use was unavailable for 5 participants. In group II, 8 participants used combination oral contraceptives, 3 used progestin-only oral contraception, and 3 used a hormonal intrauterine device. Based on primarily cross-sectional evidence, there appears to be no clear association between hormonal contraceptive use and REE (25).
We determined the menopausal status of group III women with the Stages of Reproductive Aging Workshop + 10 guidelines (26) using menstrual and serum FSH data: 11 were premenopausal (PRE), 8 were perimenopausal (PERI), 30 were postmenopausal (POST), and 10 were postmenopausal MHT users (MHT). We combined the PRE and PERI women into a PRE/PERI group to represent women with meaningful ovarian E2 production but performed a sensitivity analysis without the PERI women because E2 levels decline in perimenopause. One PRE/PERI woman used a hormonal intrauterine device. In the MHT group, 7 participants used oral E2 in combination with dydrogesterone (n = 5) or norethisterone acetate (n = 2). Two participants used an E2 patch containing norethisterone acetate or combined with oral dydrogesterone, and 1 used an E2-only patch. All participants had used MHT for at least 4 months, most having used it for years. Details concerning menopausal status determination and MHT preparations used by the women are in the Supplementary Data (27).
Sex Hormones
For sex hormone assessment, the serum was separated from fasting venous blood samples according to standard procedures and stored at −80 °C. E2 and FSH concentrations were measured for group III participants using enzyme-amplified chemiluminescence immunoassays (IMMULITE 2000 XPi, Siemens Medical Solution Diagnostics, Los Angeles, CA, USA). The analytical sensitivity for the E2 kit (catalog no. L2KE22, RRID:AB_2936944) is 0.055 nmol/L with an accurate reportable range of 0.073 to 7.342 nmol/L. The coefficient of variation in our lab using control samples has been 15%. We compared the used immunoassay method with liquid chromatography-mass spectrometry (HUSLAB, Helsinki University Hospital, Helsinki, Finland) and found a good correlation in all test samples (n = 166, r = 0.91). However, when the comparison was restricted to samples with E2 concentrations less than 0.1 nmol/L, as determined by with liquid chromatography-mass spectrometry, the correlation between methods was lower (n = 76, r = 0.42). The analytical sensitivity of the FSH kit (catalog no. L2KFS2, RRID:AB_2756389) is 0.1 IU/L, and the coefficient of variation in our lab has been 5%.
Body Composition
Body composition was assessed with dual-energy X-ray absorptiometry (DXA; DXA Prodigy, GE Lunar Corp., Madison, WI, USA). We calculated the appendicular lean mass index (ALMI) by scaling appendicular lean mass (kg) to height (m) squared to estimate the level of muscularity among participants.
Resting and Total Energy Expenditure
REE was measured in all studies using the same Vmax Encore 92 metabolic cart (Sensormedics, Yorba Linda, CA, USA) and ventilated hood in the same thermoneutral laboratory; the cart was calibrated accordingly before each measurement. The REE assessment details are in the Supplementary Data (27). Measurements were performed in the morning after overnight fasting, with resting periods of 0 to 30 minutes and measurement periods of 15 to 30 minutes. We excluded at least the first 5 minutes of measurement data; for all participants, we located a steady-state period of at least 5 minutes during which the coefficient of variation was 10% or less for V̇O2 and V̇CO2, and we calculated REE with the modified Weir equation (28). We made REE comparable between different-sized participants using residuals—the differences between measured and predicted values—from a linear regression model generated using the data of the study sample [Supplementary Table S1 (27)]:
Herein, we refer to residual REE as REEadj. We also built 3 alternative explanatory models [Supplementary Table S1 (27)]: the first included age as a covariate with FFM and FM; the second added ALMI to account for differences in muscularity (29); and, in the third, we added the study data collection period as a covariate to examine potential biases introduced by including data from 2 different time periods.
To assess TEE in the Calex study, the overnight fasting participants gave a urine sample and ingested a doubly labeled water dose of 1 g per kg of body mass (21). A second urine sample was collected after 4 to 6 hours and a third 14 days later. The samples were analyzed in triplicate using mass spectrometry (Metabolic Solutions Inc., Merrimack, NH, USA) at the University of Alabama. TEE was calculated as in Schoeller et al (30).
Statistical Analyses
We performed the statistical analyses using R 4.2.1 (31). The analytic code is available in the Supplementary Data (27). We report descriptive statistics as means with standard deviations or as medians with first and third quartiles, but we did not test group differences in order to preserve statistical power. We verified the model's assumptions before accepting the results and used an alpha level of .05 for statistical significance.
We estimated the association between age and REEadj and compared the measured REE and REEadj between the age groups with linear mixed-effect models using the nlme package (32), with family identification as a random effect. We also performed a sensitivity analysis using FFM, FM, and ALMI-adjusted REE residuals as the outcome. For the mother-daughter pairs, we first compared the REEadj and then TEE using the measured TEE as the outcome and FFM and FM as covariates. We estimated intraclass correlation coefficients using the psych package (33) with one-way random-effects models—intraclass correlation compares within- and between-pair variations, thereby expressing how strongly the mothers and daughters resemble each other.
Last, we used linear regression to compare the measured REE and REEadj between the menopause groups using the POST group as the reference. Given that body composition parameters may explain REE differently among women of different ages, we performed supporting analyses using measured REE as the outcome; menopause status or sex hormone concentrations as the explanatory variable; and FFM, FM, and age as covariates in separate regression models.
Results
Participant Characteristics
Table 1 shows participant characteristics and energy expenditures across age and menopause groups, and Supplementary Table S2 (27) shows the same in the mother-daughter pairs. Based on the descriptive statistics, group II women had higher FFM and lower FM. In group III, sex hormone concentrations varied between the menopause groups, as expected. As groups I and II included naturally menstruating women in different menstrual cycle phases and hormonal contraceptive users, we did not compare hormone levels between age groups.
Table 1.
Participant characteristics and energy expenditures according to age and menopause groups
| Age groups (n = 120) | Menopause groups (n = 59) | |||||
|---|---|---|---|---|---|---|
| I | II | III | PRE/PERI | MHT | POST | |
| Variable | n = 26 | n = 35 | n = 59 | n = 19 | n = 10 | n = 30 |
| Age, year | 19.8 (1.1) | 28.2 (4.3) | 53.8 (3.5) | 50.7 (4.3) | 55.1 (1.7) | 55.3 (1.9) |
| Sex hormone concentrations | ||||||
| E2, nmol/L | 0.29 (0.18-0.55) | 0.29 (0.17-0.38) | 0.09 (0.06-0.12) | |||
| FSH, IU/L | 8 (7-25) | 38 (34-61) | 80 (71-102) | |||
| Anthropometrics | ||||||
| Height, cm | 165.6 (5.6) | 165.3 (5.8) | 166.4 (5.0) | 168.1 (4.6) | 166.5 (5.6) | 165.3 (4.8) |
| Body mass, kg | 65.0 (10.5) | 64.5 (7.9) | 68.5 (9.1) | 69.1 (9.0) | 71.3 (12.3) | 67.1 (7.9) |
| BMI, kg/m2 | 23.7 (3.5) | 23.6 (2.0) | 24.7 (3.1) | 24.5 (3.2) | 25.7 (4.2) | 24.5 (2.7) |
| Fat-free mass, kg | 44.2 (5.0) | 49.8 (5.3) | 44.2 (4.2) | 45.6 (4.1) | 43.4 (4.2) | 43.6 (4.1) |
| Fat mass, kg | 20.9 (8.2) | 14.7 (5.8) | 24.2 (7.3) | 23.6 (7.8) | 27.9 (8.4) | 23.4 (6.4) |
| Appendicular lean mass, kg | 18.5 (2.6) | 21.8 (2.7) | 17.9 (1.9) | 18.4 (2.0) | 17.6 (1.9) | 17.6 (1.9) |
| Appendicular lean mass index | 6.7 (0.7) | 8.0 (0.8) | 6.5 (0.6) | 6.5 (0.5) | 6.4 (0.6) | 6.4 (0.6) |
| Body fat percentage, % | 31 (8) | 23 (7) | 36 (7) | 35 (8) | 40 (5) | 36 (6) |
| Resting energy expenditure | ||||||
| Measured, kcal/d | 1430 (110) | 1474 (146) | 1286 (124) | 1323 (105) | 1249 (143) | 1276 (128) |
| PredictedFFM & FM, kcal/d | 1339 (104) | 1442 (107) | 1345 (87) | 1372 (84) | 1335 (98) | 1332 (85) |
| PredictedFFM, FM & age, kcal/d | 1440 (103) | 1467 (94) | 1286 (92) | 1322 (86) | 1283 (115) | 1264 (84) |
| Total energy expenditure | n = 10 | n = 10 | ||||
| Measured, kcal/d | 2162 (310) | 2148 (236) | ||||
Data as means (SD) or medians (interquartile range).
Abbreviations: BMI, body mass index, E2, estradiol; MHT, menopausal hormone therapy; POST, postmenopause; PRE/PERI, pre- or perimenopause.
Age and Energy Expenditure
FFM and FM explained 47% of the REE variance, while the inclusion of age increased the adjusted R2 to 68%. Figure 2 illustrates how age impacts REE estimation by presenting the relationships between the predicted and measured REE values. Neither the ALMI nor information on the study data collection period improved the explanatory value. Supplementary Table S1 (27) presents the full results.
Figure 2.
The relationship between measured REE and predicted REE among the 120 participants. (A) REE predicted with FFM and FM and (B) also with age.Abbreviations: FFM, fat-free mass; FM, fat mass; REE, resting energy expenditure.
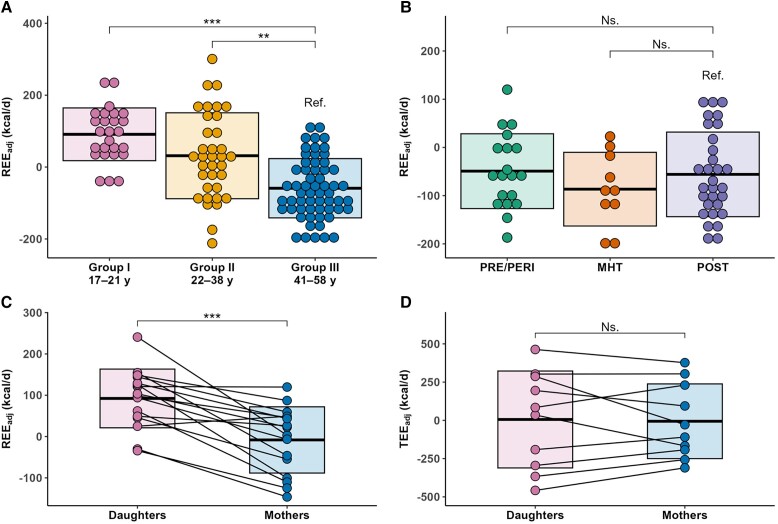
Age was inversely associated with REEadj (B = −3.9; 95% CI: −4.8 to −3.1; P < .001). Group I had 140 kcal/d (95% CI: 82-199) higher measured REE and 126 kcal/d (95% CI: 93-160) higher REEadj (Fig. 3A) than group III, while group II had 187 kcal/d (95% CI: 133-240) higher measured REE and 88 kcal/d (95% CI: 49-127) higher REEadj (P < .001 for all). The group differences in the FFM, FM, and ALMI-adjusted REE were slightly smaller [Supplementary Table S3 (27)].
Figure 3.
The association of age and fat-free mass and REEadj and TEEadj. (A) REEadj across age groups; (B) REEadj across menopause groups; (C) REEadj in 16 mother-daughter pairs; (D) TEEadj in 10 mother-daughter pairs. The boxplots show the mean and SD of each group.Abbreviations: REEadj, fat-free mass and fat mass–adjusted resting energy expenditure; TEEadj, fat-free mass and fat mass–adjusted total energy expenditure.
In the 16 mother-daughter pairs, the daughters had 100 kcal/d (95% CI: 63-138; P < .001) higher REEadj than their mothers (Fig. 3C). In the 10 pairs with REE and TEE measurements, the daughters had 85 kcal/d (95% CI: 45-125; P = .003) higher REEadj than their mothers, but there was no significant difference in TEEadj (B = 26 kcal/d; 95% CI: −128-180; P = .75, Fig. 3D). Intraclass correlation coefficients were 0.05 (95% CI: −0.43-0.52; P = .42) for REEadj, 0.83 (95% CI: 0.49-0.96; P < .001) for TEE and 0.92 (95% CI: 0.71-0.98; P < .001) for TEEadj [Supplementary Fig. S1 (27)].
Resting Energy Expenditure in Midlife
Compared with the POST group, the measured REE was not significantly different in either the PRE/PERI (B = 46 kcal/d; 95% CI: −26-119; P = .21) or MHT groups (B = −27 kcal/d; 95% CI: −118-63; P = .55); neither was REEadj (PRE/PERI; B = 7 kcal/d; 95% CI: −42-55; P = .78; MHT: B = −31 kcal/d; 95% CI: −91-30; P = .31, Fig. 3B). The exclusion of PERI women did not alter the results (data not shown). In the specific models generated for the middle-aged subsample that controlled for FFM, FM, and age, the MHT group had a lower REE than the POST group (Table 2). Furthermore, neither E2 nor FSH showed a statistically significant association with REE.
Table 2.
The associations between body composition, age, menopausal status, serum sex hormone concentrations, and resting energy expenditure in midlife (n = 59)
| Mass and age | Menopause status | E2 | FSH | |||||
|---|---|---|---|---|---|---|---|---|
| B | P-value | B | P-value | B | P-value | B | P-value | |
| Intercept | 850.1 | <.001 | 976.3 | <.001 | 838.6 | <.001 | 881.4 | <.001 |
| Fat-free mass, kg | 17.2 | <.001 | 17.2 | <.001 | 17.2 | <.001 | 17.4 | <.001 |
| Fat mass, kg | 6.6 | <.001 | 7.3 | <.001 | 6.7 | <.001 | 6.8 | <.001 |
| Age, year | −9.0 | .001 | −11.2 | <.001 | −8.9 | .005 | −10.1 | .001 |
| Menopause status | ||||||||
| PRE/PERI | −39.7 | .11 | ||||||
| MHT | −58.4 | .025 | ||||||
| E2, nmol/L | 5.2 | .89 | ||||||
| FSH, IU/L | 0.2 | .43 | ||||||
| R 2/adjusted R2 | 0.70/0.68 | 0.73/0.71 | 0.70/0.68 | 0.70/0.68 | ||||
Abbreviations: E2, estradiol; MHT, menopausal hormone therapy; POST, postmenopause; PRE/PERI, pre- or perimenopause.
Discussion
Aging through adulthood and menopause are thought to slow basal metabolism, potentially predisposing women to obesity. This study demonstrates that increasing age is associated with a decline in REEadj among young and middle-aged women, also after partly controlling for genetic background. However, menopause did not contribute to the age-associated decline in REEadj. Furthermore, E2 or FSH concentrations were not related to REEadj in middle-aged women.
The decline in age-associated REEadj aligns with previous cross-sectional studies by Pontzer et al (1), Geisler et al (3), and Siervo et al (4), in which the decline began in women at 46.5, 35.2, and 47 years of age, respectively. The turning point for REEadj therefore may occur before 60 years of age (1), but whether the phenomenon represents an actual slowing of cellular metabolism remains unclear. Low metabolic rate organs contribute more to FFM as we age (34), but aging also causes tissue quality changes; for example, the brain grey–white matter ratio (35) and skeletal muscle density (36) decline from young to middle adulthood, meaning that each kilogram of the brain or muscle tissue has fewer metabolically active cells as aging proceeds. Current body composition assessment methods, like DXA, cannot detect such changes (34), so FFM adjustments that assume FFM composition and quality are constant may overestimate the age-associated decline in REEadj. Indeed, Roubenoff et al (37) found no association between age and FFM-adjusted REE when assessing FFM using total body potassium analysis, which directly estimates cell mass. Therefore, the direct influence of age on REEadj may be less impactful than initially observed, but it may enhance REE estimation (as shown in Fig. 2B) by enabling adjustments for age-related changes in body composition within the model. If slowing cellular metabolism contributes to the observed decline in REEadj, it may result from altered systemic hormone and cytokine stimulation (34), with intrinsic changes in hormone responsiveness, protein synthesis, maintenance of membrane potentials, and mitochondrial function (38).
We also compared the TEEadj of middle-aged mothers and their daughters and found that they were similar, despite differences in their REEadj, indicating that TEE is highly heritable (39). This also suggests that the possible age-associated decline in REEadj may have a negligible effect on TEE before late adulthood, as also reported by Pontzer et al (1).
Our findings concur with previous research that menopausal status and sex hormone levels do not robustly determine REEadj during midlife. Although ovarian hormone suppression studies in premenopausal women have shown some yet not fully convincing evidence of a REEadj decline (40, 41), MHT interventions show no clear effect on REE in postmenopause (15, 17, 18). In the present study, the MHT users had even lower REE than postmenopausal women who did not use MHT after adjusting for FFM, FM, and age. However, given that the MHT group was the smallest in our study, this difference is unlikely to be attributed to MHT use per se. Observational evidence also indicates that menopause has a minimal impact on REEadj. For instance, Lovejoy et al (11) found that sleeping energy expenditure and TEEadj decreased in women transitioning from premenopause to postmenopause during a 4-year follow-up, but the changes were no different from participants who remained premenopausal, which suggests that the decreases were related to aging, not menopause. Furthermore, the menopausal transition was not associated with REE in the longitudinal MONET study (12), and cross-sectional studies have shown no association between menopausal status and measured REE (14, 16), FFM-adjusted REE (13, 15), or FFM-adjusted TEE (42), while a study that did show a higher REE in MHT users than nonusers (14) failed to adjust the analyses for differing tissue masses. Finally, Pontzer et al (1) found no differences in REEadj and TEEadj trajectories between middle-aged women and men, indicating that sex-specific changes in energy expenditure are not observed during midlife.
The lack of a clear association between menopause and REEadj is unexpected because the cessation of reproductive functions and altered hormonal profile should decrease basal metabolism. There are at least 3 potential explanations; first, with the limitations of body composition assessment and indirect calorimetry methods (34), the energy expenditure of female reproductive processes may be so small relative to other functions contributing to REE that its loss is difficult to detect. The second explanation is that the effects of menopause cannot be disentangled from the effects of aging, especially because aging progresses differently between individuals, although this is insufficient to explain why MHT interventions do not increase REE (15, 17, 19). The third, more speculative explanation is that women reallocate energy during menopause from reproduction to other purposes; Hazda hunter-gatherer women, for example, increase the time spent on gathering resources for their offspring (43), potentially reallocating the freed energy to movement and maintenance of the locomotor system (44). Women in industrialized nations live differently and may therefore use the energy to build energy reserves and bodily defense mechanisms, reallocating the freed energy inside the REE component (44). Increased FM, especially to the trunk, may further promote metabolic deterioration (45), inflammation (46), and sympathetic nervous system activity (47), thereby further elevating the REE (48–51). Such trade-offs could explain why women's REEadj does not decline and their cardiometabolic risk profile worsens after menopause in industrialized societies (52).
Finally, it should be mentioned that a decrease in absolute REE following skeletal muscle loss could also contribute to menopause-associated FM accumulation if women do not match the drop by reducing energy intake. We previously reported that a peri- to postmenopausal transition during a mean follow-up of 14 months resulted in a 0.2 kg lean mass loss, likely from skeletal muscle (53); but, assuming that the mass-specific metabolic rate of skeletal muscle is 13 kcal/d at rest (54), the loss would reduce REE by 2.6 kcal/d, which cannot explain the 0.8 kg increase in FM (6), especially as the tissue changes happen gradually.
The main limitations of this study are its cross-sectional and secondary nature. We pooled existing studies to generate a sufficiently large sample and cannot entirely exclude a clustering effect. Considering the impact of sex hormones, we did not control for the use of hormonal products or menstrual cycle phases. Furthermore, serum E2 concentrations were analyzed with immunoassays, whose accuracy is limited for the low E2 levels seen post-menopause. Although we could not compare physical activity differences across datasets due to the lack of a uniform assessment method, we assume that group II had the highest physical activity levels. Long-term physical activity may lower REE (55), but the effects of exercise training are unclear (56). If long-term physical activity reduces REE, the REEadj of group II would be an underestimation. However, REEadj was still higher in group II than in group III, suggesting that physical activity differences are unlikely to affect the validity of our conclusions.
In conclusion, REE adjusted for DXA-measured FFM and FM declines in women from young to middle adulthood, likely due to aging rather than menopause, but whether falling cellular metabolic rates contribute is unclear. Current evidence does not support the inference that menopause reduces REE. Longitudinal data from middle-aged women with differing sex hormone trajectories are needed to reconcile whether menopause truly affects REE in a meaningful way.
Acknowledgments
We thank the participants for their time and effort and the staff of the Faculty of Sport and Health Sciences of the University of Jyväskylä.
Contributor Information
Jari E Karppinen, Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland.
Petri Wiklund, Huawei Helsinki Research Center, Huawei Technologies Oy (Finland) Co. Ltd, Helsinki, Finland.
Johanna K Ihalainen, Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland.
Hanna-Kaarina Juppi, Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland; Gerontology Research Center, University of Jyväskylä, Jyväskylä, Finland.
Ville Isola, Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland.
Matti Hyvärinen, Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland; Gerontology Research Center, University of Jyväskylä, Jyväskylä, Finland.
Essi K Ahokas, Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland.
Urho M Kujala, Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland.
Jari Laukkanen, Central Finland Health Care District, Jyväskylä, Finland; Institute of Clinical Medicine, University of Eastern Finland, Kuopio, Finland.
Juha J Hulmi, Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland.
Juha P Ahtiainen, Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland.
Sulin Cheng, Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland; Exercise Translational Medicine Centre, Shanghai Jiao Tong University, Shanghai, China.
Eija K Laakkonen, Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland; Gerontology Research Center, University of Jyväskylä, Jyväskylä, Finland.
Funding
Supported by the Academy of Finland Grants 309504, 314181, 335249 (to E.K.L.) and 135038 (to S.C.) and by the Ministry of Education and Culture of Finland Grants OKM/54/627/2006, OKM/77/627/2008 (to S.C.) and OKM/10/626/2021 (to J.K.I.). The manuscript preparation was supported by the Academy of Finland Grant 330281 (to E.K.L.). The contents of this article are solely the responsibility of the authors.
Author Contributions
The authors’ responsibilities were as follows. J.E.K. conducted EsmiRs study measurements, gave guidance for the Physique and NO RED-S studies, analyzed data, and wrote the manuscript. E.K.L. led and designed the EsmiRs study with the help of J.E.K., H.-K.J., M.H., U.M.K, and J.L. P.W. conducted Calex study measurements and gave guidance for the EsmiRs study. S.C. led and designed the Calex study with help from P.W. J.P.A. and J.H. led and designed the Physique study with the help of V.I., who also participated in data collection. J.K.I. led, designed, and conducted data in the NO RED-S study with the help of E.K.A. All authors revised the manuscript and approved its final version.
Disclosures
The authors report no conflicts of interest.
Data Availability
Datasets generated during and/or analyzed during the current study are not publicly available but are available from the principal investigators of each study [Calex: C.S. and E.K.L., EsmiRs (doi.10.17011/jyx/dataset/83491): E.K.L., Physique: J.P.A., and NO RED-S: J.K.I.] on reasonable request.
References
- 1. Pontzer H, Yamada Y, Sagayama H, et al. Daily energy expenditure through the human life course. Science. 2021;373(6556):808‐812. doi: 10.1126/science.abe5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Müller MJ, Bosy-Westphal A, Klaus S, et al. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am J Clin Nutr. 2004;80(5):1379‐1390. doi:10.1093/ajcn/80.5.1379 [DOI] [PubMed] [Google Scholar]
- 3. Geisler C, Braun W, Pourhassan M, et al. Gender-specific associations in age-related changes in resting energy expenditure (REE) and MRI measured body composition in healthy Caucasians. J Gerontol A Biol Sci Med Sci. 2016;71(7):941‐946. doi: 10.1093/gerona/glv211 [DOI] [PubMed] [Google Scholar]
- 4. Siervo M, Oggioni C, Lara J, et al. Age-related changes in resting energy expenditure in normal weight, overweight and obese men and women. Maturitas. 2015;80(4):406‐413. doi: 10.1016/j.maturitas.2014.12.023 [DOI] [PubMed] [Google Scholar]
- 5. Greendale GA, Sternfeld B, Huang M, et al. Changes in body composition and weight during the menopause transition. JCI Insight. 2019;4(5):e124865. doi: 10.1172/jci.insight.124865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juppi HK, Sipilä S, Fachada V, et al. Total and regional body adiposity increases during menopause-evidence from a follow-up study. Aging Cell. 2022;21(6):e13621. doi: 10.1111/acel.13621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu Y, López M. Central regulation of energy metabolism by estrogens. Mol Metab. 2018;15:104‐115. doi: 10.1016/j.molmet.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klinge CM. Estrogenic control of mitochondrial function. Redox Biol. 2020;31:e101435. doi: 10.1016/j.redox.2020.101435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu P, Ji Y, Yuen T, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546(7656):107‐112. doi: 10.1038/nature22342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mattick LJ, Bea JW, Singh L, et al. Serum follicle-stimulating hormone and 5-year change in adiposity in healthy postmenopausal women. J Clin Endocrinol Metab. 2022;107(8):e3455‐e3462. doi: 10.1210/clinem/dgac238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). 2008;32(6):949‐958. doi: 10.1038/ijo.2008.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duval K, Prud’homme D, Rabasa-Lhoret R, et al. Effects of the menopausal transition on energy expenditure: a MONET group study. Eur J Clin Nutr. 2013;67(4):407‐411. doi: 10.1038/ejcn.2013.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hodson L, Harnden K, Banerjee R, et al. Lower resting and total energy expenditure in postmenopausal compared with premenopausal women matched for abdominal obesity. J Nutr Sci. 2014;3:e3. doi: 10.1017/jns.2013.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Monda V, Salerno M, Fiorenzo M, et al. Role of sex hormones in the control of vegetative and metabolic functions of middle-aged women. Front Physiol. 2017;8:e773. doi: 10.3389/fphys.2017.00773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bessesen DH, Cox-York KA, Hernandez TL, et al. Postprandial triglycerides and adipose tissue storage of dietary fatty acids: impact of menopause and estradiol. Obesity (Silver Spring). 2015;23(1):145‐153. doi: 10.1002/oby.20935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gould LM, Gordon AN, Cabre HE, et al. Metabolic effects of menopause: a cross-sectional characterization of body composition and exercise metabolism. Menopause. 2022;29(4):377‐389. doi: 10.1097/GME.0000000000001932 [DOI] [PubMed] [Google Scholar]
- 17. Marlatt KL, Lovre D, Beyl RA, et al. Effect of conjugated estrogens and bazedoxifene on glucose, energy and lipid metabolism in obese postmenopausal women. Eur J Endocrinol. 2020;183(4):439‐452. doi: 10.1530/EJE-20-0619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson EJ, Lavoie HB, Strauss CC, Hubbard JL, Sharpless JL, Hall JE. Body composition and energy balance: lack of effect of short-term hormone replacement in postmenopausal women. Metabolism. 2001;50(3):265‐269. doi: 10.1053/meta.2001.21015 [DOI] [PubMed] [Google Scholar]
- 19. Lwin R, Darnell B, Oster R, et al. Effect of oral estrogen on substrate utilization in postmenopausal women. Fertil Steril. 2008;90(4):1275‐1278. doi: 10.1016/j.fertnstert.2007.07.1317 [DOI] [PubMed] [Google Scholar]
- 20. Ojanen X, Cheng R, Törmäkangas T, et al. Towards early risk biomarkers: serum metabolic signature in childhood predicts cardio-metabolic risk in adulthood. EBioMedicine. 2021;72:e103611. doi: 10.1016/j.ebiom.2021.103611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu Z, Völgyi E, Wang R, et al. Comparison of heart rate monitoring with indirect calorimetry for energy expenditure evaluation. J Sport Health Sci. 2012;1(3):178‐183. doi: 10.1016/j.jshs.2012.07.004 [DOI] [Google Scholar]
- 22. Karppinen JE, Juppi HK, Hintikka J, et al. Associations of resting and peak fat oxidation with sex hormone profile and blood glucose control in middle-aged women. Nutr Metab Cardiovasc Dis. 2022;32(9):2157‐2167. doi: 10.1016/j.numecd.2022.06.001 [DOI] [PubMed] [Google Scholar]
- 23. Isola V, Hulmi JJ, Petäjä P, Helms ER, Karppinen JE, Ahtiainen JP. Weight loss induces changes in adaptive thermogenesis in female and male physique athletes. Appl Physiol Nutr Metab. 2023;48(4):307‐320. doi: 10.1139/apnm-2022-0372 [DOI] [PubMed] [Google Scholar]
- 24. Benton MJ, Hutchins AM, Dawes JJ. Effect of menstrual cycle on resting metabolism: a systematic review and meta-analysis. PLoS One. 2020;15(7):e0236025. doi: 10.1371/journal.pone.0236025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Metz L, Isacco L, Redman LM. Effect of oral contraceptives on energy balance in women: a review of current knowledge and potential cellular mechanisms. Metabolism. 2022;126:e154919. doi: 10.1016/j.metabol.2021.154919 [DOI] [PubMed] [Google Scholar]
- 26. Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19(4):387‐395. doi: 10.1097/gme.0b013e31824d8f40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karppinen JE, Wiklund P, Ihalainen JK, et al. Supplemental data for: Age but not menopausal status is linked to lower resting energy expenditure. Uploaded May 12, 2023. doi: 10.5281/zenodo.7927490 [DOI] [PMC free article] [PubMed]
- 28. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition. 1990;6(3):213‐221. doi: 10.1113/jphysiol.1949.sp004363 [DOI] [PubMed] [Google Scholar]
- 29. Heymsfield SB, Smith B, Chung EA, et al. Phenotypic differences between people varying in muscularity. J Cachexia Sarcopenia Muscle. 2022;13(2):1100‐1112. doi: 10.1002/jcsm.12959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jéquier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol. 1986;250(5 Pt 2):R823‐R830. 10.1152/ajpregu.1986.250.5.R823 [DOI] [PubMed] [Google Scholar]
- 31. R Core Team . R: a language and environment for statistical computing. Published online 2022.
- 32. Pinheiro J, Bates D, DebRoy S, et al. nlme: linear and nonlinear mixed effects models. Published online March 25, 2022. Accessed May 16, 2022. https://CRAN.R-project.org/package=nlme
- 33. Revelle W. psych: procedures for psychological, psychometric, and personality research. Published online May 10, 2022. Accessed May 16, 2022. https://CRAN.R-project.org/package=psych
- 34. Müller MJ, Geisler C, Hübers M, Pourhassan M, Braun W, Bosy-Westphal A. Normalizing resting energy expenditure across the life course in humans: challenges and hopes. Eur J Clin Nutr. 2018;72(5):628‐637. doi: 10.1038/s41430-018-0151-9 [DOI] [PubMed] [Google Scholar]
- 35. Giorgio A, Santelli L, Tomassini V, et al. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51(3):943‐951. doi: 10.1016/j.neuroimage.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lauretani F, Bandinelli S, Bartali B, et al. Axonal degeneration affects muscle density in older men and women. Neurobiol Aging. 2006;27(8):1145‐1154. doi: 10.1016/j.neurobiolaging.2005.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roubenoff R, Hughes VA, Dallal GE, et al. The effect of gender and body composition method on the apparent decline in lean mass-adjusted resting metabolic rate with age. J Gerontol A Biol Sci Med Sci. 2000;55(12):M757‐M760. doi: 10.1093/gerona/55.12.m757 [DOI] [PubMed] [Google Scholar]
- 38. Heymsfield SB, Smith B, Dahle J, et al. Resting energy expenditure: from cellular to whole-body level, a mechanistic historical perspective. Obesity (Silver Spring). 2021;29(3):500‐511. doi: 10.1002/oby.23090 [DOI] [PubMed] [Google Scholar]
- 39. Joosen AMCP, Gielen M, Vlietinck R, Westerterp KR. Genetic analysis of physical activity in twins. Am J Clin Nutr. 2005;82(6):1253‐1259. doi: 10.1093/ajcn/82.6.1253 [DOI] [PubMed] [Google Scholar]
- 40. Melanson EL, Gavin KM, Shea KL, et al. Regulation of energy expenditure by estradiol in premenopausal women. J Appl Physiol (1985). 2015;119(9):975‐981. doi: 10.1152/japplphysiol.00473.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gavin KM, Melanson EL, Hildreth KL, Gibbons E, Bessesen DH, Kohrt WM. A randomized controlled trial of ovarian suppression in premenopausal women: no change in free-living energy expenditure. Obesity (Silver Spring). 2020;28(11):2125‐2133. doi: 10.1002/oby.22978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tooze JA, Schoeller DA, Subar AF, Kipnis V, Schatzkin A, Troiano RP. Total daily energy expenditure among middle-aged men and women: the OPEN study. Am J Clin Nutr. 2007;86(2):382‐387. doi: 10.1093/ajcn/86.2.382 [DOI] [PubMed] [Google Scholar]
- 43. Hawkes K, O’Connell JF, Blurton Jones NG. Hadza women’s time allocation, offspring provisioning, and the evolution of long postmenopausal life spans. Curr Anthropol. 1997;38(4):551‐577. doi: 10.1086/204646 [DOI] [Google Scholar]
- 44. Lieberman DE, Kistner TM, Richard D, Lee IM, Baggish AL. The active grandparent hypothesis: physical activity and the evolution of extended human healthspans and lifespans. Proc Natl Acad Sci U S A. 2021;118(50):e2107621118. doi: 10.1073/pnas.2107621118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol. 1997;145(7):614‐619. doi: 10.1093/oxfordjournals.aje.a009158 [DOI] [PubMed] [Google Scholar]
- 46. Timpson NJ, Nordestgaard BG, Harbord RM, et al. C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. Int J Obes (Lond). 2011;35(2):300‐308. doi: 10.1038/ijo.2010.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scherrer U, Randin D, Tappy L, Vollenweider P, Jéquier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89(6):2634‐2640. doi: 10.1161/01.cir.89.6.2634 [DOI] [PubMed] [Google Scholar]
- 48. Geisler C, Braun W, Pourhassan M, et al. Age-dependent changes in resting energy expenditure (REE): insights from detailed body composition analysis in normal and overweight healthy Caucasians. Nutrients. 2016;8(6):E322. doi: 10.3390/nu8060322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bosy-Westphal A, Wolf A, Bührens F, et al. Familial influences and obesity-associated metabolic risk factors contribute to the variation in resting energy expenditure: the Kiel Obesity Prevention Study. Am J Clin Nutr. 2008;87(6):1695‐1701. doi: 10.1093/ajcn/87.6.1695 [DOI] [PubMed] [Google Scholar]
- 50. Calton EK, Pathak K, Soares MJ, et al. Vitamin D status and insulin sensitivity are novel predictors of resting metabolic rate: a cross-sectional analysis in Australian adults. Eur J Nutr. 2016;55(6):2075‐2080. doi: 10.1007/s00394-015-1021-z [DOI] [PubMed] [Google Scholar]
- 51. Soares MJ, Zhao Y, Calton EK, Pathak K. Triglycerides and systolic blood pressure negatively mediate the direct relationship of vitamin D status to resting energy expenditure: a cross sectional analysis. Diabetes Metab Syndr. 2022;16(12):102664. doi: 10.1016/j.dsx.2022.102664 [DOI] [PubMed] [Google Scholar]
- 52. Maas AHEM, Rosano G, Cifkova R, et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur Heart J. 2021;42(10):967‐984. doi: 10.1093/eurheartj/ehaa1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Juppi HK, Sipilä S, Cronin NJ, et al. Role of menopausal transition and physical activity in loss of lean and muscle mass: a follow-up study in middle-aged Finnish women. J Clin Med. 2020;9(5):E1588. doi: 10.3390/jcm9051588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Elia M. Organ and tissue contribution to metabolic rate. In: Kinney JM, Tucker HN eds. Energy Metabolism: Tissue Determinants and Cellular Corollaries. Raven Press; 1992:61‐79. [Google Scholar]
- 55. Pontzer H. Energy constraint as a novel mechanism linking exercise and health. Physiology (Bethesda). 2018;33(6):384‐393. doi: 10.1152/physiol.00027.2018 [DOI] [PubMed] [Google Scholar]
- 56. MacKenzie-Shalders K, Kelly JT, So D, Coffey VG, Byrne NM. The effect of exercise interventions on resting metabolic rate: a systematic review and meta-analysis. J Sports Sci. 2020;38(14):1635‐1649. doi: 10.1080/02640414.2020.1754716 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets generated during and/or analyzed during the current study are not publicly available but are available from the principal investigators of each study [Calex: C.S. and E.K.L., EsmiRs (doi.10.17011/jyx/dataset/83491): E.K.L., Physique: J.P.A., and NO RED-S: J.K.I.] on reasonable request.